The shoot and root growth of Brachypodium and its potential as a model for wheat and other cereal crops
Michelle Watt A C , Katharina Schneebeli A , Pan Dong A B and Iain W. Wilson AA CSIRO Plant Industry, GPO Box 1600, Canberra, ACT 2601, Australia.
B Triticeae Research Institute, Sichuan Agricultural University, Yaan, Sichuan 625014, China.
C Corresponding author. Email: michelle.watt@csiro.au
Functional Plant Biology 36(11) 960-969 https://doi.org/10.1071/FP09214
Submitted: 11 August 2009 Accepted: 18 September 2009 Published: 5 November 2009
Abstract
The grass genetic model Brachypodium (Brachypodium distachyon (L.) Beauv., sequenced line Bd 21) was studied from germination to seed production to assess its potential as a phenotypic model for wheat (Triticum aestivum L.) and other cereal crops. Brachypodium and wheat shoot and root development and anatomy were highly similar. Main stem leaves and tillers (side shoots) emerged at the same time in both grasses in four temperature and light environments. Both developed primary and nodal axile roots at similar leaf stages with the same number and arrangement of vascular xylem tracheary elements (XTEs). Brachypodium, unlike wheat, had an elongated a mesocotyl above the seed and developed only one fine primary axile root from the base of the embryo, while wheat generally has three to five. Roots of both grasses could develop first, second and third order branches that emerged from phloem poles. Both developed up to two nodal axile roots from the coleoptile node at leaf 3, more than eight nodal axile roots from stem nodes after leaf 4, and most (97%) of the deepest roots at flowering were branches. In long days Brachypodium flowered 30 days after emergence, and root systems ceased descent 42 cm from the soil surface, such that mature roots can be studied readily in much smaller soil volumes than wheat. Brachypodium has the overwhelming advantage of a small size, fast life cycle and small genome, and is an excellent model to study cereal root system genetics and function, as well as genes for resource partitioning in whole plants.
Additional keywords: Arabidopsis, architecture, genome, monocotyledons, photoperiod, root anatomy, tillering.
Introduction
This paper presents a description of Brachypodium distachyon (L.) Beauv. (Brachypodium) sequenced line Bd 21 shoot and root systems from the seedling to grain filling stages. The aim was to assess its potential to identify genes associated with the development and function of wheat (Triticum aestivum L.) root systems. Brachypodium has been developed as a model for the genetics of cool climate grasses for fuel and crop cereals (Draper et al. 2001; Opanowicz et al. 2008; Vogel and Bragg 2009). It has all the desirable features of a genetic model including a small genome, short generation time between emergence and seed yield, and easy growth requirements. It is an annual, inbreeding and can be readily transformed (Vogel et al. 2006). The entire genome of diploid line Bd 21 is sequenced and publically available (J. Vogel, unpubl. data; http://www.Brachypodium.org/, accessed 30 September 2009).
Brachypodium originates from the Fertile Crescent, the geographic origin of wheat, and is considered a temperate grass suited to cooler conditions (Vogel et al. 2009). On the phylogeny trees of the grass family Poaceae, the genus Brachypodium is positioned with the temperate cereal genera Triticum, Hordeum and Avena in the subfamily Pooideae (Kellogg 2001; Opanowicz et al. 2008). It can be expected that the environmental and soil conditions favourable to wheat growth are similar to Brachypodium, their root–rhizosphere interactions may be similar, as well as root architectures for different moisture and nutrient availabilities. Importantly, at maturity Brachypodium is much smaller than cereal crops, including other genetic models such as rice (Oryza sativa L.) and maize (Zea mays L.) and it can be grown in controlled conditions in small volumes of soil without greatly crowding roots and compromising their growth and function (Fig. 1). Brachypodium presents a new opportunity to identify genes associated with the growth and function of the roots of important cereal crops such as wheat, especially at more mature plant stages that are very important in field grown cereals.

