Control of glycerol biosynthesis under high salt stress in Arabidopsis
Ahmed Bahieldin A B G , Jamal S. M. Sabir A , Ahmed Ramadan A C , Ahmed M. Alzohairy D , Rania A. Younis B , Ahmed M. Shokry A C , Nour O. Gadalla A E , Sherif Edris A B F , Sabah M. Hassan A B , Magdy A. Al-Kordy A E , Khalid B. H. Kamal A , Samar Rabah A , Osama A. Abuzinadah A and Fotouh M. El-Domyati A BA Department of Biological Sciences, Faculty of Science, King Abdulaziz University (KAU), PO Box 80141, Jeddah 21589, Saudi Arabia.
B Department of Genetics, Faculty of Agriculture, Ain Shams University, Cairo, Egypt.
C Agricultural Genetic Engineering Research Institute (AGERI), Agriculture Research Center (ARC), Giza, Egypt.
D Genetics Department, Faculty of Agriculture, Zagazig University, Zagazig 44511, Egypt.
E Genetics and Cytology Department, Genetic Engineering and Biotechnology Division, National Research Center, Dokki, Egypt.
F Princess Al-Jawhara Al-Brahim Centre of Excellence in Research of Hereditary Disorders (PACER-HD), Faculty of Medicine, King Abdulaziz University (KAU), Jeddah, Saudi Arabia.
G Corresponding author. Email: bahieldin55@gmail.com
Functional Plant Biology 41(1) 87-95 https://doi.org/10.1071/FP13005
Submitted: 4 February 2013 Accepted: 27 June 2013 Published: 24 July 2013
Abstract
Loss-of-function and gain-of-function approaches were utilised to detect the physiological importance of glycerol biosynthesis during salt stress and the role of glycerol in conferring salt tolerance in Arabidopsis. The salt stress experiment involved wild type (WT) and transgenic Arabidopsis overexpressing the yeast GPD1 gene (analogue of Arabidopsis GLY1 gene). The experiment also involved the Arabidopsis T-DNA insertion mutants gly1 (for suppression of glycerol 3-phosphate dehydrogenase or G3PDH), gli1 (for suppression of glycerol kinase or GK), and act1 (for suppression of G3P acyltransferase or GPAT). We evaluated salt tolerance levels, in conjunction with glycerol and glycerol 3-phosphate (G3P) levels and activities of six enzymes (G3PDH, ADH (alcohol dehydrogenase), ALDH (aldehyde dehydrogenase), GK, G3PP (G3P phosphatase) and GLYDH (glycerol dehydrogenase)) involved in the glycerol pathway. The GPD1 gene was used to overexpress G3PDH, a cytosolic NAD+-dependent key enzyme of cellular glycerol biosynthesis essential for growth of cells under abiotic stresses. T2 GPD1-transgenic plants and those of the two mutants gli1 and act1 showed enhanced salt tolerance during different growth stages as compared with the WT and gly1 mutant plants. These results indicate that the participation of glycerol, rather than G3P, in salt tolerance in Arabidopsis. The results also indicate that the gradual increase in glycerol levels in T2 GPD1-transgenic, and gli1 and act1 mutant plants as NaCl level increases whereas they dropped at 200 mM NaCl. However, the activities of the G3PDH, GK, G3PP and GLYDH at 150 and 200 mM NaCl were not significantly different. We hypothesise that mechanism(s) of glycerol retention/efflux in the cell are affected at 200 mM NaCl in Arabidopsis.
Additional keywords: abiotic stress, osmoprotection, T-DNA insertion.
Introduction
A plant’s life depends on its ability to sense and positively respond to environmental stresses (Padamsee et al. 2012). Under saline condition, cells respond to osmolarity changes through multiple signalling pathways (Hohmann 2009). Eukaryotic cells are able to respond to environmental stresses by changing their protein repertoire (Melamed et al. 2008). Several adaptations are required in the cell in order to survive osmotic stress. These adaptations involve osmoregulation, ion homeostasis, accumulation of solutes, and possibly modifications of cell morphology (Gunde-Cimerman et al. 2009; Kralj Kunčič et al. 2010).
There is reported evidence for the existence of a specific signal transduction pathway that senses the osmotic situation of the cell and eventually induces physiological changes to adapt to the new growth condition (Brewster et al. 1993). A candidate element of this pathway is the product of the high osmolarity glycerol 1 (HOG1) gene, which is a mitogen-activated protein kinase or MAPK (Lawrence et al. 2004). This protein may be involved in signal transduction after the change in the osmotic environment has been sensed.
The most prominent physiological effect in osmotically-stressed cells is the enhanced production of intracellular compatible solutes to counterbalance the osmotic pressure. Glycerol seems to be an important compatible solute produced in yeast, and mechanisms exist to specifically retain glycerol in the cytosol under abiotic stress (Albertyn et al. 1994). Glycerol 3-phosphate (G3P) generated by the glycerol pathway can be transported between the cytosol and plastidial stroma. The plastidial ACT1 gene product catalyses the acylation of oleic acid (18 : 1) by the action of G3P acyltransferase (GPAT) on the generated G3P backbone (Kunst et al. 1988). This is a shift of G3P utilisation in the direction of lipid biosynthetic pathway. Plants impaired in utilisation of plastidial G3P (act1 mutants) are expected to accumulate elevated levels of G3P (Venugopal et al. 2009). The pathway for glycerol production starting from dihydroxyacetone phosphate (DHAP) is shown in Fig. S1, available as Supplementary Material to this paper.
