Coal chemical industry membrane concentrates: characterisation and treatment by ozonation and catalytic ozonation processes
Xiangtong Kong A , Shikha Garg A , Guifeng Chen B , Wenbo Li B , Yuan Wang A C , Jikun Wang B , Jinxing Ma A , Yuting Yuan A C and T. David Waite A C *
A C *
A Water Research Centre, School of Civil and Environmental Engineering, The University of New South Wales, Sydney, NSW 2052, Australia.
B China Coal Research Institute, Beijing 100013, P. R. China.
C UNSW Centre for Transformational Environmental Technologies (CTET), Yixing, Jiangsu Province 214206, P. R. China.
Environmental Chemistry 19(4) 156-166 https://doi.org/10.1071/EN22042
Submitted: 30 April 2022 Accepted: 5 July 2022 Published: 8 August 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Environmental context. Reverse osmosis (RO) is widely used for the treatment of hazardous wastewaters produced from the coal chemical industry (CCI) to achieve zero liquid discharge however the use of RO inevitably results in accumulation of refractory organic matter in the RO membrane concentrate, the treatment of which is challenging. This work provides useful insights into the organic composition of RO concentrates obtained from a range of real CCI wastewaters. The efficacy of treatment of these concentrates by ozonation processes is assessed as is the cost effectiveness of such treatment.
Rationale. The enactment of increasingly stringent regulations has prompted the implementation of membrane technologies such as reverse osmosis (RO) in the management of coal chemical industry (CCI) wastewaters with the goal of achieving zero liquid discharge (ZLD). However, this practice inevitably results in the production of high salinity concentrates containing refractory organic matter.
Methodology. In this study, we characterised the organic composition of RO concentrates obtained from the CCI using a variety of methods including liquid chromatography–organic carbon/nitrogen detection (LC-OCND) and investigated the degradability of organic compounds present in these concentrates by ozonation and catalytic ozonation processes.
Results and discussion. Organic analysis using LC-OCND revealed that humic-like substances and low molecular weight neutral compounds were the dominant constituents in the CCI concentrates examined. Measurement of degradability of the CCI concentrate by a pure ozonation process showed low treatment efficiency (~20% dissolved organic carbon (DOC) removal) as a result of the refractory nature of the organic compounds present in the wastewater. The degradation of these organics by a catalytic ozonation process employing a commercially available Fe-oxide based catalyst was higher than that observed by pure ozonation although the extent of organics removal (DOC removal ~47%) is still low due to the refractory nature of the organics as well as the influence of salts on the catalyst performance. Techno-economic analysis of the pure ozonation and catalytic ozonation processes indicated that the total cost of implementation of the ozonation processes (homogeneous or heterogeneous) for CCI concentrate treatment is negligible compared with the overall cost of the complete ZLD process.
Keywords: catalytic ozonation, coal chemical wastewaters, liquid chromatography–organic carbon/nitrogen detection, low molecular weight neutrals, refractory organics, RO concentrate, technoeconomic assessment, zero liquid discharge.
Introduction
We are pleased to include this article in the Special Edition of Environmental Chemistry dedicated to Dr Graeme Batley. One of the hallmarks of Graeme’s career has been the application of analytical chemistry to the understanding and, where possible, solution of real-world problems. In this article, we use a variety of analytical methods to characterise membrane concentrates produced in the coal chemical industry (CCI) and, based on the results obtained, assess the viability of the increasingly widely used treatment technology of ozonation, both conventional and catalytic, to the removal of recalcitrant organic compounds from these troublesome wastewaters. Particular attention is given to the increasingly popular method of liquid chromatography–organic carbon/nitrogen detection (LC-OCND) as a relatively simple means of separating compounds in these complex mixtures into different groups based on their size, charge and hydrophobicity. We then assess the ability of ozonation to degrade these various groupings. Graeme’s work has been particularly impactful because of his use of analytical tools to find pragmatic solutions to environmental problems, an approach we believe is embodied in the work presented here.
The CCI plays an important role in the supply of a variety of high-value chemicals such as synthetic natural gas, methanol and ammonia (Zhang et al. 2019). However, CCI is also a leading contributor to wastewater discharge with treatment of CCI wastewater particularly challenging due to the presence of toxic and hazardous compounds such as polycyclic aromatic hydrocarbons and nitrogen heterocyclic compounds (Zhao and Liu 2016). Extensive effort has therefore been devoted to the research and development of various processes (including biological processes, coagulation processes and advanced oxidation processes (AOPs)) for the removal of organic matter (and other contaminants such as ammonia and fluoride) from CCI wastewaters. Membrane technologies are also becoming increasingly popular as a treatment option to polish the effluent from biological and/or coagulation processes to meet rigorous discharge standards and, in some instances, zero liquid discharge (ZLD) requirements (Jin et al. 2013; Li et al. 2016). Both low- and high-pressure driven membrane processes (e.g. ultrafiltration (UF) and reverse osmosis (RO)) have been used in the management of CCI wastewaters (Ji et al. 2016; Wang et al. 2019; Li et al. 2020). While UF membranes are typically integrated with coagulation, activated carbon adsorption and biological processes to enhance the removal of colloidal particles, the high-pressure technologies such as RO have smaller molecular weight cut-offs (MWCO) and produce desalinated effluents suitable for reuse in coal production and/or for cooling purposes. However, the use of RO inevitably results in the accumulation of refractory organic matter in the RO membrane concentrate, the treatment of which has been recognised as one of the critical challenges in the management of CCI wastewater and achievement of ZLD (Xiong and Wei 2017; Shi et al. 2021).
