Examining the utility of existing chemical hazard paradigms to predict future global-scale environmental impacts from emerging chemicals
Karl C. Bowles A B * and Janina Beyer C
A B * and Janina Beyer C
A RPS AAP Consulting Pty Ltd, 420 George Street, Sydney, NSW 2000, Australia.
B Queensland Alliance for Environmental Health Sciences (QAEHS), The University of Queensland, 20 Cornwall Street, Woolloongabba, Qld 4102, Australia.
C Contaminants and Risk Team, Science, Economics & Insights Division, NSW Department of Planning and Environment, 480 Weeroona Road, Lidcombe, NSW 2141, Australia.
Environmental Chemistry 19(4) 254-262 https://doi.org/10.1071/EN22046
Submitted: 2 May 2022 Accepted: 22 September 2022 Published: 2 November 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Environmental context. In previous instances of global impacts from chemicals, there were significant gaps between the onset of use and observations that triggered management. The lessons of the past have informed the development of strong paradigms for chemical management, but at some point, major impacts will again emerge, not covered by these paradigms. Holistic observation of the environment and collaborative reporting are needed to identify signals of future major issues.
Abstract. Increasing concern over per- and polyfluoroalkyl substances (PFAS) in the environment, in the last decade, has sparked an interest in emerging chemicals more broadly, leading to the development or strengthening of many useful programs for understanding and prioritising environmental hazards and risks for chemicals. While important and useful, such efforts mostly rely on comparing chemical properties with paradigms generated from previous environmental issues. The lessons of the past demonstrate that, at some point, major challenges to our existing paradigms will eventuate. Key to addressing these challenges is our ability for early identification of ‘blind spots’ not covered by our existing paradigms. Furthermore, if we only look for gross observable changes in the environment, we will only ever be able to respond with reactive measures. We suggest that while various relevant monitoring programs are in place and have been proposed, encouraging those processes to look beyond existing hazard paradigms and look for more subtle environmental signals will improve the ability to respond proactively when harm is still limited.
Keywords: chemicals, chlorofluorocarbons, environmental signals, hazard paradigms, mercury, mobility, organochlorine pesticides, PBT, Persistent Organic Pollutants, PFAS, regulation.
Introduction
In the last decade or so, there has been a growing interest in emerging chemicals among a diverse range of stakeholders outside of the academic science community (e.g. OECD 2018a; US Government 2021; US Navy 2022). This growth can be attributed at least in part to growing concerns over per- and polyfluoroalkyl substances (PFAS) in the environment. The desire to predict the next major chemical issue is understandable. While most stakeholders such as politicians and communities want to avoid environmental disasters for a range of obvious reasons, commercial stakeholders such as environmental consultants, waste contractors and analytical laboratories use such knowledge to target growth strategies to capitalise on future business opportunities. The burden of providing useful information, guidance and policy typically falls on scientists and regulators who rely on hazard and risk paradigms for matters such as approving chemicals for use in products, licensing discharges to environment and responding to spills or legacy contamination.
Specifically, for the purposes of this article, the term ‘paradigm of the day’ is being used to mean related scientific principles that are generally accepted by the scientific establishment in relevant fields of study and incorporated into environmental regulation and guidance. Examples of current chemical paradigms include:
Hazard characterisation such as Persistence, Bioaccumulation and Toxicity (PBT) for organic chemicals, along with long-range transport potential (LRTP) (Stockholm Convention 2019)
Ecotoxicological paradigms such as the free ion activity model (FIAM) (Campbell 1995) and biotic ligand model (BLM) (Paquin et al. 2002) for understanding metal bioavailability in aquatic systems
Toxicological groupings such as Carcinogenic, Mutagenic, Reproductive (CMR) toxins (eurostat 2020) and endocrine active/disruptive chemicals (EACs/EDCs/EDs) (e.g. EC 2022).
While screening chemical hazard against such paradigms is certainly necessary to prioritise current concerns, it is questionable whether such efforts will identify future issues requiring establishment of new global intervention on the same scale as the Stockholm Convention for Persistent Organic Pollutants (POPs) or the Minamata Convention for Mercury. To understand why, it helps to look to the past and understand how current paradigms for chemical prioritisation have developed. This article uses examples of groups of chemicals that have challenged or are challenging chemical hazard paradigms of the day and considers possible actions that may reduce the risk from relying too heavily on our existing paradigms. The examples given below are but a few and the reader is referred to other examples in the EEA (2013) report on early warnings, late action.
Looking to the past: examples of major chemical environmental impacts
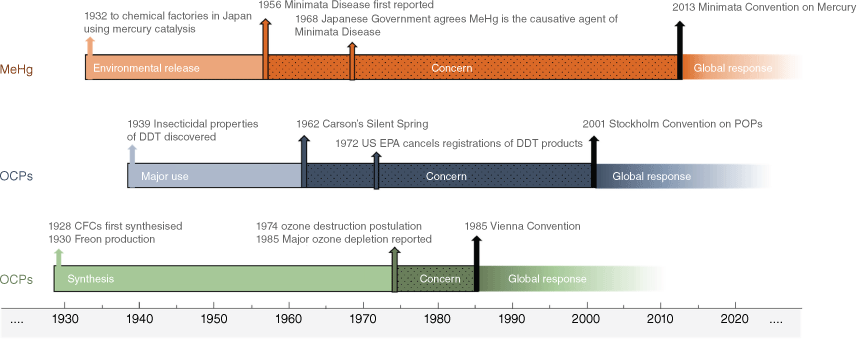
Over the twentieth century, several groups of chemicals emerged with issues so remarkable as to warrant global response. Three major examples are methylmercury (MeHg), organochlorine pesticides (OCPs) and chlorofluorocarbons (CFCs). A common factor for these was widespread and long-term use (or inadvertent production in the case of MeHg), before environmental issues were understood and significant attempts to manage were instigated. Looking at how these three classes of chemicals emerged as major environmental issues is instructive to understanding the nature of our chemical assessment paradigms. Fig. 1 gives a brief timeline of key events for these examples.