In conjunction with the induction of glycerol 3-phosphate dehydrogenase (G3PDH), the activities of ADH and ALDH enzymes – involved in NADH : NAD+ recycling – also change, resulting in an enhanced production of NADH, and consequently production of G3P and glycerol (Blomberg and Adler 1989). G3PDH is a NAD+-dependent key enzyme of cellular glycerol biosynthesis essential for growth of cells under abiotic stresses in which ADH is repressed, and ALDH is induced, but actions of both enzymes result in enhanced production of NADH, and consequently G3P and glycerol. The pyridine nucleotides NAD+ and NADH are the primary redox carriers involved in cell metabolism. Analysis of mutants in Arabidopsis deficient in G3PDH (namely gly1) showed an elevated NADH : NAD+ ratio under standard growth conditions resulting in constitutively increased level of reactive oxygen species (ROS) (Shen et al. 2006). A yeast mutant lacking GPP2 gene activity (homologue of AtGPP2), which encodes glycerol 3-phosphatase (G3PP) for glycerol production in yeast, is devoid of G3PP activity, and produces only a small amount of glycerol. However, overproduction of G3PP in yeast did not significantly enhance glycerol production, indicating that the enzyme is not rate-limiting for glycerol production (Påhlman et al. 2001). Expression of GPP2 gene was shown to be induced under hyperosmotic stress and this induction partially depends on the high osmolarity glycerol (HOG) pathway.
There are at least two avenues for glycerol metabolism. The first is initiated upon conversion (phosphorylation) of glycerol to glycerol 3-phosphate (G3P) via the action of glycerol kinase (GK) (Chanda et al. 2008). The second is initiated upon conversion (oxidation) of glycerol to dihydroxyacetone (DHA) (Mandel et al. 2011). The gene GLI (analogue of GUT gene in yeast) in Arabidopsis is known for its effect in glycerol utilisation and a knockout mutant of this gene (namely gli) is glycerol-insensitive (Eastmond 2004). The latter was reported for the overproduction of glycerol and consequently resistance to abiotic stresses. Glycerol dehydrogenase (GLYDH) is known for its effect on catalysing the oxidation of glycerol to DHA with concomitant reduction of NAD+ to NADH (Ruzheinikov et al. 2001; Rawls et al. 2011). This confirms that the combined action of the enzymes ADH, ALDH and GLYDH is recycling of NADH : NAD+ to allow continuous operation of glycerol production and metabolism.
In previous work (Edris et al. 2012), we investigated the possible production and subsequent role of glycerol in osmostress response by expressing the yeast (Saccharomyces cerevisiae) GPD1 gene (homologue of AtGLY1) in Escherichia coli and analysing the response of the modified E. coli to osmostress. The results led us to examine expression of this gene in Arabidopsis thaliana (L. Heynh.) to determine its effect on salt tolerance and to study the genetic control of the glycerol pathway. G3P was shown lately to be a regulator of plant defence signalling (Chanda et al. 2008; Venugopal et al. 2009), but its role in plant salt tolerance was not detected. Here we report observations of a reduction in glycerol production when salt concentration reached 200 mM. As the activity of the enzymes in the corresponding pathway remained essentially unchanged, we speculate that a salt-dependent change in the permeability of membranes for glycerol may occur at this salt level. This result is in accordance with the finding that the facilitators (e.g. MIPs) that transport glycerol across the membrane (Luyten et al. 1995) change as a function of osmotic stress.
Materials and methods
Construction of recombinant genes and Agrobacterium transformation
The coding nucleotide sequence of the yeast (Saccharomyces cerevisiae) glycerol 3-phosphate dehydrogenase (GPD1) cDNA was isolated using the GeneRacer kit (Invitrogen Life Tech, Grand Island, NY, USA) and cloned into pCR4TOPO vector (Invitrogen Life Tech). The complete cDNA sequence (861 bp) contained 105 bp of a 5′ untranslated region, 645 bp of an open reading frame (ORF), and 111 bp of a 3′ untranslated region. An 881 bp EcoRI fragment containing the full-length cDNA was filled using Klenow fragment and blunt-end ligated to the previously digested and filled BamHI site of pAHC17 (Christensen and Quail 1996). A 3.16-kb GPD1 gene cassette was recovered via partial digestion with HinDIII/EcoRI, filled using Klenow fragment and blunt-end ligated to the previously digested and filled NcoI/BglII sites of the binary vector pCAMBIA1302 (~10.55 kb, CAMBIA, Brisbane, Qld, Australia) to obtain pGPD1 (13.71 kb, Fig. S2). The plasmid was mobilised into Agrobacterium tumefaciens strain GV3101 and used for genetic transformation of Arabidopsis thaliana (L. Heynh.) wild type A. thaliana (accession Columbia) and the three T-DNA insertion mutants (Schubert et al. 2004), namely gly1, gli1 and act1 with codes of At2 g41540 (NACS ID: N520437), At1 g80460 (NACS ID: N567205) and At1 g32200 (NACS ID: N627295), respectively, were obtained from the Nottingham Arabidopsis Stock Centre (NASC, the European Arabidopsis Stock Center, University of Nottingham, Loughborough, UK). A single-copy T2 GPD1-transgenic plant was selected for the salt stress experiments. This selection was based on the plant’s ability to set seeds, its overall morphological similarity to wild-type, transgene copy number and level of transgene expression. The WT plant was used as a negative control. The gly1, gli1 and act1 mutants were also used to examine elements of the glycerol pathway and possible participation of G3P and/or glycerol in conferring salt tolerance in Arabidopsis.
Plant transformation
WT A. thaliana (accession Columbia) plants (5 weeks old) of were exposed to an A. tumefaciens strain harbouring the pGPD1 by using the vacuum infiltration method (Bechtold et al. 1993) and grown in the greenhouse. Seeds were collected and transformants were screened in MS medium (Murashige and Skoog 1962) supplemented with 20 μg mL–1 hygromycin. Transgenic plants were transferred to soil with mixture of vermiculite : peat moss : perlite with equal volumes and left to grow until seed set in the greenhouse under 16 h light/8 h dark cycle at 21°C. Three GPD1 homozygous lines at the second generation (T2) whose overall morphology looked like WT plants were selected for subsequent analyses based on the ability to set viable seeds.