Recently, implementation of AOPs in the treatment of RO concentrates from industrial wastewater reclamation plants has gained interest (Cai et al. 2020). However, there is limited understanding of the nature of the refractory organic compounds present in the RO concentrate produced following treatment of CCI wastewaters and how this might influence the overall degradability and treatment efficiency. In this study, we characterised the organic matter present in a variety of RO concentrates from full-scale plants treating CCI wastewaters (referred as CCI concentrates from hereon) and investigated the treatment of one of these concentrates by pure ozonation and catalytic ozonation processes (Miklos et al. 2018; Du et al. 2020; Zheng et al. 2022). We used liquid chromatography–organic carbon/nitrogen detection (LC-OCND) (Huber et al. 2011) to fractionate the organic matter present in the concentrate into different groups and subsequently assessed the treatability of these various groups by pure ozonation and catalytic ozonation. We also characterised the changes in the excitation–emission fluorescence (EEM) spectra of the CCI concentrate following treatment by pure ozonation and catalytic ozonation processes although it should be noted that this technique only depicts the fraction of organic matter that exhibits fluorescence (Yang et al. 2013; Yu et al. 2016; Li et al. 2019). We have also performed a detailed technical economic analysis of the pure ozonation and catalytic ozonation processes to determine the economic viability of these to achieve ZLD. This is one of very few studies that have performed detailed analysis of the organic nature of CCI concentrates from various coal chemical processes to provide insights into the organic composition of CCI concentrates and the treatability of these concentrates using ozonation processes.
Experimental
CCI concentrate sampling
Five CCI concentrate samples (i.e. C1#, C2#, C13#, M1# and CW1#) were collected from four wastewater treatment plants. C1# and C2# are essentially duplicate samples taken on the same day of an RO concentrate from a coal gasification wastewater treatment plant (Xintian) with the location from which these samples were taken shown in the flow diagram of the treatment unit in Fig. 1a. C13# represents an RO concentrate sample from another treatment facility for coal gasification wastewater (Fig. 1b). M1# represents a combined stream of concentrates from the ion exchange and RO units, used for treatment of methanol synthesis wastewater (Zhongmei Yuanxing), which was further polished by coagulation and ultrafiltration prior to collection (Fig. 1c). CW1# represents a sample of mixed concentrates from RO and electrodialysis processes from a coking wastewater treatment plant in Qian-an (Fig. 1d). The concentrate used in this study has undergone multistage ultrafiltration prior to the RO process and hence contains no particulate organic carbon (>0.22 µm).

|
Reagents
All chemicals (NaCl, Na2SO4, tert-butyl alcohol (TBA, SKU 360538) and humic acid (HA, SKU 53680)) used in this work were of analytical grade and purchased from Sigma–Aldrich unless stated otherwise. All solutions were prepared in Milli-Q (MQ) ultrapure water with a resistivity of 18.2 MΩ cm−1 (Millipore). To avoid contamination by trace metals, all glassware was soaked in 5% v/v nitric acid prior to use. A 1.0 g C L–1 stock solution of HA (Aldrich) was prepared in MQ and filtered using 0.22 µm PVDF filters (Millipore) prior to use. Gas phase ozone was produced from an ozone generator (Yuan et al. 2020) (T4000, 5.0 g L−1, Oxyzone Pty Ltd, Australia) with pure oxygen used as the gas source.
Catalyst description and characterisation
The Fe-loaded Al2O3 catalyst used for the catalytic ozonation study was provided by the Coal Chemical Research Institute (CCRI, China). Upon receipt, the catalyst was prewashed with MQ water until the supernatant was clear and then dried at 60°C prior to use. Detailed characterisation of the surface properties and composition of the catalyst was performed in our earlier work (Yuan et al. 2022) and showed that the major constituents of the catalyst were hematite (01-084-9870), Al2O3 (00-046-1131) and MnO2 (04-009-8106). The pHzpc of the catalyst was around 8.4 suggesting that the catalyst surface will be positive under the pH conditions investigated in this study (pH 7.5–7.6).
CCI concentrate analysis
Methods used for analysis of CCI concentrate samples are summarised in Supplementary Table S1. The concentration of organics present in the CCI concentrates was quantified using dissolved organic carbon (DOC) and chemical oxygen demand (COD) measurements. DOC and COD were measured using a Shimadzu TOC analyser and Hach COD reactor respectively. COD measurements were corrected for the presence of high Cl− concentrations in the CCI concentrates by subtracting the COD contributed from Cl− (CODCl−) from the measured sample COD values. For calculation of CODCl−, the Cl− concentration in the samples was measured using ion chromatography (IC) and then converted into CODCl− values using the calibration curve developed by measuring the COD value of pure NaCl solutions. Fractionation of the organic matter present in the CCI concentrates was conducted using a LC-OCND size-exclusion chromatography system (DOC-LABOR) according to the standard protocols for this instrument (Huber 2017). The signals of the principal components including biopolymers (BP, molecular weight (MW) >20 000 Da), humic substances (HS, MW of 500–10 000 Da), building blocks (BB, MW of 300–500 Da), low molecular weight (LMW) acids (MW <350 Da), LMW uncharged species (or ‘neutrals’; MW < 350 Da) and hydrophobic organic carbon were integrated using the proprietary software ChromCalc (DOC-LABOR, Karlsruhe, Germany) (Huber et al. 2011). Note that the NaCl concentrations in the concentrate were much lower than the salt concentrations (i.e. 35 g L–1) at which LC-OCND analysis may be impacted due to formation of hypochlorite on UV irradiation. The similarity in total DOC concentrations determined by TOC analysis and LC-OCND analysis further supports the conclusion that the influence of salts on LC-OCND measurements was negligible.