The so-called Minamata incident occurred following long-term release of mercury (Hg) and MeHg from chemical plants in Minamata and Niigata in Japan from the early 1900s (NIMD 2014). Fish contaminated with MeHg were consumed, leading to major congenital deformities and deaths over a period of decades. Toxicity of inorganic and elemental Hg had been observed since ancient times, with well-known symptoms, however symptoms of Minamata Disease were different. This led to an initial failure to understand the cause, which was originally thought to be pathogenic, hence the term ‘disease’ (Hachiya 2006). This was compounded by the prevailing perception that toxic chemicals don’t pass the placenta (Hachiya 2006) and therefore congenital effects from methylmercury would not have been expected or understood. Also, biomagnification was an important factor in the exposure to humans. The principle of biomagnification would remain unknown until after OCPs emerged as an environmental issue in 1960s, and even then, the paradigm to explain biomagnification of OCPs did not apply to the organometal MeHg. The Japanese government did not fully accept MeHg as the cause until 1968 (Hachiya 2006), noting that this was as much a political decision as revision of a scientific paradigm. Improved analytical techniques resulted in a significant improvement of the sensitivity of mercury analysis and speciation of environmentally relevant forms of mercury (Bloom and Crecelius 1983; Fitzgerald and Watras 1989; Mason and Fitzgerald 1991). These changes allowed a greatly improved understanding of the biogeochemistry of mercury from the late 1980s (Watras and Huckabee 1994), leading eventually to the adoption of the Minamata Convention from 2013 (UNEP 2021).
Organochlorine pesticides (OCPs) had been widely used from the 1940s and considered as ‘safe’ pesticides with low human toxicity in agricultural and domestic settings (Moore and Ramamoorthy 1984). It wasn’t until Rachel Carson and others observed and reported environmental effects, in particular lack of birds, and attributed this to chemical contamination that the widespread use of OCPs was widely questioned (Carson 1962; Paull 2013). Initially Carson faced staunch opposition from both scientists and industry who reasoned that chemicals with low acute toxicity could not cause these impacts (McLaughlin 2010). The fact that effects were on reproduction, as opposed to ‘typical’ mortality expected for pesticides, hindered understanding, despite earlier observations on toxicity and persistence (Fitzhugh et al. 1950; Lichtenstein and Medler 1958). As for methylmercury, also critical was the lack of an existing paradigm to explain biomagnification. The idea that chemicals occurring in the environment at low concentrations (part per billion and lower) could be responsible for impacts on higher trophic birds and mammals was not conceivable until biomagnification based on the tendency of hydrophobic chemicals to accumulate in fat tissue became known even if not fully understood (Gobas et al. 1999). The understanding of multiple properties that combine to cause a much greater environmental hazard ultimately led to the Stockholm Convention on POPs from 2001 (UNEP 2019a). This provides global regulation and guidance for organic pollutants that cross certain thresholds for persistence (P), bioaccumulation (B), toxicity (T) and long-range transport potential (LRTP).
Chlorofluorocarbons (CFCs) were another class of chemical where widespread use resulted in environmental impacts justifying a global response, following a significant delay. These quickly became refrigerant gases of choice in the 1930s due to their extremely low chemical reactivity and acute toxicity, making them ideal to replace other refrigerants such as ammonia. The first hint that CFCs may not be environmentally benign came when Molina and Rowland in 1974 published in Nature a mechanism by which CFCs could be degraded in the presence of high energy solar radiation such as might occur on the stratosphere leading to catalytic ozone destruction (Molina and Rowland 1974). Scientists recognised that an ozone hole would cause increased cancer rates and environmental degradation (EU 1994), but at this time, transport to the stratosphere and accumulation at the poles was generally underestimated and some aspects of Molina’s and Rowland’s work were criticised (Baum 2017). The actual observation of an ozone hole over Antarctica in 1985 (Farman et al. 1985) quickly led to the Vienna Convention for the Protection of the Ozone Layer in 1985 (UNEP 2020) and the Montreal Protocol in 1987 (UNEP 1987), starting the successful phase out of CFCs. As with the OCPs, the significant delay between widespread use of CFCs, and eventual understanding of the environmental impacts, was a consequence of the lack of paradigms at the time to assess the hazard.
Table 1 summarises information relevant to the three examples above. In each case, an understanding of the toxicology and the (biogeo)chemistry/physics explains the processes leading to major environmental impacts. The problem for regulation at the time, such as it existed, was that some of the issues were not known and not foreseeable when the chemicals were developed and first used. The existing paradigms of the day for chemical assessment were not sufficient to predict the impacts eventually observed. In other words, the understanding of the science followed the identification of the issue.

|
The present: how have we learned from the past?
The lessons of the past have been built into our current paradigms for chemical assessment at different regulatory levels: chemical approval processes by continents/countries such as EU’s REACH (ECHA 2022a) and the Australian AICIS (AICIS 2022) and environmental regulation at a state and local level, including licensing of industrial premises and clean-up of legacy contamination.
As noted above, the Stockholm Convention on POPs plays an important role in regulating those chemicals specifically identified as hazardous according to the PBT and LRTP paradigm. Originally listing the so-called ‘Dirty Dozen’, the Convention has since listed other organic chemicals. Examples of currently listed POPs include:
Chlorinated pesticides: aldrin, chlordane, DDT, etc.
Polychlorinated dibenzodioxins and furans (PCDD, PCDF) and polychlorinated biphenyls (PCBs)
Various polybrominated diphenylethers (PBDEs) and hexabromocyclododecane (HBCDD)
Perfluorooctane sulfonate (PFOS), its salts, and PFOSF, perfluorooctanoic acid (PFOA), its salts and related compounds, and perfluorohexane sulfonate (PFHxS) its salts and related compounds
Some chlorinated hydrocarbons such as short chain chlorinated paraffins (SCCPs) and polychlorinated naphthalenes, etc.
Even a brief examination reveals that these chemicals have something in common: they are all halogenated organic compounds. An interesting exception is UV-328, a substituted phenolic benzotriazole used as an ultraviolet radiation absorber in many products. UV-328 has been proposed for listing and meets the Stockholm criteria (UNEP 2019a) but contains no halogen atoms in its structure (IPCP 2022).
Halogenation often confers a critical property, persistence, due to the relative lack of enzymes capable of degrading halogenated organic chemicals and contributes to the tendency to bioaccumulate, especially for fluorinated compounds such as some PFAS. While some PFAS are listed in the Annexes, these chemicals were not initially recognised as being likely to biomagnify, even once the PBT paradigm was developed and applied to other chemicals. PFAS are therefore considered in more detail in this paper to understand why chemicals with such clear PBT properties were not regulated until long after the OCPs and some other chlorinated chemicals.
