Metal contaminants of emerging concern in aquatic systems
Graeme E. Batley A * and Peter G. C. Campbell
A * and Peter G. C. Campbell  B
B
A CSIRO Land and Water, Locked Bag 2007, Kirrawee, NSW 2232, Australia.
B Institut National de la Recherche Scientifique, Centre Eau Terre Environnement, Quebec City, QC G1K 9A9, Canada.
Environmental Chemistry 19(1) 23-40 https://doi.org/10.1071/EN22030
Submitted: 11 April 2022 Accepted: 6 May 2022 Published: 20 June 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Environmental context. There is potential for a range of metals being used in emerging industries to pose a risk if they reach aquatic environments. This is assessed by evaluating known environmental concentrations against available toxicity data. In most instances risks are low with current usage. Areas are identified where additional data are needed.
Abstract. The environmental concentrations and aquatic toxicity of a range of technology-critical metals comprising platinum group and rare earth group elements, together with gallium, germanium, indium, lithium, niobium, rhenium, tantalum, tellurium and thallium, have been reviewed to determine whether they pose a risk to aquatic ecosystem health. There is a reasonable body of toxicity data for most, but the quality is quite variable, and more data are required. Chronic toxicity EC10 or NOEC values are generally in the low mg L–1 range, far higher than the current environmental concentrations in the ng L–1 range, meaning that the existing risks to ecosystem health are extremely low. Missing are reliable toxicity data for niobium and tantalum, while confounding results for lanthanum toxicity need to be resolved. There is a likelihood that the currently low concentrations of most of these elements will increase in future years. Whether these concentrations are in bioavailable forms remains to be reliably determined. For most of the elements, measured speciation information is scarce, and unfortunately the thermodynamic data required to calculate their speciation are incomplete. In addition to this problem of uncertain speciation for some of these metals, notably those present in oxidation states of III or higher, there is also a need to explore the links between speciation and bioavailability for these higher valence metals. For circumneutral solutions, the calculated concentrations of the free metal ion tend to be very low for these metals and under such conditions the link between metal speciation and bioavailability is unclear.
Keywords: contaminant, gallium, germanium, indium, lithium, niobium, platinum group elements, rare earth elements, rhenium, tantalum, tellurium, thallium.
Introduction
The term ‘emerging contaminant’ is usually associated with new synthetic organic molecules that have been recently identified as environmental contaminants. Critical readers of the title of this article may well be wondering how an inorganic contaminant could qualify as ‘emerging’, given that we are necessarily constrained by the Periodic Table of the Elements. In effect, the use of the term ‘emerging’ here refers not to the element itself but rather to the new and emerging uses of the element and the concern that these pose in the aquatic environment. To quote from Gulley et al. (2018), ‘While previous ages of human history can broadly be defined by a single metal or alloy (i.e. Iron Age or Bronze Age), the material compositions of today’s emerging technologies encompass almost the entire periodic table and are constantly evolving.’
Over the past 50 years, when the issue of environmental concern for metal contaminants in waters and sediments was raised, those metals of primary concern were typically restricted to cadmium, copper, lead, zinc and mercury. Of secondary concern were chromium, cobalt, nickel and silver, largely related to their mining sources or to their subsequent use in industry. More ubiquitous metals such as iron, manganese and aluminium have also been investigated but only recently has their ecotoxicity been the subject of more detailed evaluations to allow derivation of water quality guideline values.
Recent publications have listed what are termed technology-critical elements (TCEs) (Cobelo-García et al. 2015), a term that includes those metals listed in Table 1. With the increasing use of these metals, attention is now focusing on the potential environmental impacts of elements that were previously minimally exploited. For the purposes of discussion, the TCEs have been subdivided into platinum group elements, rare earth elements and assorted other elements. An assessment of their environmental impact will require knowledge of their behaviour and fate in aquatic systems. This will include understanding their speciation in solution. There is a lack of thermodynamic data to input into speciation modelling, unlike the situation for the common metals listed above. Other laboratory or field-based measures of speciation have yet to be fully applied to TCEs. In addition, ecotoxicological studies are needed to better understand the bioavailability and toxicity of TCEs and the potential threats that they might pose to the aquatic environment.
|
In this review, we have focused on waterborne metals rather than those that are accumulated via dietary sources, noting that there are a number of papers (e.g. Amyot et al. 2017; Cardon et al. 2019) that cover the trophic transfer of TCEs in some detail, although this route appears to be less critical to the health of aquatic ecosystems than is the case for elements such as mercury and selenium. In the following pages, we first provide a brief overview of the data that would be needed to carry out a prospective ecological risk assessment of the TCEs and then review the available toxicity data and compare these with recent measured data on actual environmental concentrations to assess the current risk that is posed by these contaminants should they enter aquatic ecosystems.