We also used EEM fluorescence spectroscopy to investigate the transformation of organic compounds in CCI concentrate CW1# (Yang et al. 2013; Yu et al. 2016; Li et al. 2019) following treatment by pure ozonation and catalytic ozonation. All EEM spectra were measured using a fluorescence spectrometer (Cary Eclipse) with scanning emission spectra from 250 to 600 nm at a data interval of 5 nm and excitation wavelength from 200 to 450 nm at an increment of 5 nm. Both the excitation and emission slits were set at 5 nm and the scan speed was set at 600 nm min–1. The spectrum of Milli-Q water was used as the baseline. Note that the concentrate samples were diluted 1000 times to enable analysis by EEM fluorescence spectroscopy, but some degree of inner filter effects remained due to matrix complexity. Classification of EEM signals into categories of humic acid-like and fulvic acid-like substances was performed according to the method documented elsewhere (Chen et al. 2003; Li et al. 2019).
Ozonation and catalytic ozonation setup
Investigation of the degradability of the organic compounds in the concentrate obtained from the Qian-an treatment plant (CW1#) by pure ozonation and catalytic ozonation processes was carried out in a semi-batch system (Supplementary Fig. S1). The volume of the concentrate was fixed at 150 mL and the initial pH of the concentrate was adjusted to 7.5–7.6 using 1 M HCl or 1 M NaOH solutions. The initial DOC and total dissolved solids contents of the concentrate were ~30 mg L–1 and 9 ± 1 g L–1, respectively. In these studies, O3 gas was sparged into the wastewater at a controlled flow rate of 600 mL min–1 using a gas-phase ozone concentration of 51 mg L–1. For catalytic ozonation, 33.3 g L–1 of the catalyst was added to the reactor. At predetermined time intervals (i.e. 10, 20, 30, 40, 60 and 120 min), 5 mL of sample was withdrawn from the reactor. Following sparging with N2 gas for 1 min, the samples were filtered using 0.22 µm PVDF filters (Millipore) and the COD value, DOC concentration and LC-OCND and EEM spectra were measured according to the aforementioned methods.
Results and discussion
Composition of CCI concentrates
The composition of the various CCI concentrate samples is provided in Table 1. The accuracy of major ion measurements was evaluated by checking the charge balance using Visual MINTEQ with the results of these analyses given in Supplementary Table S2. As shown, the charge balance errors are between 5 and 20% for these samples with this error considered acceptable.

|
It can be seen from Table 1 that the composition of C1# and C2# are quite comparable, reflecting the accuracy of these analysis results given the source similarity of these samples. The sum of cation and anion concentrations (>14 000 mg L–1) is largely equal to the total dissolved solid concentrations for each sample. The high hardness value of these concentrates is due to the presence of Mg ions. Other multivalent elements including Fe, Al and Mn are present at relatively low concentrations. Cl− and SO42− are the dominant anions in C1# and C2# samples. The high concentration of Cl− (~200 mM) may potentially create challenges in application of ozonation and catalytic ozonation processes. As shown in our recent work (Yuan et al. 2022), scavenging of ˙OH by chloride ions (particularly in the case of the catalytic ozonation process) as well as transformation of humic-like moieties to a more compact hydrophobic form in the presence of cations (such as Na+/Ca2+) affects the removal of organics by both pure ozonation and catalytic ozonation processes. Since the presence of divalent cations results in more charge shielding between adjacent functional groups in humic compounds compared to that induced by monovalent ions (Baalousha et al. 2006), we expect the transformation of humics to a hydrophobic form to be pronounced in the case of C1# and C2# due to the high hardness of these wastewaters.
As in the case of C1# and C2#, Na+ (~250 mM) and Cl− (~250 mM) are the dominant ionic species in the C13# sample although COD is present at a much higher concentration and SO42− at a much lower concentration (~5 mM) compared to other CCI concentrates. These results are generally in accord with the use of nanofiltration prior to the RO unit (Fig. 1b). In addition, bicarbonate and ammonia are present at much higher concentrations in C13# than in the other samples. Since ammonia can react with ozone (Haag et al. 1984; Khuntia et al. 2012) and bicarbonate ions scavenge ˙OH (Ruffino and Zanetti 2019), matrix effects are likely to be more prominent in the case of C13# compared to other CCI concentrates. As in the case of C1# and C2#, humic-like moieties in this concentrate (if any) are likely to be present in compact hydrophobic form due to the high hardness of these waters which will affect the removal of these moieties by both pure ozonation and catalytic ozonation processes.
