Hans Charles Freeman 1929–2008
Trevor W. Hambley A * and Ian D. Rae BA School of Chemistry, University of Sydney, NSW 2006, Australia.
B School of Chemistry, University of Melbourne, Vic. 3010, Australia.
Historical Records of Australian Science 33(2) 180-190 https://doi.org/10.1071/HR21011
Published: 7 June 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of the Australian Academy of Science. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Hans Freeman was born in Germany and arrived in Australia with his parents in 1938. A brilliant student at the University of Sydney, he spent a seminal year at the California Institute of Technology before joining the staff at Sydney and initiating research on bioinorganic chemistry, studying metal ion complexes of compounds of biological significance such as amino acids, peptides and proteins. In his use of X-ray crystallography he was a pioneer in Australia, constructing his first crystallographic apparatus and mastering the necessary computing, at first by hand but soon with electronic computers. The culmination of his work with a series of collaborators was the structure of the blue, copper-containing metalloprotein, plastocyanin. Freeman also employed another advanced technique—X-ray spectroscopy and the study of X-ray absorption fine structure. He was a leading figure in Australia and internationally, and played an important role in gaining access for Australian scientists to international facilities such as synchrotron radiation sources at the dawning of the era of ‘Big Science’.
Keywords: bioinorganic chemistry, copper proteins, metal complexes, plastocyanin, synchrotron radiation, XAFS, X‐ray crystallography.
Introduction1
Hans Freeman was born in 1929 in Breslau, Germany,2 to Karl Heinz Friedmann (later Karl Hans Freeman) (1901–58) and his wife Friederike Pauline Lotte née Friedensohn (1902–53) daughter of Eugen and Johanna née Prager. Hans’ sister Evie (Eva) was born in 1932. Their father, Karl, was an expert in the chemistry and technology of detergents, a then-modern field, in which he was established in Germany. Being Jewish, however, the Friedmann family would have been increasingly threatened, and following advice from a local Nazi Party member, they emigrated to Australia in 1938. Karl’s skills quickly gained him work in the same industry in Australia, where he contributed to the war effort by devising a way to remove blood from soiled blankets that would otherwise have been discarded. After the war he went on to establish the soap and detergent manufacturing company, K. H. Freeman Pty Ltd. After his father’s death in 1958, Hans and his friend Ken Seale became directors of the company.3
The family members were quick to adapt to the lifestyle in Australia, different in many ways to the one they had left behind. Hans was soon proficient in English, so much so that he was dux of his year at Double Bay Public School. He later headed his class at Sydney Boys High School, where his final-year studies were guided by Len Basser, a legendary teacher whose protégés included ten students who later became professors, eight of them Fellows of the Royal Society of London.4 Hans began his chemical career as a week-end assistant in the family business making detergents and other chemicals.
Enrolling for a science degree at the University of Sydney, Freeman graduated BSc in 1949 with First Class Honours and the University Medal in chemistry (Fig. 1). Under the supervision of R. J. W. Le Fèvre he undertook research with organic chemical substances and was co-author of seven papers published in the Journal of the Chemical Society.5 His MSc degree was awarded in 1952,6 and in October of that year he was awarded a Rotary Foundation Fellowship for postgraduate study abroad that enabled him to spend a year at the California Institute of Technology (Caltech) at Pasadena, California.

|
At Caltech
Most Australian chemists at that time, seeking overseas experience, went to Great Britain,7 a choice fostered by traditional ties including the fact many chemistry staff members had been recruited from that country, including Freeman’s supervisor, Le Fèvre. Freeman may well have taken advice from another staff member at Sydney, D. P. Mellor, who spent a sabbatical leave at Caltech 1937–8 and returned to Sydney with a fresh outlook on chemistry that he attributed to his interactions with Linus Pauling.8 It was there, some 15 years later, that Pauling, whose PhD thesis at Caltech in 1925 was entitled ‘The determination with X-rays of the structure of crystals’ and who retained a lifelong interest in the technique, suggested to Freeman that he take up the study of X-ray crystallography and work with his associate, the crystallographer E. W. (Eddie) Hughes. Hughes was a pioneer of numerical methods in crystallography, introducing the method of least squares using high-speed computers for structure refinement, and later developing direct methods for solving crystal structures.9 Freeman’s work with Hughes, published some years later, was on the structure of biuret (NH2–CO–NH–CO–NH2) and its interactions with copper ions that were the basis of the ‘biuret test’ that involved the binding of copper ions to the peptide group (–NH–CO–) in proteins.10
The interest of the Pauling laboratory in the determination of crystal structures by X-ray diffraction was enriched, for Freeman, by two significant meetings. The first was a workshop held to inform industrial associates of Caltech about progress in the elucidation of protein structures, and the second was a Pasadena conference on the structure of proteins held in September 1953 that was attended by luminaries (and eventual Nobel laureates), Pauling, Bragg, Wilkins, Watson, Crick, Perutz and Kendrew and described as ‘the most successful ever held in this field’.11
Return to Sydney
Freeman returned to Sydney to a lectureship in chemistry and the opportunity to undertake research for his PhD degree under the supervision of La Fèvre. It was expected that, on his return, Freeman would resume research in the field of his MSc, but his time at Caltech was transformative and he was determined to continue with crystallographic studies. This was despite a lack of suitable equipment and some resistance from those who felt that such major undertakings were beyond the resources accessible to Australian scientists. His aim with the crystallographic work was to explore the function of metals in biological systems by studying the crystal structures of metal complexes with simple biological ligands, continuing his work with biuret that was published in 1959.12 Freeman’s work at Caltech and in his first few years back in Sydney, where he completed his PhD degree,13 was to set the pattern for much of his later research involving proteins and metal ions. A short paper in Nature in 1959 included structures for potassium bisbiuretocuprate(II) tetrahydrate, K2[Cu(NHCONHCONH)2]·4H2O, and bis(biuret)copper(II) dichloride, [Cu(NH2CONHCONH2)2Cl2], the details of which were published later.14 At first, the necessary calculations were carried out by hand, but Freeman was able to write programs for SILLIAC, a vacuum-tube computer based on designs from the University of Illinois and constructed at the University of Sydney that became operational in 1956.15 Freeman constructed his original diffractometer, based on Weissenberg geometry and driven by a PDP8-5 computer that was programmed by his students. Structures were solved by hand and with limited computing facilities at the university, but by the late 1960s access was gained to Commonwealth Scientific and Industrial Research Organisation (CSIRO) computers. What followed, and continued throughout the next decades, was a sustained effort to develop ever newer and better crystallographic facilities. Collecting and building equipment to conduct research at the highest level was expensive and Freeman received generous support in a series of grants from the United States National Institutes of Health.
