Mass spectrometric approaches for chemical characterisation of atmospheric aerosols: critical review of the most recent advances
Alexander Laskin A D , Julia Laskin B and Sergey A. Nizkorodov CA Environmental Molecular Sciences Laboratory, Pacific Northwest National Laboratory, Richland, WA 99352, USA.
B Chemical and Materials Sciences Division, Pacific Northwest National Laboratory, Richland, WA 99352, USA. Email: julia.laskin@pnnl.gov
C Department of Chemistry, University of California, Irvine, CA 92697, USA. Email: nizkorod@uci.edu
D Corresponding author. Email: alexander.laskin@pnnl.gov

Alexander Laskin is a Senior Research Scientist at Pacific Northwest National Laboratory (PNNL). He received his M.Sc. degree (physics) in 1991 from the Leningrad Polytechnical Institute, Russia, and Ph.D. degree (physical chemistry) in 1998 from the Hebrew University of Jerusalem, Israel. Following postdoctoral research appointments at the University of Delaware (1998–99) and PNNL (1999–2001), he became a permanent PNNL scientist in 2001. His present and past research interests include: physical and analytical chemistry of environmental aerosols, environmental and atmospheric effects of aerosols, combustion-related aerosols, combustion chemistry and chemical kinetics. |

Julia Laskin is a Laboratory Fellow at PNNL. She received her M.Sc. degree in physics from the Leningrad Polytechnical Institute (1990) and her Ph.D. degree in physical chemistry from the Hebrew University of Jerusalem (1998). She was a postdoctoral fellow at the University of Delaware (1998–99) and PNNL (2000–2002). She became a research scientist at PNNL in 2002. Her research is focussed on understanding activation and dissociation following collisions of complex ions with surfaces, selective surface modification using ion beams and developing new approaches for characterisation of the chemical composition of organic aerosols and biological materials. |

Sergey A. Nizkorodov is an Associate Professor at the University of California, Irvine. He received his M.Sc. degree in biochemistry from Novosibirsk State University, Russia (1993) and Ph.D. degree in chemical physics from Basel University, Switzerland (1997). After doing his postdoctoral research in chemical kinetics and reaction dynamics at the University of Colorado at Boulder and in atmospheric chemistry at the California Institute of Technology, he joined the faculty at the University of California, Irvine Chemistry Department in 2002. His current research interests include atmospheric photochemistry, air pollution in the outdoor and indoor environments and aerosol science. |
Environmental Chemistry 9(3) 163-189 https://doi.org/10.1071/EN12052
Submitted: 4 April 2012 Accepted: 16 May 2012 Published: 29 June 2012
Journal Compilation © CSIRO Publishing 2012 Open Access CC BY-NC-ND
Environmental context. Atmospheric aerosols have profound effects on the environment through several physicochemical processes, such as absorption and scattering of sunlight, heterogeneous gas–particle reactions and adverse effects on the respiratory and cardiovascular systems. Understanding aerosol atmospheric chemistry and its environmental impact requires comprehensive characterisation of the physical and chemical properties of particles. Results from mass spectrometry provide important new insights into the origin of atmospheric aerosols, the evolution of their physicochemical properties, their reactivity and their effect on the environment.
Abstract. This manuscript presents an overview of the most recent instrument developments for the field and laboratory applications of mass spectrometry (MS) to investigate the chemistry and physics of atmospheric aerosols. A range of MS instruments, employing different sample introduction methods, ionisation and mass detection techniques are used both for ‘online’ and ‘offline’ characterisation of aerosols. Online MS techniques enable detection of individual particles with simultaneous measurement of particle size distributions and aerodynamic characteristics and are ideally suited for field studies that require high temporal resolution. Offline MS techniques provide a means for detailed molecular-level analysis of aerosol samples, which is essential to gain fundamental knowledge regarding aerosol chemistry, mechanisms of particle formation and atmospheric aging. Combined, complementary MS techniques provide comprehensive information on the chemical composition, size, morphology and phase of aerosols – data of key importance for evaluating hygroscopic and optical properties of particles, their health effects, understanding their origins and atmospheric evolution. Over the last few years, developments and applications of MS techniques in aerosol research have expanded remarkably as evident by skyrocketing publication statistics. The goal of this review is to present the most recent developments in the field of aerosol mass spectrometry for the time period of late 2010 to early 2012, which have not been conveyed in previous reviews.
Introduction
Chemistry and microphysics of aerosols (airborne suspensions of fine particles) have been the most active areas in modern environmental science because of their critical role in air-quality issues,[1,2] gas–particle atmospheric reactions,[3–6] ecosystem–atmosphere interactions,[7,8] climate change[9,10] and human health.[11,12] Aerosols have both natural and anthropogenic sources and are either directly emitted into the atmosphere (primary particles) or formed from gas-phase species following nucleation, heterogeneous gas–particle reactions or condensation processes (secondary particles). Airborne particles continuously evolve in the atmosphere as a result of physical and chemical transformations that are cumulatively referred to as ‘atmospheric aging.’ In turn, these processes change the chemical composition of both particles and gas-phase atmospheric constituents and have a complex feedback to the Earth’s land and aqueous ecosystems through biogeochemical cycling of carbon, nitrogen, sulfur and other elements.[7,8] Aerosols influence radiative forcing of climate by scattering and absorbing incoming solar radiation (direct effect). They also affect climate indirectly by acting as atmospheric ice and cloud condensation nuclei (IN and CCN). Clouds reflect radiation back to space and, therefore, cool the planet. The overall extent, and even sign, of radiative forcing by aerosols is a major uncertainty for predictive understanding of climate change.[13] Submicrometre airborne particles may have substantial toxicity and adverse effects on human health upon inhalation. For example, epidemiological studies have shown consistent correlation of cardiovascular diseases with exposure to airborne particulates.[14]
The air pollution issues of rapidly expanding megacities are probably the most eloquent examples of aerosols’ environmental impact.[15,16] Fig. 1 shows photographs of some typical ‘haze’-reduced visibility events observed by the authors in Shanghai and Mexico City (both metropolitan areas exceeding 20 million in population). The economic growth and industrial developments in these areas over the last few decades has led to increased consumption of energy, as well as expansion and intensification of the transportation infrastructure. In turn, these developments have resulted in anthropogenic emissions of unprecedented severity and extent, including emissions of primary and formation of secondary aerosols responsible for the grave pollution events illustrated in the photographs (Fig. 1).

|
Ambient aerosols are very complex mixtures of particles with diverse variations in their size, and with chemical composition that commonly is multi-component within individual particles (internal mixing) or distinguished between separate particles (external mixing). Characteristic sizes of ambient particles span a range from a few nanometres to tens of micrometres and commonly are described in terms of three characteristic modes: (1) nucleation mode (5–100 nm), corresponding to new particle formation; (2) accumulation mode (100 nm–2 μm), produced by subsequent particle growth and coagulation and (3) coarse mode (>2 μm), particles produced by direct emissions, such as windblown dust, sea salt spray, plant pollens, etc. A large fraction of both anthropogenic and biogenic aerosols contains organic material with composition that is only partly understood.[17] Organic aerosols (OAs) can be classified into primary organic aerosols (POAs), which are emitted directly by the sources, and secondary organic aerosols (SOAs), which are formed in the atmosphere through gas-phase and aqueous chemistry. However, the distinction between POAs and SOAs is blurred by the exchange of materials between particles through various physical and chemical processes. Urban aerosols are of particular complexity and concern due to the range of emission sources, high concentrations of pollutants, numerous photochemical aging mechanisms, and global impact on large groups of human populations and substantial geographic areas. Fig. 2 shows microscopy images of typical ambient particles sampled in recent field studies conducted in Mexico City[18] (a and b) and in central California[19] (c and d). Fig. 2a shows an external mixture of soot and elongated inorganic Pb- and Zn-containing particles apportioned to industrial waste incineration.[20] Fig. 2b and c show internally mixed particles containing inorganic cores (mostly black carbon and sulfates of anthropogenic origin) and outer shells of photochemically formed SOA material.[19,21] Fig. 2d shows similar internally mixed morphologies of aged marine particles, where inorganic sea salt crystals (cores) are coated with organics (outer shell areas) of anthropogenic origin.[22] Comprehensive analysis of such a diverse mixture of aerosols requires multi-dimensional measurements and applications of complementary analytical methods that provide experimental data, ranging from bulk measurements such as mass loadings of simplified aerosol classes (e.g. black carbon, sulfates, nitrates, organics, etc.) to in-depth properties of individual particles and advanced molecular-level characterisation of complex molecules comprising organic particulate matter.

|
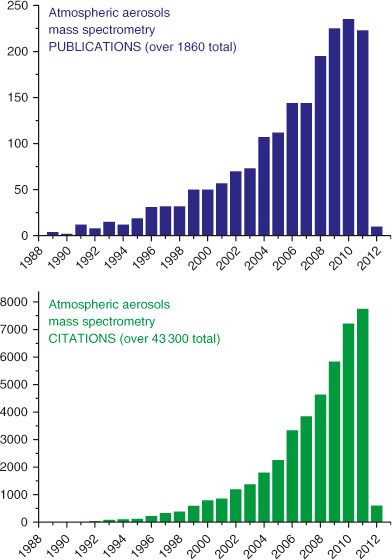
Mass spectrometry (MS) techniques have an overall versatility of sensitive detection of a range of chemical species, providing high-throughput qualitative and quantitative data on physicochemical properties of aerosols in laboratory, test facilities, and field studies. Owing to a broad range of MS analytical capabilities, complementary variations in sample introduction approaches, ionisation techniques and mass detectors, a field of ‘Mass Spectrometry of Atmospheric Aerosols’ has been established and has quickly become the most essential and fastest growing area of aerosol research. Fig. 3 shows publication and citation statistics indicating dynamics of the MS applications for aerosol characterisation over the last 25 years. Remarkably, nearly half of the records are the most recent studies published within the last 3 years. Among them, there are numerous review manuscripts and book chapters featuring instrument development, analytical methodologies and application advances in the field of MS of atmospheric aerosols, including both offline and online approaches. Offline MS techniques typically are applied to bulk (e.g. filter) samples of aerosols, and their unique advances are in the area of molecular characterisation of organic aerosols.[23–28] Online techniques employ rapid sampling of particles followed by evaporation and ionisation of the resulting vapour.[29–36] Critical advantages of the online techniques are simultaneous measurements of particle composition, as well as size, high temporal resolution, and ability to distinguish between internally and externally mixed particle components. A comprehensive account of the developments and applications in the entire field recently was presented in a two-part review of Pratt and Prather[37,38] written in August 2010. However, since the publication of that review, there have already been more than 250 new manuscripts published in the peer-reviewed literature (Fig. 3).
|
This review intends to convey the most recent developments and applications in the field of mass spectrometry of aerosols that took place in a time period between late 2010 and the preparation of this manuscript (January 2012). We note that additional papers have appeared in press since January 2012, which are not included in this review. The manuscript is organised into five sections. Following the introduction, we present the most significant developments in novel MS instrumentation and relevant analytical methodologies. Specific applications and new findings that resulted from field and laboratory studies of aerosols using MS techniques are presented in the next two sections. Summary and future outlooks are provided in the final section.
Advances in instrumentation and analytical methods
Online MS methods
Several groups reported the development of compact single particle mass spectrometers (SPMS) and improved sizing and morphology characteristics inherent to SPMS performance. For example, Brands et al.[39] and Li et al.[40] independently reported the design and performance of lightweight, field-deployable SPMS systems that use bipolar time-of-flight (TOF) mass analysers for simultaneous detection of positive and negative ions produced using laser ablation or laser desorption ionisation. Steele et al.[41] described the performance of an SPMS system specially designed for rapid aerosol threat detection. Zelenyuk et al. reported improved approaches for high-throughput measurements of particle number concentrations, size distributions, asphericity and particle density.[42,43] The same group also demonstrated that the combination of an aerosol particle mass analyser with a differential mobility analyser enables separation of aspherical particles of the same size and mass.[44]
Soft ionisation aerosol MS techniques that enable the formation of intact ions in SPMS systems also have gained significant attention. Current advances in aerosol MS instrumentation involving tunable vacuum ultraviolet (VUV) light from a synchrotron source, resonant capture of low-energy electrons and chemical ionisation have recently been reviewed.[35] More recently, Zimmermann et al.[45] described the first field application of resonance-enhanced multiphoton ionisation (REMPI) using a UV laser combined with thermal desorption of the particulate matter. REMPI enables facile and selective multiphoton ionisation of aromatic compounds, resulting in sensitive detection of particle-bound polycyclic aromatic hydrocarbons (PAHs). In that study, PAH signals were used as unique signatures of different combustion sources. Adam et al.[46] used this instrument in combination with other techniques for detailed characterisation of heavy-duty vehicle exhaust emissions. In another study, REMPI–TOFMS was coupled to a carbon analyser for the analysis of thermally desorbed organic compounds from collected aerosols in parallel with bulk characterisation of the organic and elemental carbon.[47] Single-photon ionisation using synchrotron radiation enables ionisation of a range of compounds in laboratory-generated aerosol samples with minimal fragmentation. Fang et al. used VUV photoionisation in a single-particle aerosol time-of-flight mass spectrometer (ATOFMS) for characterisation of the composition of SOAs produced through photooxidation of toluene in a smog chamber.[48,49] Robinson et al.[50] reported coupling of a softer ionisation source with an Aerodyne aerosol mass spectrometer (AMS). In that study, a metastable atom bombardment (MAB) ionisation source was incorporated so the system could be readily switched between MAB and traditionally used electron ionisation (EI). In the MAB source, metastable atoms produced using an electrical discharge in N2, Ar or Kr are directed at the ionisation volume. Penning ionisation produces significantly less fragmentation than EI with considerably lower ionisation efficiency (<0.1 % compared with EI). The authors indicated further optimisation of the MAB source may result in significantly enhanced ion signals.
Development of new techniques related to the AMS-TOF family of instruments focussed on faster data acquisition, improved quantitation[51–54] and data visualisation. For example, Meddlebrook et al.[51] developed an algorithm for estimating the instrument collection efficiency for field data based on measured aerosol chemical composition and sampling line relative humidity (RH). Ng et al.[55] reported the development of a simplified version of the AMS – the aerosol chemical speciation monitor (ACSM) – capable of routine characterisation of mass and chemical composition of submicrometre particles in real time. Kimmel et al.[56] developed a new data acquisition system for AMS that enables rapid acquisition of mass spectra with an acquisition rate of 1 kHz. The new data system supports real-time AMS acquisition with millisecond resolution. During the BEARPEX-2007 campaign, Farmer et al.[57] used a combination of the standard and a new data acquisition mode to enable eddy covariance flux measurements. Isobaric overlaps present a significant challenge for the interpretation of the AMS-TOF data. Müller et al.[58] developed a method for improved spectroscopic analysis of the AMS data that enables separation of isobaric peaks at m/z 43 (e.g. C2H3O+ at m/z 43.015 and C2H5N+ at m/z 43.043) – 50-ppm mass accuracy was achieved, which enabled efficient identification of the relative abundance of the isobaric peaks.
Characterisation of the chemical composition of particles smaller than 50 nm is hindered by difficulties in transmission of ultra-small particles through aerodynamic lenses and the limitations of the optical detection methods. To alleviate these problems, Zauscher et al.[59] coupled a growth tube to an ultrafine ATOFMS. Small particles (40–60 nm) grow to larger sizes in the growth tube in the presence of water vapour, which enables their detection using the standard optical detection scheme. A comparison between the particle types detected for 175-nm particles characterised using ATOFMS with and without the growth tube indicated that particle growth in the growth tube did not have a measurable effect on the appeared spectra. This result was attributed to the short residence time of the aerosol material in the droplets. It has been demonstrated that this system enables characterisation of the chemical composition of individual particles down to 38 nm in diameter. In the future, it will be used to probe changes in the chemical composition of CCN as a function of meteorological conditions.
The elemental composition of nanoparticles (<50 nm) can be characterised using a nano aerosol mass spectrometer (NAMS), developed by Johnston et al.[60] In this system, singly charged particles are stored in an ion trap and analysed using high-power laser ablation that results in a complete disintegration of a particle. Singly and multiply charged atomic ions produced by laser ablation are subsequently analysed using a TOF mass analyser. Accurate elemental composition is obtained from the TOF-MS data using a deconvolution procedure described by Zordan et al.[61] Assuming the charge state distribution of a particular element is independent of the particle composition, the ratio between different charge states is calibrated using particles of known composition (e.g. sucrose and ammonium sulfate). Isobaric peaks (e.g. O4+ and C3+; O2+ and S4+) are subsequently separated based on the expected ratios. It has been demonstrated that this approach enables accurate measurement of elemental ratios of C, N, O and S atoms in aerosol particles of 10–25 nm. Furthermore, carbon mole fraction plots constructed by plotting the elemental composition of individual particles provide a means to explore the effect of the presence of inorganic constituents on the degree of oxidation of organic molecules in field-collected aerosol particles. For example, Klems et al.[62] showed correlation between the degree of oxidation of organic compounds and the presence of inorganic species in ambient particles.
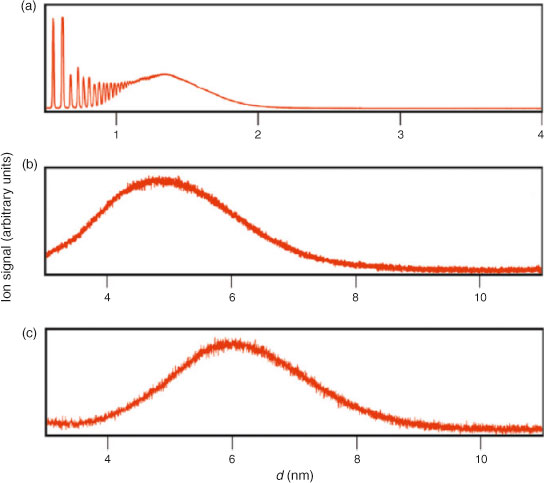
Yoder et al.[63] developed a new experimental approach for accurate determination of the size distributions of ultrafine particles with sizes ranging from subnanometres to several nanometres. The approach uses sodium doping of aerosol particles followed by UV photoionisation MS. In that study, clusters formed in a supersonic beam were transferred into a reaction chamber filled with Na vapour. The resulting clusters were later ionised using either a 223-nm (5.56 eV) light from a tunable UV laser or a fixed 266-nm (4.66 eV) light from an Nd:YAG laser. Mass analysis of the ionised clusters was performed using a linear TOF mass spectrometer. Because of the low ionisation energy of sodium-containing clusters, sodium doping enables facile photoionisation of ultrafine particles using UV light. A comparison between the cluster size distribution, determined using UV photoionisation of Na-doped clusters, with the relatively soft ionisation of undoped clusters, using a tunable extreme ultraviolet (EUV) laser, demonstrated that EUV results in significant fragmentation of the clusters. The degree of fragmentation is determined by the binding energy of molecules in the clusters. For example, less fragmentation was observed for the more strongly bound water clusters as compared with clusters of acetic acid or dimethyl ether. Sodium doping is an attractive approach because of the high Na capture efficiency, resulting in efficient conversion of the precursor particles. In addition, Na-doping results in evaporation of only a few molecules from the cluster’s surface, which has a negligible effect on the overall composition of nanometre-sized particles. Fig. 4 shows size distributions obtained using this technique for 1.3-, 4.8- and 6-nm ammonia particles. Future studies will focus on improving the instrument’s sensitivity and development of an ambient aerosol sampling inlet.
|
Offline MS methods
Several research groups reported on the development of new approaches for more sensitive quantitative offline characterisation of a broader range of organic compounds in aerosol samples collected on filters using gas-chromatography mass spectrometry (GC/MS). For example, González et al.[64] developed a new chiral GC/MS method for separation of enantiomers of 2-methyltetrols (2-methylthreitol and 2-methylerythritol), products of isoprene oxidation. The results demonstrated enhanced abundance of one enantiomer in aerosol samples collected from a boreal forest, whereas laboratory generated isoprene SOA contained a racemic mixture of 2-methyltetrols. These results indicate that the 2-methyltetrols in ambient aerosol samples exhibit chirality as observed for 2-methylerythritol present in the leaves of certain plant species.
Schnelle-Kreis et al.[65] demonstrated that improved sensitivity of thermal desorption (TD) GC/MS analysis of non-polar organic molecules in field-collected aerosol samples enables chemical characterisation of such compounds with better temporal resolution. When combined with in situ derivatisation,[66] TD-GC/MS enables identification of both polar and non-polar constituents of OAs. Furthermore, Kowalewski et al.[67] developed a three-step derivatisation for identifying multifunctional oxygenated compounds in SOAs. In this method, carbonyl groups are converted into methyloximes, carboxy groups converted into methyl esters and hydroxy groups converted into trimethylsilyl ethers. The method was demonstrated for several model multifunctional compounds. In another study, Özel et al.[68] used GC/MS equipped with a nitrogen chemiluminescence detection system for characterisation of water-soluble organic nitrogen in field-collected aerosol samples. The analysis enabled quantification of nitriles, amides, alkyl nitro compounds, nitrophenols, nitrosamines and nitro-PAHs.
Although GC/MS enables quantitative characterisation of organic nitrogen (ON) compounds in particulate phase, the proton-transfer reaction mass spectrometer (PTRMS) is ideally suited for sensitive detection and quantification of gas-phase ON. Hanson et al.[69] developed an ambient pressure PTRMS method for quantification of gaseous amines. In this technique, H3O+(H2O)n clusters are generated using a radioactive source in the presence of water vapour. Ionisation of amines occurs in a drift tube, where reagent ions are mixed with the sampled air. The ions are then transferred into a vacuum system and analysed using a quadrupole mass analyser. This technique is capable of detecting amines at parts per thousand by volume levels.
Although GC/MS is a robust technique for quantitative characterisation of organic molecules in aerosol samples, it is limited to fairly small, thermally stable molecules. Liquid chromatography mass spectrometry (LC/MS), although less quantitative, enables detection and identification of a broader range of analytes in complex mixtures. Kitanovski et al.[70] reported a new approach for LC separation of carboxylic acids, which are commonly present in OA samples. In this study, hydrophilic interaction normal phase LC combined with electrospray ionisation mass spectrometry (ESI/MS) analysis was developed as a tool for separation and identification of aliphatic, alicyclic and aromatic carboxylic acids. Recoveries higher than 90 % and limits of detection (LOD) from 0.03 to 16 μg L–1 were reported. Kampf et al.[71] developed a method for the quantitative detection of two other common OA constituents, glyoxal and methylglyoxal. The approach involves filter extraction followed by chemical derivatisation, solid-phase extraction and reversed-phase high-performance liquid chromatography (HPLC) coupled with ESI/MS and tandem mass spectrometry (MS/MS) in an ion trap mass spectrometer. Multiple reaction monitoring enabled sensitive detection of both compounds with a LOD of 0.51 and 0.62 μg L–1 for glyoxal and methylglyoxal. Samy et al.[72] described an advanced LC/MS technique using high-resolution mass analysis in a quadrupole-TOF (Q-TOF) instrument for quantification of amino acids in aerosols collected in a south-eastern United States forest environment. High mass accuracy of a Q-TOF (<2 ppm) is essential for unambiguous identification of amino acids and other organonitrogen compounds in aerosol samples.
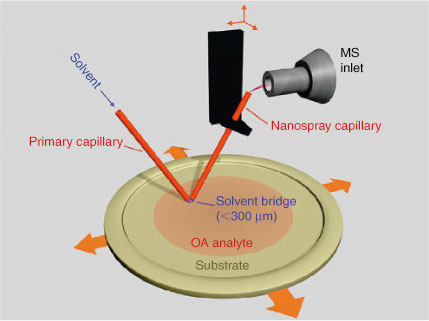
Ambient pressure surface ionisation techniques have attracted significant attention because they enable analysis of compounds in laboratory-generated and field-collected OA samples without sample pretreatment. Bruns et al.[73] demonstrated the utility of atmospheric solids analysis probe MS (ASAP-MS) for identification of organic compounds in laboratory-generated SOA and field-collected OA samples. In ASAP-MS, thermal desorption of molecules from the probe using a heated stream of N2 is followed by atmospheric pressure chemical ionisation and mass analysis of the resulting ions. OA constituents were identified based on the accurate mass measurement. Another advantage of ambient surface ionisation techniques is the ability to detect molecules that cannot be observed using ESI/MS. For example, it has been demonstrated that desorption electrospray ionisation (DESI) enables detection of chemically labile analytes in OA samples that are not observed in ESI/MS.[74] Similarly, chemically labile compounds were detected in laboratory-generated aerosol samples using recently developed nanospray desorption electrospray ionisation (nano-DESI) MS.[75,76] This technique relies on localised liquid extraction using a probe composed of two fused silica capillaries (shown in Fig. 5). The analyte molecules are desorbed into an appropriate solvent and transferred to a mass spectrometer inlet where they are ionised through nanospray ionisation. It has been demonstrated that nano-DESI enables sensitive analysis of aerosol samples collected on Teflon or aluminium substrates. Furthermore, it is ideally suited for monitoring chemical changes in aerosol samples subjected to chemical aging.[75,77,78]
|
Secondary ion mass spectrometry (SIMS) and X-ray photoelectron spectroscopy (XPS) enable sensitive characterisation of inorganic and organic materials on surfaces. Tyler et al.[79] demonstrated the utility of laser secondary neutral mass spectrometry (Laser-SNMS) for detection of PAHs in individual aerosol particles. In this technique, molecules sputtered using a primary ion beam are subsequently ionised using a 157-nm laser, resulting in a several orders of magnitude improvement in the sensitivity. High ionisation yields in single-photon post-ionisation enabled detection of PAHs on 2-μm ambient aerosol particles. This approach can be used for detailed characterisation of organic material on surfaces of individual particles. Brender et al.[80] used a combination of temperature-programmed desorption (TPD) and XPS to examine the surface chemistry of organic materials containing different functional groups. Particles of 10–20 nm containing graphitic material decorated with carbonyl, carboxyl and hydroxyl groups were used in that study. TPD-MS was used for quantitative analysis of the released volatile compounds as a function of temperature, whereas XPS provided complementary characterisation of the remaining functional groups on the surface. Although this methodology has not been applied to aerosols, it can be readily extended to studies of chemistry on surfaces of ambient particles collected on substrates.