Southern blot hybridisation
Plant genomic DNA (30 ug) was extracted from leaves of WT plant and the three selected T2 GPD1-transgenics as described by Dellaporta et al. (1983). Genomic Southern hybridisation was conducted using the non-radioactive digoxigenin (DIG) High Prime DNA Labelling and Detection Starter kit (Roche Applied Science, Penzberg, Germany). Hybridisation was conducted to demonstrate the integration and copy number of the transgene and to confirm independence of the different transgenic events. Genomic DNA was digested with SalI to release a 1.41 kb fragment involving a portion of the P-ubi promoter, the ubi intron and the 5′ untranslated region of GPD1 gene and probed with a PstI/EcoRI fragment (1.38 kb) of the P-ubi promoter and portion of the ubi intron of pGPD1. A genomic Southern blot was hybridised and processed according to manufacturer’s instructions. The three T2 GPD1 homozygous lines were selected for real-time PCR to detect the event with the highest expression level of GPD1 transgene.
qRT-PCR
qRT-PCR was conducted for the WT and the three selected T2 transgenic plants using the Agilent Mx3000P QPCR Systems (Agilent Technologies, Santa Clara, CA, USA). All cDNA-synthesised samples were diluted (1 : 10) before amplification. The reaction (25 μL) components were 12.5 μL Maxima SYBR Green/ROX qPCR master mix, 0.2 μM of each gene forward (5′ TGTCTCGGTGGTGTCCCTAT 3′) and reverse (5′ ACCATGGCTGATGGAAGACT 3′) primers, and PCR-grade water was added up to 22.5 μL. Primers were designed using the Universal ProbeLibrary Assay Design Center (Roche, www.roche-applied-science.com). Finally, 2.5 μL of diluted cDNA template was added to the reaction mix. Amplification for each sample was conducted in three replicates along with a no-template control (NTC, PCR-grade water). The thermal cycling conditions consisted of 1 cycle of pre-incubation at 95°C for 5 min (hot-start), denaturation at 95°C for 10 s, annealing at 59°C for 10 s and extension at 72°C for 10 s followed by 40 cycles of denaturation at 95°C for 15 s, annealing at 59°C for 30 s and extension at 72°C for 2 min. Data were collected and amplification plots of ΔRn versus cycle number were generated for analysis. Amplicons were further analysed by agarose gel electrophoresis. Actin gene was used as a reference to show that equal amounts of RNA were used in the analysis. To avoid false positives due to DNA contamination, PCR reaction was carried out for all RNA samples and results were negative (data not shown).
Data analysis
Real-time PCR data were analysed and amplification plots were generated using the software program MxPro QPCR (Agilent Mx3000P QPCR Systems, Santa Clara, CA, USA). The standard curves were generated using both the software programs followed by Microsoft Excel (Microsoft, Redmond, WA, USA). Calculations of ΔΔCT for the relative quantification of target DNA were done as follows: (ΔΔCT = (CT, target gene – CT, Actin) x – (CT, target gene – CT, Actin) y), where x is the treated sample and y is the control sample. Validation and normalisation to cr-Actin relative to the copy number of the target gene in control were done following work by Livak and Schmittgen (2001). Two experiments were carried out for each sample in which individual samples were run in triplicates and the average value was accepted when the s.d. was lower than 0.38 (Pfaffl 2001).
Salt stress experiment
Experiments were conducted to observe the performances of different genotypes (WT, GPD1-transgenic plant, and gly1, gli1 and Act1 mutants) at 0, 100, 150 or 200 mM NaCl. We determined levels of glycerol, as well as G3P at different NaCl concentrations and activities of four enzymes involved directly in the glycerol pathway (namely G3PDH, G3PP, GK and G3PDH) and two for NAD+/NADH recycling (ADH and ALDH).
Germination studies
For germination studies, 50 seeds/replicate/treatment/genotype were surface sterilised, sown in Petri dishes containing either MS medium (Murashige and Skoog 1962) or MS medium with different concentrations of NaCl (100, 150 or 200 mM). Experiments were conducted in four replicates and analysed using a randomised complete block design. The plates were kept in the dark at 4°C for 2 days and then shifted to 21 ± 2°C (day/night) under a 16-h-light/8-h-dark cycle. Germination was recorded up to day 6. Emergence of the radicle from seed coat was considered as completion of germination.
Growth measurements
Two-week-old plants of different genotypes, grown at 0 mM NaCl under a 16-h-light/8-h-dark cycle at 21 ± 2°C (day/night), were transferred to MS agar plates with different concentrations of NaCl (0, 100, 150 or 200 mM). Light intensity was ~175 μmol m–2 s–1 for the 16-h photoperiod. Plants were allowed to grow on these media for 2 more weeks. Then, individual plant root length, number of leaves per plant and the rosette area were scored. The experiment was conducted using a randomised complete block design with three replicates in which 50 plants were utilised for each replicate, genotype and treatment.
Measurements of enzyme activities, and glycerol and G3P levels
For analysis of glycerol and G3P levels and enzyme activities at different salt concentrations, 5 g of 4-week-old Arabidopsis leaves of genotypes grown at different salt concentrations for 2 weeks were homogenised in liquid nitrogen to a fine powder or collected as leaf extracts. Protein content was determined as described by Bradford (1976) using bovine serum albumin as a standard.
Cellular glycerol and G3P levels were determined enzymatically as described by Wei et al. (2004). Glycerol and G3P concentrations were given in μmol per gram leaf tissue (FW) of different genotypes grown at different salt concentrations (0, 100, 150 or 200 mM NaCl). Assays of G3PDH, G3PP and GLYDH enzyme activities were done according to work by Chen et al. (2012). Glycerol kinase assay was performed using the method described by Liu et al. (1994), ADH assay was performed according to work by Benz et al. (2007), and ALDH assay was performed according to Li et al. (2004). Statistical analyses of different experiments were performed following the procedure outlined by Gomez and Gomez (1984).
Results
Molecular characterisation of transgenic plants
A total of 20 putatively transgenic To plants were generated from transformation experiments, as indicated by hygromycin selection and PCR analyses for the presence of GPD1 gene (data not shown). Of the 20 plant lines, 10 with morphological similarities to WT were selected for further characterisation. Among the T1 transgenic lines, only three (lines 1, 5 and 6) segregated for the presence of GPD1 gene in a ratio of 3 : 1 indicating the insertion of a single copy of the GPD1 gene cassette. This result was supported by genomic Southern hybridisation of T2 transgenics, which also demonstrated independence of the three transgenic events (Fig. S3).
