Soil–air partitioning of volatile organic compounds into soils with high water content
Jeonghyeon Ahn A , Guiying Rao A , Mustafa Mamun A and Eric P. Vejerano A B
A B
A Center for Environmental Nanoscience and Risk, Department of Environmental Health Sciences, University of South Carolina, Columbia, SC 29208, USA.
B Corresponding author. Email: vejerano@mailbox.sc.edu
Environmental Chemistry 17(8) 545-557 https://doi.org/10.1071/EN20032
Submitted: 28 February 2020 Accepted: 22 May 2020 Published: 11 June 2020
Environmental context. Assessing environmental and human health impacts of chemical spills relies on information about how chemicals move across multiple environments. We measured volatile contaminants in the air above soil saturated with water to provide estimates of air concentrations of selected chemicals released to soil from an oil refinery in Texas during Hurricane Harvey. Estimated concentrations were below recommended exposure limits, even in a worst-case scenario.
Abstract. The emission of volatile organic compounds (VOCs) from soil into air is affected by soil moisture dynamics, soil temperature, solar irradiance and carbon availability. The high amount of water in soil can modify its properties, which changes how VOCs interact. We conducted a comprehensive measurement of the soil–air partition coefficient (KSA) of VOCs into water-saturated soil with both low and high water contents for polar, weakly polar and nonpolar VOCs into a mineral soil (S-clay) and soil containing a high amount of organic matter (S-om) under a water-saturated condition. Partitioning of non-polar substituted aromatics (1,2-dichlorobenzene and toluene) was sensitive to the organic matter content in water-saturated soil. 1,2-Dichlorobenzene and toluene had higher affinities to S-om than to S-clay at all investigated water contents because of their strong interaction with the organic matter in soil. KSA decreased with elevated water content only for non-polar substituted aromatic VOCs. Less hydrophobic VOCs (benzene and trichloroethylene) exhibited similar partitioning into both soils by sorbing onto the air-water interface and dissolving in soil water, while the organic matter did not affect partitioning. The weakly polar and polar VOCs (methyl tert-butyl ether and 1-butanol) showed similar partitioning into both soils by dissolving in soil water while sorption to the organic matter was significant only at high soil water contents. KSA of VOCs on soil with high organic matter content correlated strongly with psat and Koa, but not on mineral soil. Estimates of the air concentrations for a subset of VOCs released from one refinery during Hurricane Harvey in 2017 in Harris County, Texas were lower than the recommended exposure limits, even under a worst-case scenario.
Additional keywords: clay, Hurricane Harvey, octanol-air partitioning constant, organic matter, silt, sorption.
Introduction
Predicting the environmental and human health impact of accidental chemical spills and emissions of contaminants moving across different environmental compartments (soil, air and airborne particles) hinges on acquiring partitioning data that closely mimic environmental conditions. Volatile organic compounds (VOCs) emitted from biological and anthropogenic sources have dominated the class of organic contaminants present in the atmosphere (Breus and Mishchenko 2006; Dumanoglu et al. 2014; Rao and Vejerano 2018). While VOC emission from mobile sources has been declining, the contribution of volatile chemical products has been increasing, which suggests that VOCs are being manufactured at increasing quantities (McDonald et al. 2018). Approximately 50–90 million tons of VOCs have contaminated soil annually worldwide from accidental spills and leaks (Breus and Mishchenko 2006). In the USA alone, of the 479 contaminated sites, 84 % of the soil contaminants were VOCs (Breus and Mishchenko 2006). VOCs in soil will eventually partition into the atmosphere since they preferentially distribute into the air (Hwang et al. 2019; Rao and Vejerano 2018). In 2016, ~16 million tons of VOCs were emitted into the atmosphere in the USA alone (Statista 2018).
VOC emission from the soil depends on the soil moisture dynamics, soil temperature, solar irradiance and carbon availability (i.e. organic matter content) (Rossabi et al. 2018). Previous studies that investigated the soil-to-air partitioning of VOCs were mainly performed on unsaturated soil that contained extremely low water (oven-dried and air-dried soil, which are in equilibrium at a relative humidity (RH) of <90 %) and low organic matter content (Asensio et al. 2007; Goss 2004; Kim et al. 2003; Sanscartier et al. 2009; Shih and Wu 2005). These soil were mainly composed of minerals that contained minimal organic matter (e.g. <3 % by weight for most mineral soil (Chiou 2003). Results from such studies may have limited applicability in assessing the emission of VOCs since most soil in diverse environments will likely contain a high amount of water and organic matter. What is lacking is a comprehensive investigation of the partitioning for a broader class of VOCs of varying polarities into water-saturated soil (in equilibrium with an RH of nearly 100 %) (Hoff et al. 1993).
Entrainment and transport of VOCs as they move through a soil column will depend on the soil properties, physicochemical properties of the VOCs and the condition at which these interactions are occurring. Soil moisture dynamics strongly affect the emission of VOCs in the soil (Rossabi et al. 2018). The behaviour of VOCs partitioning into water-saturated soil (i.e. surface and internal pores are coated or filled by water vapour) (Seneviratne et al. 2010; SU et al. 2014) will substantially differ from unsaturated soil (Ong and Lion 1991a). A small amount of water easily saturates soil (e.g. <1 wt-% by mass for sand-dominated soil (Batterman et al. 1995; Hoff et al. 1993). The soil–air partition coefficient (KSA) decreases significantly with increasing water content because water competes strongly with VOCs to occupy the available sorption sites in soil (Kim et al. 2005; Shih and Wu 2005). Some VOCs are highly soluble in water; a large mass fraction of these VOCs in the water-saturated soil migrates to groundwater while those that interact poorly will partition preferentially into the air or interact actively with components in soil (Rivett et al. 2011). For some VOCs, their concentrations in groundwater have exceeded the United States Environmental Protection Agency drinking water standards (Fram and Belitz 2011; Moran et al. 2007).
Additionally, soil may contain considerable amounts of organic matter. Studies on the partitioning of VOCs into unsaturated soil are dominated by adsorption onto the mineral surface, while the organic matter content of the soil is deemed as not important for partitioning (Rivett et al. 2011; Shih and Wu 2005). However, different functional groups that are present on the VOCs will interact to a varying extent with water, as well as with the hydrophobic and hydrophilic domains in the organic matter fraction of the soil affecting the emission of VOCs into the air.
Here, we report a part of our investigation on the partitioning of VOCs as they move across the soil, air and airborne particles. We conducted a systematic measurement of the KSA of VOCs into water-saturated soil with both low and high water contents for three classes of VOCs (polar, weakly polar and nonpolar) into a surrogate mineral soil and soil containing a high amount of organic matter under water-saturated conditions. The objective of this study is to determine the physicochemical properties of the VOCs that can be used to predict their partitioning constants. Results will inform the prediction of the KSA, which can then be used as proxies to measure the emissions of VOCs from the soil into the air when air measurement is not readily available or difficult to obtain, such as after chemical spills resulting from natural disasters.
Experimental
VOCs
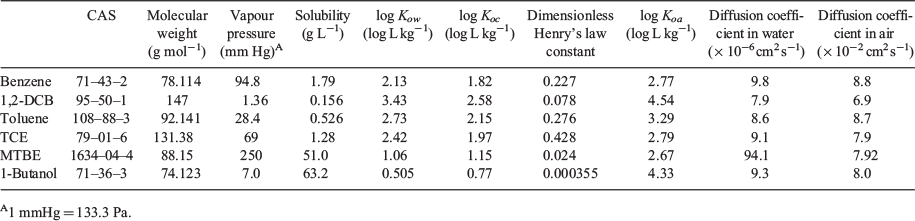
All standards and solvents used in this study were analytical grade reagents. Chemicals were purchased from Sigma-Aldrich, unless otherwise noted, and were used as received. Six VOCs were purchased. Nonpolar VOCs include the aromatic VOCs (benzene (99.8 %), 1,2-dichlorobenzene (1,2-DCB, 99 %), and toluene (≥99.8 %, BeanTown Chemical)) and the chlorinated alkane VOC (trichloroethylene (TCE, ≥99.5 %)). 1-Butanol (≥99.4 %) and methyl tert-butyl ether (MTBE, 99.9 %) were used as representatives of polar and weakly polar VOCs respectively. The deuterated VOCs, benzene-d6, 1,2-DCB-d4, toluene-d8, 1-butanol-d10, MTBE-d3 and TCE-d were used as the internal standards to quantify the VOCs. All deuterated chemicals were purchased from AccuStandard except for 1-butanol-d10 and TCE-d. Methanol (>99.99 %, Fisher Chemical) was used as a solvent for the calibration standards. The physicochemical properties of VOCs are listed in Table 1. The VOCs in this study included a wide range for each parameter, thus were good representatives in the statistical analysis for identifying the parameters that best correlated with KSA.