Field and test facilities studies
Anthropogenic aerosols
Chemical characterisation of anthropogenic aerosols present in urban and industrial areas is one of the most challenging analytical tasks[81] because of a variety of emission sources, complex internal and external mixing of particles that also may change rapidly at any sampling location, and moment of time. Online mass spectrometers provide an important capability to report on aerosol composition, size and their variations with high time resolution. In recent years, these instruments have been increasingly used in all major field studies.
The AMS instruments, with different modifications developed by Aerodyne Research Inc,[29] are likely the most widely used commercial online instruments. The AMS sizes particle ensembles sampled over short periods of time (seconds to minutes) and simultaneously records cumulative spectra of non-refractory components detected in each ensemble. The AMS spectra comprise a complex mixture of ions, corresponding to hundreds of individual molecules and their numerous fragments, which complicate spectra interpretation. Methods of multivariate factor analysis have been developed and applied to deconvolute AMS spectra into several orthogonal component spectra (factors) that indicate different aerosol sources, and can be used to represent their averaged chemical properties, relative contributions of individual factors to the total aerosol, its source apportionment and atmospheric history.[82] In recent years, positive matrix factorisation (PMF) analysis has become a common tool for AMS data analysis and provided valuable information about sources of OAs and their atmospheric evolution at many locations affected by anthropogenic emissions.[83] Specifically, a hydrocarbon-like OA (HOA) factor was attributed to primary aerosols emissions associated with urban traffic,[84–90] residential heating using solid fuel and wood,[86–88] fossil fuel combustion and biomass burning[84] and food cooking and charbroiling.[87] The HOA factor was minor in AMS datasets collected in remote and rural areas.[91,92] Photochemically formed SOAs are associated with oxygenated OA (OOA) factors distinguished by consistent variations in AMS spectra. Semi-volatile and low-volatility OOA (SV-OOA and LV-SOA) factors were commonly reported and apportioned to fresh and atmospherically aged anthropogenic SOAs respectively.[82–92] Narrowly focussed, source-specific factors, such as cooking OAs,[87] biomass burning OAs (BBOAs),[84,88] solid fuel OAs[86] and amine-containing OAs,[91] were also reported in some studies. Furthermore, Sun et al.[93] recently conducted analysis of water-soluble organic carbon (WSOC), where the AMS measurements and the PMF data analysis were applied to interrogate aerosolised mist of aqueous extracts from filter samples.
In addition, AMS instruments were used in several experimental projects conducted at test facilities,[94–98] where mixtures of particles and gases from exhaust plumes of commercial engines were sampled into a smog chamber and exposed to a simulated photochemical oxidation environment. Continuous, real-time AMS measurements then were used to monitor the concentration of OAs in the smog chamber and evolution of its chemical properties. Consistently, all of these studies showed an overall increase in the OA mass and its oxidation extent (O/C ratio) after aging. These results underscore the dynamic evolution of anthropogenic OAs that has not been accounted for in modelling efforts.
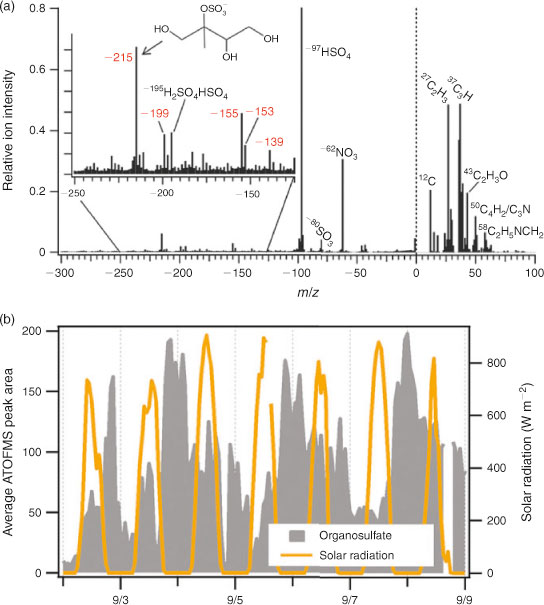
In field studies, SPMS instruments provide a valuable opportunity to probe the chemical composition of individual particles with high-throughput data acquisition, and then correlate properties of specific particles with other measurements and meteorology data, providing a unique approach to study chemical changes in individual ambient particles as they evolve in the atmosphere. Fig. 6 highlights recent results reported by Hatch et al. on the detection[99] and temporal variability[100] of organosulfate species in individual particles from an urban area in Atlanta. Although the presence and significance of organosulfates has been previously reported in several works using bulk analysis of aerosol samples, the work of Hatch et al.[99,100] was the first study where single-particle measurements were used to investigate specific formation mechanisms occurring at ambient conditions. In particular, a group of peaks with the most prominent peak at m/z = –215 was ascribed to organosulfates derived from isoprene epoxydiol, found to be ubiquitous in ambient aerosol during two separate field studies in the Atlanta area. Real-time ATOFMS measurements revealed that their highest concentrations were reproducibly observed at night when the RH was measured at 80–90 %. These observations suggested that efficient heterogeneous chemistry of organosulfate precursors and their subsequent aqueous-phase chemistry play an important role in enhancing SOA formation in the areas with mixed biogenic and anthropogenic emissions. Similarly, the significance of gas-particle uptake and aqueous-phase chemistry in deliquesced particles and fog droplets was reported in a separate SPMS study that focussed on the ambient processing of trimethylamine conducted at urban and rural areas of Southern Ontario, Canada.[101]
|
Particle type classification, source apportionment and temporal trends of individual classes were elucidated based on statistical sorting of combined SPMS and meteorological datasets collected in two unrelated field studies conducted in Shanghai[102] and Southern Ontario.[103] In Shanghai, ambient measurements by ATOFMS indicated a persistent contribution of biomass-burning particles from multiple regional sources, and several particle classes were apportioned to local emission sources using a neural network algorithm.[102] PMF analysis of the ATOFMS dataset from the Southern Ontario study identified several factors associated with regional and long-range transport of biomass-burning aerosols, aged carbonaceous particles, and pyrotechnical particles from fireworks, as well as several factors ascribed to local emission sources.[103] Other recent studies demonstrated complementary use of datasets from co-deployed SPMS and ion chromatography techniques[104] and SPMS and AMS instruments[105] that allowed a quantitative analysis of the neutralisation process of ambient sulfate aerosol.
Offline MS techniques with either GC or LC separation stages were used to characterise and quantify selected organic constituents in bulk samples of anthropogenic aerosols and provided important insights on molecular speciation of particulate matter characteristic for specific areas of field studies.[106–110] Yu et al.[108] examined non-polar organic compounds in ambient aerosol collected in Hong Kong using TD-GC/MS. For the area of study, hopanes and alkanes were apportioned to local emissions from gasoline vehicles, whereas PAHs were ascribed to regional emissions from rice straw burning and coal combustion. The analysis of PAH was also a focus of the field study in Spain, conducted in both urban and rural areas, where the PAH content of aerosol was attributed to vehicle emissions and crude oil combustion.[107]
Accelerator MS is commonly used in environmental studies to distinguish between fossil and non-fossil carbon based on quantitative measurements of 14C radiocarbon.[111] Because of a radioactive decay on an ~5700-year time scale, fossil carbon is devoid of 14C. Therefore, the isotopic ratio of 14C/12C is used to apportion carbonaceous aerosols to either anthropogenic emissions from fossil fuel combustion or modern carbon sources that can be relevant to both natural (e.g. biogenic SOAs) and man-made (e.g. biomass combustion, cooking) emissions. Recent field studies used radiocarbon measurements to ascribe emission sources contributing to black and organic carbon,[112–114] SOA[115] and WSOC[116] at specific geographic locations in Europe, the United Kingdom and North America.
Combined datasets from online and offline MS analysis of aerosols, cross-correlated with meteorology records and emission inventories, were also used to apportion origins and composition of regional plumes of anthropogenic aerosols transported over long ranges.[117–120]
Biomass burning organic aerosols (BBOAs)
Physico-chemical properties of BBOAs and its atmospheric transformations were investigated in recent field[116,121–123] and test facility[124–128] studies. Hennigan et al.[127] reported results on the physicochemical transformation of BBOA particles from photooxidation experiments conducted in a smog chamber. In their work, smoke emissions (both particles and gases) from separate controlled burns of 12 different types of wood were introduced into the smog chamber, and the aging transformations were monitored by AMS and PTRMS instruments for particles and gases respectively. The results showed, under atmospherically relevant conditions, that BBOA particles undergo extensive chemical processing that results in a substantial increase of OA mass in aged smoke plumes. The increase in the aerosol mass averaged over all experiments was a factor of 1.7 with variations between individual experiments in a range of 1.1–2.8. Heringa et al.[129] independently reported similar results, where an increase in the OA mass by a factor as high as 4 was also observed. In both studies, changes in chemical composition were inferred from the AMS datasets that showed increasing oxygenation and lower volatility of the aged particles. An increase in the OA mass in regional biomass burning plumes was also supported by AMS field measurements taken aboard the NASA DC-8 research aircraft following plume advection.[122] These highly dynamic transformations of BBOAs challenge traditional assumptions of chemical transport models and emission inventories, where BBOA is usually assumed to be non-reactive.
Bateman et al.[130] demonstrated the utility of a particle-into-liquid-sampler (PILS) for high-resolution mass spectrometry (HR-MS) analysis of water-soluble organics in chamber-generated and BBOAs. Chang-Graham et al.[124] used the PILS technique to sample the WSOC fraction of BBOAs followed by ESI/HR-MS that enabled accurate mass measurements and assignments of elemental formulae in a mass range of 70–1000 Da. In that work, BBOA samples from test facility burns of biomass fuels collected at the USA sites managed by prescribed fires were analysed. A substantial presence of N-, S- and P-containing organics, as a well as organometallic species containing alkaline earth metals (Ca, Ba and Mg) and transition metals (Mn, Fe, Ni, Cu and Zn) were detected in the WSOC extracts. The results suggest the potential importance of BBOAs in the dispersion and persistence of airborne metals – a potential environmental issue that requires future focussed studies.
The emission factors and occurrence of selected compounds in BBOA emissions typical for residential wood burning, agricultural field burning and wildfires in Portugal were studied using GC/MS and additional bulk sample techniques in a set of interrelated studies.[121,125,126] Several unique chemical markers characteristic for the BBOA samples were identified and apportioned to specific sources of biomass fuel. A separate study by Iinuma et al.[131] reported on the detection of methyl-nitrocatechols using LC/(–)ESI-MS analysis. The authors suggested these molecules may serve as potential tracers of SOA-related wood combustion. These tracers are formed by oxidation of m-cresol emitted during wood combustion. In ambient samples collected at a rural site in Germany affected by wood combustion, these tracers were found in particulate matter less than 10 μm in size (PM10) in significant concentrations (on average, 29 ng m–3), and they were well correlated with levoglucosan, a primary BBOA tracer.
Biogenic organic aerosols
Chemical speciation, physical properties, sources and fluxes of biogenic organic aerosols were reported in several recent field studies conducted in rural,[132] forest[133–138] and marine[133–142] environments. Rural site measurements by co-deployed AMS and PTRMS instruments were employed to evaluate the role of different volatile organic compounds (VOCs) for SOA formation in Southern Ontario, Canada. Systematic comparison of the diurnal profiles of SOA mass concentrations and mixing ratios of individual VOCs led to the conclusion that biogenic precursors dominate SOA production in the area of study.[132] In another study at the same geographical region, AMS measurements were taken at a tower above a mixed forest as a part of a field experiment to quantify aerosol fluxes with limited chemical speciation. The results indicated interesting diurnal dynamics between the air masses above and below the canopy, whereby SOA formed within the canopy is accumulated during the night-time then is driven upward during the day through mixing with air above the canopy.[133] Leaitch et al.[135] compiled data of long-term, multi-seasonal measurements from AMS, ACSM and other aerosol instruments deployed at forest sites in Canada with the goal to assess the temperature dependence of SOA production in forests. The results indicated an exponential dependence of SOA mass concentrations with temperature increases in a range of 7 to 34 °C. Two other studies employed AMS measurements in tropical rainforests in Amazonia[137] and Borneo,[136] where PMF analysis of the obtained datasets produced site-specific SOA factors identified by trace compounds unique to the areas of study.
HPLC/ESI/MS measurements were employed for chemical characterisation of SOA samples collected at European forest sites.[134,138,143,144] Yasmeen et al.[138] reported an extended dataset on molecular and structural characterisation for a range of isobaric terpene-derived acids, distinguished based on MS/MS fragmentation patterns. Fig. 7 illustrates representative, structure-specific MS/MS spectra of two isobaric cis-pinic and cis-caric acids ([M – H]–, m/z = 185 Da) – distinct tracers of α-pinene SOA and Δ3-carene SOA. The study provided the reference data necessary for molecular-level speciation of biogenic SOA that also will be useful for more detailed descriptions of SOA sources and aging. Kristensen and Glasius[134] performed molecular speciation and quantitative analysis of a range of individual molecules in forest SOA samples, particularly those including organosulfates and nitrooxy organosulfates. Gómez-González et al.[143] conducted a comprehensive analysis of SOA field samples collected at a Belgian forest site influenced by urban emissions and measured the particulate matter less than 2.5 μm in size (PM2.5) concentrations of a range of biogenic tracers relevant to the oxidation of isoprene, α-pinene, β-pinene, Δ3-carene and D-limonene, including organosulfates and nitrooxy organosulfates. They also examined temperature-dependent trends in their concentrations. The study revealed the highest concentrations for 3-methyl-1,2,3-butanetricarboxylic acid and the lowest ones for cis-pinonic acid at high ambient temperatures >22 °C, indicative of an aged biogenic aerosol.
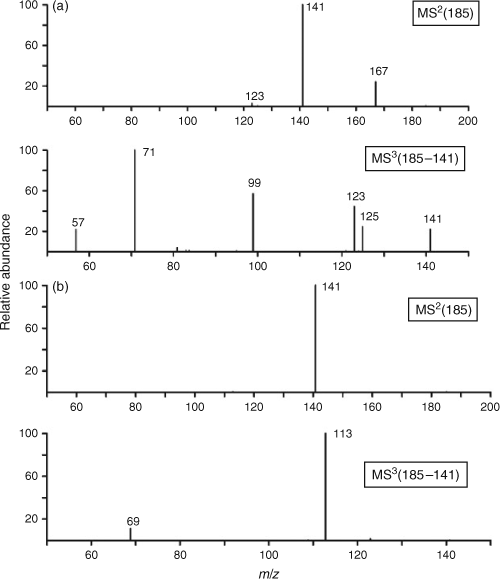
|
AMS[142] and ATOFMS[141] field measurements of marine OAs revealed unique fingerprints of submicrometre primary organic particles generated by sea spray and bubble-bursting in the areas of phytoplankton bloom. Mass concentrations of these particles suggested that the ocean might be a substantial source of OA.[142] Conversely, extensive filter sampling and GC/MS analysis of tracer compounds in marine aerosol during an around-the-world cruise study suggested that long-range transport of continental aerosol is the most important factor determining chemical composition of OAs in marine areas.[140] Given the large areas covered by the oceans and variability in OA sources, the overall effect of marine OA emissions remains uncertain and more field and laboratory studies are needed to address this issue.[139]
New particle formation (NPF)
Understanding the physico-chemical processes of NPF is one of the most challenging topics in the physical and analytical chemistry of aerosols. NPF may be viewed as a two-step process that includes nucleation of neutral or ion clusters within the size of a few nanometres or less (<~3 nm) and growth of nucleated clusters into stable particles of ultrafine sizes (<100 nm). The difficulties in investigating NPF processes are inherent to the small amount of available material in the particles. Furthermore, approaches for sample collection, introduction, and ionisation are quite different for clusters and ultrafine particles. Therefore, two separate MS methodologies are usually employed to study NPF events: (1) MS methods for cluster detection and analysis that can be regarded as gas-phase MS approaches pushed to the limits of larger molecules and clusters and (2) MS methods of aerosol detection and analysis pushed to the limits of small, ultrafine particles.
Chemical composition and dynamics of atmospheric clusters relevant to NPF events have been discussed in several recent field studies conducted at forest,[145] boreal[146] and other rural[147] sites. Kanawabe et al.[145] performed a month-long field study at a remote site in a Michigan forest and concluded that NPF can be efficiently suppressed by elevated emissions of isoprene. Kiendler-Scharr et al.[148] also reported the same effect in an earlier laboratory study. However, the mechanistic understanding of this suppression effect remains unclear. A recently developed atmospheric pressure interface-TOF (APi-TOF) MS instrument, capable of detecting ambient ions,[149] was deployed for a field study at a boreal forest site in Finland.[146] Elevated concentrations of ambient sulfuric acid clusters and number concentrations of neutral nanoparticles (1.3–5 nm) were well correlated, indicating an ion-induced nucleation mechanism. Zhao et al.[147] reported field measurements using a novel chemical ionisation mass spectrometer (CIMS) developed for sensitive detection of ambient neutral clusters during NPF events. The results indicated that clustering of sulfuric acid and amines was a dominant nucleation process for the area of study. The broader relevance of amines to NPF events at other geographic locations also was suggested by independent measurements of ultrafine particles grown to sizes of 25–100 nm. In particular, the presence of amines detected by ATOFMS measurements was reported by Creamen et al.[150] for the field study in the Sierra Nevada Mountains, California, and Laitinen et al.[151] reported detection of amines using modified AMS with laser-assisted desorption and ionisation of bulk particle samples. Significant amounts of ammonium nitrate and organic material, including amines, also were detected with the NAMS instrument in freshly nucleated particles (<20 nm).[152]
Climate-related properties or aerosols
MS and complementary measurements correlating aerosol composition and its climate-related properties, such as hygroscopicity, CCN and IN activity, absorption and scattering of light, were commonly reported in recent field studies. CCN activity is quantified as the fraction of particles of a given size that activate at a particular supersaturation. Often, it is parameterised using semi-empirical hygroscopicity parameter κ introduced by Petters et al.[153] (smaller κ implies less hygroscopic material that needs higher supersaturation for CCN activation). Measurements from collocated AMS and CCN counters at different geographic locations[151,154–161] were used to provide field data for simplified parameterisation of aerosol effective κ as a function of AMS-reported organic and inorganic mass fractions (forg, finorg) that would be practical for climate modelling efforts. Overall, κ values were reasonably estimated from AMS data using a mixing rule of κ = κorg·forg + κinorg·finorg, where κorg = ~0.1 and κinorg = ~0.7 for most of the studies. In some instances, improved parameterisation was also achieved by incorporating additional subfractions of organics in to the mixing rule, such as highly hygroscopic WSOC[154] and largely insoluble LV-SOA.[160] The possible positive correlation of κorg with O/C ratio measured by AMS was also suggested.[162]
Slowik et al.[163] and Zelenyuk et al.[164] respectively used AMS and SPMS instruments with the inlets connected to counterflow virtual impactors that segregate dry residuals of cloud and fog droplets from inactivated background aerosol. In their studies, cloud droplet residuals were probed directly by online MS techniques, yielding explicit data on the chemical composition of CCN active particles present in ambient air.
Closure studies on optical properties and the inferred mixing state of black carbon were reported in independent studies based on combined datasets from an AMS and a single-particle soot photometer[165]; AMS and a laser-induced incandescence instrument[166] and AMS and a set of instruments containing a nephelometer, an aethalometer and a passive cavity aerosol spectrometer.[167]
Laboratory studies
Clusters and NPF
MS is the method of choice for laboratory investigations of the initial steps in NPF. In the case of ion-induced nucleation, the charged clusters can be detected directly. Neutral precursors to the nucleation and nucleating clusters can be ionised by appropriate CIMS methods. For example, recent investigation of binary nucleation in H2SO4–H2O[168] and ternary nucleation in H2SO4–H2O–NH3[169] mixtures took advantage of CIMS to quantify the concentration of H2SO4 and NH3. One of the most comprehensive investigations of neutral and ion-induced particle nucleation rates in H2SO4–H2O–NH3 (or urea) ternary mixtures was published recently by the CLOUD (cosmics leaving outdoor droplets) team at CERN.[170] They used an APi-TOF instrument to observe negatively charged clusters of the type [(NH3)m(H2SO4)n(HSO4)]– with n and m up to 16. They concluded that the rate of binary H2SO4–H2O nucleation, a subject of long-standing controversy, is too slow for this process to occur in the boundary layer. Although NH3 enhances the nucleation rate, the enhancement is not sufficient to explain the high nucleation rates observed in field experiments.[170] Amines have long been the primary suspects behind this discrepancy. Bzdek et al.[171] took advantage of the high resolving power of Fourier transform ion cyclotron resonance MS (FTICR-MS) to investigate addition and displacement reactions of amines with negatively charged sulfuric acid clusters. Dimethylamine was shown to displace ammonia from [(NH4HSO4)x(H2SO4)3(HSO4)]– at collision limited rates, but its addition to small [(HSO4)(H2SO4)x]– clusters was slow. The same group also showed that dimethylamine efficiently displaces ammonia from the salts of methanesulfonic acid, believed to be important in nucleation of marine aerosols.[172] The important role of amines in nucleation was confirmed in a study by Erupe et al.[173] who found that trimethylamine enhances nucleation rates much more efficiently than ammonia, especially under dry conditions. Efficient displacement of ammonium by ammonium ions was also observed in amine and bulk ammonium sulfate and bisulfate reactions.[174]
Following the initial nucleation, particles grow by capturing low-volatility compounds from the air. Recent advances in MS have made it possible to analyse the chemical composition of growing particles in the 1–20-nm size range. Using nanoparticle tandem mobility methods, Wang et al.[175] showed that homogeneously nucleated H2SO4 nanoparticles efficiently take up other organic compounds in addition to amines, such as methylglyoxal, heptanol, decanol and even volatile molecules such as ethanol. We expect several exciting new developments in this area in the near future.
Phase state of organic particles and gas–particlepartitioning
Advanced MS methods are increasingly applied to measurement of vapour pressures of low-volatility organics that can partition into growing particles. For example, Booth et al.[176] used Knudsen effusion MS to quantify vapour pressures of cyclic dicarboxylic acids, as well as cis-pinonic acid and levoglucosan. Isaacman et al.[177] described a novel method for investigating the volatility distribution of SOA organics. They used two-dimensional thermal desorption aerosol gas chromatography (2D-TAG) coupled to an AMS to investigate the temporal evolution in the volatility of nearly 200 oxidation products produced during oxidation of the sesquiterpene, longifolene. In a related study, Salo et al.[178] examined the volatility of α-pinene and O3 SOA and D-limonene and O3 SOA, which were subsequently aged with OH, using a tandem particle mobility approach. They observed a decrease in particle volatility during OH aging and attributed most of this decrease to gas-phase OH oxidation of organics in equilibrium with particles and small contributions from the particle-phase chemistry. Cappa and Wilson used synchrotron VUV photoionisation aerosol MS to compare the thermal evaporation of aerosols composed of lubricating oil and products of the α-pinene and O3 reaction.[179] They found that lubricating oil particles evaporated as predicted by gas–particle partitioning theory, whereas α-pinene SOA did not, suggesting they were in a glassy state. Using SPMS, Vaden et al.[180,181] also observed exceptionally slow evaporation of α-pinene SOA (~24 h instead of the expected minutes at room temperature) and ambient OA particles. These observations support the emerging picture of a highly viscous glassy organic phase in certain types of OA.[182]
Molecular-level characterisation of SOA
Several new papers deconstructing chamber-generated SOA at a molecular level with help from various MS-based methods have appeared within the time period covered by this review. Because of its large contributions to the global OA budget, SOA prepared by oxidation of isoprene has received considerable attention. Nguyen et al.[183] examined the composition of isoprene and O3 SOA using HR-MS methods and found several highly oxidised oligomeric compounds containing peroxy and carbonyl groups. Fig. 8 reproduces the mass spectrum obtained in that work and also illustrates the utility of HR-MS in the assignment of individual aerosol compounds. A study of high-NOx SOA from isoprene by the same group[184] revealed similarly extensive oligomerisation, as well as the presence of –ONO2 groups in one-third of the products. Nguyen et al.[78] and Zhang et al.[185] independently investigated the effect of RH on the chemical composition of high-NOx isoprene SOA. Dry conditions significantly enhanced the fraction of oligomeric compounds in SOA formed by condensation reactions.[78] For example, the amount of 2-methylglyceric acid and the average chain length of its oligomers were reduced under humid conditions.[78,185] Organonitrates were suppressed by high RH,[78] whereas organosulfates were enhanced.[185] Galloway et al.[186] quantified yields of several low-yield (<5 %) first-generation products of isoprene photooxidation (glyoxal, methylglyoxal, glycolaldehyde, hydroxyacetone, etc.) using a combination of spectroscopic, CIMS, and chromatographic methods, and investigated the effect of inclusion of these products in the master chemical mechanism (MCM). A reexamination of the MCM and other kinetic mechanisms of isoprene–NOx photooxidation[187] resulted in improved predictions of chamber ozone and identified the low-NOx oxidation regime as an area where further experiments are urgently needed.