In the pGPD1 plasmid (Fig. S2), the restriction enzyme SalI has several cleavage sites: one of them is located within the ubi1 promoter and the second is located at the end of the 5′ untranslated region of GPD1 gene. This SalI cleavage results in the liberation of a 1.41 kb fragment in the three transgenic lines (Fig. S3). One other hybridising high-molecular-weight fragment of different size was observed in each of the three lines suggesting unique transgene insertions for each event and confirming the insertion of a single copy of the transgene. The results for the positive control (plasmid DNA) indicate the presence of the 1.41 kb fragment as well as a fragment for the rest of the plasmid (~11.65 kb), except for the 645 bp of the GPD1 ORF that was cleaved with SalI at several sites and consequently, not hybridised to the probe (Fig. S3).
T2 families of the three GPD1-transgenic events were further characterised for overexpression of the transgene as detected by real-time PCR (Fig. S4). The results indicated that the transgene was most highly expressed in line 6 followed by lines 5 and 1. Line 6 was chosen as the GPD1-transgenic event for further analyses, along with the three T-DNA insertion mutants gly1, gli1 and act1 representing genes involved in the glycerol pathway. These genotypes were utilised in evaluating salt tolerance in conjunction with the overproduction of glycerol and G3P as well as for determining the activities of six enzymes in the glycerol pathway.
Salt stress experiments
Growth parameters under salt stress
We first examined the effects of NaCl, at different concentrations, on germination of seeds of T2 GPD1-transgenic family of line 6 and the three mutants versus the WT as shown in Figs 1 and 2. We observed that seed germination initiated at day 4 under the control condition (0 mM NaCl), whereas it began at day 3 in all of the salt stress treatments (Fig. 1). Thus, NaCl treatments stimulated germination of seeds compared with the controls. Essentially, no further changes in germination efficiency across genotypes and salt conditions were observed after day 5. Although germination rates of the lines varied somewhat, no statistically significant differences were observed at day 6 across genotypes under different conditions except at 150 mM NaCl (Figs 1 and 2). In that condition, germination percentages for the GPD1-transgenic line and the two mutants gli1 and act1 were significantly higher than those of the WT and gly1 mutant. NaCl at the highest concentration (200 mM) severely affected seed germination percentages and rates for all genotypes and was thus excluded. Maintenance of the plants for an additional week at 150 mM NaCl, revealed the transgenic line and the two gli1 and act1 mutants continued to grow better and appear more vigorous than the WT and gly1 mutant (Fig. S5).
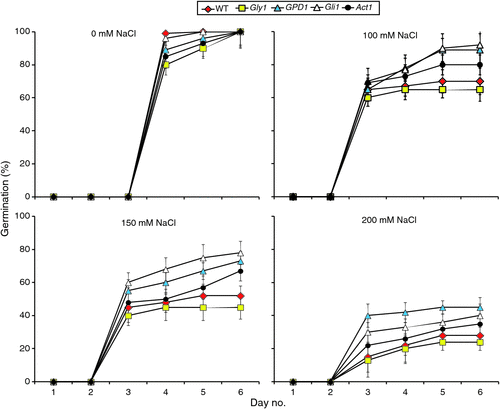
|
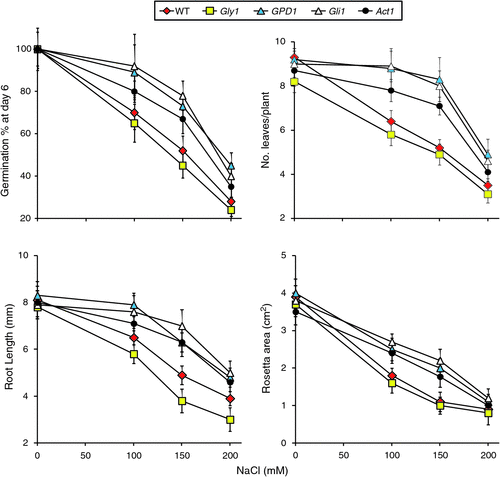
|
After 2 weeks of growth at different salt stress levels, the mean root length (mm), number of leaves per plant and rosette areas (cm2) were measured for the different genotypes (Fig. 2). The results for these three parameters indicated better performance of transgenic line GPD1 and the two mutants gli1 and act1 at 100 and 150 mM NaCl as compared with the WT and the mutant gly1. The latter showed absence of greening under salt stress and started dying soon after being transferred to the soil, whereas the other three genotypes were still green at 100 and 150 mM NaCl and were able to continue growth up to maturity (data not shown). However, none of the genotypes, including those expected to overproduce glycerol, were able to continue growth up to maturity at 200 mM NaCl. It is worth noting that the morphological and visual growth characteristics of transgenic and mutant plants were similar to the WT plants under normal growth conditions.
Glycerol and G3P levels and cellular enzyme activities as affected by salt stress
The different lines utilised in salt stress experiment, were next subjected to biochemical analysis. The abundance of glycerol in the genotypes at different NaCl concentrations correlated with the growth performances under the same conditions (Fig. 3). Glycerol levels across treatments were lowest for the gly1 mutant followed by WT, and highest for mutant gli1 followed by GPD1-transgenic and act1 mutant. For the last two genotypes, glycerol levels significantly increased as NaCl concentration increased until 150 mM, and subsequently dropped at 200 mM.
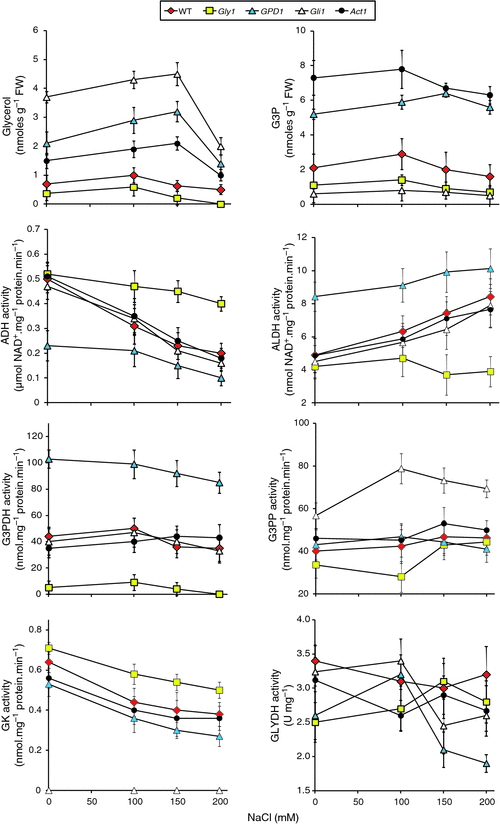
|
Discussion
The act1 mutant, followed by the transgenic plants, accumulated higher G3P levels at 100 mM NaCl as compared with the other genotypes. However, no statistically significant difference in G3P levels were scored across genotypes and treatments, indicating that G3P level might be insensitive to salt stress. The overall results of glycerol measurements indicate its association with tolerance to salt stress in Arabidopsis. Eastmond (2004) indicated that overproduction of glycerol in gli mutants resulted in higher resistance to hyperosmotic and salt stresses in Arabidopsis. Mandel et al. (2011) reported that G3P contributed to resistance to biotic stress and to play a role in inducing systemic acquired resistance (SAR), however, our data do not suggest a possible role for G3P during salt stress.