|
Soil and soil characterisation
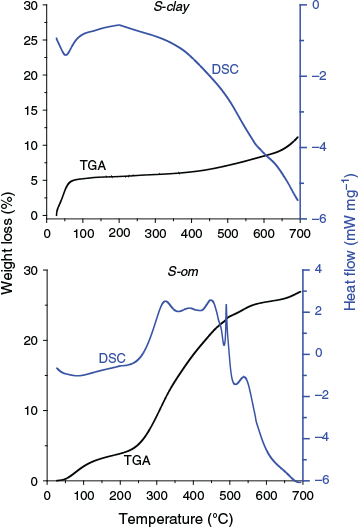
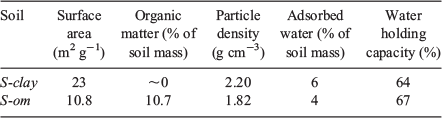
In our initial experiment, we found that the soil collected from the environment contained significant concentration levels of 1,2-DCB; therefore, we purchased soil to lessen the presence of organic contaminants contained in the soil. Two types of soil were purchased and used without further treatment to compare KSA of VOCs according to the soil water content and physicochemical properties of VOCs: clay (470025–200, VWR), which we refer to as S-clay, and silt with high organic matter content (470025–202, VWR), which we refer to as S-om. The Brunauer–Emmett–Teller (BET) test was used to determine the specific surface area and porosity of the soil through nitrogen adsorption at 77 K using a surface area and porosity analyser (Micromeritics ASAP 2020). The BET surface area and the total pore volume of each soil were determined by a multipoint BET method using the adsorption data at the relative pressure (p/p0) within 0.5. The percent of the adsorbed water and organic matter of each soil were characterised by simultaneously performing thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) thermal analyses from 25 to 700 °C (298 to 973 K) with a heating rate of 10 °C min−1 (0.167 K s−1) in air. The results are shown in Fig. 1. Weight loss from 25 to 150 °C (298 to 423 K) was taken as a measure of the adsorbed water content in the soil, and the weight loss from 250 to 370 °C (523 to 643 K) for the amount of the organic matter (Post and Henderson 2012). The soil particle density was measured to calculate the partition coefficient, which was estimated by dividing the mass of the soil by the volume of the particles measured using a graduated cylinder. The maximum water holding capacity (WHC) of each soil was determined by first adding water into the soil sample to form a mixture and then centrifuging the mixture at 5600× g for 3 min to separate the unabsorbed water. The difference in mass of the water added into the soil and the water extracted from it after centrifuging was the maximum amount of water the soil could hold (water holding capacity (WHC)).
|
The results of the soil characterisation are summarised in Table 2. The clay mineral (S-clay) contained negligible amounts, ~0.5 wt-%, of organic matter. S-clay also had a much larger surface area (~2 times) than that of S-om, which was similar to silt. In contrast, S-om contained a large amount of organic matter (~10.7 wt-%). Organic matter in the soil can absorb water efficiently, which enhances the WHC of the soil (Brown and Wherrett 2018). The amount of adsorbed water in the original soil (4 % and 6 % of the soil mass) without any treatment generated nearly an RH level of 100 % in the sealed glass bottle during the partitioning experiment. Therefore, both of the as-received soils from the vendor were already saturated with water before the test. The RH level inside the bottle containing the soil was measured after inserting a temperature and humidity probe (USBQTENKI-T-RH-CC2, Dracal Technologies, Inc.), then sealing it, and equilibrating it at 25 °C (298 K) in a temperature-controlled chamber for 20 min.
|
Measurement of soil–air equilibrium partitioning constant
The KSA was determined using Eqn 1:

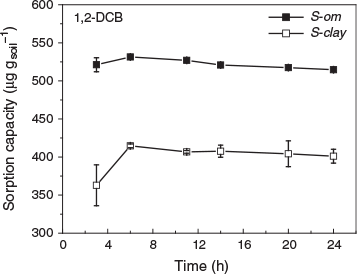
where Cis and Cia are the concentrations of VOCs, i, in soil (µg g−1) and air (µg m−3) respectively, and ds is the density of the soil particle (g m−3). The batch sorption experiments were carried out in 10-mL borosilicate glass bottles; each contained 1 g of soil (S-clay or S-om) and different amounts of de-ionised water to achieve 5, 20, 40, 60 and 80 % of the WHC of the soil. The glass bottles were shaken on a mini orbital shaker (VWR International) at 0.54 × g for 6 h to mix the water with the soil thoroughly. The temperature was kept at 25 ± 0.2 °C (298 ± 273.4 K) by installing the mini shaker in a temperature-controlled chamber (CEO932, Lunaire Environmental, New Columbia, PA). After that, the pure liquid VOC (0.4 µL for 1-butanol, benzene, toluene and 1,2-DCB; 1 µL for MTBE and TCE) was injected into the glass bottle through the Teflon-lined rubber septum and immediately sealed with an aluminium crimp cap. The glass bottle that contained the water and liquid VOC was agitated for 24 h at 25 ± 0.2 °C. While temperature affects partitioning (Ranjan et al. 2012; Wei et al. 2016), we conducted the experiment only at this temperature because the mass transfer of VOCs from the soil into the air depends primarily on the subsurface temperature, which fluctuates sinusoidally with depth but has a maximum temperature close to 25 °C (Nofziger 2003). Steady-state was established in less than 24 h for the VOC with the lowest vapour pressure (1,2-DCB) (Fig. 2), thus we deemed that 24 h was sufficient for other VOCs to reach steady-state, which is consistent with the times that are frequently observed and reported in the literature (Rogers et al. 1980; Shimizu et al. 1992; Tekrony and Ahlert 2001).
|
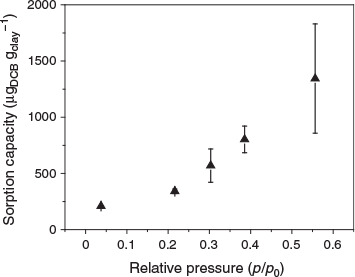
The volumes of the pure VOCs injected into the glass bottles were set at 0.4 µL (1 µL = 10−9 m3) for 1-butanol, benzene, toluene and 1,2-DCB, and 1 µL for MTBE and TCE to produce a relative vapour pressure (p/po) within 0.1 at equilibrium (except for 1,2-DCB partitioning into S-clay). Within such a low range of the relative vapour pressure, adsorption can be approximated to be linearly related to the vapour pressure of the VOC; thus, the concept of a partition coefficient applies (Petersen et al. 1995). At high vapour pressures, BET theory can be used to describe the whole adsorption isotherm over the full range of the relative vapour pressures (Petersen et al. 1995). Our measurement of the adsorption isotherm suggested a linear increase of the partitioning of 1,2-DCB into S-clay at the investigated p/po range (Fig. 3); thus, the partition coefficient concept can also be applied to describe the partitioning of 1,2-DCB into S-clay.
|
VOC sampling and analysis
We used the head-space sampling method to take gas samples from the borosilicate glass bottles at the end of the sorption experiment (Kremser et al. 2016; Mead et al. 2017). A volume of 30 µL of gas was taken from the headspace of the bottle using a 50-µL gas-tight syringe (Hampton 1705SL). Since we used borosilicate glass bottles, wall loss of the gaseous VOCs was negligible (Ahlberg et al. 2017; Kim and Kim 2015).
A gas chromatograph (Clarus 680, PerkinElmer, Waltham, MA) and mass spectrometer (Clarus SQ-8T, PerkinElmer, Waltham, MA) (GC/MS) system equipped with an Agilent DB-5ms capillary column (30 m × 0.25 mm ID, 0.25-µm film coated with 5 %-(phenylmethylpolysiloxane) were used to quantify the VOCs. Ultrapure helium was used as the carrier gas at a flow rate of 1 mL min−1 (1.67 × 10−8 m3 s−1). For each measurement, immediately after injecting the 30 µL of headspace sample, 1 µL of 500 ppb of the internal standard dissolved in methanol was also injected into the GC/MS. The ratio of the peak areas of the target VOCs and the internal standard (500 ppb) was measured. Five different concentrations of the VOCs and 500 ppb of their corresponding internal standard were prepared in methanol solutions to generate the calibration curve. Next, 1 µL of each solution was injected into the GC/MS, and the peak areas of both the VOC and internal standard were recorded as the response ratio. The response ratio against the concentration of the analyte to that of the internal standard was plotted to generate the calibration curves (r2 = 0.996). The mass of VOC that partitioned into the soil was calculated by deducting the measured mass of the gas-phase VOC in the GC/MS analysis from the mass of the injected VOC (by multiplying the injection volume with the density of the pure liquid VOC). The mass spectrometer was operated in the single-ion monitoring mode.
