Compound-specific bromine isotope compositions of one natural and six industrially synthesised organobromine substances
Daniel Carrizo A , Maria Unger A B , Henry Holmstrand A E , Per Andersson C , Örjan Gustafsson A , Sean P. Sylva D and Christopher M. Reddy DA Department of Applied Environmental Science, Stockholm University, SE-106 91 Stockholm, Sweden.
B Department of Materials and Environmental Chemistry, Stockholm University, SE-106 91 Stockholm, Sweden.
C Laboratory for Isotope Geology, Swedish Museum of Natural History, SE-104 05 Stockholm, Sweden.
D Department of Marine Chemistry and Geochemistry, Woods Hole Oceanographic Institution, Woods Hole, MA 02543, USA.
E Corresponding author. Email: henry.holmstrand@itm.su.se
Environmental Chemistry 8(2) 127-132 https://doi.org/10.1071/EN10090
Submitted: 10 August 2010 Accepted: 17 December 2010 Published: 2 May 2011
Journal Compilation © CSIRO Publishing 2011 Open Access CC BY-NC-ND
Environmental context. Brominated organic compounds of both natural and anthropogenic origin are commonly found in the environment. Bromine has two stable isotopes and the isotopic composition of brominated compounds may vary depending on production pathways and degradation processes. These variations are a result of isotope fractionation effects, when heavy isotopes react slower than lighter isotopes. We apply compound-specific bromine isotope analysis to industrial brominated organic compounds, and one naturally produced analogue, to test the feasibility of the technique to investigate the source and environmental fate of these compounds.
Abstract. The stable bromine isotopic composition (δ81Br) was determined for six industrially synthesised brominated organic compounds (BOCs) and one natural BOC by gas-chromatography multi-collector inductively coupled plasma mass spectrometry (GC-mcICP-MS). The δ81Br compositions of brominated benzenes, phenols (both natural and industrial), anisoles, and naphthalenes were constrained with the standard differential measurement approach using as reference a monobromobenzene sample with an independently determined δ81Br value (–0.39‰ v. Standard Mean Ocean Bromide, SMOB). The δ81Br values for the industrial BOCs ranged from –4.3 to –0.4‰. The average δ81Br value for the natural compound (2,4-dibromophenol) was 0.2 ± 1.6‰ (1 s.d.), and for the identical industrial compound (2,4-dibromophenol) –1.1 ± 0.9‰ (1 s.d.), with a statistically significant difference of ~1.4 (P < 0.05). The δ81Br of four out of six industrial compounds was found to be significantly different from that of the natural sample. These novel results establish the bromine isotopic variability among the industrially produced BOCs in relation to a natural sample.
Additional keywords: brominated organic compounds (BOCs), compound-specific isotope analysis (CSIA), gas-chromatography multi-collector inductively coupled plasma mass spectrometry (GC-mcICP-MS), pollutants.
Introduction
Brominated organic compounds (BOCs) are found worldwide in various matrices and are known to have both natural and anthropogenic sources. More than 1600 organobromine compounds are naturally produced.[1] For example, mono-, di- and tribrominated phenols are excreted by marine organisms such as algae, polychaetes, and hemichordates.[2] BOCs produced in situ are a consistent feature of pristine marine soft-bottom habitats, and their spatial and temporal abundance correlates with the abundance of infauna that produces these metabolites.[3] The physiological and ecological function of BOCs may be to deter feeding, hinder or inhibit settlement of competitors, and regulate internal salt or hydrogen peroxide levels.[4–6] Natural BOCs can be formed via photochemical,[7] abiotic, Fe-mediated,[8,9] and enzymatic reactions.[10,11] Many anthropogenic BOCs are (or have until recently been) produced in large quantities for uses such as disinfectants, flame retardants, pesticides and gasoline additives.[12] Industrial BOCs are mostly produced by bromination of hydrocarbon precursors using bromine gas and by addition reactions of already brominated precursors. These compounds exhibit persistence in the environment, long-range transport and possibly toxic effects in humans and biota.[13] There is thus a need to comprehend the environmental dynamics of BOCs, and knowledge of the relative magnitude of natural and industrial sources will be an important contribution to that end.
