Possible contribution of triboelectricity to snow–air interactions
Ekaterina Y. Tkachenko A C and Sergey G. Kozachkov BA Department of Antarctic Geoecology, Institute of Geological Science, NAS of Ukraine, 55b Gonchara Street, Kyiv, 01054, Ukraine.
B Donau Lab, 73 Laureatska Street, Kyiv, 03026, Ukraine.
C Corresponding author. Email: ktkachenko@igs-nas.org.ua
Environmental Chemistry 9(2) 109-115 https://doi.org/10.1071/EN10074
Submitted: 10 July 2010 Accepted: 29 June 2011 Published: 13 September 2011
Journal Compilation © CSIRO Publishing 2012 Open Access CC BY-NC-ND
Environmental context. Polar near-surface snow can act as a chemical reactor that alters the composition and chemistry of snow and the overlying air. Although the mechanisms and driving forces of these reactions have long been debated, triboelectrification (production of electrostatic charges by friction) of snow by wind has not yet been considered as a factor. It is proposed that in polar regions, triboelectrification could significantly influence the composition and chemistry of snow.
Abstract. Reactions that proceed in polar snow cover may significantly affect the chemistry of the overlying atmosphere, but mechanisms and driving forces of these reactions are still under discussion. The proposed hypothesis attempts to explain some experimental data that cannot be fully understood (e.g. the effect of wind on OH radicals, ozone and persistent organic pollutants levels) by taking into account the influence of electrical phenomena on the snow surface. We assumed that a combination of such factors as low humidity, high wind speed and low temperatures makes the influence of triboelectrification of snow significant for polar areas, where purity and the depth of snow cover prevent fast charge dissipation. The major points of the hypothesis are: (1) when the electric field reaches a value sufficient for the onset of corona discharge, various free radical processes are initiated resulting in changes in the concentrations of ozone, OH radical, nitrate, etc., and the decomposition of pollutant molecules; (2) the high electric field can stimulate transport of ions (such as bromide and nitrate) from the condensed phase to the gas phase; and (3) the ageing of charged snow can increase its electrical potential.
Introduction
It is well known that some volatile organic compounds can reach the polar regions by long-range atmospheric transport where they can be detected in polar snow.[1] Their accumulation in these regions is powered by the so-called ‘cold condensation mechanism’[2] which manifests itself in an increased efficiency of trapping by snowflakes of large non-polar organic molecules, including persistent organic pollutants, under low temperature conditions.[3] What is the fate of pollutants in polar snowpack? Is snowpack a long-term reservoir for contaminants? Can pollutants be chemically destroyed in snow? These questions are of fundamental importance because polar glaciers and ice-sheets store ~75 % of the Earth’s fresh water.
To clarify the fate of pollutants in polar areas we need to have a clear understanding of the processes that take place in snow cover and in the overlying atmosphere. Discussions about mechanisms and driving forces of snow–air interactions started with the discovery by Mauldin et al.[4] of surprisingly high concentrations of some radicals and intermediates of radical reactions at the South Pole. Subsequently, it became clear that observed levels of these substances arise from chemical reactions in the snowpack and at the snow–air interface.
Photochemistry is assumed to be the major factor in snow chemistry processes (Grannas et al.[5] and references therein), but in many cases[6–8] calculations that take into account only the photochemical factor give levels lower than those observed. This observation has led to speculation that some additional coreactant(s) or unknown factor(s) play an important role in snow chemistry processes. Here, we present our view about the role of electrical phenomena in snow–air interactions. Although the electrification of drifting snow is a well known phenomenon,[9–14] the possibility that the polar snow’s electrical energy could be transformed into chemical energy has not previously been discussed.
Snow chemistry experimental data: gaps in our understanding
Why do pollutant levels decrease so rapidly under the influence of wind?
There is evidence (Herbert et al.[15] and references therein) that the decline in concentrations of persistent organic pollutants (POPs) and other volatile chloro-organic compounds during fresh snow pack metamorphosis is quite rapid taking place over a period of several months, which is much faster than values predicted by snow models.[16] Herbert et al.[15] found a correlation between decreases in the levels of POPs in snow and the snow specific surface area (SSA). According to Halsall,[17] these changes are even more significant when wind speed is high: the loss of hexachlorocyclohexane over a 247-h period from a 0.5-m snow layer was 75 % under conditions of high wind speed (10 m s–1), but only 5 % when there was no wind. At some point in their re-volatilisation from snow, POPs have to rise, first, because of the advection-controlled mechanism in POP transfer under wind[18] and, second, because of intensification of snow dry sublimation which results in faster re-partitioning of POPs into interstitial pore spaces and then to the atmosphere.[19] However, existing models of the wind pumping process are poor and a better understanding of the parameters is necessary. It has never been considered in the models that, under conditions of high electrification arising from the wind, the decomposition of POPs might also become a significant factor.