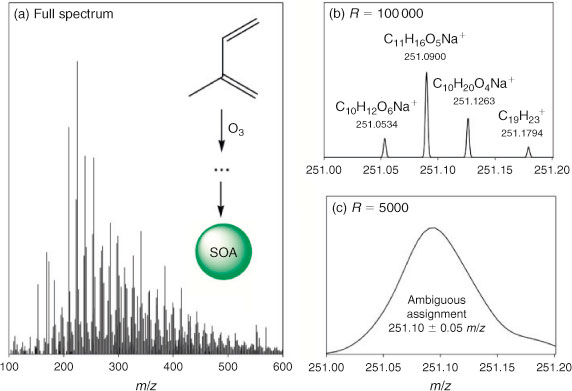
|
Monoterpenes and sesquiterpenes have lower emissions compared with isoprene, but they contribute significantly to global SOAs because their oxidation produces low-volatility products in higher yields. Hall and Johnston[188] were able to estimate that oligomers account for more than 50 % by mass in α-pinene SOA using ESI/FTICR-MS and the method of standard addition. By experimenting with OH scavengers in chamber ozonolysis of α-pinene, Putman et al.[189] concluded that OH does not significantly alter the distribution of particle-phase products as observed by ESI/FTICR-MS. Chan et al.[190] took advantage of ultra performance liquid chromatography (UPLC) coupled to ESI/TOF-MS to investigate the effect of aerosol acidity on the chemical composition of SOAs from photooxidation of the sesquiterpene, β-caryophyllene. They were able to identify several products, including multifunctional carbonyls and carboxylic acids, as well as products of particle-phase acid-catalysed reactions, such as organosulfates. Li et al.[191] also used UPLC/ESI/TOF-MS to investigate SOA from ozonolysis of β-caryophyllene. They positively identified 15 new particle-phase products, including highly substituted compounds, and concluded that second-generation products make a significant contribution to the aerosol mass. Hamilton et al.[192] used SOA from β-caryophyllene as a seed for the condensation of oxidised monoterpenes, such as D-limonene. The fraction of cross-products between D-limonene and β-caryophyllene identified by HR-MS was minimal, suggesting that β-caryophyllene SOA is a suitable seed material for chamber studies. Yasmeen et al.[193] elucidated the chemical structure of the m/z 358 α-pinene SOA product, which is also observed in ambient aerosol from a boreal forest site as a diester formed between cis-pinic and diaterpenylic acid, a hydrolysis product of terpenylic acid. Of note, this molecule received considerable attention from several groups in previous studies, but its structure remained unresolved until the Yasmeen et al. study. The m/z 358 compound could be structurally identified mainly because of the prior structure characterisation[194] of the precursor of one the composing monomers, terpenylic acid, a first-generation lactone-containing product in α-pinene SOA.
Aromatic precursors contribute significantly to SOA production, especially in urban areas. Nakao et al.[195] investigated photooxidation of a series of phenolic compounds using a novel online PILS coupled with a TOF-MS instrument and identified multiple products consistent with OH addition to the aromatic ring. The same group investigated photooxidation of diesel exhaust in a smog chamber.[96] Ofner et al.[196] described a comprehensive study of SOA production from catechol and guaiacol, which relied on FTICR-MS, temperature-programmed-pyrolysis MS, Fourier transform infrared (FTIR) spectroscopy, and ultraviolet and visible (UV-vis) spectroscopy. They concluded that SOA produced from these precursors may serve as a good proxy for humic-like substances (HULIS). Gratien et al.[197] reported a rather unexpected formation of an aromatic product, p-cymene, in the oxidation of α-pinene by OH, O3 and NO3. Such conversion of non-aromatic precursors into aromatic ones may potentially complicate source apportionment, which relies on specific tracers.
A recent review of Ziemann[198] summarises the present state of the knowledge on contributions of long-chain saturated hydrocarbons to SOA formation from vehicular emissions in urban areas. Despite their relative simplicity (compared with terpenoid and aromatic VOCs), the chemical composition of SOAs from long-chain saturated hydrocarbons is quite complex. Kessler et al.[199] conducted chamber experiments with the reaction initiated by direct photolytic production or alkylperoxy radicals from alkyl iodides in air (as opposed to the more conventional OH or O3 oxidation of VOC). Although the AMS mass spectra of the resulting SOAs were still complex, this approach holds promise for mechanistic investigations of the initial stages of SOA formation. Chacon-Madrid and Donahue[200] examined the role of fragmentation versus functionalisation by cleverly selecting either straight-chain saturated hydrocarbons or corresponding aldehydes and ketones as aerosol precursors. They found the aerosol yield depended sensitively on the location of the carbonyl group within the precursor.
Isotope-resolved studies help constrain the mechanisms of atmospheric reactions and increase the accuracy of global VOC emission estimates. Using thermal desorption gas chromatography isotope ratio mass spectrometry (TD/GC/IRMS), Gensch et al.[201] studied the temperature dependence of the 12C→13C kinetic isotope effect in the ozonolysis of β-pinene. They showed that neglecting the temperature dependence of the kinetic isotope effect led to a significant underestimation of the average β-pinene age in the atmosphere. Moukhtar et al.[202] developed a GC/IRMS-based method to determine the stable carbon isotope ratio of methylnitrophenols in atmospheric particulate matter.
Organosulfate and organonitrate compounds
It is well established that organic compounds containing nitrogen, sulfur and other hetero elements can be incorporated into OAs by several mechanisms. For example, esters of nitric acids (organonitrates) are produced in high yields in reactions between peroxy radicals (RO2) and NO. Auld and Hastie[203] examined formation of keto- and hydroxy-nitrates in high-NOx oxidation of β-pinene using atmospheric pressure ionisation MS. Fry et al.[204] found that oxidation of limonene by NO3 produces particle-phase nitrates in high yields (~30 %). A HR-MS study by Nguyen et al.[77] found that organosulfates form efficiently during evaporation of an aqueous solution of OA acidified with sulfuric acid. Although organosulfates and organonitrates are thermodynamically unstable with respect to hydrolysis, bulk kinetic measurements found that only tertiary organonitrates hydrolyse quickly.[205–207] This is consistent with the high frequency of organosulfate and organonitrate observations in field studies of OAs (refer to the examples in the field section of this review).
Aqueous-phase chemistry of OAs
A recent review by Ervens et al.[208] presented convincing evidence from field, laboratory and modelling work indicating that a significant fraction of OAs can be produced through aqueous photochemistry and dark chemistry occurring in cloud droplets, fog droplets and aerosol water. MS methods have been invaluable in elucidating the mechanistic details of atmospheric aqueous chemistry. Lee et al.[209,210] described a novel approach for studying aqueous OH chemistry, wherein the solution is atomised directly from a photochemical reactor and the resulting aerosol is analysed using AMS. They observed the formation of highly oxidised, oligomeric products in the OH oxidation of glyoxal solutions,[210] as well as aqueous extracts of ambient aerosol and cloud water.[209] In addition to the aqueous OH oxidation, direct photolysis of dissolved organics is an important mechanism of cloud-processing as demonstrated recently in a HR-MS study by Bateman et al.[211] Fig. 9 shows the evolution of the HR mass spectrum of an aqueous aerosol extract of D-limonene SOA resulting from 2 and 24 h of irradiation by 300–400-nm light. The changes in the mass spectrum are driven primarily by the direct photolysis of carbonyls and peroxides. The OH-driven chemistry becomes even more complex in ice. For example, Gao et al.[212] found that an aqueous reaction of OH with dicarboxylic acids slowed down upon freezing, largely as a result of freezing-induced phase separation of the organic and aqueous phase.
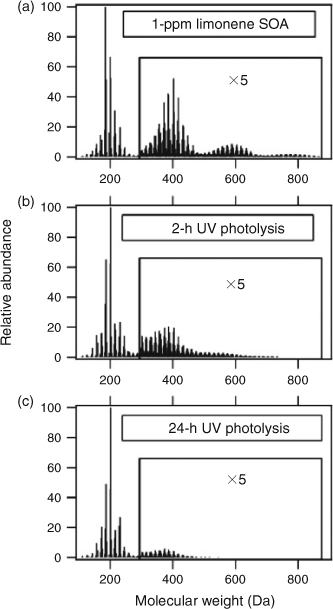
|
Although the OH-driven oxidation is believed to dominate the atmospheric aqueous chemistry,[213] dark reactions of carbonyls, such as glyoxal with nitrogen-containing compounds, may contribute to SOA formation at night.[214] For example, De Haan et al.[215] observed light-absorbing, nitrogen-containing oligomers upon simulated evaporation of cloud droplets containing glyoxal and methylglyoxal using a combination of AMS and ESI/MS methods. Formation of light-absorbing compounds was also observed upon evaporation of aqueous mixtures of ammonium sulfate and SOA.[77] Galloway et al.[216] investigated uptake of glyoxal onto deliquesced ammonium sulfate aerosol under both dark and irradiated conditions. Surprisingly, the glyoxal uptake did not depend on the presence of radiation, suggesting that the dark chemistry may, in fact, dominate in this case. Trainic et al.[217] examined the dark glyoxal and ammonium sulfate aerosol reaction at different RH levels using AMS and cavity ring-down spectroscopy methods. The growth of glyoxal oligomers depended on RH in a complicated way and was the most efficient at ~40 % RH, below the deliquescence point of AS. In contrast, the fraction of nitrogen-containing absorbing species increased with RH. These examples clearly demonstrate the high degree of sensitivity of aqueous reactions to the amount of available water in particles.
Simplified approaches for SOA characterisation
The high level of molecular complexity of OAs has prompted attempts to correlate various physical and chemical properties of OAs to parameters that are straightforward to measure with a mass spectrometer. These parameters include average elemental ratios O/C and H/C, which can be measured with an AMS,[218] and a more chemically intuitive metric ‘average carbon oxidation state' (OSC) introduced by Kroll et al.[219] Ng et al.[52] introduced a ‘triangle plot’, which graphs normalised the m/z 44 signal (f44, mostly from CO2+) against the m/z 43 signal (f43, mostly from C2H3O+), as a way visualising the evolution of AMS data. Most existing field data on OAs appear to occupy a limited triangle-shaped area in the f44–f43 space with the most oxidised (aged) SOAs converging to the top corner of the triangle. Chhabra et al.[220] studied how different types of chamber-generated SOAs evolve in the f44–f43 space. They found that SOAs, formed from already oxidised precursors, can achieve a higher placement on the ‘triangle plot’ (i.e. closer to the field observations) not easily accessible to non-oxidised precursors. Chen et al.[221] examined the SOA yields and O/C and H/C ratios measured with AMS in the photooxidation of isoprene, ozonolysis of α-pinene and ozonolysis of β-caryopyhllene. They found that an MCM-based model did a reasonable job in predicting the SOA yield, but it produced significant deviations from the average particle composition, implying missing pathways in the mechanism.
Gas–solid heterogeneous kinetics, interfacial chemistry and aerosol aging
Chemistry at the air–particle interface continues to enjoy increased attention because several unique and important chemical and physical processes are believed to occur right at or close to the interface. Laboratory studies of gas–surface reactive uptake can be conducted using either bulk samples or aerosolised materials. MS methods are instrumental in providing information on the composition of the surface, particles and gas-phase compounds interacting with the surface and particles. Net et al.[222] showed that the heterogeneous reaction of ozone with surfaces coated by phenolic compounds accelerated under irradiated conditions implied the existence of photosensitised reactions on the surface. Nájera et al.[223] identified several reaction products by atmospheric pressure CIMS in reactions of ozone with anthracene-coated surfaces and ammonium sulfate particles. Oldrgide and Abbatt[224] investigated the interaction of ozone with frozen and liquid solutions of NaCl/NaBr with the ozone and released halogens detected by negative-ion CIMS and found evidence for the surface and bulk reactions occurring in parallel. Gallimore et al.[225] found a surprisingly strong effect of RH on reactive uptake of ozone by maleic acid aerosol with considerably more reaction products observed above 50 % RH. The preceding examples amply illustrate the complexity of ozone reactions with surfaces with phase state, radiation and humidity all affecting reaction rates and mechanisms.
Due to its important role in controlling NOx levels in the atmosphere and aerosol aging chemistry, reactive uptake of nitrogen oxides by various surfaces has been studied by multiple groups. Using ESI/MS, Kinugawa et al.[226] investigated disproportionation of NO2, leading to HONO and NO3– on aqueous surfaces containing cationic, anionic, and neutral surfactants. They observed significant enhancement in the uptake of NO2 in the presence of cationic surfactants, an important finding in view of the widespread occurrence of surface-active organics in the environment. Knopf et al.[227] carried out an extensive study on the uptake of O3, NO2, NO3 and N2O5 by mixtures of levoglucosan, abietic acid and nitroguaiacol (surrogates for BBOA particles) over a range of RH values in a wall-coated flow reactor. They observed deactivation of the surface with respect to the NO3 uptake in all cases – except for the pure nitroguaiacol surface. The estimated lifetimes of these organic compounds under typical atmospheric conditions imply significant processing of BBOAs on a time scale of minutes to hours. Using an aerosol-CIMS approach, Zhao et al.[228] examined the particle-phase products formed in reactive uptake of NO3 by unsaturated fatty acid aerosols and found that the reaction mechanism depends on the presence of O2 (reactive uptake of chlorine atoms by squalane aerosol[229] also exhibited a strong dependence on the presence of O2). Measurements of the NO3 uptake by mixed solid organics and liquid unsaturated fatty acids by Xiao and Bertram[230] confirmed that lifetimes of unsaturated organics should be short in liquid particles and increase when the unsaturated organics are embedded in a solid matrix. The kinetics of reactions of NO3 with aerosolised insecticide (Phosmet)[231] and pesticide (Carbaryl)[232] was investigated using a VUV ATOFMS method. The results imply surprisingly short lifetimes of these compounds (0.3–1.3 h) with respect to oxidation by atmospheric NO3. The same group also investigated reaction of NO3 with various nitro-, oxy- and hydroxy-PAHs coated onto azelaic acid particles[233] and observed extensive nitration of the aromatics with both VUV ATOFMS and GC/MS.
The uptake of organics by atmospherically relevant solutions has also been investigated in the review period. Liu et al. examined uptake of 2-methyl-3-buten-2-ol[234] and methacrolein[235] into fairly concentrated aqueous solutions of H2O2 and H2SO4 in a rotating wall flow reactor coupled to a single-photon-ionisation TOFMS instrument. Acetone, acetaldehyde and isoprene were observed as products. Reactive uptake of nonanal by acidic aerosols (sulfuric acid by itself or mixed with levoglucosan or oleic acid) was measured with the help of an electrodynamic balance. The conclusion was that the acid-catalysed uptake of carbonyls is highly dependent on the presence of organics in particles.[236]
CCN and IN propensities of aerosols
The ability of aerosol particles to serve as CCN and IN is the primary mechanism behind their indirect effect on climate. Laboratory CCN studies have benefitted greatly from parallel characterisation of particles by various MS-based methods. For example, the expectation that more oxidised organic material should be more hygroscopic has prompted several attempts to correlate κ with the average level of oxidation in the particle material, expressed as either f44 (normalised CO2+ signal) or average O/C, measured by AMS. Engelhart et al.[237] reported κ = 0.12 for isoprene photooxidation SOA and found that CCN activation kinetics was similar to that for pure ammonium sulfate aerosol. CCN measurements by Frosch et al.[238] for α-pinene photooxidation SOA found that κ increased insignificantly with the oxidation state in the O/C range of 0.3–0.6. Kuwata et al.[239] conducted a related study for α-pinene ozonolysis SOA. The measured κ did not depend on the particle mass loading in the chamber or the average O/C ratio in the 0.38–0.50 range. However, particles subjected to thermodenuder treatment before the CCN measurements did have mass-loading-dependent hygroscopicity, and their CCN activity decreased. This effect was attributed to the higher fraction of less hygroscopic oligomeric compounds in particles exposed to elevated temperature. Using a potential aerosol mass flow reactor, Lambe et al.[240] were able to achieve a significantly wider range of O/C ratios (0.05–1.42) for chamber-generated SOAs and oxidised POAs. Before the CCN measurements, the particles were exposed in the potential aerosol mass flow reactor to OH or O3 for an equivalent of 1–20 days in ambient air. The hygroscopicity of the aged particles ranged from κ = 8.4 × 10–4 to 0.28 and was reasonably correlated with the average O/C (shown in Fig. 10). Another paper by the same group[241] investigated the complicated (and still unanswered) question regarding to what extent the accelerated oxidation in various types of aging reactors approximate the slower oxidation in ambient air. Meanwhile, Kang et al.[242] examined the effect of OH exposure in a potential aerosol mass flow reactor on the measured degree of oxidation. To complete the story, we should note that the oxidation of organics in particles does not always increase their CCN activities. For example, ozone oxidation of particles containing oleic acid and sodium oleate internally mixed with sodium chloride or ammonium sulfate has little effect on their critical diameters.[243]
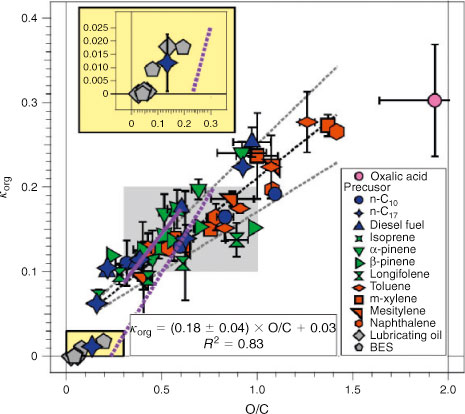
|
In contrast to the moderate level of understanding of the CCN activity of organic particles, which can be parameterised using κ and correlated with the average O/C, there are no simple correlations between IN activity and particle composition. However, IN studies also benefit greatly from MS-based particle characterisation. Friedman et al.[244] investigated whether carboxylic acid coatings (adipic acid, malic acid, oleic acid and oleic acid exposed to ozone) can improve the IN ability of soot particles. The composition and morphology of particles were characterised by a SPMS method. Although the coatings aided in water uptake, they did not induce heterogeneous IN by the core soot particles. Reitz et al.[245] studied the effect of exposure of mineral dust particles to sulfuric acid, humidity and ammonia vapours on their IN activities. The change in particle composition was characterised by ATOFMS and AMS. Generally, surface modification by chemical exposure was found to decrease the IN activity of dust particles.
Once the cloud droplets are activated, they can scavenge additional aerosol particles. Ladino et al.[246] measured scavenging efficiencies by crossing a laminar flow of lithium metaborate particles (0.05–0.3 μm) and free-falling droplets (12–20 μm) with the droplets collected and analysed by an inductively coupled plasma MS instrument. They were able to observe the so-called ‘Greenfield gap’, the particle size resistant to scavenging, at ~0.24 μm.
Optical and hygroscopic properties of aerosols
In addition to the indirect effect of aerosols on climate through cloud and ice nucleation, aerosols can also affect the climate directly by scattering and absorbing solar radiation. As particle absorption (σa), scattering (σs) and extinction (σext = σa + σs) coefficients depend sensitively on the presence of adsorbed water, laboratory studies of the optical properties of aerosols are often conducted in parallel with particle hygroscopic growth measurements at sub-saturated RH (<100 %). MS methods provide invaluable information for interpreting the optical and hygroscopic properties of OAs and relating them to chemical composition. Cappa et al.[247] measured σext (500 nm) for particles of squalane and azelaic acid as they were oxidised by OH. In both cases, there was an increase in σext with oxidation correlated with the average O/C ratio. At the same time, there was a significant increase in hygroscopicity for the oxidised azelaic acid particles but not for the squalane ones. Redmond and Thomson[248] proposed a promising approach for correlating σext with the molecular structure by means of structure–polarisability relationships. Correlations of this type should be especially useful for aerosols characterised by HR-MS methods. Duplissy et al.[249] were able to correlate hygroscopicity of chamber-generated SOAs with the average degree of oxidation and proposed a correlation between the effective hygroscopicity parameter and f44 (previously defined): κorganic = 2.2 · f44 – 0.13. Tritscher et al.[250] examined how hygroscopicity and volatility of α-pinene SOA evolve with initial formation, ripening and OH-induced chemical aging. In their measurements, κorganic initially increased with OH-aging then reached a plateau, whereas the particle volatility continued to drop. Lu et al.[251] quantified σext, using cavity ring-down spectroscopy, and σa, using photoacoustic spectroscopy for benzo[a]pyrene-coated particles during their reaction with NO2 and NO3. When some benzo[a]pyrene in particles was nitrated, evident by the parallel AMS measurements, both the scattering and absorption coefficients increased.
Internally mixed organic–inorganic particles offer an additional layer of complexity in interpreting their hygroscopic and optical properties. Work by Trainic et al.,[217] which has already been mentioned in conjunction with the aqueous chemistry of SOAs, characterised optical properties of ammonium sulfate aerosols interacting with glyoxal vapour at different RH. Zhong and Jang[252] developed a promising method for measurements of σa using filter samples of aerosols in an integrated sphere and applied it to internally mixed ammonium sulfate and SOA particles. A study by Smith et al.[253] examined deliquescence and efflorescence phase transitions in ammonium sulfate coated with α-pinene SOA organic material. The surprising result of this study was that the organic coating had an insignificant effect on the phase transition in the ammonium sulfate core. Even in the deliquesced state, the phase separation between the organic and inorganic fractions appeared to persist. This represents a significant change in how the phase states of internally mixed particles are viewed. Several interesting new developments can be expected in this research area. To make matters even more interesting, Nguyen et al. observed chemical reactions between ammonium ions and SOA carbonyls occurring during evaporation of ammonium sulphate–SOA solutions[184] leading, among other things, to light-absorbing, nitrogen-containing compounds. Once again, these examples emphasise the role of RH in controlling not only the phase state but the chemistry of internally mixed aerosols.
Summary and outlook to the Special Issue
Developments and applications of novel MS methodologies discussed herein are at the forefront of aerosol chemistry and physics research. Over a span of a few years, we have witnessed tremendous expansion and growth in this research area, and this trend likely will continue. Several scientific challenges related to complex, multi-phase chemistry and physics of aerosols that are yet to be resolved would benefit from experimental capabilities of complementary MS techniques. The synopsis that we present is not intended to convey an inclusive list of all possible topics. Rather, it embraces selected areas where the authors of this special issue are currently working in parallel with other groups.
-
Elucidation of the effect of OA chemistry and changes in its molecular composition on the increased viscosity and formation of glassy SOA particles is particularly important for understanding how these changes affect the atmospheric lifetime of particles, their heterogeneous reactions, optical and hygroscopic properties. Coupled experimental and modelling studies in this area are needed to develop a parameterisation framework to include these effects in aerosol and cloud models. Although this research topic is not explicitly presented in the collection of manuscripts for this special issue, advances in this area have been discussed in great detail in a recent perspective manuscript by Koop et al.[182] and references therein.
-
Identification of ‘brown carbon’ chromophores in OAs, as well as a molecular-level understanding of their composition, light-absorption properties, atmospheric sources, possible formation and aging mechanisms, is also important. The work of Claeys et al.[254] illustrates efforts in this area and reports on the chemical speciation of HULIS in field-collected aerosol samples ascribing chromophoric substances to nitrocatechols based on LC/UVVIS/MS measurements.
-
Identification of molecular markers unique to POA and SOA from different biogenic and anthropogenic sources is needed to improve aerosol emission inventories, including source-specific attribution of OA in the atmospheric environment. Detection and molecular-level speciation of OA markers are commonly used in focussed laboratory studies designed to investigate different aspects of aerosol chemistry inferred from field observations. In this issue, Zhang et al.[255] use GC/MS and UPLC/ESI/MS techniques to analyse samples of laboratory-generated and ambient aerosols and systematically evaluate the role of environmental conditions, such as RH, aerosol, acidity and NOx level, on yields and composition of SOA derived from the photochemical oxidation of isoprene. A separate study by Yasmeen et al.[256] focussed on the structural characterisation of molecular markers and the reaction mechanism of α-pinene SOA aging. Stone et al.[257] evaluates biogenic contributions to SOAs at a specific geographic location (Kathmandu Valley, Nepal) based on the GC/MS analysis of field samples and quantitative analysis of targeted molecular markers.
