A review of the potential risks associated with mercury in subsea oil and gas pipelines in Australia
Francesca Gissi A * , Darren Koppel
A * , Darren Koppel  B C , Alexandra Boyd A , Fenny Kho
B C , Alexandra Boyd A , Fenny Kho  C , Rebecca von Hellfeld
C , Rebecca von Hellfeld  D E , Stuart Higgins C , Simon Apte F and Tom Cresswell
D E , Stuart Higgins C , Simon Apte F and Tom Cresswell  A
A
A Australian Nuclear Science and Technology Organisation, Lucas Heights, NSW 2234, Australia.
B Australian Institute of Marine Science, Crawley, WA 6009, Australia.
C Curtin University Oil and Gas Innovation Centre, Faculty of Science and Engineering, Perth, WA 6009, Australia.
D National Decommissioning Centre, Newburgh, Ellon AB41 6AA, UK.
E School of Biological Sciences, University of Aberdeen, AB24 3UL, Aberdeen, UK.
F Commonwealth Scientific Industrial Research Organisation Land and Water, Lucas Heights, NSW 2234, Australia.
Environmental Chemistry 19(4) 210-227 https://doi.org/10.1071/EN22048
Submitted: 5 May 2022 Accepted: 17 August 2022 Published: 1 November 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Environmental context. The oil and gas industry has a significant liability in decommissioning offshore infrastructure. Following decommissioning, subsea pipelines could be left on the seabed to provide artificial reefs. Mercury is a contaminant of concern which could remain within pipelines. There are gaps in our knowledge on how mercury moves through the marine environment. We review the current science and identify future research needs to understand potential impacts from mercury in subsea pipelines which will better inform decommissioning activities globally.
Abstract. In the coming years, the oil and gas industry will have a significant liability in decommissioning offshore infrastructure such as subsea pipelines. The policies around decommissioning vary depending on regional policies and laws. In Australia, the ‘base case’ for decommissioning is removal of all property and the plugging and abandonment of wells in line with the Offshore Petroleum and Greenhouse Gas Storage (OPGGS) Act 2006. Options other than complete removal may be considered where the titleholder can demonstrate that the alternative decommissioning activity delivers equal or better environmental outcomes compared to complete removal and meets all requirements under the OPGGS Act and regulations. Recent research has demonstrated that decommissioning in situ can have significant environmental benefits by forming artificial reefs, increasing marine biodiversity, and providing a potential fishery location. An issue, which has been given less attention, is around contaminants remaining within decommissioned infrastructure and their potential risks to the marine environment. Mercury is a contaminant of concern known to be present in some oil and gas pipelines, but the potential long-term impacts on marine ecosystems are poorly understood. We present a synthesis of information on mercury cycling in the marine environment including key drivers of methylation in sediments and ocean waters, existing models to predict methylmercury concentrations in sediments, and toxicological effects to marine biota. We discuss the applicability of existing water and sediment quality guidelines, and the associated risk assessment frameworks to decommissioning offshore infrastructure contaminated with mercury. Globally, research is needed to provide a comprehensive risk assessment framework for offshore infrastructure decommissioning. We recommend future areas of research to improve our understanding of the potential risks associated with mercury in subsea oil and gas pipelines.
Keywords: decommissionineg, marine, methylmercury, offshore infrastructure, petroleum, risk assessment, sediments, toxicity.
Introduction
Mercury is a global pollutant found in all ecosystems (Li et al. 2020). While mercury occurs naturally, its concentration in the environment has increased in the last 200 years due to anthropogenic activities (Poulain and Barkay 2013). This has prompted global action to limit its release and to better understand its impacts on the environment and human health. Primary natural sources include the mercury emitted from topsoil, geothermal and volcanic sources (Li et al. 2020). Factors such as land use change, biomass burning, changing meteorological conditions and exchange mechanisms of gaseous mercury can result in the re-emission of previously deposited sources (Pirrone et al. 2010). Anthropogenic sources of mercury to the atmosphere include fossil-fuel power plants, artisanal scale gold mining, non-ferrous metals manufacturing, cement production, waste disposal and caustic soda production (Pirrone et al. 2010).
Atmospheric deposition is the primary pathway by which mercury enters the ocean (Li et al. 2020). Elemental mercury (Hg0) is the dominant form of natural and anthropogenic emissions. Owing to its volatility, Hg0 has long residence times in the atmosphere (6–12 months) allowing it to be transported long distances and deposited in all environments including remote locations (Li et al. 2020). In the atmosphere, Hg0 is photo-oxidised to Hg2+ and returned to the land or oceans in rainfall (Mason et al. 1994; Mason and Sheu 2002; Sunderland and Mason 2007; Soerensen et al. 2010). Riverine, submarine groundwater discharges and deep-sea hydrothermal vents are additional sources of mercury into oceans (Lamborg et al. 2006; Ganguli et al. 2012; Amos et al. 2014). Sea-based anthropogenic sources of mercury pollution include seabed mining, shipwrecks and accidental releases from oil and gas activities (e.g. drilling wastes, produced water, accidental oil spills) (Tornero and Hanke 2016). Estuarine and coastal sediments are often contaminated with mercury from historical and new inputs, and dredging of contaminated sediments can release mercury back into the water column (Tornero and Hanke 2016).
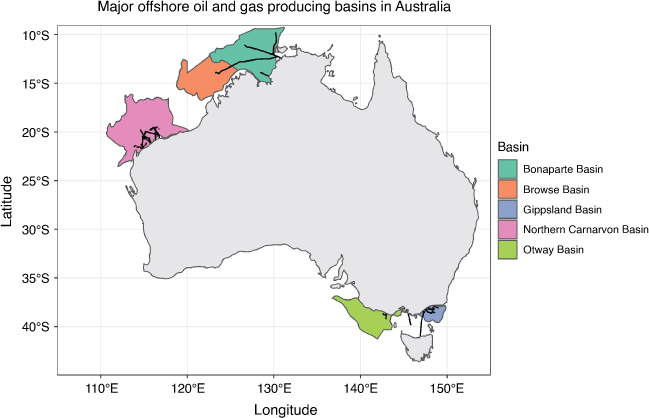
Mercury naturally presents in some oil and gas reservoirs. Various species of mercury coexist in production fluids (oil, gas, water) from these reservoirs including; Hg0, inorganic forms (e.g. HgCl2), cinnabar or metacinnabar (α-HgS, ß-HgS, respectively), or organic mercury (dimethylmercury or methylmercury) (Kho et al. 2022). Over the production life of an oil or gas system, the various forms of mercury interact differently with the infrastructure used to transport and process the production fluids. Subsea oil and gas infrastructure includes subsea Christmas trees (a pressure-containing barrier between the well and the surrounding environment), well head jumpers and spools, flexible and rigid risers, and pipelines (Koppel et al. 2022). In pipelines, particularly gas-export pipelines, elemental mercury can adsorb to surfaces, bind to corrosion products (e.g. iron sulfides), and form sulfide species such as metacinnabar, forming a scale on the internal walls of pipelines. During production and prior to decommissioning, technologies such as pigging or chemical cleaning are used to remove the scale (Kho et al. 2022). Before cleaning options are employed, mercury concentrations in pipelines can be above 1 g Hg/m length of pipeline (Wilhelm and Nelson 2010), depending on the pipeline material, form of mercury, and presence of any internal coatings. Subsea pipelines are believed to contain the largest inventory of contaminants due to the large number of pipelines and their direct contact with production fluids (Kho et al. 2022; Koppel et al. 2022). In Australia, the total length of subsea pipelines is estimated at 8160 km. In the Gulf of Mexico there are over 29 000 km of pipelines that have already been decommissioned in situ (Koppel et al. 2022).
Once an oil or gas field reaches the end of its productive life, the associated infrastructure will need to be decommissioned. In Australia, the ‘base case’ for decommissioning is removal of all property and the plugging and abandonment of wells, as described in the Australian Offshore Petroleum and Greenhouse Gas Storage (OPGGS) Act 2006. Options other than complete removal may be considered where the titleholder can demonstrate that the alternative decommissioning activity delivers equal or better environmental outcomes compared to complete removal and meets all requirements under the OPGGS Act and regulations (DISER 2022). One possible option currently being explored in Australia is to decommission in situ, whereby certain parts of the infrastructure are left on the seabed. Previous studies have demonstrated the benefits of decommissioning in situ through the provision of artificial reefs that increase biodiversity and improve ecosystem connectivity (McLean et al. 2017, 2022; Bond et al. 2018; Fowler et al. 2018). However, few studies to date have assessed the potential risks due to contaminants associated with the infrastructure (MacIntosh et al. 2021; Melbourne-Thomas et al. 2021; Koppel et al. 2022).
Currently, 97 countries, including Australia, have ratified the Minamata Convention. The Convention holds all parties accountable for reducing the release of mercury into the environment (UNEP 2021). This includes the release of mercury from waste products, which can include decommissioned infrastructure, and stipulates that ‘Parties are required to ensure that mercury waste is managed in an environmentally sound manner’ (UNEP 2020). Implementation of the Minamata Convention, as well as other existing international treaties (London Protocol, Basel Convention, United Nations Convention on the Law of the Sea) will likely guide decision making around decommissioning activities in Australia. Policies around mercury management are based on interdisciplinary science with well accepted principles around mercury methylation, methylmercury cycling and bioaccumulation, and food web/trophic transfer to humans (Bank 2020). However, fine-scale aspects of mercury cycling processes, especially in the marine environment remain poorly understood (Bank 2020). This increases the difficulty when assessing the potential long-term risk from mercury associated with oil and gas infrastructure.
A comprehensive risk assessment of offshore decommissioning activities requires an understanding of the potential release of mercury from subsea pipelines and its subsequent fate in the marine environment (Melbourne-Thomas et al. 2021). Models suggest the major pathway for mercury release into the environment will be due to corrosive breakthrough of subsea pipelines, expected to occur >100 years after decommissioning (Kho et al. 2022). The offshore environment around Australia where much of this infrastructure is located is very different to the environments where most research on mercury has focused (freshwater, estuarine, coastal), which has predominantly been in the Northern Hemisphere. Key differences in the Australian marine environment include low sedimentation rates, tropical and temperate ecosystems, different species compositions and low primary productivity (Melbourne-Thomas et al. 2021).
Current knowledge on the formation of mercury in oil and gas production systems was recently reviewed by (Kho et al. 2022). This paper follows on from that review and presents a synthesis of information on mercury cycling in the marine environment including key drivers of methylation in sediments and ocean waters, existing models to predict methylmercury concentrations in sediments, and toxicological effects to marine biota. We discuss the applicability of the Australian and New Zealand water and sediment quality guidelines and risk assessment framework to mercury in decommissioned offshore infrastructure. Globally, research is needed to provide a comprehensive risk assessment framework for decommissioning offshore infrastructure. We recommend future areas of research to better understand potential long-term risks associated with mercury in subsea pipelines.
Mercury in the marine environment
Mercury undergoes chemical and biochemical processes which facilitate its speciation and transport between solid and aqueous phases (Gworek et al. 2016). There are five main species of mercury detected in the environment, inorganic mercury (Hg0, Hg2+) and the organic forms, monomethylmercury, dimethylmercury and monoethylmercury (Li and Cai 2013). In most aquatic systems >95% of total mercury load is associated with sediments (Craig 1986), because dissolved mercury is effectively scavenged from solution by organic matter and mineral phases. Of the dissolved mercury, typically 90% is inorganic (i.e. Hg2+), which is the most stable species of mercury in oxygenated waters. The remainder is found as elemental mercury and methylated forms.
In aquatic systems, the adsorption and desorption processes involving benthic sediments are critical to the distribution of mercury and its transport, transformation, uptake, and toxicity. Whilst the methylation and demethylation of mercury tends towards equilibrium, the dynamic nature of aquatic environments, including the stability of the various forms of mercury, results in continuous shifts in the balance of these reactions (Hintelmann et al. 2000). The additional complexity of oceanic processes further increases the difficulty in accurately determining the fate of mercury in the ocean and its accumulation in marine biota.