Unknown sources of OH• formation
Hydroxyl radicals (OH•) play a key role in controlling the oxidising power of the atmospheric boundary layer in polar regions.[4,20] Because radicals are short-lived, their concentrations are regarded as the difference between the rate of formation and decay. Concentrations of OH• measured recently at the South Pole[4] and at Summit, Greenland,[6] were found to be comparable with concentrations of this radical in the tropics ((3–6) × 106 cm–3 [21]) and 3–5 times higher than values measured at Palmer and Halley Research Stations (Table 1). It has been suggested that recorded levels of OH• are a product of photochemical transformations at the snow–air interface. Molecules and radicals such as HCHO,[22] NOx,[23] HONO,[24] hydrogen peroxide,[25] higher aldehydes,[26] and HO2 radicals,[27] produced by other photochemical processes taking place in the snowpack, are considered as sources of OH• formation Measured levels of these compounds were used in calculations by a steady-state photochemical box model.[27,28] For data obtained in 2000 at the South Pole, model predictions of OH• were accurate only in a certain range of NO levels.[4,27] For data obtained at Summit, the discrepancy between model and measured values was found to depend strongly on meteorology:[6] when the wind was light (<6 m s–1) the measured value was twice the calculated values, whereas in high wind (>6 m s–1) and blowing snow the measured value was an order of magnitude higher than the calculated value.[6] Thus, the model calculations that have used only photochemical reactions have underestimated the level of OH• in all cases. Possibly, some unknown additional coreactant(s)[7] or unknown factor(s)[6] might be playing a substantial role in OH• formation.

|
Increase of ozone level under the wind at Summit, Greenland
In recent years it has been discovered[29] that ozone is present in the interstitial air of the polar snowpack at levels comparable with those in ambient air. At Summit, up to 90 % of ambient ozone levels were observed at a depth of up to 1 m.[30] According to Helmig et al.[30] photochemical destruction is the dominant mechanism for ozone loss at Summit, and maximum loss is in the near surface layer, where ozone depletion follows both the diurnal and seasonal cycle in solar radiation. Ambient ozone is practically uninfluenced by diurnal cycles. For the investigation of gas exchange processes taking place at the atmosphere–snowpack boundary, Helmig et al.[30] used such parameters as the ozone concentration gradient, which was estimated as the ambient air ozone level at 2 m minus the snowpack ozone concentration at –30 cm. When the snowpack ozone decomposed, the gradient increased. When radiation was <100 W m–2 the gradient was small (<5 ppbv), and the largest gradients (>20 ppbv) were observed at high levels of solar radiation (>500 W m–2) and low wind (<4 m s–1). But as the wind speed increased, the ozone gradient tended to zero. When the wind speed was >8 m s–1, a dramatic increase of snowpack ozone levels occurred which practically compensated the destructive influence of the photochemical factor.[30] We suppose that the wind pumping mechanism cannot completely explain this phenomenon.
Ozone depletion events (ODEs) under high wind in the sea ice zone
ODEs describe a phenomenon that takes place in spring during polar sunrise when boundary layer ozone values drop rapidly from background levels to instrumental detection limits.[31] The mechanism of ODEs is not understood completely, but it is known that the phenomenon is associated with the polar sea ice zone and coincides with enhancement of BrO levels (a ‘bromine explosion’). Current understanding of ODE chemical processes may be represented by the following equations

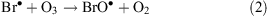
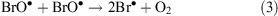

where Reaction 4 is the sum of Reactions 2 and 3.
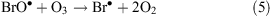
In the presence of ClO•, interhalogen compounds can form
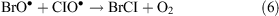
Spectroscopic detection of Br2 (up to 25 ppt) and BrCl (up to 35 ppt) have been reported by Foster et al.[32] during an ODE at Alert, Nunavut, Canada. Hydroxyl and superoxide radicals as well as atmospheric nitrogen oxides, carbon monoxide, methane, and hydrogen also participate in radical reaction cycles. Bromine compounds, however, are considered separately because they are very effective ozone depletion catalysts. But the mechanism of bromine release to the gas phase is not fully understood, nor has it been physically interpreted yet.
ODEs are usually associated with stable or low wind, i.e. ‘fair weather’ conditions. But in the investigation of Jones et al.,[33] enhanced BrO and depleted boundary layer ozone over the Weddell Sea coincided with high wind and saline-blowing snow. Vertical column densities of BrO were over 1 × 1014 molecules cm–2 for the majority of the Weddell Sea (its area is ~2.8 × 106 km2) and BrO was detected even at Halley Station. The role of wind in triggering ODEs and in the release of bromine-containing compounds to the atmosphere is not clear yet.
Triboelectrification processes under high wind
Although the electrification of drifting snow under the influence of wind is well known to meteorologists,[9–14] the chemical consequences of such a phenomenon has never been discussed. Dry snow is a loose material that undergoes triboelectrification under the action of the wind when drifting snow particles rub against each other and strike the underlying surface. Similar triboelectrification of sand can be observed during sandstorms in deserts.[34] When wind blowing over hard snow is particle free, changes in electric field strength are small.[14] With the addition of snow particles, however, the field strength increases significantly, frequently changing polarity, reaching values of 30 kV m–1 at a height of 4 cm above the eroding snow surface, and exceeding fair-weather field values by four orders of magnitude.[14] Both blowing particles and surfaces accumulate some electric charge. Negatively charged snow particles predominate in drifting snow.[13] Freshly fallen, blowing snow can have the opposite sign to snow that has lain for some time. Attractions of dissimilar charges may explain the high adhesion of freshly fallen blowing snow, i.e. its ability to form snowdrifts, dunes, and cornices.[35] We propose that electric phenomena on the snow surface may explain the empirical relationship found by Lipenkov et al.[36] between snow blanket density and wind speed for a region of the East Antarctic ice slope, where the mean wind speed is more than 6 m s–1 and temperatures range from –47 to –20 °C.
