Flux chamber study of particle formation from Durvillaea potatorum
Jill M. Cainey A E , Melita Keywood B , E. Keith Bigg C , Michael R. Grose D , Rob W. Gillett B and Mick Meyer BA Cape Grim Baseline Air Pollution Station, Bureau of Meteorology, 159 Nelson Street, Smithton, Tas. 7330, Australia.
B CSIRO Marine and Atmospheric Research, Aspendale, Vic. 3195, Australia.
C Castle Hill, NSW 2154, Australia.
D IASOS, University of Tasmania, Hobart, Tas. 7001, Australia.
E Corresponding author. Email: j.cainey@bom.gov.au
Environmental Chemistry 4(3) 151-154 https://doi.org/10.1071/EN07006
Submitted: 24 January 2007 Accepted: 6 June 2007 Published: 22 June 2007
Environmental context. Kelp at Mace Head, Ireland, produces large quantities of iodine when exposed to sunlight at low tide and this iodine results in the rapid production of particles. Cape Grim, Tasmania, also has large colonies of kelp (Durvillaea potatorum) but its role in particle formation appears limited. A flux chamber was used to better understand the response of Durvillaea potatorum to light stress and ozone.
Abstract. Brown kelp, in particular Laminara digitata at Mace Head, Ireland, has been shown to emit iodine when under stress, resulting in new particle formation. The Cape Grim Baseline Air Pollution Station, Tasmania, is surrounded by rocky reefs that support large colonies of the brown kelp Durvillaea potatorum. During an intensive campaign in February 2006 at Cape Grim, levels of IO, OIO and methyl iodide remained at background levels and no particle formation events could be associated with locally generated precursor iodine species.
In order to better understand the limitations of the local kelp to provide a source of precursor species, samples of Durvillaea potatorum were collected from the beach below the Cape Grim Station and tested for their capacity to initiate particle formation using a flux chamber technique. Particles were observed only when the kelp was exposed to both very high levels (>100 ppb) of ozone and natural solar radiation. There was a high correlation between ozone level and particles produced. The particles resulting from exposure to high levels of ozone were aromatic and volatile.
Durvillaea potatorum appears to plays a very limited role in contributing to particle formation at Cape Grim, but it does represent a source of atmospheric iodine under photo-oxidative stress, of 18 pmol g–1 (fresh weight) min–1 and is likely to have a significant role in atmospheric chemistry at this site.
The role of the brown kelp, Laminaria digitata, in providing the necessary precursors to nucleation events at Mace Head, Ireland, has been well studied,[1–3] and it had been expected that the brown kelp, Durvillaea potatorum, at Cape Grim, Australia, would also provide a source of iodine and iodated gases that would initiate particle formation.
Previous measurements at the Cape Grim Baseline Air Pollution Station of a range of alkyl halides, including IO and OIO, had shown that these gases were either at low levels or below detection limits,[4–6] and this was suggested to be the result of the distance of the Cape Grim Station from the source region on the beach 94 m below.[7] Measurements of particle numbers, both at the Station and on the beach below had previously shown that beach cast Durvillaea potatorum was a source of particles[8,9] and at the same magnitude as observed at Mace Head, 20 000 and 57 000 respectively.[1,9]