Statistical analysis
We measured the KSA at least in triplicate, which we then averaged with the uncertainty reported as one standard deviation. Regression analysis and statistical calculations were performed using SigmaPlot (Systat Software Inc.). We used Eqn 2 to perform regression analyses to determine which physicochemical parameters best correlated with the measured KSA; A and B are fitting constants.
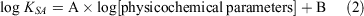
We used these physicochemical parameters: log psat, log Kow, log Koa, log [water solubility], log [Henry’s law constant], log [diffusion coefficient in air], log [diffusion coefficient in water] and log [molecular weight].
Results
Partitioning of VOCs in water-saturated soil
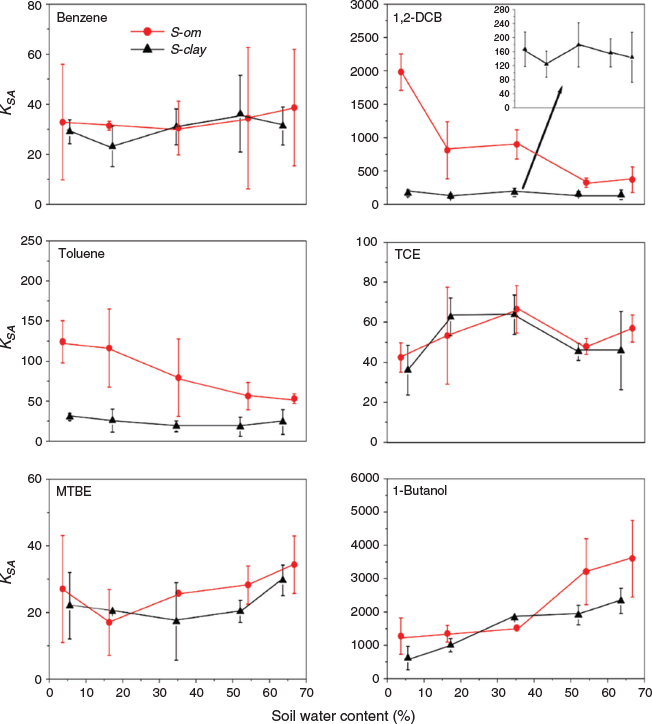
The KSA of VOCs in S-clay and S-om at different soil water contents are shown in Fig. 4. We observed different partitioning patterns for the VOCs that we investigated, but we did not observe significant differences in the KSA for similar VOC partitioning into both types of soil (Fig. 4), except 1,2-DCB and toluene, which partitioned preferentially into S-om (red curve) than into S-clay. The average KSA of 1,2-DCB on S-om was 2.6× to 10.9× higher than the average KSA on S-clay. In contrast, the average KSA of toluene on S-om was 2.3× to 4.1× the average KSA on S-clay. Benzene had a similar KSA on both types of soil. Although benzene was aromatic, it partitioned to a lesser extent into S-om compared with the substituted aromatic VOCs (1,2-DCB and toluene). We observed a similar phenomenon for the chlorinated alkane VOC (TCE) and polar VOCs (MTBE and 1-butanol), in which the extent of their partitioning was identical in both soil despite S-om containing higher organic matter (10.7 %).
Relationship between KSA of the VOCs and soil water content
We observed an almost linear decrease of log KSA with soil water content for 1,2-DCB and toluene partitioning into S-om (Fig. 5), while that of the other nonpolar VOCs (MTBE and TCE) exhibited poor linearity into both soils (not shown). As the water content of S-om increased, the KSA of 1,2-DCB and toluene decreased by 80 % and 55 % respectively over the entire soil water content range. Partitioning of TCE into both soils increased with an increasing water content of the soil to ~35 % by mass (~40 % of the WHC for each soil) and then gradually declined. In contrast, for both soils, MTBE exhibited an opposite partitioning trend: initially, the KSA slightly decreased, which then increased as the water content of the soil increased. Also, the KSA was 15–20 % greater on S-om than on S-clay at a soil water content of >50 % by mass, which suggested that partitioning into the soil organic matter also plays an important role for MTBE. For 1-butanol, the KSA increased almost linearly for all water contents in both soils. Similar to MTBE, when the water content in soil was relatively high (>50 % by mass), the KSA on S-om was nearly twice that of the KSA on S-clay. As the water content of S-om increased, the KSA of 1,2-DCB and toluene decreased by 80 % and 55 % respectively over the entire soil water content range.
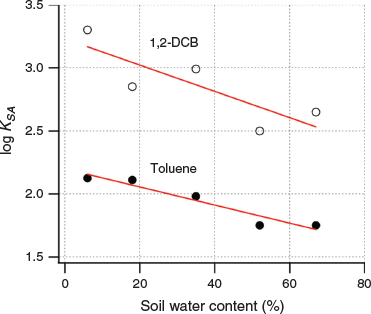
|
Correlation of the physicochemical parameters with log KSA
We investigated which physicochemical properties of the VOCs best correlated with the measured log KSA for both soils under varying water contents for predicting KSA using linear regression. For this analysis (performed under a confidence interval of 95 %), we used the following physicochemical properties of the VOCs: log psat, log Kow, log Koa, log [water solubility], log [Henry’s law constant], log [diffusion coefficient in air], log [diffusion coefficient in water] and log [molecular weight]. Since the organic carbon partitioning parameter (Koc) was strongly correlated with Kow (r2 = 0.9996), we only used Kow for our analysis. Among the physicochemical parameters, psat and Koa have been extensively used as the correlation parameters in modelling studies to predict the KSA of VOCs partitioning into soil with a small amount of water (Goss and Schwarzenbach 1999, 1998; Pankow 1998; Rao and Vejerano 2018). The Kow has also previously been incorporated in modelling (Hippelein and Mclachlan 1998). The Henry’s law constant describes the dissolution of VOCs into soil water (Goss and Eisenreich 1996; Ong and Lion 1991a) and the partitioning of VOCs into soil is described as the diffusion of VOCs into the soil micropores (Cheng et al. 2012; Jochum et al. 2015); thus, these parameters were investigated in this study as well. Since Koc was strongly correlated with Kow (r2 = 0.9996), we only used Kow in the analysis. The physicochemical parameters of log Kow, log [water solubility], log [Henry’s law constant], log [diffusion coefficient in air], log [diffusion coefficient in water] and log [molecular weight] were weakly correlated with log KSA with r2 < 0.23, 0.18, 0.30, 0.44, 0.24 and 0.23 respectively, compared with the other parameters regardless of soil type.
Correlation of KSA with psat and Koa in different soil types
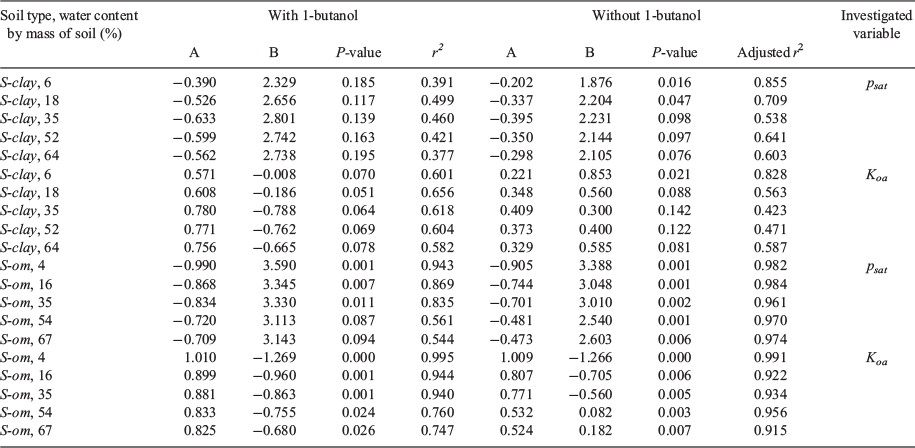
Only log psat and log Koa correlated strongly with log KSA compared with the other physicochemical parameters that we investigated but this was dependent on soil class. The summary of the results of the correlation analysis for the two parameters (log psat and log Koa) is given in Table 3. The graphs of the analysis depicting the correlation between log KSA and log psat or log Koa are shown in Fig. 6 and Fig. 7. Both parameters weakly correlated with log KSA for S-clay at all water contents of the soil (P > 0.05, r2 = 0.377–0.499) for log psat and log Koa (P > 0.05, r2 = 0.582–0.656) (Table 3) if all the VOCs were included.