Factors affecting triboelectrification
Factors affecting triboelectrification have been analysed quantitatively by Petrenko et al.[37,38] Under their experimental conditions, when the temperature decreased from –10 to –35 °C, the density of charge I increased by an order of magnitude and I collected from the surface increased non-linearly depending on the sliding velocity (v). At t = –10 °C, I is proportional to v, at –14 °C, I increases as v1.5 and at t = –25 °C it increases as v2.[38] Thus, triboelectrification of snow grains by wind increases when the temperature is low and the wind speed is high. With a decrease in humidity, triboelectrification increases because dry air is a poor conductor of electrical current. It is worth noting that ‘Antarctica is the coldest, the windiest, and the driest place on Earth’ (Table 2).

|
Another factor that promotes triboelectrification is a friability of polar snow. Firnification of snow in the Antarctic generally has a reverse direction in comparison with the same process in the middle latitudes.[39] As temperatures of upper layers decrease, evaporation or sublimation goes from the top down, transforming snow of deep layers to ice and making snow of the upper layer more friable. In addition, triboelectricity accumulated during snow drifting cannot be grounded on most of the Antarctic area because of the high thickness of snow cover (on average 2040 m). We suggest that this electrical energy partially converts into chemical energy.
Triboelectrification mechanism
The mechanism of ‘asymmetrical rubbing’ was proposed to interpret ice frictional electrification.[40] According to this mechanism, the charge separation caused by ice-to-ice rubbing occurs as a result of temperature and concentration gradients.[40–42] During friction between two ice pellets, each of them heats up in a different way. A constant rubbing point becomes warmer than a variable rubbing point. When the temperature increases, the concentrations of H+ and OH– in the ice increase. Because H+ ions move more rapidly to colder regions than do OH– ions,[41] a temperature gradient leads to the appearance of an ion concentration gradient. Thus, the colder parts of the ice and snow particles become positively charged whereas the warmer parts take on a negative charge. Hobbs[43] termed this phenomenon the ‘thermoelectric effect’. Besides protons and hydroxide ions, charge can also be carried by defects in the ice, so called protonic point defects,[44] that deviate locally from the ice lattice. Petrenko[37,38] described the ice as a protonic semiconductor.
Measured charge-to-mass ratio for individual blowing snow particles: possibility of corona discharge
Direct measurements of charge-to-mass ratio for individual drifting snow particles have been carried out by Wishart[13] on Byrd Station during the Antarctic summer in wind speeds ranging from 8 to 15 m s–1. The average value obtained by Wishart[13] was –50 μC kg–1. The author noted that during the experiment ‘very high wind conditions and intense electrical effects did not occur. Thus the results obtained may not be applicable to these winter and spring blizzard conditions’. At the same time, Wishart pointed out that some snow particles carried charges ‘close to the maximum values deduced by Wishart and Radok[45] from corona discharge considerations’. A comparable value of average charge-to-mass ratio (–10 μC kg–1) has been reported by Latham and Mongtagne[35] who measured the electrification of drifting snow in Montana. These measurements were carried out using drift traps constructed on the principle of Faraday’s Cage.[13,35] In the 1990s, Schmidt et al.[14] carried out quantitative measurements of charge-to-mass ratio for blowing snow particles and of the electric field gradient near the snow surface in south-east Wyoming with the aim to evaluate the influence of electrostatic force on the trajectory of snow particles moving by saltation. It was found that this influence is comparable with the effect of gravitational and fluid forces. Schmidt et al.[14] concluded that all earlier reported data were under-estimated because drifting particles with opposing charge coexist in saltation, and thus the Faraday cage most likely registered averaged values.[14] Even in the conditions of the Wyoming experiment, where the wind speed was not high (11–14 m s–1), measured particle charge-to-mass ratios (ranging from 72 to –208 μC kg–1) were substantially higher than values reported earlier. The electric field strength fluctuated, reaching +30 kV m–1 at a height of 4 cm above the snow surface and increasing towards the surface of the snowpack. More recent measurements were provided in February 2008 during an Arctic blizzard in Iqaluit, Nunavut, Canada, by Gordon et al.;[46] the measured electric field strength was 26.2 kV m–1 at a height of 0.5 m, which corresponded to model predictions given by the same authors.[47]
According to Dunin,[39] when the electric field strength achieves the value 10 kV m–1 electric energy starts to dissipate through corona discharges that most likely occur on sharp edges where electric field gradients have maximum values. During snow blizzards, blue and violet flashes and glows are often observed, and the noise level in radio signals increases. We expect that the needle-like morphology of snowflakes, which is typical for precipitations of central Antarctica,[48] can also promote the occurrence of corona discharges. Furthermore, we propose that under high winds with high triboelectrification, corona discharge becomes a significant factor in snow chemistry processes.