PFAS are a family of chemicals that runs into the 1000s. As the family is extremely diverse in chemical and other properties, this discussion will be limited to the perfluoroalkyl acids (PFAAs, including PFOS and PFOA) which have relatively similar structures, with hazard generally related to the fluorinated carbon chain length, although the terminal functional group also plays a part.
Perhaps the first warning signs that PFAAs were not as benign as previously believed was in the mid-1970s when unidentified organofluorine compounds were found in the blood of 3M factory workers (Ubel et al. 1980). It was not until the development of liquid chromatography – tandem mass spectrometry in the 1990s (Olsen et al. 2000; Hansen et al. 2001) that accurate determination at relevant concentrations was possible in blood serum and environmental matrices. Tissues from stranded aquatic mammals collected over the 1990s frequently contained PFOS when analysed between 1999 and 2001 (Giesy et al. 2001) and also human blood serum (Hansen et al. 2001). Some interventions resulted, such as 3M withdrawing PFOS manufacture in 2000 (Buck et al. 2011) and the US EPA 2010/2015 PFOA Stewardship Program (US EPA 2022a), but other PFAS manufacture continues globally. Investigation of environmental contamination from PFAAs has exploded in the twenty-first century, initially largely related to AFFF use (Australian Defence 2022; US DoD 2022), but now recognising the vast range of industrial, commercial and domestic products in which PFAS are used (Glüge et al. 2020).
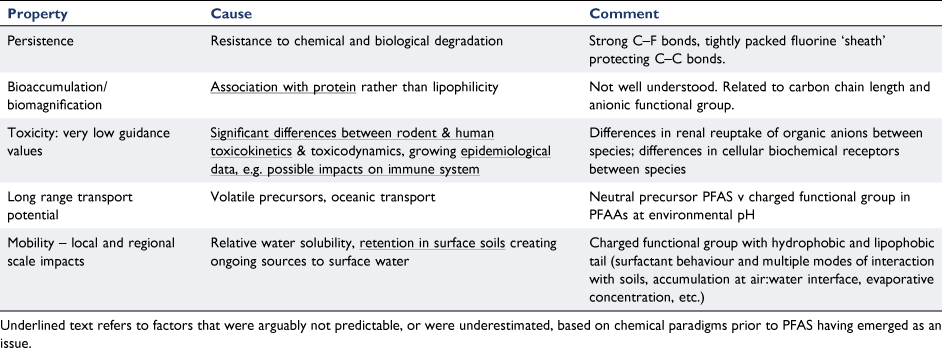
Table 2 summarises factors relating to the specific hazard properties of long-chain PFAAs such as PFOS and PFOA. Some, but not all, of these factors were predictable based on chemical paradigms prior to PFAS concerns emerging in the late 1990s. Some that may not have been predictable are underlined in the table, noting this analysis is subjective and some of these assignments are debatable. For example, it could be argued that the chemical resistance of these chemicals was certainly recognised early on, as it was a contributing reason for important uses such as in firefighting foams, but the protection conferred specifically by the tight geometry of the fluorine sheath around the carbon chain, on both radical and enzymatic attack, may have been underestimated when these chemicals were first used.
|
The information in Table 2 demonstrates that while some attributes of PFAAs leading to environmental hazard were predictable, such as persistence as noted above, the degree and importance of some other chemical properties and hazards were not yet understood based on the paradigms of the day. In particular, the notion that chemicals would biomagnify based on protein-association, and not lipophilicity, was apparently not recognised, even after the biomagnification of OCPs as a concept was understood. Methylmercury also biomagnifies based on association with proteins, but in a completely different manner (for MeHg it is affinity for reduced sulfur in cysteine). In short, no one expected organic chemicals with surfactant properties to biomagnify, and this would have been compounded by reliance on measuring octanol–water partitioning, as a surrogate to predict potential to bioaccumulate but not relevant for predicting protein binding. This demonstrates that focussing too much on individual paradigms can be distracting and lead to unwarranted confidence considering the global scale of use of many chemicals.
A specific property shared by PFAAs also worth considering is environmental mobility. This is not to be confused with the concept of LRTP included in the Stockholm definition for POPs, which is a more specific concept, although environmental mobility can contribute to that tendency. While LRTP is a critical hazard for reacting to global impacts, mobility at local and regional scales can result in exposures at higher concentrations, with consequences for potential human health and ecotoxic impacts. Considering mobility as a key property is becoming more recognised. For example, the mobility of PFAS in groundwater has contributed to exposures via drinking water in large parts of the US and Sweden (Gobelius et al. 2018; Brown et al. 2019), and the German Environment Agency has led efforts to identify Persistent, Mobile and Toxic (PMT) and very Persistent and very Mobile substances (vPvM) under the EU chemicals regulation REACH (Neumann and Schliebner 2019; Rüdel et al. 2020). This reflects the importance of groundwater as a drinking water source for large populations in some parts of the world, and contrasts with the conventional PBT paradigm which is focused more on food-based exposures.
An example to highlight the importance of considering mobility beyond LRTP is comparing PFAS to the polybrominated diphenyl ethers (PBDEs). Specific PBDEs have been listed on the Stockholm Convention for POPs (UNEP 2019b). Like PFOS and PFOA (and related chemicals), the listed PBDEs qualify as PBT chemicals, and have been widely used in products and articles. However, the PBDEs have not received nearly the same attention as PFAS at local and regional scales, particularly in effort spent on investigations and remediation, and focus from environmental regulators, media and politicians. The answer for this may lie largely in the much greater mobility of PFAAs. People in the general population will be exposed to both PFAS and PBDEs in homes and workplaces from consumer products. However, the much greater water solubility of PFAAs (mg L–1 to g L–1) compared to PBDEs (typically low µg L–1), results in exposures for certain groups of people, above that of the general population, whether via surface water, groundwater sourced drinking water or via bioaccumulation into food (e.g. Cousins et al. 2022).