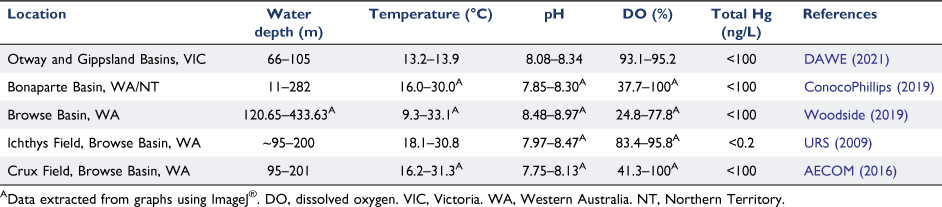
Surveys of the Atlantic, Pacific, Arctic and Southern Oceans have reported low concentrations of total mercury (in the picomolar (10−12) range; as reviewed by Bowman et al. (2020)). The proportion of methylmercury relative to total mercury in surface oceanic waters (0–2 m) ranged from 3 to 34%. At the thermocline (150–1000 m), methylmercury ranged from 3 to 41% of total mercury (Bowman et al. 2020). Limited data exists for total mercury and methylmercury in Australian oceanic waters. Two studies have reported total mercury in marine waters of the North West Shelf of Australia ranging from 0.2 to 0.4 ng/L (Wenziker et al. 2006). Only one data set could be found for methylmercury in Australian waters. Cossa et al. (2011) measured methylmercury in the Southern Ocean with data for the south of Tasmania; the concentration (mean ± s.d.) was 0.06 ± 0.04 ng/L (n = 71). Concentrations of mercury measured in oceanic waters in offshore basins around Australia were reported to be <100 ng/L (Table 1).
|
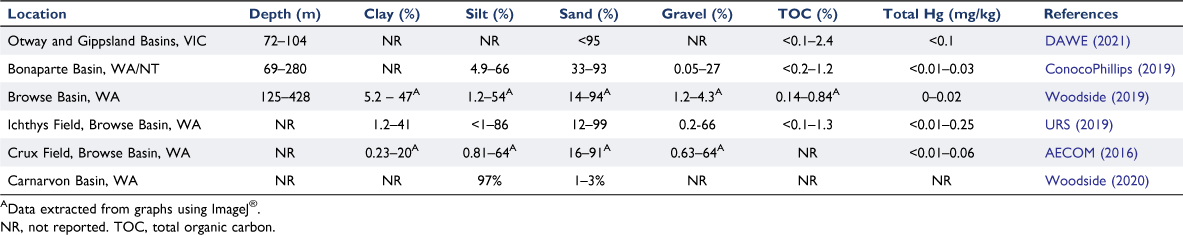
Data on mercury concentrations in offshore sediments is lacking globally and especially around Australia. Chen and Li (2019) reviewed mercury cycling in the Pacific Ocean and cited total mercury concentrations in sediments ranged from 0.02 mg/kg in the Gulf of Thailand to 0.24 mg/kg in Kagoshima Bay in Japan. The proportion of methylmercury relative to total mercury in sediments from the Pacific Ocean, sampled around San Francisco Bay, Malaysia and East China Sea ranged from 0.2 to 7% (Conaway et al. 2003; Shi et al. 2005; Chen and Li 2019). In the open ocean of the northwest Pacific, total mercury in surface sediments ranged from 0.02 to 0.16 mg/kg (Sattarova and Aksentov 2018). This study sampled sediments at >700 m and found that mercury concentrations in sandy sediments (>20% grain fraction of 0.063–2.0 mm) was negligible compared to clayey/silty sediments (>50% grain fraction of 0.004–0.063 mm), which were enriched with organic matter and the remains of silicate phytoplankton (Sattarova and Aksentov 2018). These studies were conducted in bays or deep trenches where the sediment composition is predominantly of a clayey/silty nature, unlike Australian offshore marine sediments, which are predominantly sandy (Table 2). However, total organic carbon (TOC) (0.2–2.2%, Conaway et al. 2003; Shi et al. 2005) in these few studies was similar to what was reported for Australian offshore sediments (Table 2). A handful of publicly accessible industry reports and environmental plans have reported on mercury concentrations in sediments in the offshore environment around Australia. Total mercury concentrations measured in offshore basins around Australia ranged from <0.01 to 0.25 mg/kg (Table 2). Off the North West Shelf of Australia, mercury concentrations were below the limit of detection, <0.01 mg/kg (DEC 2006).
|
Baseline data for total mercury and methylmercury in Australian oceanic waters and sediments are lacking. Such data are required to better understand the sources, transport, and fate of mercury in the offshore environment. Data on physico-chemical properties of offshore sediments (e.g. grain size, redox potential, organic carbon content, pH, sulfide concentrations) are also required to better assess the potential for mercury methylation in these environments.
Methylmercury and mercury methylation
Methylmercury is the most common form of organic mercury in the environment. For over half a century, scientists and regulators have been aware of the toxicity of methylmercury and its potential for biomagnification through aquatic food webs (Bloom 1992; Sørensen et al. 1999; Sunderland 2007). The average proportion of methylmercury to total mercury increases from about 10% in the water column to 15% in phytoplankton, 30% in zooplankton, and 95% in fish (Luoma and Rainbow 2008). The accumulation of methylmercury in higher trophic level organisms results mainly from the ingestion of methylmercury-containing food rather than the direct uptake of methylmercury from the water.
Methylation is a result of abiotic and biotic processes. Abiotic methylation of mercury can occur through reactions with methylcobalamin, methyltin compounds and humic matter (Weber 1993). Under laboratory conditions, methylcobalamin readily methylates mercury. In the marine environment, methyltin and humic matter are the main compounds responsible for abiotic methylation (Weber 1993). This is considered a minor contribution to the total environmental methylmercury pool compared to biotic processes.
Biotic methylation of mercury in sediments is facilitated by methylating microbes (including sulfate and iron reducing bacteria) (Compeau and Bartha 1985; Kerin et al. 2006). Strong environmental controls on this process are provided by pH, redox potential, temperature, the presence of sulfates and the availability of organic carbon (Gilmour et al. 2018). These parameters can increase rates of mercury methylation by either increasing the bioavailability of mercury to methylating microbes, increasing the activity of these microbes, or both (Regnell and Watras 2019). In freshwater systems, it is widely accepted that the dominant source of methylmercury is anoxic sediments by the activity of anaerobic microbes (Regnell and Watras 2019). In these environments, methylation is favoured by low oxygen, high TOC, and fine silty/clayey sediments. Although in stratified lake water columns, methylmercury production is highest just below the oxic-anoxic boundary near the sulfide/sulfate transition zone, possibly reflecting the migration of sulfate reducing bacteria to the sulfate source (Watras and Bloom 1994). Demethylation of methylmercury occurs simultaneously due to photochemical reactions as well as microbial processes (Wang et al. 2020; Li et al. 2022). It is the net result of methylation and demethylation reactions that regulate the concentrations of methylmercury available for uptake by organisms (Paranjape and Hall 2017).
Monomethylmercury appears to be the principal methylated mercury species in freshwater systems while dimethylmercury is more prevalent, and at times is the dominant species, in open ocean waters (Black et al. 2009; Munson et al. 2015). In marine systems, the processes driving methylation are less well understood. Sediments, particularly in the open ocean, are not believed to be a dominant source of methylmercury (Hammerschmidt and Bowman 2012). Offshore marine sediments typically have a sandier texture, with a lower organic matter content and higher dissolved oxygen concentrations compared to coastal, estuarine, or freshwater sediments (Table 2, Supplementary Table S2). There is also minimal evidence to link methylmercury production in marine sediments to the high concentrations observed in some marine organisms, particularly fish. This issue is illustrated by the situation in the open oceans where mercury concentrations in pelagic marine organisms reach parts-per-million range in spite of there being no food web connection with benthic sediments, which lie typically >3 km beneath the ocean surface (Monperrus et al. 2007). However, it is important to note that in the scenario of decommissioning oil and gas infrastructure, subsea pipelines could be a point source of mercury release into the surrounding sediment (Kho et al. 2022).
Anaerobic microbes possessing the hgcAB gene pair that encodes proteins for mercury methylation (Parks et al. 2013) have been identified and well described in anaerobic environments including coastal ‘dead zones’, sediments and extreme environments like deep sea trenches (Podar et al. 2015). The hgcAB gene has been identified in iron reducing bacteria and methanogens, but more often sulfate reducing bacteria are reported to be the dominant drivers of methylmercury production in freshwater, estuarine and coastal sediments (Merritt and Amirbahman 2009; Regnell and Watras 2019). However, methylmercury has been measured in the open ocean without clear links to a sediment source (Mason and Fitzgerald 1990; Cossa et al. 2009, 2011; Sunderland et al. 2009; Munson et al. 2018). It is believed that methylation of mercury in the oceans is largely biologically mediated, but the organisms involved are poorly understood (Lin et al. 2021). Lin and co-authors conducted a large-scale multi-omics study along defined redox gradients in a coastal environment in Canada. Several new marine microorganisms with the hgcAB gene pair and a greater oxygen tolerance and habitat range were identified (Lin et al. 2021).
There is growing evidence that methylation can also occur in oxic environments such as the oxygenated surface waters of the ocean (Monperrus et al. 2007; Heimbürger et al. 2010; Sonke et al. 2013) and polar marine waters (Lehnherr et al. 2011; Sonke et al. 2013). Liu et al. (2015) found no relationship between methylmercury concentrations and trends in water column hypoxia in the Gulf of Mexico, although potential rates of both methylation and demethylation were higher than in other coastal systems. Other research has demonstrated positive correlations between oxygenic conditions such as those that exist in photosynthetic blooms in marine waters and methylmercury concentrations (Cossa et al. 2009; Sunderland et al. 2009; Heimbürger et al. 2010).
Recent developments in the use of stable isotope tracers and genomic work have begun to examine the interplay between multiple environmental factors. For example, Kucharzyk et al. (2015) found that methylation rates in marine sediments were highest when mixed cell cultures were amended with carbon, regardless of whether inorganic mercury was added as dissolved nitrate salt or as nanoparticles of mercuric sulfide (HgS) (as also observed by Graham et al. 2013). Similarly, nutrient loading stimulated microbial activity indirectly by increasing phytoplankton biomass, which was as important as the bioavailability of mercury to methylators (Liem-Nguyen et al. 2016). The conclusion was that methylmercury production is limited by microbial productivity, regardless of mercury bioavailability, and that there is a threshold over which inorganic mercury speciation becomes a contributing control on methylation.
Short-term laboratory measurements of potential rates of methylation and demethylation have been found to be unrelated to gross measures of long-term methylmercury accumulation in sediments (Paranjape and Hall 2017). However, significant positive correlations have been found between the rate of methylation and the proportion of methylmercury in many environments (Drott et al. 2008), suggesting some predictive utility of these measurements.
The relative importance of methylation in the ocean and offshore sediments are no doubt important and require further investigation. Data on the ranges of physico-chemical parameters (particle size for sediments, TOC, dissolved oxygen etc. for waters and sediments) in offshore environments is required. Fundamental studies on how variations in these parameters influence mercury methylation are also needed. An ecological assessment of the microbial community in sediments around subsea oil and gas pipelines could identify the presence of the hgcAB gene pair and elucidate the ability of local microbes to methylate mercury from scale if it were to be released from subsea pipelines.
Solubility and methylation of different mercury species
Mercury solubility and speciation are important variables controlling methylation. Inorganic (Hg0 and Hg2+) and organic (methyl- and dimethyl-) forms of mercury move between sediments, porewaters and overlying waters depending on local environmental conditions. Typically, only a small fraction of inorganic mercury is present in the aqueous phase with the majority partitioned to the solid phase (Jonsson et al. 2014). For example, partition coefficients (Kd) derived from empirical measurements of mercury in estuarine and coastal waters have been reported in the range of 104–106 L/kg (Benoit et al. 1999; Turner et al. 2001; Fitzgerald et al. 2007; Schartup et al. 2014).
Divalent mercury (Hg2+) has a very strong affinity for chloride and reduced sulfur-containing ligands, particularly sulfide. The control that sulfide exerts on mercury bioavailability is not well understood but has been the focus of research in the past 30 years. In reducing environments, most of the mercury is likely to be present as mercuric sulfide, which can include both solid and aqueous species (Benoit et al. 1999). It is also possible that, even in oxygenated surface waters, some or much of Hg2+ might be bound to sulfides, which have been measured at nanomolar concentrations in surface seawater (Morel et al. 1998). At low sulfide concentrations, soluble neutral mercury sulfide (HgS0) complexes are formed, which are capable of diffusing through cell membranes and are therefore more bioavailable (Morel et al. 1998). At moderate to high concentrations of sulfide, insoluble solid HgS as well as HgS0 can form. Higher concentrations of sulfide can result in charged mercury sulfide and polysulfide complexes (HgHS2−) that may still be bioavailable (Benoit et al. 1999; Jay et al. 2000). In the marine environment, it is suggested that the HgS0 species is the form of mercury most bioavailable to methylating microbes (An et al. 2019).
Solid HgS species such as cinnabar or metacinnabar are considered to be sinks for mercury in sediments, due to the very low solubility of HgS (Oliveri et al. 2016). However, the solubility of HgS can increase under anoxic conditions where there is high sulfide activity (Benoit et al. 1999). Oxidation of HgS may also occur in suboxic environments. Oliveri et al. (2016) found irregular vertical distributions of sulfate (SO42−) and enrichment in the iron-manganese (Fe–Mn) reduction zone in coastal marine sediments off Sicily, Italy. Results also showed varying concentrations of dissolved Hg2+ in sediment porewaters between 0 and 6 cm depth profile. The authors suggested this indicated mechanisms of sulfate generation by sulfide oxidation and hence mercury mobilisation. Within these sediments, the authors also identified specific microbial populations dominated by sulfur oxidising bacteria (Oliveri et al. 2016).
Inorganic mercury also exhibits a very high affinity for natural organic matter as is evidenced by strong correlations between organic matter and total mercury content of sediments (Mazrui et al. 2016; Oliveri et al. 2016). A fraction of Hg2+ will be bound to humic and fulvic acids, the assemblage of poorly defined organic compounds that constitute 50–90% of the dissolved organic carbon (DOC) in natural waters (Wu et al. 1997). Mercury-organic matter complexes may not be bioavailable for methylating microbes (Zhao et al. 2017). However, it has also been shown that the presence of dissolved organic matter (DOM) can increase the dissolution of some mercury complexes, including cinnabar (αHg-S) (Waples et al. 2005; Mazrui et al. 2016). DOM can easily adsorb to the surface of HgS nanoparticles and cause changes in the micro-environment around the Hg atom (Lei et al. 2022). This can break the Hg–S bond and dissolve Hg (possibly as a Hg-DOM complex) into the surrounding solution (Lei et al. 2022). Organic matter may also increase methylation of mercury by providing a food source to methylating microbes (Regnell and Watras 2019).