Chemical processes that can be initiated by corona discharge
Corona discharges appear in regions with a high gradient of electric field as channels of energy dissipation. Obviously, reactions that proceed in the field of a corona discharge take place in the gas phase and at the snow–air interface. Under the influence of a corona discharge, molecules become excited and the degradation of the excited states leads to the formation of reactive species such as radicals, atomic oxygen, ozone, etc.
Under the influence of a corona discharge, oxygen transforms to atomic oxygen and ozone as shown in reactions 7–9.

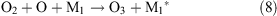
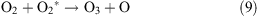
The water molecules that become excited by the corona discharge decay to yield OH• and H• radicals (reaction 10)
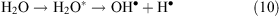
In oxidative conditions H• radicals are oxidised according to reaction 11:
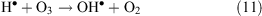
or with assistance of a third particle M (reaction 12)
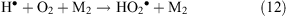
It might be expected that if organic compounds present in the gas phase or at the snow–air interface come under the influence of the corona discharge, they could undergo ionisation and degradation to fragments, or be chemically transformed by the action of reactive species. Indeed, a corona discharge is used both as a standard way of manufacturing ozone and for the removal of unwanted volatile organics, such as solvents, pesticides, and chemical weapons from the atmosphere and water.[49]
Collisions of free radicals with each other, a third particle or with the surface lead to their decay (reactions 13 and 14).
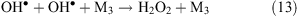
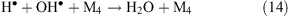
Thus, in places that are affected by a corona discharge, the levels of OH•, atomic oxygen, ozone, and hydrogen peroxide are expected to increase. Nitrogen present in the ambient air under the influence of a corona produces atomic nitrogen, NO3– and NH4+ ions. The influence of a corona discharge can be a possible explanation for the above-mentioned increase in the level of OH• under wind (see ‘Unknown sources of OH• formation’ section above) and the increase in ozone concentration in the interstitial air of snowpacks (see ‘Increase of ozone level under the wind at Summit, Greenland’ section above). In the later case, we propose that ozone production under wind occurs in quantities that are comparable with its photochemical loss and this is the reason why the concentration gradient decreases.
In the investigation of Jones et al.,[33] however, high wind initiated an ozone depletion event. To understand this phenomenon, a distinction should be made between pure Summit snow in the experiment of Helmig[30] and the saline snow of Weddell Sea blizzards described by Jones et al.[33] We propose that if a corona discharge occurs in the case of saline snow blizzards, a mechanism becomes apparent whereby halogens (including bromine) can be released from the condensed phase to the gas phase.
We propose that under a positive potential both the oxidation of bromide anions and the formation of volatile brominated compounds can proceed and the direct emission of negative bromide ions to the gas phase can occur under negative potential.
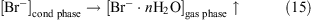
As mentioned above, bromine is an extremely effective ozone depletion catalyst (reactions 1–6), so any ozone present in this environment would be destroyed. In this way a ‘bromine explosion’ and a consequent ODE could be triggered under the influence of wind that comes from the sea ice zone.
‘Ion shooting’ in an electric field: another possible mechanism of ion transport from the condensed phase to the gas phase
‘Shooting’ of charged particles from charged frosty surfaces under high electric potentials has been reported. In the experiment of Latham,[50] a frost specimen was exposed to airstreams of different temperatures resulting in charged ice splinters being blown away. The colder airstreams charged the blowing splinters positively, whereas warmer airstreams charged them negatively. This effect was explained[50] as a manifestation of the thermoelectric effect: positive ions (H+) diffuse down the temperature gradient faster than OH– ions (see ‘Triboelectrification mechanism’ section above). Such temperature gradients are produced down a frost needle owing to the difference in temperature between the air-stream and the frost deposit causing the outer and inner tips of the needle to acquire equal and opposite charges. If the needle was then broken and blown off by the air-stream, it would carry away a charge of one sign leaving that of the other sign on the frost deposit on which it had grown.
Ejection of multiply charged ice fragments from the frosty surface of a growing ice crystal have been also described by Schaefer and Cheng,[51] where electric potential occurred as a result of ice growth (so-called ‘freezing potential’[52]). On the basis of microscopic observations, those authors suggested that strong electric effects were involved with ice dendrites twisting and turning and occasionally ‘shooting off‘.[51] This effect of ‘spraying’ of charged particles into a gas phase is a way to decrease the total energy of the system.
For liquid media, a similar phenomenon of charged particles being ejected from droplets under high electric potential is observed in electrospray ionisation, which is commonly used in mass spectrometry to produce gas-phase ions.[53] Under the conditions of electrospray ionisation, charged micro-drops decrease in size as a result of solvent evaporation until they reach a critical size when surface tension becomes less than the Coulomb repulsion forces (Rayleigh limit) whereupon they burst to form new smaller droplets. This process repeats until the formation of single charged particles. If a similar mechanism could be invoked for charged ice pellets, it might explain how ions such as nitrate and halogens could be transported from the condensed phase to the gas-phase. This hypothesis needs verification.
Electric potential on the snow: ways of transformation
Using physical reasoning it can be shown that when the charge on a body is constant while the surface of the body decreases, the potential increases. We believe that the same effect could be occurring in charged snow. The ageing of charged snow can result in an increase in its electrical potential. This conclusion also follows from the work of Eroshenko[54] who studied the thermodynamics of interfacial areas with the aim of analysing the dispersion, accumulation and transformation of energy. He showed that during the isothermal compression of a heterogeneous system with highly developed interphase boundaries, part of the energy is transformed into electrical energy.[54] Because snow is a thermodynamically non-equilibrium system, its specific surface area decreases as the snow ages, and energy is released partly as heat and partly as electrical energy.