During the Precursors to Particles Campaign 2006 (P2P 2006),[10] the expected nucleation events due to the local macroalgae, Durvillaea potatorum, were not observed, suggesting differences between the processes at Cape Grim and at Mace Head. Initial tests during P2P 2006 showed that beach cast Durvillaea potatorum produced particles only when exposed to high levels of ozone (>450 ppb) and light. To better understand the possible role of the local kelp as a source of particles, a limited assessment of Durvillaea potatorum was made using a flux chamber, in an approach similar to that used by Palmer et al.[11]
Bull Kelp, Durvillaea potatorum, was collected from the beach 94 m below the Cape Grim Baseline Air Pollution Station by cutting a frond from a rocky outcrop at high tide to minimise stress and it was stored in seawater before use in the flux chamber.
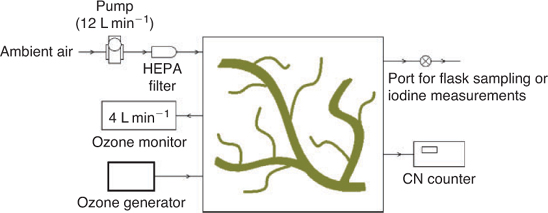
The kelp (3.15 kg fresh wet weight) was placed in a 250-L flux chamber, with mylar walls, transparent to UV-B radiation.[12] The flux chamber was sited on a bench on the Cape Grim instrument deck. The flux chamber also contained an ozone generator (Rollex HV-206A, New Zealand) and the ozone concentrations and particle number concentrations were monitored continuously. Ozone concentration in the chamber was measured using an ozone calibrator (ML9811, Monitor Laboratories, USA). Particle number concentrations were measured using a TSI 3776 CPC, which was connected to the flux chamber using a short (<1 m) length of antistatic tubing to minimise particle loss.
The flux chamber was not leak tight and to ensure that ambient air did not enter the chamber a small diaphragm pump (Dynavac OD1, Australia) was used to provide airflow at 12 L min–1 to maintain the chamber at a positive pressure. A HEPA filter (Pall Gelman #12144, USA) was fitted to the outlet of the pump to remove any particles. This flow of particle-free air also flushed the chamber between each change in ozone level. The experimental set up is shown in Fig. 1.
|
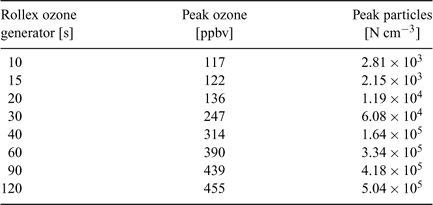
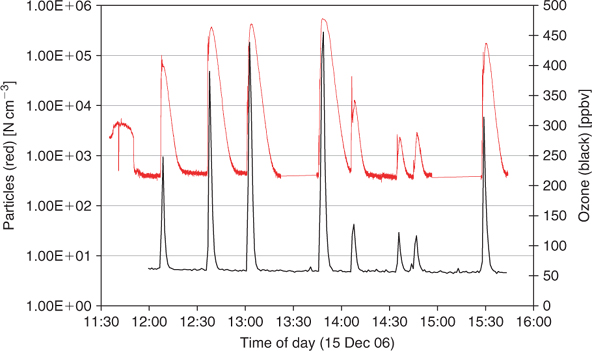
The ozone generator was turned on for a specified period (see Table 1) and then turned off. Particle numbers showed an immediate response to the addition of ozone and the concentration of both was allowed to increase and then decay back to ambient levels before restarting the generator (Fig. 2).
|
|
Iodine measurements could not be made on the flux chamber simultaneously due to the high levels of water vapour in the chamber, which contaminated the trap measurements. However a small 1-L chamber was used to assess the iodine emissions from subsamples of Durvillaea potatorum, when exposed to ambient light, using an ethanol trap as described by Palmer et al.,[11] but with the addition of an ice and sodium chloride (–15°C) water trap in line between the chamber and the ethanol sparger. The blank for iodine was assessed by determining the iodine emissions from the empty 1-L flask, which demonstrated that there was no iodine without the presence of kelp. Measurements were made on several fresh strands of kelp (n = 9) and iodine emissions from kelp exposed to ambient sunlight were 18 ± 11 pmol g–1 (FW) min–1.
During P2P 2006, as part of the initial trials, six transmission electron microscopy grids were placed in the chamber, immediately before exposure to ~450 ppb of ozone, and left for 24 h. It was expected that very large numbers of the small newly formed particles would be collected on the grids; however, an untreated grid was apparently devoid of particles when examined, while those exposed to decane, decanol, or dimethylsulfoxide vapours showed only a few. Only the grid exposed to xylene vapour showed many particles like that of Fig. 3. This suggests that the particles evaporated before they could be examined in the electron microscope unless previously stabilised by dissolving in xylene.