To our knowledge, there are no published studies investigating the solubility and methylation of different forms of mercury under marine conditions, so the consequence of these speciation differences is not well understood. However, several studies have been conducted in estuarine environments. Jonsson et al. (2012) determined the methylation rates of five different types of geochemically important mercury species in estuarine sediments. Adsorbed and solid forms of stable Hg2+ isotope tracers were added to estuarine sediments and incubated under anoxic conditions. Rates of methylation were determined at 24 h, and 7, 14, and 21 days. Methylation rates of the more insoluble mercuric sulfides (metacinnabar and cinnabar) were two orders of magnitude lower than the aqueous mercury (II) nitrate (Hg(NO3)2). In sediments spiked with metacinnabar, the proportion of methylmercury relative to total mercury after 21 days was ~0.5%. In comparison, sediments spiked with Hg(NO3)2 isotope tracer resulted in ~10% methylmercury (relative to total mercury) after 21 days (Jonsson et al. 2012). The authors concluded that the rate of mercury methylation in sediments is controlled by thermodynamic and kinetic effects of Hg2+ solid-phase dissolution and surface desorption control (Jonsson et al. 2012).
Zhang et al. (2012) demonstrated that nanoparticulate forms of metacinnabar are far more amenable to methylation by sulfate reducing bacteria than microparticulate forms, likely due to a substantially increased surface area to volume ratio. They also demonstrated that ageing of particles over periods of days reduced the methylation potential of the nanoparticulate metacinnabar. Tian et al. (2021) investigated the influence of ageing on methylation potential of nanoparticulate metacinnabar. They found that ageing altered the nano-scale surface structure while particle size and surface area remained relatively unchanged. The nano-scale crystalline structure was interrogated by X-ray diffraction. The facet or the surfaces of the crystalline structures identified as (111) are octahedral in shape (Tian et al. 2021). The nano-scale surface structure of the facet identified as (111) had a larger binding affinity to methylating bacteria and hence increased methylation rates. During the ageing process/nanocrystal growth, the (111) facet decreased and therefore so did mercury methylation (Tian et al. 2021). The speciation, particle size and age of solid-phase mercury are important factors that influence mercury solubility and methylation (Zhang et al. 2012; Tian et al. 2021).
The methylation and bioaccumulation of different forms of mercury was further explored by Jonsson et al. (2014). In mesocosm experiments using Hg2+ and methylmercury isotope tracers, the authors simulated recent mercury inputs in the water phase and mercury in sediment bound to organic matter or as metacinnabar. The experiment measured the formation of methylmercury and bioaccumulation within a model estuarine ecosystem. The results indicated that methylmercury from terrestrial and atmospheric sources bioaccumulated more readily in estuarine biota than methylmercury formed within the local sediment (Jonsson et al. 2014).
The studies discussed above have focused on understanding methylation under estuarine conditions. Further research is needed to understand the oxidation, solubility, and rate of methylation of different forms of mercury (e.g. HgS, Hg0, Hg2+) under marine sediment conditions relevant to the Australian offshore marine environments. The mechanisms and reactions behind mercury oxidation, solubility and bioaccumulation by microorganisms are not yet fully understood. We should also investigate the mobility and bioavailability of atmospheric/aqueous mercury versus solid phase/point source additions of mercury, such as HgS or Hg0, which could be released from subsea pipelines into surrounding sediments. This will provide an indication of potential concentrations of inorganic and methylmercury in sediment porewaters and contribute to assessing risk of mercury in subsea oil and gas pipelines.
Models to predict mercury methylation in sediments
Previous studies have aimed to determine if methylation rates are proportional to inorganic mercury concentrations (i.e. is methylation a first-order reaction). This was examined in the context of freshwater ecosystems by Krabbenhoft et al. (1999). Methylmercury production appeared proportional to total mercury concentrations at low total mercury concentrations in sediments; but at high total mercury levels little additional methylmercury was produced with additional total mercury suggesting a saturation effect (Krabbenhoft et al. 1999). A similar relationship was found for mercury in sediments from a range of environments including marine and estuarine systems (Cossa et al. 2014).
Cossa et al. (2014) proposed a Michaelis–Menten type model to relate total mercury to methylmercury and validated the model for a range of estuarine and marine sediments. Other studies have similarly attempted to model mercury methylation or predict methylation potential in sediments including Beldowski et al. (2015) and Dai et al. (2021). The model proposed by Beldowski et al. includes labile mercury, organic matter, redox potential, and abundance of sulfate reducing bacteria as inputs. The model was developed based on field surveys in the Baltic Sea with sediments predominantly under anoxic conditions. The inclusion of sulfate reducing bacteria (SRB) in the model equation is questionable given not all SRBs have the hgcAB gene, therefore the activity of SRB is not an ideal indicator for methylation (Heyes et al. 2006; Paranjape and Hall 2017). Microbes of other groups (iron reducers, methanogens and aerobic microbes) also have the ability to methylate mercury (Paranjape and Hall 2017). In the development of this model, it appears that some of the parameters co-vary, e.g. organic matter and SRB. However, there is no clear demonstration of which parameters are the key drivers in predicting methylation potential.
Dai et al. (2021) used structural equation modelling to investigate global distribution and environmental drivers of methylmercury production in sediments. Marine sediments were found to have the highest methylation potential, compared to paddy soils, wetlands, lakes, and forest soils. After accounting for local drivers of methylation, mercury availability and sediment geochemistry, temperature and precipitation were important regulators of methylmercury production in sediments. This study provided a valuable and comprehensive analysis of global data, although data for marine sediments in the Southern Hemisphere were considerably lacking. To determine the applicability of these models to the Australian offshore environment, research is needed to understand site-specific sediment geochemistry, mercury speciation and methylation rates.
Toxicity of mercury to marine organisms
It is well established that mercury is one of the most toxic metals in the environment (Chen et al. 2008; Mason et al. 2012). Both organic and inorganic forms of mercury have been reported to have a range of effects on marine organisms including reduced growth/development and reproduction, behavioural changes and lethality in acute exposures. Chen and Dong (2021) exposed an estuarine copepod, Tigriopus japonicus to mercury (as HgCl2) for 24 h. The authors reported 50% lethality (LC50) at 1000 µg Hg2+/L at 22°C and 520 µg Hg2+/L at 25°C (Chen and Dong 2021). Lee et al. (2017) assessed lethality of aqueous methylmercury exposure to T. japonicus for 48 h and reported an LC10 (concentration of methylmercury to cause 10% lethality) of 3.8 µg/L. Development time and reproduction were also inhibited at 0.5 and 0.1 μg/L, respectively, however, toxicity estimates were not calculated for these chronic endpoints (Lee et al. 2017). Harayashiki et al. (2016) reported reduced swimming activity in prawns, Penaeus monodon, fed a diet containing 0.6 and 1.2 µg/g of HgCl2 for 96 h. This could indicate an increased risk for prawns in mercury contaminated environments due to the increased risk of predation (Harayashiki et al. 2016).
In a marine flounder, Paralichthys olivaceus, morphological abnormalities and mortality of embryos significantly increased following aqueous exposure to 13 and 11 µg/L of methylmercury (48 h exposure) (Ren et al. 2019). Embryos of another marine fish, Pseudoscianaena crocea, appeared to be less sensitive than the flounder with 50% lethality following 48 h exposure to aqueous methylmercury reported to be 28 µg/L (Yu et al. 2019). Yu et al. also reported a higher rate of morphological abnormalities at ≥1 µg/L. In contrast, larvae of female fish (Cyprinodon variegatus) fed a methylmercury contaminated diet (4.8 mg/kg wet weight) for 2 months were not impacted with respect to survival or growth (Ye and Fisher 2020). The studies discussed so far represent estuarine environments with test species exposed to mercury (inorganic or organic) either through water or diet at salinities between 27–32 psu. It is also noted that many of the reported concentrations are substantially higher than are found in the marine environment and are short term/acute exposures.
Limited chronic mercury toxicity data, for example those including key endpoints such as development and reproduction, exists for marine species (salinities >33 psu). The Australian and New Zealand marine water quality guidelines report lethal effects ranging from 1.8 µg Hg2+/L, in a crustacean to 800 µg Hg2+/L in a fish following exposures for >30 days (ANZG 2018). This data, along with other mercury toxicity data is summarised in Supplementary Table S3. We note that most of this data are acute endpoints and used species or tests which are more relevant to the Northern Hemisphere and/or estuarine environments (see above and section “Application of the ANZG guidelines and framework to the offshore decommissioning scenario”, for further discussion).
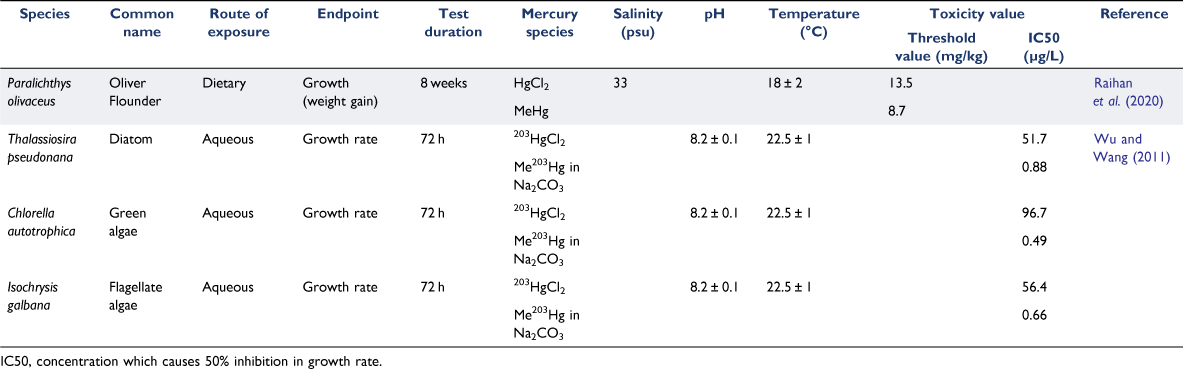
Limited data exists on the toxicity of organic mercury compounds to marine organisms. ANZG and United States Environmental Protection Authority (USEPA) reported that organomercury compounds appear to be 4–31 times more toxic than inorganic mercury (ANZG 2018). We searched for recent studies which directly compared the toxicity of inorganic and methylmercury to marine species. This was done using the databases and search strings as described in Supplementary Table S4. This review paper focuses on understanding impacts of mercury in the offshore marine environment. Therefore, we identified studies where exposures were conducted at salinities ≥33 psu, or where test species were identified as having a coastal/oceanic distribution. We identified two publications which are summarised in Table 3. Raihan et al. (2020) fed adult olive flounder a diet spiked with HgCl2 (at 10, 20, 40 and 160 mg/kg Hg2+) or methyl mercury (10, 20, 40 and 160 mg/kg) for eight weeks and measured growth (weight gain). Food spiked with methylmercury reduced weight gain 1.5 times compared to fish with a diet containing HgCl2 (Raihan et al. 2020). The second study by Wu and Wang (2011) used mercury radioisotope tracers (203Hg) to investigate the effects of HgCl2 versus methylmercury exposure on marine microalgae. They observed changes in growth rate and bioaccumulation of mercury depending on the algal species and the form of mercury (Wu and Wang 2011). Based on growth rate, methylmercury was ~60–200 times more toxic than HgCl2. Chlorella autotrophica (green alga) was the most and least sensitive species to methylmercury and HgCl2, respectively. Thalassiosira pseudonana (diatom) was more sensitive to HgCl2 but less sensitive to methylmercury, when compared to the other two algal species. The authors reported that the differences in sensitivity to methylmercury could be explained by the total or intracellular accumulation of methylmercury. Isochrysis galbana (haptophyte) and T. pseudonana showed similar sensitivities to HgCl2 but I. galbana accumulated about half the amount of total cellular Hg2+ compared to T. pseudonana.
|
A critical knowledge gap exists for the toxicity of mercury to sediment organisms. Conder et al. (2015) compiled sediment quality values (SQVs) for mercury based on ‘co-occurrence’ data on the effects of co-occurring contaminants on benthic invertebrates from field collected sediments. In most cases, mercury concentrations were at background levels and the derived guideline values did not reflect cause-effect or concentration-response relationships for mercury because of the potential effects from multiple stressors. The co-occurrence SQV associated with a lack of effects was 0.16 (0.13–66) mg/kg (median ± interquartile range). The SQV with a potential for effects was 0.88 (0.50–1.4) mg/kg. Mercury-specific effects data were also compiled from laboratory studies using mercury-spiked sediments and field studies at mercury-contaminated sites. Data from these studies resulted in no observable effect concentrations that were orders of magnitude higher than the co-occurrence SQVs; 3.3 (1.1–9.4) mg/kg (median ± interquartile range) for mercury-spiked sediment toxicity tests and 22 (3.8–66) mg/kg for mercury site investigations (Conder et al. 2015). The authors recommended that further research is required to understand what measures of mercury exposure best reflect difference in mercury bioavailability and toxicity of mercury in sediments. The authors recommended development of a more robust exposure-response dataset for bioavailable mercury in sediments. This data could be generated from further studies investigating toxic effects to benthic organisms in laboratory studies using mercury-spiked sediments, or in field-studies at mercury contaminated sites (Conder et al. 2015).
