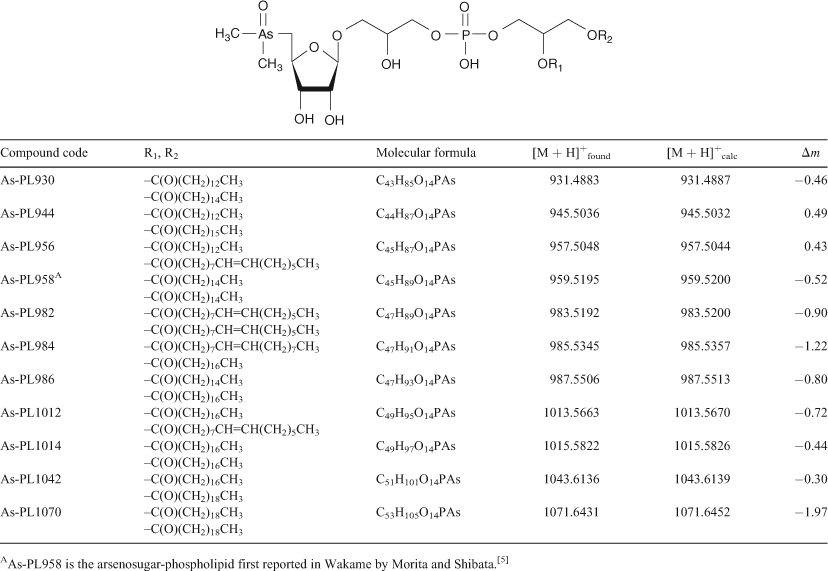
Arsenosugar phospholipids and arsenic hydrocarbons in two species of brown macroalgae
Sara García-Salgado A , Georg Raber B D , Reingard Raml C , Christoph Magnes C and Kevin A. Francesconi BA School of Civil Engineering, Department of Hydraulic and Energy Technology, University College for the Technical Engineering of Public Works, Polytechnic University of Madrid, E-28014 Madrid, Spain.
B Institute of Chemistry – Analytical Chemistry, Karl-Franzens University Graz, A-8010 Graz, Austria.
C HEALTH – Institute for Biomedicine and Health Sciences, Joanneum Research, A-8036 Graz, Austria.
D Corresponding author. Email: georg.raber@uni-graz.at
Environmental Chemistry 9(1) 63-66 https://doi.org/10.1071/EN11164
Submitted: 20 December 2011 Accepted: 17 January 2012 Published: 14 February 2012
Journal Compilation © CSIRO Publishing JYEAR Open Access CC BY-NC-ND
Environmental context. Although organoarsenic compounds occur in marine organisms at high concentrations, the origin and role of these compounds is unknown. Arsenic-containing lipids (arsenolipids) are newly discovered compounds in fish. We identify a range of arsenolipids in algae and propose that algae are the origin of these unusual arsenic compounds in marine ecosystems.
Abstract. Fourteen arsenolipids, including 11 new compounds, were identified and quantified in two species of brown algae, Wakame (Undaria pinnatifida) and Hijiki (Hizikia fusiformis), by high resolution mass spectrometry, high performance liquid chromatography–mass spectrometry and gas chromatography–mass spectrometry. Both algal species contained arsenosugar-phospholipids as the major type of arsenolipid, and arsenic-hydrocarbons were also significant components, particularly in Hijiki. The origin of the various arsenolipids, and the possible significance of their relative quantities, is briefly discussed.
Arsenic-containing organic compounds are abundant in marine ecosystems where they are thought to play a pivotal role in the cycling and detoxification of potentially toxic inorganic arsenic (arsenate) present in seawater.[1] Although most of the arsenic compounds identified so far have been water-soluble species, the early work on arsenic marine chemistry focussed on lipid-soluble compounds, so called arsenolipids.[2–4] Identification of these arsenolipids proved difficult, however, and it was not until 1988 that an arsenolipid was first rigorously characterised and identified as an arsenosugar-containing phospholipid[5] (see Table 1, compound As-PL958).