Here we apply compound-specific bromine isotope analysis (CSIA-Br) to investigate the plausibility of apportioning the aforementioned natural and anthropogenic sources, as a baseline for further CSIA-Br studies of the environmental fate of BOCs. Similar to other stable isotope systems, it is reasonable to expect that different synthetic pathways in some cases give rise to distinguishable bromine isotope effects and thus imprint distinct and potentially diagnostic δ81Br values. Methods have recently been developed to allow environmental applications of CSIA-Br[14–16] by measuring the ratio of the two stable bromine isotopes (81Br and 79Br; average 81Br/79Br = 0.97277) using gas-chromatography multi-collector inductively coupled plasma mass spectrometry (GC-mcICP-MS). The composition of bromine isotopes in a sample is usually expressed in δ81Br notation – the permille deviation of the ratio 81Br/79Br in the sample (RSample) relative to the corresponding ratio (RReference) in the reference material (standard mean ocean bromide, SMOB):
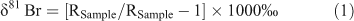
The isotopic composition of organic compounds can vary because of mass-dependent isotope fractionation where one isotope may react faster or be incorporated preferentially in one or several phases.[17] Kinetic isotope effects, where the lighter isotope usually reacts faster than the heavier isotope, are generally more pronounced than equilibrium isotope effects and arise due to slight differences in the chemical bond energy of the isotopes.[18] The isotopic difference in bond energy, and thus reaction rate, is correlated with the relative mass difference between the isotopes. Thus, the isotopes of lighter elements are more likely to exhibit strong isotope fractionation than those of heavier elements. For instance, Reddy et al.[19] found a Cl isotope effect of 11‰ depletion in 37Cl relative to the chloride reagent stock during the enzymatic chlorination of organic substrates via haloperoxidase; the effect for Br is expected to be less (e.g. ~4‰[14]). Very few data of δ81Br-BOC have been reported in the literature. Nevertheless, this methodology has the potential to yield important information about sources as well as degradation and other environmental-fate processes of BOCs in analogy to that of chlorinated organic compounds.[20–24]
The aim of this study was to investigate the stable bromine isotope composition of industrial BOCs by GC-mcICP-MS. This work provides the necessary baseline for forthcoming attempts towards source and fate studies of anthropogenic and naturally produced BOCs based on their bromine isotope composition.
Experimental
Chemicals
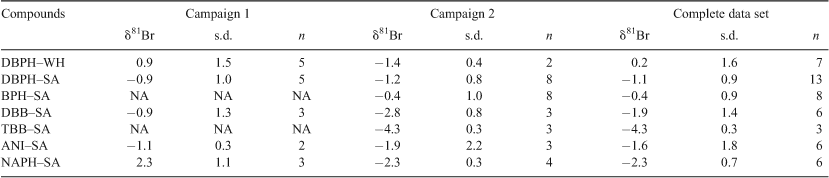
The analytical standard solutions were prepared in n-hexane from the following pure reagents, purchased from Sigma-Aldrich Chemie GmbH, Steinheim, Germany (the –SA suffix refers to Sigma Aldrich): monobromobenzene (MBB–SA); 1,4-dibromobenzene (DBB–SA); 1,3,5-tribromobenzene (TBB–SA); 2,4-dibromoanisole (ANI–SA); 4-bromophenol (BPH–SA); 2,4-dibromophenol (DBPH–SA); and 2-bromonaphthalene (NAPH–SA). Isolation of the natural product 2,4-dibromophenol (DBPH–WH) from gut-voided Saccoglossus bromophenolosus, collected at Lowes Cove (Maine, USA), was performed at the Woods Hole (WH) Oceanographic Institution (Table 1) as detailed in the previous work on these samples.[2]
Derivatisation of bromophenols
Brominated phenols were derivatised by silylation before injection using bis(trimethylsilyl)-trifluoroacetamide (BSTFA). Briefly, the brominated phenols were dried under a gentle stream of N2, redissolved with 200 μL of BSTFA and placed in an oven at 90°C for 1 h. Next 500 μL of n-hexane and 500 μL of acetonitrile were added, the samples were mixed and the acetonitrile was removed. This step was repeated twice. The n-hexane fraction was transferred to a graded vial and reconcentrated to the original volume under a gentle stream of nitrogen. The chosen derivatisation reaction does not affect the bonds to the bromine substituents and is therefore unlikely to cause any bromine isotope fractionation. Any such effects would be secondary and of no significance to the obtained isotope data.