X-ray diffraction studies: amino acids and peptides
Over the next decade, Freeman and his students determined the structures of many metal complexes of amino acids and peptides (a full bibliography is available in ‘Supplementary material’), adopting new developments in X-ray diffraction as they became available. The subjects of the investigations were increasingly described as model compounds for metal–protein interactions and they formed a major part of the review of metal complexes of amino acids and peptides he published in 1967.16 This field of chemistry was attracting much attention and Freeman established an international presence through his published work and his appointment as a George Ellery Hale Research Fellow at Caltech (1958), visiting professor at the University of Göteborg (1966) and at Yale University (1968). He was successively promoted, becoming a reader in 1964 and in 1971, upon the retirement of Le Fèvre, Freeman was appointed to the newly-created Chair of Inorganic Chemistry at the University of Sydney. He remained in this role until his retirement during which time he established and developed the Department of Inorganic Chemistry within the School of Chemistry. The department was initially staffed by a small number of established inorganic chemists and by former students of Le Fèvre who redirected their research efforts toward inorganic chemistry with Freeman’s support. In the following years Freeman recruited numerous staff who would go on to take leadership roles in chemistry nationally and internationally. Freeman served for some years as the head of school, and following his retirement he was president of the University of Sydney Association of Professors, 2006–7.
X-ray diffraction studies: metallo-proteins
In his 1967 review of crystal structures of metal–peptide complexes, Freeman raised the question of whether the structure of a molecule in a crystalline solid was the same as that of the same molecule in solution, and observed that ‘at first sight this imposes a severe handicap on those crystallographers who claim that their work is biochemically motivated’, especially those who had made ‘the assumption that such complexes act as models for the metal-binding sites of proteins’.17 Few crystal structures of biological molecules were available against which the question could be tested, but their value as models was a theme that he developed further in 1970, in a major review of the field of metal complexes of amino acids and peptides in which it can be read that he saw the need to tackle the more difficult crystallography of metal containing proteins,18 as follows:
The claim has often been made that complexes of the type which have been discussed in this chapter are ‘of biological interest’. In a few cases it is true that a particular complex turns up in some biological system. In many more cases the phrase ‘of biological interest’ expresses the hope that the systematic study of many complexes will contribute to the discovery of the rules which govern the interactions between metal ions and naturally occurring ligands. Many naturally occurring ligands are tremendously complicated molecules. The ligands whose complexes can be systematically studied are relatively simple. A metal–peptide complex may have some properties in common with a metal–protein complex. So far as these properties alone are concerned, the simple complex is a ‘model compound’ for the biological interaction. But we must remember that it is a model—not a replica.19
Freeman attributed this warning to Professor B. G. Malmström who had mentioned it in his plenary address to the ‘International conference on coordination chemistry’ held in Sydney in 1969.20 Referring to his own and others’ studies of enzymes, Malmström suggested ‘that in these enzymes the “unique” coordination imparts properties to the metal ion facilitating its role in the catalytic reaction,’21 thus amply illustrating ‘the thesis that the metal-ion coordination in the active sites of metalloenzymes shows many unusual features compared to the simple complexes generally studied by coordination chemists … but the view seems only gradually to have won wider acceptance … [until] … recently some of the investigators earlier relying most heavily on model arguments have also expressed similar ideas’.22 Freeman went on to examine the reported arrangements of the ligands around a zinc atom in the enzyme carboxypeptidase A that was more fully described in another chapter in Eichhorn’s book.23
Freeman continued the study of the model compounds—metal complexes of small molecules of biological relevance—while the group prepared for the major assault on the structure of the copper-containing protein, plastocyanin, which was found in many plants species but at such low concentrations that its intense blue colour, due to an absorption maximum near 600 nm with an extinction coefficient one or two orders of magnitude greater than those of other Cu(II) complexes, goes unnoticed. Its molecular weight is relatively low (approximately 10 500 Dalton) and there is only one copper atom per molecule, which is involved in electron transfer reactions mediated by changes between Cu(I) and Cu(II).24
In pursuit of protein crystallography, Freeman made two important appointments to his group in 1975. The first was Mitchell Guss, who followed his PhD studies with Freeman (1967–9) with postdoctoral appointments in Britain and the United States before rejoining Freeman as a professional officer whose responsibilities included equipment and computing in the X-ray diffraction laboratory, a role he continued as a research fellow from 1991 until 1994 when Freeman retired. Guss then moved to the Department of Biochemistry, where he established his own X-ray diffraction laboratory but also continued to collaborate with Freeman. He became Professor of Structural Biology in the university’s School of Molecular Bioscience in 2006. The second was Peter Colman, an Adelaide graduate and already an experienced crystallographer who was studying as a postdoctoral fellow at the Max Planck Institute in Munich when in 1973 he attended a conference of the International Union for Biochemistry in Stockholm at which he met Freeman, who suggested that he consider joining him in Sydney where work had begun on obtaining crystals of plastocyanin suitable for X-ray crystallography.25 ‘I had more-or-less given up on the idea of a return to Australia’, Colman said, ‘believing that the capital investment needed to support a protein crystallography lab was still beyond’ the reach of organisations supporting research in Australia such as the Australian Research Council and the National Health and Medical Research Council.26 With support of a Queen Elizabeth II fellowship, Colman moved to Sydney in 1975, continuing his own work and collaborating with Freeman and Guss, until in 1978 he moved to the CSIRO Division of Protein Chemistry, becoming the inaugural chief of the Division of Biomolecular Engineering.27