Subsequently, the range of naturally occurring arsenolipids has been extended with the discovery of arsenic-containing fatty acids in fish oils,[6] and arsenic-containing hydrocarbons in fish oils,[7] fish liver,[8] sashimi tuna[9] and fish meal.[10] The origin of these compounds was presumed to be algae. We report the arsenolipid profiles of two species of brown algae, determined mainly by high performance liquid chromatography–mass spectrometry (HPLC-MS), and we briefly discuss the possible biosynthetic origin of these unusual compounds.
Samples of Wakame (Undaria pinnatifida, 40 ± 3 µg As g–1 dry mass) and Hijiki (Hizikia fusiformis, 113 ± 5 µg As g–1 dry mass),A obtained from a Japanese commercial source, were extracted with a mixture of chloroform and methanol using a modificationB of the classical procedure of Bligh and Dyer.[11] The lipid fraction containing 6.7 % (Wakame) and 1.6 % (Hijiki) of the initial total arsenic was passed through a small plug of silica, which removed 90 % of the sample mass with little loss of arsenic. Both the pre- and post-silica fractions were then analysed by reversed-phase HPLC with simultaneous detection of arsenic and organo-arsenic compounds by using inductively coupled plasma mass spectrometry (ICPMS) and electrospray mass spectrometry (ESMS) respectively.C The clean-up step with silica was essential to obtain good ESMS data. ICPMS, a more robust detection method, was capable of measuring the arsenolipids in both pre- and post-silica fractions, and provided data showing that the pattern of arsenolipids was essentially unchanged by the silica clean-up step.
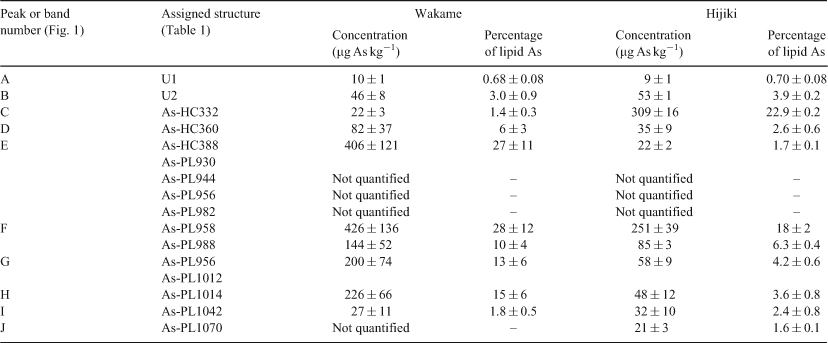
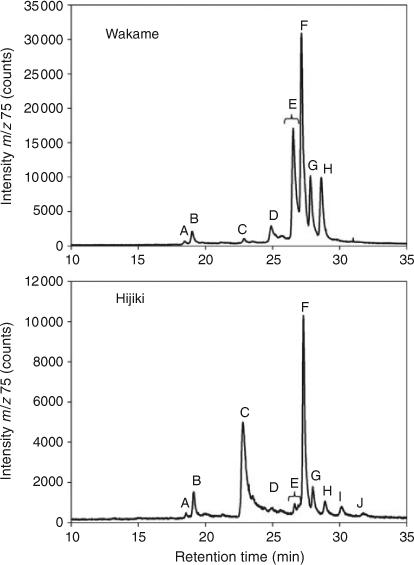
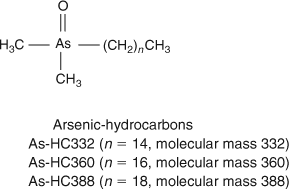
HPLC-MS revealed the presence of at least 10 arsenic-containing peaks or bands (Fig. 1). The peaks eluted at ~23 to 25 min from the HPLC column were shown to contain two known arsenic-hydrocarbons (Fig. 2, As-HC332 and As-HC360) by accurate mass measurements and gas chromatography (GC)-MS analysis (see Supplementary material, Fig. S1).[12] In addition, a new arsenic-hydrocarbon (retention time (RT) 26.5 min; Fig. 2, As-HC388) was identified in Wakame by accurate mass measurements and GC-MS. A major peak in the HPLC chromatogram (RT = 27.5 min) for both Wakame and Hijiki had a protonated molecular mass of 959, which matched that for the arsenosugar-phospholipid previously characterised by Morita and Shibata.[5] In addition, there was a series of peaks before and after the peak with m/z 959 separated by 28, 26 or 24 mass units, which indicated the presence of a homologous series of saturated and unsaturated arsenosugar-phospholipids (Fig. 1). These assignments were strongly supported by accurate mass measurementsD performed on 11 of the compounds, which agreed in every case to within 2 ppm of the calculated molecular masses (Table 1). The possible presence in algae of a range of arsenosugar-phospholipids differing only in their fatty acid content had been predicted in 1988 by Morita and Shibata[5]; our data confirm their astute early prediction.