Instrumental analysis
A Hewlett-Packard 5890 Series II GC (Agilent, Santa Clara, CA, USA) with a split/splitless injector was coupled to an Isoprobe ICP-mcMS (GV Instruments, Manchester, UK, formerly Micromass), which features a hexapole collision cell and multi-collector detection system. Gas-chromatographic separation was performed on a 30-m mega-bore column to facilitate the analysis of large samples (Factor Four VF-5 ms: 0.53-mm internal diameter, 0.5-µm film thickness, 5% phenyl–95% dimethylpolysiloxane, Varian Inc., Walnut Creek, CA, USA) with the following temperature programming: 90°C, hold 1.5 min, 15°C min–1 to 270°C, hold 7 min. The injector was operated at 240°C with a splitless time of 1.5 min. Helium was used as carrier-gas at a flow of 6 mL min–1. A deactivated fused silica (FS) capillary (0.25-mm internal diameter) without stationary phase was joined to the analytical column with a standard press-fit union (Siltek Press-Tight, Scantec Lab AB, Sweden) and used for the final length of the transferline. A detailed description of the instrument set-up is given in Holmstrand et al.[15]
Quality control
Signal intensities in the range 2–8 V were obtained from injecting a sample amount equivalent of 30–150 ng of Br (Table 1). This variation in sensitivity depends on the precise position of the capillary within the torch injector tube and on the exact torch position relative to the MS cone aperture. Another source of variation could be due to differences in the ionisation yield of the target compounds in the plasma. Initial tests where an independently determined monobromobenzene (MBB) isotope standard (see below) was measured over several days showed variations of 1.16‰ (1σ, n = 75) in the obtained δ81Br values.[15] The target BOCs were therefore co-injected with the monoaromatic MBB, which was used as a near-authentic reference material in order to reduce the effect of the instrument drift using the standard bracketing procedure. The MBB isotope standard brackets (δ81Br of –0.39‰ v. SMOB) were subsequently used for the calculation of the δ81Br for all compounds as detailed by Holmstrand et al.[15] Multiple injections of samples together with standard brackets were performed for each sample in every measurements campaign (n = 3 to 5 sample injections). The same sets of samples were analysed during the two measurement campaigns and derivatisation of phenolic compounds was performed immediately before injection.
Mass spectrometry peak integration
The mass spectrometry data files were analysed using a Matlab script (version 7.9.0, The MathWorks, Natick, MA, USA). The position of the data peaks were manually selected using an interactive plot. A non-peak region was used to fit a second degree polynomial to account for baseline drift. The baseline corrected data was subsequently used for peak integration. The peak integration was made using a cumulative addition of the intensities in the region of –200 data points to +300 data points relative to the peak position (a data range corresponding to 85 s). As it is the ratios of the calculated integrals for two different isotopes that are used to calculate the δ values, it is the relative values of the integral (rather than the absolute) that are most important. As the cumulative integration scales directly with the intensity of the data, this integration method provides an accurate estimate of the intensity ratio. Both peaks (m/z 79 and 81) were integrated over the same time period, no significant time difference was found for the start, centroid or end of the m/z 79 and 81 peaks.
Data analysis
Standard descriptive statistics were calculated for δ81Br values for the industrial and natural compounds analysed. Student’s t-test was used to examine δ81Br differences between natural and industrial BOCs. Statistical significance was considered at P < 0.05 (two sided). Statistical analysis was performed with the SPSS version 15.0 statistical program (SPSS Inc., Chicago, IL, USA).
Results and discussion
δ81Br values of industrial BOCs
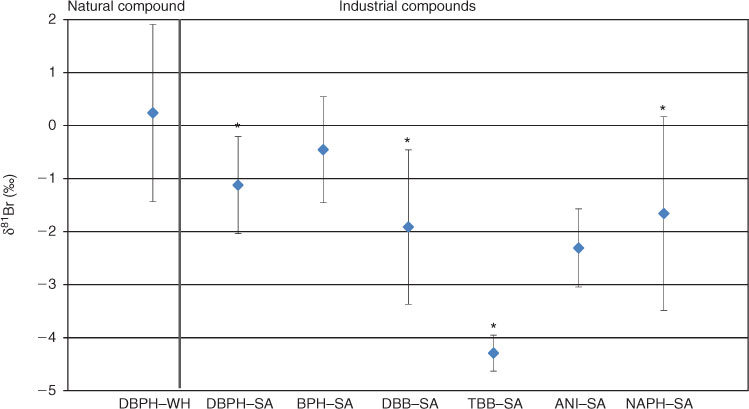
The average δ81Br value for the industrial compounds ranged from –4.3 to –0.4‰ (1 s.d.) (Table 2, Fig. 1). This places initial constraints on the extent of the Br isotopic variability among the industrially produced BOCs in view of the scarce compound-specific δ81Br data reported in the literature.