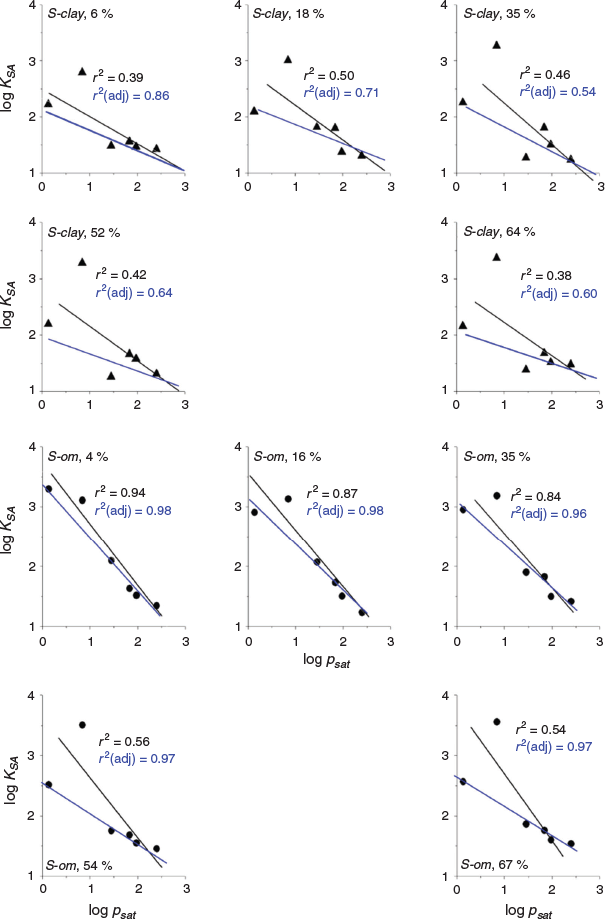
|
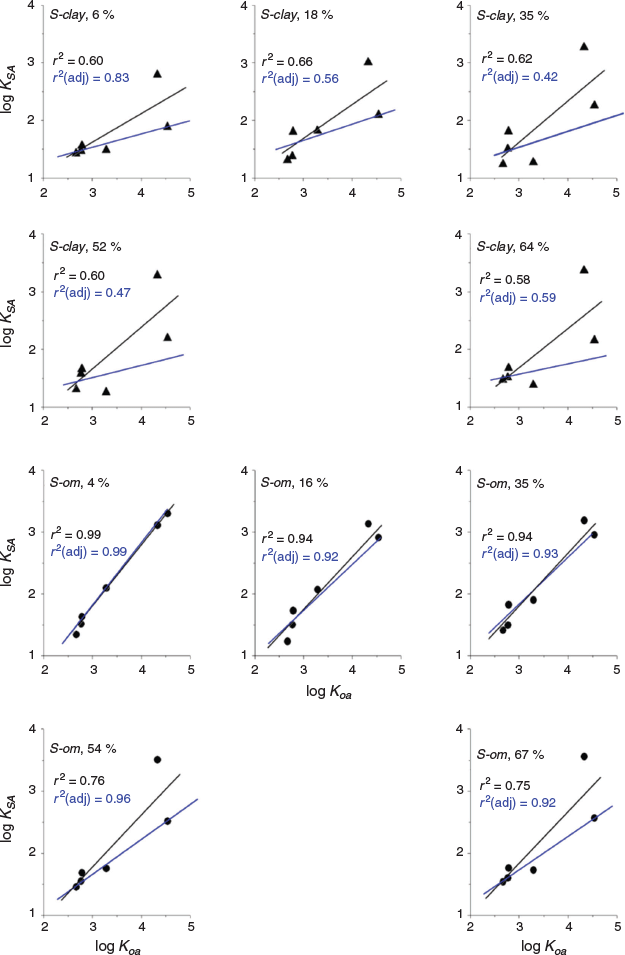
|
For S-om, which contained a high amount of organic matter, the log KSA was strongly correlated with log Koa at 4–35 % soil water content (corresponding to 5–40 % WHC) (r2 ≥ 0.94, P ≤ 0.001; Table 3). The log psat also showed a relatively strong correlation with log KSA at these soil water content levels (r2 of 0.835–0.943, P < 0.011). However, at the soil water content of 54–67 % (corresponding to a WHC of ~80–100 %), both parameters correlated weakly with log KSA (r2 ≤ 0.760).
Among the VOCs, the highly polar 1-butanol was an outlier in most cases. Excluding 1-butanol from the analysis, the r2 improved slightly, but the log KSA was still weakly correlated with both log psat and log Koa for S-clay. However, for S-om, excluding 1-butanol significantly improved the adjusted r2 from 0.747 to 0.974 (P ≤ 0.007, Table 3).
Discussion
The emission of VOCs from the soil and into the air is primarily affected by the soil moisture dynamics and carbon availability (i.e. the presence of organic matter) (Rossabi et al. 2018). The high mass fraction of water and organic matter affect VOC partitioning, and ultimately the emission of VOCs into the air. We measured the soil–air partition constant for three classes of VOCs: non-polar aromatic, slightly polar alkanes and highly polar compounds, as representatives of chemicals that may be present in chemical spills and environmental contamination. Our results regarding 1,2-DCB and toluene partitioning more into S-om than into S-clay indicate that aromatic VOCs preferentially partitioned into the organic matter fraction rather than into the mineral fraction of the water-saturated soil, which is consistent with those in the literature (Costanza and Brusseau 2000; Han et al. 2013). These results indicate that the chlorine substituents likely enhanced the affinity of the VOC to sorb onto the organic matter fraction (e.g. the hydrophobic sites) of the water-saturated soil, which is consistent with the finding that chlorinated aromatic VOCs partition into soil with high organic matter suspended in aqueous water (Pierce et al. 1974). We expect that the association of the chlorinated aromatic VOCs with soil organic matter may be enhanced if the soil is saturated with water.
In soil that contained high amounts of organic matter, we attributed the decline in KSA as the water content increases to the impact of water covering the hydrophobic sites of the organic matter, which rendered them less available to interact with the VOC. Benzene had a similar KSA on both types of soil. Although benzene is aromatic, it partitioned to a lesser extent into S-om compared with the substituted aromatic VOCs (1,2-DCB and toluene). We observed a similar phenomenon for the chlorinated alkane VOC (TCE) and polar VOCs (MTBE and 1-butanol), in which the extent of their partitioning was similar in both soils, despite S-om containing higher organic matter (10.7 %). This phenomenon may likely arise because these VOCs are less hydrophobic compared with aromatic VOCs (1,2-DCB and toluene). Therefore, for the less hydrophobic nonaromatic VOCs, the organic matter fraction in soil was not the primary partitioning medium.
The comparable partition coefficients for benzene, TCE, MTBE and 1-butanol into both soils indicated that neither the mineral soil’s specific surface area nor the organic matter fraction in soil (Table 2) played a significant role on partitioning. The more dominant factor was the presence of condensed water on the soil surface at a high level of RH (~100 %) (Cabbar 1999; Goss and Eisenreich 1996). This condensed water may form a thick layer on soil that prevents the components in the soil from interacting directly with the VOCs (Goss and Eisenreich 1996) but enhances the dissolution of the VOCs. For instance, TCE has been observed to dissolve into a water film containing at least five layers of molecular water on a mineral surface (Ong and Lion 1991a).
Although benzene is highly soluble in water (~1.79 g L−1, Table 1), the dissolution of benzene into the water in soil may not be the only mechanism of its partitioning; otherwise, we would have observed an increased in KSA as the water content increased (Goss and Eisenreich 1996), but in fact, the KSA remained constant (Fig. 1). Thus, it was likely that some mass fraction of benzene adsorbed at the air-water interface (Costanza and Brusseau 2000; Heath and Valsaraj 2015). As the water content of the soil increased, more mesopores and macrospores were filled with water, which led to a decrease in the surface area of the air-water interface (Cabbar 1999). The increased dissolution of benzene with increasing soil water content might have offset the decrease in the mass of adsorbed benzene at the air-water interface, leading to an almost constant KSA. Such mechanisms may also explain the near constant KSA with increasing soil water content observed for 1,2-DCB and toluene partitioning into S-clay.