In contrast to C1#, C2# and C13#, M1# represents a combined concentrate stream of the waste from the ion exchange process and the concentrate of the second stage RO in a facility treating methanol synthesis wastewater (Fig. 1c). The low hardness in this sample is not surprising as a result of the pre-treatment by a high-loading clarifier with the addition of poly-AlCl3, Na2CO3 and NaOH. SO42− is the dominant anion in M1# due to inclusion of ion exchange waste in M1# (Fig. 1c). In summary, the COD concentration of M1# is relatively low and further mineralisation using AOPs may not be required prior to the thermal treatment of the concentrate (although the catalytic ozonation process has been installed on site).
CW1# represents a mixed RO and electrodialysis concentrate from a coking wastewater treatment facility (Qian-an, Fig. 1d). The COD and salt concentrations of these concentrate samples were relatively low (i.e. COD < 120 mg L–1, TDS < 10 g L–1). The high hardness of these concentrates is due to the presence of Ca and Mg ions. Other multivalent elements including Fe, Al and Mn are present at relatively low concentrations. Similar to the cases of C1# and C2#, Cl− and SO42− are the dominant anions in the CW1# sample as well. The high concentration of Cl− (~50 mM) and high hardness of these samples may potentially create challenges in the application of ozonation and catalytic ozonation processes (Yuan et al. 2022). Since the impact of Ca2+ on the transformation of humic substances and the strength of aggregates formed is more pronounced compared to that observed in the presence of Mg2+ (Hakim et al. 2019), the degradability of humic-like moieties is expected to be lower for this concentrate sample compared to C1# and C2#. Our results also show that F− is present at fairly high concentrations in these concentrate samples although its treatment is beyond the scope of this study.
Fractionation of the organic matter by LC-OCND
Fig. 2a indicates that for CCI concentrate samples C1#, C2#, C13# and M1#, LMW neutrals including alcohols, aldehydes, ketones, amino acids and sugars are the major components (57–88% of the DOC) followed by humic substances and biopolymers. In coking wastewater samples (CW1#), humic substances are the major component (~45%) with hydrophobic organics, building blocks and LMW neutrals present at lower concentrations. While there is no LC-OCND investigation of CCI concentrates reported in the literature, a comparable study on petroleum industrial wastewater treatment in Singapore (Cai et al. 2020) also showed the presence of ‘humic substances’ as the dominant organic fraction in the RO concentrate collected from a water reclamation facility in which the biologically treated effluents were further polished by an MF-RO system.

|
A typical EEM spectrum of the Qian-an CCI concentrate is shown in Fig. 2b with two peaks (i.e. 36.4 and 26.7 AU (mg C L–1)–1) identified at Ex/Em = 235/415 nm and 310/410 nm respectively related to fulvic-like and humic-like substances in Regions III and V of the EEM spectra (Chen et al. 2003; Li et al. 2019). Signals of aromatic proteins I and II in the EEM spectrum were very low with the fluorescence properties generally in good agreement with the LC-OCND results.
Degradability of the refractory organic matter in concentrates by pure ozonation
In this section, we investigate the degradability of the CCI concentrate by pure ozonation. Since only the concentrate from the Qian-an plant (i.e. CW1#) could be regularly collected for further analysis, only treatment of this concentrate stream was performed. Fig. 3 summarises the changes in DOC concentration, LC-OCND components, COD concentration and EEM components as a function of ozonation duration. It can be seen from Fig. 3a that the removal of DOC (~20%) is largely completed in the first 1 h of reaction with the ongoing bubbling of ozone resulting in a minimal increase in the removal efficiency. We classified the DOC based on its reactivity with ozone (Eqn 1):
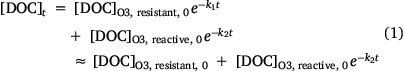
where k1 and k2 represent the apparent pseudo first order rate constants for degradation of O3-resistant DOC and O3-reactive DOC, respectively, with k1 ≪ k2. Fitting Eqn 1 to the results in Fig. 3a, we find that ~76% of the DOC in the Qian-an CCI concentrate is resistant to O3 attack with the pseudo first order degradation rate constant of the reactive component equal to 0.021 min−1. LC-OCND results provided more information on the temporal variations of the different components quantified by this technique (i.e. humic substances, building blocks and LMW neutrals; Fig. 3b). The concentrations of humic substances varied in a similar manner to the total DOC (Fig. 3a) with the O3-resistant component representing 72% of the total humic substances and the O3-reactive humic substances exhibiting a slightly higher pseudo first order degradation rate constant (k2′ = 0.033 min−1, Eqn 2) than that of the DOC. It is worthwhile noting that the oxidation of humic substances led to the formation of LMW neutrals rather than building blocks although this fraction has a molecular weight range between humic substances and LMW neutrals (Huber et al. 2011). An accumulation of LMW neutrals was noted in the first 30 min followed by a decrease through the ongoing process. Since the decrease in humic substance concentration and the initial increase in LMW neutral concentration were similar, it is plausible to conclude that the native LMW neutrals present in the Qian-an concentrate were not O3 reactive. The LMW neutral concentration as a function of time was therefore calculated according to Eqns 2 and 3. The pseudo first order rate constant of degradation of LMW neutrals formed on oxidation of humics (i.e. k3′) was calculated to be 0.005 min−1 indicating that the intermediate products of humics oxidation are more refractory to ozonation. In contrast to the DOC and LC-OCND results, the COD concentration firstly increased with the transformation of humic substances to LMW neutrals and then decreased with the ozonation of LMW neutrals (Fig. 3c). It seems that part of the native humics in the CCI concentrate could not be oxidised by the CODCr reagents but, once these moieties are oxidised on ozonation, the product(s) formed are oxidised by CODCr reagents initially resulting in an increase in the COD value followed by a small decrease on further breakdown of these oxidised products.