Marine sediment and water quality guidelines for mercury
Australia and New Zealand’s water quality management framework includes water and sediment quality guidelines for mercury (ANZG 2018). These are based on data describing where negative effects were observed to different marine organisms and so represent a toxicity impact. The origin of all the data points used in the water quality guideline is not clear, although we believe most of the data originates from the USEPA (1985). We were unable to find the original publications for all the cited data nor identify all the 43 data points used in deriving the guideline (Supplementary Table S3). Where we were able to identify the original source of the data, toxicity was typically based on exposures to HgCl2 (Supplementary Table S3). The source of the toxicity data used in the derivation of the sediment guideline value is not clear, although it is believed to originate from Long et al. (1995) and possibly includes data which was reviewed in Conder et al. (2015).
For waters in Australia and New Zealand, the default guideline value (DGV) of 0.1 µg/L total mercury is applied to pristine and slightly to moderately disturbed systems to account for the biomagnifying nature of mercury. For sediments the DGV is 0.15 mg/kg total mercury. The DGV-High is 1 mg/kg total mercury (ANZG 2018). The DGV-high provides an indication that toxicity-related adverse effects may already be observed and should only be used as an indicator of potential high-level toxicity problems, not as a guideline value to protect ecosystems (ANZG 2018).
These values should be used as part of a weight-of-evidence process where multiple lines of evidence are applied to assess risk to an ecosystem. If concentrations of mercury in the environment are measured below guideline values, it may be possible that the risk is low, and no further action is required. If concentrations are measured above these guideline values, then further investigations must be undertaken to determine risk.
Application of the ANZG guidelines and framework to the offshore decommissioning scenario
The data used to develop the Australian and New Zealand sediment and water quality guidelines originates from the Northern Hemisphere and predominantly represents estuarine/coastal, not marine species (Supplementary Table S3). This is understandable given that pollution impacts are typically located along the coast and in estuarine systems. Offshore oil and gas activities, including decommissioning, may introduce point sources of pollution in the ocean where environmental conditions are different to the coast. For example, the main Australian offshore oil and gas producing regions range from tropical (Bonaparte and Browse Basins) to sub-tropical (Carnarvon Basin), and temperate (Gippsland and Otway Basins) ecoregions (Fig. 1). Maximum water temperatures in these regions have been recorded above 30°C (Table 1).
The toxicity data used to derive the water quality guidelines were generated from tests where the salinity ranged from 20 to 33 psu and temperature ranged from 4.5 to 25°C (Supplementary Table S3). As discussed above, the transport, fate and especially methylation of mercury in marine environments varies greatly depending on the physico-chemical conditions. The current water quality guidelines may not be appropriate to apply to the offshore Australian marine environment. A significant improvement to the water quality guidelines would be the inclusion of marine/oceanic species (salinity > 33 psu), and species representative of tropical/subtropical habitats (temperature > 20°C).
The data used to derive the ANZG sediment DGVs are for predominantly estuarine or silty sediments (Long et al. 1995; Conder et al. 2015; ANZG 2018). Marine sediments in basins around Australia are typically comprised of sandy sediments (12–99%) and low TOC (<0.1–2.4%) (Table 2). Therefore, the ANZG sediment DGVs may not be applicable to sandy sediments, such as those in the offshore environment on the continental shelf.
In lieu of site-specific values, guidance is provided to tailor existing guidelines to local conditions (Simpson and Batley 2016; van Dam et al. 2019). In waters, this requires water chemistry data to account for local conditions (Warne et al. 2014). For sediments, grain size and organic carbon content are used to refine guidelines for local conditions. Sediments with finer grain size have an increased capacity to bind contaminants, including mercury. When conducting site-specific assessments, the <2 mm sediment particle size fraction should be used to determine metal concentrations and compare with DGVs so that the potential risk from contaminants is not diluted by a mass of larger particles. Contaminants in the <63 µm fraction should be analysed, as particles of this size are more readily resuspended or potentially ingested by benthic organisms (Simpson and Batley 2016).
The unique chemistry of mercury means that relationships between sediment characteristics and risk are not clear. For example, sandy sediments have a lower binding capacity compared to silty sediments, so metal contaminants may pose a greater risk to marine organisms because they are more likely to partition to the porewaters and may be more bioavailable to local organisms (ANZG 2018). However, sandy sediments also typically have unfavourable conditions for mercury methylation (low TOC, oxygenated) and are not believed to promote methylating microbial activity. This could reduce the methylation rate and mean that methylmercury may pose a lower risk in these environments.
For both waters and sediments, guideline values are based on total mercury and derived largely using toxicity data for inorganic mercury. As discussed above, it has been demonstrated that methylmercury is more toxic (Table 3). Future iterations of these guideline values should consider the inclusion of toxicity data for methylmercury or the development of a guideline value specifically for methylmercury.
Additional impacts beyond toxicity to local organisms may also arise from mercury contamination. Methylmercury can biomagnify in food webs. This may cause toxic effects to organisms occupying higher trophic levels (such as seabirds and humans) and may cause other impacts to users of marine resources. The USEPA has released a water quality criterion based on 0.3 mg of methylmercury per kg of fish tissues (wet weight). This is the concentration of methylmercury in freshwater and estuarine fish and shellfish tissue which should not be exceeded to ensure protection of human consumers (USEPA 2001). This is the first time that a water quality criterion for methylmercury expresses a human health criterion as a concentration of contaminant in fish and shellfish tissue, rather than the concentration in the water column (USEPA 2001). Using a fish tissue concentration of methylmercury as the criterion could create challenges in the application to monitoring levels in waters or sediments. However, given concentrations of methylmercury in waters and sediments are often <5% of total mercury, and may be close to or below detection limits of analytical instruments, measuring concentrations of methylmercury in higher trophic organisms provides an additional line of evidence to assess impacts on the ecosystem and potential exposures to human consumers.
The Australian and New Zealand Food standards Code (FSANZ 2017) prescribes maximum levels for mercury in seafood for human consumption. In fish known to contain high levels of mercury (such as swordfish, southern bluefin tuna, barramundi, ling, orange roughy, rays and shark), a tissue concentration of 1 mg/kg of total mercury is applied. For all other species of fish, it is 0.5 mg/kg. Exceeding these values may result in impacts to recreational and commercial fishing communities and Indigenous populations that use marine resources. We are uncertain if the current FSANZ guideline values will prevent regional-scale food web impacts from local releases of mercury from oil and gas infrastructure.
It is unclear how best to predict the environmental concentrations that will cause mercury biomagnification impacts. Tissue concentration standards for metals could be adopted for contaminants such as mercury which biomagnify in marine food webs. However, models that can predict tissue concentrations in any given food web based on exposure concentrations are still in their infancy.
Past examples of mercury contamination in the marine environment
This section describes two instances of historical anthropogenic mercury contamination in the marine environment. These examples highlight the local environmental controls on the subsequent fate of that mercury in sediments, overlying water, and accumulation in marine food webs.
German submarine U-864 wreck, Norway
Lying in approximately 150 m of water off the Norwegian North Sea Island of Fedje is the German World War II submarine (U-864), which was estimated to be carrying 67 tonnes of elemental (liquid) mercury when it was sunk in 1945. The mercury was believed to be stored in >1860 steel canisters to provide ballast within the submarine’s keel (Ndungu et al. 2017). This has contributed to an elevation of up to 108 g/kg dry weight (d.w.) inorganic mercury within the sediment (gravelly sand and sandy clay overlying glacial debris deposited on bedrock) at the wreckage hotspot to approximately 1 mg/kg d.w. at 100 m from the wreck, sampled in 2005 (Uriansrud et al. 2005). Ndungu et al. (2017) collected 2–3 m length sediment core samples and found that the average mercury concentration at 0.1–3 m depth sampled close to the wreck was 0.13 mg/kg, suggesting that the majority of mercury was still present in the surficial layers within the sediments. The concentration of methylmercury within sediments around the wreck was very low (<0.05% of total mercury; Ndungu et al. 2016).
Ndungu et al. (2016) conducted a laboratory study using sediments collected near the U-864 wreck, with the addition of organic carbon (in the form of Chlorella algae), to determine the formation of methylmercury within the sediments and in the overlying water. Total mercury concentration measured in the sediment was 8.9 ± 1.8 mg/kg d.w. (mean ± s.d. n = 3). The study was conducted over 6 months in the absence of bioturbating biota (i.e. burrowing invertebrates) and found that methylmercury production was limited by the amount of organic carbon available within the sediment. For those sediments without any additional organic carbon (1% organic matter as per field conditions), the methylmercury fraction (as a % of total mercury) in the sediment was very low (approx. 0.02%) for the duration of the 6-month experiment. The study also found a strong positive correlation (R2 = 0.92) between organic carbon within the sediment and the flux of both total mercury (up to 1000 ng/m2/day) and methylmercury (up to 100 ng/m2/day) to the overlying water (Ndungu et al. 2016). Though the results of this study demonstrated the methylation potential of the mercury-contaminated sediment, the study was conducted under controlled laboratory conditions, and it is unknown how this would translate to field conditions.
While mercury concentrations in surficial sediment around the submarine U-864 were substantial, the mean concentration in the muscle tissue of tusk fish caught in 2009 (0.21 ± 0.08 mg/kg wet weight (w.w.); Brosme brosme, the main fish species around the island of Fedje at 150 m) were similar or lower than the mean tissue concentration of tusk caught at nine other stations along the Norwegian coast in the same period (0.37 mg/kg w.w.; Kvangarsnes et al. 2012). These values were below the European Commission’s upper regulatory limit of mercury in fish muscle meant for human consumption of 0.5 mg/kg w.w. (European Commission (EC) 2006).
Rua-Ibarz et al. (2016) conducted analyses of the stable mercury isotope composition of elemental mercury recovered from an intact steel container and from sediments around the U-864 wreck in 2013. They also collected crabs (Cancer pargurus) from the wreck location and at 4 nautical miles (nm) north and south of the wreck. Due to the use of stable mercury isotopes, the sediment mercury contamination and the mercury within the brown meat (mainly the digestive organ/hepatopancreas and gonads) could be unequivocally linked with the elemental mercury from the wreck. Furthermore, the stable mercury isotope signatures of the crab’s brown meat suggested that direct ingestion of the elemental mercury from the wreck was occurring (Rua-Ibarz et al. 2016). No other wreck-originating mercury was found in the marine food chain.
Mean concentrations of total mercury in claw meat (muscle tissue) were 0.11 mg/kg w.w. and in brown meat were 0.094 mg/kg w.w. (Rua-Ibarz et al. 2016). These concentrations were not substantially higher than tissues of the same species caught elsewhere along the Norwegian coast with means of 0.095 and 0.067 mg/kg w.w. for claw and brown meat respectively. Rua-Ibarz et al. (2016) noted that the low organic matter content of the sediments around U-864 (approximately 1%; Ndungu et al. 2016) likely means that the majority of total mercury within the sediments was present as elemental mercury, which is less bioavailable than other mercury species. The data suggest that even when exposed to significant concentrations of total mercury in sediments (as elemental mercury) the total concentrations of mercury within crabs was not substantially above background concentrations found in non-contaminated sites.
Studies conducted around this wreck where significant concentrations of elemental mercury were deposited within the sediment suggest that there was minimal bioaccumulation of mercury to the broad marine food chain. When mercury was found to have bioaccumulated in benthic species (e.g. in crabs and tusk fish), concentrations were below human consumption regulatory limits and not substantially above mercury concentrations found in the same species collected away from the wreck. These findings are contrary to other studies where lower mercury concentrations within sediments were found to have bioaccumulated to a greater degree. This suggests that the speciation of mercury (elemental mercury in the case of this wreck) within marine sediments is a governing factor driving the methylation potential and bioavailability of mercury to marine organisms. It is therefore important to understand the site-specific mercury speciation when assessing the potential for bioaccumulation in marine biota.
Minamata Bay, Japan
Between 1932 and 1968, industrial wastewater containing methylmercury and other mercury compounds was discharged directly into Minamata Bay, Japan, seriously contaminating waters, sediments, and biota (Balogh et al. 2015). Subsequent studies suggested that 70–150 t of mercury had been discharged to Minamata Bay, including 0.6–6 t of methylmercury. Total mercury concentrations in the bottom sediments of Minamata Bay were found to exceed 25 μg/g d.w. over a large area (2.1 km2). Concentrations over 100 μg/g were not uncommon, and contamination to a sediment depth of 4 m was observed. As part of a comprehensive remediation project initiated in 1977, the portion of the bay closest to the discharge outlet was sealed off from the rest of the bay. The enclosed area (0.58 km2) isolated the most highly contaminated sediments. Sediments outside this area, with total mercury concentrations exceeding 25 μg/g, were suction-dredged and deposited within the enclosure. Upon project completion in 1990, the enclosed area was capped and covered with clean topsoil and developed for recreational use. In samples taken in 2002, concentrations of total mercury in surface sediments in Minamata Bay were generally less than 6 μg/g and varied little within the top 6–8 cm, indicating that the most highly contaminated sediments had been removed but suggesting also that substantial sediment resuspension, mixing, and subsequent re-deposition had occurred since the dredging project (Balogh et al. 2015).
Methylmercury concentrations in the Minamata Bay sediments were measured by Matsuyama et al. (2016) in samples collected between June 2013 and October 2014, 28 years after dredging operations ceased. The Bay sediments consisted of sandy silt, and the average loss-on-ignition organic carbon concentration in surface sediment was 7.0 ± 2.3%. The average methylmercury concentrations in the upper sediment layers in Minamata Bay were significantly higher than those in the lower sediment layers. The average concentration of methylmercury in surface sediment was 1.74 ± 1.0 ng/g, d.w. (n = 107, maximum 5.50, minimum 0.01 μg/kg). This was almost 16 times higher than that in surface sediment from a nearby control site, which was 0.11 ± 0.045 ng/g (n = 5). The ratio of the methylmercury concentration to the total-mercury concentration was 0.06% (Matsuyama et al. 2016).