The increase of KSA at the beginning was likely a result of a larger mass fraction of TCE dissolving at higher soil water content levels and less adsorption at the air-water interface, and the dissolution partly contributed to the overall partitioning (Ong and Lion 1991a, 1991b). The chlorine substituents of the alkane VOC may also increase the sorption by interacting strongly with sites on the water film that have high interfacial energy through induced electrostatic forces (Tekrony and Ahlert 2001). As the water content increased up to the WHC, the area at the air-water interface and the availability of high interfacial energy sites on the water film were significantly reduced, which resulted in the overall decline in the KSA. We attributed the slight decrease of KSA to the adsorption-dominated partitioning mechanism at the air-water interface when the water content of the soil was relatively low. Although the weakly polar VOCs (e.g. MTBE) have stronger tendency to adsorb at the air-water interface than the nonpolar VOCs, the high aqueous solubility of MTBE (Table 1) may have limited the relative importance of interfacial sorption to the overall MTBE partitioning (Costanza and Brusseau 2000), in which we observed an increase in KSA at higher soil water content. Also, the KSA was 15–20 % greater on S-om than on S-clay at a soil water content of >50 % by mass, which suggested that partitioning into the soil organic matter also played a vital role in the overall MTBE partitioning.
Dissolution of 1-butanol into the water in soil was likely the dominant partitioning mechanism because this VOC is highly polar (log Kow < 1, Table 1), and because we observed an almost linear increase in the KSA under all water contents for both soil. However, because 1-butanol has the lowest dimensionless Henry’s law constant that is two to three orders magnitude lower than the other VOCs (Table 1), the air-water partitioning mechanism might have also contributed to the overall partitioning of 1-butanol into soil. Similar to MTBE, when the water content in soil was relatively high (>50 % by mass), the KSA on S-om was nearly twice that on S-clay, which suggests the importance of the organic matter on partitioning for this class of VOC.
We are interested in determining if psat and Koa are good predictors of KSA for VOCs sorbing in soil that are saturated with water or those that contain water close to the WHC. Although the psat and Koa parameters have been extensively applied in modelling studies to predict the partitioning of VOCs into unsaturated soil, they are not applicable for mineral soil that contains almost no organic matter under a water-saturated condition. Both parameters were weakly correlated with log KSA for S-clay at all water contents of the soil (P > 0.05, r2 = 0.377–0.499) for log psat and log Koa (P > 0.05, r2 = 0.582–0.656) (Table 2). The adjusted r2 rather than r2 was reported when we excluded 1-butanol from the analysis because this regression contained only a part of the targeted VOC parameters (Miles 2005). Therefore, in this case, psat and Koa may be used to predict KSA of VOCs partitioning into S-om. However, using these parameters may underestimate the partitioning of highly polar VOCs (e.g. 1-butanol, log Kow << 1) when the soil water content is relatively high.
We also compared our correlation analysis results with the work reported by Hippelein and Mclachlan (Hippelein and Mclachlan 1998), where the partitioning of semivolatile organic compounds (SVOCs; chlorinated benzenes, PCBs and PAHs) into the water-saturated soil that contained mainly sand with 1 % of organic carbon and 1.9 % of soil water was studied. In their study, the log KSA correlated well with log Koa (r2 = 0.972, A = 0.987, B = −1.686), which is comparable to the results of our analysis of VOCs partitioning into the as-received S-om (Table 2; r2 = 0.995, A = 1.01, B = −1.269). Hippelein and McLachlan (Hippelein and Mclachlan 1998) also found a strong correlation between log KSA and log psat (r2 = 0.974, A = −0.912, B = 4.304) after excluding PAHs from the regression, which again was comparable to our analysis for VOCs partitioning into the as-received S-om (Table 2; r2 = 0.943, A = −0.99, B = 3.59). Therefore, soil with an organic matter as low as 1 wt-% may be considered as an S-om type soil for SVOCs partitioning. However, it is unclear if a similar organic matter content of 1 wt-% is also applicable to VOC partitioning since our S-om contained 10.7 % organic matter by soil mass. Also, the VOCs may partition differently from SVOCs (e.g. PAHs), thus models explicitly developed for VOC-soil partitioning are desirable.
Based on these results, we have three recommendations: (1) psat or Koa is not applicable to predict KSA for mineral-type soil that is water-saturated and contains minimal organic matter; (2) for water-saturated soil that contains a relatively high amount of organic matter, psat and Koa are good parameters (Koa is slightly better than psat) in modelling studies to predict KSA if the soil contains a low mass fraction of water; (3) for soil with a high water content (>50 % of soil mass using Koa and >35 % of soil mass using psat in this study), highly polar VOCs need to be separated from other types of VOCs in modelling studies to predict KSA. Therefore, for most soils in the environment that contain a high amount of organic matter and are saturated with water, psat or Koa can be used to predict KSA.
Only a limited number of protocols exist for estimating VOC emission from the soil into the air. A predictive equation, as in this case, can be used to determine the average long-term emissions of VOCs released into the air. Because it is relatively easier to measure the concentration of contaminants in water and soil, our results can be used to estimate the emission of a broad class of non-polar and slightly polar VOCs into the atmosphere if air quality measurement is difficult to obtain or unavailable, especially after a natural disaster. Here, we described an application scenario of our results. The estimations described here illustrate a worst-case scenario with several assumptions and simplifications. The most important, but not limited to these, were: (1) the contaminants were ultimately deposited into the soil at a high concentration, (2) emission was homogenous over time, (3) the soil and air temperatures were assumed to be isothermal at 25 °C, (4) the contaminants accumulated in the air and were stagnant. Of course, the actual air concentrations of the VOCs would be lower than these estimates since the prevailing meteorological conditions disperse the contaminants. Also, emission rates into the air will decline as the VOCs in the soil are metabolised by microorganisms or undergo biogeochemical processes over time.
In August 2017, Hurricane Harvey dumped massive rain damaging chemical and industrial plants across south-eastern Texas. During and following the storm, 46 chemical plants and refineries released 4.6 million pounds of hazardous chemicals into the air, land and water over a 13-county area (Nicole 2018). Harris County, a highly-populated area with a large number of industrial or chemical facilities and refineries, had 63 % of the accidents (Qin et al. 2020). During the hurricane, 40 % of the spilled chemicals was released into the air, 35 % into the water and 6 % into the soil (Misuri et al. 2019). Harris County has a total area of 4600 km2, of which 4410 km2 consists of land and the rest is covered with water. Dark gumbo clay is the dominant soil type covering half of the non-impervious surface in Harris county (USDA 2019).
In our calculation, we considered the mass concentration of chemicals deposited in the soil and those in the water, which we assumed would eventually sorb in the soil. We only considered one refinery that released 106 781 kg of chemicals during the hurricane (Qin et al. 2020). The amount released into the soil was 43 780 kg (41 %). The refinery usually emits 2.8–7.8 wt-% of benzene and 3.1–5.9 wt-% of toluene (Mo et al. 2015; Wei et al. 2014). If each VOC (benzene, toluene, ethylbenzene and xylenes (BTEX)) contained only 5 wt-% in the spilled chemical, each VOC was present at ~2189 kg in soil. We calculated the concentration of VOCs that partitioned into the air using Eqn 3:
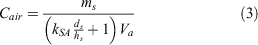
where Cair (µg m−3) is the concentration in air, ms (µg) is the mass of the VOC in soil, ds (m) is the depth of the soil, hs (m) is the depth of the surface layer and Va (m3) is the volume of air. We used a volume of 4.42 × 1010 m3. The depth of the surface layer, the region of the atmosphere in which heat and momentum fluxes are negligible (<10 %), is ≤ 100 m during a typical daytime unstable condition and ≤ 10 m during a typical nighttime situation (Zannetti 2013). In the calculations, we used hs at 10 m, which depicted a worst-case scenario. For the surface area, we used the percent of non-impervious surface in Harris County, which was estimated to be 50 % (2205 km2) (Han and Burian 2009). We used a soil depth of 0.2 m as it contained a high concentration of organic matter (Kramer and Gleixner 2008). We calculated the concentrations BTEX using the experimental KSA derived from our study, which was 40 for benzene and 56.2 for toluene under S-om at 67 % WHC. For ethylbenzene, xylenes and other VOCs that may be present during the chemical spill, we used the Koa to determine the KSA using log KSA = 0.524 × log Koa + 0.182 in Table 3, which we obtained from our correlation analysis. The conditions used in the calculation and the air concentration for BTEX are depicted in Fig. 8d.