Data needed for the environmental risk assessment of the TCEs
For most waterborne metals, there is good evidence that the free-metal ion concentration or activity is a good predictor of the metal’s bioavailability, with due consideration of such toxicity modifying factors as water hardness and pH (Adams et al. 2020). To apply this approach to the TCEs, one would need to be able to measure the free metal ion concentration/activity, or to calculate it using chemical equilibrium software. Unfortunately, as is discussed in the following sections, measured speciation results are very scarce and for many of the TCEs the thermodynamic data needed to calculate their speciation are incomplete.
In addition, the experimental evidence for the importance of the free-metal ion as a bioavailability predictor has been derived almost entirely for divalent cations (Cd2+, Cu2+, Ni2+, Pb2+, Zn2+) and there is good evidence that non-essential elements such as cadmium and lead can masquerade as essential metals and enter living cells in that manner (Bridges and Zalups 2005). However, as indicated in the following sections, many of the TCEs exist in higher oxidation states and little is known about how they enter cells. Additionally, in some cases the calculated free-ion concentration in aqueous solution for these higher oxidation states is vanishingly low – in such cases, does the free metal ion [Mz+] remain a useful bioavailability predictor? Indeed, in some cases toxicity may be induced by interaction of a metal at the surface of an epithelial cell surface (i.e. without involving trans-membrane transport). In such cases, the link between metal speciation and metal toxicity remains to be explored in detail.
Finally, the TCEs are also data-poor with respect to their intrinsic toxicity (Le Faucheur et al. 2021). This lack of reliable toxicological data reflects in part the recent surge in use of these elements and the accompanying lag time before the necessary toxicological data can be generated. However, another complicating factor is the limited aqueous solubility of many of the TCEs in the common toxicity test exposure media and the resulting limits on the concentration ranges that can be explored with the standard test protocols.
Platinum group elements
The environmental impacts of platinum group elements (PGEs) have been comprehensively reviewed by both Fortin et al. (2011) and Zereini and Wiseman (2015). Mining is a significant localised source of PGEs, as indicated by a limited number of reports of high measured concentrations (Rauch and Fatoki 2015). More widespread sources include their use as automotive catalysts for emission control, while their medical usage (anticancer drugs, implanted medical devices) represents 3–12% of the automotive source (Rauch and Peucker-Ehrenbrink 2015). Particulate emissions eventually reach aquatic systems as documented in the many papers by Rauch and coworkers (e.g. Rauch and Morrison 1999, 2000, 2008; Rauch et al. 2004, 2005). The use of PGEs as automobile catalysts dates back to the 1970s in Europe with adoption in other jurisdictions happening later in the 1980s and 1990s. There is evidence that environmental concentrations are increasing (Fortin et al. 2011).
Environmental concentrations and speciation
Little is known of the routes by which particulate emissions are transformed to soluble ionic, complexed or adsorbed forms in aquatic environments (Fortin et al. 2011). Analytical data indicate that aqueous concentrations are normally extremely low (< 1 ng L–1) and are only elevated as a consequence of anthropogenic activities, e.g. in areas with high vehicular traffic. Sediment concentrations are high in such areas, suggesting that many of the PGEs are transported there, possibly in relatively unavailable forms. A good discussion of the speciation of platinum in natural waters has been provided by Reith et al. (2014).