There are other aspects to exposure to PFAS of which we are only just becoming fully aware, related to mobility. For some time, there have been empirical observations that certain PFAS from legacy use of AFFF (aqueous film-forming foams), especially PFOS, persist in near-surface vadose zone soils, for periods much longer than would intuitively be expected for chemicals with reasonable water solubility (for example refer to Australian Department of Defence PFAS Investigation and Management Program (Australian Defence 2022)). This is consistent with similar observations in an experimental study where > 99% of PFOS in a biosolids amended soil was still present under normal climatic conditions after 5 years (Stahl et al. 2013). This retention can be attributed in part to accumulation of PFAS at the air/water interface in soil pores (Brusseau 2018), retarding downward movement to groundwater. More recently, researchers have identified evaporative concentration as an important phenomenon for PFAAs such as PFOS (Davis et al. 2021). This phenomenon results in availability of PFAAs at the soil surface, which is then carried away in surface water runoff, in contrast to many other contaminants where transport in groundwater is regarded as a more important pathway. While both accumulation at the air/water interface and evaporative concentration have been noted for other chemicals (e.g. ionisable pesticides for the former and salts for the latter), these concepts are only more recently being applied to PFAS. This suggests that focussing too much on individual hazard paradigms can be distracting and could potentially lead to delays before important environmental impacts may be uncovered.
Looking to the future: will our current paradigms be enough?
Examples such as those above continue to strengthen our paradigms for chemical hazard. However, given the complexity of chemicals currently being developed and introduced to market, we should assume that at some point major challenges to our existing paradigms will eventuate. The scale of chemical issues globally, and the need for more preventive and precautionary hazard-based approaches is highlighted in the recent publication on planetary boundaries (Persson et al. 2022). Key to addressing these challenges is our ability towards early identification of ‘blind spots’ not covered by our existing paradigms. Furthermore, if we only look for gross, observable changes in the environment, we will only ever be able to respond with reactive measures. If we also look for more subtle signals and encourage precautionary measures, there will be better ability to respond proactively when harm is still limited.
As Wang and co-authors (Wang et al. 2021) importantly pointed out, ‘there is a lack of horizon scanning and early warning mechanisms: Most existing interface bodies are not tasked, on a regular basis, with monitoring scientific developments and providing early warnings on risks related to chemicals and waste in their specific areas.’ Existing programs that search for ‘blind spots’ and aim to provide early warning systems are few (e.g. EEA 2001, 2013; Bakker et al. 2017; Dulio et al. 2018; SamTox 2018; OECD 2019), and often cover either very specific or broad topics. Some focus on specific topics, for example persistent chemicals in food waste (US EPA 2021b), while others publish high-level position statements, e.g., the Scientific Committee on Health, Environmental and Emerging Risks (SCHEER) reports on emerging health and environmental issues (SCHEER 2022).
Within government agencies in different countries, there is increasing awareness of the need to identify signals for emerging risks and issues. For example, the Netherlands has developed an approach to identify New and Emerging Risks of Chemicals (NERCS) (Hogendoorn et al. 2014; Palmen 2015), and in Australia, the NSW government is developing an approach to identify and evaluate emerging chemical issues (Tran et al. 2022a, 2022b). The concepts for these programs to look for signals as early warning systems are similar, although differ in scope, scale and methodologies employed.
These programs provide the first steps to develop approaches for identifying signals and early warning systems. However, several challenges remain for us to look holistically for early signals of major new chemical impacts. To ensure these kinds of programs and activities are effective we need to further develop our capabilities in:
Transparent data for chemical production, chemicals used in products/articles and how they are used and disposed of, with consideration for life cycle assessment accounting for all phases and focusing on routes to the environment.
Multiple lines of evidence including current and advancing methods in (eco)toxicology, analytical chemistry and omics, ecology and epidemiology, targeted and untargeted monitoring of chemical occurrence in the environment and in people (biomonitoring) and inclusion of new and innovative approaches when searching for signals.
Accessible global information platforms that allow integration and sharing of data systems and schemes.
Of the above points, many calls for the first have been made but the conflict with commercial interests inevitably remains a roadblock. However, we can collectively continue to strengthen and support collaborative and transparent efforts between industry, governments, researchers and communities for monitoring and reporting data, and incorporating life cycle assessments that include reliable data for production, use and disposal of chemicals and products (e.g. Bucknall 2020).
For the second point, many programs already incorporate these ideas, approaches and technologies particularly in research, although uptake of such data in regulatory frameworks and when searching for signals is required. Examples of such approaches include the use of high resolution mass spectrometry (Brack et al. 2019; Hollender et al. 2019), omics approaches (Martyniuk and Simmons 2016; Mortimer et al. 2022), high throughput in vitro and in silico testing (Dix et al. 2007; Judson et al. 2013; Villeneuve et al. 2019; US EPA 2021a) and adverse outcome pathways (OECD 2017, 2022a). Interdisciplinary approaches are needed (e.g. Xia et al. 2013), and as highlighted by Maertens et al. (2021) on avoiding regrettable chemical substitutions, there is a need for an integrated approach using qualitative and quantitative data and using methods for analysing cumulative and interactive effects. For example, there is now a strong body of literature identifying behavioural effects to aquatic fauna from chemical mixtures, but this remains difficult to incorporate into existing water quality guidelines, even for individual chemicals (Ford et al. 2021). Similarly, the use of tools such as omics can provide important insights in understanding potential environmental impacts as a line of evidence for signals (Beale et al. 2022). Therefore, conducting environmental monitoring or managing chemicals only where hazards are understood with strong certainty, will result in continued delayed responses in managing chemicals.
The third point, data sharing and reporting, is an area that could and should be strengthened (Dulio et al. 2018; OECD 2018b, 2019). Examples of existing chemical hazard platforms include eChemPortal (OECD 2022b), PubChem (NIH 2022) and the CompTox database (US EPA 2022b). These are accessible online to allow users to search for chemicals, although automated data searching within such platforms remains a challenge, and working towards using common data formats would help link such databases (e.g. OECD 2019; ECHA 2022b). Furthermore, the example databases above are for chemical hazards, and not focused on environmental monitoring of chemicals (e.g. Bopp et al. 2020) or ecosystem impacts. Expanding the breadth and diversity of the information available will be critical for identifying early warning signals, outside of existing hazard paradigms.
Observation of past major chemical issues also demonstrates that there is a human element to advancing paradigms for environmental issues. In most cases, both commercial interests and existing science paradigms provided resistance to embracing new information and delayed effective management. This element will likely always exist to some degree, and this strengthens the need for open and collaborative approaches to reporting and interpreting information. We refer here to the call by Wang and co-authors (Wang et al. 2021) for ‘an overarching international body to facilitate and foster broad bidirectional science–policy interactions on chemicals and waste.’ Based on the analysis presented here, we would add that part of the obligation of such a body could be to look beyond current chemical hazard paradigms, to better enable identification of early warning signals, as part of its function of horizon scanning.