-
Molecular-level understanding of the interactions of anthropogenic and biogenic/geogenic emissions and their effects on the physicochemical properties of aerosols is a topic of intensive ongoing research in many groups and has a direct effect on air quality management and emission regulation in urban areas. In this issue, the Huang et al.[258] field study presents ATOFMS measurements from an urban area of Shanghai, discusses the formation mechanism of amine-containing OAs and describes plausible environmental factors enhancing their formation and growth. Kroll et al.[259] presents key chemical characteristics and oxidation trends of anthropogenic diesel exhaust particles generated in a test laboratory study and investigated using an AMS instrument coupled to the synchrotron-based VUV ionisation source.
-
The investigation of the aqueous-phase chemistry of organics relevant to processes in cloud and fog droplets and deliquesced aerosols has been the subject of many studies.[208] Although condensed water is an abundant medium in atmospheric processes, the importance of the aqueous processes in the formation and aging of SOAs was not considered until recently. To explore the molecular composition of organics in cloud and fog water, more studies are needed, which, in turn, would facilitate laboratory studies with selected model systems. Ge et al.[260] report results of AMS measurements during fog episodes in the Central Valley, California, and indicates significant oxidation of organics through aqueous-phase atmospheric chemistry.
-
One of the most challenging tasks in aerosol chemistry research is experimental studies of NPF events that require development and application of MS techniques capable of detection and chemical characterisation of the smallest, nucleation mode particles and sensitive measurements of their gas-phase precursors. This research area is illustrated by the work of Yu and Lee,[108] which presents a novel CIMS instrument uniquely configured for sensitive detection of gaseous amines and its first field application.
-
Methods of HR-MS (>50 000 m/Δm) coupled to soft ionisation methods, such as ESI and its variations, arguably are among the most powerful techniques to probe the molecular-level composition of individual molecules in complex OA mixtures.[26] The ESI/HR-MS analysis can detect thousands of individual molecules at once and provide their elemental composition, and additional structural information can be obtained from fragmentation experiments using MS/MS. Applications of these techniques to unravel the chemical complexity of aerosols have increased remarkably over the last few years for analysis of both laboratory and field-collected aerosols – with many more novel developments and applications in this area expected in the near future. In this issue, Mazzoleni et al.[261] used HR-MS analysis to characterise individual constituents of the WSOC fraction of ambient aerosol, whereas Rincón et al.[262] used HR-MS to investigate seasonal variations in the composition of ambient OAs collected at an urban site.
Abbreviations used
-
2D-TAG, two-dimensional thermal desorption aerosol gas chromatography
-
ACSM, aerosol chemical speciation monitor
-
AMS, aerosol mass spectrometer
-
APi-TOF, atmospheric pressure interface time-of-flight (mass spectrometer)
-
ASAP, atmospheric solids analysis probe
-
ATOFMS, aerosol time-of-flight mass spectrometer
-
BBOA, biomass burning organic aerosol
-
CIMS, chemical ionisation mass spectrometry
-
CCN, cloud condensation nucleus
-
DESI, desorption electrospray ionisation
-
EI, electron ionisation
-
ESI, electrospray ionisation
-
EUV, extreme ultraviolet
-
FTIR, Fourier transform infrared (spectroscopy)
-
FTICR, Fourier transform ion cyclotron resonance
-
GC/MS, gas chromatography mass spectrometry
-
HPLC, high-performance liquid chromatography
-
HR-MS, high-resolution mass spectrometry
-
HOA, hydrocarbon-like organic aerosol (as observed by AMS)
-
HULIS, humic-like substances
-
IN, ice nucleus
-
IRMS, isotope ratio mass spectrometry
-
LC/MS, liquid chromatography mass spectrometry
-
LOD, limit of detection
-
LV-OOA, low-volatility oxygenated organic aerosol (as observed by AMS)
-
MAB, metastable atom bombardment
-
MCM, master chemical mechanism
-
MS, mass spectrometry
-
NAMS, nano aerosol mass spectrometer
-
NPF, new particle formation
-
OA, organic aerosol
-
OOA, oxygenated organic aerosol (as observed by AMS)
-
PAH, polycyclic aromatic hydrocarbon
-
PILS, particle-into-liquid sampler
-
PMF, positive matrix factorisation
-
POA, primary organic aerosol
-
PTRMS, proton-transfer reaction mass spectrometer
-
REMPI, resonance enhanced multi-photon ionisation
-
Q-TOF, (hybrid) quadrupole time-of-flight
-
SIMS, secondary ion mass spectrometry
-
SNMS, secondary neutral mass spectrometry
-
SPMS, single particle mass spectrometer
-
SOA, secondary organic aerosol
-
SV-OOA, semi-volatile oxygenated organic aerosol (as observed by AMS)
-
TD, thermal desorption
-
TOF, time-of-flight
-
UPLC, ultra performance liquid chromatography
-
UV-vis, ultraviolet and visible (spectroscopy)
-
VOC, volatile organic compound
-
VUV, vacuum ultraviolet
-
WSOC, water-soluble organic carbon
-
XPS, X-ray photoelectron spectroscopy
Acknowledgements
The authors acknowledge financial support from the US Department of Energy’s (DOE) Office of Biological and Environmental Research (BER) Atmospheric System Research program; the Division of Chemical Sciences, Geosciences & Biosciences, DOE Office of Basic Energy Sciences; the National Science Foundation (grants ATM-0831518 and CHE-0909227); and the W.R. Wiley Environmental Molecular Sciences Laboratory’s (EMSL) intramural research and development program. EMSL is a national scientific user facility sponsored by DOE-BER and located at PNNL. PNNL is operated for DOE by Battelle Memorial Institute under contract number DE-AC06–76RL0 1830.
References
[1] S. Menon, N. Unger, D. Koch, J. Francis, T. Garrett, I. Sednev, D. Shindell, D. Streets, Aerosol climate effects and air quality impacts from 1980 to 2030. Environ. Res. Lett. 2008, 3, 024004.| Aerosol climate effects and air quality impacts from 1980 to 2030.Crossref | GoogleScholarGoogle Scholar |
[2] U. Pöschl, Atmospheric aerosols: composition, transformation, climate and health effects. Angew. Chem. Int. Ed. 2005, 44, 7520.
| Atmospheric aerosols: composition, transformation, climate and health effects.Crossref | GoogleScholarGoogle Scholar |
[3] I. J. George, J. P. D. Abbatt, Heterogeneous oxidation of atmospheric aerosol particles by gas-phase radicals. Nat. Chem. 2010, 2, 713.
| Heterogeneous oxidation of atmospheric aerosol particles by gas-phase radicals.Crossref | GoogleScholarGoogle Scholar |
[4] U. Pöschl, Gas-particle interactions of tropospheric aerosols: kinetic and thermodynamic perspectives of multiphase chemical reactions, amorphous organic substances, and the activation of cloud condensation nuclei. Atmos. Res. 2011, 101, 562.
| Gas-particle interactions of tropospheric aerosols: kinetic and thermodynamic perspectives of multiphase chemical reactions, amorphous organic substances, and the activation of cloud condensation nuclei.Crossref | GoogleScholarGoogle Scholar |
[5] W. L. Chang, P. V. Bhave, S. S. Brown, N. Riemer, J. Stutz, D. Dabdub, Heterogeneous atmospheric chemistry, ambient measurements, and model calculations of N2O5: a review. Aerosol Sci. Technol. 2011, 45, 665.
| Heterogeneous atmospheric chemistry, ambient measurements, and model calculations of N2O5: a review.Crossref | GoogleScholarGoogle Scholar |
[6] C. E. Kolb, R. A. Cox, J. P. D. Abbatt, M. Ammann, E. J. Davis, D. J. Donaldson, B. C. Garrett, C. George, P. T. Griffiths, D. R. Hanson, M. Kulmala, G. McFiggans, U. Pöschl, I. Riipinen, M. J. Rossi, Y. Rudich, P. E. Wagner, P. M. Winkler, D. R. Worsnop, C. D. O’Dowd, An overview of current issues in the uptake of atmospheric trace gases by aerosols and clouds. Atmos. Chem. Phys. 2010, 10, 10 561.
| An overview of current issues in the uptake of atmospheric trace gases by aerosols and clouds.Crossref | GoogleScholarGoogle Scholar |
[7] D. Fowler, K. Pilegaard, M. A. Sutton, P. Ambus, M. Raivonen, J. Duyzer, D. Simpson, H. Fagerli, S. Fuzzi, J. K. Schjoerring, C. Granier, A. Neftel, I. S. A. Isaksen, P. Laj, M. Maione, P. S. Monks, J. Burkhardt, U. Daemmgen, J. Neirynck, E. Personne, R. Wichink-Kruit, K. Butterbach-Bahl, C. Flechard, J. P. Tuovinen, M. Coyle, G. Gerosa, B. Loubet, N. Altimir, L. Gruenhage, C. Ammann, S. Cieslik, E. Paoletti, T. N. Mikkelsen, H. Ro-Poulsen, P. Cellier, J. N. Cape, L. Horvath, F. Loreto, U. Niinemets, P. I. Palmer, J. Rinne, P. Misztal, E. Nemitz, D. Nilsson, S. Pryor, M. W. Gallagher, T. Vesala, U. Skiba, N. Brueggemann, S. Zechmeister-Boltenstern, J. Williams, C. O’Dowd, M. C. Facchini, G. de Leeuw, A. Flossman, N. Chaumerliac, J. W. Erisman, Atmospheric composition change: ecosystems–atmosphere interactions. Atmos. Environ. 2009, 43, 5193.
| Atmospheric composition change: ecosystems–atmosphere interactions.Crossref | GoogleScholarGoogle Scholar |
[8] S. Wu, L. J. Mickley, J. O. Kaplan, D. J. Jacob, Impacts of changes in land use and land cover on atmospheric chemistry and air quality over the 21st century. Atmos. Chem. Phys. 2012, 12, 1597.
| Impacts of changes in land use and land cover on atmospheric chemistry and air quality over the 21st century.Crossref | GoogleScholarGoogle Scholar |
[9] M. Chin, R. A. Kahn, S. E. Schwartz, Atmospheric Aerosol Properties and Climate Impacts 2009 (Aeronautics and Space Administration: Washington, DC).
[10] S. J. Ghan, S. E. Schwartz, Aerosol properties and processes – a patha from field and laboratory measurements to global climate models. Bull. Am. Meteorol. Soc. 2007, 88, 1059.
| Aerosol properties and processes – a patha from field and laboratory measurements to global climate models.Crossref | GoogleScholarGoogle Scholar |
[11] A. Nel, Air pollution-related illness: effects of particles. Science 2005, 308, 804.
| Air pollution-related illness: effects of particles.Crossref | GoogleScholarGoogle Scholar |
[12] R. Zimmermann, Ambient aerosols and human health: working towards a combined analytical and toxicological approach. Anal. Bioanal. Chem. 2011, 401, 3041.
| Ambient aerosols and human health: working towards a combined analytical and toxicological approach.Crossref | GoogleScholarGoogle Scholar |
[13] IPCC, Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, in Climate Change 2007: The Physical Scientific Basis (Eds S. Solomon, D. Qin, M. Manning, Z. Chen, M. Marquis, K. B. Averyt, M. Tignor, H. L. Miller) 2007 (Cambridge University Press: Cambridge, UK, and New York).
[14] R. D. Brook, S. Rajagopalan, C. A. Pope, J. R. Brook, A. Bhatnagar, A. V. Diez-Roux, F. Holguin, Y. L. Hong, R. V. Luepker, M. A. Mittleman, A. Peters, D. Siscovick, S. C. Smith, L. Whitsel, J. D. Kaufman, Particulate matter air pollution and cardiovascular disease an update to the scientific statement from the American heart association. Circulation 2010, 121, 2331.
| Particulate matter air pollution and cardiovascular disease an update to the scientific statement from the American heart association.Crossref | GoogleScholarGoogle Scholar |
[15] L. T. Molina, M. J. Molina, Air Quality in the Mexico Megacity: An Integrated Assessment 2002 (Kluwer Academic Publishers: Dordrecht, the Netherlands).
[16] M. G. Lawrence, T. M. Butler, J. Steinkamp, B. R. Gurjar, J. Lelieveld, Regional pollution potentials of megacities and other major population centers. Atmos. Chem. Phys. 2007, 7, 3969.
| Regional pollution potentials of megacities and other major population centers.Crossref | GoogleScholarGoogle Scholar |
[17] M. Hallquist, J. C. Wenger, U. Baltensperger, Y. Rudich, D. Simpson, M. Claeys, J. Dommen, N. M. Donahue, C. George, A. H. Goldstein, J. F. Hamilton, H. Herrmann, T. Hoffmann, Y. Iinuma, M. Jang, M. E. Jenkin, J. L. Jimenez, A. Kiendler-Scharr, W. Maenhaut, G. McFiggans, T. F. Mentel, A. Monod, A. S. H. Prevot, J. H. Seinfeld, J. D. Surratt, R. Szmigielski, J. Wildt, The formation, properties and impact of secondary organic aerosol: current and emerging issues. Atmos. Chem. Phys. 2009, 9, 5155.
| The formation, properties and impact of secondary organic aerosol: current and emerging issues.Crossref | GoogleScholarGoogle Scholar |
[18] L. T. Molina, S. Madronich, J. S. Gaffney, E. Apel, B. de Foy, J. Fast, R. Ferrare, S. Herndon, J. L. Jimenez, B. Lamb, A. R. Osornio-Vargas, P. Russell, J. J. Schauer, P. S. Stevens, R. Volkamer, M. Zavala, An overview of the MILAGRO 2006 Campaign: Mexico City emissions and their transport and transformation. Atmos. Chem. Phys. 2010, 10, 8697.
| An overview of the MILAGRO 2006 Campaign: Mexico City emissions and their transport and transformation.Crossref | GoogleScholarGoogle Scholar |
[19] R. A. Zaveri, W. J. Shaw, D. J. Cziczo, B. Schmid, R. A. Ferrare, M. L. Alexander, M. Alexandrov, W. P. Arnott, D. Atkinson, J. C. Barnard, L. K. Berg, J. Beranek, F. Brechtel, J. F. Cahill, B. Cairns, C. D. Cappa, S. China, J. Comstock, M. K. Dubey, R. C. Easter, M. H. Erickson, J. D. Fast, C. Floerchinger, B. A. Flowers, E. Fortner, J. S. Gaffney, M. K. Gilles, K. Gorkowski, W. I. Gustafson, M. Gyawali, J. Hair, J. W. Harworth, S. C. Herndon, C. Hostetler, J. M. Hubbe, J. T. Jayne, H. Jeong, B. T. Jobson, E. Kassianov, L. I. Kleinman, K. R. Kolesar, C. Kluzek, B. Knighton, A. Kubátová, C. Kuang, A. Laskin, N. Laulainen, C. Mazzoleni, F. Mei, R. C. Moffet, D. Nelson, M. Obland, T. B. Onasch, M. Ottaviani, M. Pekour, K. A. Prather, J. G. Radney, A. Sedlacek, G. I. Senum, A. Setyan, J. E. Shilling, M. Shrivastava, C. Song, S. R. Springston, R. Subramanian, K. Suski, J. Tomlinson, H. W. Wallace, J. Wang, D. R. Worsnop, A. Zelenyuk, Q. Zhang, Overview of the 2010 carbonaceous aerosols and radiative effects study (CARES). Atmos. Chem. Phys. Discuss. 2012, 12, 1299.
| Overview of the 2010 carbonaceous aerosols and radiative effects study (CARES).Crossref | GoogleScholarGoogle Scholar |
[20] R. C. Moffet, Y. Desyaterik, R. J. Hopkins, A. V. Tivanski, M. K. Gilles, Y. Wang, V. Shutthanandan, L. T. Molina, R. G. Abraham, K. S. Johnson, V. Mugica, M. J. Molina, A. Laskin, K. A. Prather, Characterization of aerosols containing Zn, Pb, and Cl from an industrial region of Mexico City. Environ. Sci. Technol. 2008, 42, 7091.
| Characterization of aerosols containing Zn, Pb, and Cl from an industrial region of Mexico City.Crossref | GoogleScholarGoogle Scholar |
[21] R. C. Moffet, T. R. Henn, A. V. Tivanski, R. J. Hopkins, Y. Desyaterik, A. L. D. Kilcoyne, T. Tyliszczak, J. Fast, J. Barnard, V. Shutthanandan, S. S. Cliff, K. D. Perry, A. Laskin, M. K. Gilles, Microscopic characterization of carbonaceous aerosol particle aging in the outflow from Mexico City. Atmos. Chem. Phys. 2010, 10, 961.
| Microscopic characterization of carbonaceous aerosol particle aging in the outflow from Mexico City.Crossref | GoogleScholarGoogle Scholar |
[22] A. Laskin, R. C. Moffet, M. K. Gilles, J. D. Fast, R. A. Zaveri, B. Wang, J. Shutthanandan, Tropospheric chemistry of internally mixed sea salt and organic particles: surprising reactivity of NaCl with weak organic acids. J. Geophys. Res. – Atmos. 2012, in press
| Tropospheric chemistry of internally mixed sea salt and organic particles: surprising reactivity of NaCl with weak organic acids.Crossref | GoogleScholarGoogle Scholar |
[23] C. Arsene, D. Vione, N. Grinberg, R. I. Olariu, GC × GC-MS hyphenated techniques for the analysis of volatile organic compounds in air. J. Liquid Chromatogr. Relat. Technol. 2011, 34, 1077.
| GC × GC-MS hyphenated techniques for the analysis of volatile organic compounds in air.Crossref | GoogleScholarGoogle Scholar |
[24] R. Duarte, A. C. Duarte, A critical review of advanced analytical techniques for water-soluble organic matter from atmospheric aerosols. TRAC – Trends in Analytical Chemistry 2011, 30, 1659.
| A critical review of advanced analytical techniques for water-soluble organic matter from atmospheric aerosols.Crossref | GoogleScholarGoogle Scholar |
[25] M. D. Hays, R. J. Lavrich, Developments in direct thermal extraction gas chromatography-mass spectrometry of fine aerosols. TRAC – Trends in Analytical Chemistry 2007, 26, 88.
| Developments in direct thermal extraction gas chromatography-mass spectrometry of fine aerosols.Crossref | GoogleScholarGoogle Scholar |
[26] S. A. Nizkorodov, J. Laskin, A. Laskin, Molecular chemistry of organic aerosols through the application of high resolution mass spectrometry. Phys. Chem. Chem. Phys. 2011, 13, 3612.
| Molecular chemistry of organic aerosols through the application of high resolution mass spectrometry.Crossref | GoogleScholarGoogle Scholar |
[27] T. Reemtsma, Determination of molecular formulas of natural organic matter molecules by (ultra-) high-resolution mass spectrometry: status and needs. J. Chromatogr. A 2009, 1216, 3687.
| Determination of molecular formulas of natural organic matter molecules by (ultra-) high-resolution mass spectrometry: status and needs.Crossref | GoogleScholarGoogle Scholar |
[28] B. Zielinska, S. Samy, Analysis of nitrated polycyclic aromatic hydrocarbons. Anal. Bioanal. Chem. 2006, 386, 883.
| Analysis of nitrated polycyclic aromatic hydrocarbons.Crossref | GoogleScholarGoogle Scholar |
[29] M. R. Canagaratna, J. T. Jayne, J. L. Jimenez, J. D. Allan, M. R. Alfarra, Q. Zhang, T. B. Onasch, F. Drewnick, H. Coe, A. Middlebrook, A. Delia, L. R. Williams, A. M. Trimborn, M. J. Northway, P. F. DeCarlo, C. E. Kolb, P. Davidovits, D. R. Worsnop, Chemical and microphysical characterization of ambient aerosols with the aerodyne aerosol mass spectrometer. Mass Spectrom. Rev. 2007, 26, 185.
| Chemical and microphysical characterization of ambient aerosols with the aerodyne aerosol mass spectrometer.Crossref | GoogleScholarGoogle Scholar |
[30] K. Hartonen, T. Laitinen, M. L. Riekkola, Current instrumentation for aerosol mass spectrometry. TRAC – Trends in Analytical Chemistry 2011, 30, 1486.
| Current instrumentation for aerosol mass spectrometry.Crossref | GoogleScholarGoogle Scholar |
[31] M. V. Johnston, Sampling and analysis of individual particles by aerosol mass spectrometry. J. Mass Spectrom. 2000, 35, 585.
| Sampling and analysis of individual particles by aerosol mass spectrometry.Crossref | GoogleScholarGoogle Scholar |
[32] D. M. Murphy, P. K. Hudson, D. J. Cziczo, S. Gallavardin, K. D. Froyd, M. V. Johnston, A. M. Middlebrook, M. S. Reinard, D. S. Thomson, T. Thornberry, A. S. Wexler, Distribution of lead in single atmospheric particles. Atmos. Chem. Phys. 2007, 7, 3195.
| Distribution of lead in single atmospheric particles.Crossref | GoogleScholarGoogle Scholar |
[33] D. G. Nash, T. Baer, M. V. Johnston, Aerosol mass spectrometry: an introductory review. Int. J. Mass Spectrom. 2006, 258, 2.
| Aerosol mass spectrometry: an introductory review.Crossref | GoogleScholarGoogle Scholar |
[34] C. A. Noble, K. A. Prather, Real-time single particle mass spectrometry: a historical review of a quarter century of the chemical analysis of aerosols. Mass Spectrom. Rev. 2000, 19, 248.
| Real-time single particle mass spectrometry: a historical review of a quarter century of the chemical analysis of aerosols.Crossref | GoogleScholarGoogle Scholar |
[35] J. Zahardis, S. Geddes, G. A. Petrucci, Improved understanding of atmospheric organic aerosols via innovations in soft ionization aerosol mass spectrometry. Anal. Chem. 2011, 83, 2409.
| Improved understanding of atmospheric organic aerosols via innovations in soft ionization aerosol mass spectrometry.Crossref | GoogleScholarGoogle Scholar |
[36] A. Zelenyuk, D. Imre, Beyond single particle mass spectrometry: multidimensional characterisation of individual aerosol particles. Int. Rev. Phys. Chem. 2009, 28, 309.
| Beyond single particle mass spectrometry: multidimensional characterisation of individual aerosol particles.Crossref | GoogleScholarGoogle Scholar |
[37] K. A. Pratt, K. A. Prather, Mass spectrometry of atmospheric aerosols. Recent developments and applications – Part I. Off-line mass spectrometry techniques. Mass Spectrom. Rev. 2012, 31, 1.
| Mass spectrometry of atmospheric aerosols. Recent developments and applications – Part I. Off-line mass spectrometry techniques.Crossref | GoogleScholarGoogle Scholar |
[38] K. A. Pratt, K. A. Prather, Mass spectrometry of atmospheric aerosols. Recent developments and applications – Part II. On-line mass spectrometry techniques. Mass Spectrom. Rev. 2012, 31, 17.
| Mass spectrometry of atmospheric aerosols. Recent developments and applications – Part II. On-line mass spectrometry techniques.Crossref | GoogleScholarGoogle Scholar |
[39] M. Brands, M. Kamphus, T. Bottger, J. Schneider, F. Drewnick, A. Roth, J. Curtius, C. Voigt, A. Borbon, M. Beekmann, A. Bourdon, T. Perrin, S. Borrmann, Characterization of a newly developed aircraft-based laser ablation aerosol mass spectrometer (ALABAMA) and first field deployment in urban pollution plumes over Paris during MEGAPOLI 2009. Aerosol Sci. Technol. 2011, 45, 46.
| Characterization of a newly developed aircraft-based laser ablation aerosol mass spectrometer (ALABAMA) and first field deployment in urban pollution plumes over Paris during MEGAPOLI 2009.Crossref | GoogleScholarGoogle Scholar |
[40] L. Li, Z. X. Huang, J. G. Dong, M. Li, W. Gao, H. Q. Nian, Z. Fu, G. H. Zhang, X. H. Bi, P. Cheng, Z. Zhou, Real time bipolar time-of-flight mass spectrometer for analyzing single aerosol particles. Int. J. Mass Spectrom. 2011, 303, 118.
| Real time bipolar time-of-flight mass spectrometer for analyzing single aerosol particles.Crossref | GoogleScholarGoogle Scholar |
[41] P. T. Steele, G. R. Farquar, A. N. Martin, K. R. Coffee, V. J. Riot, S. I. Martin, D. P. Fergenson, E. E. Gard, M. Frank, Autonomous, broad-spectrum detection of hazardous aerosols in seconds. Anal. Chem. 2008, 80, 4583.
| Autonomous, broad-spectrum detection of hazardous aerosols in seconds.Crossref | GoogleScholarGoogle Scholar |
[42] T. D. Vaden, D. Imre, J. Beranek, A. Zelenyuk, Extending the capabilities of single particle mass spectrometry: I. Measurements of aerosol number concentration, size distribution, and asphericity. Aerosol Sci. Technol. 2011, 45, 113.
| Extending the capabilities of single particle mass spectrometry: I. Measurements of aerosol number concentration, size distribution, and asphericity.Crossref | GoogleScholarGoogle Scholar |
[43] T. D. Vaden, D. Imre, J. Beranek, A. Zelenyuk, Extending the capabilities of single particle mass spectrometry: II. Measurements of aerosol particle density without DMA. Aerosol Sci. Technol. 2011, 45, 125.
| Extending the capabilities of single particle mass spectrometry: II. Measurements of aerosol particle density without DMA.Crossref | GoogleScholarGoogle Scholar |
[44] J. Beranek, A. Imre, A. Zelenyuk, Real-time shape-based particle separation and detailed in situ particle shape characterization. Anal. Chem. 2012, 84, 1459.