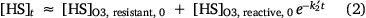
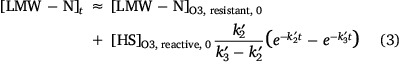

|
The removal of fluorescence signals has been widely used as an indicator of treatability of refractory organic matter from coking wastewaters and concentrates (Yang et al. 2013; Yu et al. 2016; Li et al. 2019). Results shown in Fig. 3d confirm that the fluorescent compounds present in CCI concentrates are readily oxidised by the ozonation process with the specific fluorescence significantly decreasing within 30 min of reaction. The complete EEM spectra of the CCI concentrate at various reaction times is shown in Supplementary Fig. S2. These results agree with a recent study which showed that ozonation caused a significant reduction in aromaticity of humic substances resulting in a decrease in the fluorescence intensity of the local peaks measured using synchronous scanning fluorescence spectroscopy (Sadrnourmohammadi et al. 2020). However, the use of EEM spectroscopy to depict the removal of the refractory organic matter from CCI concentrates is questionable since the results shown in Fig. 3 confirm that the fluorescent compounds likely account for only a small portion of the total humic substances present. While the fulvic acid-like and humic acid-like substances shown in the EEM spectra are likely O3 reactive, the O3-resistant fraction is non fluorescent and is the dominant fraction of organics present in the CCI concentrate (Fig. 3b). As such, it is clear that the LC-OCND technique provides more insight into the organic composition and degradability of CCI concentrates compared to fluorescence spectroscopy.
The low degradability of DOC in the CCI concentrate by the pure ozonation process is possibly related to the high salinity and/or refractory nature of the organics. Measurement of the degradation of synthetic wastewaters containing humic acid (Aldrich) at a salinity similar to that of the CCI concentrates showed that ~46% of the DOC of HA was removed after 1 h of ozonation (Supplementary Fig. S3a). The COD of the HA solution decreased significantly with only 10% COD remaining after 1 h of treatment (Supplementary Fig. S3b). LC-OCND analysis of treated samples clearly show that the humic substances (i.e. HA) had been largely transformed to LMW neutrals despite the high salinity of the solution matrix (Supplementary Fig. S3c). These results thus suggest that matrix effects are not the primary reason for the low removal of humic substances from the Qian-an concentrate by the ozonation process. The low degradability of the CCI concentrate organic constituents is related more to the refractory nature of the organics. As such, further understanding of the structure and properties of the O3-resistance humic substances present in these concentrates would benefit the design of treatment processes for removal of these refractory organic compounds from the wastewater concentrate.
Degradability of the refractory organic matter in concentrates by catalytic ozonation
Results from pure ozonation studies indicated that the organic matter in the CCI concentrates is not readily oxidised by O3 but may possibly degrade under stronger oxidant (e.g. ˙OH) attack. Thus, we investigated the degradation of CCI concentrate by catalytic ozonation employing an Fe-oxide@Al2O3 catalyst. In our earlier work (Yuan et al. 2022), we investigated the mechanism of the catalytic ozonation process using Fe-oxide@Al2O3 and showed that O3 decays on the Fe-oxide@Al2O3 surface to form surface-located ˙OH which then diffuse away from the surface and interact with organic compounds present in the interfacial boundary layer and/or bulk solution. The formation of surface-located ˙OH on catalyst-O3 interaction was confirmed in our earlier work (Yuan et al. 2022) using fluorescence microscopy imaging analysis.
As shown in Fig. 4, treatment of the CCI concentrate by catalytic ozonation results in higher DOC removal (47%) compared to that observed by pure ozonation (20%). Fitting Eqn 1 to the results shown in Fig. 4a, we find that ~60% of the DOC in the Qian-an CCI concentrate is resistant to degradation by catalytic ozonation with the pseudo first order degradation rate constant of the reactive component equal to 0.040 min−1; a value nearly two-fold higher than the rate constant determined in the pure ozonation studies. LC-OCND results show that the concentration of humic substances (Fig. 4b) varied in a similar manner to the total DOC concentration (Fig. 4a) with the O3-resistant component representing 64% of the total humic substances and the O3-reactive humic substances exhibiting a slightly lower pseudo first order degradation rate constant (k2′ = 0.012 min−1, Eqn 2) than that of the DOC. This is in contrast with the results obtained from pure ozonation studies wherein k2′ > k1. This observation suggests that catalytic ozonation is more effective in oxidising other organic components (i.e. LMW neutral and/or building block) while humic substances are more readily degraded by direct O3 attack. Fitting Eqns 2 and 3 to the LMW neutral concentration, we obtain a pseudo first order degradation rate constant of 0.017 min−1 which is much higher than the value determined for the pure ozonation process (0.005 min−1). This result supports the conclusion that the intermediate products of humics oxidation are more readily oxidised by the oxidants generated on catalytic ozonation compared to pure ozonation. As observed in the case of pure ozonation, specific fluorescence of the CCI concentrate significantly decreased within 30 min of treatment (Fig. 4c) confirming that the fluorescent compounds present in CCI concentrates are readily oxidised by ozone with no further enhancement in the rate of removal of these fluorescent compounds by the catalytic ozonation process. Our earlier results indicated that the accumulation of chloride ions in the electrical double layer near the catalyst surface, particularly when pH < pHpzc, results in significant scavenging of surface associated ˙OH. As such, the influence of salts present in CCI concentrate on the performance of this catalyst will be significant. Use of a halide-resistant catalyst is thus expected to further enhance the performance of the catalytic ozonation process. Furthermore, employing a two-stage process involving pure ozonation (to degrade humic-like substances) followed by catalytic ozonation (to degrade oxidation products of these substances) may be more effective than use of catalytic ozonation alone.