At the same time, the system tends to compensate for this potential increase by equalising the charge in two ways: first, by aggregation of oppositely charged grains, and, second, by the creation of local currents that flow from positive to negative areas thereby initiating electrochemical processes.
Experimental validation: where to now?
To gather experimental support for the proposed hypothesis, we have started to construct a model device, a modification of a vertical mechano-turbine activator with two contra-directional closed circuit snow flows, to simulate friction and attrition of snow grains under blizzard conditions. The interior volume of the reactor needs to be covered with inert material, such as Teflon, or glazed with ice. In such an experimental device – a mechano-activation reactor – with integrated sensors we propose to study the friction and collision of ice particles, snow friction on ice and the chemical consequences of snow triboelectrification. Under these experimental conditions, natural snow as well as artificial snow generated by a snow-making unit can be investigated. Test compounds could be added to the snow to study their behaviour as well as free radicals and ozone levels.
We hope that our discussion may stimulate polar researchers to find evidence for or against the presented hypothesis.
Acknowledgement
The authors express their appreciation to Dr Oleg Varzatskii for professional advice and interesting discussions, and to Prof Petro Gozhyk for support.
References
[1] R. W. Risebrough, W. Walker, T. T. Schmidt, B. W. DeLappe, C. W. Connors, Transfer of chlorinated biphenyls to Antarctica. Nature 1976, 264, 738.| Transfer of chlorinated biphenyls to Antarctica.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE2sXkt1Kgtbs%3D&md5=6383ab130811394d1f2875f0bfd6ebc2CAS |
[2] F. Wania, D. Mackay, A global distribution model for persistent organic chemicals. Sci. Total Environ. 1995, 160–161, 211.
| A global distribution model for persistent organic chemicals.Crossref | GoogleScholarGoogle Scholar |
[3] F. Wania, D. Mackay, Global fractionation and cold condensation of low volatile organochlorine compounds in polar regions. Ambio 1993, 22, 10.
[4] R. L. Mauldin, F. L. Eisele, D. J. Tanner, E. Kosciuch, R. Shetter, B. Lefer, S. R. Hall, J. B. Nowak, M. Buhr, G. Chen, P. Wang, D. David, Measurements of OH, H2SO4 and MSA at the South Pole during ISCAT. Geophys. Res. Lett. 2001, 28, 3629.
| Measurements of OH, H2SO4 and MSA at the South Pole during ISCAT.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXns1ykt7o%3D&md5=682b9af05d1bf063bcd469b8abce29a4CAS |
[5] A. M. Grannas, A. E. Jones, J. Dibb, M. Ammann, C. Anastasio, H. J. Beine, M. Bergin, J. Bottenheim, C. S. Boxe, G. Carver, G. Chen, J. H. Crawford, F. Dominé, M. M. Frey, M. I. Guzmán, D. E. Heard, D. Helmig, M. R. Hoffmann, R. E. Honrath, L. G. Huey, M. Hutterli, H. W. Jacobi, P. Klán, B. Lefer, J. McConnell, J. Plane, R. Sander, J. Savarino, P. B. Shepson, W. R. Simpson, J. R. Sodeau, R. von Glasow, R. Weller, E. W. Wolff, T. Zhu, An overview of snow photochemistry: evidence, mechanisms and impacts. Atmos. Chem. Phys. 2007, 7, 4329.
| An overview of snow photochemistry: evidence, mechanisms and impacts.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtlWiurbJ&md5=e6223eb83c019413ce3c8bce528a5512CAS |
[6] S. J. Sjostedt, L. G. Huey, D. J. Tanner, J. Peischl, G. Chen, J. E. Dibb, B. Lefer, M. A. Hutterli, A. J. Beyersdorf, N. J. Blake, D. R. Blake, D. Sueper, T. Ryerson, J. Burkhart, A. Stohl, Observations of hydroxyl and the sum of peroxy radicals at Summit, Greenland during summer 2003. Atmos. Environ. 2007, 41, 5122.
| Observations of hydroxyl and the sum of peroxy radicals at Summit, Greenland during summer 2003.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXns1Kru7c%3D&md5=aef639c3cafb7d7cf33504e11e7bdaa2CAS |
[7] W. J. Bloss, J. D. Lee, D. E. Heard, R. A. Salmon, S. J.-B. Bauguitte, H. K. Roscoe, A. E. Jones, Observations of OH and HO2 radicals in coastal Antarctica. Atmos. Chem. Phys. 2007, 7, 4171.
| Observations of OH and HO2 radicals in coastal Antarctica.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtlWiurnP&md5=3896db0b3e6c52afe9cdd56b835bcf5eCAS |
[8] A. Jefferson, D. J. Tanner, F. L. Eisele, D. D. Davis, G. Chen, J. Crawford, J. W. Huey, A. L. Torres, H. Berresheim, OH Photochemistry and methane sulphonic acid formation in the coastal Antarctic boundary layer. J. Geophys. Res. 1998, 103, 1647.
| OH Photochemistry and methane sulphonic acid formation in the coastal Antarctic boundary layer.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXpsleksw%3D%3D&md5=8712aa286376ef998383cfcf53c0030aCAS |
[9] P. C. Pearce, B. W. Currie, Some qualitative results on the electrification of snow. Can. J. Res. A 1949, 27, 1.