| Real-time shape-based particle separation and detailed in situ particle shape characterization.Crossref | GoogleScholarGoogle Scholar |
[45] M. Oster, M. Elsasser, J. Schnelle-Kreis, R. Zimmermann, First field application of a thermal desorption resonance-enhanced multiphoton-ionisation single particle time-of-flight mass spectrometer for the on-line detection of particle-bound polycyclic aromatic hydrocarbons. Anal. Bioanal. Chem. 2011, 401, 3173.
| First field application of a thermal desorption resonance-enhanced multiphoton-ionisation single particle time-of-flight mass spectrometer for the on-line detection of particle-bound polycyclic aromatic hydrocarbons.Crossref | GoogleScholarGoogle Scholar |
[46] T. W. Adam, R. Chirico, M. Clairotte, M. Elsasser, U. Manfredi, G. Martini, M. Sklorz, T. Streibel, M. F. Heringa, P. F. DeCarlo, U. Baltensperger, G. De Santi, A. Krasenbrink, R. Zimmermann, A. S. H. Prevot, C. Astorga, Application of modern online instrumentation for chemical analysis of gas and particulate phases of exhaust at the European commission heavy-duty vehicle emission laboratory. Anal. Chem. 2011, 83, 67.
| Application of modern online instrumentation for chemical analysis of gas and particulate phases of exhaust at the European commission heavy-duty vehicle emission laboratory.Crossref | GoogleScholarGoogle Scholar |
[47] J. Grabowsky, T. Streibel, M. Sklorz, J. C. Chow, J. G. Watson, A. Mamakos, R. Zimmermann, Hyphenation of a carbon analyzer to photo-ionization mass spectrometry to unravel the organic composition of particulate matter on a molecular level. Anal. Bioanal. Chem. 2011, 401, 3153.
| Hyphenation of a carbon analyzer to photo-ionization mass spectrometry to unravel the organic composition of particulate matter on a molecular level.Crossref | GoogleScholarGoogle Scholar |
[48] W. Z. Fang, L. Gong, X. B. Shan, F. Y. Liu, Z. Y. Wang, L. S. Sheng, Thermal desorption/tunable vacuum-ultraviolet time-of-flight photoionization aerosol mass spectrometry for investigating secondary organic aerosols in chamber experiments. Anal. Chem. 2011, 83, 9024.
| Thermal desorption/tunable vacuum-ultraviolet time-of-flight photoionization aerosol mass spectrometry for investigating secondary organic aerosols in chamber experiments.Crossref | GoogleScholarGoogle Scholar |
[49] W. Z. Fang, L. Gong, X. B. Shan, Y. J. Zhao, F. Y. Liu, Z. Y. Wang, L. S. Sheng, Photoionization and dissociation of the monoterpene limonene: mass spectrometric and computational investigation. J. Mass Spectrom. 2011, 46, 1152.
| Photoionization and dissociation of the monoterpene limonene: mass spectrometric and computational investigation.Crossref | GoogleScholarGoogle Scholar |
[50] C. B. Robinson, J. R. Kimmel, D. E. David, J. T. Jayne, A. Trimborn, D. R. Worsnop, J. L. Jimenez, Thermal desorption metastable atom bombardment ionization aerosol mass spectrometer. Int. J. Mass Spectrom. 2011, 303, 164.
| Thermal desorption metastable atom bombardment ionization aerosol mass spectrometer.Crossref | GoogleScholarGoogle Scholar |
[51] A. M. Middlebrook, R. Bahreini, J. L. Jimenez, M. R. Canagaratna, Evaluation of composition-dependent collection efficiencies for the Aerodyne aerosol mass spectrometer using field data. Aerosol Sci. Technol. 2012, 46, 258.
| Evaluation of composition-dependent collection efficiencies for the Aerodyne aerosol mass spectrometer using field data.Crossref | GoogleScholarGoogle Scholar |
[52] N. L. Ng, M. R. Canagaratna, J. L. Jimenez, P. S. Chhabra, J. H. Seinfeld, D. R. Worsnop, Changes in organic aerosol composition with aging inferred from aerosol mass spectra. Atmos. Chem. Phys. 2011, 11, 6465.
| Changes in organic aerosol composition with aging inferred from aerosol mass spectra.Crossref | GoogleScholarGoogle Scholar |
[53] N. L. Ng, M. R. Canagaratna, J. L. Jimenez, Q. Zhang, I. M. Ulbrich, D. R. Worsnop, Real-time methods for estimating organic component mass concentrations from aerosol mass spectrometer data. Environ. Sci. Technol. 2011, 45, 910.
| Real-time methods for estimating organic component mass concentrations from aerosol mass spectrometer data.Crossref | GoogleScholarGoogle Scholar |
[54] A. A. Mensah, A. Buchholz, T. F. Mentel, R. Tillmann, A. Kiendler-Scharr, Aerosol mass spectrometric measurements of stable crystal hydrates of oxalates and inferred relative ionization efficiency of water. J. Aerosol Sci. 2011, 42, 11.
| Aerosol mass spectrometric measurements of stable crystal hydrates of oxalates and inferred relative ionization efficiency of water.Crossref | GoogleScholarGoogle Scholar |
[55] N. L. Ng, S. C. Herndon, A. Trimborn, M. R. Canagaratna, P. L. Croteau, T. B. Onasch, D. Sueper, D. R. Worsnop, Q. Zhang, Y. L. Sun, J. T. Jayne, An aerosol chemical speciation monitor (ACSM) for routine monitoring of the composition and mass concentrations of ambient aerosol. Aerosol Sci. Technol. 2011, 45, 780.
| An aerosol chemical speciation monitor (ACSM) for routine monitoring of the composition and mass concentrations of ambient aerosol.Crossref | GoogleScholarGoogle Scholar |
[56] J. R. Kimmel, D. K. Farmer, M. J. Cubison, D. Sueper, C. Tanner, E. Nemitz, D. R. Worsnop, M. Gonin, J. L. Jimenez, Real-time aerosol mass spectrometry with millisecond resolution. Int. J. Mass Spectrom. 2011, 303, 15.
| Real-time aerosol mass spectrometry with millisecond resolution.Crossref | GoogleScholarGoogle Scholar |
[57] D. K. Farmer, J. R. Kimmel, G. Phillips, K. S. Docherty, D. R. Worsnop, D. Sueper, E. Nemitz, J. L. Jimenez, Eddy covariance measurements with high-resolution time-of-flight aerosol mass spectrometry: a new approach to chemically resolved aerosol fluxes. Atmos. Meas. Tech. 2011, 4, 1275.
| Eddy covariance measurements with high-resolution time-of-flight aerosol mass spectrometry: a new approach to chemically resolved aerosol fluxes.Crossref | GoogleScholarGoogle Scholar |
[58] M. Müller, C. George, B. D’Anna, Enhanced spectral analysis of C-TOF aerosol mass spectrometer data: iterative residual analysis and cumulative peak fitting. Int. J. Mass Spectrom. 2011, 306, 1.
| Enhanced spectral analysis of C-TOF aerosol mass spectrometer data: iterative residual analysis and cumulative peak fitting.Crossref | GoogleScholarGoogle Scholar |
[59] M. D. Zauscher, M. J. K. Moore, G. S. Lewis, S. V. Hering, K. A. Prather, Approach for measuring the chemistry of individual particles in the size range critical for cloud formation. Anal. Chem. 2011, 83, 2271.
| Approach for measuring the chemistry of individual particles in the size range critical for cloud formation.Crossref | GoogleScholarGoogle Scholar |
[60] S. Y. Wang, C. A. Zordan, M. V. Johnston, Chemical characterization of individual, airborne sub-10-nm particles and molecules. Anal. Chem. 2006, 78, 1750.
| Chemical characterization of individual, airborne sub-10-nm particles and molecules.Crossref | GoogleScholarGoogle Scholar |
[61] C. A. Zordan, M. R. Pennington, M. V. Johnston, Elemental composition of nanoparticles with the nano aerosol mass spectrometer. Anal. Chem. 2010, 82, 8034.
| Elemental composition of nanoparticles with the nano aerosol mass spectrometer.Crossref | GoogleScholarGoogle Scholar |
[62] J. P. Klems, C. A. Zordan, M. R. Pennington, M. V. Johnston, Chemical composition of ambient nanoparticles on a particle-by-particle basis. Anal. Chem. 2012, 84, 2253.
| Chemical composition of ambient nanoparticles on a particle-by-particle basis.Crossref | GoogleScholarGoogle Scholar |
[63] B. L. Yoder, J. H. Litman, P. W. Forysinski, J. L. Corbett, R. Signorell, Sizer for neutral weakly bound ultrafine aerosol particles based on sodium doping and mass spectrometric detection. J. Phys. Chem. Lett. 2011, 2, 2623.
| Sizer for neutral weakly bound ultrafine aerosol particles based on sodium doping and mass spectrometric detection.Crossref | GoogleScholarGoogle Scholar |
[64] N. J. D. González, A. K. Borg-Karlson, J. P. Redeby, B. Noziere, R. Krejci, Y. X. Pei, J. Dommen, A. S. H. Prevot, New method for resolving the enantiomeric composition of 2-methyltetrols in atmospheric organic aerosols. J. Chromatogr. A 2011, 1218, 9288.
| New method for resolving the enantiomeric composition of 2-methyltetrols in atmospheric organic aerosols.Crossref | GoogleScholarGoogle Scholar |
[65] J. Schnelle-Kreis, J. Orasche, G. Abbaszade, K. Schafer, D. P. Harlos, A. D. A. Hansen, R. Zimmermann, Application of direct thermal desorption gas chromatography time-of-flight mass spectrometry for determination of nonpolar organics in low-volume samples from ambient particulate matter and personal samplers. Anal. Bioanal. Chem. 2011, 401, 3083.
| Application of direct thermal desorption gas chromatography time-of-flight mass spectrometry for determination of nonpolar organics in low-volume samples from ambient particulate matter and personal samplers.Crossref | GoogleScholarGoogle Scholar |
[66] J. Orasche, J. Schnelle-Kreis, G. Abbaszade, R. Zimmermann, Technical Note: In-situ derivatization thermal desorption GC-TOFMS for direct analysis of particle-bound non-polar and polar organic species. Atmos. Chem. Phys. 2011, 11, 8977.
| Technical Note: In-situ derivatization thermal desorption GC-TOFMS for direct analysis of particle-bound non-polar and polar organic species.Crossref | GoogleScholarGoogle Scholar |
[67] K. Kowalewski, T. Gierczak, Multistep derivatization method for the determination of multifunctional oxidation products from the reaction of α-pinene with ozone. J. Chromatogr. A 2011, 1218, 7264.
| Multistep derivatization method for the determination of multifunctional oxidation products from the reaction of α-pinene with ozone.Crossref | GoogleScholarGoogle Scholar |
[68] M. Z. Özel, J. F. Hamilton, A. C. Lewis, New sensitive and quantitative analysis method for organic nitrogen compounds in urban aerosol samples. Environ. Sci. Technol. 2011, 45, 1497.
| New sensitive and quantitative analysis method for organic nitrogen compounds in urban aerosol samples.Crossref | GoogleScholarGoogle Scholar |
[69] D. R. Hanson, P. H. McMurry, J. Jiang, D. Tanner, L. G. Huey, Ambient pressure proton transfer mass spectrometry: detection of amines and ammonia. Environ. Sci. Technol. 2011, 45, 8881.
| Ambient pressure proton transfer mass spectrometry: detection of amines and ammonia.Crossref | GoogleScholarGoogle Scholar |
[70] Z. Kitanovski, I. Grgic, M. Veber, Characterization of carboxylic acids in atmospheric aerosols using hydrophilic interaction liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2011, 1218, 4417.
| Characterization of carboxylic acids in atmospheric aerosols using hydrophilic interaction liquid chromatography tandem mass spectrometry.Crossref | GoogleScholarGoogle Scholar |
[71] C. J. Kampf, B. Bonn, T. Hoffmann, Development and validation of a selective HPLC-ESI-MS/MS method for the quantification of glyoxal and methylglyoxal in atmospheric aerosols (PM2.5). Anal. Bioanal. Chem. 2011, 401, 3115.
| Development and validation of a selective HPLC-ESI-MS/MS method for the quantification of glyoxal and methylglyoxal in atmospheric aerosols (PM2.5).Crossref | GoogleScholarGoogle Scholar |
[72] S. Samy, J. Robinson, M. D. Hays, An advanced LC-MS (Q-TOF) technique for the detection of amino acids in atmospheric aerosols. Anal. Bioanal. Chem. 2011, 401, 3103.
| An advanced LC-MS (Q-TOF) technique for the detection of amino acids in atmospheric aerosols.Crossref | GoogleScholarGoogle Scholar |
[73] E. A. Bruns, V. Perraud, J. Greaves, B. J. Finlayson-Pitts, Atmospheric solids analysis probe mass spectrometry: a new approach for airborne particle analysis. Anal. Chem. 2010, 82, 5922.
| Atmospheric solids analysis probe mass spectrometry: a new approach for airborne particle analysis.Crossref | GoogleScholarGoogle Scholar |
[74] J. Laskin, A. Laskin, P. J. Roach, G. W. Slysz, G. A. Anderson, S. A. Nizkorodov, D. L. Bones, L. Q. Nguyen, High-resolution desorption electrospray ionization mass spectrometry for chemical characterization of organic aerosols. Anal. Chem. 2010, 82, 2048.
| High-resolution desorption electrospray ionization mass spectrometry for chemical characterization of organic aerosols.Crossref | GoogleScholarGoogle Scholar |
[75] P. J. Roach, J. Laskin, A. Laskin, Molecular characterization of organic aerosols using nanospray desorption/electrospray ionization mass spectrometry. Anal. Chem. 2010, 82, 7979.
| Molecular characterization of organic aerosols using nanospray desorption/electrospray ionization mass spectrometry.Crossref | GoogleScholarGoogle Scholar |
[76] P. J. Roach, J. Laskin, A. Laskin, Nanospray desorption electrospray ionization: an ambient method for liquid-extraction surface sampling in mass spectrometry. Analyst (Lond.) 2010, 135, 2233.
| Nanospray desorption electrospray ionization: an ambient method for liquid-extraction surface sampling in mass spectrometry.Crossref | GoogleScholarGoogle Scholar |
[77] T. B. Nguyen, P. B. Lee, K. M. Updyke, D. L. Bones, J. Laskin, A. Laskin, S. A. Nizkorodov, Formation of nitrogen- and sulfur-containing light-absorbing compounds accelerated by evaporation of water from secondary organic aerosols. J. Geophys. Res. – Atmos. 2012, 117, D01207.
| Formation of nitrogen- and sulfur-containing light-absorbing compounds accelerated by evaporation of water from secondary organic aerosols.Crossref | GoogleScholarGoogle Scholar |
[78] T. B. Nguyen, P. J. Roach, J. Laskin, A. Laskin, S. A. Nizkorodov, Effect of humidity on the composition of isoprene photooxidation secondary organic aerosol. Atmos. Chem. Phys. 2011, 11, 6931.
| Effect of humidity on the composition of isoprene photooxidation secondary organic aerosol.Crossref | GoogleScholarGoogle Scholar |
[79] B. J. Tyler, S. Dambach, S. Galla, R. E. Peterson, H. F. Arlinghaus, Investigation of the utility of laser-secondary neutral mass spectrometry for the detection of polyaromatic hydrocarbons in individual atmospheric aerosol particles. Anal. Chem. 2012, 84, 76.
| Investigation of the utility of laser-secondary neutral mass spectrometry for the detection of polyaromatic hydrocarbons in individual atmospheric aerosol particles.Crossref | GoogleScholarGoogle Scholar |
[80] P. Brender, R. Gadiou, J.-C. Rietsch, P. Fioux, J. Dentzer, A. Ponche, C. Vix-Guterl, Characterization of carbon surface chemistry by combined temperature programmed desorption with in situ X-ray photoelectron spectrometry and temperature programmed desorption with mass spectrometry analysis. Anal. Chem. 2012, 84, 2147.
| Characterization of carbon surface chemistry by combined temperature programmed desorption with in situ X-ray photoelectron spectrometry and temperature programmed desorption with mass spectrometry analysis.Crossref | GoogleScholarGoogle Scholar |
[81] S.-H. Lee, H. C. Allen, Analytical measurements of atmospheric urban aerosol. Anal. Chem. 2012, 84, 1196.
| Analytical measurements of atmospheric urban aerosol.Crossref | GoogleScholarGoogle Scholar |
[82] Q. Zhang, J. L. Jimenez, M. R. Canagaratna, I. M. Ulbrich, N. L. Ng, D. R. Worsnop, Y. L. Sun, Understanding atmospheric organic aerosols via factor analysis of aerosol mass spectrometry: a review. Anal. Bioanal. Chem. 2011, 401, 3045.
| Understanding atmospheric organic aerosols via factor analysis of aerosol mass spectrometry: a review.Crossref | GoogleScholarGoogle Scholar |
[83] I. M. Ulbrich, M. R. Canagaratna, M. J. Cubison, Q. Zhang, N. L. Ng, A. C. Aiken, J. L. Jimenez, Three-dimensional factorization of size-resolved organic aerosol mass spectra from Mexico City. Atmos. Meas. Tech. 2012, 5, 195.
| Three-dimensional factorization of size-resolved organic aerosol mass spectra from Mexico City.Crossref | GoogleScholarGoogle Scholar |
[84] L. Y. He, X. F. Huang, L. Xue, M. Hu, Y. Lin, J. Zheng, R. Y. Zhang, Y. H. Zhang, Submicron aerosol analysis and organic source apportionment in an urban atmosphere in Pearl River Delta of China using high-resolution aerosol mass spectrometry. J. Geophys. Res. – Atmos. 2011, 116, D12304.
| Submicron aerosol analysis and organic source apportionment in an urban atmosphere in Pearl River Delta of China using high-resolution aerosol mass spectrometry.Crossref | GoogleScholarGoogle Scholar |
[85] S. P. Hersey, J. S. Craven, K. A. Schilling, A. R. Metcalf, A. Sorooshian, M. N. Chan, R. C. Flagan, J. H. Seinfeld, The Pasadena Aerosol Characterization Observatory (PACO): chemical and physical analysis of the Western Los Angeles basin aerosol. Atmos. Chem. Phys. 2011, 11, 7417.
| The Pasadena Aerosol Characterization Observatory (PACO): chemical and physical analysis of the Western Los Angeles basin aerosol.Crossref | GoogleScholarGoogle Scholar |
[86] D. Liu, J. Allan, B. Corris, M. Flynn, E. Andrews, J. Ogren, K. Beswick, K. Bower, R. Burgess, T. Choularton, J. Dorsey, W. Morgan, P. I. Williams, H. Coe, Carbonaceous aerosols contributed by traffic and solid fuel burning at a polluted rural site in Northwestern England. Atmos. Chem. Phys. 2011, 11, 1603.
| Carbonaceous aerosols contributed by traffic and solid fuel burning at a polluted rural site in Northwestern England.Crossref | GoogleScholarGoogle Scholar |
[87] C. Mohr, R. Richter, P. F. DeCarlo, A. S. H. Prevot, U. Baltensperger, Spatial variation of chemical composition and sources of submicron aerosol in Zurich during wintertime using mobile aerosol mass spectrometer data. Atmos. Chem. Phys. 2011, 11, 7465.
| Spatial variation of chemical composition and sources of submicron aerosol in Zurich during wintertime using mobile aerosol mass spectrometer data.Crossref | GoogleScholarGoogle Scholar |
[88] A. Richard, M. F. D. Gianini, C. Mohr, M. Furger, N. Bukowiecki, M. C. Minguillon, P. Lienemann, U. Flechsig, K. Appel, P. F. DeCarlo, M. F. Heringa, R. Chirico, U. Baltensperger, A. S. H. Prevot, Source apportionment of size and time resolved trace elements and organic aerosols from an urban courtyard site in Switzerland. Atmos. Chem. Phys. 2011, 11, 8945.
| Source apportionment of size and time resolved trace elements and organic aerosols from an urban courtyard site in Switzerland.Crossref | GoogleScholarGoogle Scholar |
[89] J. G. Slowik, J. Brook, R. Y. W. Chang, G. J. Evans, K. Hayden, C. H. Jeong, S. M. Li, J. Liggio, P. S. K. Liu, M. McGuire, C. Mihele, S. Sjostedt, A. Vlasenko, J. P. D. Abbatt, Photochemical processing of organic aerosol at nearby continental sites: contrast between urban plumes and regional aerosol. Atmos. Chem. Phys. 2011, 11, 2991.
| Photochemical processing of organic aerosol at nearby continental sites: contrast between urban plumes and regional aerosol.Crossref | GoogleScholarGoogle Scholar |
[90] Y. L. Sun, Q. Zhang, J. J. Schwab, K. L. Demerjian, W. N. Chen, M. S. Bae, H. M. Hung, O. Hogrefe, B. Frank, O. V. Rattigan, Y. C. Lin, Characterization of the sources and processes of organic and inorganic aerosols in New York city with a high-resolution time-of-flight aerosol mass apectrometer. Atmos. Chem. Phys. 2011, 11, 1581.
| Characterization of the sources and processes of organic and inorganic aerosols in New York city with a high-resolution time-of-flight aerosol mass apectrometer.Crossref | GoogleScholarGoogle Scholar |
[91] L. Hildebrandt, E. Kostenidou, V. A. Lanz, A. S. H. Prevot, U. Baltensperger, N. Mihalopoulos, A. Laaksonen, N. M. Donahue, S. N. Pandis, Sources and atmospheric processing of organic aerosol in the Mediterranean: insights from aerosol mass spectrometer factor analysis. Atmos. Chem. Phys. 2011, 11, 12 499.
| Sources and atmospheric processing of organic aerosol in the Mediterranean: insights from aerosol mass spectrometer factor analysis.Crossref | GoogleScholarGoogle Scholar |
[92] X. F. Huang, L. Y. He, M. Hu, M. R. Canagaratna, J. H. Kroll, N. L. Ng, Y. H. Zhang, Y. Lin, L. Xue, T. L. Sun, X. G. Liu, M. Shao, J. T. Jayne, D. R. Worsnop, Characterization of submicron aerosols at a rural site in Pearl River Delta of China using an Aerodyne high-resolution aerosol mass spectrometer. Atmos. Chem. Phys. 2011, 11, 1865.
| Characterization of submicron aerosols at a rural site in Pearl River Delta of China using an Aerodyne high-resolution aerosol mass spectrometer.Crossref | GoogleScholarGoogle Scholar |
[93] Y. L. Sun, Q. Zhang, M. Zheng, X. Ding, E. S. Edgerton, X. M. Wang, Characterization and source apportionment of water-soluble organic matter in atmospheric fine particles (PM2.5) with high-resolution aerosol mass spectrometry and GC-MS. Environ. Sci. Technol. 2011, 45, 4854.
| Characterization and source apportionment of water-soluble organic matter in atmospheric fine particles (PM2.5) with high-resolution aerosol mass spectrometry and GC-MS.Crossref | GoogleScholarGoogle Scholar |
[94] S. M. Li, J. Liggio, L. Graham, G. Lu, J. Brook, C. Stroud, J. Zhang, P. Makar, M. D. Moran, Condensational uptake of semivolatile organic compounds in gasoline engine exhaust onto pre-existing inorganic particles. Atmos. Chem. Phys. 2011, 11, 10 157.
| Condensational uptake of semivolatile organic compounds in gasoline engine exhaust onto pre-existing inorganic particles.Crossref | GoogleScholarGoogle Scholar |
[95] M. A. Miracolo, C. J. Hennigan, M. Ranjan, N. T. Nguyen, T. D. Gordon, E. M. Lipsky, A. A. Presto, N. M. Donahue, A. L. Robinson, Secondary aerosol formation from photochemical aging of aircraft exhaust in a smog chamber. Atmos. Chem. Phys. 2011, 11, 4135.
| Secondary aerosol formation from photochemical aging of aircraft exhaust in a smog chamber.Crossref | GoogleScholarGoogle Scholar |
[96] S. Nakao, M. Shrivastava, A. Nguyen, H. J. Jung, D. Cocker, Interpretation of secondary organic aerosol formation from diesel exhaust photooxidation in an environmental chamber. Aerosol Sci. Technol. 2011, 45, 964.
| Interpretation of secondary organic aerosol formation from diesel exhaust photooxidation in an environmental chamber.Crossref | GoogleScholarGoogle Scholar |
[97] A. A. Presto, N. T. Nguyen, M. Ranjan, A. J. Reeder, E. M. Lipsky, C. J. Hennigan, M. A. Miracolo, D. D. Riemer, A. L. Robinson, Fine particle and organic vapor emissions from staged tests of an in-use aircraft engine. Atmos. Environ. 2011, 45, 3603.
| Fine particle and organic vapor emissions from staged tests of an in-use aircraft engine.Crossref | GoogleScholarGoogle Scholar |
[98] M. Ranjan, A. A. Presto, A. A. May, A. L. Robinson, Temperature dependence of gas-particle partitioning of primary organic aerosol emissions from a small diesel engine. Aerosol Sci. Technol. 2012, 46, 13.