Overall, our results show that the catalytic ozonation process is effective in degrading CCI concentrate. With improved understanding of the nature of the refractory organics and use of a halide-resistant catalyst, the treatment efficiency of CCI concentrates by a catalytic ozonation process may be further increased.
Techno-economic analysis
In order to explore the viability of use of ozonation processes at an industrial scale, we also performed a detailed technical-economic analysis of the ozonation and catalytic ozonation processes for a treatment capacity of 5000 m3 day–1 and water quality the same as that of the concentrate from the Qian-an plant (i.e. CODfeed = 150 mg L–1). For the technical-economic analysis, we calculated total capital investment and operating costs for both the pure and catalytic ozonation processes. For total capital investment, we included the cost of procuring and installing all process equipment (referred to as the inside investment cost (ISBL)), offsite investment cost (OSBL), design and engineering cost, contingency cost and working capital (Kinney and Gauche 2006). For the operating cost calculation, we included the costs of raw materials, transportation, utilities use and waste disposal. Detailed descriptions of the calculation of total capital investment and operation costs are provided in Section S3 in the Supplementary material. The breakdown of the various components of the total capital investment and operation costs are shown in Supplementary Figs S5, S6 respectively. As shown in Fig. 5, compared to the pure ozonation process, the overall capital cost and operating cost (annual) of the catalytic ozonation process is only slighter higher (~$0.08 and ~$0.35 M, respectively), while the DOC removal (%) improved significantly from ~20 to 47% with this result demonstrating that application of catalytic ozonation rather than pure ozonation alone is justified for the treatment of CCI concentrates. The cost difference between the pure ozonation and catalytic ozonation processes is mainly due to the cost of raw material, catalyst delivery and the extra process of catalyst regeneration. The daily operating cost of the catalytic ozonation process, which mainly constitutes the raw material cost and electricity cost for process equipment, is estimated to be only $0.22 m–3. The overall cost of installation and operation of the catalytic ozonation process is much less than the cost of a complete ZLD process (~$200 M) (Tong and Elimelech 2016) suggesting that the use of the catalytic ozonation process for concentrate treatment is economically viable.

|
Conclusions
The integration of membrane technologies has gained increasing popularity in ZLD management of CCI wastewaters with this practice generating high-salinity concentrates containing refractory organic matter. Results of this study indicate that the pH of CCI concentrates were largely circumneutral with NaCl and/or Na2SO4 being the dominant salts present although other cations/anions (e.g. Ca2+/Mg2+) were present at significant concentrations in some cases. LC-OCND results showed that humic substances and LMW neutrals were the dominant components in all CCI concentrates. Degradation of the organic matter in CCI concentrate from the Qian-an treatment plant by ozonation and catalytic ozonation processes showed that nearly 20 and 47% of the DOC could be removed following 1 h of treatment in the absence and presence of catalyst, respectively. The low removal of DOC by the pure ozonation process from Qian-an concentrate was mainly ascribed to the refractory nature of the humics and LMW neutrals present in the CCI concentrate with salinity effects (i.e. scavenging of O3 and ˙OH by chloride ions) having no significant influence on the pure ozonation performance. The refractory nature of the humics is potentially due to the presence of a high concentration of Ca2+ in these samples which is known to transform humics to compact and unreactive forms (Baalousha et al. 2006; Yuan et al. 2022). Catalytic ozonation showed better removal of DOC (47%) with the generation of stronger oxidants (particularly ˙OH) presumably facilitating the degradation of LMW neutrals formed on oxidation of humic-like substances. Cost analysis of the catalytic ozonation process indicated that the total cost of this process is negligible compared with the cost of the complete ZLD process, suggesting that the use of catalytic ozonation represents a viable method for the degradation of the refractory organics in CCI concentrates. It should be noted however that CCI membrane concentrates are likely to vary widely in composition with any one technology (such as catalytic ozonation) unlikely to be effective in treating all types of concentrate with the efficacy of catalytic ozonation and, indeed, other oxidation technologies expected to be dependent on the particular contaminants present.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This work was financially supported by the National Key Research and Development Program of China: Research of Key Technologies for the Treatment of Highly Salinated Coal Chemical Wastewater (2019YFE0103300) and International Cooperation Project of Innovation and Entrepreneurship of Tiandi Technology Co., Ltd. (Funding number 2019-TD-GH002). Assistance from the Coal Chemical Research Institute (CCRI) in provision of wastewater concentrate samples from various coal processing plants is gratefully acknowledged.
Supplementary material
Supplementary material is available online.