Data availability
All data used in preparation of this paper are included in the paper.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research did not receive any specific funding
Acknowledgements
The authors are greatly indebted to the peer reviewers, whose thorough comments significantly improved the focus and quality of this article.
References
AICIS (2022) ‘Australian Industrial Chemicals Introduction Scheme (AICIS).’ (Australian Government Department of Health) Available at https://www.industrialchemicals.gov.au/ [accessed 25 April 2022]Australian Defence (2022) ‘PFAS Investigation & Management Program.’ (Australian Department of Defence) Available at https://defence.gov.au/environment/pfas/ [accessed 6 August 2022]
Bakker J, Bruinen de Bruin Y, Hogendoorn E, Palmen N, Soeteman-Hernandez L (2017) ‘Study for the strategy for a non-toxic environment of the 7th EAP’. Sub-study g: Early Warning Systems for emerging chemical risks. Prepared by the Institute for Public Health and the Environment (RIVM). (European Commission)
Baum R (2017) ‘Chlorofluorocarbons and Ozone Depletion.’ (American Chemical Society, University of California, American Chemical Society, National Historic Chemical Landmarks Program: Washington, D.C.)
Beale DJ, Sinclair GM, Shah R, Paten AM, Kumar A, Long SM, Vardy S, Jones OAH (2022). A review of omics-based PFAS exposure studies reveals common biochemical response pathways. Science of The Total Environment 845, 157255
| A review of omics-based PFAS exposure studies reveals common biochemical response pathways.Crossref | GoogleScholarGoogle Scholar |
Bloom NS, Crecelius EA (1983). Determination of mercury in seawater at sub-nanogram per liter levels. Marine Chemistry 14, 49–59.
| Determination of mercury in seawater at sub-nanogram per liter levels.Crossref | GoogleScholarGoogle Scholar |
Bopp S, Franco A, Cusinato A, Kephalopoulos S, Ceridono M (2020) ‘Information Platform for Chemical Monitoring (IPCHEM)’. Update on the state of play of IPCHEM. (European Commission)
Brack W, Hollender J, de Alda ML, Müller C, Schulze T, Schymanski E, Slobodnik J, Krauss M (2019). High-resolution mass spectrometry to complement monitoring and track emerging chemicals and pollution trends in European water resources. Environmental Sciences Europe 31, 62–67.
| High-resolution mass spectrometry to complement monitoring and track emerging chemicals and pollution trends in European water resources.Crossref | GoogleScholarGoogle Scholar |
Brown P, Cordner A, Richter L, Andrews D, Naidenko O (2019) Mapping the PFAS Contamination Crisis. Research by the Environmental Working Group (EWG) and the PFAS Project at Northeastern University’s Social Science Environmental Health Research Institute (SSEHRI). The PFAS Project lab. Available at https://pfasproject.com/mapping-the-pfas-contamination-crisis/ [accessed 25 April 2022]
Brusseau ML (2018). Assessing the potential contributions of additional retention processes to PFAS retardation in the subsurface. Science of The Total Environment 613–614, 176–185.
| Assessing the potential contributions of additional retention processes to PFAS retardation in the subsurface.Crossref | GoogleScholarGoogle Scholar |
Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, Jensen AA, Kannan K, Mabury SA, van Leeuwen SP (2011). Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integrated Environmental Assessment and Management 7, 513–541.
| Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins.Crossref | GoogleScholarGoogle Scholar |
Bucknall DG (2020). Plastics as a materials system in a circular economy. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 378, 20190268
Campbell PGC (1995) Interactions between trace metals and aquatic organisms: a critique of the free-ion activity model. In ‘Metal Speciation and Bioavailability in Aquatic Systems’. (Eds A Tessier, DR Turner). pp. 45–102. (John Wiley & Sons: London, UK)
Carson R (1962) ‘Silent Spring.’ (Houghton: Boston, MA, USA)
Cousins IT, Johansson JH, Salter ME, Sha B, Scheringer M (2022). Outside the safe operating space of a new planetary boundary for per- and polyfluoroalkyl substances (PFAS). Environmental. Science & Technology 56, 11172–11179.
| Outside the safe operating space of a new planetary boundary for per- and polyfluoroalkyl substances (PFAS).Crossref | GoogleScholarGoogle Scholar |
Davis GB, Wallis I, Kookana R, Navarro D, Rayner JL, Prommer H (2021) Key Unsaturated Zone Soil Processes for PFAS Mobility and Retention: Principles and Understanding. (CSIRO, Australia Report for the Department of Defence)
Dix DJ, Houck KA, Martin MT, Richard AM, Setzer RW, Kavlock RJ (2007). The ToxCast Program for prioritizing toxicity testing of environmental chemicals. Toxicological Sciences 95, 5–12.
| The ToxCast Program for prioritizing toxicity testing of environmental chemicals.Crossref | GoogleScholarGoogle Scholar |
Dulio V, van Bavel B, Brorström-Lundén E, Harmsen J, Hollender J, Schlabach M, Slobodnik J, Thomas K, Koschorreck J (2018). Emerging pollutants in the EU: 10 years of NORMAN in support of environmental policies and regulations. Environmental Sciences Europe 30, 5–17.
| Emerging pollutants in the EU: 10 years of NORMAN in support of environmental policies and regulations.Crossref | GoogleScholarGoogle Scholar |
EC (2022) Which substances are of concern? Endocrine Disruptors - Chemicals - Environment - European Commission. Directorate-General for Environment. Available at https://ec.europa.eu/environment/chemicals/endocrine/strategy/substances_en.htm [accessed 24 April 2022]
ECHA (2022a) Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH). European Chemicals Agency (ECHA). Available at https://echa.europa.eu/regulations/reach/understanding-reach [accessed 25 April 2022]
ECHA (2022b) International Uniform Chemical Information Database (IUCLID). European Chemicals Agency (ECHA). Available at https://iuclid6.echa.europa.eu/ [accessed 25 Apr 2022]
EEA (2001) ‘Late lessons from early warnings: the precautionary principle, 1896-2000.’ ISBN 92-9167-323-4. (European Environment Agency (EEA))
EEA (2013) ‘Late lessons from early warnings: science, precaution, innovation. Summary.’ ISSN 1725‑9177. (European Environment Agency (EEA): Denmark) Available at https://data.europa.eu/doi/10.2800/70069 [accessed 10 April 2022]
EU (1994) Toxic Substances Control Act: Legislative Changes Could Make the Act More Effective (Chapter Report, 09/26/94, GAO/RCED-94-103). Toxic Substances Control Act. Available at https://www.govinfo.gov/content/pkg/GAOREPORTS-RCED-94-103/html/GAOREPORTS-RCED-94-103.htm [accessed 25 April 2022]
eurostat (2020) ‘Statistics Explained. Glossary: Carcinogenic, mutagenic and reprotoxic (CMR).’ ISSN 2443-8219. (European Commission) Available at https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Glossary:Carcinogenic,_mutagenic_and_reprotoxic_(CMR)# [accessed 24 April 2022]
Farman JC, Gardiner BG, Shanklin JD (1985). Large losses of total ozone in Antarctica reveal seasonal ClOx/NOx interaction. Nature 315, 207–210.