[10] P. Pluvinage, Some results of atmospheric electricity measurements on the Greenland ice cap. Geophys. Res. Papers 1955, 42, 109.
[11] C. Magono, N. Sakurai, Elektrische Ladungen während Schneefegen im Hochgebirge. Pure Appl. Geophys. 1970, 83, 142.
[12] J. R. Herman, On the electrical properties of blowing snow. Ann. Geophys. 1964, 20, 235.
[13] R. Wishart, Electrification of Antarctic drifting snow, in Proceedings International Symposium on Antarctic Glaciological Exploration (ISAGE Symposium), Hanover, NH, USA, 3–7 September 1968 (Eds A. J. Gow, C. Keeler, C. C. Langway, W. F. Weeks) 1970, IASH Publication Number 86, pp. 316–324. Available at http://iahs.info/redbooks/a086/086036.pdf [Verified 19 July 2011].
[14] D. S. Schmidt, R. A. Schmidt, J. D. Dent, Electrostatic force in blowing snow. Boundary-Layer Meteorol. 1999, 93, 29.
| Electrostatic force in blowing snow.Crossref | GoogleScholarGoogle Scholar |
[15] B. M. J. Herbert, C. J. Halsall, S. Villa, K. C. Jones, R. Kallenborn, Rapid changes in PCB and OC pesticide concentrations in Arctic snow. Environ. Sci. Technol. 2005, 39, 2998.
| Rapid changes in PCB and OC pesticide concentrations in Arctic snow.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXisFOgu7k%3D&md5=322cf29b2f0425194cda7cd04375e372CAS |
[16] F. Wania, Modeling the fate of non-polar organic chemicals in an ageing snow pack Chemosphere 1997, 35, 2345.
| Modeling the fate of non-polar organic chemicals in an ageing snow packCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXnt1artrw%3D&md5=ad3a35c2bf1dad7383e10e5f3ad5bd64CAS |
[17] C. J. Halsall, Investigation and occurrence of persistent organic pollutants (POPs) in the arctic: their atmospheric behaviour and interaction with the seasonal snow pack. Environ. Pollut. 2004, 128, 163.
| Investigation and occurrence of persistent organic pollutants (POPs) in the arctic: their atmospheric behaviour and interaction with the seasonal snow pack.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXps1Cjt7k%3D&md5=4340dea92cf3039773834632d6ea243aCAS |
[18] M. R. Albert, E. F. Shulz, Snow and firn properties and air-snow transport processes at Summit, Greenland. Atmos. Environ. 2002, 36, 2789.
| Snow and firn properties and air-snow transport processes at Summit, Greenland.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XktlKmt78%3D&md5=f30b7f8a662c127c34d91b3c4d0455bcCAS |
[19] A. Cabanes, L. Legagneux, F. Dominé, Evolution of the specific surface area and morphology of Arctic fresh snow during the ALERT 2000 campaign. Atmos. Environ. 2002, 36, 2767.
| Evolution of the specific surface area and morphology of Arctic fresh snow during the ALERT 2000 campaign.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XktlKmtrc%3D&md5=66ac05b815e0367d4ef53f271995bd28CAS |
[20] F. Dominé, P. B. Shepson, Air–snow interactions and atmospheric chemistry. Science 2002, 297, 1506.
| Air–snow interactions and atmospheric chemistry.Crossref | GoogleScholarGoogle Scholar |
[21] R. L. Mauldin, D. J. Tanner, F. L. Eisele, Measurement of OH during PEM-Tropics A. J. Geophys. Res. 1999, 104, 5817.
| Measurement of OH during PEM-Tropics A.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXis1Cns7k%3D&md5=6f98f5fea73423dcc082abb65b0f1339CAS |
[22] A.-L. Sumner, P. B. Shepson, Snowpack production of formaldehyde and its effect on the Arctic troposphere. Nature 1999, 398, 230.
| Snowpack production of formaldehyde and its effect on the Arctic troposphere.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXitVGksro%3D&md5=28ef57bd58cd09abd28b92da29052dc3CAS |
[23] A. E. Jones, R. Weller, E. W. Wolf, H.-W. Jacobi, Speciation and rate of photochemical NO and NO2 production in Antarctic snow. Geophys. Res. Lett. 2000, 27, 345.
| Speciation and rate of photochemical NO and NO2 production in Antarctic snow.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXhtV2lurk%3D&md5=8e4122cf12df648873fa477bcf5090e9CAS |
[24] X. Zhou, H. Beine, R. E. Honrath, J. D. Fuentes, W. Simpson, P. B. Shepson, J. W. Bottenheim, Snowpack photochemical production of HONO: a major source of OH in the Arctic Boundary Layer in springtime. Geophys. Res. Lett. 2001, 28, 4087.