Dissolved platinum concentrations in a pristine Croatian river were found to be as low as 0.03 ng L–1 (Padan et al. 2020) and in East Asian rivers at a median concentration of 0.10 ng L–1 (Soyol-Erdene and Huh 2012). In freshwaters, platinum is largely present as the Pt(OH)20 complex, but in estuarine and marine waters, PtIV becomes dominant, predicted to be largely as PtCl5(OH)2− (Cobelo-García et al. 2013). Dissolved platinum concentrations increased with salinity and in estuarine areas of Tokyo Bay were as high as 7 ng L–1 (Obata et al. 2006). Needless to say, detection of picomolar platinum concentrations has required the development of specialised analytical detection methods (e.g. Obata et al. 2006; Padan et al. 2020).
Few measurements have been reported for palladium and rhodium in waters, although these too are used in automotive catalysts. Liu et al. (2021) discuss the transport of atmospheric sources of palladium via rainfall leading to dissolved concentrations near 5 ng L–1 in receiving waters and decreasing to 2.3 ng L–1 in downstream estuarine waters near the city of Haikou in Hainin Province, China, not dissimilar to the concentrations found for platinum. In an earlier report, Hu et al. (2005) reported respective concentrations of 5.2, 4.1 and 1.9 ng L–1 for platinum, palladium and rhodium in an unspecified Chinese river's water. In the water column, the concentrations of particle-associated platinum group metals (expressed as ng L–1) are typically lower than the dissolved concentrations (Fortin et al. 2011). Bioaccumulation mostly occurs from waters via dissolved forms although the links with dissolved metal speciation need further investigation (Fortin et al. 2011).
Like platinum, palladium exists as PdII and PdIV although in natural waters the former is dominant (Cobelo-Garcia et al. 2007). Rhodium is present only as RhIII so its chemistry and adsorptive behaviour will be different.
Aquatic toxicity
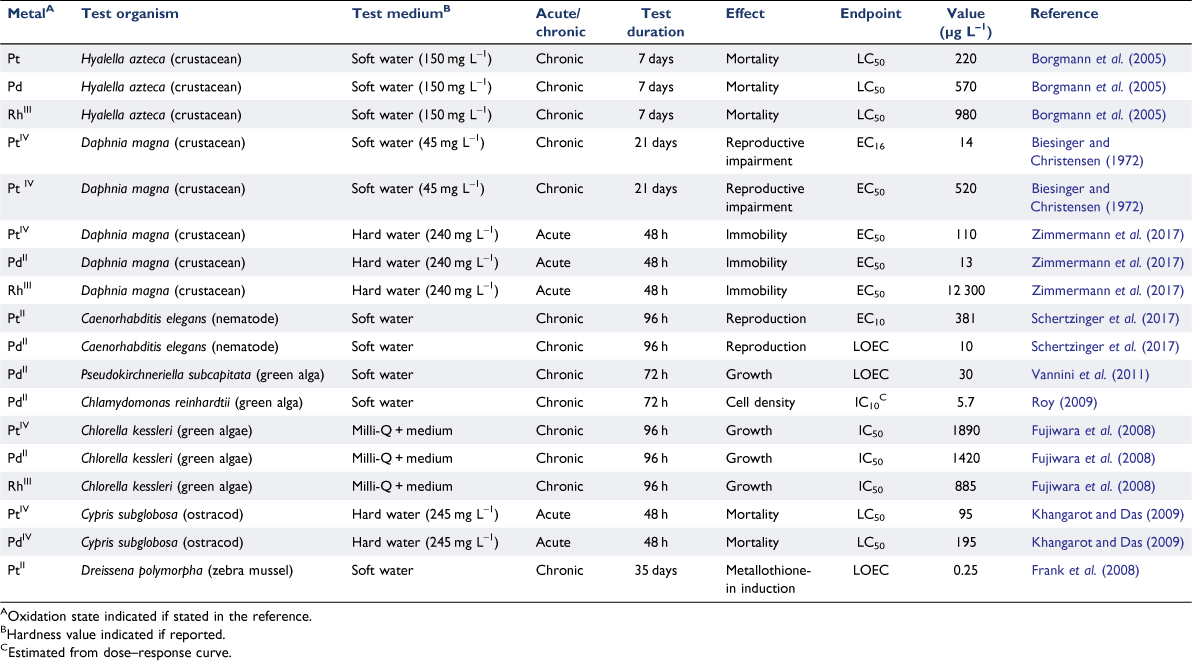
There are quite a few reports of aquatic toxicity testing of platinum group metals, although many of these are based only on acute toxicity (as 50% lethal concentration, LC50, values) rather than the chronic 10% effective concentration (EC10) data that are favoured for the development of water quality guideline values, as summarised by Fortin et al. (2011) and Sures et al. (2015). These and more recent data are shown in Table 2.