The group, which also included biochemists skilled in the separation of proteins from complex mixtures, extracted plastocyanin from thirteen plant species, among them common vegetables and garden plants, and both Cu(I) and Cu(II) species were purified by standard methods (Fig. 2). Only three plants yielded crystalline products—French bean, cucumber and oleander—but in none of the six cases was a crystal obtained that was suitable for diffraction experiments, a dramatic example of the importance of the art of crystal-growing to the success of X-ray studies.28 Finally, crystals suitable for structure analysis (Fig. 3) were obtained from leaves collected by John Ramshaw from common poplar trees (Populus nigra var. italica) growing at St John’s College oval adjacent to the Sydney campus. The preliminary three-dimensional crystallographic data (resolution of 2.5 Å) for a Cu(II) plastocyanin and isomorphous heavy-atom derivatives were published (Fig. 4).29

|
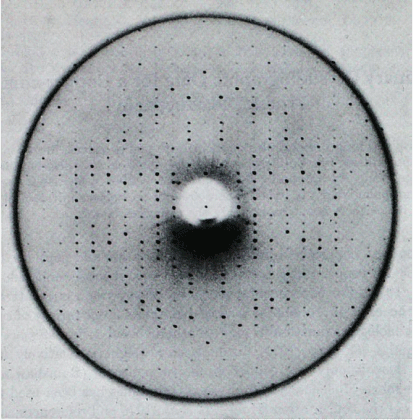
|
A preliminary structure of the Cu(II) species was reported at a conference in September 1977,30 prompting a comment that ‘Plastocyanin now seems to be fully accepted as a major electron carrier in chloroplasts and at the meeting the results of high resolution X-ray analysis was reported for the first time by H. C. Freeman (University of Sydney)’.31 A sketch of the protein structure (Fig. 5), drawn by Freeman during a telephone conversation with Mitchell Guss, was presented at the conference, and the published article contains a version of the sketch with some amino-acid locations identified. Further details appeared the following year.32 Plastocyanin was described as a blue or type-I copper protein in which ‘the copper atom has highly distorted tetrahedral coordination geometry’, coordinated by two sulfur atoms, one the thiol of a cysteine residue and the other a methionine thioether, and two histidine imidazole nitrogen atoms. The geometry and the nature of the ligands were described by the authors as ‘not surprising’ and were consistent with physical and spectroscopic properties that had been reported for plastocyanin by other researchers. Refinement of the plastocyanin structure at 1.6 Å resolution produced more precise interatomic distances that were further improved when resolution of 1.3 Å was achieved some years later with advanced techniques.33

|
At the outset of Freeman’s work the nature and geometrical disposition of the functional groups bonded to the metal was uncertain, but as the research into this continued over the next two decades, it became clear that the situation was extremely complex. For example, the crystal structure of the copper (I) form of plastocyanin was determined at six pH values ranging from 3.8 to 7.8.34 This study, heroic in scale at its time, revealed that one of the histidine ligands was protonated at the lower pH values, leading to the tetrahedral four-coordinate arrangement around the copper becoming trigonal planar three coordinate.
The Freeman group used a range of scientific techniques—optical spectroscopy,35 electrochemistry, nuclear magnetic resonance,36 electron spin resonance, amino-acid sequencing and optical rotatory dispersion—to examine the structures of the proteins that formed the core of their X-crystallographic studies (see ‘Supplementary material’). They also studied the blue copper protein in cucumber (Cucumus sativus), obtaining 10 mg of protein from 100 kg of cucumbers. This feat attracted the attention of Sydney journalist Gavin Gilchrist, who wrote in a magazine piece entitled ‘Peel me a cucumber, Hans’ that Freeman ‘became interested in the cucumber protein after meeting an American scientist over a beer at a conference in California’.37 Preliminary studies were published in 1977 and 1983,38 but complete elucidation of the structure proved very difficult until the then new multiple wavelength anomalous dispersion (MAD) method was applied,39 yielding a structure to 3.0 Å resolution, and a final refinement of the structure at 1.8 Å resolution was published in 1996.40
X-ray spectroscopy
While ancillary information about metallo-proteins, often pertaining to specific residues or sub-structures, was available from magnetic resonance, electron spin resonance, ultraviolet absorption and other techniques, the main technique for structure determination remained diffraction of a single-frequency X-ray beam by regular arrays of atoms in well-formed crystals. The positions and intensities of the scattered radiation were analysed to reveal the positions of the atoms in the crystal. With the advent in the 1980s of synchrotrons providing intense radiation, it was possible to develop X-ray spectroscopy that was conceptually akin to spectroscopies based on different parts of the radiation spectrum (infrared, visible and ultraviolet) and to apply it to chemical and structural analysis. Using variable monochromators, a crystal could be exposed to a range of X-ray frequencies for which the absorption coefficients could be measured: perfect crystals were not required and spectra could be recorded for molecules in solution. The position of the X-ray absorption maximum was less important than the fine structure (XAFS) of those peaks which could be interpreted in terms of the oxidation state of an atom, its coordination, and the influence of surrounding atoms.41
Freeman turned to X-ray spectroscopy to explore the role of the methionine sulfur that was, in crystallographic terms, a long way (2.90 Å) from the copper atom. Facilities for such experiments were not available in Australia, so Freeman entered into collaboration with Keith Hodgson, deputy director of the synchrotron division of the Stanford Linear Accelerator Center, a pioneer of the use of synchrotron X-radiation in spectroscopic studies with a special interest in biological molecules containing metal ions.42 Hodgson’s research group had measured the XAFS spectra of copper in plastocyanin and a related metallo-protein, azurin, in solution at room temperature and concluded that the methionine sulfur made no contribution to the absorption curve.43 With Freeman, Hodgson examined oriented single crystals of plastocyanin,44 but again concluded that the methionine sulfur makes no contribution to the XAFS. Suspecting that this negative result may have been due to thermal disorder in the crystals, the experiment was repeated at cryogenic temperatures, again yielding a negative result that the authors concluded meant that ‘the Cu–S(Met) interaction must therefore be extremely weak’.45
Organisations of crystallographers
Freeman was the driving force behind the formation of the Society of Crystallographers in Australia and New Zealand that grew from a series of informal meetings of crystallographers. He was the inaugural president (1976–8) of the society and he was also involved in the connections between local organisations and international bodies of crystallographers. At the time of its formation in 1954, the Australian Academy of Science (AAS) took over from the Australian National Research Council (ANRC) a number of national committees that were the links between Australian science and the bodies that made up the International Council of Scientific Unions (ICSU).46 The link with the International Union of Crystallography (IUCr) was Australia’s National Committee on Crystallography, of which Freeman was a member from 1970 and the chair 1984–92. During that time, he was a delegate to the international congresses of crystallography held in 1984, 1987 (in Perth, when he chaired the congress program committee) and 1990.