Despite a knockout mutation in the gly1 gene, this mutant still produces a low level of glycerol across treatments indicating the possible presence of another G3PDH-encoding gene(s) or the occurrence of other avenue(s) for glycerol production in Arabidopsis. Another possibility is the occurrence of incomplete loss-of-function in this particular mutant. This may also indicate the presence of a closely related gene, analogous to the yeast GPD2 gene (Albertyn et al. 1994; Hubmann et al. 2011), in Arabidopsis for glycerol production. The act1 mutant overproduced G3P, but glycerol levels were not higher than in the WT. This result indicates that the G3P pool might not be directly accessible to glycerol production due to compartmentalisation. Glycerol levels decreased for gli1, act1 and GPD1 lines when salt concentration increased from 150 to 200 mM. As the levels of the precursor G3P did not change significantly this suggests that mechanism(s) of glycerol retention in the cell might be impaired at 200 mM NaCl in Arabidopsis. During osmotic stress, Bohnert et al. (1999) indicated that salt-tolerant Zygosaccharomyces rouxii accumulated glycerol by increasing its retention within the cell. They suggested the occurrence of active uptake of glycerol, rather than increased production of glycerol, during this stress condition. Further, they reported that in Saccharomyces cerevisiae, glycerol biosynthesis increases during osmotic stress, although cells do not alter membrane permeability. They hypothesise that holding high glycerol concentration in the cell is a difficult task as it requires high energy cost that limits further increases in salt tolerance. The latter hypothesis seems to match ours in which mechanism(s) of glycerol retention in the cell of Arabidopsis might be impaired at high NaCl concentration.
Activities of enzymes of the glycerol pathway during salt stress were analysed in order to detect the performance of enzymes in this pathway at different NaCl concentrations (Fig. 3). The activities of ADH and ALDH in the Gly1 mutant indicate that both enzymes are insensitive to salt concentrations whereas ADH activity decreased and ALDH activity increased with increasing salt concentration in the other lines. This result suggests that G3PDH might have a role in decreasing activity of ADH and increasing activity of ALDH. The latter response results in enhanced production of NADH and, consequently, of glycerol (Blomberg and Adler 1989). Shen et al. (2006) indicated that the specific activity of the NADH-dependent G3PDH is induced by osmotic stress up to 8-fold. The results of the present study indicate no substantial increase in enzyme activity of the GPD1 transgenic across different growth conditions.
Chen et al. (2012) conducted a comparative analysis in Dunaliella salina, a halotolerant green algae, on four key enzymes of the glycerol cycle metabolic pathway, e.g. G3PDH, G3PP, GLYDH (or DHAR) and dihydroxyacetone kinase (DHAK) under different salt stress conditions. Their results showed that NaCl could stimulate the activities of G3PDH and G3PP and consequently glycerol production in agreement with the results of the present finding up to 150 mM NaCl. Chen et al. (2012) also emphasised the role of G3PDH in the regulation of glycerol metabolism by directing its flow under different salt stresses. They found that the G3PDH activity in D. salina reached highest at continuous 3.5 M NaCl exposure, but decreased at higher concentrations. Upon glycerol excessive accumulation due to increased salinities, G3PDH activity tends opposite to the direction of glycerol synthesis. In other words, it is the net rate of glycerol synthesis rather than the net accumulation that is responding to the external osmoticum. These results confirm our finding on the trilateral relationships comprising the activity of this enzyme, glycerol production and salt tolerance.
The results of G3PP and GK enzyme activities (both are functioning in opposite directions in the glycerol pathway) indicated that G3P (substrate) is a limiting factor for the direction of the pathway (Fig. 3). GK activity in gly1 mutant (G3P-deficient) was high across treatments, while low in gli1 mutant (for G3P overproduction). The results of G3PP activities for the same two mutants were controversial. The results of GLYDH activities for different genotypes during salt stress treatments were arbitrary - with no specific pattern. The results of the present study indicate the possible combined action of the enzymes ADH, ALDH and GLYDH in recycling NADH : NAD+ to allow continuous operation of glycerol production and metabolism. However, scoring NADH : NAD+ levels during salt stress is required to support this hypothesis. The results also indicate that GLYDH is insensitive to salt stress, to the activities of the five upstream enzymes in the pathway or to the level of glycerol or G3P in different genotypes. Furthermore, the results of enzyme activities during salt stress do not justify the drop of glycerol level at 200 mM NaCl. The results also indicate that this drop is not due to its conversion to DHA via the action of GLYDH or to G3P via the action of GK. The activities of the four enzymes, directly related to glycerol pathway, at 150 and 200 mM NaCl were not different.
The permeability of plant membranes is reported to be mainly controlled by the regulation of major intrinsic proteins (MIPs) accumulation (Gerbeau et al. 1999; Lee et al. 2009). MIP gating (opening and closing of the pore) is reported to respond to a wide range of stimuli such as pH, phosphorylation, programmed cell death, hydrogen peroxide and abiotic stresses (Cabello-Hurtado and Ramos 2004; Horie et al. 2011). However, Klepek et al. (2005) indicated that other types of transporters (e.g. Arabidopsis POLYOL TRANSPORTER5) can mediate the transport of several substrates including glycerol. It is also possible that the drop in glycerol level under severe salt stress is due to glycerol leakage across membranes that were damaged by the exposure to high NaCl concentration. The hypothesis for the possible impairment of glycerol retention or gain of glycerol efflux mechanism(s) in the cell of Arabidopsis under severe salt stress requires further attention.