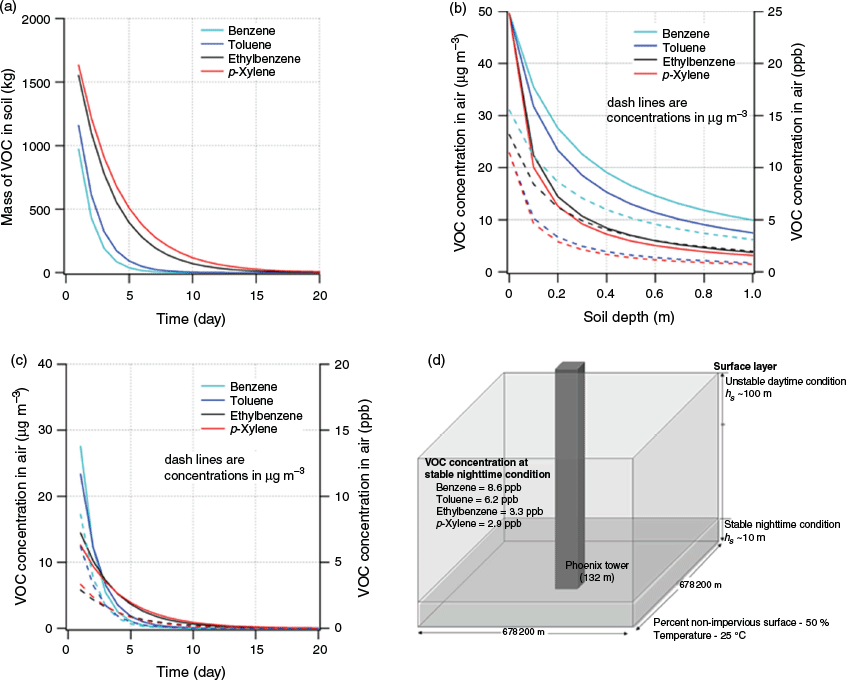
|
At the nighttime condition, the emitted VOC concentrations into the air were 8.6 ppb for benzene, 6.2 ppb for toluene, 3.3 ppb for ethylbenzene and ~3.0 ppb for isomers of xylenes. These values were well below the National Institute of Occupational Safety and Health Recommended Exposure Limits (NIOSH REL). During the daytime, the air concentrations for similar VOCs were 10× less because of the higher depth of the surface layer. The masses of the VOCs remaining in the soil were three orders of magnitude lower than the initial masses after 30 days (Fig. 8a), and the concentrations of BTEX emitted into the air were less than 10 ppb (Fig. 8b). The concentration of VOCs emitted into the air decreased with soil depth. Note, we assumed that the VOCs accumulated into the surface layer for 24 h. At a soil depth of zero, which represents the maximum emission rate, the air concentrations of BTEX at a surface layer of 10 m were ~31 ppb (Fig. 8c). This value is also the maximum concentration if we assumed that all the emitted BTEX accumulated in the air for ~30 days in the absence of a removal mechanism. Note that we estimated only from one-point source; therefore, a higher air concentration owing to a localised emission of the VOCs is expected. Air sampling in Houston after Hurricane Harvey was ~99 ppb, which suggested that other sources contributed to that spike or from localised emissions (Tabuchi 2017). A summary of the calculated air concentrations for a larger subset of VOCs is presented in Table 4.
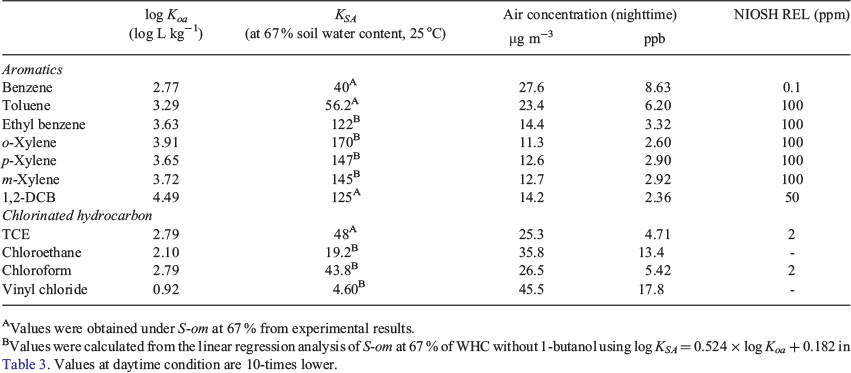
|
In summary, we assessed the partitioning of six polar, weakly polar or nonpolar VOCs into two types of soil under environmentally relevant conditions (i.e. water-saturated condition) to find a physicochemical parameter for predicting the KSA for these VOCs. The soil water content is important for the partitioning of VOCs, particularly, for highly polar VOCs and some aromatic VOCs. Partitioning of non-polar substituted aromatics was sensitive to the organic matter content in water-saturated soil. psat and Koa correlated best with KSA compared with the other physicochemical parameters; the KSA of VOCs on soil with high organic matter content correlated well with psat and Koa but not on mineral soil (clays). The VOCs partitioned into the soil by a combination of different mechanisms; associating with the soil organic matter, dissolving into the water in soil and adsorbing at the air-water interface. We applied the results of our study to calculate the air concentration of the VOCs. The air concentration for some VOCs was substantially lower than NIOSH REL even under a worst-case scenario. Results can be used to estimate emissions for similar types of VOCs emitted from the soil to the atmosphere. For future studies, we recommend developing a separate model for predicting the partition coefficient of highly polar and nonpolar or weakly polar VOCs. Also, we recommend investigating further the impact of organic matter on the partitioning of aromatic VOCs.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgement
The United States Army Research Office (GRANT11970168) supported this work.
References
Ahlberg E, Falk J, Eriksson A, Holst T, Brune WH, Kristensson A, Roldin P, Svenningsson B (2017). Secondary organic aerosol from VOC mixtures in an oxidation flow reactor. Atmospheric Environment 161, 210–220.| Secondary organic aerosol from VOC mixtures in an oxidation flow reactorCrossref | GoogleScholarGoogle Scholar |
Asensio D, Peñuelas J, Filella I, Llusià J (2007). On-line screening of soil VOCs exchange responses to moisture, temperature and root presence. Plant and Soil 291, 249–261.
| On-line screening of soil VOCs exchange responses to moisture, temperature and root presenceCrossref | GoogleScholarGoogle Scholar |
Batterman S, Kulshrestha A, Cheng H-Y (1995). Hydrocarbon vapor transport in low moisture soils. Environmental Science & Technology 29, 171–180.
| Hydrocarbon vapor transport in low moisture soilsCrossref | GoogleScholarGoogle Scholar |
Breus IP, Mishchenko AA (2006). Sorption of volatile organic contaminants by soils (a review). Eurasian Soil Science 39, 1271–1283.
| Sorption of volatile organic contaminants by soils (a review)Crossref | GoogleScholarGoogle Scholar |
Brown K, Wherrett A (2018). Bulk density – Measurement. Available at http://soilquality.org.au/factsheets/bulk-density-measurement [verified 19 February 2020]
Cabbar HC (1999). Effects of humidity and soil organic matter on the sorption of chlorinated methanes in synthetic humic-clay complexes. Journal of Hazardous Materials 68, 217–226.
| Effects of humidity and soil organic matter on the sorption of chlorinated methanes in synthetic humic-clay complexesCrossref | GoogleScholarGoogle Scholar |
Cheng H, Hu E, Hu Y (2012). Impact of mineral micropores on transport and fate of organic contaminants: A review. Journal of Contaminant Hydrology 129–130, 80–90.
| Impact of mineral micropores on transport and fate of organic contaminants: A reviewCrossref | GoogleScholarGoogle Scholar | 22055156PubMed |
Chiou CT (2003). ‘Partition and adsorption of organic contaminants in environmental systems.’ (John Wiley & Sons: Hoboken, NJ)
Costanza MS, Brusseau ML (2000). Contaminant vapor adsorption at the gas− water interface in soils. Environmental Science & Technology 34, 1–11.
| Contaminant vapor adsorption at the gas− water interface in soilsCrossref | GoogleScholarGoogle Scholar |
Dumanoglu Y, Kara M, Altiok H, Odabasi M, Elbir T, Bayram A (2014). Spatial and seasonal variation and source apportionment of volatile organic compounds (VOCs) in a heavily industrialized region. Atmospheric Environment 98, 168–178.