Australian synchrotron
Starting in the mid-1980s, the AAS, recognising that research facilities in Australia lagged behind those in some overseas countries, and made approaches to Australian governments for a scheme and funding to enable researchers to access these facilities and to practise what was known colloquially as ‘suitcase science’. Neutron beams, synchrotron science, particle physics and astronomy were the disciplines most in need of this assistance. A report to the Prime Minister of Australia by the Australian Science and Technology Council (ASTEC) on Australian participation in major international and beam facilities (Small Country—Big Science) led to a scheme that purchased access to some of these and provided support for scientists to use them.47 A synchrotron is a particle accelerator that can produce intense, coherent radiation at a range of wavelengths, and it was the X-rays that were of most interest to crystallographers. Eventually, in 1990, it was agreed that the Australian Research Council would fund the construction of a beam line for the exclusive use of Australian scientists for X-ray powder and spectroscopic studies at the ‘Photon Factory’, a synchrotron in Tsukuba, Japan.48 Funding was also provided (on a competitive basis) for travel by Australians to use the facility, that came into operation in the early 1990s.49 Further agreements followed,50 and travel by Australian researchers to international facilities was necessary until a local facility could be provided. In 2007, the Australian Synchrotron, adjacent to Monash University in Clayton, Victoria, began operations with five beamlines active and several others in construction. This served researchers in X-ray diffraction and X-ray absorption as well as those requiring access to strong coherent infrared, visible and ultraviolet light.51
Freeman promoted the adoption of the X-ray absorption spectroscopic techniques through workshops and small conferences. For example, at its forty-first annual general meeting in 1995,52 the AAS held a symposium on ‘100 years of X-rays: retrospect and prospect’, at which Freeman spoke on ‘The colour of X-rays: X-ray absorption spectra’.53 Noting that the colour of a substance is determined by the extent to which light is absorbed at different wavelengths, Freeman described spectroscopy using ‘light with extremely short wavelengths’, that is, ‘a very strong X-ray beam tunable over a wide range of wavelengths’ that allowed chemists, physicists and biologists to study the surroundings of metal atoms in a range of materials. The principal designer of Australia’s beamline at Tsukuba, Dudley Creagh, of the Australian Defence Force Academy (part of the University of New South Wales) and chairman of the Commission on Crystallographic Apparatus for the IUCr,54 led development of a short course on XFAS for use internationally.55 Freeman retained a connection with the Australian synchrotron as a member of the policy and review board for its research program.
Boden philanthropy
Since 1946, the chemistry school had received generous financial support from Alexander Boden (1913–93), a graduate of the department who had established a chemical manufacturing business.56 Shortly after Freeman’s taking up a lectureship in the department, and explaining the research path that he proposed to follow, Boden provided a very substantial sum (₤5000) that was used to support Alexander Boden Fellowships. In 1970, when Freeman was appointed to the Chair of Inorganic Chemistry, Boden, jointly with his business associates, helped establish the Foundation for Inorganic Chemistry, the funds from which still support up to two visiting scientists each year, one of whom presents the annual Hans C. Freeman Lecture. The foundation was inaugurated with a dinner in the university’s great hall in 1973 with the inaugural address delivered by Linus Pauling. Pauling, who, together with his wife, was the guest of the foundation for a 3-week visit told a reporter that his purpose in coming to Australia was ‘to help inaugurate a chemistry foundation at the University of Sydney and to lecture elsewhere’.57 The history of Sydney’s science faculty reported that he lectured on social responsibility, chemistry and peace (and no doubt of prophylaxis of the common cold by vitamin C) ‘to overflow crowds’.58
Recognition, awards and honours
Freeman joined the Royal Australian Chemical Institute in 1952 and was elected a fellow in 1968. In 1980, he was awarded the Burrows Award in inorganic chemistry, and in 1999 he was the recipient of the institute’s highest award, the Leighton Medal. The inorganic division of the Royal Australian Chemical Institute at its conference in Perth (IC’94) held a ‘Golden oldies of inorganic chemistry’ symposium, in which the ‘younger’ generation of Australian and New Zealand inorganic chemists, practising in the second half of the twentieth century, were honoured.59 The presentations were published in 1995 in a special issue of the Australian Journal of Chemistry, for which the foreword was provided by Ron Dickson (Monash University).60
Freeman became a fellow of the Royal Society of Chemistry in 1984 and in the same year he was elected to fellowship of the AAS. He was awarded the academy’s David Craig Medal in 2007 for his outstanding contribution to chemical research, and as part of the award he delivered public lectures in several Australian cities. He was made a Member of the Order of Australia (AM) in 2005 ‘for service to science and scientific research in the field of bio-inorganic chemistry, particularly through the establishment and development of the discipline of crystallography in Australia’, having in 2001 received a Centenary Medal ‘for service to Australian society and science in chemistry’.
The twenty-fifth anniversary of the determination of the plastocyanin structure was celebrated with a special evening session at the Eleventh conference on biological inorganic chemistry' (ICBIC-11), held in Cairns in 2003. The highly entertaining presentations by Mitchell Guss (Sydney), Ed. Solomon (Stanford), Harry Gray (Caltech) and Hans himself were not included in the conference proceedings volume.
