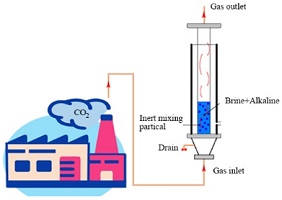
Environmental context. Atmospheric particulate NH4+, primarily produced from the reaction of NH3 and acids, is an important component of PM2.5. In this study, nitrogen stable isotope analyses and an atmospheric chemistry model were used to estimate the contribution of major NH3 sources to particulate NH4+ in Tianjin, a megacity in North China Plain (NCP). Our research has implications for investigations of NH3 emission sources and relevant pollution control in Tianjin and NCP. Photograph by Libin Wu.
This article belongs to the collection Dedication to Roy Harrison.