| Large losses of total ozone in Antarctica reveal seasonal ClOx/NOx interaction.Crossref | GoogleScholarGoogle Scholar |
Fitzgerald WF, Watras CJ (1989). Mercury in surficial waters of rural Wisconsin lakes. Science of The Total Environment 87–88, 223–232.
| Mercury in surficial waters of rural Wisconsin lakes.Crossref | GoogleScholarGoogle Scholar |
Fitzhugh OG, Nelson AA, Frawley JP (1950). The chronic toxicities of technical benzene hexachloride and its alpha, beta and gamma isomers. The Journal of Pharmacology and Experimental Therapeutics 100, 59–66.
Ford AT, Ågerstrand M, Brooks BW, Allen J, Bertram MG, Brodin T, Dang Z, Duquesne S, Sahm R, Hoffmann F, Hollert H, Jacob S, Klüver N, Lazorchak JM, Ledesma M, Melvin SD, Mohr S, Padilla S, Pyle GG, Scholz S, Saaristo M, Smit E, Steevens JA, van den Berg S, Kloas W, Wong BBM, Ziegler M, Maack G (2021). The role of behavioral ecotoxicology in environmental protection. Environmental Science & Technology 55, 5620–5628.
| The role of behavioral ecotoxicology in environmental protection.Crossref | GoogleScholarGoogle Scholar |
Giesy JP, Kannan K, Jones PD (2001). Global biomonitoring of perfluorinated organics. The Scientific World Journal 1, 627–629.
| Global biomonitoring of perfluorinated organics.Crossref | GoogleScholarGoogle Scholar |
Glüge J, Scheringer M, Cousins IT, DeWitt JC, Goldenman G, Herzke D, Lohmann R, Ng CA, Trier X, Wang Z (2020). An overview of the uses of per- and polyfluoroalkyl substances (PFAS). Environmental Science: Processes & Impacts 22, 2345–2373.
| An overview of the uses of per- and polyfluoroalkyl substances (PFAS).Crossref | GoogleScholarGoogle Scholar |
Gobas FAPC, Wilcockson JB, Russell RW, Haffner GD (1999). Mechanism of biomagnification in fish under laboratory and field conditions. Environmental Science & Technology 33, 133–141.
| Mechanism of biomagnification in fish under laboratory and field conditions.Crossref | GoogleScholarGoogle Scholar |
Gobelius L, Hedlund J, Dürig W, Tröger R, Lilja K, Wiberg K, Ahrens L (2018). Per- and polyfluoroalkyl substances in Swedish groundwater and surface water: implications for environmental quality standards and drinking water guidelines. Environmental Science & Technology 52, 4340–4349.
| Per- and polyfluoroalkyl substances in Swedish groundwater and surface water: implications for environmental quality standards and drinking water guidelines.Crossref | GoogleScholarGoogle Scholar |
Hachiya N (2006). The history and the present of Minamata Disease – entering the second half a century. Japan Medical Association Journal 49, 112–118.
Hansen KJ, Clemen LA, Ellefson ME, Johnson HO (2001). Compound-specific, quantitative characterization of organic fluorochemicals in biological matrices. Environmental Science & Technology 35, 766–770.
| Compound-specific, quantitative characterization of organic fluorochemicals in biological matrices.Crossref | GoogleScholarGoogle Scholar |
Hogendoorn E, Bakker J, Bruinen de Bruin Y, Kooi M, Palmen N, Salverda J, Traas T, Sijm D (2014) Progress report on New or Emerging Risks of Chemicals (NERCs). RIVM Letter report 2014-0040. (RIVM (National Institute for Public Health and the Environment): The Netherlands)
Hollender J, van Bavel B, Dulio V, Farmen E, Furtmann K, Koschorreck J, Kunkel U, Krauss M, Munthe J, Schlabach M, Slobodnik J, Stroomberg G, Ternes T, Thomaidis NS, Togola A, Tornero V (2019). High resolution mass spectrometry-based non-target screening can support regulatory environmental monitoring and chemicals management. Environmental Sciences Europe 31, 42
| High resolution mass spectrometry-based non-target screening can support regulatory environmental monitoring and chemicals management.Crossref | GoogleScholarGoogle Scholar |
IPCP (2022) Draft risk profile of UV absorber UV-328 adopted by the POPs Review Committee of the Stockholm Convention on Persistent Organic Pollutants. (International Panel on Chemical Pollution (IPCP)). Available at https://www.ipcp.ch/news/draft-risk-profile-of-uv-absorber-uv-328-adopted [accessed 25 April 2022]
Judson R, Kavlock R, Martin M, Reif D, Houck K, Knudsen T, Richard A, Tice RR, Whelan M, Xia M, Huang R, Austin C, Daston G, Hartung T, Fowle III JR, Wooge W, Tong W, Dix D (2013). Perspectives on validation of high-throughput assays supporting 21st Century toxicity testing. ALTEX - Alternatives to Animal Experimentation 30, 51–56.
| Perspectives on validation of high-throughput assays supporting 21st Century toxicity testing.Crossref | GoogleScholarGoogle Scholar |
Lichtenstein EP, Medler JT (1958). Persistence of aldrin and heptachlor residues on alfalfa. Journal of Economic Entomology 51, 222–226.
| Persistence of aldrin and heptachlor residues on alfalfa.Crossref | GoogleScholarGoogle Scholar |
Maertens A, Golden E, Hartung T (2021). Avoiding regrettable substitutions: green toxicology for sustainable chemistry. ACS Sustainable Chemistry & Engineering 9, 7749–7758.