| Spatial and seasonal variation and source apportionment of volatile organic compounds (VOCs) in a heavily industrialized regionCrossref | GoogleScholarGoogle Scholar |
Fram MS, Belitz K (2011). Occurrence and concentrations of pharmaceutical compounds in groundwater used for public drinking-water supply in California. The Science of the Total Environment 409, 3409–3417.
| Occurrence and concentrations of pharmaceutical compounds in groundwater used for public drinking-water supply in CaliforniaCrossref | GoogleScholarGoogle Scholar | 21684580PubMed |
Goss K-U (2004). The air/surface adsorption equilibrium of organic compounds under ambient conditions. Critical Reviews in Environmental Science and Technology 34, 339–389.
| The air/surface adsorption equilibrium of organic compounds under ambient conditionsCrossref | GoogleScholarGoogle Scholar |
Goss K-U, Eisenreich SJ (1996). Adsorption of VOCs from the gas phase to different minerals and a mineral mixture. Environmental Science & Technology 30, 2135–2142.
| Adsorption of VOCs from the gas phase to different minerals and a mineral mixtureCrossref | GoogleScholarGoogle Scholar |
Goss K-U, Schwarzenbach RP (1998). Gas/solid and gas/liquid partitioning of organic compounds: critical evaluation of the interpretation of equilibrium constants. Environmental Science & Technology 32, 2025–2032.
| Gas/solid and gas/liquid partitioning of organic compounds: critical evaluation of the interpretation of equilibrium constantsCrossref | GoogleScholarGoogle Scholar |
Goss K-U, Schwarzenbach RP (1999). Quantification of the effect of humidity on the gas/mineral oxide and gas/salt adsorption of organic compounds. Environmental Science & Technology 33, 4073–4078.
| Quantification of the effect of humidity on the gas/mineral oxide and gas/salt adsorption of organic compoundsCrossref | GoogleScholarGoogle Scholar |
Han WS, Burian SJ (2009). Determining Effective Impervious Area for Urban Hydrologic Modeling. Journal of Hydrologic Engineering 14, 111–120.
| Determining Effective Impervious Area for Urban Hydrologic ModelingCrossref | GoogleScholarGoogle Scholar |
Han C, Zhang H, Gu Q, Guo G, Li Y, Li F (2013). Toluene sorption behavior on soil organic matter and its composition using three typical soils in China. Environmental Earth Sciences 68, 741–747.
| Toluene sorption behavior on soil organic matter and its composition using three typical soils in ChinaCrossref | GoogleScholarGoogle Scholar |
Heath AA, Valsaraj KT (2015). Effects of Temperature, Oxygen Level, Ionic Strength, and pH on the Reaction of Benzene with Hydroxyl Radicals at the Air–Water Interface in Comparison to the Bulk Aqueous Phase. The Journal of Physical Chemistry A 119, 8527–8536.
| Effects of Temperature, Oxygen Level, Ionic Strength, and pH on the Reaction of Benzene with Hydroxyl Radicals at the Air–Water Interface in Comparison to the Bulk Aqueous PhaseCrossref | GoogleScholarGoogle Scholar | 26158391PubMed |
Hippelein M, Mclachlan MS (1998). Soil/air partitioning of semivolatile organic compounds. 1. Method development and influence of physical-chemical properties. Environmental Science & Technology 32, 310–316.
| Soil/air partitioning of semivolatile organic compounds. 1. Method development and influence of physical-chemical propertiesCrossref | GoogleScholarGoogle Scholar |
Hoff JT, Gillham R, Mackay D, Shiu WY (1993). Sorption of organic vapors at the air-water interface in a sandy aquifer material. Environmental Science & Technology 27, 2789–2794.
| Sorption of organic vapors at the air-water interface in a sandy aquifer materialCrossref | GoogleScholarGoogle Scholar |
Hwang H-M, Fiala MJ, Wade TL, Park D (2019). Review of pollutants in urban road dust: Part II. Organic contaminants from vehicles and road management. International Journal of Urban Sciences 23, 445–463.
| Review of pollutants in urban road dust: Part II. Organic contaminants from vehicles and road managementCrossref | GoogleScholarGoogle Scholar |
Jochum T, Michalzik B, Bachmann A, Popp J, Frosch T (2015). Microbial respiration and natural attenuation of benzene contaminated soils investigated by cavity enhanced Raman multi-gas spectroscopy. Analyst 140, 3143–3149.
| Microbial respiration and natural attenuation of benzene contaminated soils investigated by cavity enhanced Raman multi-gas spectroscopyCrossref | GoogleScholarGoogle Scholar | 25751376PubMed |
Kim Y-H, Kim K-H (2015). Test on the reliability of gastight syringes as transfer/storage media for gaseous VOC analysis: The extent of VOC sorption between the inner needle and a glass wall surface. Analytical Chemistry 87, 3056–3063.
| Test on the reliability of gastight syringes as transfer/storage media for gaseous VOC analysis: The extent of VOC sorption between the inner needle and a glass wall surfaceCrossref | GoogleScholarGoogle Scholar | 25627703PubMed |
Kim J-H, Gan J, Farmer WJ, Yates SR, Papiernik SK, Dungan RS (2003). Organic matter effects on phase partition of 1, 3-dichloropropene in soil. Journal of Agricultural and Food Chemistry 51, 165–169.
| Organic matter effects on phase partition of 1, 3-dichloropropene in soilCrossref | GoogleScholarGoogle Scholar | 12502402PubMed |
Kim H, Lee S, Moon J-W, Rao PSC (2005). Gas transport of volatile organic compounds in unsaturated soils. Soil Science Society of America Journal 69, 990–995.
| Gas transport of volatile organic compounds in unsaturated soilsCrossref | GoogleScholarGoogle Scholar |
Kramer C, Gleixner G (2008). Soil organic matter in soil depth profiles: Distinct carbon preferences of microbial groups during carbon transformation. Soil Biology & Biochemistry 40, 425–433.
| Soil organic matter in soil depth profiles: Distinct carbon preferences of microbial groups during carbon transformationCrossref | GoogleScholarGoogle Scholar |
Kremser A, Jochmann MA, Schmidt TC (2016). Systematic comparison of static and dynamic headspace sampling techniques for gas chromatography. Analytical and Bioanalytical Chemistry 408, 6567–6579.
| Systematic comparison of static and dynamic headspace sampling techniques for gas chromatographyCrossref | GoogleScholarGoogle Scholar | 27526093PubMed |
McDonald BC, de Gouw JA, Gilman JB, Jathar SH, Akherati A, Cappa CD, Jimenez JL, Lee-Taylor J, Hayes PL, McKeen SA, Cui YY, Kim S-W, Gentner DR, Isaacman-VanWertz G, Goldstein AH, Harley RA, Frost GJ, Roberts JM, Ryerson TB, Trainer M (2018). Volatile chemical products emerging as largest petrochemical source of urban organic emissions. Science 359, 760–764.
| Volatile chemical products emerging as largest petrochemical source of urban organic emissionsCrossref | GoogleScholarGoogle Scholar | 29449485PubMed |
Mead RN, Cala JM, Felix JD, Shimizu MS, Casas MS, Lathrope T, Avery GB, Kieber RJ, Willey JD (2017). A static headspace GC-MS/MS method for the determination of ethanol, iso-butanol, and n-butanol at nanomolar concentrations in aqueous environmental samples. Limnology and Oceanography, Methods 15, 1007–1014.
| A static headspace GC-MS/MS method for the determination of ethanol, iso-butanol, and n-butanol at nanomolar concentrations in aqueous environmental samplesCrossref | GoogleScholarGoogle Scholar |
Miles J (2005). R‐squared, adjusted R‐squared. In ‘Encyclopedia of statistics in behavioral science’. (Eds B Everitt, D Howell) pp. 1655–1657. (John Wiley & Sons: Chichester)
Misuri A, Moreno VC, Quddus N, Cozzani V (2019). Lessons learnt from the impact of hurricane Harvey on the chemical and process industry. Reliability Engineering & System Safety 190, 106521
| Lessons learnt from the impact of hurricane Harvey on the chemical and process industryCrossref | GoogleScholarGoogle Scholar |
Mo Z, Shao M, Lu S, Qu H, Zhou M, Sun J, Gou B (2015). Process-specific emission characteristics of volatile organic compounds (VOCs) from petrochemical facilities in the Yangtze River Delta, China. The Science of the Total Environment 533, 422–431.