References
Baalousha M, Motelica-Heino M, Coustumer PL (2006). Conformation and size of humic substances: Effects of major cation concentration and type, pH, salinity, and residence time. Colloids and Surfaces A: Physicochemical and Engineering Aspects 272, 48–55.| Conformation and size of humic substances: Effects of major cation concentration and type, pH, salinity, and residence time.Crossref | GoogleScholarGoogle Scholar |
Cai QQ, Wu MY, Li R, Deng SH, Lee BCY, Ong SL, Hu JY (2020). Potential of combined advanced oxidation – Biological process for cost-effective organic matters removal in reverse osmosis concentrate produced from industrial wastewater reclamation: Screening of AOP pre-treatment technologies. Chemical Engineering Journal 389, 123419
| Potential of combined advanced oxidation – Biological process for cost-effective organic matters removal in reverse osmosis concentrate produced from industrial wastewater reclamation: Screening of AOP pre-treatment technologies.Crossref | GoogleScholarGoogle Scholar |
Chen W, Westerhoff P, Leenheer JA, Booksh K (2003). Fluorescence Excitation–Emission Matrix Regional Integration to Quantify Spectra for Dissolved Organic Matter. Environmental Science & Technology 37, 5701–5710.
| Fluorescence Excitation–Emission Matrix Regional Integration to Quantify Spectra for Dissolved Organic Matter.Crossref | GoogleScholarGoogle Scholar |
Du J, Zhang B, Li J, Lai B (2020). Decontamination of heavy metal complexes by advanced oxidation processes: A review. Chinese Chemical Letters 31, 2575–2582.
| Decontamination of heavy metal complexes by advanced oxidation processes: A review.Crossref | GoogleScholarGoogle Scholar |
Haag WR, Hoigné J, Bader H (1984). Improved ammonia oxidation by ozone in the presence of bromide ion during water treatment. Water Research 18, 1125–1128.
| Improved ammonia oxidation by ozone in the presence of bromide ion during water treatment.Crossref | GoogleScholarGoogle Scholar |
Hakim A, Suzuki T, Kobayashi M (2019). Strength of Humic Acid Aggregates: Effects of Divalent Cations and Solution pH. ACS Omega 4, 8559–8567.
| Strength of Humic Acid Aggregates: Effects of Divalent Cations and Solution pH.Crossref | GoogleScholarGoogle Scholar |
Huber S (2017) DOC LABOR. Available at http://www.doc-labor.de/ [Accessed 3 May 2017]
Huber SA, Balz A, Abert M, Pronk W (2011). Characterisation of aquatic humic and non-humic matter with size-exclusion chromatography – organic carbon detection – organic nitrogen detection (LC-OCD-OND). Water Research 45, 879–885.
| Characterisation of aquatic humic and non-humic matter with size-exclusion chromatography – organic carbon detection – organic nitrogen detection (LC-OCD-OND).Crossref | GoogleScholarGoogle Scholar |
Ji Q, Tabassum S, Hena S, Silva CG, Yu G, Zhang Z (2016). A review on the coal gasification wastewater treatment technologies: past, present and future outlook. Journal of Cleaner Production 126, 38–55.
| A review on the coal gasification wastewater treatment technologies: past, present and future outlook.Crossref | GoogleScholarGoogle Scholar |
Jin X, Li E, Lu S, Qiu Z, Sui Q (2013). Coking wastewater treatment for industrial reuse purpose: Combining biological processes with ultrafiltration, nanofiltration and reverse osmosis. Journal of Environmental Sciences 25, 1565–1574.
| Coking wastewater treatment for industrial reuse purpose: Combining biological processes with ultrafiltration, nanofiltration and reverse osmosis.Crossref | GoogleScholarGoogle Scholar |
Khuntia S, Majumder SK, Ghosh P (2012). Removal of ammonia from water by ozone microbubbles. Industrial & Engineering Chemistry Research 52, 318–326.
| Removal of ammonia from water by ozone microbubbles.Crossref | GoogleScholarGoogle Scholar |
Kinney CL, Gauche R (2006) What’s in ISBL, OSBL, and The Factors? In ‘AACE International Transactions’. American Association of Cost Engineers. ES141–145.
Li J, Wu J, Sun H, Cheng F, Liu Y (2016). Advanced treatment of biologically treated coking wastewater by membrane distillation coupled with pre-coagulation. Desalination 380, 43–51.
| Advanced treatment of biologically treated coking wastewater by membrane distillation coupled with pre-coagulation.Crossref | GoogleScholarGoogle Scholar |
Li J, Guo S, Xu Z, Li J, Pan Z, Du Z, Cheng F (2019). Preparation of omniphobic PVDF membranes with silica nanoparticles for treating coking wastewater using direct contact membrane distillation: Electrostatic adsorption vs. chemical bonding. Journal of Membrane Science 574, 349–357.
| Preparation of omniphobic PVDF membranes with silica nanoparticles for treating coking wastewater using direct contact membrane distillation: Electrostatic adsorption vs. chemical bonding.Crossref | GoogleScholarGoogle Scholar |
Li Y, Li M, Xiao K, Huang X (2020). Reverse osmosis membrane autopsy in coal chemical wastewater treatment: Evidences of spatially heterogeneous fouling and organic-inorganic synergistic effect. Journal of Cleaner Production 246, 118964
| Reverse osmosis membrane autopsy in coal chemical wastewater treatment: Evidences of spatially heterogeneous fouling and organic-inorganic synergistic effect.Crossref | GoogleScholarGoogle Scholar |
Miklos DB, Remy C, Jekel M, Linden KG, Drewes JE, Hübner U (2018). Evaluation of advanced oxidation processes for water and wastewater treatment – A critical review. Water Research 139, 118–131.