| Temperature dependence of gas-particle partitioning of primary organic aerosol emissions from a small diesel engine.Crossref | GoogleScholarGoogle Scholar |
[99] L. E. Hatch, J. M. Creamean, A. P. Ault, J. D. Surratt, M. N. Chan, J. H. Seinfeld, E. S. Edgerton, Y. X. Su, K. A. Prather, Measurements of isoprene-derived organosulfates in ambient aerosols by aerosol time-of-flight mass spectrometry – Part 1. Single particle atmospheric observations in Atlanta. Environ. Sci. Technol. 2011, 45, 5105.
| Measurements of isoprene-derived organosulfates in ambient aerosols by aerosol time-of-flight mass spectrometry – Part 1. Single particle atmospheric observations in Atlanta.Crossref | GoogleScholarGoogle Scholar |
[100] L. E. Hatch, J. M. Creamean, A. P. Ault, J. D. Surratt, M. N. Chan, J. H. Seinfeld, E. S. Edgerton, Y. X. Su, K. A. Prather, Measurements of isoprene-derived organosulfates in ambient aerosols by aerosol time-of-flight mass spectrometry – Part 2. Temporal variability and formation Mechanisms. Environ. Sci. Technol. 2011, 45, 8648.
| Measurements of isoprene-derived organosulfates in ambient aerosols by aerosol time-of-flight mass spectrometry – Part 2. Temporal variability and formation Mechanisms.Crossref | GoogleScholarGoogle Scholar |
[101] P. J. G. Rehbein, C. H. Jeong, M. L. McGuire, X. H. Yao, J. C. Corbin, G. J. Evans, Cloud and fog processing enhanced gas-to-particle partitioning of trimethylamine. Environ. Sci. Technol. 2011, 45, 4346.
| Cloud and fog processing enhanced gas-to-particle partitioning of trimethylamine.Crossref | GoogleScholarGoogle Scholar |
[102] S. K. Tao, X. N. Wang, H. Chen, X. Yang, M. Li, L. Li, Z. Zhou, Single particle analysis of ambient aerosols in Shanghai during the World Exposition, 2010: two case studies. Front. Environ. Sci. Eng. China 2011, 5, 391.
| Single particle analysis of ambient aerosols in Shanghai during the World Exposition, 2010: two case studies.Crossref | GoogleScholarGoogle Scholar |
[103] M. L. McGuire, C. H. Jeong, J. G. Slowik, R. Y. W. Chang, J. C. Corbin, G. Lu, C. Mihele, P. J. G. Rehbein, D. M. L. Sills, J. P. D. Abbatt, J. R. Brook, G. J. Evans, Elucidating determinants of aerosol composition through particle_type-based receptor modeling. Atmos. Chem. Phys. 2011, 11, 8133.
| Elucidating determinants of aerosol composition through particle_type-based receptor modeling.Crossref | GoogleScholarGoogle Scholar |
[104] X. H. Yao, P. J. G. Rehbein, C. J. Lee, G. J. Evans, J. Corbin, C. H. Jeong, A study on the extent of neutralization of sulphate aerosol through laboratory and field experiments using an ATOFMS and a GPIC. Atmos. Environ. 2011, 45, 6251.
| A study on the extent of neutralization of sulphate aerosol through laboratory and field experiments using an ATOFMS and a GPIC.Crossref | GoogleScholarGoogle Scholar |
[105] J. H. Xing, K. Takahashi, A. Yabushita, T. Kinugawa, T. Nakayama, Y. Matsumi, K. Tonokura, A. Takami, T. Imamura, K. Sato, M. Kawasaki, T. Hikida, A. Shimono, Characterization of aerosol particles in the Tokyo metropolitan area using two different particle mass spectrometers. Aerosol Sci. Technol. 2011, 45, 315.
| Characterization of aerosol particles in the Tokyo metropolitan area using two different particle mass spectrometers.Crossref | GoogleScholarGoogle Scholar |
[106] E. Barbaro, R. Zangrando, I. Moret, C. Barbante, P. Cescon, A. Gambaro, Free amino acids in atmospheric particulate matter of Venice, Italy. Atmos. Environ. 2011, 45, 5050.
| Free amino acids in atmospheric particulate matter of Venice, Italy.Crossref | GoogleScholarGoogle Scholar |
[107] M. S. Callén, J. M. López, A. M. Mastral, Characterization of PM10-bound polycyclic aromatic hydrocarbons in the ambient air of Spanish urban and rural areas. J. Environ. Monit. 2011, 13, 319.
| Characterization of PM10-bound polycyclic aromatic hydrocarbons in the ambient air of Spanish urban and rural areas.Crossref | GoogleScholarGoogle Scholar |
[108] H. Yu, S. H. Lee, Development of chemical ionization mass spectrometry for the measurement of atmospheric amines. Environ. Chem. 2012, 9, 190.
| Development of chemical ionization mass spectrometry for the measurement of atmospheric amines.Crossref | GoogleScholarGoogle Scholar |
[109] M. Zheng, Y. Cheng, L. M. Zeng, Y. H. Zhang, Developing chemical signatures of particulate air pollution in the Pearl River Delta region, China. J. Environ. Sci. (China) 2011, 23, 1143.
| Developing chemical signatures of particulate air pollution in the Pearl River Delta region, China.Crossref | GoogleScholarGoogle Scholar |
[110] M. Zheng, F. Wang, G. S. W. Hagler, X. M. Hou, M. Bergin, Y. A. Cheng, L. G. Salmon, J. J. Schauer, P. K. K. Louie, L. M. Zeng, Y. H. Zhang, Sources of excess urban carbonaceous aerosol in the Pearl River Delta Region, China. Atmos. Environ. 2011, 45, 1175.
| Sources of excess urban carbonaceous aerosol in the Pearl River Delta Region, China.Crossref | GoogleScholarGoogle Scholar |
[111] S. Szidat, Radiocarbon analysis of carbonaceous aerosols: recent developments. Chimia (Aarau) 2009, 63, 157.
| Radiocarbon analysis of carbonaceous aerosols: recent developments.Crossref | GoogleScholarGoogle Scholar |
[112] M. R. Heal, P. Naysmith, G. T. Cook, S. Xu, T. R. Duran, R. M. Harrison, Application of 14C analyses to source apportionment of carbonaceous PM2.5 in the UK. Atmos. Environ. 2011, 45, 2341.
| Application of 14C analyses to source apportionment of carbonaceous PM2.5 in the UK.Crossref | GoogleScholarGoogle Scholar |
[113] I. El Haddad, N. Marchand, B. Temime-Roussel, H. Wortham, C. Piot, J. L. Besombes, C. Baduel, D. Voisin, A. Armengaud, J. L. Jaffrezo, Insights into the secondary fraction of the organic aerosol in a Mediterranean urban area: Marseille. Atmos. Chem. Phys. 2011, 11, 2059.
| Insights into the secondary fraction of the organic aerosol in a Mediterranean urban area: Marseille.Crossref | GoogleScholarGoogle Scholar |
[114] I. El Haddad, N. Marchand, H. Wortham, C. Piot, J. L. Besombes, J. Cozic, C. Chauvel, A. Armengaud, D. Robin, J. L. Jaffrezo, Primary sources of PM2.5 organic aerosol in an industrial Mediterranean city, Marseille. Atmos. Chem. Phys. 2011, 11, 2039.
| Primary sources of PM2.5 organic aerosol in an industrial Mediterranean city, Marseille.Crossref | GoogleScholarGoogle Scholar |
[115] M. Glasius, A. la Cour, C. Lohse, Fossil and nonfossil carbon in fine particulate matter: a study of five European cities. J. Geophys. Res. – Atmos. 2011, 116, D11302.
| Fossil and nonfossil carbon in fine particulate matter: a study of five European cities.Crossref | GoogleScholarGoogle Scholar |
[116] A. S. Wozniak, J. E. Bauer, R. M. Dickhut, Characteristics of water-soluble organic carbon associated with aerosol particles in the eastern United States. Atmos. Environ. 2012, 46, 181.
| Characteristics of water-soluble organic carbon associated with aerosol particles in the eastern United States.Crossref | GoogleScholarGoogle Scholar |
[117] A. A. Frossard, P. M. Shaw, L. M. Russell, J. H. Kroll, M. R. Canagaratna, D. R. Worsnop, P. K. Quinn, T. S. Bates, Springtime Arctic haze contributions of submicron organic particles from European and Asian combustion sources. J. Geophys. Res. – Atmos. 2011, 116, D05205.
| Springtime Arctic haze contributions of submicron organic particles from European and Asian combustion sources.Crossref | GoogleScholarGoogle Scholar |
[118] H. Furutani, J. Y. Jung, K. Miura, A. Takami, S. Kato, Y. Kajii, M. Uematsu, Single-particle chemical characterization and source apportionment of iron-containing atmospheric aerosols in Asian outflow. J. Geophys. Res. – Atmos. 2011, 116, D18204.
| Single-particle chemical characterization and source apportionment of iron-containing atmospheric aerosols in Asian outflow.Crossref | GoogleScholarGoogle Scholar |
[119] B. Quennehen, A. Schwarzenboeck, J. Schmale, J. Schneider, H. Sodemann, A. Stohl, G. Ancellet, S. Crumeyrolle, K. S. Law, Physical and chemical properties of pollution aerosol particles transported from North America to Greenland as measured during the POLARCAT summer campaign. Atmos. Chem. Phys. 2011, 11, 10947.
| Physical and chemical properties of pollution aerosol particles transported from North America to Greenland as measured during the POLARCAT summer campaign.Crossref | GoogleScholarGoogle Scholar |
[120] D. R. Worton, A. H. Goldstein, D. K. Farmer, K. S. Docherty, J. L. Jimenez, J. B. Gilman, W. C. Kuster, J. de Gouw, B. J. Williams, N. M. Kreisberg, S. V. Hering, G. Bench, M. McKay, K. Kristensen, M. Glasius, J. D. Surratt, J. H. Seinfeld, Origins and composition of fine atmospheric carbonaceous aerosol in the Sierra Nevada Mountains, California. Atmos. Chem. Phys. 2011, 11, 10 219.
| Origins and composition of fine atmospheric carbonaceous aerosol in the Sierra Nevada Mountains, California.Crossref | GoogleScholarGoogle Scholar |
[121] C. A. Alves, A. Vicente, C. Monteiro, C. Gonçalves, M. Evtyugina, C. Pio, Emission of trace gases and organic components in smoke particles from a wildfire in a mixed-evergreen forest in Portugal. Sci. Total Environ. 2011, 409, 1466.
| Emission of trace gases and organic components in smoke particles from a wildfire in a mixed-evergreen forest in Portugal.Crossref | GoogleScholarGoogle Scholar |
[122] M. J. Cubison, A. M. Ortega, P. L. Hayes, D. K. Farmer, D. Day, M. J. Lechner, W. H. Brune, E. Apel, G. S. Diskin, J. A. Fisher, H. E. Fuelberg, A. Hecobian, D. J. Knapp, T. Mikoviny, D. Riemer, G. W. Sachse, W. Sessions, R. J. Weber, A. J. Weinheimer, A. Wisthaler, J. L. Jimenez, Effects of aging on organic aerosol from open biomass burning smoke in aircraft and laboratory studies. Atmos. Chem. Phys. 2011, 11, 12049.
| Effects of aging on organic aerosol from open biomass burning smoke in aircraft and laboratory studies.Crossref | GoogleScholarGoogle Scholar |
[123] A. Wonaschütz, S. P. Hersey, A. Sorooshian, J. S. Craven, A. R. Metcalf, R. C. Flagan, J. H. Seinfeld, Impact of a large wildfire on water-soluble organic aerosol in a major urban area: the 2009 Station Fire in Los Angeles County. Atmos. Chem. Phys. 2011, 11, 8257.
| Impact of a large wildfire on water-soluble organic aerosol in a major urban area: the 2009 Station Fire in Los Angeles County.Crossref | GoogleScholarGoogle Scholar |
[124] A. L. Chang-Graham, L. T. M. Profeta, T. J. Johnson, R. J. Yokelson, A. Laskin, J. Laskin, Case study of water-soluble metal containing organic constituents of biomass burning aerosol. Environ. Sci. Technol. 2011, 45, 1257.
| Case study of water-soluble metal containing organic constituents of biomass burning aerosol.Crossref | GoogleScholarGoogle Scholar |
[125] C. Gonçalves, C. Alves, A. P. Fernandes, C. Monteiro, L. Tarelho, M. Evtyugina, C. Pio, Organic compounds in PM2.5 emitted from fireplace and woodstove combustion of typical Portuguese wood species. Atmos. Environ. 2011, 45, 4533.
| Organic compounds in PM2.5 emitted from fireplace and woodstove combustion of typical Portuguese wood species.Crossref | GoogleScholarGoogle Scholar |
[126] C. Gonçalves, M. Evtyugina, C. Alves, C. Monteiro, C. Pio, M. Tomé, Organic particulate emissions from field burning of garden and agriculture residues. Atmos. Res. 2011, 101, 666.
| Organic particulate emissions from field burning of garden and agriculture residues.Crossref | GoogleScholarGoogle Scholar |
[127] C. J. Hennigan, M. A. Miracolo, G. J. Engelhart, A. A. May, A. A. Presto, T. Lee, A. P. Sullivan, G. R. McMeeking, H. Coe, C. E. Wold, W. M. Hao, J. B. Gilman, W. C. Kuster, J. de Gouw, B. A. Schichtel, J. L. Collett, S. M. Kreidenweis, A. L. Robinson, Chemical and physical transformations of organic aerosol from the photo-oxidation of open biomass burning emissions in an environmental chamber. Atmos. Chem. Phys. 2011, 11, 7669.
| Chemical and physical transformations of organic aerosol from the photo-oxidation of open biomass burning emissions in an environmental chamber.Crossref | GoogleScholarGoogle Scholar |
[128] M. D. Hays, B. Gullett, C. King, J. Robinson, W. Preston, A. Touati, Characterization of carbonaceous aerosols emitted from outdoor wood boilers. Energy Fuels 2011, 25, 5632.
| Characterization of carbonaceous aerosols emitted from outdoor wood boilers.Crossref | GoogleScholarGoogle Scholar |
[129] M. F. Heringa, P. F. DeCarlo, R. Chirico, T. Tritscher, J. Dommen, E. Weingartner, R. Richter, G. Wehrle, A. S. H. Prevot, U. Baltensperger, Investigations of primary and secondary particulate matter of different wood combustion appliances with a high-resolution time-of-flight aerosol mass spectrometer. Atmos. Chem. Phys. 2011, 11, 5945.
| Investigations of primary and secondary particulate matter of different wood combustion appliances with a high-resolution time-of-flight aerosol mass spectrometer.Crossref | GoogleScholarGoogle Scholar |
[130] A. P. Bateman, S. A. Nizkorodov, J. Laskin, A. Laskin, High-resolution electrospray ionization mass spectrometry analysis of water-soluble organic aerosols collected with a particle into liquid sampler. Anal. Chem. 2010, 82, 8010.
| High-resolution electrospray ionization mass spectrometry analysis of water-soluble organic aerosols collected with a particle into liquid sampler.Crossref | GoogleScholarGoogle Scholar |
[131] Y. Iinuma, O. Boge, R. Grafe, H. Herrmann, Methyl-nitrocatechols: atmospheric tracer compounds for biomass burning secondary organic aerosols. Environ. Sci. Technol. 2010, 44, 8453.
| Methyl-nitrocatechols: atmospheric tracer compounds for biomass burning secondary organic aerosols.Crossref | GoogleScholarGoogle Scholar |
[132] S. J. Sjostedt, J. G. Slowik, J. R. Brook, R. Y. W. Chang, C. Mihele, C. A. Stroud, A. Vlasenko, J. P. D. Abbatt, Diurnally resolved particulate and VOC measurements at a rural site: indication of significant biogenic secondary organic aerosol formation. Atmos. Chem. Phys. 2011, 11, 5745.
| Diurnally resolved particulate and VOC measurements at a rural site: indication of significant biogenic secondary organic aerosol formation.Crossref | GoogleScholarGoogle Scholar |
[133] M. Gordon, R. M. Staebler, J. Liggio, A. Vlasenko, S. M. Li, K. Hayden, Aerosol flux measurements above a mixed forest at Borden, Ontario. Atmos. Chem. Phys. 2011, 11, 6773.
| Aerosol flux measurements above a mixed forest at Borden, Ontario.Crossref | GoogleScholarGoogle Scholar |
[134] K. Kristensen, M. Glasius, Organosulfates and oxidation products from biogenic hydrocarbons in fine aerosols from a forest in North West Europe during spring. Atmos. Environ. 2011, 45, 4546.
| Organosulfates and oxidation products from biogenic hydrocarbons in fine aerosols from a forest in North West Europe during spring.Crossref | GoogleScholarGoogle Scholar |
[135] W. R. Leaitch, A. M. Macdonald, P. C. Brickell, J. Liggio, S. J. Sjostedt, A. Vlasenko, J. W. Bottenheim, L. Huang, S. M. Li, P. S. K. Liu, D. Toom-Sauntry, K. A. Hayden, S. Sharma, N. C. Shantz, H. A. Wiebe, W. D. Zhang, J. P. D. Abbatt, J. G. Slowik, R. Y. W. Chang, L. M. Russell, R. E. Schwartz, S. Takahama, J. T. Jayne, N. L. Ng, Temperature response of the submicron organic aerosol from temperate forests. Atmos. Environ. 2011, 45, 6696.
| Temperature response of the submicron organic aerosol from temperate forests.Crossref | GoogleScholarGoogle Scholar |
[136] N. H. Robinson, J. F. Hamilton, J. D. Allan, B. Langford, D. E. Oram, Q. Chen, K. Docherty, D. K. Farmer, J. L. Jimenez, M. W. Ward, C. N. Hewitt, M. H. Barley, M. E. Jenkin, A. R. Rickard, S. T. Martin, G. McFiggans, H. Coe, Evidence for a significant proportion of secondary organic aerosol from isoprene above a maritime tropical forest. Atmos. Chem. Phys. 2011, 11, 1039.
| Evidence for a significant proportion of secondary organic aerosol from isoprene above a maritime tropical forest.Crossref | GoogleScholarGoogle Scholar |
[137] J. Schneider, F. Freutel, S. R. Zorn, Q. Chen, D. K. Farmer, J. L. Jimenez, S. T. Martin, P. Artaxo, A. Wiedensohler, S. Borrmann, Mass- spectrometric identification of primary biological particle markers and application to pristine submicron aerosol measurements in Amazonia. Atmos. Chem. Phys. 2011, 11, 11 415.
| Mass- spectrometric identification of primary biological particle markers and application to pristine submicron aerosol measurements in Amazonia.Crossref | GoogleScholarGoogle Scholar |
[138] F. Yasmeen, R. Szmigielski, R. Vermeylen, Y. Gómez-González, J. D. Surratt, A. W. H. Chan, J. H. Seinfeld, W. Maenhaut, M. Claeys, Mass spectrometric characterization of isomeric terpenoic acids from the oxidation of α-pinene, β-pinene, d-limonene, and Δ3-carene in fine forest aerosol. J. Mass Spectrom. 2011, 46, 425.
| Mass spectrometric characterization of isomeric terpenoic acids from the oxidation of α-pinene, β-pinene, d-limonene, and Δ3-carene in fine forest aerosol.Crossref | GoogleScholarGoogle Scholar |
[139] S. Decesari, E. Finessi, M. Rinaldi, M. Paglione, S. Fuzzi, E. G. Stephanou, T. Tziaras, A. Spyros, D. Ceburnis, C. O’Dowd, M. Dall’Osto, R. M. Harrison, J. Allan, H. Coe, M. C. Facchini, Primary and secondary marine organic aerosols over the North Atlantic Ocean during the MAP experiment. J. Geophys. Res. – Atmos. 2011, 116, D22210.
| Primary and secondary marine organic aerosols over the North Atlantic Ocean during the MAP experiment.Crossref | GoogleScholarGoogle Scholar |
[140] P. Q. Fu, K. Kawamura, K. Miura, Molecular characterization of marine organic aerosols collected during a round-the-world cruise. J. Geophys. Res. – Atmos. 2011, 116,
| Molecular characterization of marine organic aerosols collected during a round-the-world cruise.Crossref | GoogleScholarGoogle Scholar |
[141] C. J. Gaston, H. Furutani, S. A. Guazzotti, K. R. Coffee, T. S. Bates, P. K. Quinn, L. I. Aluwihare, B. G. Mitchell, K. A. Prather, Unique ocean-derived particles serve as a proxy for changes in ocean chemistry. J. Geophys. Res. – Atmos. 2011, 116, D18310.
| Unique ocean-derived particles serve as a proxy for changes in ocean chemistry.Crossref | GoogleScholarGoogle Scholar |
[142] J. Ovadnevaite, C. O’Dowd, M. Dall’Osto, D. Ceburnis, D. R. Worsnop, H. Berresheim, Detecting high contributions of primary organic matter to marine aerosol: a case study. Geophys. Res. Lett. 2011, 38, L02807.
| Detecting high contributions of primary organic matter to marine aerosol: a case study.Crossref | GoogleScholarGoogle Scholar |
[143] Y. Gómez-González, W. Wang, R. Vermeylen, X. Chi, J. Neirynck, I. A. Janssens, W. Maenhaut, M. Claeys, Chemical characterisation of atmospheric aerosols during a 2007 summer field campaign at Brasschaat, Belgium: sources and source processes of biogenic secondary organic aerosol. Atmos. Chem. Phys. 2012, 12, 125.
| Chemical characterisation of atmospheric aerosols during a 2007 summer field campaign at Brasschaat, Belgium: sources and source processes of biogenic secondary organic aerosol.Crossref | GoogleScholarGoogle Scholar |
[144] K. E. Yttri, D. Simpson, J. K. Nojgaard, K. Kristensen, J. Genberg, K. Stenstrom, E. Swietlicki, R. Hillamo, M. Aurela, H. Bauer, J. H. Offenberg, M. Jaoui, C. Dye, S. Eckhardt, J. F. Burkhart, A. Stohl, M. Glasius, Source apportionment of the summer time carbonaceous aerosol at Nordic rural background sites. Atmos. Chem. Phys. 2011, 11, 13 339.
| Source apportionment of the summer time carbonaceous aerosol at Nordic rural background sites.Crossref | GoogleScholarGoogle Scholar |
[145] V. P. Kanawade, B. T. Jobson, A. B. Guenther, M. E. Erupe, S. N. Pressley, S. N. Tripathi, S. H. Lee, Isoprene suppression of new particle formation in a mixed deciduous forest. Atmos. Chem. Phys. 2011, 11, 6013.
| Isoprene suppression of new particle formation in a mixed deciduous forest.Crossref | GoogleScholarGoogle Scholar |
[146] K. Lehtipalo, M. Sipila, H. Junninen, M. Ehn, T. Berndt, M. K. Kajos, D. R. Worsnop, T. Petaja, M. Kulmala, Observations of nano-CN in the nocturnal boreal forest. Aerosol Sci. Technol. 2011, 45, 499.
| Observations of nano-CN in the nocturnal boreal forest.Crossref | GoogleScholarGoogle Scholar |
[147] J. Zhao, J. N. Smith, F. L. Eisele, M. Chen, C. Kuang, P. H. McMurry, Observation of neutral sulfuric acid-amine containing clusters in laboratory and ambient measurements. Atmos. Chem. Phys. 2011, 11, 10 823.
| Observation of neutral sulfuric acid-amine containing clusters in laboratory and ambient measurements.Crossref | GoogleScholarGoogle Scholar |
[148] A. Kiendler-Scharr, J. Wildt, M. Dal Maso, T. Hohaus, E. Kleist, T. F. Mentel, R. Tillmann, R. Uerlings, U. Schurr, A. Wahner, New particle formation in forests inhibited by isoprene emissions. Nature 2009, 461, 381.
| New particle formation in forests inhibited by isoprene emissions.Crossref | GoogleScholarGoogle Scholar |
[149] H. Junninen, M. Ehn, T. Petäjä, L. Luosujärvi, T. Kotiaho, R. Kostiainen, U. Rohner, M. Gonin, K. Fuhrer, M. Kulmala, D. R. Worsnop, A high-resolution mass spectrometer to measure atmospheric ion composition. Atmos. Meas. Tech. 2010, 3, 1039.
| A high-resolution mass spectrometer to measure atmospheric ion composition.Crossref | GoogleScholarGoogle Scholar |
[150] J. M. Creamean, A. P. Ault, J. E. Ten Hoeve, M. Z. Jacobson, G. C. Roberts, K. A. Prather, Measurements of aerosol chemistry during new particle formation events at a remote rural mountain site. Environ. Sci. Technol. 2011, 45, 8208.
| Measurements of aerosol chemistry during new particle formation events at a remote rural mountain site.Crossref | GoogleScholarGoogle Scholar |
[151] T. Laitinen, M. Ehn, H. Junninen, J. Ruiz-Jimenez, J. Parshintsev, K. Hartonen, M. L. Riekkola, D. R. Worsnop, M. Kulmala, Characterization of organic compounds in 10-to 50-nm aerosol particles in boreal forest with laser desorption-ionization aerosol mass spectrometer and comparison with other techniques. Atmos. Environ. 2011, 45, 3711.