Acknowledgements
The authors of this paper sincerely thank Professor Gregory Martin (Professor of Plant Pathology and Plant-Microbe Biology, Boyce Schulze Downey Research Chair, Boyce Thompson Institute Cornell University, Tower Road Ithaca, NY 14853–1801, USA) and Professor Lothar Willmitzer (Director of Max-Planck-Institut für Molekulare Pflanzenphysiologie, Am Mühlenberg 1 14476 Potsdam-Golm, Germany) for revisions and making valuable comments. This paper was generated through a project funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, under Grant no. (4-3-1432/HiCi). The authors, therefore, acknowledge with thanks DSR technical and financial support.
References
Albertyn J, Hohmann S, Prior BA (1994) Characterization of the osmotic stress response in Saccharomyces cerevisiae, osmotic stress and glucose repression regulate glycerol-3-phosphate dehydrogenase independently. Current Genetics 25, 12–18.| Characterization of the osmotic stress response in Saccharomyces cerevisiae, osmotic stress and glucose repression regulate glycerol-3-phosphate dehydrogenase independently.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXksFWnt7s%3D&md5=f1160e84686a42345aaae049ff61711bCAS | 8082159PubMed |
Bechtold N, Ellis J, Pelletier G (1993) In planta Agrobacterium mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. Comptes Rendus de l’Académie des Sciences 316, 1194–1199.
Benz BR, Rhode JM, Cruzan MB (2007) Aerenchyma development and elevated alcohol dehydrogenase activity as alternative responses to hypoxic soils in the Piriqueta caroliniana complex. American Journal of Botany 94, 542–550.
| Aerenchyma development and elevated alcohol dehydrogenase activity as alternative responses to hypoxic soils in the Piriqueta caroliniana complex.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXlt1Ggs70%3D&md5=d2c898638a929fbbfc2b0cef9a43178dCAS | 21636424PubMed |
Blomberg A, Adler L (1989) Roles of glycerol and glycerol-3-phosphate dehydrogenase (NAD+) in acquired osmotolerance of Saccharomyces cerevisiae. Journal of Bacteriology 171, 1087–1092.
Bohnert HJ, Su H, Shen B (1999) Molecular mechanisms of salinity tolerance. In ‘Cold, drought, heat, and salt stress: molecular responses in higher plants’. (Ed. K Shinozaki) pp. 29–60. (RG Landes: Austin, TX)
Bradford MM (1976) A rapid and sensitive for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72, 248–254.
| A rapid and sensitive for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE28XksVehtrY%3D&md5=bead43c1adb6f57157bea3eab849e63cCAS | 942051PubMed |
Brewster JL, de Valoir T, Dwyer ND, Winter E, Gustin MC (1993) An osmosensing signal transduction pathway in yeast. Science 259, 1760–1763.
| An osmosensing signal transduction pathway in yeast.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXksVCqs7Y%3D&md5=dbad38c95cfc1cf288a3fcfecdf82400CAS | 7681220PubMed |
Cabello-Hurtado F, Ramos J (2004) Isolation and functional analysis of the glycerol permease activity of two new nodulin-like intrinsic proteins from salt stressed roots of the halophyte Atriplex nummularia. Plant Science 166, 633–640.
| Isolation and functional analysis of the glycerol permease activity of two new nodulin-like intrinsic proteins from salt stressed roots of the halophyte Atriplex nummularia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhtFWgsLc%3D&md5=c17b3ffa3b349b3a25379745232e711fCAS |
Chanda B, Venugopal SC, Kulshrestha S, Navarre DA, Downie B, Vaillancourt L, Kachroo A, Kachroo P (2008) Glycerol-3-phosphate levels are associated with basal resistance to the emibiotrophic fungus Colletotrichum higginsianum in Arabidopsis. Plant Physiology 147, 2017–2029.
| Glycerol-3-phosphate levels are associated with basal resistance to the emibiotrophic fungus Colletotrichum higginsianum in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtVSrtrrI&md5=d419fb0cdd1c9e34948cad7b8eb67826CAS | 18567828PubMed |
Chen H, Lu Y, Jiang J-G (2012) Comparative analysis on the key enzymes of the glycerol cycle metabolic pathway in Dunaliella salina under osmotic stresses. PLoS ONE 7, e37578
| Comparative analysis on the key enzymes of the glycerol cycle metabolic pathway in Dunaliella salina under osmotic stresses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XosFWrt7g%3D&md5=61b3b62809e622bcbf0225b5a9d92f08CAS | 22675484PubMed |
Christensen AH, Quail P (1996) Ubiquitin promoter-based vectors for high level expression of selectable and/or screenable marker genes in monocotyledonous plant. Transgenic Research 5, 213–218.
| Ubiquitin promoter-based vectors for high level expression of selectable and/or screenable marker genes in monocotyledonous plant.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XjsFamsbo%3D&md5=b42afa42c807b08971648de7a2bc4008CAS | 8673150PubMed |
Dellaporta SL, Wood J, Hicks JB (1983) A plant DNA minipreparation, Version II. Plant Molecular Biology Reporter 1, 19–21.
| A plant DNA minipreparation, Version II.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2cXksFWhtrk%3D&md5=cb2ccf6382903bcf5f3af1622173bd02CAS |
Eastmond PJ (2004) Glycerol-insensitive Arabidopsis mutants, gli seedlings lack glycerol kinase, accumulate glycerol and are more resistant to abiotic stress. The Plant Journal 37, 617–625.
| Glycerol-insensitive Arabidopsis mutants, gli seedlings lack glycerol kinase, accumulate glycerol and are more resistant to abiotic stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXisVWhtr8%3D&md5=b15e74b493d1c531dee00e63943d6d13CAS | 14756771PubMed |
Edris S, Mutwakil MHZ, Sabir JSM, Ramadan AM, Gadalla NO, Shokry AM, Al-Kordy MA, Abo-Aba SEM, El-Domyati FM, Bahieldin A (2012) Identification of GPD1 gene from yeast via fluorescence differential display-polymerase chain reaction (FDD-PCR). African Journal of Biotechnology 11, 8653–8664.