Total mercury and methylmercury were determined in core samples taken at selected locations in Minamata Bay in 2002 by Tomiyasu et al. (2006). Both total mercury and methylmercury were highest in the upper layers (top 10–20 cm) of the sediment profile. Fish mercury concentrations decreased following remediation but remained near the Japanese regulatory limit of 0.4 μg/g w.w. and are higher than those found in many other coastal Japanese fisheries (Balogh et al. 2015). Overall, these studies indicate that Minamata Bay provides very valuable information on the fate of mercury in a contaminated marine environment. The linkages between mercury methylation in sediments and bioaccumulation in fish are worthy of further investigation.
Understanding potential risks of mercury from subsea pipelines
Studies of inputs of metal contaminants to the marine environment from offshore oil and gas activities have largely been concerned with rock cuttings from drilling and formation water (produced water) extracted with hydrocarbons (Bakke et al. 2013). In a review of chemical contaminants entering the marine environment from sea-based sources, including offshore oil and gas exploration and production (Tornero and Hanke 2016), the only mention of risks from subsea pipelines is from the mobilisation of contaminants held within the sediment when the seabed is disturbed i.e. via pipeline laying during asset development or during scour due to seabed currents during operation. Residual scale (containing mercury and other contaminants) within pipelines was not mentioned in the review.
In a paper reviewing offshore decommissioning regulations in five countries, Fam et al. (2018) mentioned that there is high mercury content in crude oil in the region of South East Asia and that consequently, equipment and structures ‘may be contaminated with higher levels of mercury and require special waste processing.’ Where contaminated infrastructure is removed from the seabed and transported across boundaries for cleaning/disposal, the Basel Convention must be adhered to in order to provide operational and safety boundaries of the shipment of contaminated items. Furthermore, management plans in the receiving country need to be in place (Fam et al. 2018). However, nothing further is discussed in the publication around the management or fate of mercury within offshore infrastructures.
The Gulf publication entitled ‘The Petroleum Engineering Handbook: Sustainable Operations’ lists specific national standards for decommissioning of subsea pipelines: in Norway a preferred option is to abandon pipelines in situ provided they do not ‘impede other users of the sea’; in the United Kingdom, pipelines are addressed case by case with major pipelines considered for in situ abandonment; in the United States, pipelines can be abandoned in situ provided they do not ‘constitute a hazard to navigation or commercial fishing’ (Khan and Islam 2007). There are no other mentions of pipeline scale/contaminants within this publication.
A recent review by Sommer et al. (2019) did not mention mercury or pipeline scales and made only a single mention of metals (vis bioaccumulation) in the context of considerations of environmental criteria for decommissioning assessment. Another contemporary review of practices and reefing options for global oil and gas platform decommissioning by Bull and Love (2019) provides a comprehensive summary of decommissioning and multiple case studies. However, the review focuses primarily on the ecological aspects of ‘rigs to reefs’ and has no mention of mercury and only mentions metals in the context of scrap infrastructure and drilling fluids. In the last year, a handful of review papers have highlighted the need to understand the risks associated with any contaminants (Melbourne-Thomas et al. 2021; Schläppy et al. 2021) within subsea infrastructure, especially naturally occurring radioactive materials (MacIntosh et al. 2021; Koppel et al. 2022), and mercury (Kho et al. 2022).
The available literature demonstrates that there has previously been a lack of appreciation for the management of contaminants within offshore petroleum infrastructure. Critical knowledge gaps around mercury cycling in marine environments need to be addressed to better facilitate risk assessments and decommissioning practices into the future. In addition, to assess potential risk of mercury from subsea pipelines, data on mercury inventories in oil and gas infrastructure are needed. We also require standardised methods to calculate and model dispersion and mixing of mercury from pipeline scale into sediments to estimate mercury concentrations in surrounding sediments.
Recommendations
Scientific principles related to mercury methylation, methylmercury cycling, bioaccumulation and trophic transfer to humans are relatively well established (Bank 2020). However, certain aspects on the biogeochemistry, transfer, and fate of mercury in the marine environment remain poorly understood. It was just under 10 years ago that the hgcAB gene pair for mercury methylation was identified (Parks et al. 2013). But the diversity of microorganisms which possess this gene and the habitats in which they are found are yet to be fully uncovered. The presence and bioaccumulation of methylmercury in the marine food chain is one example where we still do not completely understand the source of the methylmercury.
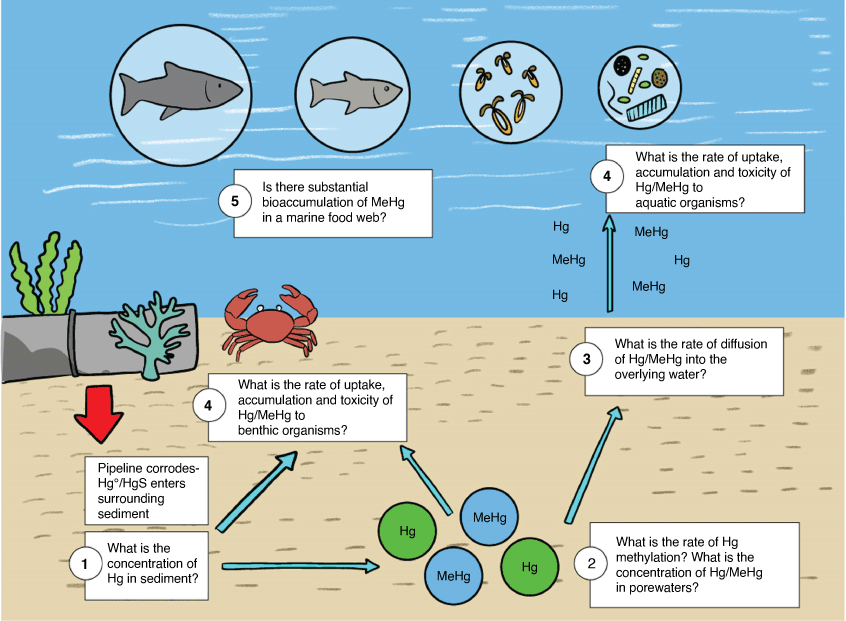
In the context of decommissioning, we need to better understand the biogeochemistry, transport, and fate of mercury in the marine environment so that we can appropriately assess the risk of potential contamination from mercury within subsea oil and gas pipelines. Fig. 2 is a conceptual model outlining some of the research questions that require further investigation:
What is the likely spatial extent of mercury contamination following pipeline decommissioning? We require an understanding of the mercury inventories within subsea pipelines and standardised methods/models to estimate mercury contamination in the sediment once corrosive breakthrough of a pipeline occurs. Further information is required on background concentrations of total- and methylmercury in Australian marine sediments to assess when contamination has occurred, or to detect concentrations above a site-specific threshold value that may be of concern.
What are the likely rates of mercury methylation in the Australian marine environment and how do local environmental factors and mercury speciation affect these rates? What are the concentrations of total- and methyl mercury in porewaters? Laboratory and field experiments are required to understand the oxidation, solubility, and rates of methylation of different forms of mercury (HgS and Hg0, for example) under various sediment conditions relevant to the Australian marine environment. Data on sediment physico-chemical properties and biomonitoring of local microbial communities are also required to provide better assessments on methylation potential.
What are the likely dispersion and diffusion rates of different mercury species from solid to aqueous and particulate phases? Laboratory and field studies should aim to understand the rate of diffusion of total and methylmercury from marine sediments to the overlying water accounting for various sediment and water conditions relevant to the offshore environment.
What is the impact (bioaccumulation and toxicity) of inorganic- and methyl-mercury to Australian marine organisms? The sediment and water quality guidelines were derived using data for predominantly northern hemisphere/estuarine species. Toxicity data and guidelines which are more relevant to the offshore environment are required.
At what concentration do local mercury releases (i.e. from a subsea pipeline) result in substantial biomagnification of inorganic- and methyl-mercury in a marine food web? If subsea pipelines are decommissioned in situ with the justification of forming an artificial reef, which supports enriched biodiversity and a higher biomass of commercially important fishery species, then further research is required to understand the potential risk of mercury accumulation within a food-web and possible outcomes to human consumers. Food safety standards for mercury in fish tissue should be considered in a risk assessment framework, alongside chemical and toxicological data.
|
Supplementary material
Supplementary material is available online.
Data availability
The data that support this study are available in the article and accompanying online supplementary material.
Declaration of funding
No funding sources to declare.
Conflicts of interest
All authors declare that there are no conflicts of interest.
Author contributions
Conceptualisation – FG, TC; Lit review, paper screening – FG, TC, AB; Data compilation, figures, tables – FG, AB, DK; Manuscript preparation – FG, TC, AB, DK; Manuscript review/editing – FG, TC, AB, DK, RvH, FK, SA, SH.
References
AECOM (2016) Appendix A: Water Quality Study. Available at https://www.nopsema.gov.au/sites/default/files/documents/2021-04/A737169.pdf [accessed 1 November 2021]Amos HM, Jacob DJ, Kocman D, Horowitz HM, Zhang Y, Dutkiewicz S, Horvat M, Corbitt ES, Krabbenhoft DP, Sunderland EM (2014). Global biogeochemical implications of mercury discharges from rivers and sediment burial. Environmental Science & Technology 48, 9514–9522.
| Global biogeochemical implications of mercury discharges from rivers and sediment burial.Crossref | GoogleScholarGoogle Scholar |
An J, Zhang L, Lu X, Pelletier DA, Pierce EM, Johs A, Parks JM, Gu B (2019). Mercury uptake by Desulfovibrio desulfuricans ND132: passive or active?. Environmental Science & Technology 53, 6264–6272.
| Mercury uptake by Desulfovibrio desulfuricans ND132: passive or active?.Crossref | GoogleScholarGoogle Scholar |
ANZG (2018) Australian and New Zealand Guidelines for Fresh and Marine Water Quality. Canberra ACT, Australia. Available at www.waterquality.gov.au/anz-guidelines [accessed 1 October 2021]
Bakke T, Klungsøyr J, Sanni S (2013). Environmental impacts of produced water and drilling waste discharges from the Norwegian offshore petroleum industry. Marine Environmental Research 92, 154–169.
| Environmental impacts of produced water and drilling waste discharges from the Norwegian offshore petroleum industry.Crossref | GoogleScholarGoogle Scholar |
Balogh SJ, Tsui MT-K, Blum JD, Matsuyama A, Woerndle GE, Yano S, Tada A (2015). Tracking the fate of mercury in the fish and bottom sediments of Minamata Bay, Japan, using stable mercury isotopes. Environ Sci Technol 49, 5399–406.
| Tracking the fate of mercury in the fish and bottom sediments of Minamata Bay, Japan, using stable mercury isotopes.Crossref | GoogleScholarGoogle Scholar |
Bank MS (2020). The mercury science-policy interface: History, evolution and progress of the Minamata Convention. Science of The Total Environment 722, 137832
| The mercury science-policy interface: History, evolution and progress of the Minamata Convention.Crossref | GoogleScholarGoogle Scholar |
Beldowski J, Miotk M, Pempkowiak J (2015). Methylation index as means of quantification of the compliance of sedimentary mercury to be methylated. Environmental Monitoring and Assessment 187, 498
| Methylation index as means of quantification of the compliance of sedimentary mercury to be methylated.Crossref | GoogleScholarGoogle Scholar |
Benoit JM, Gilmour CC, Mason RP, Heyes A (1999). Sulfide controls on mercury speciation and bioavailability to methylating bacteria in sediment pore waters. Environmental Science & Technology 33, 951–957.
| Sulfide controls on mercury speciation and bioavailability to methylating bacteria in sediment pore waters.Crossref | GoogleScholarGoogle Scholar |
Black FJ, Conaway CH, Flegal AR (2009). Stability of dimethyl mercury in seawater and its conversion to monomethyl mercury. Environmental Science & Technology 43, 4056–4062.
| Stability of dimethyl mercury in seawater and its conversion to monomethyl mercury.Crossref | GoogleScholarGoogle Scholar |
Bloom NS (1992). On the chemical form of mercury in edible fish and marine invertebrate tissue. Canadian Journal of Fisheries and Aquatic Sciences 49, 1010–1017.
| On the chemical form of mercury in edible fish and marine invertebrate tissue.Crossref | GoogleScholarGoogle Scholar |
Bond T, Langlois TJ, Partridge JC, Birt MJ, Malseed BE, Smith L, Mclean DL (2018). Diel shifts and habitat associations of fish assemblages on a subsea pipeline. Fisheries Research 206, 220–234.
| Diel shifts and habitat associations of fish assemblages on a subsea pipeline.Crossref | GoogleScholarGoogle Scholar |
Bowman KL, Lamborg CH, Agather AM (2020). A global perspective on mercury cycling in the ocean. Science of The Total Environment 710, 136166
| A global perspective on mercury cycling in the ocean.Crossref | GoogleScholarGoogle Scholar |
Bull AS, Love MS (2019). Worldwide oil and gas platform decommissioning: a review of practices and reefing options. Ocean & Coastal Management 168, 274–306.
| Worldwide oil and gas platform decommissioning: a review of practices and reefing options.Crossref | GoogleScholarGoogle Scholar |
Chen Y, Dong W (2021). Predicted near-future oceanic warming enhances mercury toxicity in marine copepods. Bulletin of Environmental Contamination and Toxicology 108, 824–829.