|
Arabidopsis thaliana (L.) is a small-sized genetic model, and has been the source of most genetic information for roots for nearly 20 years (Schiefelbein and Benfey 1991; Osmont et al. 2007). However, it is a dicotyledon and member of the Brassicaceae family. Dicotyledons develop a tap root system with vascular cambia that produce new xylem and phloem with development (Esau 1977). Monocotyledons like Brachypodium, and all members of the grass family, develop a ‘fibrous’ root system with no vascular cambia, and roots that emerge from the base of the embryo within the seed (primary or seminal axile roots), and from successive nodes along the stem (nodal axile roots) (Klepper et al. 1984; Wenzel et al. 1989; Hochholdinger et al. 2004; Watt et al. 2008). The water transport through dicotyledons and monocotyledons is different due, in part, to their development and anatomy (Bramley et al. 2009).
Other cereal models such as rice and maize are also very useful for identifying root-associated genes (Hochholdinger et al. 2004; Hochholdinger and Zimmermann 2008). However, they grow in warm conditions. Rice, in particular, is adapted to waterlogged conditions, unlike wheat. Their large adult size means both are difficult to screen in the laboratory to maturity. The root systems quickly reach the bottom of pots and become crowded and impeded, and their deep root development and physiology may not be relevant to that of field roots. Mature root system architecture and function are valuable to yield because they determine the water that can be taken up around flowering and grain filling, which contributes directly to final yield and crop water use efficiency (Passioura 1983; Manschadi et al. 2006; Kirkegaard et al. 2007). Directly selecting and validating mature root systems traits in crops, especially deep in the soil, is challenging. In the field, this involves direct coring or time-consuming neutron moisture metre readings. By the time of flowering, many crop root systems extend more than 1 m below ground, and it is impossible to capture entire root systems in the field unless constrained in buried tubes. In controlled environments, root systems can be grown to maturity in root boxes greater than 1 m deep with clear faces, to map the spatial distribution of the entire root system to yield (Manschadi et al. 2006). However, these boxes are time-consuming and would be impractical to use on large numbers of lines in a genetics program. Most screening is performed on seedling roots in very small volumes or on agar or other artificial media in the laboratory, and it is unclear if these seedling traits contribute to mature root system traits and yield in the field. Here, we grew Brachypodium alongside wheat in soil in four environments and tracked the shoot and root systems growth and anatomical development, from germination to grain filling, to assess the potential of Brachypodium to identify genes regulating the root systems of wheat (cv. Janz), especially at adult plant stages.
Materials and methods
Growing environments
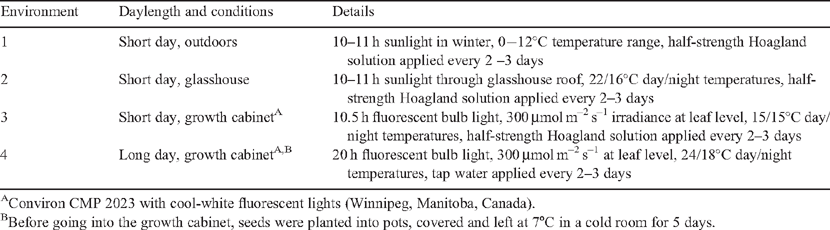
Brachypodium distachyon (L.) Beauv. (line Bd 21) seeds were kindly provided by Dr D. F. Garvin (USDA-ARS Plant Science Research Unit, University of Minnesota, MN, USA). Brachypodium was grown alongside wheat (Triticum aestivum L. cv. Janz) in the four environments described in Table 1 to compare their timing of root and shoot development, and to establish conditions that resulted in mature Brachypodium root systems that did not touch the bottoms of pots at grain filling. Three of the environments were short day, cooler conditions similar to those that occur in the field during wheat establishment and vegetative growth in Australia and other regions where wheat is grown in cool winter months; the fourth environment had long days and warmer temperatures to promote more rapid flowering (from Vogel and Bragg 2009).
|
For all experiments, seeds of Brachypodium and wheat were planted with the embryo of the seed facing the base of the pot, ~2 cm below the soil surface. For studies in the three short day environments, the husks were removed from the Brachypodium seeds and the seeds planted directly into soil and allowed to germinate in the conditions described above. For the long day environment studies, the husks were not removed, the seeds planted, soil covered and pots vernalised at 7°C in a cold room for 5 days, before transfer to the growth cabinet where the plastic cover was removed. Husk removal may have sped up germination but the effect was marginal. Plants were grown in a soil that was a mix of 50% river sand and 50% recycled potting soil (organic matter, loam and sand supplemented with lime, 14.4% N, 6.6% P and 5% K). It was chosen because it drains well and washes off roots easily and was used to characterise wheat, barley (Hordeum vulgare L.) and triticale (x Triticosecale) root systems in Watt et al. (2008). Plants were grown in PVC tubes of loosely packed soil that were 7 cm wide, either 25 cm or 50 cm deep, and sealed at the bottom with a Petri dish lid with holes for drainage. All plants were watered with tap water or half strength Hoagland’s nutrient solution from the tops of the pots. Plants in environments 1 to 3 in the short day conditions received nutrients approximately twice a week; plants in environment 4 in long days did not receive nutrients additional to those in the soil at sowing.