Gerbeau P, Güçlü J, Ripoche P, Maurel C (1999) Aquaporin Nt-TIPa can account for the high permeability of tobacco cell vacuolar membrane to small neutral solutes. The Plant Journal 18, 577–587.
| Aquaporin Nt-TIPa can account for the high permeability of tobacco cell vacuolar membrane to small neutral solutes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXlsFGhsbw%3D&md5=26ae554b9676fbef78954842dee2e344CAS | 10417709PubMed |
Gomez KA, Gomez AA (1984) ‘Statistical procedures for agricultural research.’ 2nd edn. (John Wiley & Sons: New York)
Gunde-Cimerman N, Ramos J, Plemenitas A (2009) Halotolerant and halophilic fungi. Mycological Research 113, 1231–1241.
| Halotolerant and halophilic fungi.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXkvF2nug%3D%3D&md5=da60ec1347e2ea6edb042958a2d255ecCAS | 19747974PubMed |
Hohmann S (2009) Control of high osmolarity signaling in the yeast Saccharomyces cerevisiae. FEBS Letters 583, 4025–4029.
| Control of high osmolarity signaling in the yeast Saccharomyces cerevisiae.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFams7fI&md5=e1e2790318067184296d51efbbe3376cCAS | 19878680PubMed |
Horie T, Kaneko T, Sugimoto G, Sasano S, Panda SK, Shibasaka M, Katsuhara M (2011) Mechanisms of water transport mediated by PIP aquaporins and their regulation via phosphorylation events under salinity stress in barley roots. Plant & Cell Physiology 52, 663–675.
| Mechanisms of water transport mediated by PIP aquaporins and their regulation via phosphorylation events under salinity stress in barley roots.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXksFKju7s%3D&md5=a3127f9bb00c4c30834f87753d5e3ccfCAS |
Hubmann G, Guillouet S, Nevoigt E (2011) Gpd1 and Gpd2 fine-tuning for sustainable reduction of glycerol formation in Saccharomyces cerevisiae. Applied and Environmental Microbiology 77, 5857–5867.
| Gpd1 and Gpd2 fine-tuning for sustainable reduction of glycerol formation in Saccharomyces cerevisiae.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXht1OjurvN&md5=78b0973e161d38a9c3a18eacbd586316CAS | 21724879PubMed |
Klepek YS, Geiger D, Stadler R, Klebl F, Landouar-Arsivaud L, Lemoine R, Hedrich R, Sauer N (2005) Arabidopsis POLYOL TRANSPORTER5, a new member of the monosaccharide transporter-like superfamily, mediates H+-symport of numerous substrates, including myo-inositol, glycerol, and ribose. The Plant Cell 17, 204–218.
| Arabidopsis POLYOL TRANSPORTER5, a new member of the monosaccharide transporter-like superfamily, mediates H+-symport of numerous substrates, including myo-inositol, glycerol, and ribose.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXotlantg%3D%3D&md5=ba67114fb70fcc193280645ed8a92fcfCAS | 15598803PubMed |
Kunst L, Browse J, Somerville C (1988) Altered regulation of lipid biosynthesis in a mutant of Arabidopsis deficient in chloroplast glycerol-3-phosphate acyltransferase activity. Proceedings of the National Academy of Sciences of the United States of America 85, 4143–4147.
| Altered regulation of lipid biosynthesis in a mutant of Arabidopsis deficient in chloroplast glycerol-3-phosphate acyltransferase activity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1cXkvV2ru7Y%3D&md5=5fcd1886003fb5bd07c69698af2cc3fbCAS | 16593939PubMed |
Lawrence CL, Botting CH, Antrobus R, Coote PJ (2004) Evidence of a new role for the high-osmolarity glycerol mitogen-activated protein kinase pathway in yeast: regulating adaptation to citric acid stress. Molecular and Cellular Biology 24, 3307–3323.
| Evidence of a new role for the high-osmolarity glycerol mitogen-activated protein kinase pathway in yeast: regulating adaptation to citric acid stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXjtFKjtrc%3D&md5=07550b279555da0e251dfb19b576654cCAS | 15060153PubMed |
Lee HK, Cho SK, Son O, Xu Z, Hwang I, Kim WT (2009) Drought stress-induced Rma1H1, a RING membrane-anchor E3 ubiquitin ligase homolog, regulates aquaporin levels via ubiquitination in transgenic Arabidopsis plants. The Plant Cell 21, 622–641.
| Drought stress-induced Rma1H1, a RING membrane-anchor E3 ubiquitin ligase homolog, regulates aquaporin levels via ubiquitination in transgenic Arabidopsis plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXksVKrt7o%3D&md5=a23aa29d71f90027b590a4160762d8deCAS | 19234086PubMed |
Li SY, Gomelsky M, Duan J, Zhang Z, Gomelsky L, Zhang X, Epstein PN, Ren J (2004) Overexpression of Aldehyde Dehydrogenase-2 (ALDH2) Transgene prevents acetaldehyde-induced cell injury in human umbilical vein endothelial cells. The Journal of Biological Chemistry 279, 11 244–11 252.
| Overexpression of Aldehyde Dehydrogenase-2 (ALDH2) Transgene prevents acetaldehyde-induced cell injury in human umbilical vein endothelial cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXitFyhsLY%3D&md5=180732e03bc70adeca04bafbaec15266CAS |
Liu WZ, Faber R, Feese M, Remington SJ, Pettigrew DW (1994) Escherichia coli glycerol kinase, role of a tetramer interface in regulation by fructose 1,6-biphosphate and phosphotransferase system regulatory protein IIIglc. Biochemistry 33, 10 120–10 126.
| Escherichia coli glycerol kinase, role of a tetramer interface in regulation by fructose 1,6-biphosphate and phosphotransferase system regulatory protein IIIglc.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXltVarsLg%3D&md5=30ffb6bf324c9d4730a7c2f825343dd3CAS |
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔ C t method. Methods 25, 402–408.
| Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔ C t method.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XhtFelt7s%3D&md5=cda22da73d6034ad2e4c74827f633baaCAS | 11846609PubMed |
Luyten K, Albertyn J, Skibbe WF, Prior BA, Ramos J, Thevelein JM, Hohmann S (1995) Fps1, a yeast member of the MIP family of channel proteins, is a facilitator for glycerol uptake and efflux and is inactive under osmotic stress. EMBO Journal 14, 1360–1371.