|
An aromatic is perhaps the most common class of compound that would be readily soluble in xylene but not in decane, decanol or dimethylsulfoxide vapours. Some of the crystals formed exceeded 1 µm in length but had widths <200 nm and their thickness, while indeterminate, appeared to be much lower. Growth of airborne particles in a small chamber to a size large enough to create such crystals is very improbable. Instead, a deposit of fine droplets on the collecting surface, which coagulated to form larger drops, is indicated. The fact that the large drops were not observed in the electron microscope unless stabilised by xylene suggests a compound with a relatively high vapour pressure, which includes some organics and aromatic compounds. The atmospheric lifetime of very small, nucleated particles of this material would, therefore, probably be short when the gas from which they formed became subsaturated, even if they were to be nucleated in the presence of ambient ozone conditions.
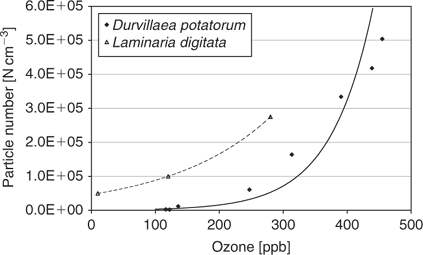
The results from the flux chamber in December 2006 show a very strong relationship between ozone added and particle production from Durvillaea potatorum, with an exponential fit giving an R2 of 0.93 (Fig. 4). If this fit is used to extrapolate back to ambient ozone levels at Cape Grim, it implies that between 900 and 1300 N cm–3 can result from the interaction of the kelp and ozone. The ambient ozone concentration during P2P 2006 and in December 2006 was 15–16 ppb, suggesting that ambient particle generation would be at the lower end of the predicted range and in agreement with the particle number concentrations measured on the beach, directly over beach cast Durvillaea potatorum of 843 ± 205 N cm–3.[10]
|
The response of Laminaria digitata to a variety of ozone levels varied markedly between samples of the kelp,[11] with particle numbers reaching 650 N cm–3 with 500 ppb of ozone, but also showing little or no response to subsequent additions of ozone at 500 ppb (see fig. 8, Palmer et al.[11]). In their fig. 8, Laminaria digitata produced >275 000 particles with only the addition of 280 ppb of ozone. For one sample of Laminaria digitata, there was a strong relationship (R2 of 1) between ozone added and particle number generated.[11] These points are shown here in Fig. 4 and clearly Laminaria digitata produces significant numbers of particles at much lower levels of ozone than Durvillaea potatorum.
Ambient levels of ozone at Cape Grim range seasonally between 15 ppbv in summer and 30 ppbv in winter,[13] so the levels used in the flux chamber experiment are not likely to occur in the clean marine boundary layer. The lowest level of ozone achieved in the flux chamber was close to 100 ppb and this was at the limit of controlling the operation of the generator.
While the kelp beds 94 m below the Cape Grim Station contain a much higher biomass than the beds at Mace Head, 82 kg m–2 and 2.5 kg m–2 respectively,[14,15] Durvillaea potatorum has a far lower iodine content than Laminaria digitata. Preliminary measurements indicate that it contains in the order of 90 mg kg–1 of iodine[16] compared with Laminaria digitata, which contains 2500–12 000 mg kg–1.[17]
In contrast to the iodine content of the kelp, the emission of iodine from Durvillaea potatorum at 18 pmol g–1 (FW) min–1 is consistent with emissions from unstressed or mildly stressed Laminaria digitata, which emitted iodine in the range 3–130 pmol g–1 (FW) min–1.[11] This suggests that the kelp at Cape Grim still represents a potential source of iodine given its high biomass and iodine emissions under moderate photo-oxidative stress.
Durvillaea potatorum is not likely to be a major source of new particles at Cape Grim, but could represent an important source of iodine, which may induce limited particle formation and also contribute to the iodine in aged aerosols. Emissions of iodine and iodated species, including methyl iodide, from Durvillaea potatorum are likely to have an important role in atmospheric chemistry at Cape Grim[15,18] and iodine has previously been measured in individual particles at Cape Grim.[19]
Further work at Cape Grim is needed to extend the limited study reported here by assessing other types of macroalgae found locally at Cape Grim for their potential to produce the precursor gases needed for particle generation and the robustness of the relationship between particles produced and ozone concentration. In addition, a range of precursor gases should be continuously monitored, along with particle numbers, to better determine the identity of any precursors to particle formation. A campaign is planned at Cape Grim for late 2007 (Austral spring/summer) to address the points above.
Coastal regions and the open ocean support high biological activity and occupy a large surface area.[20] If the processes that are important at Mace Head, where the local biology (Laminaria digitata) provides nucleating precursors to particles, are also important in Tasmania then these regions are likely to be highly critical for particle production and the understanding of particle production. However, the specific conditions and coastal ecology of the ocean near Tasmania are very different to the environment of the Northern Hemisphere coastlines and oceans. The assessment of the local kelp, Durvillaea potatorum, at Cape Grim suggests that while it may contribute to new particle numbers it does so at close to three orders of magnitude lower than that observed for Laminaria digitata at Mace Head. Clearly, further work is needed to assess a range of macroalgae along a variety of coastlines, in a variety of conditions, before the processes observed at Mace Head can be assumed to be applicable globally.[21]
[1]
C. D. O’Dowd ,
K. Hämeri ,
J. M. Mäkelä ,
L. Pirjola ,
M. Kulmala ,
S. G. Jennings ,
H. Berresheim ,
H.-C. Hansson ,
G. de Leeuw ,
G. J. Kunz ,
A. G. Allen ,
C. N. Hewitt ,
A. Jackson ,
Y. Viisanen ,
T. Hoffmann ,
A dedicated study of new particle formation and fate in the coastal environment (PARFORCE): overview of objectives and achievements.
J. Geophys. Res. 2002
, 107, 8108.
| Crossref | GoogleScholarGoogle Scholar |