| Characterization of organic compounds in 10-to 50-nm aerosol particles in boreal forest with laser desorption-ionization aerosol mass spectrometer and comparison with other techniques.Crossref | GoogleScholarGoogle Scholar |
[152] B. R. Bzdek, C. A. Zordan, G. W. Luther, M. V. Johnston, Nanoparticle chemical composition during new particle formation. Aerosol Sci. Technol. 2011, 45, 1041.
| Nanoparticle chemical composition during new particle formation.Crossref | GoogleScholarGoogle Scholar |
[153] M. D. Petters, S. M. Kreidenweis, A single parameter representation of hygroscopic growth and cloud condensation nucleus activity. Atmos. Chem. Phys. 2007, 7, 1961.
| A single parameter representation of hygroscopic growth and cloud condensation nucleus activity.Crossref | GoogleScholarGoogle Scholar |
[154] A. Asa-Awuku, R. H. Moore, A. Nenes, R. Bahreini, J. S. Holloway, C. A. Brock, A. M. Middlebrook, T. B. Ryerson, J. L. Jimenez, P. F. DeCarlo, A. Hecobian, R. J. Weber, R. Stickel, D. J. Tanner, L. G. Huey, Airborne cloud condensation nuclei measurements during the 2006 Texas Air Quality Study. J. Geophys. Res. – Atmos. 2011, 116, D11201.
| Airborne cloud condensation nuclei measurements during the 2006 Texas Air Quality Study.Crossref | GoogleScholarGoogle Scholar |
[155] K. M. Cerully, T. Raatikainen, S. Lance, D. Tkacik, P. Tiitta, T. Petaja, M. Ehn, M. Kulmala, D. R. Worsnop, A. Laaksonen, J. N. Smith, A. Nenes, Aerosol hygroscopicity and CCN activation kinetics in a boreal forest environment during the 2007 EUCAARI campaign. Atmos. Chem. Phys. 2011, 11, 12 369.
| Aerosol hygroscopicity and CCN activation kinetics in a boreal forest environment during the 2007 EUCAARI campaign.Crossref | GoogleScholarGoogle Scholar |
[156] S. S. Gunthe, D. Rose, H. Su, R. M. Garland, P. Achtert, A. Nowak, A. Wiedensohler, M. Kuwata, N. Takegawa, Y. Kondo, M. Hu, M. Shao, T. Zhu, M. O. Andreae, U. Pöschl, Cloud condensation nuclei (CCN) from fresh and aged air pollution in the megacity region of Beijing. Atmos. Chem. Phys. 2011, 11, 11 023.
| Cloud condensation nuclei (CCN) from fresh and aged air pollution in the megacity region of Beijing.Crossref | GoogleScholarGoogle Scholar |
[157] C. L. Martin, J. D. Allan, J. Crosier, T. W. Choularton, H. Coe, M. W. Gallagher, Seasonal variation of fine particulate composition in the centre of a UK city. Atmos. Environ. 2011, 45, 4379.
| Seasonal variation of fine particulate composition in the centre of a UK city.Crossref | GoogleScholarGoogle Scholar |
[158] M. Martin, R. Y. W. Chang, B. Sierau, S. Sjogren, E. Swietlicki, J. P. D. Abbatt, C. Leck, U. Lohmann, Cloud condensation nuclei closure study on summer arctic aerosol. Atmos. Chem. Phys. 2011, 11, 11 335.
| Cloud condensation nuclei closure study on summer arctic aerosol.Crossref | GoogleScholarGoogle Scholar |
[159] R. H. Moore, R. Bahreini, C. A. Brock, K. D. Froyd, J. Cozic, J. S. Holloway, A. M. Middlebrook, D. M. Murphy, A. Nenes, Hygroscopicity and composition of Alaskan Arctic CCN during April 2008. Atmos. Chem. Phys. 2011, 11, 11 807.
| Hygroscopicity and composition of Alaskan Arctic CCN during April 2008.Crossref | GoogleScholarGoogle Scholar |
[160] D. Rose, S. S. Gunthe, H. Su, R. M. Garland, H. Yang, M. Berghof, Y. F. Cheng, B. Wehner, P. Achtert, A. Nowak, A. Wiedensohler, N. Takegawa, Y. Kondo, M. Hu, Y. Zhang, M. O. Andreae, U. Pöschl, Cloud condensation nuclei in polluted air and biomass burning smoke near the mega-city Guangzhou, China – Part 2. Size-resolved aerosol chemical composition, diurnal cycles, and externally mixed weakly CCN-active soot particles. Atmos. Chem. Phys. 2011, 11, 2817.
| Cloud condensation nuclei in polluted air and biomass burning smoke near the mega-city Guangzhou, China – Part 2. Size-resolved aerosol chemical composition, diurnal cycles, and externally mixed weakly CCN-active soot particles.Crossref | GoogleScholarGoogle Scholar |
[161] C. M. Berkowitz, L. K. Berg, X. Y. Yu, M. L. Alexander, A. Laskin, R. A. Zaveri, B. T. Jobson, E. Andrews, J. A. Ogren, The influence of fog and airmass history on aerosol optical, physical and chemical properties at Pt. Reyes National Seashore. Atmos. Environ. 2011, 45, 2559.
| The influence of fog and airmass history on aerosol optical, physical and chemical properties at Pt. Reyes National Seashore.Crossref | GoogleScholarGoogle Scholar |
[162] T. Mihara, M. Mochida, Characterization of solvent-extractable organics in urban aerosols based on mass spectrum analysis and hygroscopic growth measurement. Environ. Sci. Technol. 2011, 45, 9168.
| Characterization of solvent-extractable organics in urban aerosols based on mass spectrum analysis and hygroscopic growth measurement.Crossref | GoogleScholarGoogle Scholar |
[163] J. G. Slowik, D. J. Cziczo, J. P. D. Abbatt, Analysis of cloud condensation nuclei composition and growth kinetics using a pumped counterflow virtual impactor and aerosol mass spectrometer. Atmos. Meas. Tech. 2011, 4, 1677.
| Analysis of cloud condensation nuclei composition and growth kinetics using a pumped counterflow virtual impactor and aerosol mass spectrometer.Crossref | GoogleScholarGoogle Scholar |
[164] A. Zelenyuk, D. Imre, M. Earle, R. Easter, A. Korolev, R. Leaitch, P. Liu, A. M. Macdonald, M. Ovchinnikov, W. Strapp, In situ characterization of cloud condensation nuclei, interstitial, and background particles using the single particle mass spectrometer, SPLAT II. Anal. Chem. 2010, 82, 7943.
| In situ characterization of cloud condensation nuclei, interstitial, and background particles using the single particle mass spectrometer, SPLAT II.Crossref | GoogleScholarGoogle Scholar |
[165] G. R. McMeeking, W. T. Morgan, M. Flynn, E. J. Highwood, K. Turnbull, J. Haywood, H. Coe, Black carbon aerosol mixing state, organic aerosols and aerosol optical properties over the United Kingdom. Atmos. Chem. Phys. 2011, 11, 9037.
| Black carbon aerosol mixing state, organic aerosols and aerosol optical properties over the United Kingdom.Crossref | GoogleScholarGoogle Scholar |
[166] T. W. Chan, J. R. Brook, G. J. Smallwood, G. Lu, Time-resolved measurements of black carbon light absorption enhancement in urban and near-urban locations of southern Ontario, Canada. Atmos. Chem. Phys. 2011, 11, 10 407.
| Time-resolved measurements of black carbon light absorption enhancement in urban and near-urban locations of southern Ontario, Canada.Crossref | GoogleScholarGoogle Scholar |
[167] Y. Cai, D. C. Montague, T. Deshler, Comparison of measured and calculated scattering from surface aerosols with an average, a size-dependent, and a time-dependent refractive index. J. Geophys. Res. – Atmos. 2011, 116, D02202.
| Comparison of measured and calculated scattering from surface aerosols with an average, a size-dependent, and a time-dependent refractive index.Crossref | GoogleScholarGoogle Scholar |
[168] D. Brus, K. Neitola, A. P. Hyvarinen, T. Petaja, J. Vanhanen, M. Sipila, P. Paasonen, M. Kulmala, H. Lihavainen, Homogenous nucleation of sulfuric acid and water at close to atmospherically relevant conditions. Atmos. Chem. Phys. 2011, 11, 5277.
| Homogenous nucleation of sulfuric acid and water at close to atmospherically relevant conditions.Crossref | GoogleScholarGoogle Scholar |
[169] D. R. Benson, J. H. Yu, A. Markovich, S. H. Lee, Ternary homogeneous nucleation of H2SO4, NH3, and H2O under conditions relevant to the lower troposphere. Atmos. Chem. Phys. 2011, 11, 4755.
| Ternary homogeneous nucleation of H2SO4, NH3, and H2O under conditions relevant to the lower troposphere.Crossref | GoogleScholarGoogle Scholar |
[170] J. Kirkby, J. Curtius, J. Almeida, E. Dunne, J. Duplissy, S. Ehrhart, A. Franchin, S. Gagné, L. Ickes, A. Kürten, A. Kupc, A. Metzger, F. Riccobono, L. Rondo, S. Schobesberger, G. Tsagkogeorgas, D. Wimmer, A. Amorim, F. Bianchi, M. Breitenlechner, A. David, J. Dommen, A. Downard, M. Ehn, R. C. Flagan, S. Haider, A. Hansel, D. Hauser, W. Jud, H. Junninen, F. Kreissl, A. Kvashin, A. Laaksonen, K. Lehtipalo, J. Lima, E. R. Lovejoy, V. Makhmutov, S. Mathot, J. Mikkilä, P. Minginette, S. Mogo, T. Nieminen, A. Onnela, P. Pereira, T. Petaja, R. Schnitzhofer, J. H. Seinfeld, M. Sipilä, Y. Stozhkov, F. Stratmann, A. Tomé, J. Vanhanen, Y. Viisanen, A. Vrtala, P. E. Wagner, H. Walther, E. Weingartner, H. Wex, P. M. Winkler, K. S. Carslaw, D. R. Worsnop, U. Baltensperger, M. Kulmala, Role of sulphuric acid, ammonia and galactic cosmic rays in atmospheric aerosol nucleation. Nature 2011, 476, 429.
| Role of sulphuric acid, ammonia and galactic cosmic rays in atmospheric aerosol nucleation.Crossref | GoogleScholarGoogle Scholar |
[171] B. R. Bzdek, D. P. Ridge, M. V. Johnston, Amine reactivity with charged sulfuric acid clusters. Atmos. Chem. Phys. 2011, 11, 8735.
| Amine reactivity with charged sulfuric acid clusters.Crossref | GoogleScholarGoogle Scholar |
[172] B. R. Bzdek, D. P. Ridge, M. V. Johnston, Reactivity of methanesulfonic acid salt clusters relevant to marine air. J. Geophys. Res. – Atmos. 2011, 116, D03301.
| Reactivity of methanesulfonic acid salt clusters relevant to marine air.Crossref | GoogleScholarGoogle Scholar |
[173] M. E. Erupe, A. A. Viggiano, S. H. Lee, The effect of trimethylamine on atmospheric nucleation involving H2SO4. Atmos. Chem. Phys. 2011, 11, 4767.
| The effect of trimethylamine on atmospheric nucleation involving H2SO4.Crossref | GoogleScholarGoogle Scholar |
[174] C. Qiu, L. Wang, V. Lal, A. F. Khalizov, R. Y. Zhang, Heterogeneous reactions of alkylamines with ammonium sulfate and ammonium bisulfate. Environ. Sci. Technol. 2011, 45, 4748.
| Heterogeneous reactions of alkylamines with ammonium sulfate and ammonium bisulfate.Crossref | GoogleScholarGoogle Scholar |
[175] L. Wang, W. Xu, A. F. Khalizov, J. Zheng, C. Qiu, R. Y. Zhang, Laboratory investigation on the role of organics in atmospheric nanoparticle growth. J. Phys. Chem. A 2011, 115, 8940.
| Laboratory investigation on the role of organics in atmospheric nanoparticle growth.Crossref | GoogleScholarGoogle Scholar |
[176] A. M. Booth, W. J. Montague, M. H. Barley, D. O. Topping, G. McFiggans, A. Garforth, C. J. Percival, Solid state and sub-cooled liquid vapour pressures of cyclic aliphatic dicarboxylic acids. Atmos. Chem. Phys. 2011, 11, 655.
| Solid state and sub-cooled liquid vapour pressures of cyclic aliphatic dicarboxylic acids.Crossref | GoogleScholarGoogle Scholar |
[177] G. Isaacman, D. R. Worton, N. M. Kreisberg, C. J. Hennigan, A. P. Teng, S. V. Hering, A. L. Robinson, N. M. Donahue, A. H. Goldstein, Understanding evolution of product composition and volatility distribution through in-situ GC × GC analysis: a case study of longifolene ozonolysis. Atmos. Chem. Phys. 2011, 11, 5335.
| Understanding evolution of product composition and volatility distribution through in-situ GC × GC analysis: a case study of longifolene ozonolysis.Crossref | GoogleScholarGoogle Scholar |
[178] K. Salo, M. Hallquist, Å. M. Jonsson, H. Saathoff, K. H. Naumann, C. Spindler, R. Tillmann, H. Fuchs, B. Bohn, F. Rubach, T. F. Mentel, L. Müller, M. Reinnig, T. Hoffmann, N. M. Donahue, Volatility of secondary organic aerosol during OH radical induced ageing. Atmos. Chem. Phys. 2011, 11, 11 055.
| Volatility of secondary organic aerosol during OH radical induced ageing.Crossref | GoogleScholarGoogle Scholar |
[179] C. D. Cappa, K. R. Wilson, Evolution of organic aerosol mass spectra upon heating: implications for OA phase and partitioning behavior. Atmos. Chem. Phys. 2011, 11, 1895.
| Evolution of organic aerosol mass spectra upon heating: implications for OA phase and partitioning behavior.Crossref | GoogleScholarGoogle Scholar |
[180] T. D. Vaden, D. Imre, J. Beranek, M. Shrivastava, A. Zelenyuk, Evaporation kinetics and phase of laboratory and ambient secondary organic aerosol. Proc. Natl. Acad. Sci. USA 2011, 108, 2190.
| Evaporation kinetics and phase of laboratory and ambient secondary organic aerosol.Crossref | GoogleScholarGoogle Scholar |
[181] T. D. Vaden, C. Song, R. A. Zaveri, D. Imre, A. Zelenyuk, Morphology of mixed primary and secondary organic particles and the adsorption of spectator organic gases during aerosol formation. Proc. Natl. Acad. Sci. USA 2010, 107, 6658.
| Morphology of mixed primary and secondary organic particles and the adsorption of spectator organic gases during aerosol formation.Crossref | GoogleScholarGoogle Scholar |
[182] T. Koop, J. Bookhold, M. Shiraiwa, U. Pöschl, Glass transition and phase state of organic compounds: dependency on molecular properties and implications for secondary organic aerosols in the atmosphere. Phys. Chem. Chem. Phys. 2011, 13, 19 238.
| Glass transition and phase state of organic compounds: dependency on molecular properties and implications for secondary organic aerosols in the atmosphere.Crossref | GoogleScholarGoogle Scholar |
[183] T. B. Nguyen, A. P. Bateman, D. L. Bones, S. A. Nizkorodov, J. Laskin, A. Laskin, High-resolution mass spectrometry analysis of secondary organic aerosol generated by ozonolysis of isoprene. Atmos. Environ. 2010, 44, 1032.
| High-resolution mass spectrometry analysis of secondary organic aerosol generated by ozonolysis of isoprene.Crossref | GoogleScholarGoogle Scholar |
[184] T. B. Nguyen, J. Laskin, A. Laskin, S. A. Nizkorodov, Nitrogen-containing organic compounds and oligomers in secondary organic aerosol formed by photooxidation of isoprene. Environ. Sci. Technol. 2011, 45, 6908.
[185] H. Zhang, J. D. Surratt, Y. H. Lin, J. Bapat, R. M. Kamens, Effect of relative humidity on SOA formation from isoprene/NO photooxidation: enhancement of 2-methylglyceric acid and its corresponding oligoesters under dry conditions. Atmos. Chem. Phys. 2011, 11, 6411.
| Effect of relative humidity on SOA formation from isoprene/NO photooxidation: enhancement of 2-methylglyceric acid and its corresponding oligoesters under dry conditions.Crossref | GoogleScholarGoogle Scholar |
[186] M. M. Galloway, A. J. Huisman, L. D. Yee, A. W. H. Chan, C. L. Loza, J. H. Seinfeld, F. N. Keutsch, Yields of oxidized volatile organic compounds during the OH radical initiated oxidation of isoprene, methyl vinyl ketone, and methacrolein under high-NOx conditions. Atmos. Chem. Phys. 2011, 11, 10 779.
| Yields of oxidized volatile organic compounds during the OH radical initiated oxidation of isoprene, methyl vinyl ketone, and methacrolein under high-NOx conditions.Crossref | GoogleScholarGoogle Scholar |
[187] H. F. Zhang, W. Rattanavaraha, Y. Zhou, J. Bapat, E. P. Rosen, K. G. Sexton, R. M. Kamens, A new gas-phase condensed mechanism of isoprene-NOx photooxidation. Atmos. Environ. 2011, 45, 4507.
| A new gas-phase condensed mechanism of isoprene-NOx photooxidation.Crossref | GoogleScholarGoogle Scholar |
[188] W. A. Hall, M. V. Johnston, Oligomer content of α-pinene secondary organic aerosol. Aerosol Sci. Technol. 2011, 45, 37.
| Oligomer content of α-pinene secondary organic aerosol.Crossref | GoogleScholarGoogle Scholar |
[189] A. L. Putman, J. H. Offenberg, R. Fisseha, S. Kundu, T. A. Rahn, L. R. Mazzoleni, Ultrahigh-resolution FT-ICR mass spectrometry characterization of α-pinene ozonolysis SOA. Atmos. Environ. 2012, 46, 164.
| Ultrahigh-resolution FT-ICR mass spectrometry characterization of α-pinene ozonolysis SOA.Crossref | GoogleScholarGoogle Scholar |
[190] M. N. Chan, J. D. Surratt, A. W. H. Chan, K. Schilling, J. H. Offenberg, M. Lewandowski, E. O. Edney, T. E. Kleindienst, M. Jaoui, E. S. Edgerton, R. L. Tanner, S. L. Shaw, M. Zheng, E. M. Knipping, J. H. Seinfeld, Influence of aerosol acidity on the chemical composition of secondary organic aerosol from β-caryophyllene. Atmos. Chem. Phys. 2011, 11, 1735.
| Influence of aerosol acidity on the chemical composition of secondary organic aerosol from β-caryophyllene.Crossref | GoogleScholarGoogle Scholar |
[191] Y. J. Li, Q. Chen, M. I. Guzman, C. K. Chan, S. T. Martin, Second-generation products contribute substantially to the particle-phase organic material produced by β-caryophyllene ozonolysis. Atmos. Chem. Phys. 2011, 11, 121.
| Second-generation products contribute substantially to the particle-phase organic material produced by β-caryophyllene ozonolysis.Crossref | GoogleScholarGoogle Scholar |
[192] J. F. Hamilton, M. R. Alfarra, K. P. Wyche, M. W. Ward, A. C. Lewis, G. B. McFiggans, N. Good, P. S. Monks, T. Carr, I. R. White, R. M. Purvis, Investigating the use of secondary organic aerosol as seed particles in simulation chamber experiments. Atmos. Chem. Phys. 2011, 11, 5917.
| Investigating the use of secondary organic aerosol as seed particles in simulation chamber experiments.Crossref | GoogleScholarGoogle Scholar |
[193] F. Yasmeen, R. Vermeylen, R. Szmigielski, Y. Iinuma, O. Boge, H. Herrmann, W. Maenhaut, M. Claeys, Terpenylic acid and related compounds: precursors for dimers in secondary organic aerosol from the ozonolysis of α- and β-pinene. Atmos. Chem. Phys. 2010, 10, 9383.
| Terpenylic acid and related compounds: precursors for dimers in secondary organic aerosol from the ozonolysis of α- and β-pinene.Crossref | GoogleScholarGoogle Scholar |
[194] M. Claeys, Y. Iinuma, R. Szmigielski, J. D. Surratt, F. Blockhuys, C. Van Alsenoy, O. Boge, B. Sierau, Y. Gómez-González, R. Vermeylen, P. Van der Veken, M. Shahgholi, A. W. H. Chan, H. Herrmann, J. H. Seinfeld, W. Maenhaut, Terpenylic acid and related compounds from the oxidation of α-pinene: implications for new particle formation and growth above forests. Environ. Sci. Technol. 2009, 43, 6976.
| Terpenylic acid and related compounds from the oxidation of α-pinene: implications for new particle formation and growth above forests.Crossref | GoogleScholarGoogle Scholar |
[195] S. Nakao, C. Clark, P. Tang, K. Sato, D. Cocker, Secondary organic aerosol formation from phenolic compounds in the absence of NOx. Atmos. Chem. Phys. 2011, 11, 10 649.
| Secondary organic aerosol formation from phenolic compounds in the absence of NOx.Crossref | GoogleScholarGoogle Scholar |
[196] J. Ofner, H. U. Kruger, H. Grothe, P. Schmitt-Kopplin, K. Whitmore, C. Zetzsch, Physico-chemical characterization of SOA derived from catechol and guaiacol – a model substance for the aromatic fraction of atmospheric HULIS. Atmos. Chem. Phys. 2011, 11, 1.
| Physico-chemical characterization of SOA derived from catechol and guaiacol – a model substance for the aromatic fraction of atmospheric HULIS.Crossref | GoogleScholarGoogle Scholar |
[197] A. Gratien, S. N. Johnson, M. J. Ezell, M. L. Dawson, R. Bennett, B. J. Finlayson-Pitts, Surprising formation of p-cymene in the oxidation of α-pinene in air by the atmospheric oxidants OH, O3, and NO3. Environ. Sci. Technol. 2011, 45, 2755.
| Surprising formation of p-cymene in the oxidation of α-pinene in air by the atmospheric oxidants OH, O3, and NO3.Crossref | GoogleScholarGoogle Scholar |
[198] P. J. Ziemann, Effects of molecular structure on the chemistry of aerosol formation from the OH-radical-initiated oxidation of alkanes and alkenes. Int. Rev. Phys. Chem. 2011, 30, 161.
| Effects of molecular structure on the chemistry of aerosol formation from the OH-radical-initiated oxidation of alkanes and alkenes.Crossref | GoogleScholarGoogle Scholar |
[199] S. H. Kessler, T. Nah, A. Carrasquillo, J. T. Jayne, D. R. Worsnop, K. R. Wilson, J. H. Kroll, Formation of secondary organic aerosol from the direct photolytic generation of organic radicals. J. Phys. Chem. Lett. 2011, 2, 1295.
| Formation of secondary organic aerosol from the direct photolytic generation of organic radicals.Crossref | GoogleScholarGoogle Scholar |
[200] H. J. Chacon-Madrid, N. M. Donahue, Fragmentation vs. functionalization: chemical aging and organic aerosol formation. Atmos. Chem. Phys. 2011, 11, 10 553.
| Fragmentation vs. functionalization: chemical aging and organic aerosol formation.Crossref | GoogleScholarGoogle Scholar |
[201] I. Gensch, W. Laumer, O. Stein, B. Kammer, T. Hohaus, H. Saathoff, R. Wegener, A. Wahner, A. Kiendler-Scharr, Temperature dependence of the kinetic isotope effect in β-pinene ozonolysis. J. Geophys. Res. – Atmos. 2011, 116, D20301.
| Temperature dependence of the kinetic isotope effect in β-pinene ozonolysis.Crossref | GoogleScholarGoogle Scholar |
[202] S. Moukhtar, M. Saccon, A. Kornilova, S. Irei, L. Huang, J. Rudolph, Method for determination of stable carbon isotope ratio of methylnitrophenols in atmospheric particulate matter. Atmos. Meas. Tech. 2011, 4, 2453.
| Method for determination of stable carbon isotope ratio of methylnitrophenols in atmospheric particulate matter.Crossref | GoogleScholarGoogle Scholar |
[203] J. Auld, D. R. Hastie, Investigation of organic nitrate product formation during hydroxyl radical initiated photo-oxidation of β-pinene. Atmos. Environ. 2011, 45, 26.
| Investigation of organic nitrate product formation during hydroxyl radical initiated photo-oxidation of β-pinene.Crossref | GoogleScholarGoogle Scholar |
[204] J. L. Fry, A. Kiendler-Scharr, A. W. Rollins, T. Brauers, S. S. Brown, H. P. Dorn, W. P. Dube, H. Fuchs, A. Mensah, F. Rohrer, R. Tillmann, A. Wahner, P. J. Wooldridge, R. C. Cohen, SOA from limonene: role of NO3 in its generation and degradation. Atmos. Chem. Phys. 2011, 11, 3879.
| SOA from limonene: role of NO3 in its generation and degradation.Crossref | GoogleScholarGoogle Scholar |
[205] A. I. Darer, N. C. Cole-Filipiak, A. E. O’Connor, M. J. Elrod, Formation and stability of atmospherically relevant isoprene-derived organosulfates and organonitrates. Environ. Sci. Technol. 2011, 45, 1895.
| Formation and stability of atmospherically relevant isoprene-derived organosulfates and organonitrates.Crossref | GoogleScholarGoogle Scholar |
[206] K. S. Hu, A. I. Darer, M. J. Elrod, Thermodynamics and kinetics of the hydrolysis of atmospherically relevant organonitrates and organosulfates. Atmos. Chem. Phys. 2011, 11, 8307.
| Thermodynamics and kinetics of the hydrolysis of atmospherically relevant organonitrates and organosulfates.Crossref | GoogleScholarGoogle Scholar |
[207] C. N. Olson, M. M. Galloway, G. Yu, C. J. Hedman, M. R. Lockett, T. Yoon, E. A. Stone, L. M. Smith, F. N. Keutsch, Hydroxycarboxylic acid-derived organosulfates: synthesis, stability, and quantification in ambient aerosol. Environ. Sci. Technol. 2011, 45, 6468.
| Hydroxycarboxylic acid-derived organosulfates: synthesis, stability, and quantification in ambient aerosol.Crossref | GoogleScholarGoogle Scholar |
[208] B. Ervens, B. J. Turpin, R. J. Weber, Secondary organic aerosol formation in cloud droplets and aqueous particles (aqSOA): a review of laboratory, field and model studies. Atmos. Chem. Phys. 2011, 11, 11 069.
| Secondary organic aerosol formation in cloud droplets and aqueous particles (aqSOA): a review of laboratory, field and model studies.Crossref | GoogleScholarGoogle Scholar |
[209] A. K. Y. Lee, P. Herckes, W. R. Leaitch, A. M. Macdonald, J. P. D. Abbatt, Aqueous OH oxidation of ambient organic aerosol and cloud water organics: formation of highly oxidized products. Geophys. Res. Lett. 2011, 38, L11805.
| Aqueous OH oxidation of ambient organic aerosol and cloud water organics: formation of highly oxidized products.Crossref | GoogleScholarGoogle Scholar |
[210] A. K. Y. Lee, R. Zhao, S. S. Gao, J. P. D. Abbatt, Aqueous-phase OH oxidation of glyoxal: application of a novel analytical approach employing aerosol mass spectrometry and complementary off-line techniques. J. Phys. Chem. A 2011, 115, 10517.