|
Sylva et al.[14] analysed industrial monobromobenzene (MBB) and 1,3,5-tribromobenzene (TBB), using dibromobenzene with an unknown δ81Br as the isotopic anchor point. Although not able to report results as δ81Br values relative to SMOB, that study demonstrated that the difference in the bromine isotope composition relative to a dibromobenzene was 0.24 ± 0.24‰ for MBB and 0.25 ± 0.30‰ for TBB in a single measurement campaign, indicating a very small isotopic variability among these industrially produced compounds. Gelman et al.[16] analysed 79Br/81Br ratios of industrial BOCs and found a difference of ~2‰ between dibromoethane and other brominated aromatic compounds (toluene, benzene and phenol). They related this variation to different synthetic routes used in the industrial production of the compounds. Another study performed by Holmstrand et al.[15] analysed larger BOCs such as polybrominated diphenyl ethers (PBDEs) in the technical flame retardant mixture Bromkal 70–5DE. That study, similar to the present investigation, employed standard bracketing with MBB and found δ81Br values for the Bromkal components BDE#47, #99 and #100 of –0.6 to +1.6‰. That study also analysed the difference in δ81Br for a BDE#47 and a methoxy BDE#47, both extracted from the blubber of a stranded whale and found it to be just 0.3 ± 0.7‰. Thus, putatively different natural and anthropogenic sources of those particular compounds could not be distinguished with current δ81Br techniques.
The present study found slightly negative δ81Br values for all the industrial compounds (mean value of –1.58 ± 1.44‰) (Table 1). The variability among the industrial BOCs may be related to differences in starting materials, synthesis routes, purification or storage.
δ81Br values of natural v. industrial products
Student’s t-test was used to examine differences between the δ81Br for the natural and the different industrial compounds, using a statistical significance of P < 0.05 (two sided). The average δ81Br for the natural compound (DBPH–WH) was 0.2 ± 1.6‰ (1 s.d.) and for the identical industrial compound (DBPH–SA) –1.1 ± 0.8‰ (1 s.d.). The difference between the natural product (DBPH–WH), and the identical industrial compound (DBPH–SA) was 1.4‰ and statistically significant at P < 0.05 (Accessory publication, Table A1). Furthermore, the δ81Br of the natural compound is significantly different from the mean δ81Br of the industrial compounds (Accessory publication, Table A1). However, the δ81Br of the natural compound is not significantly different from that of compounds ANI–SA and BPH–SA when tested statistically on an individual basis. Thus, the present data set reveals an overlap in the δ81Br of industrial and natural compounds. Even if a higher measurement precision might facilitate the resolution of the δ81Br values of the two groups (i.e. natural and anthropogenic sources), the dynamic range appears too small to be of practical use for source apportionment in this source spectrum.
A perspective on this difference can be had by comparison with the aforementioned experiment of enzymatic chlorination of monoaromatic compounds[19] where the end product was depleted in 37Cl by some ~11‰ relative to the starting material. The isotope effect for bromine in the analogous chemical reaction has been estimated to be one-third of that for chlorine.[25] As our brominated phenol (DBPH–WH) is believed to arise via a similar enzymatic pathway, through the action of the vanadium bromoperoxidase,[11] the expected bromine isotope effect of this bromination would be a ~4‰ depletion of 81Br compared with the bromide starting material.[14] Thus, the hypothesised enzymatic bromination cannot explain the lack of depletion in the δ81Br of the natural sample, unless complicating factors such as isotope fractionation concomitant to degradation processes are considered.