| Predicted near-future oceanic warming enhances mercury toxicity in marine copepods.Crossref | GoogleScholarGoogle Scholar |
Chen L, Li Y (2019). A review on the distribution and cycling of mercury in the Pacific Ocean. Bulletin of Environmental Contamination and Toxicology 102, 665–671.
| A review on the distribution and cycling of mercury in the Pacific Ocean.Crossref | GoogleScholarGoogle Scholar |
Chen C, Amirbahman A, Fisher N, Harding G, Lamborg C, Nacci D, Taylor D (2008). Methylmercury in marine ecosystems: spatial patterns and processes of production, bioaccumulation, and biomagnification. EcoHealth 5, 399–408.
| Methylmercury in marine ecosystems: spatial patterns and processes of production, bioaccumulation, and biomagnification.Crossref | GoogleScholarGoogle Scholar |
Compeau GC, Bartha R (1985). Sulfate-reducing bacteria: principal methylators of mercury in anoxic estuarine sediment. Applied and Environmental Microbiology 50, 498–502.
| Sulfate-reducing bacteria: principal methylators of mercury in anoxic estuarine sediment.Crossref | GoogleScholarGoogle Scholar |
Conaway CH, Squire S, Mason RP, Flegal AR (2003). Mercury speciation in the San Francisco Bay estuary. Marine Chemistry 80, 199–225.
| Mercury speciation in the San Francisco Bay estuary.Crossref | GoogleScholarGoogle Scholar |
Conder JM, Fuchsman PC, Grover MM, Magar VS, Henning MH (2015). Critical review of mercury sediment quality values for the protection of benthic invertebrates. Environmental Toxicology and Chemistry 34, 6–21.
| Critical review of mercury sediment quality values for the protection of benthic invertebrates.Crossref | GoogleScholarGoogle Scholar |
ConocoPhillips (2019) Barossa Area Development. Offshore Project Proposal. Appendices. Available at https://docs.nopsema.gov.au/A598152 [accessed 1 November 2021]
Cossa D, Averty B, Pirrone N (2009). The origin of methylmercury in open Mediterranean waters. Limnology and Oceanography 54, 837–844.
| The origin of methylmercury in open Mediterranean waters.Crossref | GoogleScholarGoogle Scholar |
Cossa D, Heimbürger L-E, Lannuzel D, Rintoul SR, Butler ECV, Bowie AR, Averty B, Watson RJ, Remenyi T (2011). Mercury in the Southern Ocean. Geochimica et Cosmochimica Acta 75, 4037–4052.
| Mercury in the Southern Ocean.Crossref | GoogleScholarGoogle Scholar |
Cossa D, Garnier C, Buscail R, Elbaz-Poulichet F, Mikac N, Patel-Sorrentino N, Tessier E, Rigaud S, Lenoble V, Gobeil C (2014). A Michaelis–Menten type equation for describing methylmercury dependence on inorganic mercury in aquatic sediments. Biogeochemistry 119, 35–43.
| A Michaelis–Menten type equation for describing methylmercury dependence on inorganic mercury in aquatic sediments.Crossref | GoogleScholarGoogle Scholar |
Craig PJ (1986) ‘Organometallic Compounds in the Environment: Principles and Reactions.’ (Wiley: New York, NY, USA)
Dai SS, Yang Z, Tong Y, Chen L, Liu SY, Pan R, Li Y, Zhang CJ, Liu YR, Huang Q (2021). Global distribution and environmental drivers of methylmercury production in sediments. Journal of Hazardous Materials 407, 124700
| Global distribution and environmental drivers of methylmercury production in sediments.Crossref | GoogleScholarGoogle Scholar |
DAWE (2021) EPBC Act Protected Matters Report. Available at https://docs.nopsema.gov.au/A800772 [accessed 1 November 2021]
DEC (2006) Background quality of the marine sediments of the Pilbara coast. Marine Technical Report Series. Available at https://www.epa.wa.gov.au/sites/default/files/Policies_and_Guidance/MTR1_Pilbara%20Coast_29Sept06.pdf [accessed 1 November 2021]
DISER (2022) ‘Department of Industry, Science, Energy and Resources. Guideline: Offshore petroleum decommissioning.’ (Australian Government: Canberra, ACT) Available at https://www.nopta.gov.au/_documents/guidelines/Offshore-Petroleum-Decommissioning-guideline.pdf [accessed 1 April 2022]
Drott A, Lambertsson L, Björn E, Skyllberg U (2008). Do potential methylation rates reflect accumulated methyl mercury in contaminated sediments. Environmental Science & Technology 42, 153–158.
| Do potential methylation rates reflect accumulated methyl mercury in contaminated sediments.Crossref | GoogleScholarGoogle Scholar |
European Commission (EC) (2006) Setting Maximum Levels for Certain Contaminants in Foodstuffs. Commission Regulation (EC) No 1881/2006. Available at https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:364:0005:0024:EN:PDF [accessed 1 October 2021]
Fam ML, Konovessis D, Ong LS, Tan HK (2018). A review of offshore decommissioning regulations in five countries – strengths and weaknesses. Ocean Engineering 160, 244–263.
| A review of offshore decommissioning regulations in five countries – strengths and weaknesses.Crossref | GoogleScholarGoogle Scholar |
Fitzgerald WF, Lamborg CH, Hammerschmidt CR (2007). Marine biogeochemical cycling of mercury. Chemical Reviews 107, 641–662.
| Marine biogeochemical cycling of mercury.Crossref | GoogleScholarGoogle Scholar |
Fowler AM, Jørgensen AM, Svendsen JC, Macreadie PI, Jones DOB, Boon AR, Booth DJ, Brabant R, Callahan E, Claisse JT, Dahlgren TG, Degraer S, Dokken QR, Gill AB, Johns DG, Leewis RJ, Lindeboom HJ, Linden O, May R, Murk AJ, Ottersen G, Schroeder DM, Shastri SM, Teilmann J, Todd V, Van Hoey G, Vanaverbeke J, Coolen JWP (2018). Environmental benefits of leaving offshore infrastructure in the ocean. Frontiers in Ecology and the Environment 16, 571–578.
| Environmental benefits of leaving offshore infrastructure in the ocean.Crossref | GoogleScholarGoogle Scholar |
FSANZ (2017) Australia and New Zealand Food Standards Code - Schedule 19 - Maximum levels of contaminants and natural toxicants. Commonwealth of Australia, Canberra. Available at https://www.legislation.gov.au/Details/F2017C00333 [accessed 1 October 2021].
Ganguli PM, Conaway CH, Swarzenski PW, Izbicki JA, Flegal AR (2012). Mercury speciation and transport via submarine groundwater discharge at a Southern California Coastal Lagoon System. Environmental Science & Technology 46, 1480–1488.
| Mercury speciation and transport via submarine groundwater discharge at a Southern California Coastal Lagoon System.Crossref | GoogleScholarGoogle Scholar |
Gilmour CC, Bullock AL, Mcburney A, Podar M, Elias DA (2018). Robust mercury methylation across diverse methanogenic Archaea. mBio 9, e02403-17
| Robust mercury methylation across diverse methanogenic Archaea.Crossref | GoogleScholarGoogle Scholar |
Graham AM, Aiken GR, Gilmour CC (2013). Effect of dissolved organic matter source and character on microbial Hg methylation in Hg–S–DOM solutions. Environmental Science & Technology 47, 5746–5754.
| Effect of dissolved organic matter source and character on microbial Hg methylation in Hg–S–DOM solutions.Crossref | GoogleScholarGoogle Scholar |
Gworek B, Bemowska-Kałabun O, Kijeńska M, Wrzosek-Jakubowska J (2016). Mercury in marine and oceanic waters—a review. Water, Air, & Soil Pollution 227, 371
| Mercury in marine and oceanic waters—a review.Crossref | GoogleScholarGoogle Scholar |
Hammerschmidt CR, Bowman KL (2012). Vertical methylmercury distribution in the subtropical North Pacific Ocean. Marine Chemistry 132–133, 77–82.
| Vertical methylmercury distribution in the subtropical North Pacific Ocean.Crossref | GoogleScholarGoogle Scholar |
Harayashiki CAY, Reichelt-Brushett AJ, Liu L, Butcher P (2016). Behavioural and biochemical alterations in Penaeus monodon post-larvae diet-exposed to inorganic mercury. Chemosphere 164, 241–247.
| Behavioural and biochemical alterations in Penaeus monodon post-larvae diet-exposed to inorganic mercury.Crossref | GoogleScholarGoogle Scholar |
Heimbürger L-E, Cossa D, Marty J-C, Migon C, Averty B, Dufour A, Ras J (2010). Methyl mercury distributions in relation to the presence of nano- and picophytoplankton in an oceanic water column (Ligurian Sea, North-western Mediterranean. Geochimica et Cosmochimica Acta 74, 5549–5559.
| Methyl mercury distributions in relation to the presence of nano- and picophytoplankton in an oceanic water column (Ligurian Sea, North-western Mediterranean.Crossref | GoogleScholarGoogle Scholar |
Heyes A, Mason RP, Kim EH, Sunderland E (2006). Mercury methylation in estuaries: insights from using measuring rates using stable mercury isotopes. Marine Chemistry 102, 134–147.
| Mercury methylation in estuaries: insights from using measuring rates using stable mercury isotopes.Crossref | GoogleScholarGoogle Scholar |
Hintelmann H, Keppel-Jones K, Evans RD (2000). Constants of mercury methylation and demethylation rates in sediments and comparison of tracer and ambient mercury availability. Environmental Toxicology and Chemistry 19, 2204–2211.
| Constants of mercury methylation and demethylation rates in sediments and comparison of tracer and ambient mercury availability.Crossref | GoogleScholarGoogle Scholar |
Jay JA, Morel FMM, Hemond HF (2000). Mercury speciation in the presence of polysulfides. Environmental Science & Technology 34, 2196–2200.
| Mercury speciation in the presence of polysulfides.Crossref | GoogleScholarGoogle Scholar |
Jonsson S, Skyllberg U, Nilsson MB, Westlund PO, Shchukarev A, Lundberg E, Björn E (2012). Mercury methylation rates for geochemically relevant HgII species in sediments. Environmental Science & Technology 46, 11653–11659.
| Mercury methylation rates for geochemically relevant HgII species in sediments.Crossref | GoogleScholarGoogle Scholar |
Jonsson S, Skyllberg U, Nilsson MB, Lundberg E, Andersson A, Björn E (2014). Differentiated availability of geochemical mercury pools controls methylmercury levels in estuarine sediment and biota. Nature Communications 5, 4624
| Differentiated availability of geochemical mercury pools controls methylmercury levels in estuarine sediment and biota.Crossref | GoogleScholarGoogle Scholar |
Kerin EJ, Gilmour CC, Roden E, Suzuki MT, Coates JD, Mason RP (2006). Mercury methylation by dissimilatory iron-reducing bacteria. Applied and Environmental Microbiology 72, 7919–7921.
| Mercury methylation by dissimilatory iron-reducing bacteria.Crossref | GoogleScholarGoogle Scholar |
Khan MI, Islam MR (2007) Chapter 9 - Decommissioning of drilling and production facilities. In ‘The Petroleum Engineering Handbook: Sustainable Operations’. (Eds Khan MI, Islam MR) (Gulf Publishing Company)
Kho F, Koppel DJ, Von Hellfeld R, Hastings A, Gissi F, Cresswell T, Higgins S (2022). Current understanding of the ecological risk of mercury from subsea oil and gas infrastructure to marine ecosystems. Journal of Hazardous Materials 438, 129348
| Current understanding of the ecological risk of mercury from subsea oil and gas infrastructure to marine ecosystems.Crossref | GoogleScholarGoogle Scholar |
Koppel DJ, Kho F, Hastings A, Crouch D, Macintosh A, Cresswell T, Higgins S (2022). Current understanding and research needs for ecological risk assessments of naturally occurring radioactive materials (NORM) in subsea oil and gas pipelines. Journal of Environmental Radioactivity 241, 106774
| Current understanding and research needs for ecological risk assessments of naturally occurring radioactive materials (NORM) in subsea oil and gas pipelines.Crossref | GoogleScholarGoogle Scholar |
Krabbenhoft DP, Wiener JG, Brumbaugh WG, Olson ML, Dewild JF, Sabin TJ (1999) A national pilot study of mercury contamination of aquatic ecosystems along multiple gradients. US geological survey toxic substances hydrology program. In ‘Proceedings of the Technical Meeting, Charleston, South Carolina’. pp. 147–160. (United States Geological Survey: Washington, DC, USA)
Kucharzyk KH, Deshusses MA, Porter KA, Hsu-Kim H (2015). Relative contributions of mercury bioavailability and microbial growth rate on net methylmercury production by anaerobic mixed cultures. Environmental Science: Processes & Impacts 17, 1568–1577.
| Relative contributions of mercury bioavailability and microbial growth rate on net methylmercury production by anaerobic mixed cultures.Crossref | GoogleScholarGoogle Scholar |
Kvangarsnes K, Frantzen S, Julshamn K, Sæthre LJ, Nedreaas K, Maage A (2012). Distribution of mercury in a gadoid fish species, tusk (Brosme brosme), and its implication for food safety. Journal of Food Science and Engineering 2, 603–615.