| Avoiding regrettable substitutions: green toxicology for sustainable chemistry.Crossref | GoogleScholarGoogle Scholar |
Martyniuk CJ, Simmons DB (2016). Spotlight on environmental omics and toxicology: a long way in a short time. Comparative Biochemistry and Physiology Part D: Genomics and Proteomics 19, 97–101.
| Spotlight on environmental omics and toxicology: a long way in a short time.Crossref | GoogleScholarGoogle Scholar |
Mason RP, Fitzgerald WF (1991). Mercury speciation in open ocean waters. Water Air & Soil Pollution 56, 779–789.
| Mercury speciation in open ocean waters.Crossref | GoogleScholarGoogle Scholar |
McLaughlin D (2010) Fooling with nature: silent spring revisited. Archived from the original on 10 March 2010. Frontline. PBS. Available at https://www.pbs.org/wgbh/pages/frontline/shows/nature/disrupt/sspring.html [accessed 24 April 2022]
Molina MJ, Rowland FS (1974). Stratospheric sink for chlorofluoromethanes: chlorine atom-catalysed destruction of ozone. Nature 249, 810–812.
| Stratospheric sink for chlorofluoromethanes: chlorine atom-catalysed destruction of ozone.Crossref | GoogleScholarGoogle Scholar |
Moore JW, Ramamoorthy S (1984) Chlorinated pesticides. In ‘Organic Chemicals in Natural Waters: Applied Monitoring and Impact Assessment’. (Eds JW Moore, S Ramamoorthy) pp. 88–114. (Springer New York: New York, NY)
Mortimer M, Fang W, Zhou X, Vodovnik M, Guo L-H (2022) Omics approaches in toxicological studies. In ‘Advances in Toxicology and Risk Assessment of Nanomaterials and Emerging Contaminants’. (Eds L-H Guo, M Mortimer) pp. 61–94. (Springer: Singapore)
Neumann M, Schliebner I (2019) 39 Protecting the sources of our drinking water: the criteria for identifying persistent, mobile and toxic (PMT) substances and very persistent and very mobile (vPvM) substances under EU Regulation REACH (EC) No 1907/2006. German Environment Agency. 127/2019. Available at https://www.umweltbundesamt.de/publikationen/protecting-the-sources-of-our-drinking-water-the [accessed 7 August 2022]
NIH (2022) PubChem. National Library of Medicine. (National Center for Biotechnology Information: USA) Available at https://pubchem.ncbi.nlm.nih.gov/ [accessed 6 August 2022]
NIMD (2014) Ministry of the Environment. National institute for Minamata Disease. Minamata Disease Archives. Available at http://nimd.env.go.jp/english/ [accessed 24 April 2022]
OECD (2017) Revised Guidance Document on Developing and Assessing Adverse Outcome Pathways. Series on Testing & Assessment No. 184. JT03417683. (Organisation for Economic Co-operation and Development (OECD))
OECD (2018a) Summary Note. OECD workshop on Managing Contaminants of Emerging Concern in Surface Waters: Scientific developments and cost-effective policy responses, 5 February 2018. Available at https://www.oecd.org/water/Summary%20Note%20-%20OECD%20Workshop%20on%20CECs.pdf [accessed 24 April 2022]
OECD (2018b) OECD workshop on Managing Contaminants of Emerging Concern in Surface Waters: Scientific developments and cost-effective policy responses, 5 February 2018. Summary Note. Available at https://www.oecd.org/water/Summary%20Note%20-%20OECD%20Workshop%20on%20CECs.pdf
OECD (2019) International Best Practices for Identification of Priorities within Chemicals Management Systems. Series on Testing and Assessment No. 314. JT03456238. (Organisation for Economic Co-operation and Development (OECD)) Available at https://www.oecd-ilibrary.org/environment/international-best-practices-for-identification-of-priorities-within-chemicals-management-systems_0fafd6f5-en [accessed 25 April 2022]
OECD (2022a) Adverse Outcome Pathways, Molecular Screening and Toxicogenomics. (Organisation for Economic Co-operation and Development (OECD)) Available at https://www.oecd.org/chemicalsafety/testing/adverse-outcome-pathways-molecular-screening-and-toxicogenomics.htm [accessed 25 April 2022]
OECD (2022b) eChemPortal. The Global Portal to Information on Chemical Substances. (Organisation for Economic Co-operation and Development (OECD)) Available at https://www.echemportal.org/echemportal/ [accessed 6 August 2022]
Olsen GW, Burris JM, Burlew MM, Mandel JH (2000). Plasma cholecystokinin and hepatic enzymes, cholesterol and lipoproteins in ammonium perfluorooctanoate production workers. Drug and Chemical Toxicology 23, 603–620.
| Plasma cholecystokinin and hepatic enzymes, cholesterol and lipoproteins in ammonium perfluorooctanoate production workers.Crossref | GoogleScholarGoogle Scholar |
Palmen NGM (2015) Early warning systems to detect new and emerging risks in Europe. RIVM Letter report 2016-0022. (RIVM (National Institute for Public Health and the Environment): The Netherlands)
Paquin PR, Gorsuch JW, Apte S, Batley GE, Bowles KC, Campbell PGC, Delos CG, Di Toro DM, Dwyer RL, Galvez F, Gensemer RW, Goss GG, Hogstrand C, Janssen CR, McGeer JC, Naddy RB, Playle RC, Santore RC, Schneider U, Stubblefield WA, Wood CM, Wu KB (2002). The biotic ligand model: a historical overview. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 133, 3–35.
| The biotic ligand model: a historical overview.Crossref | GoogleScholarGoogle Scholar |
Paull J (2013). The Rachel Carson letters and the making of Silent Spring. SAGE Open 3, 215824401349486
| The Rachel Carson letters and the making of Silent Spring.Crossref | GoogleScholarGoogle Scholar |
Persson L, Carney Almroth BM, Collins CD, Cornell S, de Wit CA, Diamond ML, Fantke P, Hassellöv M, MacLeod M, Ryberg MW, Søgaard Jørgensen P, Villarrubia-Gómez P, Wang Z, Hauschild MZ (2022). Outside the safe operating space of the planetary boundary for novel entities. Environmental Science & Technology 56, 1510–1521.
| Outside the safe operating space of the planetary boundary for novel entities.Crossref | GoogleScholarGoogle Scholar |
Rüdel H, Körner W, Letzel T, Neumann M, Nödler K, Reemtsma T (2020). Persistent, mobile and toxic substances in the environment: a spotlight on current research and regulatory activities. Environmental Sciences Europe 32, 5–15.