[2]
C. D. O’Dowd ,
T. Hoffmann ,
Coastal new particle formation: a review of current state-of-the-art.
Environ. Chem. 2005
, 2, 245.
| Crossref | GoogleScholarGoogle Scholar |

[3]
C. D. O’Dowd ,
M. Geever ,
M. K. Hill ,
M. H. Smith ,
S. G. Jennings ,
New particle formation: nucleation rates and spatial scales in the clean marine coastal environment.
Geophys. Res. Lett. 1998
, 25, 1661.
| Crossref | GoogleScholarGoogle Scholar |

[4]
L. J. Carpenter ,
W. T. Sturges ,
S. A. Penkett ,
P. S. Liss ,
B. Alicke ,
K. Hebestreit ,
U. Platt ,
Short-lived alkyl iodides and bromides at Mace Head, Ireland: links to biogenic sources and halogen oxide production.
J. Geophys. Res. 1999
, 104, 1679.
| Crossref | GoogleScholarGoogle Scholar |

[5]
B. J. Allan ,
G. McFiggans ,
J. M. C. Plane ,
Observations of iodine monoxide in the remote marine boundary layer.
J. Geophys. Res. 2000
, 105, 14 363.
| Crossref | GoogleScholarGoogle Scholar |

[6]
B. J. Allan ,
J. M. C. Plane ,
G. Mcfiggans ,
Observations of OIO in the remote marine boundary layer.
Geophys. Res. Lett. 2001
, 28, 1945.
| Crossref | GoogleScholarGoogle Scholar |