| Distribution of mercury in a gadoid fish species, tusk (Brosme brosme), and its implication for food safety.Crossref | GoogleScholarGoogle Scholar |
Lamborg CH, Von Damm KL, Fitzgerald WF, Hammerschmidt CR, Zierenberg R (2006). Mercury and monomethylmercury in fluids from Sea Cliff submarine hydrothermal field, Gorda Ridge. Geophysical Research Letters 33, L17606
| Mercury and monomethylmercury in fluids from Sea Cliff submarine hydrothermal field, Gorda Ridge.Crossref | GoogleScholarGoogle Scholar |
Lee YH, Kang H-M, Kim D-H, Wang M, Jeong C-B, Lee J-S (2017). Adverse effects of methylmercury (MeHg) on life parameters, antioxidant systems, and MAPK signaling pathways in the copepod Tigriopus japonicus. Aquatic Toxicology 184, 133–141.
| Adverse effects of methylmercury (MeHg) on life parameters, antioxidant systems, and MAPK signaling pathways in the copepod Tigriopus japonicus.Crossref | GoogleScholarGoogle Scholar |
Lehnherr I, St. Louis VL, Hintelmann H, Kirk JL (2011). Methylation of inorganic mercury in polar marine waters. Nature Geoscience 4, 298–302.
| Methylation of inorganic mercury in polar marine waters.Crossref | GoogleScholarGoogle Scholar |
Lei P, Zou N, Liu Y, Cai W, Wu M, Tang W, Zhong H (2022). Understanding the risks of mercury sulfide nanoparticles in the environment: formation, presence, and environmental behaviors. Journal of Environmental Sciences 119, 78–92.
| Understanding the risks of mercury sulfide nanoparticles in the environment: formation, presence, and environmental behaviors.Crossref | GoogleScholarGoogle Scholar |
Li YB, Cai Y (2013). Progress in the study of mercury methylation and demethylation in aquatic environments. Chinese Science Bulletin Science 58, 177–185.
| Progress in the study of mercury methylation and demethylation in aquatic environments.Crossref | GoogleScholarGoogle Scholar |
Li C, Sonke JE, Le Roux G, Piotrowska N, Van Der Putten N, Roberts SJ, Daley T, Rice E, Gehrels R, Enrico M, Mauquoy D, Roland TP, De Vleeschouwer F (2020). Unequal anthropogenic enrichment of mercury in Earth’s northern and southern hemispheres. ACS Earth and Space Chemistry 4, 2073–2081.
| Unequal anthropogenic enrichment of mercury in Earth’s northern and southern hemispheres.Crossref | GoogleScholarGoogle Scholar |
Li Y, Li D, Song B, Li Y (2022). The potential of mercury methylation and demethylation by 15 species of marine microalgae. Water Research 215, 118266
| The potential of mercury methylation and demethylation by 15 species of marine microalgae.Crossref | GoogleScholarGoogle Scholar |
Liem-Nguyen V, Jonsson S, Skyllberg U, Nilsson MB, Andersson A, Lundberg E, Björn E (2016). Effects of nutrient loading and mercury chemical speciation on the formation and degradation of methylmercury in estuarine sediment. Environmental Science & Technology 50, 6983–6990.
| Effects of nutrient loading and mercury chemical speciation on the formation and degradation of methylmercury in estuarine sediment.Crossref | GoogleScholarGoogle Scholar |
Lin H, Ascher DB, Myung Y, Lamborg CH, Hallam SJ, Gionfriddo CM, Holt KE, Moreau JW (2021). Mercury methylation by metabolically versatile and cosmopolitan marine bacteria. The ISME Journal 15, 1810–1825.
| Mercury methylation by metabolically versatile and cosmopolitan marine bacteria.Crossref | GoogleScholarGoogle Scholar |
Liu B, Schaider LA, Mason RP, Shine JP, Rabalais NN, Senn DB (2015). Controls on methylmercury accumulation in northern Gulf of Mexico sediments. Estuarine, Coastal and Shelf Science 159, 50–59.
| Controls on methylmercury accumulation in northern Gulf of Mexico sediments.Crossref | GoogleScholarGoogle Scholar |
Long ER, Macdonald DD, Smith SL, Calder FD (1995). Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environmental Management 19, 81–97.
| Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments.Crossref | GoogleScholarGoogle Scholar |
Luoma SN, Rainbow PS (2008) ‘Metal Contamination in Aquatic Environments: Science and Lateral Management.’ (Cambridge University Press: Cambridge)
Macintosh A, Dafforn K, Penrose B, Chariton A, Cresswell T (2021). Ecotoxicological effects of decommissioning offshore petroleum infrastructure: a systematic review. Critical Reviews in Environmental Science and Technology 52, 3283–3321.
| Ecotoxicological effects of decommissioning offshore petroleum infrastructure: a systematic review.Crossref | GoogleScholarGoogle Scholar |
Mason RP, Fitzgerald WF (1990). Alkylmercury species in the equatorial Pacific. Nature 347, 457–459.
| Alkylmercury species in the equatorial Pacific.Crossref | GoogleScholarGoogle Scholar |
Mason RP, Sheu G-R (2002). Role of the ocean in the global mercury cycle. Global Biogeochem. Cycles 16, 1093
| Role of the ocean in the global mercury cycle.Crossref | GoogleScholarGoogle Scholar |
Mason RP, Fitzgerald WF, Morel FMM (1994). The biogeochemical cycling of elemental mercury: anthropogenic influences. Geochimica et Cosmochimica Acta 58, 3191–3198.
| The biogeochemical cycling of elemental mercury: anthropogenic influences.Crossref | GoogleScholarGoogle Scholar |
Mason RP, Choi AL, Fitzgerald WF, Hammerschmidt CR, Lamborg CH, Soerensen AL, Sunderland EM (2012). Mercury biogeochemical cycling in the ocean and policy implications. Environmental Research 119, 101–117.
| Mercury biogeochemical cycling in the ocean and policy implications.Crossref | GoogleScholarGoogle Scholar |
Matsuyama A, Yano S, Hisano A, Kindaichi M, Sonoda I, Tada A, Akagi H (2016). Distribution and characteristics of methylmercury in surface sediment in Minamata Bay. Marine Pollution Bulletin 109, 378–385.
| Distribution and characteristics of methylmercury in surface sediment in Minamata Bay.Crossref | GoogleScholarGoogle Scholar |
Mazrui NM, Jonsson S, Thota S, Zhao J, Mason RP (2016). Enhanced availability of mercury bound to dissolved organic matter for methylation in marine sediments. Geochimica et Cosmochimica Acta 194, 153–162.
| Enhanced availability of mercury bound to dissolved organic matter for methylation in marine sediments.Crossref | GoogleScholarGoogle Scholar |
McLean DL, Partridge JC, Bond T, Birt MJ, Bornt KR, Langlois TJ (2017). Using industry ROV videos to assess fish associations with subsea pipelines. Continental Shelf Research 141, 76–97.
| Using industry ROV videos to assess fish associations with subsea pipelines.Crossref | GoogleScholarGoogle Scholar |
McLean DL, Ferreira LC, Benthuysen JA, Miller KJ, Schläppy M-L, Ajemian MJ, Berry O, Birchenough SNR, Bond T, Boschetti F, Bull AS, Claisse JT, Condie SA, Consoli P, Coolen JWP, Elliott M, Fortune IS, Fowler AM, Gillanders BM, Harrison HB, Hart KM, Henry L-A, Hewitt CL, Hicks N, Hock K, Hyder K, Love M, Macreadie PI, Miller RJ, Montevecchi WA, Nishimoto MM, Page HM, Paterson DM, Pattiaratchi CB, Pecl GT, Porter JS, Reeves DB, Riginos C, Rouse S, Russell DJF, Sherman CDH, Teilmann J, Todd VLG, Treml EA, Williamson DH, Thums M (2022). Influence of offshore oil and gas structures on seascape ecological connectivity. Global Change Biology 28, 3515–3536.
| Influence of offshore oil and gas structures on seascape ecological connectivity.Crossref | GoogleScholarGoogle Scholar |
Melbourne-Thomas J, Hayes KR, Hobday AJ, Little LR, Strzelecki J, Thomson DP, Van Putten I, Hook SE (2021). Decommissioning research needs for offshore oil and gas infrastructure in Australia. Frontiers in Marine Science 8, 711151
| Decommissioning research needs for offshore oil and gas infrastructure in Australia.Crossref | GoogleScholarGoogle Scholar |
Merritt KA, Amirbahman A (2009). Mercury methylation dynamics in estuarine and coastal marine environments – a critical review. Earth-Science Reviews 96, 54–66.
| Mercury methylation dynamics in estuarine and coastal marine environments – a critical review.Crossref | GoogleScholarGoogle Scholar |
Monperrus M, Tessier E, Amouroux D, Leynaert A, Huonnic P, Donard OFX (2007). Mercury methylation, demethylation and reduction rates in coastal and marine surface waters of the Mediterranean Sea. Marine Chemistry 107, 49–63.
| Mercury methylation, demethylation and reduction rates in coastal and marine surface waters of the Mediterranean Sea.Crossref | GoogleScholarGoogle Scholar |
Morel FMM, Kraepiel AML, Amyot M (1998). The chemical cycle and bioaccumulation of mercury. Annual Review of Ecology and Systematics 29, 543–566.
| The chemical cycle and bioaccumulation of mercury.Crossref | GoogleScholarGoogle Scholar |
Munson KM, Lamborg CH, Swarr GJ, Saito MA (2015). Mercury species concentrations and fluxes in the central tropical Pacific Ocean. Global Biogeochemical Cycles 29, 656–676.
| Mercury species concentrations and fluxes in the central tropical Pacific Ocean.Crossref | GoogleScholarGoogle Scholar |
Munson KM, Lamborg CH, Boiteau RM, Saito MA (2018). Dynamic mercury methylation and demethylation in oligotrophic marine water. Biogeosciences 15, 6451–6460.
| Dynamic mercury methylation and demethylation in oligotrophic marine water.Crossref | GoogleScholarGoogle Scholar |
Ndungu K, Schaanning M, Braaten HFV (2016). Effects of organic matter addition on methylmercury formation in capped and uncapped marine sediments. Water Research 103, 401–407.
| Effects of organic matter addition on methylmercury formation in capped and uncapped marine sediments.Crossref | GoogleScholarGoogle Scholar |
Ndungu K, Beylich BA, Staalstrøm A, Øxnevad S, Berge JA, Braaten HFV, Schaanning M, Bergstrøm R (2017). Petroleum oil and mercury pollution from shipwrecks in Norwegian coastal waters. Science of the Total Environment 593-594, 624–633.
| Petroleum oil and mercury pollution from shipwrecks in Norwegian coastal waters.Crossref | GoogleScholarGoogle Scholar |
Oliveri E, Salvagio Manta D, Bonsignore M, Cappello S, Tranchida G, Bagnato E, Sabatino N, Santisi S, Sprovieri M (2016). Mobility of mercury in contaminated marine sediments: biogeochemical pathways. Marine Chemistry 186, 1–10.
| Mobility of mercury in contaminated marine sediments: biogeochemical pathways.Crossref | GoogleScholarGoogle Scholar |
Paranjape AR, Hall BD (2017). Recent advances in the study of mercury methylation in aquatic systems. FACETS 2, 85–119.
| Recent advances in the study of mercury methylation in aquatic systems.Crossref | GoogleScholarGoogle Scholar |
Parks JM, Johs A, Podar M, Bridou R, Hurt Jr RA, Smith SD, Tomanicek SJ, Qian Y, Brown SD, Brandt CC, Palumbo AV, Smith JC, Wall JD, Elias DA, Liang L (2013). The genetic basis for bacterial mercury methylation. Science 339, 1332–1335.
| The genetic basis for bacterial mercury methylation.Crossref | GoogleScholarGoogle Scholar |
Pirrone N, Cinnirella S, Feng X, Finkelman RB, Friedli HR, Leaner J, Mason R, Mukherjee AB, Stracher GB, Streets DG, Telmer K (2010). Global mercury emissions to the atmosphere from anthropogenic and natural sources. Atmospheric Chemistry and Physics 10, 5951–5964.
| Global mercury emissions to the atmosphere from anthropogenic and natural sources.Crossref | GoogleScholarGoogle Scholar |
Podar M, Gilmour CC, Brandt CC, Soren A, Brown SD, Crable BR, Palumbo AV, Somenahally AC, Elias DA (2015). Global prevalence and distribution of genes and microorganisms involved in mercury methylation. Science Advances 1, e1500675–e1500675.
| Global prevalence and distribution of genes and microorganisms involved in mercury methylation.Crossref | GoogleScholarGoogle Scholar |
Poulain AJ, Barkay T (2013). Cracking the mercury methylation code. Science 339, 1280–1281.
| Cracking the mercury methylation code.Crossref | GoogleScholarGoogle Scholar |
Raihan SM, Moniruzzaman M, Park Y, Lee S, Bai SC (2020). Evaluation of dietary organic and inorganic mercury threshold levels on induced mercury toxicity in a marine fish model. Animals 10, 405
| Evaluation of dietary organic and inorganic mercury threshold levels on induced mercury toxicity in a marine fish model.Crossref | GoogleScholarGoogle Scholar |
Regnell O, Watras CJ (2019). Microbial mercury methylation in aquatic environments: a critical review of published field and laboratory studies. Environmental Science & Technology 53, 4–19.
| Microbial mercury methylation in aquatic environments: a critical review of published field and laboratory studies.Crossref | GoogleScholarGoogle Scholar |
Ren Z, Cao L, Huang W, Liu J, Cui W, Dou S (2019). Toxicity test assay of waterborne methylmercury on the Japanese flounder (Paralichthys olivaceus) at embryonic-larval stages. Bulletin of Environmental Contamination and Toxicology 102, 770–777.