| Persistent, mobile and toxic substances in the environment: a spotlight on current research and regulatory activities.Crossref | GoogleScholarGoogle Scholar |
SamTox (2018) Report 1/18. Progress report for the Toxicological Council 2017-2018. Organisation and start up (The Toxicological Council - body of experts for advice and consultation on toxicological issues: Sweden)
SCHEER (2022) Scientific Committee on Health, Environmental and Emerging Risks (SCHEER). Statement II on emerging health and environmental issues. (European Commission)
Stahl T, Riebe RA, Falk S, Failing K, Brunn H (2013). Long-term lysimeter experiment to investigate the leaching of perfluoroalkyl substances (PFASs) and the carry-over from soil to plants: results of a pilot study. Journal of Agricultural and Food Chemistry 61, 1784–1793.
| Long-term lysimeter experiment to investigate the leaching of perfluoroalkyl substances (PFASs) and the carry-over from soil to plants: results of a pilot study.Crossref | GoogleScholarGoogle Scholar |
Stockholm Convention (2019) What are Persistent Organic Pollutants (POPs)? Available at http://www.pops.int/TheConvention/ThePOPs/tabid/673/Default.aspx [accessed 24 April 2022]
Tran O, Merrington G, Peters A, Manning T, Ramarosandratana A, Cattle J, Beyer J (2022a) Framework for evaluating emerging chemical issues. In ‘Presented at the SETAC Copenhagen’. (NSW EPA: Sydney, NSW, Australia)
Tran O, Merrington G, Peters A, Manning T, Ramarosandratana A, Cattle J, Beyer J (2022b) Global approaches to identifying and prioritising apparent emerging chemical issues. In ‘Presented at the SETAC Copenhagen’. (NSW EPA: Sydney, NSW, Australia)
Ubel FA, Sorenson SD, Roach DE (1980). Health status of plant workers exposed to fluorochemicals – a preliminary report. American Industrial Hygiene Association Journal 41, 584–589.
| Health status of plant workers exposed to fluorochemicals – a preliminary report.Crossref | GoogleScholarGoogle Scholar |
UNEP (1987) ‘The Montreal Protocol on Substances that Deplete the Ozone Layer’. p. 15. (United Nations Environmental Programme (UNEP): New York) Available at https://ozone.unep.org/treaties/montreal-protocol [accessed 25 April 2022]
UNEP (2019a) Stockholm Convention. Protecting human health and the environment from persistent organic pollutants. (UN environment programme) Available at http://chm.pops.int/ [accessed 24 April 2022]
UNEP (2019b) The new POPs under the Stockholm Convention. (UN environment programme) Available at http://www.pops.int/TheConvention/ThePOPs/TheNewPOPs/tabid/2511/Default.aspx [accessed 25 April 2022]
UNEP (2020) The Vienna Convention for the Protection of the Ozone Layer. (UN environment programme) Available at https://ozone.unep.org/treaties/vienna-convention [accessed 6 August 2022]
UNEP (2021) Minamata Convention on Mercury. (UN environment programme 1972–2022) Available at https://www.mercuryconvention.org/en [accessed 24 April 2022]
US DoD (2022) PFAS: A National Issue That Needs National Solutions. (U.S. Department of Defense) Available at https://www.defense.gov/Spotlights/pfas/ [accessed 6 August 2022]
US EPA (2021a) Toxicity Forecaster (Toxcast). Advancing the Next Generation of Chemical Evaluation. Available at https://www.epa.gov/chemical-research/toxicity-forecasting [accessed 25 April 2022]
US EPA (2021b) Emerging issues in Food Waste management. Persistent Chemical Contaminants. EPA/600/R-21/115. (U.S. Environmental Protection Agency)
US EPA (2022a) Assessing and Managing Chemicals under TSCA. Fact Sheet: 2010/2015 PFOA Stewardship Program. Available at https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/fact-sheet-20102015-pfoa-stewardship-program [accessed 6 August 2022]
US EPA (2022b) CompTox Chemicals Dashboard. Available at https://comptox.epa.gov/dashboard/ [accessed 6 August 2022]
US Government (2021) FACT SHEET: Biden-Harris Administration Launches Plan to Combat PFAS Pollution. October 18, 2021. Statement and Releases. Available at https://www.whitehouse.gov/briefing-room/statements-releases/2021/10/18/fact-sheet-biden-harris-administration-launches-plan-to-combat-pfas-pollution/ [accessed 24 April 2022]
US Navy (2022) Emerging Contaminants. Naval Facilities Engineering Systems Command (NAVFAC), Engineering and Expeditionary Warfare Center. Available at https://exwc.navfac.navy.mil/Products-and-Services/Environmental-Security/NAVFAC-Environmental-Restoration-and-BRAC/Focus-Areas/Emerging-Contaminants/[accessed 25 October 2022]
Villeneuve DL, Coady K, Escher BI, Mihaich E, Murphy CA, Schlekat T, Garcia-Reyero N (2019). High-throughput screening and environmental risk assessment: state of the science and emerging applications. Environmental Toxicology and Chemistry 38, 12–26.
| High-throughput screening and environmental risk assessment: state of the science and emerging applications.Crossref | GoogleScholarGoogle Scholar |
Wang Z, Altenburger R, Backhaus T, Covaci A, Diamond ML, Grimalt JO, Lohmann R, Schäffer A, Scheringer M, Selin H, Soehl A, Suzuki N (2021). We need a global science-policy body on chemicals and waste. Science 371, 774–776.
| We need a global science-policy body on chemicals and waste.Crossref | GoogleScholarGoogle Scholar |
Watras CJ, Huckabee JW (Eds) (1994) ‘Mercury Pollution Integration and Synthesis.’ (Lewis Publishers)
Xia T, Malasarn D, Lin S, Ji Z, Zhang H, Miller RJ, Keller AA, Nisbet RM, Harthorn BH, Godwin HA, Lenihan HS, Liu R, Gardea-Torresdey J, Cohen Y, Mädler L, Holden PA, Zink JI, Nel AE (2013). Implementation of a multidisciplinary approach to solve complex nano EHS problems by the UC Center for the Environmental Implications of Nanotechnology. Small 9, 1428–1443.
| Implementation of a multidisciplinary approach to solve complex nano EHS problems by the UC Center for the Environmental Implications of Nanotechnology.Crossref | GoogleScholarGoogle Scholar |