In 2012, the Journal of Inorganic Biochemistry published a special issue in honour of Hans Freeman edited by two colleagues, Peter Lay and Trevor Hambley, who wrote that they ‘benefitted enormously from both the rigor and insights he provided as a colleague and the facilitated introduction of new methods and techniques to our research’.61 The issue included a number of research papers by authors who dedicated them to the memory of Freeman, and a personal tribute by his long-term partner in the protein work, Mitchell Guss.62
Personal
Outside the laboratory Freeman had a wide range of interests, beginning with his involvement in the scouting movement that grew into membership of the University of Sydney bushwalking club. On the campus he was visible as someone who dressed casually and drove a sports car. On many of his overseas trips he managed to include the enjoyment of opera performances at cities such as Milan, Glyndebourne, Berlin or Santa Fe, an interest that grew from his long membership of the university choral society. His wife, Edith née Siou, of Tahitian and Chinese descent whom he married in 1966, shared his musical interests and often travelled with him. Their children, Maeva and Philip, were born in 1972 and 1974 respectively and continue to live and work in Sydney.
Freeman died peacefully on 9 November 2008. He was remembered as ‘a friend, colleague and collaborator’ to many people, ‘always a delightful host and an enthusiastic organiser of opportunities to talk science’, and a pioneer of bioinorganic chemistry.63
The hallmarks of his work with Mitchell Guss on poplar plastocyanin and the cucumber basic protein were published in the Handbook of Metalloproteins and (after Freeman’s death) in the online Encyclopedia of Inorganic and Bioinorganic Chemistry.64
Data availability
Data sharing is not applicable as no new data were generated or analysed during this study.
Conflicts of interest
The authors declare no conflict of interest. TWH was a colleague of Hans Freeman at the University of Sydney and collaborated with him in research. IDR is an editor of Historical Records of Australian Science but was blinded from the peer-review process for this paper.
Declaration of funding
This research did not receive any specific funding.
Supplementary material
Supplementary material is available online.
Acknowledgements
The authors acknowledge the help received from Dr John Bell, Professor Peter Colman, Emeritus Professor Mitchell Guss, Professor Michael Parker, Dr John Webb, Professor John White, and the Freeman family.
References
Anonymous (1973) Man of science for all seasons, Australian Women’s Weekly, 25 April, 2.Anonymous (1990) Small Country—Big Science, Canberra. Available at https://nla.gov.au:443/tarkine/nla.obj-1745265283 [Viewed November 2020]
Anonymous (1992) Scientists to benefit from XAFS international short-course, Uniken, 6 November, 7. Available at https://nla.gov.au:443’tarkine/nla.obj-253834480 [Viewed November 2020]
Anonymous (2020) ‘History of the Australian synchrotron’. Available at http://archive.synchrotron.org.au/about‐us/history [Viewed November 2020]
Aroney, M. J., and Buckingham, A. D. (1988) Raymond James Wood Le Fevre 1905–1986, Historical Records of Australian Science, 7, 273–297.
Baker, A. T. (2021) David Mellor at the California Institute of Technology, 1937–8, the beginnings of Australian magnetochemistry, Historical Records of Australian Science, 32, 29–40.
| David Mellor at the California Institute of Technology, 1937–8, the beginnings of Australian magnetochemistryCrossref | GoogleScholarGoogle Scholar |
Barber, J., and Halliwell, B. (1977) Photosynthesis at Reading, Nature, 270, 104–105.
| Photosynthesis at ReadingCrossref | GoogleScholarGoogle Scholar |
Boldeman, J. W. (1997) ‘Access to major overseas research facilities’. Available at https://inis.iaea.org/collection/NCLCollectionStore/_Public/29/016/29016319.pdf?r==1&r==1 [viewed June 2021]
Branagan, D., and Holland, G. (eds) (1985) Ever Reaping Something New: a Science Centenary, Sydney.
Chapman, G. V., Colman, P. M., Freeman, H. C., Guss, J. M., Murata, M., Norris, V. A., Ramshaw, J. A., and Venkatappa, M. P. (1977) Preliminary crystallographic data for a copper-containing protein, plastocyanin, Journal of Molecular Biology, 110, 187–189.
| Preliminary crystallographic data for a copper-containing protein, plastocyaninCrossref | GoogleScholarGoogle Scholar | 845945PubMed |
Colman, P. M., Freeman, H. C., Guss, J. M., Murata, M., Norris, V. A., Ramshaw, J. A., Venkatappa, M. P., and Vickery, L. E. (1977) Preliminary crystallographic data for a basic copper-containing protein from cucumber seedling, Journal of Molecular Biology, 112, 649–650.
| Preliminary crystallographic data for a basic copper-containing protein from cucumber seedlingCrossref | GoogleScholarGoogle Scholar | 875035PubMed |
Colman, P. M., Freeman, H. C., Guss, J. M., Murata, M., Norris, V. A., Ramshaw, J. A., and Venkatappa, M. P. (1978a) ‘The structure of plastocyanin determined by x-ray diffraction at 2.7Å resolution’, in Photosynthesis 77—Proceedings of the Fourth International Congress on Photosynthesis, eds D. O. Hall, J. Coombs, T. W. Goodwin, London, pp. 810–813.
Colman, P. M., Freeman, H. C., Guss, J. M., Murata, M., Norris, V. A., Ramshaw, J. A. M., and Venkatappa, M. P. (1978b) X-ray crystal structure analysis of plastocyanin at 2.7 Å resolution, Nature, 272, 319–324.
| X-ray crystal structure analysis of plastocyanin at 2.7 Å resolutionCrossref | GoogleScholarGoogle Scholar |
Creagh, D. (2018) ‘Personal profile’. Available at https://researchprofiles.canberra.edu.au/en/persons/dudley‐creagh[viewed November 2020]
Dickson, R. (1995) The golden oldies of inorganic chemistry, Australian Journal of Chemistry, 48, 689.
| The golden oldies of inorganic chemistryCrossref | GoogleScholarGoogle Scholar |
Elkin, A. P. (1954) The Australian National Research Council, The Australian Journal of Science, 16, 203–211.