| Toxicity test assay of waterborne methylmercury on the Japanese flounder (Paralichthys olivaceus) at embryonic-larval stages.Crossref | GoogleScholarGoogle Scholar |
Rua-Ibarz A, Bolea-Fernandez E, Maage A, Frantzen S, Valdersnes S, Vanhaecke F (2016). Assessment of Hg pollution released from a WWII submarine wreck (U-864) by Hg isotopic analysis of sediments and Cancer pagurus tissues. Environmental Science & Technology 50, 10361–10369.
| Assessment of Hg pollution released from a WWII submarine wreck (U-864) by Hg isotopic analysis of sediments and Cancer pagurus tissues.Crossref | GoogleScholarGoogle Scholar |
Sattarova VV, Aksentov KI (2018). Geochemistry of mercury in surface sediments of the Kuril Basin of the Sea of Okhotsk, Kuril-Kamchatka Trench and adjacent abyssal plain and northwest part of the Bering Sea. Deep Sea Research Part II: Topical Studies in Oceanography 154, 24–31.
| Geochemistry of mercury in surface sediments of the Kuril Basin of the Sea of Okhotsk, Kuril-Kamchatka Trench and adjacent abyssal plain and northwest part of the Bering Sea.Crossref | GoogleScholarGoogle Scholar |
Schartup AT, Balcom PH, Mason RP (2014). Sediment-porewater partitioning, total sulfur, and methylmercury production in estuaries. Environmental Science & Technology 48, 954–960.
| Sediment-porewater partitioning, total sulfur, and methylmercury production in estuaries.Crossref | GoogleScholarGoogle Scholar |
Schläppy M-L, Robinson LM, Camilieri-Asch V, Miller K (2021). Trash or treasure? Considerations for future ecological research to inform oil and gas decommissioning. Frontiers in Marine Science 8, 642539
| Trash or treasure? Considerations for future ecological research to inform oil and gas decommissioning.Crossref | GoogleScholarGoogle Scholar |
Shi J-B, Liang L-N, Yuan C-G, He B, Jiang G-B (2005). Methylmercury and total mercury in sediments collected from the East China Sea. Bulletin of Environmental Contamination and Toxicology 74, 980–987.
| Methylmercury and total mercury in sediments collected from the East China Sea.Crossref | GoogleScholarGoogle Scholar |
Simpson SL, Batley GE (2016) ‘Sediment Quality Assessment; a Practical Handbook’. (CSIRO Publishing: Melbourne, Vic., Australia)
Soerensen AL, Sunderland EM, Holmes CD, Jacob DJ, Yantosca RM, Skov H, Christensen JH, Strode SA, Mason RP (2010). An improved global model for air-sea exchange of mercury: high concentrations over the North Atlantic. Environmental Science & Technology 44, 8574–8580.
| An improved global model for air-sea exchange of mercury: high concentrations over the North Atlantic.Crossref | GoogleScholarGoogle Scholar |
Sommer B, Fowler AM, Macreadie PI, Palandro DA, Aziz AC, Booth DJ (2019). Decommissioning of offshore oil and gas structures – environmental opportunities and challenges. Science of the Total Environment 658, 973–981.
| Decommissioning of offshore oil and gas structures – environmental opportunities and challenges.Crossref | GoogleScholarGoogle Scholar |
Sonke JE, Heimbürger L-E, Dommergue A (2013). Mercury biogeochemistry: paradigm shifts, outstanding issues and research needs. Comptes Rendus Geoscience 345, 213–224.
| Mercury biogeochemistry: paradigm shifts, outstanding issues and research needs.Crossref | GoogleScholarGoogle Scholar |
Sørensen N, Murata K, Budtz-Jørgensen E, Weihe P, Grandjean P (1999). Prenatal methylmercury exposure as a cardiovascular risk factor at seven years of age. Epidemiology 10, 370–375.
| Prenatal methylmercury exposure as a cardiovascular risk factor at seven years of age.Crossref | GoogleScholarGoogle Scholar |
Sunderland EM (2007). Mercury exposure from domestic and imported estuarine and marine fish in the U.S. seafood market. Environmental Health Perspectives 115, 235–242.
| Mercury exposure from domestic and imported estuarine and marine fish in the U.S. seafood market.Crossref | GoogleScholarGoogle Scholar |
Sunderland EM, Mason RP (2007). Human impacts on open ocean mercury concentrations. Global Biogeochemical Cycles 21, GB4022
| Human impacts on open ocean mercury concentrations.Crossref | GoogleScholarGoogle Scholar |
Sunderland EM, Krabbenhoft DP, Moreau JW, Strode SA, Landing WM (2009). Mercury sources, distribution, and bioavailability in the North Pacific Ocean: Insights from data and models. Global Biogeochemical Cycles 23, GB2010
| Mercury sources, distribution, and bioavailability in the North Pacific Ocean: Insights from data and models.Crossref | GoogleScholarGoogle Scholar |
Tian L, Guan W, Ji Y, He X, Chen W, Alvarez PJJ, Zhang T (2021). Microbial methylation potential of mercury sulfide particles dictated by surface structure. Nature Geoscience 14, 409–416.
| Microbial methylation potential of mercury sulfide particles dictated by surface structure.Crossref | GoogleScholarGoogle Scholar |
Tomiyasu T, Matsuyama A, Eguchi T, Fuchigami Y, Oki K, Horvat M, Rajar R, Akagi H (2006). Spatial variations of mercury in sediment of Minamata Bay, Japan. Science of The Total Environment 368, 283–290.
| Spatial variations of mercury in sediment of Minamata Bay, Japan.Crossref | GoogleScholarGoogle Scholar |
Tornero V, Hanke G (2016). Chemical contaminants entering the marine environment from sea-based sources: a review with a focus on European seas. Marine Pollution Bulletin 112, 17–38.
| Chemical contaminants entering the marine environment from sea-based sources: a review with a focus on European seas.Crossref | GoogleScholarGoogle Scholar |
Turner A, Millward GE, Le Roux SM (2001). Sediment-water partitioning of inorganic mercury in estuaries. Environ. Sci. Technol 35, 4648–4654.
| Sediment-water partitioning of inorganic mercury in estuaries.Crossref | GoogleScholarGoogle Scholar |
UNEP (2020) Progress report 2020. Overview of the Minamata Convention on Mercury Activities. Available at https://www.mercuryconvention.org/sites/default/files/2021-06/Minamata-Progress-report-2020.pdf [accessed 1 April 2022]
UNEP (2021) UN Environment Program. Minamata Convention on Mercury. Available at https://www.mercuryconvention.org/en [accessed 1 April 2022]
Uriansrud F, Kskei K, Schoyen M (2005) Miljøkonsekvensvurdering av kvikksølv ved sunket ubåt U-864, Fedje i Hordaland. Fase 1. Kvikksølvkartlegging. NIVA Report No. 5022-2005 (Norwegian)
URS (2009) Ichthys Gas Field Development Project. Nearshore Marine Water Quality and Sediment Study. Available at https://ntepa.nt.gov.au/__data/assets/pdf_file/0005/287483/draft_eis_appendix_9.pdf [accessed 1 November 2021]
URS (2019) Appendix 9 Nearshore marine water quality and sediment study. Available at https://ntepa.nt.gov.au/__data/assets/pdf_file/0005/287483/draft_eis_appendix_9.pdf [accessed 1 November 2021]
USEPA (1985) Guidelines for deriving numerical national water quality criteria for the protection of aquatic organisms and their uses. Available at https://www.epa.gov/wqc/guidelines‐deriving‐numerical‐national‐water‐quality‐criteria‐protection‐aquatic‐organisms‐and [accessed 1 October 2021]
USEPA (2001) Guidance for implementing the January 2001 methylmercury water quality criterion. Available at https://www.epa.gov/sites/default/files/2019-02/documents/guidance-implement-methylmercury-2001.pdf [accessed 1 October 2021]
van Dam RA, Hogan AC, Harford AJ, Humphrey CL (2019). How specific is site‐specific? A review and guidance for selecting and evaluating approaches for deriving local water quality benchmarks. Integrated Environmental Assessment and Management 15, 683–702.
| How specific is site‐specific? A review and guidance for selecting and evaluating approaches for deriving local water quality benchmarks.Crossref | GoogleScholarGoogle Scholar |
Wang K, Munson KM, Armstrong DA, Macdonald RW, Wang F (2020). Determining seawater mercury methylation and demethylation rates by the seawater incubation approach: A critique. Marine Chemistry 219, 103753–103753.
| Determining seawater mercury methylation and demethylation rates by the seawater incubation approach: A critique.Crossref | GoogleScholarGoogle Scholar |
Waples JS, Nagy KL, Aiken GR, Ryan JN (2005). Dissolution of cinnabar (HgS) in the presence of natural organic matter. Geochimica et Cosmochimica Acta 69, 1575–1588.
| Dissolution of cinnabar (HgS) in the presence of natural organic matter.Crossref | GoogleScholarGoogle Scholar |
Warne MSJ, Batley GE, Braga O, Chapman JC, Fox DR, Hickey CW, Stauber JL, Van Dam R (2014). Revisions to the derivation of the Australian and New Zealand guidelines for toxicants in fresh and marine waters. Environmental Science and Pollution Research 21, 51–60.
| Revisions to the derivation of the Australian and New Zealand guidelines for toxicants in fresh and marine waters.Crossref | GoogleScholarGoogle Scholar |
Watras CJ, Bloom N (1994) The vertical distribution of mercury species in Wisconsin lakes: accumulation in plankton layers. In ‘Mercury Pollution: Integration and Synthesis’. Limnology Library 730163. (Lewis Publishers: Chelsea, MI, USA)
Weber JH (1993). Review of possible paths for abiotic methylation of mercury(II) in the aquatic environment. Chemosphere 26, 2063–2077.
| Review of possible paths for abiotic methylation of mercury(II) in the aquatic environment.Crossref | GoogleScholarGoogle Scholar |
Wenziker K, Mcalpine K, Apte S, Masini R (2006) Background quality for coastal marine waters of the North West Shelf, Western Australia. Technical Report. Available at https://www.epa.wa.gov.au/sites/default/files/Policies_and_Guidance/NWSJEMS%20Technical%20Report-NWS%20BG%20WaterQual.pdf [accessed 1 November 2021]
Wilhelm SM, Nelson M (2010) Interaction of Elemental Mercury with Steel Surfaces. The Journal of Corrosion Science and Engineering. Volume 13, preprint 38, University of Manchester.
Woodside (2019) Proposed Browse to NWS Project. Draft EIS/ERD. Available at https://www.woodside.com.au/docs/default-source/current-consultation-activities/australian-activties/propsed-browse-to-north-west-shelf-project---draft-eis-erd.pdf [accessed 1 November 2021]
Woodside (2020) Scarborough Offshore Project Proposal. Available at https://www.woodside.com.au/docs/default-source/our-business---documents-and-files/burrup-hub---documents-and-files/scarborough---documents-and-files/scarborough-offshore-project-proposal.pdf [accessed 1 April 2022]
Wu Y, Wang W-X (2011). Accumulation, subcellular distribution and toxicity of inorganic mercury and methylmercury in marine phytoplankton. Environmental Pollution 159, 3097–3105.
| Accumulation, subcellular distribution and toxicity of inorganic mercury and methylmercury in marine phytoplankton.Crossref | GoogleScholarGoogle Scholar |
Wu Q, Apte SC, Batley GE, Bowles KC (1997). Determination of the mercury complexation capacity of natural waters by anodic stripping voltammetry. Analytica Chimica Acta 350, 129–134.
| Determination of the mercury complexation capacity of natural waters by anodic stripping voltammetry.Crossref | GoogleScholarGoogle Scholar |
Ye X, Fisher NS (2020). Minor effects of dietary methylmercury on growth and reproduction of the sheepshead minnow Cyprinodon variegatus and toxicity to their offspring. Environmental Pollution 266, 115226
| Minor effects of dietary methylmercury on growth and reproduction of the sheepshead minnow Cyprinodon variegatus and toxicity to their offspring.Crossref | GoogleScholarGoogle Scholar |
Yu X, Wu FZ, Xu XQ, Chen QZ, Huang L, Tesfai BT, Cao L, Xu XD, Dou SZ, Huang W (2019). Effects of short term methylmercury exposure on growth and development of the large yellow croaker embryos and larvae. Frontiers in Marine Science 6, 754
| Effects of short term methylmercury exposure on growth and development of the large yellow croaker embryos and larvae.Crossref | GoogleScholarGoogle Scholar |
Zhang T, Kim B, Levard C, Reinsch BC, Lowry GV, Deshusses MA, Hsu-Kim H (2012). Methylation of mercury by bacteria exposed to dissolved, nanoparticulate, and microparticulate mercuric sulfides. Environmental Science & Technology 46, 6950–6958.
| Methylation of mercury by bacteria exposed to dissolved, nanoparticulate, and microparticulate mercuric sulfides.Crossref | GoogleScholarGoogle Scholar |
Zhao L, Chen H, Lu X, Lin H, Christensen GA, Pierce EM, Gu B (2017). Contrasting effects of dissolved organic matter on mercury methylation by Geobacter sulfurreducens PCA and Desulfovibrio desulfuricans ND132. Environmental Science & Technology 51, 10468–10475.
| Contrasting effects of dissolved organic matter on mercury methylation by Geobacter sulfurreducens PCA and Desulfovibrio desulfuricans ND132.Crossref | GoogleScholarGoogle Scholar |