Shoot observations
Leaf and tiller (side shoot) emergence were scored every 2 to 3 days according to the code developed by Zadoks et al. (1974). Flowering times were also noted, judged to be at the time that the stems had elongated, heads had emerged partially and a few whitish stamens could be seen emerged from the florets. Leaf lengths and plant heights were measured with a ruler.
Brachypodium root system measurements
Tubes were harvested at leaf 1, 2, 3, 4–5 and 7, and roots gently washed by first saturating the soil in the pot, then removing the bottom and tilting the pots to slide out the soil and roots onto a mesh tray to avoid breaking any fine roots. The remaining soil was washed away with a gentle spray of water and the entire shoot and intact root system floated in a large tray with water and a black background. The roots were gently disentangled and spread out to identify the positions of emergence of the axile roots around the seed and stem, and these were recorded in diagrams. The lengths of the axile roots and maximum root system depth were measured with a ruler, noting which types of roots penetrated deepest in plants at the onset of flowering and at grain filling. The root systems were then preserved in 50% ethanol until stained, scanned using an Epson 1680 flatbed scanner (Regent Instruments, Quebec, Canada) at 800 dpi resolution and analysed for total root length (axile plus branch roots) using WINRhizo software (Regent Instruments) (Watt et al. 2005) or used for the detailed morphological and anatomical studies described below.
Detailed morphological and anatomical studies of Brachypodium root systems
The orders of branching along primary and nodal axile roots were scored under a dissecting microscope in a shallow dish of water. The diameters of different root types at their base and tip were measured whole-mounted on slides using an eyepiece micrometer with brightfield optics with a compound microscope. Anatomies of the different axile and branch root types were determined from transverse sections of pieces of root (~2 cm long) excised from the basal regions of axile roots and from excised branch roots using the methods described in Watt et al. (2008). Briefly, the pieces were frozen in liquid nitrogen, mounted in low-temperature mounting medium on stubs in the chamber of a cryo-microtome (Leica CM 1850; Leica Microsystems, Wetzlar, Germany), sections ~4 to 8 μm thick were made, mounted in water on adhesive slides (Star-Frost Poly-L-lysine slides; ProSci Tech, Thuringowa, Qld, Australia), dried on a warm plate, stained with Rhodamine B (0.0005% w/w in water) and viewed with UV optics using a Leica DMR (Leica Microsystems) compound microscope. Rhodamine B is a metachromatic dye that fluoresces differently when excited by UV illumination depending on the wall compound it binds to. The method results in sections with easily distinguishable central and peripheral xylem tracheary elements (XTEs). Their number and arrangement were quantified to classify root types similar to the approach used for wheat, barley and triticale roots (Watt et al. 2008).
Results
Shoots
The number and timing of emergence of leaves along the main stems of Brachypodium and wheat were nearly identical within each of the four growing environments (Fig. 2). Tiller emergence relative to main stem leaf emergence was also nearly identical for the two grasses in the shorter and longer day environments (Fig. 3) with each developing four main stem tillers at similar stages of the development of six main stem leaves. The shoots of the two grasses appeared similar with the exception that Brachypodium was shorter (~3.5 times shorter in the longer day, cool conditions; and ~2 times shorter in the long day, warmer conditions), more prostrate and the leaves were smaller. The time to flowering strongly depended on the growing environment for both grasses. By 100 days post sowing, flowering had not occurred in the grasses outdoors (environment 1, Table 1) and they developed numerous secondary tillers. In the glasshouse (environment 2), Brachypodium flowered by 85 days and wheat by 90 days post sowing. In the growth cabinet with cool, short days (environment 3), Brachypodium and wheat flowered by ~70 days post sowing. In contrast, the time to flowering in the long day conditions (environment 4) was much shorter in Brachypodium, which flowered ~30 days post emergence; wheat flowered ~60 days post emergence. Brachypodium line Bd 21 appeared to have strong photoperiod sensitivity, although this shorter time to flowering in the long day conditions may have also depended on the cold treatment before transfer to the growth cabinet that was unique to this treatment.