[7]
L. J. Carpenter ,
P. S. Liss ,
S. A. Penkett ,
Marine organohalogens in the atmosphere over the Atlantic and Southern Oceans.
J. Geophys. Res. 2003
, 108, 4256.
| Crossref | GoogleScholarGoogle Scholar |

[8]
[9]
E. K. Bigg ,
D. E. Turvey ,
Sources of atmospheric particles over Australia.
Atmos. Environ. 1978
, 12, 1643.
| Crossref | GoogleScholarGoogle Scholar |

[10]
J. M. Cainey ,
M. Keywood ,
M. R. Grose ,
P. Krummel ,
I. E. Galbally ,
P. Johnston ,
R. Gillett ,
M. Meyer ,
P. Fraser ,
P. Steele ,
M. Harvey ,
K. Kreher ,
T. Stein ,
O. Ibrahim ,
Z. D. Ristovski ,
G. Johnson ,
C. Fletcher ,
E. K. Bigg ,
J. Gras ,
Precursors to Particles (P2P) at Cape Grim 2006: campaign overview.
Environ. Chem. 2007
, 4, 143.

[11]
C. J. Palmer ,
T. L. Anders ,
L. J. Carpenter ,
F. C. Küpper ,
G. B. McFiggans ,
Iodine and halocarbon response of Laminaria digitata to oxidative stress and links to atmospheric new particle production.
Environ. Chem. 2005
, 2, 282.
| Crossref | GoogleScholarGoogle Scholar |

[12]
[13]
G. P. Ayers ,
S. A. Penkett ,
R. W. Gillett ,
B. Bandy ,
I. E. Galbally ,
C. P. Meyer ,
C. M. Elsworth ,
T. S. Bentley ,
B. W. Forgan ,
The annual cycle of peroxides and ozone in marine air at Cape Grim, Tasmania.
J. Atmos. Chem 1996
, 23, 221.
| Crossref | GoogleScholarGoogle Scholar |

[14]
A. C. Cheshire ,
N. D. Hallam ,
Biomass and density of native stands of Durvillaea potatorum (southern bull-kelp) in south eastern Australia.
Mar. Ecol. Prog. Ser. 1988
, 48, 277.

[15]
M. R. Grose ,
J. M. Cainey ,
A. McMinn ,
J. A. E. Gibson ,
Coastal marine methyl iodide source and links to new particle formation at Cape Grim during February 2006.
Environ. Chem. 2007
, 4, 172.

[16]
[17]
E. A. Gall ,
C. K. Frithjof ,
B. Kloareg ,
A survey of iodine content in Laminaria digitata.
Bot. Mar. 2004
, 47, 30.
| Crossref | GoogleScholarGoogle Scholar |

[18]
R. von Glasow ,
P. Crutzen ,
Model study of multiphase DMS oxidation with a focus on halogens.
Atmos. Chem. Phys. 2004
, 4, 589.

[19]
D. M. Murphy ,
D. S. Thomson ,
A. M. Middlebrook ,
Bromine, iodine, and chlorine in single aerosol particles at Cape Grim.
Geophys. Res. Lett. 1997
, 24, 3197.
| Crossref | GoogleScholarGoogle Scholar |

[20]
P. Vaattovaara ,
P. E. Huttunen ,
Y. J. Yoon ,
J. Joutsensaari ,
K. E. J. Lehtinen ,
C. D. O’Dowd ,
A. Laaksonen ,
The composition of nucleation and Aitken mode particles during coastal nucleation events: evidence for marine secondary organic contribution.
Atmos. Chem. Phys. 2006
, 6, 4601.

[21]
R. Von Glasow ,
Seaweed, iodine, new particles and atmospheric chemistry – the current state of play.
Environ. Chem. 2005
, 2, 243.
| Crossref | GoogleScholarGoogle Scholar |