|
|
Quantification of the major arsenic compounds in the two algal species was achieved by HPLC-ICPMS using a modification of reported methods (Table 2).[13,14] Although both species contained arsenosugar-phospholipid As-PL958 as a major compound, Wakame also contained significant quantities of other arsenosugar-phospholipids, whereas Hijiki contained a higher proportion of arsenic-hydrocarbons. The possible significance of these differences awaits studies with more samples of these two algae and other algal species from various locations. Such studies would be best carried out with samples collected directly from the natural environment, rather than with commercial products, thereby excluding the possibility of artefact formation.
The presence of both arsenic-hydrocarbons and arsenosugar-phospholipids in algae raises questions as to how these unusual compounds are synthesised, and what their possible roles in algae might be. The early experiments of Benson and co-workers provide some information on the biochemical processes involved.[3] By using radiolabelled arsenic and monitoring products by radiochromatography, they showed that unicellular algae take up arsenate from seawater and convert it into lipid- and water-soluble arsenic species. By selective use of enzymes (e.g. phospholipases A and D), they also demonstrated that the lipid-soluble arsenic comprised three types of lipids: phospholipids, lyso-phospholipids and an unknown lipid class. Although the water-soluble products from the enzyme treatments were incorrectly assigned at that time, they were later correctly ascribed to arsenosugars.[1,15–17] Our definitive results on the structures of the major arsenolipids in algae are consistent with the earlier observations. Collectively, the data suggest that the arsenosugar-phospholipids might be the target arsenic species synthesised by algae, and that the arsenosugars are merely degradation products of compounds excess to the algae’s requirements.
The third, unknown, class of lipid reported by Benson and co-workers[3] did not degrade with the enzymes they tested. Possibly, that group of compounds comprised the arsenic-hydrocarbons found in our study. These compounds are likely to have a biosynthetic pathway quite different from that of the arsenosugar-phospholipids, although their properties might be similar since they both contain polar head groups and long non-polar tails. We speculate that both these types of arsenolipids could be used by algae in membrane chemistry.
Possibly, arsenolipids impart a particular useful property to cell membranes not attainable from normal membrane lipids. In this regard, the recent work by Van Mooy et al.[18] is relevant because they reported that algae can utilise S and N in place of P to form membrane lipids. In that case, however, the driving force was considered to be the need for algae to conserve phosphate for use in other essential cell biochemistry. Thus, the relative quantities of arsenosugar-phospholipids and arsenic-hydrocarbons in the algae might reflect phosphate levels in water or the relative phosphate requirements of algal species; i.e. when phosphate levels are limiting, arsenic-hydrocarbons are preferentially synthesised by algae for membrane chemistry. We are currently investigating these biological questions, building on the preliminary quantitative results reported here for different types of arsenolipids in algae.
Supplementary material
The following information is available as Supplementary material:
-
GC/MS of arsenic-hydrocarbons in Wakame and Hijiki.