Influence of precursory δ81Br on BOCs
Natural variation in the δ81Br values of inorganic bromide has been reported, such as for bromide in deep groundwater samples from the Norwegian Oseberg field having δ81Br values of +0.08 to +1.27‰.[26] The same set of samples shows negative values for Cl isotopes, in a range from –0.27 to –4.96‰ relative to SMOC, indicating that the isotopic fractionation of Br and Cl isotopes in nature may be driven by different processes. Later work by Shouakar-Stash et al.[26] expanded the known range of δ81Br in natural bromide to –0.80 to + 3.35‰. Taking this natural variability into account, it cannot be excluded that the δ81Br of the analysed samples of both natural and anthropogenic origin could be primarily determined by the δ81Br of the precursors (i.e. the inorganic bromide source).
Comparative evaluation of δ81Br and δ37Cl of industrial products
Several processes can cause fractionation of Br and Cl isotopes during industrial synthesis. Information about the bromine isotopic composition of industrial products is scarce; more work has been done on the chlorine isotopic composition of industrial products. Reddy et al.[27] found a narrow range in the δ37Cl values (–3.37 to –2.11‰) for industrial mixtures of polychlorinated biphenyls (PCBs). Later work by Drenzek et al.[28] expanded the study to other industrialised semi-volatile organochlorines (SVOCs) and found δ37Cl values from –5.10 to +1.22‰. An explanation for this difference was based on the different chemical reactions and precursors involved in the synthesis of the different SVOCs. A similar range in the δ37Cl of compounds with various properties (i.e. chlorophenols and chlorinated ethenes) was found by Aeppli et al.[29]
In the bromine industry, the bromine comes from bromide-rich brine wells and from the Dead Sea waters (up to 50 000 ppm Br). The brines are treated through flushing with chlorine gas admixed with air. Bromide anions are thereby oxidised to bromine by the chlorine gas and can be extracted as bromine gas.[11] Considering the δ81Br-BOC composition in the context of the chemical reactions and precursors involved in their synthesis, it is difficult to explain the observed variability in the δ81Br of the probed industrial BOCs of this study. The industrial compounds were presumably all synthesised under a similar route, direct bromination of the precursors (phenol, benzene or naphthalene) with elemental bromine. However, the contrast between the range of δ81Br for the industrial BOCs (–4.3 to –0.4‰) and the reported range for natural bromide sources (–0.8 to +3.5‰)[26,30,31] indicates that discernable isotope fractionation is indeed occurring during the synthesis of organobromines, in analogy to the effects found for organochlorines.
Conclusions
The herein studied set of seven industrially produced brominated organic compounds (BOCs) exhibited a small variability in their δ81Br values, falling in the range –4.3 to –0.4‰ (average: –1.6 ± 1.4‰, 1 s.d.) relative to standard mean ocean bromine. A statistically significant difference (P < 0.05) of 1.4‰ was found between a natural sample of dibromophenol and its industrial counterpart of identical chemical structure. It was also found that the range in δ81Br of the six analysed industrial compounds overlaps to some extent with the δ81Br of the natural sample. This study provides a baseline for future work employing δ81Br-BOCs to study the sources and degradation of BOCs in the environment. It is conceivable that ambient microbial and photochemical degradation of brominated flame retardants, for example, induces a Br isotope effect so that bromine isotope analysis can be used to assess the extent of environmental degradation of such chemicals, in analogy to the known isotope fractionation for chlorine in organochlorine compounds.
Acknowledgements
We are grateful for instrumental support by Hans Schöberg (Laboratory for Isotope Geology). August Andersson is acknowledged for his help with the Matlab program. The Swedish Environmental Research Council FORMAS funded this work and salary for M. Unger according to (grant contract # 216–2006–565). Ö. Gustafsson also received support as an Academy Researcher from the Royal Swedish Academy of Sciences through a grant from the Knut and Alice Wallenberg Foundation (grant no 629–2002–2309), and P. Andersson was supported by a grant from the Swedish Research Council VR (grant 621–2006–538). Additional support was provided by a grant from the Woods Hole Oceanographic Institution’s Cecil H. and Ida M. Green Technology Edwin W. Hiam Endowed fund for Ocean Science and Technology Innovation Award and the National Science Foundation (OCE-0550486). The GC-mcICP-MS analyses were conducted at the Laboratory for Isotope Geology at the Swedish Museum of Natural History. This study was supported by the Delta Facility, a core facility of the Faculty of Science, Stockholm University.