| Snowpack photochemical production of HONO: a major source of OH in the Arctic Boundary Layer in springtime.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXotl2rs7w%3D&md5=21650450ae11ecab5ef1235df7ff25ceCAS |
[25] M. A. Hutterli, J. R. McConnell, R. W. Stewart, H.-W. Jacobi, R. C. Bales, Impact of temperature-driven cycling of hydrogen peroxide (H2O2) between air and snow on the planetary boundary layer. J. Geophys. Res. Atmos. 2001, 106, 15395.
| Impact of temperature-driven cycling of hydrogen peroxide (H2O2) between air and snow on the planetary boundary layer.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXmtVyrs7Y%3D&md5=313777bfaa70336fb4994fc5c23aa772CAS |
[26] A. M. Grannas, P. B. Shepson, C. Guimbaud, A. L. Sumner, M. Albert, W. Simpson, F. Dominé, H. Boudries, J. Bottenheim, H. J. Beine, R. E. Honrath, X. Zhou, A study of photochemical and physical processes affecting carbonyl compounds in the Arctic atmospheric boundary layer. Atmos. Environ. 2002, 36, 2733.
| A study of photochemical and physical processes affecting carbonyl compounds in the Arctic atmospheric boundary layer.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XktlKmtrg%3D&md5=d7b96059b684b9f22666a775785b0c8fCAS |
[27] G. Chen, D. Davis, J. Crawford, L. M. Hutterli, L. G. Huey, D. Slusher, L. Mauldin, F. Eisele, D. Tanner, J. Dibb, M. Buhr, J. McConnell, B. Lefer, R. Shetter, D. Blake, C. H. Song, K. Lombardo, J. Arnoldy A reassessment of HOx South Pole chemistry based on observations recorded during ISCAT 2000. Atmos. Environ. 2004, 38, 5451.
| J. Arnoldy A reassessment of HOx South Pole chemistry based on observations recorded during ISCAT 2000.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXntVGjsrw%3D&md5=5e902f6a36dede4c5f269fa50dc2b4dcCAS |
[28] J. Yang, R. E. Honrath, M. C. Peterson, J. E. Dibb, A. L. Sumner, P. B. Shepson, M. Frey, H.-W. Jacobi, A. Swanson, N. Blake, Impacts of snowpack emissions on deduced levels of OH and peroxy radicals at Summit, Greenland. Atmos. Environ. 2002, 36, 2523.
| Impacts of snowpack emissions on deduced levels of OH and peroxy radicals at Summit, Greenland.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XktlKmsLk%3D&md5=1f6f9b2f3fea12abd07a46f583b673a2CAS |
[29] M. C. Peterson, R. E. Honrath, Observations of rapid photochemical destruction of ozone in snowpack interstitial air. Geophys. Res. Lett. 2001, 28, 511.
| Observations of rapid photochemical destruction of ozone in snowpack interstitial air.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXhtlahsLk%3D&md5=0ddaefac90516b439228e3bbe7adb666CAS |
[30] D. Helmig, F. Bocquet, L. Cohen, S. J. Oltmans, Ozone uptake to the polar snowpack at Summit, Greenland. Atmos. Environ. 2007, 41, 5061.
| Ozone uptake to the polar snowpack at Summit, Greenland.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXns1Kru7o%3D&md5=fce17964d92ba329f316d23982cfd7f6CAS |
[31] W. R. Simpson, R. von Glasow, K. Riedel, P. Anderson, P. Ariya, J. Bottenheim, J. Burrows, L. J. Carpenter, U. Frieß, M. E. Goodsite, D. Heard, M. Hutterli, H.-W. Jacobi, L. Kaleschke, B. Neff, J. Plane, U. Platt, A. Richter, H. Roscoe, R. Sander, P. Shepson, J. Sodeau, A. Steffen, T. Wagner, E. Wolff, Halogens and their role in polar boundary-layer ozone depletion Atmos. Chem. Phys. 2007, 7, 4375.
| Halogens and their role in polar boundary-layer ozone depletionCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtlWiurbK&md5=310626f7a40ff7118e48eda58fb804deCAS |
[32] K. L. Foster, R. A. Plastridge, J. W. Bottenheim, P. B. Shepson, B. J. Finlayson-Pitts, C. W. Spicer, The role of Br2 and BrCl in surface ozone destruction at polar sunrise. Science 2001, 291, 471.
| The role of Br2 and BrCl in surface ozone destruction at polar sunrise.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXlslegtg%3D%3D&md5=6e9bfd0c844663ad9078db35570ea5bcCAS |
[33] A. E. Jones, P. S. Anderson, M. Begoin, N. Brough, M. A. Hutterli, G. J. Marshall, A. Richter, H. K. Roscoe, E. W. Wolff, BrO, blizzards, and drivers of polar tropospheric ozone depletion events Atmos. Chem. Phys. 2009, 9, 4639.
| BrO, blizzards, and drivers of polar tropospheric ozone depletion eventsCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFGns7bJ&md5=915b68e50fbab0262b429310d8005380CAS |
[34] J. Latham, The electrification of snowstorms and sandstorms. Q. J. R. Meteorol. Soc. 1964, 90, 91.
| The electrification of snowstorms and sandstorms.Crossref | GoogleScholarGoogle Scholar |
[35] J. Latham, J. Montagne, The possible importance of electrical forces in the development of cornices. J. Glaciol. 1970, 9, 375.