-
High resolution mass spectra of arsenic-hydrocarbons found in Wakame or Hijiki.
-
High resolution mass spectra of arsenosugar-phospholipids found in Hijiki.
Acknowledgements
The authors thank the Austrian Science Fund (FWF): project number P23761-N17 for support. S. García-Salgado thanks the Social Council and Civil Engineering: Hydraulic and Energy Technology Department from the Polytechnic University of Madrid for financial support.
References
[1] K. A. Francesconi, J. S. Edmonds, Arsenic and marine organisms. Adv. Inorg. Chem. 1997, 44, 147.| 1:CAS:528:DyaK2sXhsVajtbs%3D&md5=d94bd17c5ae977ea49c0ef689ca97d1eCAS |
[2] G. Lunde, The synthesis of fat and water soluble arseno organic compounds in marine and limnetic algae. Acta Chem. Scand. 1973, 27, 1586.
| The synthesis of fat and water soluble arseno organic compounds in marine and limnetic algae.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE3sXltlCitbY%3D&md5=9c0809c72eb7cd3334de3cf0beb4e343CAS |
[3] R. V. Cooney, R. O. Mumma, A. A. Benson, Arsoniumphospholipid in algae. Proc. Natl. Acad. Sci. USA 1978, 75, 4262.
| Arsoniumphospholipid in algae.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1MXhvFShtQ%3D%3D&md5=0f71fc9f2c9a87c3149032d0fc1f5a30CAS |
[4] J. J. Wrench, R. F. Addison, Reduction, methylation, and incorporation of arsenic into lipids by the marine-phytoplankton Dunaliella tertiolecta. Can. J. Fish. Aquat. Sci. 1981, 38, 518.
| Reduction, methylation, and incorporation of arsenic into lipids by the marine-phytoplankton Dunaliella tertiolecta.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3MXksFGkt74%3D&md5=27d6839c715f100d37e9448d8a12ec0aCAS |
[5] M. Morita, Y. Shibata, Isolation and identification of arseno-lipid from a brown alga, Undaria pinnatifida (Wakame). Chemosphere 1988, 17, 1147.
| Isolation and identification of arseno-lipid from a brown alga, Undaria pinnatifida (Wakame).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1cXkvVOlt78%3D&md5=cd17541ee1fd9c0b7f3ecbad743a2e69CAS |
[6] A. Rumpler, J. S. Edmonds, M. Katsu, K. B. Jensen, W. Goessler, G. Raber, H. Gunnlaugsdottir, K. A. Francesconi, Arsenic-containing long-chain fatty acids in cod liver oil: a result of biosynthetic infidelity. Angew. Chem. Int. Ed. 2008, 47, 2665.
| Arsenic-containing long-chain fatty acids in cod liver oil: a result of biosynthetic infidelity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXkvFejsL4%3D&md5=d28e6f01dd610ac43a22cc186ab76f07CAS |
[7] M. S. Taleshi, K. B. Jensen, G. Raber, J. S. Edmonds, H. Gunnlaugsdottir, K. A. Francesconi, Arsenic-containing hydrocarbons: Natural compounds in oil from the fish capelin, Mallotus villosus. Chem. Commun. 2008, 39, 4706.
| Arsenic-containing hydrocarbons: Natural compounds in oil from the fish capelin, Mallotus villosus.Crossref | GoogleScholarGoogle Scholar |
[8] U. Arroyo-Abad, J. Mattusch, S. Mothes, M. Moeder, R. Wennrich, M. P. Elizalde-Gonzalez, F. M. Matysik, Detection of arsenic-containing hydrocarbons in canned cod liver tissue. Talanta 2010, 82, 38.
| Detection of arsenic-containing hydrocarbons in canned cod liver tissue.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXntVyktbo%3D&md5=9f0f1132bab9829fcf02cf1670e5d3f4CAS |
[9] M. S. Taleshi, J. S. Edmonds, W. Goessler, M. J. Ruiz-Chancho, G. Raber, K. B. Jenson, K. A. Francesconi, Arsenic-containing lipids are natural constituents of sashimi tuna. Environ. Sci. Technol. 2010, 44, 1478.