Fenner, F. (2005) The Australian Academy of Science: the First Fifty Years, Canberra.
Freeman, H. C. (1957) Crystallographic calculations on the silliac electronic digital computer. I. Fourier syntheses, Australian Journal of Chemistry, 10, 95–99.
| Crystallographic calculations on the silliac electronic digital computer. I. Fourier synthesesCrossref | GoogleScholarGoogle Scholar |
Freeman, H. C. (1958) Crystallographic calculations on the silliac electronic digital computer: II: structure factors, Australian Journal of Chemistry, 11, 99–103.
| Crystallographic calculations on the silliac electronic digital computer: II: structure factorsCrossref | GoogleScholarGoogle Scholar |
Freeman, H. C. (1967) Crystal structures of metal peptide complexes, Advances in Protein Chemistry, 22, 257–424.
| Crystal structures of metal peptide complexesCrossref | GoogleScholarGoogle Scholar | 4882247PubMed |
Freeman, H. C. (1970) Proceedings of the XII International Conference on Coordination Chemistry, Sydney.
Freeman, H. C. (1973) ‘Metal complexes of amino acids and peptides’, in Inorganic Biochemistry, ed. G. L. Eichhorn,vol. 1, Amsterdam, pp. 121–166.
Freeman, H. C. (1981) Electron transfer in ‘blue’ copper proteins, Coordination Chemistry, 21, 29–51.
Freeman, H. C., and Guss, J. M. (2001a) ‘Plastocyanin’, in Handbook of Metalloproteins, ed. A. Messerschmidt, vol. 2, Boca Raton, pp. 1153–1169.
Freeman, H. C., and Guss, J. M. (2001b) ‘Cucumber basic protein’, in Handbook of Metalloproteins, ed. A. Messerschmidt, vol. 2, Boca Raton, pp. 1215–1218.
Freeman, H. C., and Smith, J. E. W. L. (1966) Crystallographic studies of the biuret reaction: II: structure of bis-biuret-copper(II) dichloride, Cu(NH2CONHCONH2)2Cl2, Acta Crystallographica, 20, 153–159.
| Crystallographic studies of the biuret reaction: II: structure of bis-biuret-copper(II) dichloride, Cu(NH2CONHCONH2)2Cl2Crossref | GoogleScholarGoogle Scholar |
Freeman, H. C., Smith, J. E. W. L., and Taylor, J. C. (1959) Crystallographic studies of the biuret reaction, Nature, 184, 707–710.
| Crystallographic studies of the biuret reactionCrossref | GoogleScholarGoogle Scholar | 13824733PubMed |
Freeman, H. C, Smith, J. E. W. L., and Taylor, J. C. (1961) Crystallographic studies of the biuret reaction: I. potassium bis-biuret cuprate(II) tetrahydrate, K2[Cu(NHCONHCONH)2].4H2O, Acta Crystallographica, 14, 407–418.
| Crystallographic studies of the biuret reaction: I. potassium bis-biuret cuprate(II) tetrahydrate, K2[Cu(NHCONHCONH)2].4H2OCrossref | GoogleScholarGoogle Scholar |
Freeman, H. C., Norris, V. A., Ramshaw, J. A. M., and Wright, P. E. (1978) ‘High resolution proton magnetic resonance studies of plastocyanin’, in Photosynthesis 77—Proceedings of the fourth international congress on photosynthesis, eds. D. O. Hall, J. Coombs, T. W. Goodwin, London, pp. 805–809.
Freeman, H. C., Garrett, T. P J., Guss, J. M., Murata, M., Yoshizaki, F., Sugimura, Y., and Shimokoriyama, M. (1983) Preliminary crystallographic data for plastocyanins from an alga (Enteromorpha prolifera) and from cucumber (Cucumis sativus), Journal of Molecular Biology, 164, 351–353.
| Preliminary crystallographic data for plastocyanins from an alga (Enteromorpha prolifera) and from cucumber (Cucumis sativus)Crossref | GoogleScholarGoogle Scholar | 6842595PubMed |
Garrett, R. F., Cookson, D. J., Foran, G., Creagh, D. C., and Wilkins, S. W. (1995) The Australian national beamline facility at the Photon Factory (abstract), Review of Scientific Instruments, 66, 1687–1687.
| The Australian national beamline facility at the Photon Factory (abstract)Crossref | GoogleScholarGoogle Scholar |
Gilchrist, G. (1988) Peel me a cucumber, Hans, Sydney Morning Herald, Good Weekend, 10 December, 77.
Glusker, J. P. (1988) Edward Wesley Hughes 1904–1987, Journal of Applied Crystallography, 21, 283–284.
| Edward Wesley Hughes 1904–1987Crossref | GoogleScholarGoogle Scholar |
Guss, J. M. (2012) Hans Charles Freeman (1929–2008): a scientific journey from dipole moments to protein crystallography, Journal of Inorganic Biochemistry, 115, 114–118.
| Hans Charles Freeman (1929–2008): a scientific journey from dipole moments to protein crystallographyCrossref | GoogleScholarGoogle Scholar | 22554388PubMed |
Guss, J. M., and Freeman, H. C. (1983) Structure of oxidized poplar plastocyanin at 1.6 A resolution, Journal of Molecular Biology, 169, 521–563.
| Structure of oxidized poplar plastocyanin at 1.6 A resolutionCrossref | GoogleScholarGoogle Scholar | 6620385PubMed |
Guss J. M., and Freeman, H. C. (2011a) ‘Plastocyanin’, Encyclopedia of Inorganic and Bioinorganic Chemistry’, p. 10. Available at https://www.onlinelibrary.wiley.com/doi/10.1002/9781119951438.eibc0611 [viewed November 2020]
Guss, J. M., and Freeman, H. C. (2011b) ‘Cucumber basic protein’, Encyclopedia of Inorganic and Bioinorganic Chemistry, p. 10. Available at https://www.onlinelibrary.wiley.com/doi/10.1002/9781119951438.eibc0615 [viewed November 2020]
Guss, J. M., Harrowell, P. R., Murata, M., Norris, V. A., and Freeman, H. C. (1986) Crystal structure analyses of reduced (CuI) poplar plastocyanin at six pH value, Journal or Molecular Biology, 192, 361–387.