|

|
Root system development
Brachypodium axile root development relative to leaf and tiller development is illustrated in Fig. 4. This sequence was observed in the short and long day conditions and is summarised from observations, drawing and measurements from over 50 plants of each species grown in eight separate experiments. The Brachypodium plants grown in the long days reached maturity, flowered and produced grain before the root systems reached the bottoms of the 50-cm tubes (Fig. 5), so most of the deep root observations are from mature plants in those conditions.

|

|
Brachypodium developed one primary axile root that emerged from the base of the embryo at germination and was the only axile root of the plant until leaf 3. This root had no pith and generally one central XTE and four to eight peripheral XTEs (Fig. 6d). Wheat, as is well known, developed three to five primary axile roots from the base of the embryo. This difference was observed in all experiments, including two supplemental experiments to confirm that the difference did not depend on growing conditions. At 15°C in the dark on moist filter paper, Brachypodium germinated within 2 days growing a primary axile root from the base of the embryo; within 4 days, wheat had germinated and grown three primary axile roots while Brachypodium had one; within 7 days, Brachypodium had one primary axile root while wheat had three to four. In the supplemental experiment in the long day, warmer conditions (environment 4, Table 1) in soil, wheat developed five primary axile roots by 2 to 2.3 leaves, ranging from 0.2 to 30.3 cm long, while Brachypodium had two to three leaves at the same time from sowing and had grown only one primary axile root 10.1–12.8 cm long. Brachypodium did not develop a scutellar node axile root in these experiments, which can emerge in wheat from within the seed when the plant is between leaf 1 and leaf 2 (Klepper et al. 1984; Watt et al. 2008). At leaf 3, up to two axile roots emerged from a node usually ~1 cm above the Brachypodium seed and below the soil surface. Occasionally, no roots emerged from this node and in some conditions, the node was just above or within the seed. The anatomy at the base of the root was generally typical of nodal roots, and comprised of pith surrounded by up to six central XTEs and seven to nine peripheral XTEs (e.g. Fig. 6e). But occasionally no pith was present at the base and this root appeared as a primary axile root. We were unclear which stem node this root emerged from, and although it may be the node associated with leaf 1, we think likely it is the node associated with the coleoptile and term these roots coleoptile node axile roots, similar to those in wheat. Cross-sections above this node indicated stem tissue with vascular bundles around a pith of parenchyma cells (Fig. 6a). Cross-sections through the node (Fig. 6b) and below the node (Fig. 6c) revealed complex vascular arrangements of cells in transition from the arrangement in the stem above with discrete vascular bundles, and that in the seminal axile root below the seed, with a central xylem tracheary element (XTE) (Fig. 6d). This elongated internode is likely a mesocotyl which did not elongate in wheat in the experimental conditions in this study. By leaf 5, axile roots had emerged from the stem nodes associated with the main stem leaves, and the base of these roots had central piths with four to seven central XTEs and 8 to 15 peripheral XTEs (Fig. 6e). By leaf 6 to 7, many roots had emerged from the stem nodes. In the long day conditions, when flowering commenced at six to seven leaves, about nine leaf node axile roots were present per plant (8.8 ± 1.1, mean ± s.d.; n = 5 plants). An example of the shifting contribution to total root system length from the primary to the nodal systems is quantified in Table 2. At leaf 6, the seminal and coleoptile node axile roots comprised a large (80%) percentage of the total root length and the leaf node axile roots only 20% of the total root length; at leaf 8.5, the leaf node axile roots comprise 56% of the total root length and the number increased from 8 to 22. The primary axile root was noticeably finer (mean diameter 220 µm) than the nodal axile roots (mean diameter 361 µm for coleoptile nodal roots; 354 µm for leaf node roots; Table 3), and by leaf 3, had many long first order branch roots, plus second and occasionally third order branch roots. It contributed a large root length with high specific root length for Brachypodium growth and functioning until about leaf 4. Between leaf 5 and leaf 7, nodal system growth relative to the primary root was rapid (Fig. 4).