| Arsenic-containing lipids are natural constituents of sashimi tuna.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXptlymtg%3D%3D&md5=62e81b7eae7f468593e1c7eb8d089359CAS |
[10] K. O. Amayo, A. Petursdottir, C. Newcombe, H. Gunnlaugsdottir, A. Raab, E. M. Krupp, J. Feldmann, Identification and quantification of arsenolipids using reversed-phase HPLC coupled simultaneously to High-Resolution ICPMS and High-Resolution Electrospray MS without Species-Specific Standards. Anal. Chem. 2011, 83, 3589.
| 1:CAS:528:DC%2BC3MXksFaktL4%3D&md5=416d9d52be689503f7dcb2996b5ee2d6CAS |
[11] E. G. Bligh, W. J. Dyer, A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911.
| A rapid method of total lipid extraction and purification.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaG1MXhtVSgt70%3D&md5=1787b0c250b0b5bc032e6143e23adf51CAS |
[12] G. Raber, S. Khoomrung, M. S. Taleshi, J. S. Edmonds, K. A. Francesconi, Identification of arsenolipids with GC/MS. Talanta 2009, 78, 1215.
| Identification of arsenolipids with GC/MS.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXjtVSnsL8%3D&md5=62321dda92c7528f428cc6960fd07dc3CAS |
[13] G. Raber, R. Raml, W. Goessler, K. A. Francesconi, Quantitative speciation of arsenic compounds when using organic solvent gradients in HPLC-ICPMS. J. Anal. At. Spectrom. 2010, 25, 570.
| Quantitative speciation of arsenic compounds when using organic solvent gradients in HPLC-ICPMS.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXjslWisL4%3D&md5=4409bb12de54221f8639395e137257cdCAS |
[14] M. J. Ruiz-Chancho, M. S. Taleshi, W. Goessler, K. A. Francesconi, A method for screening arsenolipids in fish oils by HPLC-ICPMS. J. Anal. At. Spectrom. 2011, [Published online ahead of print 13 December 2011]
| A method for screening arsenolipids in fish oils by HPLC-ICPMS.Crossref | GoogleScholarGoogle Scholar |
[15] A. Benson, Arsonium compounds in algae. Proc. Natl. Acad. Sci. USA 1989, 86, 6131.
| Arsonium compounds in algae.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1MXmtFWmu74%3D&md5=77a9746596fe273a04dc8f73e37d3edbCAS |
[16] J. S. Edmonds, Y. Shibata, K. A. Francesconi, J. Yoshinaga, M. Morita, Arsenic lipids in the digestive gland of the western rock lobster Panulirus cygnus: an investigation by HPLC ICP-MS. Sci. Total Environ. 1992, 122, 321.
| Arsenic lipids in the digestive gland of the western rock lobster Panulirus cygnus: an investigation by HPLC ICP-MS.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XmtV2itL4%3D&md5=57446df7fc7377bd9be8a4a54eee4dbfCAS |
[17] S. Devalla, J. Feldmann, Determination of lipid-soluble arsenic species in seaweed-eating sheep from Orkney. Appl. Organomet. Chem. 2003, 17, 906.
| Determination of lipid-soluble arsenic species in seaweed-eating sheep from Orkney.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXpvV2hs7k%3D&md5=ed883f7d0a1003daa663bc8f326b1c2bCAS |
[18] B. A. S. Van Mooy, H. F. Fredricks, B. E. Pedler, S. T. Dyhrman, D. M. Karl, M. Koblizek, M. W. Lomas, T. J. Mincer, L. R. Moore, T. Moutin, M. S. Rappe, E. A. Webb, Phytoplankton in the ocean use non-phosphorus lipids in response to phosphorus scarcity. Nature 2009, 458, 69.