Mandel MK, Chanda B, Xia Y, Yu K, Sekine K-T, Gao Q-M, Selote D, Kachroo A, Kachroo P (2011) Glycerol-3-phosphate and systemic immunity. Plant Signaling & Behavior 6, 1–4.
Melamed D, Pnueli L, Arava Y (2008) Yeast translational response to high salinity: global analysis reveals regulation at multiple levels. RNA 14, 1337–1351.
| Yeast translational response to high salinity: global analysis reveals regulation at multiple levels.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXot1amtL8%3D&md5=c8314ef0aa8f40af7c39b3a7678f1c2dCAS | 18495938PubMed |
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum 15, 473–497.
| A revised medium for rapid growth and bio assays with tobacco tissue cultures.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF3sXksFKm&md5=00e945f2329f915ef516a05110061063CAS |
Padamsee M, Kumar TKA, Riley R, Binder M, Boyd A, Calvo AM, Furukawa K, Hesse C, Hohmann S, James TY, LaButti K, Lapidus A, Lindquist E, Lucas S, Miller K, Shantappa S, Grigoriev IV, Hibbett DS, McLaughlin DJ, Spatafora JW, Aime MC (2012) The genome of the xerotolerant mold Wallemia sebi reveals adaptations to osmotic stress and suggests cryptic sexual reproduction. Fungal Genetics and Biology 49, 217–226.
| The genome of the xerotolerant mold Wallemia sebi reveals adaptations to osmotic stress and suggests cryptic sexual reproduction.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XjslemsLs%3D&md5=3d828d2d3527a693b629e38cc161ce6cCAS | 22326418PubMed |
Påhlman A-K, Granath K, Ansell R, Hohmann S, Adler L (2001) The yeast glycerol 3-phosphatases Gpp1p and Gpp2p are required for glycerol biosynthesis and differentially involved in the cellular responses to osmotic, anaerobic, and oxidative stress. Journal of Biological Chemistry 276, 3555–3563.
| The yeast glycerol 3-phosphatases Gpp1p and Gpp2p are required for glycerol biosynthesis and differentially involved in the cellular responses to osmotic, anaerobic, and oxidative stress.Crossref | GoogleScholarGoogle Scholar | 11058591PubMed |
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research 29, e45
| A new mathematical model for relative quantification in real-time RT-PCR.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD38nis12jtw%3D%3D&md5=aa80c9eea3a1151f965b4bfe903672daCAS | 11328886PubMed |
Rawls KS, Martin JH, Maupin-Furlow JA (2011) Activity and transcriptional regulation of bacterial protein-like glycerol-3-phosphate dehydrogenase of the haloarchaea in Haloferax volcanii. Journal of Bacteriology 193, 4469–4476.
| Activity and transcriptional regulation of bacterial protein-like glycerol-3-phosphate dehydrogenase of the haloarchaea in Haloferax volcanii.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtV2gtbrK&md5=9f4b0a83750a7f3baa39f91806602818CAS | 21725010PubMed |
Ruzheinikov SN, Burke J, Sedelnikova S, Baker PJ, Taylor R, Bullough PA, Muir NM, Gore MG, Rice DW (2001) Glycerol dehydrogenase, structure, specificity, and mechanism of a family III polyol dehydrogenase. Structure 9, 789–802.
| Glycerol dehydrogenase, structure, specificity, and mechanism of a family III polyol dehydrogenase.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXntVajsrY%3D&md5=56116602632265f85b7691285b5ae209CAS | 11566129PubMed |
Schubert D, Lechtenberg B, Forsbach A, Gils M, Bahadur S, Schmidt R (2004) Silencing in Arabidopsis T-DNA transformants, the predominant role of a gene-specific RNA sensing mechanism versus position effects. The Plant Cell 16, 2561–2572.
| Silencing in Arabidopsis T-DNA transformants, the predominant role of a gene-specific RNA sensing mechanism versus position effects.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXptVSiu7o%3D&md5=898d022c275dda1543852e6b0c9d74d3CAS | 15367719PubMed |
Shen W, Wei Y, Dauk M, Tan Y, Taylor DC, Selvaraj G, Zou J (2006) Involvement of a glycerol-3-phosphate dehydrogenase in modulating the NADH/NAD+ ratio provides evidence of a mitochondrial glycerol-3-phosphate shuttle in Arabidopsis. The Plant Cell 18, 422–441.
| Involvement of a glycerol-3-phosphate dehydrogenase in modulating the NADH/NAD+ ratio provides evidence of a mitochondrial glycerol-3-phosphate shuttle in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xhs1ensr4%3D&md5=b5314890660680a171580d9f7ff8ec48CAS | 16415206PubMed |
Venugopal SC, Chanda B, Vaillancourt L, Kachroo A, Kachroo P (2009) The common metabolite glycerol-3-phosphate is a novel regulator of plant defense signaling. Plant Signaling & Behavior 4, 746–749.
| The common metabolite glycerol-3-phosphate is a novel regulator of plant defense signaling.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhs1Knsbc%3D&md5=4d2e63645c86056130c7bba475b3ca92CAS |
Wei Y, Shen W, Dauk M, Wang F, Selvaraj G, Zou J (2004) Targeted gene disruption of glycerol-3-phosphate dehydrogenase in Colletotrichum gloeosporioides reveals evidence that glycerol is a significant transferred nutrient from host plant to fungal pathogen. Journal of Biological Chemistry 279, 429–435.
| Targeted gene disruption of glycerol-3-phosphate dehydrogenase in Colletotrichum gloeosporioides reveals evidence that glycerol is a significant transferred nutrient from host plant to fungal pathogen.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXhtVSqt7%2FL&md5=630027f9446ebaf8cc141fc2e4f7c2a2CAS | 14563847PubMed |