References
[1] G. W. Gribble, The diversity of naturally occurring organobromine compounds. Chem. Soc. Rev. 1999, 28, 335.| The diversity of naturally occurring organobromine compounds.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXls1WjsLs%3D&md5=7dedfece3b0b373583c434eccaf0886aCAS |
[2] E. L. Teuten, G. M. King, C. M. Reddy, Natural 14C in Saccoglossus bromophenolosus compared to 14C in surrounding sediments. Mar. Ecol. Prog. Ser. 2006, 324, 167.
| Natural 14C in Saccoglossus bromophenolosus compared to 14C in surrounding sediments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXptlSlsA%3D%3D&md5=52e18282e20660aa708d9609763dc97aCAS |
[3] K. T. Fielman, S. A. Woodin, M. D. Walla, D. E. Lincoln, Widespread occurrence of natural halogenated organics among temperate marine infauna. Mar. Ecol. Prog. Ser. 1999, 181, 1.
| Widespread occurrence of natural halogenated organics among temperate marine infauna.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXhs1eqsg%3D%3D&md5=324e82906d961c46cd7d563545639ee2CAS |
[4] G. W. Gribble, The diversity of naturally produced organohalogens. Chemosphere 2003, 52, 289.
| The diversity of naturally produced organohalogens.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXjsVGnsLo%3D&md5=1e744cc5e10e17e0ef5212892c95be9dCAS | 12738253PubMed |
[5] N. Winterton, Chlorine: the only green element-towards a wider acceptance of its role in natural cycles. Green Chem. 2000, 2, 173.
| Chlorine: the only green element-towards a wider acceptance of its role in natural cycles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXntlSisb8%3D&md5=67889005c58743c70bc5030f1f533d52CAS |
[6] C. E. Kicklighter, J. Kubanek, M. E. Hay, Do brominated natural products defend marine worms from consumers? Some do, most don’t. Limnol. Oceanogr. 2004, 49, 430.
| Do brominated natural products defend marine worms from consumers? Some do, most don’t.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXjtFWgu78%3D&md5=6d5bb56bcad2580ce0ddd5d8a26b8485CAS |
[7] E. Pelizzetti, P. Calza, Chemistry of Marine Water and Sediment 2002 (Springer: Berlin).
[8] F. Keppler, R. Eiden, V. Niedan, J. Pracht, H. F. Schöler, Halocarbons produced by natural oxidation processes during degradation of organic matter. Nature 2000, 403, 298.
| Halocarbons produced by natural oxidation processes during degradation of organic matter.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXns1ChsA%3D%3D&md5=b5667c64ef8900f6346f1cbdb5bed64dCAS | 10659846PubMed |
[9] L. Huang, N. C. Sturchio, T. Abrajano, L. J. Heraty, B. D. Holt, Carbon and chlorine isotope fractionation of chlorinated aliphatic hydrocarbons by evaporation. Org. Geochem. 1999, 30, 777.
| Carbon and chlorine isotope fractionation of chlorinated aliphatic hydrocarbons by evaporation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXmtFynu7s%3D&md5=a4c1df8a7eadc38610e196b03cebb882CAS |
[10] R. Theiler, J. C. Cook, L. P. Hager, Haloydrocarbon synthesis by bromoperoxidase. Science 1978, 202, 1094.
| Haloydrocarbon synthesis by bromoperoxidase.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1MXot1yitg%3D%3D&md5=45ea2da2fd4dbd840ee771822b5e0700CAS | 17777960PubMed |
[11] A. Butler, J. N. Carter-Franklin, The role of vanadium bromoperoxidase in the biosynthesis of halogenated marine natural products. Nat. Prod. Rep. 2004, 21, 180.
| The role of vanadium bromoperoxidase in the biosynthesis of halogenated marine natural products.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXisFWgtb0%3D&md5=f7d65c387daaa04c8c15ad526788da79CAS | 15039842PubMed |
[12] M. Alaee, P. Arias, A. Sjödin, A. Bergman, An overview of commercially used countries/regions and possible modes of release. Environ. Int. 2003, 29, 683.