| Crystal structure analyses of reduced (CuI) poplar plastocyanin at six pH valueCrossref | GoogleScholarGoogle Scholar | 3560221PubMed |
Guss, J. M., Merritt, E. A., Phizackerley, R. P., Hedman, B., Murata, M., Hodgson, K. O., and Freeman, H. C. (1988) Phase determination by multiple-wavelength x-ray diffraction: crystal structure of a basic “blue” copper protein from cucumbers, Science, 241, 806–811.
| Phase determination by multiple-wavelength x-ray diffraction: crystal structure of a basic “blue” copper protein from cucumbersCrossref | GoogleScholarGoogle Scholar | 3406739PubMed |
Guss, J. M., Bartunik, H. D., and Freeman, H. C. (1992) Accuracy and precision in protein structure analysis: restrained least-squares refinement of the structure of poplar plastocyanin at 1.33 Å resolution, Acta Crystallographica, Section B: Structural Science, B48, 790–811.
| Accuracy and precision in protein structure analysis: restrained least-squares refinement of the structure of poplar plastocyanin at 1.33 Å resolutionCrossref | GoogleScholarGoogle Scholar |
Guss, J. M., Merritt, E. A., Phizackerley, R. P., and Freeman, H. C. (1996) The structure of a phytocyanin, the basic blue protein from cucumber, refined at 1.8 Å resolution, Journal of Molecular Biology, 262, 686–705.
| The structure of a phytocyanin, the basic blue protein from cucumber, refined at 1.8 Å resolutionCrossref | GoogleScholarGoogle Scholar | 8876647PubMed |
Hambley, T. (1995) Hans C. Freeman, Australian Journal of Chemistry, 45, 697–699.
Hambley, T. (2009) Hans Freeman 1929–2008, Journal of Biological Inorganic Chemistry, 14, 327–328.
| Hans Freeman 1929–2008Crossref | GoogleScholarGoogle Scholar |
Hughes, E. W, Yakel, H. L., and Freeman, H. C. (1961) The crystal structure of biuret hydrate, Acta Crystallographica, 14, 345–352.
| The crystal structure of biuret hydrateCrossref | GoogleScholarGoogle Scholar |
Hunter, R. J. (2017) ‘Boden, Alexander (1913–1993)’, Australian Dictionary of Biography. Available at http://adb.anu.edu.au/biography/boden-alexander-17438/text29161 [viewed November 2020]
Ji, J. (2006) ‘Celebrating 50 years of computing at Sydney’. Available at https://www.sydney.edu.au/news/84.hmtl?newsstoryid=1254 [viewed November 2020]
Kam, Z., Shore, H. B., and Feher, G. (1978) On the crystallization of proteins, Journal of Molecular Biology, 123, 539–555.
| On the crystallization of proteinsCrossref | GoogleScholarGoogle Scholar | 691056PubMed |
Kendrew, J. C. (1954) Structure of proteins, Nature, 173, 57–59.
| Structure of proteinsCrossref | GoogleScholarGoogle Scholar |
Lay, P. A., and Hambley, T. W. (2012) Hans C. Freeman preface, Journal of Inorganic Biochemistry, 115, 113.
| Hans C. Freeman prefaceCrossref | GoogleScholarGoogle Scholar | 23026017PubMed |
Ludwig, M. L., and Lipscomb, W. N. (1973) ‘Carboxypeptidase A and other peptidases’, in Inorganic Biochemistry, ed. G. L. Eichhorn, vol. 1, Amsterdam, pp. 439–487.
Malmström, B. G. (1970) The ‘unique’ metal-binding properties of metalloenzymes, Pure and Applied Chemistry, 24, 393–406.
| The ‘unique’ metal-binding properties of metalloenzymesCrossref | GoogleScholarGoogle Scholar |
Malmström, B. G., and Rosenberg, A. (1959) Mechanism of metal ion activation of enzymes, Advances in Enzymology—and Related Areas of Molecular Biology, 21, 131–167.
Newville, M. (2008) ‘Fundamentals of XAFS’. Available at https://www.lehigh.edu/imi/teched/GlassCSC/SuppReading/Tutorials.pdf [viewed November 2020]
Penfield, K. W., Gay, R. R., Himmelwright, R. S., Eickman, N. C., Norris, V. A., Freeman, H. C., and Solomon, E. I. (1981) Spectroscopic studies on plastocyanin single crystals: a detailed electronic structure determination of the blue copper active site, Journal of the American Chemical Society, 103, 4382–4388.
| Spectroscopic studies on plastocyanin single crystals: a detailed electronic structure determination of the blue copper active siteCrossref | GoogleScholarGoogle Scholar |
Penner-Hahn, J. E., Murata, M., Hodgson, K. O., and Freeman, H. C. (1989) Low-temperature x-ray absorption spectroscopy of plastocyanin: evidence for copper-site photoreduction at cryogenic temperatures, Inorganic Chemistry, 28, 1826–1832.
| Low-temperature x-ray absorption spectroscopy of plastocyanin: evidence for copper-site photoreduction at cryogenic temperaturesCrossref | GoogleScholarGoogle Scholar |
Rae, I. D. (2018) ‘Australian chemists crossing the Pacific to the promised land’, in Igniting the Chemical Ring of Fire, ed. S. C. Rasmussen, New Jersey, pp. 43–71.
Ross, I. (1996) Alexander Boden 1913–1993, Historical Records of Australian Science, 11, 523–540.
| Alexander Boden 1913–1993Crossref | GoogleScholarGoogle Scholar |
Scopes, R. K. (1982) Protein Purification: Principles and Practice, 3rd edn, New York, pp. 335–342.
Scott, R. A., Hahn, J. E., Doniach, S., Freeman, H. C., and Hodgson, K. O. (1982) Polarized X-ray absorption spectra of oriented plastocyanin single crystals. Investigation of methionine-copper coordination, Journal of the American Chemical Society, 104, 5364–5369.