[36] V. Y. Lipenkov, A. A. Ekaykin, N. I. Barkov, M. O. Purshe, On the relation between surface snow density in Antarctica and wind speed. Data Glaciol. Stud. 1998, 85, 148.[in Russian]
[37] V. F. Petrenko, S. C. Colbeck, Generation of electric fields by ice and snow friction. J. Appl. Phys. 1995, 77, 4518.
| Generation of electric fields by ice and snow friction.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXlt1Cmu7k%3D&md5=f6ff6f17b032844071cd65b3ff9b425aCAS |
[38] V. F. Petrenko, Electromechanical Phenomena in Ice. Special Report 96–2 1996 (Cold Regions Research & Engineering Laboratory, US Army Corps of Engineers).
[39] A. K. Dunin, In the Kingdom of Snow 1983 (Nauka: Novosibirsk) [in Russian].
[40] J. Latham, Electrification produced by the asymmetric rubbing of ice on ice. Br. J. Appl. Phys. 1963, 14, 488.
| Electrification produced by the asymmetric rubbing of ice on ice.Crossref | GoogleScholarGoogle Scholar |
[41] J. Latham, B. J. Mason, Electric charge transfer association with temperature gradients in ice. Proc. R. Soc. Lond. 1961, A260, 523.
[42] J. Latham, C. D. Stow, A laboratory investigation of the electrification of snowstorms. Q. J. R. Meteorol. Soc. 1967, 93, 55.
| A laboratory investigation of the electrification of snowstorms.Crossref | GoogleScholarGoogle Scholar |
[43] P. V. Hobbs, Ice Physics 1974, p. 179 (Clarendon Press: Oxford, UK).
[44] N. Bjerrum, Structure and properties of ice. Science 1952, 115, 385.
| Structure and properties of ice.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaG38XksVaqsQ%3D%3D&md5=1d726b794e1b7b2942214d0b8c28ba17CAS |
[45] E. R. Wishart, U. Radok, Electrostatic charging of aerial wires during Antarctic blizzards Polar Meteorology, Technical Note 87, WMO-No. 211.TP.111 1967, pp. 492–529 (World Meteorological Organisation: Geneva).
[46] M. Gordon, S. Biswas, P. A. Taylor, J. Hanesiak, M. Albarran-Melzer, S. Fargey, Measurements of drifting and blowing snow at Iqaluit, Nunavut, Canada during the STAR project. Atmos.-ocean 2010, 48, 81.
| Measurements of drifting and blowing snow at Iqaluit, Nunavut, Canada during the STAR project.Crossref | GoogleScholarGoogle Scholar |
[47] M. Gordon, P. Taylor, The electric field during blowing snow events. Boundary-Layer Meteorol. 2009, 130, 97.
| The electric field during blowing snow events.Crossref | GoogleScholarGoogle Scholar |
[48] A. A. Ekaykin, Meteorological regime of Central Antarctic and his role in the formation of isotope composition of snow mass 2003 PhD Dissertation, Arctic and Antarctic Institute, Saint Petersburg [in Russian].
[49] M. G. Sobacchi, A. V. Saveliev, A. A. Fridman, A. F. Gutsol, L. A. Kennedy, Experimental assessment of pulsed corona discharge for treatment of VOC emissions. Plasma Chem. Plasma Process. 2003, 23, 347.
| Experimental assessment of pulsed corona discharge for treatment of VOC emissions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXitl2jtb8%3D&md5=bf8267eb43949ba300398f0949a50294CAS |
[50] J. Latham, The electrification of frost deposits. Q. J. R. Met. Soc. 1963, 89, 265.
| The electrification of frost deposits.Crossref | GoogleScholarGoogle Scholar |
[51] V. J. Schaefer, R. J. Cheng, The production of ice crystals fragments by sublimation and electrification. J. Rech. Atmos. 1971, 5, 5.
[52] E. J. Workman, S. E. Reynolds, Electrical phenomena occurring during the freezing of dilute aqueous solutions and their possible relationship to thunderstorm electricity. Phys. Rev. 1950, 78, 254.
| Electrical phenomena occurring during the freezing of dilute aqueous solutions and their possible relationship to thunderstorm electricity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaG3cXjsFSqsw%3D%3D&md5=a16e7ca615cc6cfe390cfb3b4e71d441CAS |
[53] M. Yamashita, J. B. Fenn, Negative ion production with the electrospray ion source. J. Phys. Chem. 1984, 88, 4671.
| Negative ion production with the electrospray ion source.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2cXlsVahs7Y%3D&md5=32ee64546e875e98b894d437ec13e104CAS |
[54] V. A. Eroshenko, Influence of the specific interface in a lyophobic heterogeneous system upon observable thermal effects during the isothermal compression. J. Russ. Chem. Soc. 2002, 46, 31.[in Russian]
| 1:CAS:528:DC%2BD38XnslShtbc%3D&md5=61417f8bacc3ef14a6a9de51605f8691CAS |
[55] I. M. Dolgin, L. S. Petrov (Eds), Atmospheric temperature. Atmospheric Pressure, Wind, Atmospheric Humidity, Nebulosity, Precipitations, Meteors, Visibility. Vol. 2 1977 (Gidrometeoizdat: Leningrad) [in Russian].