| Evaluation of advanced oxidation processes for water and wastewater treatment – A critical review.Crossref | GoogleScholarGoogle Scholar |
Ruffino B, Zanetti M (2019). Orthophosphate vs. bicarbonate used as a buffering substance for optimizing the bromide-enhanced ozonation process for ammonia nitrogen removal. Science of The Total Environment 692, 1191–1200.
| Orthophosphate vs. bicarbonate used as a buffering substance for optimizing the bromide-enhanced ozonation process for ammonia nitrogen removal.Crossref | GoogleScholarGoogle Scholar |
Sadrnourmohammadi M, Brezinski K, Gorczyca B (2020). Ozonation of natural organic matter and aquatic humic substances: the effects of ozone on the structural characteristics and subsequent trihalomethane formation potential. Water Quality Research Journal 55, 155–166.
| Ozonation of natural organic matter and aquatic humic substances: the effects of ozone on the structural characteristics and subsequent trihalomethane formation potential.Crossref | GoogleScholarGoogle Scholar |
Shi J, Xu C, Han Y, Han H (2021). Case study on wastewater treatment technology of coal chemical industry in China. Critical Reviews in Environmental Science and Technology 51, 1003–1044.
| Case study on wastewater treatment technology of coal chemical industry in China.Crossref | GoogleScholarGoogle Scholar |
Tong T, Elimelech M (2016). The Global Rise of Zero Liquid Discharge for Wastewater Management: Drivers, Technologies, and Future Directions. Environmental Science & Technology 50, 6846–6855.
| The Global Rise of Zero Liquid Discharge for Wastewater Management: Drivers, Technologies, and Future Directions.Crossref | GoogleScholarGoogle Scholar |
Wang S, Xiao K, Huang X (2019). Characterizing the roles of organic and inorganic foulants in RO membrane fouling development: The case of coal chemical wastewater treatment. Separation and Purification Technology 210, 1008–1016.
| Characterizing the roles of organic and inorganic foulants in RO membrane fouling development: The case of coal chemical wastewater treatment.Crossref | GoogleScholarGoogle Scholar |
Xiong R, Wei C (2017). Current status and technology trends of zero liquid discharge at coal chemical industry in China. Journal of Water Process Engineering 19, 346–351.
| Current status and technology trends of zero liquid discharge at coal chemical industry in China.Crossref | GoogleScholarGoogle Scholar |
Yang W, Li X, Pan B, Lv L, Zhang W (2013). Effective removal of effluent organic matter (EfOM) from bio-treated coking wastewater by a recyclable aminated hyper-cross-linked polymer. Water Res 47, 4730–4738.
| Effective removal of effluent organic matter (EfOM) from bio-treated coking wastewater by a recyclable aminated hyper-cross-linked polymer.Crossref | GoogleScholarGoogle Scholar |
Yu X, Xu R, Wei C, Wu H (2016). Removal of cyanide compounds from coking wastewater by ferrous sulfate: Improvement of biodegradability. Journal of Hazardous Materials 302, 468–474.
| Removal of cyanide compounds from coking wastewater by ferrous sulfate: Improvement of biodegradability.Crossref | GoogleScholarGoogle Scholar |
Yuan Y, Xing G, Garg S, Ma J, Kong X, Dai P, Waite TD (2020). Mechanistic insights into the catalytic ozonation process using iron oxide-impregnated activated carbon. Water Research 177, 115785–115797.
| Mechanistic insights into the catalytic ozonation process using iron oxide-impregnated activated carbon.Crossref | GoogleScholarGoogle Scholar |
Yuan Y, Garg S, Wang Y, Li W, Chen G, Gao M, Zhong J, Wang J, Waite TD (2022). Influence of salinity on the heterogeneous catalytic ozonation process: Implications to treatment of high salinity wastewater. Journal of Hazardous Materials 423, 127255–127265.
| Influence of salinity on the heterogeneous catalytic ozonation process: Implications to treatment of high salinity wastewater.Crossref | GoogleScholarGoogle Scholar |
Zhang Y, Yuan Z, Margni M, Bulle C, Hua H, Jiang S, Liu X (2019). Intensive carbon dioxide emission of coal chemical industry in China. Applied Energy 236, 540–550.
| Intensive carbon dioxide emission of coal chemical industry in China.Crossref | GoogleScholarGoogle Scholar |
Zhao Q, Liu Y (2016). State of the art of biological processes for coal gasification wastewater treatment. Biotechnology Advances 34, 1064–1072.
| State of the art of biological processes for coal gasification wastewater treatment.Crossref | GoogleScholarGoogle Scholar |
Zheng H, Hou Y, Li S, Ma J, Nan J, Li T (2022). Recent advances in the application of metal organic frameworks using in advanced oxidation progresses for pollutants degradation. Chinese Chemical Letters 33, 5013–5022.
| Recent advances in the application of metal organic frameworks using in advanced oxidation progresses for pollutants degradation.Crossref | GoogleScholarGoogle Scholar |