|

|
All Brachypodium axile roots had the potential to develop first, second and third order branch roots that ranged in diameter from 47 to 375 µm (Table 3). These belonged to one of seven types based on their stele anatomy and number and arrangement of XTEs. They ranged from the most complex type with seven XTEs (Fig. 6f) to the simplest type with one central XTE (Fig. 6g). Branch roots emerged from the phloem poles from axile (Fig. 6h) and branch roots (Fig. 6i). The different orders of branch root could be one of the seven types of branch root, regardless of the axile root type.
Deepest roots around flowering and grain filling
In the longer day conditions that promoted quicker flowering, the root systems ceased descent around 42 cm from the soil surface (Fig. 5). The root systems descended initially at 0.86 cm per day until plants had four to five leaves, then descended at more than double that rate at 2.45 cm per day, to leaf 6 to 7 and flowering then greatly decreased descent rate during grain filling to 0.15 cm per day (not significant). On average from the beginning to the end of vegetative growth, the root system descended at ~1.5 cm per day but the rate varied through plant growth.
The bottom third of the root systems of plants post flowering were dominated by branch roots which made up 97 ± 3% (mean ± s.d., n = 7 plants) of the total roots present. First order branch roots made up 58 ± 30% of total roots; second order branch roots made up 39 ± 33% of the total roots; third order roots were not observed in deep layers. The deepest axile roots were a mixture of the primary root and coleoptile and leaf node axile roots (ratio of ~1 primary axile root to 4 nodal axile roots).
Discussion
The similarities and differences between Brachypodium and wheat root systems are summarised in Fig. 7. Key similarities are that Brachypodium and wheat root systems had: (1) the same types of axile roots; (2) nearly identical timing of main stem leaf and tiller emergence and timing of emergence of axile roots; (3) very similar branch root types, sharing five types, with Brachypodium having two extra types; and (4) deep roots comprising of mainly branch roots. The cereals had the same vascular anatomies especially the number and arrangement of the XTEs in axile and branch roots. Branch roots in Brachypodium emerged from the phloem poles, in contrast to Arabidopsis where they emerge from the xylem poles. It is very useful that the vascular anatomies of the axile and branch roots are common to wheat and to maize (compare with reports in Hoppe et al. 1986; Varney et al. 1991; Watt et al. 2008). This provides opportunities to understand water and carbohydrate flow through the entire and intact root systems, relative to shoot development in grasses and to contrast those with the dicotyledon genetic model Arabidopsis.