| Process-specific emission characteristics of volatile organic compounds (VOCs) from petrochemical facilities in the Yangtze River Delta, ChinaCrossref | GoogleScholarGoogle Scholar | 26179779PubMed |
Moran MJ, Zogorski JS, Squillace PJ (2007). Chlorinated solvents in groundwater of the United States. Environmental Science & Technology 41, 74–81.
| Chlorinated solvents in groundwater of the United StatesCrossref | GoogleScholarGoogle Scholar |
Nicole W (2018). Wristbands for Research: Using Wearable Sensors to Collect Exposure Data after Hurricane Harvey. Environmental Health Perspectives 126, 042001
| Wristbands for Research: Using Wearable Sensors to Collect Exposure Data after Hurricane HarveyCrossref | GoogleScholarGoogle Scholar | 29863828PubMed |
Nofziger DL (2003). ‘Soil temperature changes with time and depth: theory.’ (Department of Plant and Soil Sciences, Oklahoma State University: Stillwater, OK)
Ong SK, Lion LW (1991a). Effects of soil properties and moisture on the sorption of trichloroethylene vapor. Water Research 25, 29–36.
| Effects of soil properties and moisture on the sorption of trichloroethylene vaporCrossref | GoogleScholarGoogle Scholar |
Ong SK, Lion LW (1991b). Mechanisms for trichloroethylene vapor sorption onto soil minerals. Journal of Environmental Quality 20, 180–188.
| Mechanisms for trichloroethylene vapor sorption onto soil mineralsCrossref | GoogleScholarGoogle Scholar |
Pankow JF (1998). Further discussion of the octanol/air partition coefficient Koa as a correlating parameter for gas/particle partitioning coefficients. Atmospheric Environment 32, 1493–1497.
| Further discussion of the octanol/air partition coefficient Koa as a correlating parameter for gas/particle partitioning coefficientsCrossref | GoogleScholarGoogle Scholar |
Petersen LW, Moldrup P, El-Farhan YH, Jacobsen OH, Yamaguchi T, Rolston DE (1995). The effect of moisture and soil texture on the adsorption of organic vapors. Journal of Environmental Quality 24, 752–759.
| The effect of moisture and soil texture on the adsorption of organic vaporsCrossref | GoogleScholarGoogle Scholar |
Pierce RH, Olney CE, Felbeck GT (1974). pp′-DDT adsorption to suspended particulate matter in sea water. Geochimica et Cosmochimica Acta 38, 1061–1073.
| pp′-DDT adsorption to suspended particulate matter in sea waterCrossref | GoogleScholarGoogle Scholar |
Post E, Henderson JB (2012). Characterization of two different clay materials by thermogravimetry (TG), differential scanning calorimetry (DSC), dilatometry (DIL) and mass spectrometry (MS) – 12215. WM2012: Waste Management 2012 conference on improving the future in waste management, Phoenix, AZ (United States), 26 February – 1 March 2012. (WM Symposia: Tempe, AZ)
Qin R, Khakzad N, Zhu J (2020). An overview of the impact of Hurricane Harvey on chemical and process facilities in Texas. International Journal of Disaster Risk Reduction 45, 101453
| An overview of the impact of Hurricane Harvey on chemical and process facilities in TexasCrossref | GoogleScholarGoogle Scholar |
Ranjan M, Presto AA, May AA, Robinson AL (2012). Temperature dependence of gasparticle partitioningof primary organic aerosol emissions from a small diesel engine. Aerosol Science and Technology 46, 13–21.
| Temperature dependence of gasparticle partitioningof primary organic aerosol emissions from a small diesel engineCrossref | GoogleScholarGoogle Scholar |
Rao G, Vejerano EP (2018). Partitioning of volatile organic compounds to aerosols: A review. Chemosphere 212, 282–296.
| Partitioning of volatile organic compounds to aerosols: A reviewCrossref | GoogleScholarGoogle Scholar | 30145420PubMed |
Rivett MO, Wealthall GP, Dearden RA, McAlary TA (2011). Review of unsaturated-zone transport and attenuation of volatile organic compound (VOC) plumes leached from shallow source zones. Journal of Contaminant Hydrology 123, 130–156.
| Review of unsaturated-zone transport and attenuation of volatile organic compound (VOC) plumes leached from shallow source zonesCrossref | GoogleScholarGoogle Scholar | 21316792PubMed |
Rogers RD, McFarlane JC, Cross AJ (1980). Adsorption and desorption of benzene in two soils and montmorillonite clay. Environmental Science & Technology 14, 457–460.
| Adsorption and desorption of benzene in two soils and montmorillonite clayCrossref | GoogleScholarGoogle Scholar |
Rossabi S, Choudoir M, Helmig D, Hueber J, Fierer N (2018). Volatile organic compound emissions from soil following wetting events. Journal of Geophysical Research. Biogeosciences 123, 1988–2001.
| Volatile organic compound emissions from soil following wetting eventsCrossref | GoogleScholarGoogle Scholar |
Sanscartier D, Zeeb B, Koch I, Reimer K (2009). Bioremediation of diesel-contaminated soil by heated and humidified biopile system in cold climates. Cold Regions Science and Technology 55, 167–173.
| Bioremediation of diesel-contaminated soil by heated and humidified biopile system in cold climatesCrossref | GoogleScholarGoogle Scholar |
Seneviratne SI, Corti T, Davin EL, Hirschi M, Jaeger EB, Lehner I, Orlowsky B, Teuling AJ (2010). Investigating soil moisture–climate interactions in a changing climate: A review. Earth-Science Reviews 99, 125–161.
| Investigating soil moisture–climate interactions in a changing climate: A reviewCrossref | GoogleScholarGoogle Scholar |
Shih Y, Wu S (2005). Distinctive sorption mechanisms of soil organic matter and mineral components as elucidated by organic vapor uptake kinetics. Environmental Toxicology and Chemistry 24, 2827–2832.
| Distinctive sorption mechanisms of soil organic matter and mineral components as elucidated by organic vapor uptake kineticsCrossref | GoogleScholarGoogle Scholar | 16398119PubMed |
Shimizu Y, Takei N, Terashima Y (1992). Sorption of organic pollutants from vapor phase: The effects of natural solid characteristics and moisture content. Water Science and Technology 26, 79–87.
| Sorption of organic pollutants from vapor phase: The effects of natural solid characteristics and moisture contentCrossref | GoogleScholarGoogle Scholar |
Statista (2018). Volume of volatile organic compounds (VOC) emissions in the U.S. from 1970 to 2016 (in 1,000 tons). Available at https://www.statista.com/statistics/501310/volume-of-volatile-organic-compounds-emissions-us/ [verified 19 February 2020]
SU SL, Singh DN, Baghini MS (2014). A critical review of soil moisture measurement. Measurement 54, 92–105.
| A critical review of soil moisture measurementCrossref | GoogleScholarGoogle Scholar |
Tabuchi H (2017). High levels of carcinogen found in Houston area after Harvey. New York Times.
Tekrony MC, Ahlert RC (2001). Adsorption of chlorinated hydrocarbon vapors onto soil in the presence of water. Journal of Hazardous Materials 84, 135–146.
| Adsorption of chlorinated hydrocarbon vapors onto soil in the presence of waterCrossref | GoogleScholarGoogle Scholar | 11406302PubMed |
USDA (2019). Web Soil Survey. Available at https://websoilsurvey.nrcs.usda.gov/app/ [verified 25 February 2020]
Wei W, Cheng S, Li G, Wang G, Wang H (2014). Characteristics of volatile organic compounds (VOCs) emitted from a petroleum refinery in Beijing, China. Atmospheric Environment 89, 358–366.
| Characteristics of volatile organic compounds (VOCs) emitted from a petroleum refinery in Beijing, ChinaCrossref | GoogleScholarGoogle Scholar |
Wei W, Mandin C, Blanchard O, Mercier F, Pelletier M, Le Bot B, Glorennec P, Ramalho O (2016). Temperature dependence of the particle/gas partition coefficient: An application to predict indoor gas-phase concentrations of semi-volatile organic compounds. The Science of the Total Environment 563–564, 506–512.
| Temperature dependence of the particle/gas partition coefficient: An application to predict indoor gas-phase concentrations of semi-volatile organic compoundsCrossref | GoogleScholarGoogle Scholar | 27152992PubMed |
Zannetti P (2013). ‘Air pollution modeling: theories, computational methods and available software.’ (Springer Science & Business Media: Berlin)