| Phytoplankton in the ocean use non-phosphorus lipids in response to phosphorus scarcity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXht1ehtbY%3D&md5=32db6bf5d2a56fd088d3080cb06469e6CAS |
A Determination of arsenic contents. Total arsenic analyses were performed on portions of the dry powders, extracts or combined fractions from the silica columns by ICP-MS (Agilent 7500ce) in helium collision cell mode following a microwave-assisted acid mineralisation step. The method was validated by analysis of reference material NIES No. 9 Sargasso (certified As content 115 ± 5 µg As g–1); we obtained 116 ± 2 µg As g–1 (n = 3).
B Extraction and purification of arsenolipids from algae. Extraction of arsenolipids from algae was performed in triplicate from two species of algae (Wakame and Hijiki). Following is a typical procedure and outcome for these extractions. A portion (1.013 g dry mass containing 40 µg of As) of Wakame was extracted with a mixture of chloroform/methanol (2 : 1 v/v; 25 g) in a mechanical shaker overnight. The mixture was centrifuged, and the supernatant was washed with bicarbonate solution (1 % w/v; 2 × 20 mL) to remove the water-soluble arsenic compounds. The chloroform layer was separated and evaporated to dryness to yield a residue (crude As-lipid fraction, 175 mg containing 2.7 µg of As), which was re-dissolved in a mixture of chloroform/acetone (1 : 1 v/v, 1 mL). A portion (500 μL) of this solution was applied to a ‘plug’ of silica (conditioned with chloroform/acetone, 1 : 1 containing 1 % formic acid), packed into a Pasteur pipette. The silica was washed with the conditioning solvent mixture (5 × 1 mL), and then methanol (3 × 1 mL) and finally methanol containing 1 % aqueous ammonia (10 × 1 mL). Arsenic was located in the fractions by using graphite furnace atomic absorption spectrometry. Five of the methanol–ammonia fractions containing >85 % of the total arsenic applied to the column were combined and evaporated to dryness (purified As-lipid fraction, 8.8 mg containing 1.2 µg of As). This material was re-dissolved in methanol (200 μL) and, together with the crude As-lipid fraction, analysed by HPLC-ICPMS and HPLC-ESMS.
C HPLC-ICPMS and -ESMS analyses. Separation of the lipid-soluble arsenic species was performed under reversed-phase HPLC conditions by using a Zorbax Eclipse XDB-C8 column (4.6 × 150 mm; 5-µm particle size; Agilent Technologies, Waldbronn, Germany) and a mobile phase comprising acetic acid (10 mmol L–1 at pH 6.0, adjusted with aqueous ammonia) and methanol under the following gradient elution conditions: 0–25 min, 50–95 % methanol; 25–40 min, 95 % methanol. The column effluent was split whereby 10 % was directed to the ICPMS and 90 % to the ESMS using an Agilent G1968D active splitter and introducing a sheath flow of 0.2 mL min–1 (5 % methanol and 0.1 % formic acid). To prevent deposition of carbon on the interface cones of the ICPMS, an optional gas (1 % oxygen in argon) was introduced. ESMS data were obtained by selected ion monitoring (SIM) in positive ion mode at a fragmentor voltage of 150 V.
D Accurate mass measurements. In a separate HPLC run, the effluent was split with 10 % going to the ICPMS and the rest collected in 100- or 200-µL fractions. Accurate mass measurements were made on these fractions by direct infusion (5 µL min–1) with a LTQ Orbitrap XL (Thermo Scientific), equipped with a heated electrospray ion source. The system was operated in Fourier transform mass spectrometry (FTMS) high resolution positive scan mode (60 000 resolution) under the following conditions: source voltage, 5.01 kV; vaporiser temperature, 36 °C; sheath gas flow, 20.08 arb; auxiliary gas, 9.98 arb; capillary voltage, 16.02–36 V; capillary temperature, 275 °C; tube lens voltage, 69–124 V.