|
A notable difference between Brachypodium and the small grain cereals wheat, barley, triticale and oats (Avena sativa L.) is that the young root systems of B. distachyon line Bd 21 are much simpler with fewer numbers and types of axile roots. Brachypodium had one primary axile root from the seed, did not produce a scutellar node axile root and the development of axile roots from the node above the seed was slightly delayed compared with that of the wheat coleoptile node roots, or may have been associated with leaf one main stem node. Brachypodium may have genes regulating early root types that are more closely related to the warm climate cereals, rice, maize, sorghum (Sorghumm bicolour L.), pearl millet (Pennisatum glaucum L.) and the forage grasses such as smooth bromegrass (Bromus inermis Leyss), which all generally have one primary axile root (Hoshikawa 1969; Wenzel et al. 1989; Ries and Hofmann 1991; Hochholdinger et al. 2004; M. Watt, unpubl. data). Brachypodium is currently positioned on phylogeny trees close to maize and sorghum and Bromus spp. (Kellogg 2001; Opanowicz et al. 2008, see fig. 7 therein). However, axile root number can vary within species including wheat and maize (reviewed in O’Toole and Bland 1987) and within Brachypodium. Hoshikawa (1969) conducted a survey of the seedling roots of over 219 species within 88 grass genera and reported that the Brachypodium genotypes examined had more than one primary axile root. Numbers of axile roots at the three-leaf stage in Brachypodium and cereal crops may be due to seed or embryo size. Mac Key (1978) reported one seminal root in the primitive wheat Aegilops mutica, up to six in Triticum durum L. and suggested more early axile roots may be a result of selection in cultivation and breeding programs for larger seeds. The low number of seedling axile roots in B. distachyon line Bd 21 is an opportunity to identify the genes associated with greater numbers of early seedling roots among the diverse Brachypodium genotypes now available (Vogel et al. 2009). This will enable us to better understand their contribution relative to nodal roots to mature plant function.
Interestingly, Brachypodium had an elongated internode between the seed and the coleoptile node in the soil-grown conditions here when planted 2 cm below the soil surface (Fig. 7). The elongated internode in Brachypodium is likely a mesocotyl and the anatomy is transitional between that of the stem above and that of the primary axile root below (Fig. 6a–d) (see also Raju and Steeves 1998). Mesocotyls are reported in other grasses including maize and oats (Radford and Key 1993) and in smooth bromegrass (Ries and Hofmann 1991). Wheat did not have an elongated internode in this position in our experiments. A mesocotyl may be a difference between Brachypodium and wheat, although wheat genotypes may elongate a mesocotyl under certain conditions such a very deep planting. Hoshikawa (1969) classified Brachypodium as having a ‘more or less’ elongated mesocotyl. The observations that Brachypodium readily develops a mesocotyl and only one axile primary root suggests that Brachypodium shares developmental pathways with the warm climate cereals maize and rice and with members of the Bromus genus (Kellogg 2001; Bossolini et al. 2007).
The mature root systems of wheat and Brachypodium were nearly identical and grew to maturity within 50-cm tubes, enabling intense study to grain filling. This provides an opportunity to use Brachypodium to identify mature root system developmental and functional genes in wheat. The pattern of penetration of the Brachypodium roots was of interest, as it was slower initially, and then sped up greatly until flowering (Fig. 5). The pattern of descent was similar to that obtained from corings of root system depth of wheat in the field over a season (see fig. 4 in Gregory et al. 1978). They report a two-phase penetration rate; the first, between the surface and 1 m depth, was three times slower than the second, between 1 and 2 m depth. They suggest that this may be due to the winter and spring seasons. Kirkegaard and Lilley (2007) reviewed root system depth measurements across southern Australia and report a mainly linear pattern of root system descent for wheat. However, penetration was slightly quicker earlier in the winter during early crop growth, between 0.3 and 0.8 m, and slower later in the season between 0.8 and 1.4 m (see fig. 3 in Kirkegaard and Lilley 2007). Working in controlled environments, Derera et al. (1969) report a three-phase penetration pattern in wheat root systems; the first slow, to 0.2 or 0.3 m; the second rapid, to 1 m, and the third slow, but with rapid nodal axile root growth and branching. Brachypodium offers an opportunity to better understand root system growth through time and shoot development, and variation due to plant genetics and field variability which has long been poorly understood for plant roots.
The mature root systems of Brachypodium were dominated by branch roots which made up 97% of the total roots present. This is identical to the composition of deep field-grown wheat roots, quantified using the anatomy of cored, washed and sectioned roots (Watt et al. 2008). The axile Brachypodium roots were a mixture of the primary root and coleoptile and leaf nodal roots and these were readily identified because it was possible to follow the deep axile roots to their position of emergence after washing from soil. The finding of nodal roots among the bottom 30% of the root system contradicts our previous findings with field-grown wheat (Watt et al. 2008) and those of Weaver (1926). Araki and Iijima (2001) reported that the nodal roots from the coleoptile and stem nodes of leaf 1 and 2 can grow as long as the primary axile roots in certain wheat cultivars. Weaver (1926) followed axile roots from the seed to depth in the field and report that the nodal roots did not reach the deepest layers. Weaver (1926) may have mistaken the scutellar, coleoptile and leaf 1 nodal roots, which emerge close to the seed if planted at a shallow depth, with the primary axile roots. Brachypodium can be used to quantify the relative contribution of the primary and nodal systems at different depths in freely-growing root systems to yield. This is an area of current investigation using Brachypodium, tracking individual root axes and water uptake through time to grain filling, using new non-invasive imaging phenotyping methods. The relative rates of growth of the different Brachypodium root axes with time and shoot development to grain filling is an important area for research to improve root system genetics, coupled with studies to see if similar processes can be enhanced in wheat.
Conclusions
Brachypodium root systems had a high degree of developmental and anatomical similarity to wheat root systems, especially at the adult plant stages. Its small stature and identical coordination between root and shoot development to wheat means the function of genes regulating partitioning and water uptake of adult root systems can be studied intensely. Because Brachypodium had fewer numbers of seedling axile roots, there are opportunities to select for more of these root types among the diverse genetic resources available in Brachypodium (Vogel et al. 2009). Brachypodium is likely to speed up cereal root system genetic and functional discoveries.
Acknowledgements
We are grateful to Dr Dave Garvin for supplying the Brachypodium distachyon line Bd 21 seed, Dr John Vogel for advice on growing conditions and Drs Frank Hochholdinger, Margaret McCully and Richard Richards for valuable suggestions to the manuscript. This study was funded in part by a CSIRO Julius Award to MW and KS, a CSIRO-Ching Scholarship to PD and the Grains Research and Development Corporation (GRDC).
Araki H, Iijima M
(2001) Deep rooting in winter wheat: rooting nodes of deep roots in two cultivars with deep and shallow root systems. Plant Production Science 4, 215–219.
|
CAS |
Crossref |
PubMed |