[211] A. P. Bateman, S. A. Nizkorodov, J. Laskin, A. Laskin, Photolytic processing of secondary organic aerosols dissolved in cloud droplets. Phys. Chem. Chem. Phys. 2011, 13, 12 199.
| Photolytic processing of secondary organic aerosols dissolved in cloud droplets.Crossref | GoogleScholarGoogle Scholar |
[212] S. S. Gao, J. P. D. Abbatt, Kinetics and mechanism of OH oxidation of small organic dicarboxylic acids in ice: comparison to behavior in aqueous solution. J. Phys. Chem. A 2011, 115, 9977.
| Kinetics and mechanism of OH oxidation of small organic dicarboxylic acids in ice: comparison to behavior in aqueous solution.Crossref | GoogleScholarGoogle Scholar |
[213] Y. B. Lim, Y. Tan, M. J. Perri, S. P. Seitzinger, B. J. Turpin, Aqueous chemistry and its role in secondary organic aerosol (SOA) formation. Atmos. Chem. Phys. 2010, 10, 10 521.
| Aqueous chemistry and its role in secondary organic aerosol (SOA) formation.Crossref | GoogleScholarGoogle Scholar |
[214] B. Ervens, R. Volkamer, Glyoxal processing by aerosol multiphase chemistry: towards a kinetic modeling framework of secondary organic aerosol formation in aqueous particles. Atmos. Chem. Phys. 2010, 10, 8219.
| Glyoxal processing by aerosol multiphase chemistry: towards a kinetic modeling framework of secondary organic aerosol formation in aqueous particles.Crossref | GoogleScholarGoogle Scholar |
[215] D. O. De Haan, L. N. Hawkins, J. A. Kononenko, J. J. Turley, A. L. Corrigan, M. A. Tolbert, J. L. Jimenez, Formation of nitrogen-containing oligomers by methylglyoxal and amines in simulated evaporating cloud droplets. Environ. Sci. Technol. 2011, 45, 984.
| Formation of nitrogen-containing oligomers by methylglyoxal and amines in simulated evaporating cloud droplets.Crossref | GoogleScholarGoogle Scholar |
[216] M. M. Galloway, C. L. Loza, P. S. Chhabra, A. W. H. Chan, L. D. Yee, J. H. Seinfeld, F. N. Keutsch, Analysis of photochemical and dark glyoxal uptake: implications for SOA formation. Geophys. Res. Lett. 2011, 38, L17811.
| Analysis of photochemical and dark glyoxal uptake: implications for SOA formation.Crossref | GoogleScholarGoogle Scholar |
[217] M. Trainic, A. A. Riziq, A. Lavi, J. M. Flores, Y. Rudich, The optical, physical and chemical properties of the products of glyoxal uptake on ammonium sulfate seed aerosols. Atmos. Chem. Phys. 2011, 11, 9697.
| The optical, physical and chemical properties of the products of glyoxal uptake on ammonium sulfate seed aerosols.Crossref | GoogleScholarGoogle Scholar |
[218] C. L. Heald, J. H. Kroll, J. L. Jimenez, K. S. Docherty, P. F. DeCarlo, A. C. Aiken, Q. Chen, S. T. Martin, D. K. Farmer, P. Artaxo, A simplified description of the evolution of organic aerosol composition in the atmosphere. Geophys. Res. Lett. 2010, 37, L08803.
| A simplified description of the evolution of organic aerosol composition in the atmosphere.Crossref | GoogleScholarGoogle Scholar |
[219] J. H. Kroll, N. M. Donahue, J. L. Jimenez, S. H. Kessler, M. R. Canagaratna, K. R. Wilson, K. E. Altieri, L. R. Mazzoleni, A. S. Wozniak, H. Bluhm, E. R. Mysak, J. D. Smith, C. E. Kolb, D. R. Worsnop, Carbon oxidation state as a metric for describing the chemistry of atmospheric organic aerosol. Nat. Chem. 2011, 3, 133.
| Carbon oxidation state as a metric for describing the chemistry of atmospheric organic aerosol.Crossref | GoogleScholarGoogle Scholar |
[220] P. S. Chhabra, N. L. Ng, M. R. Canagaratna, A. L. Corrigan, L. M. Russell, D. R. Worsnop, R. C. Flagan, J. H. Seinfeld, Elemental composition and oxidation of chamber organic aerosol. Atmos. Chem. Phys. 2011, 11, 8827.
| Elemental composition and oxidation of chamber organic aerosol.Crossref | GoogleScholarGoogle Scholar |
[221] Q. Chen, Y. J. Liu, N. M. Donahue, J. E. Shilling, S. T. Martin, Particle-phase chemistry of secondary organic material: modeled compared to measured O:C and H:C elemental ratios provide constraints. Environ. Sci. Technol. 2011, 45, 4763.
| Particle-phase chemistry of secondary organic material: modeled compared to measured O:C and H:C elemental ratios provide constraints.Crossref | GoogleScholarGoogle Scholar |
[222] S. Net, E. G. Alvarez, S. Gligorovski, H. Wortham, Heterogeneous reactions of ozone with methoxyphenols, in presence and absence of light. Atmos. Environ. 2011, 45, 3007.
| Heterogeneous reactions of ozone with methoxyphenols, in presence and absence of light.Crossref | GoogleScholarGoogle Scholar |
[223] J. J. Nájera, R. Wamsley, D. J. Last, K. E. Leather, C. J. Percival, A. B. Horn, Heterogeneous oxidation reaction of gas-phase ozone with anthracene in thin films and on aerosols by infrared spectroscopic methods. Int. J. Chem. Kinet. 2011, 43, 694.
| Heterogeneous oxidation reaction of gas-phase ozone with anthracene in thin films and on aerosols by infrared spectroscopic methods.Crossref | GoogleScholarGoogle Scholar |
[224] N. W. Oldridge, J. P. D. Abbatt, Formation of gas-phase bromine from interaction of ozone with frozen and liquid NaCl/NaBr solutions: quantitative separation of surficial chemistry from bulk-phase reaction. J. Phys. Chem. A 2011, 115, 2590.
| Formation of gas-phase bromine from interaction of ozone with frozen and liquid NaCl/NaBr solutions: quantitative separation of surficial chemistry from bulk-phase reaction.Crossref | GoogleScholarGoogle Scholar |
[225] P. J. Gallimore, P. Achakulwisut, F. D. Pope, J. F. Davies, D. R. Spring, M. Kalberer, Importance of relative humidity in the oxidative ageing of organic aerosols: case study of the ozonolysis of maleic acid aerosol. Atmos. Chem. Phys. 2011, 11, 12 181.
| Importance of relative humidity in the oxidative ageing of organic aerosols: case study of the ozonolysis of maleic acid aerosol.Crossref | GoogleScholarGoogle Scholar |
[226] T. Kinugawa, S. Enami, A. Yabushita, M. Kawasaki, M. R. Hoffmann, A. J. Colussi, Conversion of gaseous nitrogen dioxide to nitrate and nitrite on aqueous surfactants. Phys. Chem. Chem. Phys. 2011, 13, 5144.
| Conversion of gaseous nitrogen dioxide to nitrate and nitrite on aqueous surfactants.Crossref | GoogleScholarGoogle Scholar |
[227] D. A. Knopf, S. M. Forrester, J. H. Slade, Heterogeneous oxidation kinetics of organic biomass burning aerosol surrogates by O3, NO2, N2O5, and NO3. Phys. Chem. Chem. Phys. 2011, 13, 21 050.
| Heterogeneous oxidation kinetics of organic biomass burning aerosol surrogates by O3, NO2, N2O5, and NO3.Crossref | GoogleScholarGoogle Scholar |
[228] Z. J. Zhao, S. Husainy, C. T. Stoudemayer, G. D. Smith, Reactive uptake of NO3 radicals by unsaturated fatty acid particles. Phys. Chem. Chem. Phys. 2011, 13, 17 809.
| Reactive uptake of NO3 radicals by unsaturated fatty acid particles.Crossref | GoogleScholarGoogle Scholar |
[229] C. L. Liu, J. D. Smith, D. L. Che, M. Ahmed, S. R. Leone, K. R. Wilson, The direct observation of secondary radical chain chemistry in the heterogeneous reaction of chlorine atoms with submicron squalane droplets. Phys. Chem. Chem. Phys. 2011, 13, 8993.
| The direct observation of secondary radical chain chemistry in the heterogeneous reaction of chlorine atoms with submicron squalane droplets.Crossref | GoogleScholarGoogle Scholar |
[230] S. Xiao, A. K. Bertram, Reactive uptake kinetics of NO3 on multicomponent and multiphase organic mixtures containing unsaturated and saturated organics. Phys. Chem. Chem. Phys. 2011, 13, 6628.
| Reactive uptake kinetics of NO3 on multicomponent and multiphase organic mixtures containing unsaturated and saturated organics.Crossref | GoogleScholarGoogle Scholar |
[231] C. G. Liu, J. Gan, Y. Zhang, M. Liang, X. Shu, J. N. Shu, B. Yang, Heterogeneous reaction of suspended phosmet particles with NO3 radicals. J. Phys. Chem. A 2011, 115, 10 744.
| Heterogeneous reaction of suspended phosmet particles with NO3 radicals.Crossref | GoogleScholarGoogle Scholar |
[232] B. Yang, J. W. Meng, Y. Zhang, C. G. Liu, J. Gan, J. N. Shu, Experimental studies on the heterogeneous reaction of NO3 radicals with suspended carbaryl particles. Atmos. Environ. 2011, 45, 2074.
| Experimental studies on the heterogeneous reaction of NO3 radicals with suspended carbaryl particles.Crossref | GoogleScholarGoogle Scholar |
[233] Y. Zhang, B. Yang, J. Gan, C. G. Liu, X. Shu, J. N. Shu, Nitration of particle-associated PAHs and their derivatives (nitro-, oxy-, and hydroxy-PAHs) with NO3 radicals. Atmos. Environ. 2011, 45, 2515.
| Nitration of particle-associated PAHs and their derivatives (nitro-, oxy-, and hydroxy-PAHs) with NO3 radicals.Crossref | GoogleScholarGoogle Scholar |
[234] Z. Liu, M. F. Ge, W. G. Wang, S. Yin, S. R. Tong, The uptake of 2-methyl-3-buten-2-ol into aqueous mixed solutions of sulfuric acid and hydrogen peroxide. Phys. Chem. Chem. Phys. 2011, 13, 2069.
| The uptake of 2-methyl-3-buten-2-ol into aqueous mixed solutions of sulfuric acid and hydrogen peroxide.Crossref | GoogleScholarGoogle Scholar |
[235] Z. Liu, L. Y. Wu, T. H. Wang, M. F. Ge, W. G. Wang, Uptake of methacrolein into aqueous solutions of sulfuric acid and hydrogen peroxide. J. Phys. Chem. A 2012, 116, 437.
| Uptake of methacrolein into aqueous solutions of sulfuric acid and hydrogen peroxide.Crossref | GoogleScholarGoogle Scholar |
[236] L. P. Chan, C. K. Chan, Enhanced reactive uptake of nonanal by acidic aerosols in the presence of particle-phase organics. Aerosol Sci. Technol. 2011, 45, 872.
| Enhanced reactive uptake of nonanal by acidic aerosols in the presence of particle-phase organics.Crossref | GoogleScholarGoogle Scholar |
[237] G. J. Engelhart, R. H. Moore, A. Nenes, S. N. Pandis, Cloud condensation nuclei activity of isoprene secondary organic aerosol. J. Geophys. Res. – Atmos. 2011, 116, D02207.
| Cloud condensation nuclei activity of isoprene secondary organic aerosol.Crossref | GoogleScholarGoogle Scholar |
[238] M. Frosch, M. Bilde, P. F. DeCarlo, Z. Juranyi, T. Tritscher, J. Dommen, N. M. Donahue, M. Gysel, E. Weingartner, U. Baltensperger, Relating cloud condensation nuclei activity and oxidation level of alpha-pinene secondary organic aerosols. J. Geophys. Res. – Atmos. 2011, 116, D22212.
| Relating cloud condensation nuclei activity and oxidation level of alpha-pinene secondary organic aerosols.Crossref | GoogleScholarGoogle Scholar |
[239] M. Kuwata, Q. Chen, S. T. Martin, Cloud condensation nuclei (CCN) activity and oxygen-to-carbon elemental ratios following thermodenuder treatment of organic particles grown by alpha-pinene ozonolysis. Phys. Chem. Chem. Phys. 2011, 13, 14 571.
| Cloud condensation nuclei (CCN) activity and oxygen-to-carbon elemental ratios following thermodenuder treatment of organic particles grown by alpha-pinene ozonolysis.Crossref | GoogleScholarGoogle Scholar |
[240] A. T. Lambe, T. B. Onasch, P. Massoli, D. R. Croasdale, J. P. Wright, A. T. Ahern, L. R. Williams, D. R. Worsnop, W. H. Brune, P. Davidovits, Laboratory studies of the chemical composition and cloud condensation nuclei (CCN) activity of secondary organic aerosol (SOA) and oxidized primary organic aerosol (OPOA). Atmos. Chem. Phys. 2011, 11, 8913.
| Laboratory studies of the chemical composition and cloud condensation nuclei (CCN) activity of secondary organic aerosol (SOA) and oxidized primary organic aerosol (OPOA).Crossref | GoogleScholarGoogle Scholar |
[241] A. T. Lambe, A. T. Ahern, L. R. Williams, J. G. Slowik, J. P. S. Wong, J. P. D. Abbatt, W. H. Brune, N. L. Ng, J. P. Wright, D. R. Croasdale, D. R. Worsnop, P. Davidovits, T. B. Onasch, Characterization of aerosol photooxidation flow reactors: heterogeneous oxidation, secondary organic aerosol formation and cloud condensation nuclei activity measurements. Atmos. Meas. Tech. 2011, 4, 445.
| Characterization of aerosol photooxidation flow reactors: heterogeneous oxidation, secondary organic aerosol formation and cloud condensation nuclei activity measurements.Crossref | GoogleScholarGoogle Scholar |
[242] E. Kang, D. W. Toohey, W. H. Brune, Dependence of SOA oxidation on organic aerosol mass concentration and OH exposure: experimental PAM chamber studies. Atmos. Chem. Phys. 2011, 11, 1837.
| Dependence of SOA oxidation on organic aerosol mass concentration and OH exposure: experimental PAM chamber studies.Crossref | GoogleScholarGoogle Scholar |
[243] A. N. Schwier, N. Sareen, T. L. Lathem, A. Nenes, V. F. McNeill, Ozone oxidation of oleic acid surface films decreases aerosol cloud condensation nuclei activity. J. Geophys. Res. – Atmos. 2011, 116, D16202.
| Ozone oxidation of oleic acid surface films decreases aerosol cloud condensation nuclei activity.Crossref | GoogleScholarGoogle Scholar |
[244] B. Friedman, G. Kulkarni, J. Beranek, A. Zelenyuk, J. A. Thornton, D. J. Cziczo, Ice nucleation and droplet formation by bare and coated soot particles. J. Geophys. Res. – Atmos. 2011, 116, D17203.
| Ice nucleation and droplet formation by bare and coated soot particles.Crossref | GoogleScholarGoogle Scholar |
[245] P. Reitz, C. Spindler, T. F. Mentel, L. Poulain, H. Wex, K. Mildenberger, D. Niedermeier, S. Hartmann, T. Clauss, F. Stratmann, R. C. Sullivan, P. J. DeMott, M. D. Petters, B. Sierau, J. Schneider, Surface modification of mineral dust particles by sulphuric acid processing: implications for ice nucleation abilities. Atmos. Chem. Phys. 2011, 11, 7839.
| Surface modification of mineral dust particles by sulphuric acid processing: implications for ice nucleation abilities.Crossref | GoogleScholarGoogle Scholar |
[246] L. Ladino, O. Stetzer, B. Hattendorf, D. Gunther, B. Croft, U. Lohmann, Experimental study of collection efficiencies between submicron aerosols and cloud droplets. J. Atmos. Sci. 2011, 68, 1853.
| Experimental study of collection efficiencies between submicron aerosols and cloud droplets.Crossref | GoogleScholarGoogle Scholar |
[247] C. D. Cappa, D. L. Che, S. H. Kessler, J. H. Kroll, K. R. Wilson, Variations in organic aerosol optical and hygroscopic properties upon heterogeneous OH oxidation. J. Geophys. Res. – Atmos. 2011, 116, D15204.
| Variations in organic aerosol optical and hygroscopic properties upon heterogeneous OH oxidation.Crossref | GoogleScholarGoogle Scholar |
[248] H. Redmond, J. E. Thompson, Evaluation of a quantitative structure-property relationship (QSPR) for predicting mid-visible refractive index of secondary organic aerosol (SOA). Phys. Chem. Chem. Phys. 2011, 13, 6872.
| Evaluation of a quantitative structure-property relationship (QSPR) for predicting mid-visible refractive index of secondary organic aerosol (SOA).Crossref | GoogleScholarGoogle Scholar |
[249] J. Duplissy, P. F. DeCarlo, J. Dommen, M. R. Alfarra, A. Metzger, I. Barmpadimos, A. S. H. Prevot, E. Weingartner, T. Tritscher, M. Gysel, A. C. Aiken, J. L. Jimenez, M. R. Canagaratna, D. R. Worsnop, D. R. Collins, J. Tomlinson, U. Baltensperger, Relating hygroscopicity and composition of organic aerosol particulate matter. Atmos. Chem. Phys. 2011, 11, 1155.
| Relating hygroscopicity and composition of organic aerosol particulate matter.Crossref | GoogleScholarGoogle Scholar |
[250] T. Tritscher, J. Dommen, P. F. DeCarlo, M. Gysel, P. B. Barmet, A. P. Praplan, E. Weingartner, A. S. H. Prevot, I. Riipinen, N. M. Donahue, U. Baltensperger, Volatility and hygroscopicity of aging secondary organic aerosol in a smog chamber. Atmos. Chem. Phys. 2011, 11, 11 477.
| Volatility and hygroscopicity of aging secondary organic aerosol in a smog chamber.Crossref | GoogleScholarGoogle Scholar |
[251] J. W. Lu, J. M. Flores, A. Lavi, A. Abo-Riziq, Y. Rudich, Changes in the optical properties of benzo a pyrene-coated aerosols upon heterogeneous reactions with NO2 and NO3. Phys. Chem. Chem. Phys. 2011, 13, 6484.
| Changes in the optical properties of benzo a pyrene-coated aerosols upon heterogeneous reactions with NO2 and NO3.Crossref | GoogleScholarGoogle Scholar |
[252] M. Zhong, M. Jang, Light absorption coefficient measurement of SOA using a UV-visible spectrometer connected with an integrating sphere. Atmos. Environ. 2011, 45, 4263.
| Light absorption coefficient measurement of SOA using a UV-visible spectrometer connected with an integrating sphere.Crossref | GoogleScholarGoogle Scholar |
[253] M. L. Smith, M. Kuwata, S. T. Martin, Secondary organic material produced by the dark ozonolysis of alpha-pinene minimally affects the deliquescence and efflorescence of ammonium sulfate. Aerosol Sci. Technol. 2011, 45, 244.
| Secondary organic material produced by the dark ozonolysis of alpha-pinene minimally affects the deliquescence and efflorescence of ammonium sulfate.Crossref | GoogleScholarGoogle Scholar |
[254] M. Claeys, R. Vermeylen, F. Yasmeen, Y. Gómez-González, X. Chi, W. Maenhaut, T. Meszaros, I. Salma, Chemical characterization of humic-like substances from urban, rural and tropical locations using liquid chromatography in combination with UV/vis photodiode array detection and electrospray ionization mass spectrometry. Environ. Chem. 2012, 9, 273.
| Chemical characterization of humic-like substances from urban, rural and tropical locations using liquid chromatography in combination with UV/vis photodiode array detection and electrospray ionization mass spectrometry.Crossref | GoogleScholarGoogle Scholar |
[255] H. Zhang, Y.-H. Lin, Z. Zhang, X. Znang, S. L. Shaw, E. M. Knipping, R. J. Weber, A. Gold, R. M. Kamens, J. D. Surratt, Secondary organic aerosol formation from methacrolein photooxidation: roles of NOx levels and relative humidity. Environ. Chem. 2012, 9, 247.
| Secondary organic aerosol formation from methacrolein photooxidation: roles of NOx levels and relative humidity.Crossref | GoogleScholarGoogle Scholar |
[256] F. Yasmeen, R. Vermeylen, N. Maurin, E. Perraudin, J.-F. Doussin, M. Claeys, Characterisation of tracers for ageing of α-pinene secondary organic aerosol using liquid chromatography/negative ion electrospray ionisation mass spectrometry. Environ. Chem. 2012, 9, 236.
| Characterisation of tracers for ageing of α-pinene secondary organic aerosol using liquid chromatography/negative ion electrospray ionisation mass spectrometry.Crossref | GoogleScholarGoogle Scholar |
[257] E. A. Stone, T. T. Nguyen, B. B. Pradhan, P. M. Dangol, Assessment of biogenic secondary organic aerosol in the Kathmandu Valley, Nepal. Environ. Chem. 2012, 9, 263.
| Assessment of biogenic secondary organic aerosol in the Kathmandu Valley, Nepal.Crossref | GoogleScholarGoogle Scholar |
[258] Y. Huang, H. Chen, L. Wang, X. Yang, J. Chen, Single particle analysis of amines in ambient aerosol in Shanghai. Environ. Chem. 2012, 9, 202.
| Single particle analysis of amines in ambient aerosol in Shanghai.Crossref | GoogleScholarGoogle Scholar |
[259] J. H. Kroll, J. D. Smith, D. R. Worsnop, K. R. Wilson, Characterization of lightly oxidized organic aerosol formed from the photochemical aging of diesel exhaust particles. Environ. Chem. 2012, 9, 211.
| Characterization of lightly oxidized organic aerosol formed from the photochemical aging of diesel exhaust particles.Crossref | GoogleScholarGoogle Scholar |
[260] X. Ge, Q. Zhang, Y. Sun, C. R. Ruehl, Effect of aqueous-phase processing on aerosol chemistry in Fresno, California, during wintertime. Environ. Chem. 2012, 9, 221.
| Effect of aqueous-phase processing on aerosol chemistry in Fresno, California, during wintertime.Crossref | GoogleScholarGoogle Scholar |
[261] L. R. Mazzoleni, P. Saranjampour, M. M. Dalbec, V. Samburova, A. G. Hallar, B. Zielinska, D. Lowenthal, S. Kohl, Identification of water-soluble organic carbon in nonurban aerosols using ultra-high resolution FT-ICR mass spectrometry: organic anions. Environ. Chem. 2012, 9, 285.
| Identification of water-soluble organic carbon in nonurban aerosols using ultra-high resolution FT-ICR mass spectrometry: organic anions.Crossref | GoogleScholarGoogle Scholar |
[262] A. G. Rincón, A. I. Calvo, M. Dietzel, M. Kalberer, Seasonal differences of urban organic aerosol composition – an ultra-high resolution mass spectrometry study. Environ. Chem. 2012, 9, 298.
| Seasonal differences of urban organic aerosol composition – an ultra-high resolution mass spectrometry study.Crossref | GoogleScholarGoogle Scholar |