| An overview of commercially used countries/regions and possible modes of release.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXltFKksb4%3D&md5=39926489811920bd99693825688707c0CAS | 12850087PubMed |
[13] C. A. de Wit, M. Alaee, D. C. G. Muir, Levels and trends of brominated retardants in the Arctic. Chemosphere 2006, 64, 209.
| Levels and trends of brominated retardants in the Arctic.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XlsFKrtb8%3D&md5=c16ac3dddde3aedc640d603a0641f13fCAS | 16458344PubMed |
[14] S. P. Sylva, L. Ball, R. K. Nelson, C. M. Reddy, Compound-specific 81Br/79Br analysis by capillary gas chromatography/multicollector inductively coupled plasma mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 3301.
| Compound-specific 81Br/79Br analysis by capillary gas chromatography/multicollector inductively coupled plasma mass spectrometry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXht1ektLfJ&md5=31844474b50e5b93ec1499e131a6c5b8CAS | 17879393PubMed |
[15] H. Holmstrand, M. Unger, D. Carrizo, P. Andersson, Ö. Gustafsson, Compound-specific bromine isotope analysis of penta-brominated diphenyl ethers using GC-ICP-multicollector-MS. Rapid Commun. Mass Spectrom. 2010, 24, 2135.
| Compound-specific bromine isotope analysis of penta-brominated diphenyl ethers using GC-ICP-multicollector-MS.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXnvVOgtbs%3D&md5=c9c5389f5885601a5fa2bbbf9f8827d1CAS | 20552688PubMed |
[16] F. Gelman, L. Halicz, High precision determination of bromide isotope ratio by GC-MC-ICPMS. Inter. Journ. Mass Spec. 2010, 289, 167.
| High precision determination of bromide isotope ratio by GC-MC-ICPMS.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhs1SmtrjM&md5=1c0d3b498649e474558db42dd6e0b8faCAS |
[17] L. Melander, W. H. Saunders, Reaction Rates of Isotopic Molecules 1987 (Krieger Publishing Company: Malabar, FL, USA).
[18] R. Criss, Principles of Stable Isotopic Distribution 1999 (Oxford University Press: New York).
[19] C. M. Reddy, L. Xu, N. D. Drenzek, N. C. Sturchio, L. J. Heraty, C. Kimblin, A. Butler, Chlorine isotope effect for enzyme-catalyzed chlorination. J. Am. Chem. Soc. 2002, 124, 14526.
| Chlorine isotope effect for enzyme-catalyzed chlorination.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xos1Gqtro%3D&md5=27ca068c5aa674813b4411701fb74f69CAS | 12465949PubMed |
[20] N. C. Sturchio, L. J. Clausen, L. J. Heraty, L. Huang, B. D. Holt, T. A. Abrajano, Chlorine isotope investigation of natural attenuation of trichloroethene in an aerobic aquifer. Environ. Sci. Technol. 1998, 32, 3037.
| Chlorine isotope investigation of natural attenuation of trichloroethene in an aerobic aquifer.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXlsVyrt78%3D&md5=f40f70fb2546fc6c103d8bb0072f400aCAS |
[21] N. J. Drenzek, T. I. Eglinton, C. O. Wirsen, H. D. May, Q. Wu, K. R. Sowers, C. M. Reddy, The absence and application of stable carbon isotopic fractionation during the reductive dechlorination of polychlorinated biphenyls. Environ. Sci. Technol. 2001, 35, 3310.
| The absence and application of stable carbon isotopic fractionation during the reductive dechlorination of polychlorinated biphenyls.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXkvV2qtLk%3D&md5=2c058383200dbbd5576f2679fcab48bcCAS | 11529569PubMed |
[22] H. Holmstrand, D. Gadomski, M. Mandalakis, M. Tysklind, R. Irvine, P. Andersson, Ö. Gustafsson, Origin of PCDDs in ball clay assessed with compound-specific chlorine isotope analysis and radiocarbon dating. Environ. Sci. Technol. 2006, 40, 3730.
| Origin of PCDDs in ball clay assessed with compound-specific chlorine isotope analysis and radiocarbon dating.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XkslWlsbY%3D&md5=36364bad54a1cd7bf150f8f73cb3af15CAS | 16830534PubMed |
[23] H. Holmstrand, M. Mandalakis, Z. Zencak, P. Andersson, Ö. Gustafsson, First compound-specific chlorine-isotope analysis of environmentally bioaccumulated organochlorines indicates a degradation-related kinetic isotope effect for DDT. Chemosphere , 69, 1533.