Bossolini E,
Wicker T,
Knobel PA, Keller B
(2007) Comparison of orthologous loci from small grass genomes brachypodium and rice: implications for wheat genomics and grass genome annotation. The Plant Journal 49, 704–717.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Bramley H,
Turner NC,
Turner DW, Tyerman SD
(2009) Roles of morphology, anatomy, and aquaporins in determining contrasting hydraulic behavior of roots. Plant Physiology 150, 348–364.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Derera NF,
Marshall DR, Balaam NL
(1969) Genetic variability in root development in relation to drought tolerance in spring wheats. Experimental Agriculture 5, 327–337.
| Crossref | GoogleScholarGoogle Scholar |

Draper J,
Mur LAJ,
Jenkins G,
Ghosh-Biswas GC,
Bablak P,
Hasterok R, Routledge APM
(2001)
Brachypodium distachyon. A new model system for functional genomics in grasses. Plant Physiology 127, 1539–1555.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Gregory PJ,
McGowan M,
Biscoe PV, Hunter B
(1978) Water relations of winter wheat. I. Growth of the root system. The Journal of Agricultural Science 91, 91–102.
| Crossref | GoogleScholarGoogle Scholar |

Hochholdinger F, Zimmermann R
(2008) Conserved and diverse mechanisms in root development. Current Opinion in Plant Biology 11, 70–74.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Hochholdinger F,
Park WJ,
Sauer M, Woll K
(2004) From weeds to crops: genetic analysis of root development in cereals. Trends in Plant Science 9, 42–48.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Hoppe D,
McCully ME, Wenzel CL
(1986) The nodal roots of Zea: their development in relations to structural features of the stem. Canadian Journal of Botany 64, 2524–2537.