Stephens, T. (1958) From riches to rags to protein pioneer, The Sydney Morning Herald, 5 December 2009. Available at https://www.smh.com.au/from-riches-to-rags-to-protein-pioneer-20081205-gdt5d5.html [viewed October 2020]
Tullius, T. D., Frank, P., and Hodgson, K. O. (1978) Characterization of the blue copper site in oxidized azurin by extended x-ray absorption fine structure: determination of a short Cu─S distance, Proceedings of the National Academy of Sciences of the USA, 75, 4069–4073.
| Characterization of the blue copper site in oxidized azurin by extended x-ray absorption fine structure: determination of a short Cu─S distanceCrossref | GoogleScholarGoogle Scholar | 16592557PubMed |
Vallee, B. L., and Williams, R. J. (1968) Metalloenzymes: the entatic nature of their active sites, Proceedings of the National Academy of Sciences USA, 59, 498–505.
| Metalloenzymes: the entatic nature of their active sitesCrossref | GoogleScholarGoogle Scholar |
Ward, C. (2014) ‘Peter Malcolm Colman’. Available at https://csiropedia.csiro.au/colman-peter-malcolm [viewed November 2020]
Wilson, E. K. (2005) Keith Hodgson, Chemical & Engineering News, 83, 28.
1 Much information in this biographical memoir has been drawn from publications of one of the authors, Hambley (1995), Hambley (2009), Guss (2012), a newspaper article published at the time of Freeman’s death and a Wikipedia entry https://en.wikipedia.org/wiki/Hans_Freeman, viewed October 2020.
2 Border adjustments in 1945 saw the city become part of Poland and renamed Wrocław.
3 Stephens (1958).
4 Basser taught at the school for 28 years until his retirement in 1959. The University of Sydney has established in his name a leadership award for young scientists: https://www.sydney.edu.au/news/84.html?newsstoryid=606, viewed January 2021. Among students who studied with him were Graeme Clark, inventor of the bionic ear, Lord Robert May, President of the Royal Society, and 1975 Nobel Laureate chemist Sir John Cornforth.
5 Aroney and Buckingham (1988).
6 Thesis title: ‘The dielectric constants or organic vapours at high temperatures, and evidence for the existence of geometrically isomeric diazocarbonamides’.
7 Rae (2018).
8 Baker (2021).
9 Glusker (1988).
10 Hughes and others (1961).
11 Kendrew (1954).
12 Freeman and others (1959).
13 Thesis title ‘The crystal structure of biuret hydrate and X-ray crystal structure calculations on the “Silliac” high-speed electronic digital computer’ (1957).
14 Freeman and others (1961). Freeman and Smith (1966).
15 Freeman (1957). Freeman (1958). Ji (2006).
16 Freeman (1967). The review was said to have been written, in part, while Freeman and his wife were on their honeymoon.
17 Freeman (1967) p. 2.
18 Freeman (1973) p. 159.
19 Freeman (1973).
20 Freeman (1970). The plenary lectures were not included in the proceedings volume, but were published separately by the International Union of Pure and Applied Chemistry, under whose auspices the conference had been held. Malmström (1970).
21 Malmström and Rosenberg (1959).
22 Vallee and Williams (1968).
23 Ludwig and Lipscomb (1973).
24 Freeman (1981).
25 Peter Malcolm Colman, CSIROpedia, Commonwealth Scientific and Industrial Research Organisation, https://csiropedia.csiro.au/Colman-Peter-Malcolm/, viewed 2022.
26 Remarks by Colman at Freeman’s funeral: Peter Colman, private communication, December 2020.
27 Ward (2014).
28 Kam and others (1978). Scopes (1982).
29 Chapman and others (1977).
30 Colman and others (1978a).
31 Barber and Halliwell (1977).
32 Colman and others (1978b).
33 Guss and Freeman (1983). Gus and others (1992).
34 Guss and others (1986).
35 A notable example was the study electronic structure of the blue copper active site undertaken with E. I. Solomon of Stanford University: Penfield and others (1981).
36 The work was led by NMR spectroscopist Dr Peter Wright. Freeman and others (1978).
37 Gilchrist (1988).
38 Colman and others (1977). Freeman and others (1983).
39 Guss and others (1988).
40 Guss and others (1996).
41 Newville (2008).
42 Wilson (2005).
43 Tullius and others (1978).
44 Scott and others (1982).
45 Penner-Hajn and others (1989).
46 Fenner (2005). Frank Fenner’s history of the academy is the source of much material in this section of the memoir, but the full story of the development of X-ray crystallography in Australia is yet to be told. Elkin (1954). In 1998 the ICSU was renamed the International Council of Science but the acronym was retained.
47 Anonymous (1990). Freeman and John White, Professor of Physical and Theoretical Chemistry at the Australian National University led the development of this report, and Freeman’s former colleague, Peter Colman, then chief of the CSIRO Division of Biomolecular Engineering was also a member of the working party.
48 Fenner (2005) p. 128.
49 Garrett and others (1995).
50 Boldeman (1997).
51 Anonymous (2020).
52 Fenner (2005) p. 501.
53 The abstract of Freeman’s talk is held by the Australian Academy of Science.
54 Creagh (2018) Personal profile. https://personalprofiles.canberra.edu.au/en/persons/dudley-creagh, viewed November 2020.
55 Anonymous (1992).
56 Ross (1996). Hunter (2017).
57 Anonymous (1973).
58 Branagan and Holland (1985).
59 The ‘Golden Oldies’ were Alan Sargeson (Australian National University), Bruce West and Ray Martin (Monash), Hans Freeman (Sydney), Neil Curtis (Wellington, NZ), and Brian Figgis (Western Australia).
60 Dickson (1995). Dickson had studied with one of the Golden Oldies, Bruce West, at the University of Adelaide.
61 Lay and Hambley (2012).
62 Guss (2012).
63 Hambley (2009).
64 Freeman and Guss (2001a). Freeman and Guss (2001b). Guss and Freeman (2011a). Guss and Freeman (2011b).