| First compound-specific chlorine-isotope analysis of environmentally bioaccumulated organochlorines indicates a degradation-related kinetic isotope effect for DDT.Crossref | GoogleScholarGoogle Scholar |
[24] T. B. Hofstetter, C. M. Reddy, L. J. Heraty, M. Berg, N. C. Sturchio, Carbon and chlorine isotope effects during abiotic reductive dechlorination of polychlorinated ethanes. Environ. Sci. Technol. 2007, 41, 4662.
| Carbon and chlorine isotope effects during abiotic reductive dechlorination of polychlorinated ethanes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXlsFOkt7w%3D&md5=ff7ffeff8c53acf946f798511998bb65CAS | 17695912PubMed |
[25] J. F. Willey, J. W. Taylor, Capacitive integration to produce high precision isotope ratio measurements on methyl chloride and methyl bromide samples. Anal. Chem. 1978, 50, 1930.
| Capacitive integration to produce high precision isotope ratio measurements on methyl chloride and methyl bromide samples.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1cXlvFSnu7k%3D&md5=4ec56cc611fe551d60bd53fb11e9e7f3CAS |
[26] O. Shouakar-Stash, S. V. Alexeev, S. K. Frape, L. P. Alexeeva, R. J. Drimmie, Geochemistry and stable isotopic signatures, including chlorine and bromine isotopes, of deep groundwaters of the Siberian Platform, Russia. Appl. Geochem. 2007, 22, 589.
| Geochemistry and stable isotopic signatures, including chlorine and bromine isotopes, of deep groundwaters of the Siberian Platform, Russia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXitlygsLs%3D&md5=5e92ced0ba05d6ce97061f8d98865881CAS |
[27] C. M. Reddy, L. J. Heraty, B. D. Holt, N. C. Sturchio, T. I. Eglinton, N. J. Drenzek, L. Xu, J. L. Lake, K. A. Maruya, Stable chlorine isotopic compositions of Aroclors and Aroclor-contaminated sediments. Environ. Sci. Technol. 2000, 34, 2866.
| Stable chlorine isotopic compositions of Aroclors and Aroclor-contaminated sediments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXjsVGks7k%3D&md5=02b17dfccf44817d59cc444b27f2babfCAS |
[28] J. N. Drenzek, C. H. Tarr, T. I. Eglinton, L. J. Heraty, N. C. Sturchio, V. J. Shineer, C. M. Reddy, Stable chlorine and carbon isotopic compositions of selected semi-volatile organochlorines compounds. Org. Geochem. 2002, 33, 437.
| Stable chlorine and carbon isotopic compositions of selected semi-volatile organochlorines compounds.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xitlaksr0%3D&md5=dd161d7f249af3a90b922bc1a754268cCAS |
[29] C. Aeppli, H. Holmstrand, P. Andersson, Ö. Gustafsson, Direct compound-specific stable isotope analysis of organic compounds with quadrupole GC/MS using standard isotope bracketing. Anal. Chem. 2010, 82, 420.
| Direct compound-specific stable isotope analysis of organic compounds with quadrupole GC/MS using standard isotope bracketing.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFCgu7bL&md5=d9423eea88720aa5fc02dc18ed415a1eCAS | 20000586PubMed |
[30] H. G. M. Eggenkamp, M. L. Coleman, Rediscovery of classical methods and their application to the measurement of stable bromine isotopes in natural samples. Chem. Geol. 2000, 167, 393.
| Rediscovery of classical methods and their application to the measurement of stable bromine isotopes in natural samples.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXjt1Shtb0%3D&md5=d1f3fc90e4d526f16c3ff8f2cbfa5aabCAS |
[31] R. L. Stotler, S. K. Frape, O. Shouakar-Stash, An isotopic survey of δ81Br and δ37Cl of dissolved halides in the Canadian and Fennoscandian Shields. Chem. Geol. 2010, 274, 38.
| An isotopic survey of δ81Br and δ37Cl of dissolved halides in the Canadian and Fennoscandian Shields.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXms1elt7g%3D&md5=8fc93cb3d26764168464d62ebb8fac44CAS |