Hoshikawa K
(1969) Underground organs of the seedlings and the systematics of Gramineae. Botanical Gazette (Chicago, Ill.) 130, 192–203.
| Crossref | GoogleScholarGoogle Scholar |

Kellogg EA
(2001) Evolutionary history of the grasses. Plant Physiology 125, 1198–1205.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Kirkegaard JA, Lilley JM
(2007) Root penetration rate – a benchmark to identify soil and plant limitations to rooting depth in wheat. Australian Journal of Experimental Agriculture 47, 590–602.
| Crossref | GoogleScholarGoogle Scholar |

Kirkegaard JA,
Lilley JM,
Howe GN, Graham JM
(2007) Impact of subsoil water use on wheat yield. Australian Journal of Agricultural Research 58, 303–315.
| Crossref | GoogleScholarGoogle Scholar |

Klepper B,
Belford RK, Rickman RW
(1984) Root and shoot development in winter wheat. Agronomy Journal 76, 117–122.

Manschadi AM,
Christopher J,
deVoil P, Hammer GL
(2006) The role of root architectural traits in adaptation of wheat to water-limited environments. Functional Plant Biology 33, 823–837.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

O’Toole JC, Bland WL
(1987) Genotypic variation in crop plant root systems. Advances in Agronomy 41, 91–145.
| Crossref | GoogleScholarGoogle Scholar |

Opanowicz M,
Vain P,
Draper J,
Parker D, Doonan JH
(2008)
Brachypodium distachyon: making hay with wild grass. Trends in Plant Science 13, 172–177.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Osmont KS,
Sibout R, Hardtke CS
(2007) Hidden branches: developments in root system architecture. Annual Review of Plant Biology 58, 93–113.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Passioura JB
(1983) Roots and drought resistance. Agricultural Water Management 7, 265–280.
| Crossref | GoogleScholarGoogle Scholar |

Radford BJ, Key AJ
(1993) Temperature affects germination, mesocotyl length and coleoptile length of oats genotypes. Australian Journal of Agricultural Research 44, 677–688.
| Crossref | GoogleScholarGoogle Scholar |

Raju MVS, Steeves TA
(1998) Growth, anatomy and morphology of the mesocotyl and the growth of appendages of the wild oat (Avena fatua L.) seedling. Journal of Plant Research 111, 73–85.
| Crossref | GoogleScholarGoogle Scholar |

Ries RE, Hofmann L
(1991) Research observations: standardized terminology for structures resulting in emergence and crown placement of 3 perennial grasses. Journal of Range Management 44, 404–407.
| Crossref | GoogleScholarGoogle Scholar |

Schiefelbein JW, Benfey PN
(1991) The development of plant roots: new approaches to underground problems. The Plant Cell 3, 1147–1154.
|
CAS |
Crossref |
PubMed |

Varney GT,
Canny MJ,
Wang XL, McCully ME
(1991) The branch roots of Zea. I. First order branches, their number, sizes and division into classes. Annals of Botany 67, 357–364.

Vogel JP,
Garvin DF,
Leong OM, Hayden DM
(2006)
Agrobacterium-mediated transformation and inbred line development in the model grass Brachypodium distachyon. Plant Cell, Tissue and Organ Culture 84, 119–211.
| Crossref | GoogleScholarGoogle Scholar |

Vogel J,
Tuna M,
Budak H,
Huo N,
Gu T, Steinward M
(2009) Development of SSR markers and analysis of diversity in Turkish populations of Brachypodium distachyon. BMC Plant Biology 9, 88.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Watt M,
Rebetzke GJ, Kirkegaard JA
(2005) A wheat genotype developed for rapid leaf growth copes well with the physical and biological constraints of unploughed soil. Functional Plant Biology 32, 695–706.
| Crossref | GoogleScholarGoogle Scholar |

Watt M,
Magee LJ, McCully ME
(2008) Types, structure and potential for axial water flow in the deepest roots of field-grown cereals. New Phytologist 178, 135–146.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Wenzel CL,
McCully ME, Canny MJ
(1989) Development of water conducting capacity in the root systems of young plants of corn and some other C4 grasses. Plant Physiology 89, 1094–1101.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Zadoks JC,
Chang TT, Konzak FC
(1974) A decimal code for the growth stages of cereals. Weed Research 14, 415–421.
| Crossref | GoogleScholarGoogle Scholar |