|
Chronic toxicity to the crustacean Hyalella azteca in soft water varied in the order Pt > Pd > Rh (Borgmann et al. 2005), whereas for Daphnia magna, acute toxicity in a similar freshwater followed the order Pd > Pt ≫ Rh (Zimmermann et al. 2017). For the nematode Caenorhabditis elegans, chronic toxicity was significantly greater for palladium than platinum (Schertzinger et al. 2017). The order of sensitivity of palladium and platinum could be a function of organism type and the mechanism of toxicity. The oxidation state of these metals could also affect their toxic response, but the oxidation state prevailing under the conditions of the toxicity tests was not always clearly indicated in the publications.
Many of the tests in Table 2 report nominal rather than measured concentrations, so the quoted toxicity values may be in error. In toxicity tests with a freshwater alga, Chlamydomonas reinhardtii, losses of metals from test solutions were shown to be a major concern by Roy (2009), with up to 50% loss over 96 h. The data reported by Roy (2009) for the toxicity of palladium to C. reinhardtii, revealed a very low IC50 value and a steep dose–response curve from which a low IC10 could be estimated. This alga was far more sensitive than Chlorella kessleri tested by Fujiwara et al. (2008), as shown in Table 2.
From Table 2, the results for palladium indicate that concentrations above 6 µg L–1 might be a concern for some sensitive species, while for platinum, that value might be 10 µg L–1. There is some evidence that hardness may protect against platinum and palladium toxicity (Fortin et al. 2011), but the data in Table 2 are largely derived for low hardness samples. There were very few data for marine waters, particularly chronic data for sensitive species, and these results have not been tabulated here. Based on these limited data, the overall findings to date are that environmental concentrations in the low ng L–1 range are well below concentrations that are likely to affect sensitive aquatic biota. So saying, it is notable that sub-chronic biomarker responses have been seen in the high ng L–1 range. For example, Frank et al. (2008) reported metallothionein induction in the freshwater zebra mussel Dreissena polymorpha following exposure to platinum(ii) at a lowest observed effect concentration (LOEC) value of 250 ng L–1. It follows that although a reliably determined water quality guideline value could be as low as ≥ 1 µg L–1, there might be a concern if dissolved platinum metal concentrations increased above 100 ng L–1.
Clearly there is a need for more toxicity testing, with careful quality control, for a range of taxonomic groups and for chronic endpoints. Equally important would be more mechanistic information regarding the uptake pathways exploited by the various platinum group elements.
The overall findings to date are that environmental concentrations are well below concentrations that are likely to affect sensitive aquatic biota. Toxicity data suggest that palladium is more toxic than platinum and rhodium, however, these data are very preliminary and many more results are required before reliable predicted no-effect concentration (PNEC) or water quality guideline values can be determined.
Rare earth elements
Environmental concentrations and speciation
Rare earth elements (REEs) comprise the 15 lanthanide elements: La, Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Yb and Lu, together with yttrium and scandium. These are well-known constituents of soils and sediments as a consequence of their natural geological abundance, and their weathering and dissolution is the source of their presence in fresh and marine waters. The distributions of lanthanide element concentrations in soils and waters decrease in a log-linear fashion with atomic number, but due to the Oddo–Harkins effect, elements with even atomic numbers are more abundant than adjacent odd numbered elements, forming a saw-tooth pattern (Byrne and Sholkovitz 1996). Well-described anomalies arise for both cerium and europium due to differences in chemical behaviour. A negative anomaly for cerium is thought to be due to oxidation of CeIII to the less soluble CeIV. A positive europium anomaly arises from the reduction of EuIII to the more mobile EuII.
While the above behaviour is a function of rare earth mineralogy, distortion of the log-linear distribution can be seen due to anthropogenic impacts. Rare earth elements are increasingly used in consumer products including catalysts, magnets, phosphors and alloys as well as in fertilisers (Yin et al. 2021). The effects of this increased use are seen for example in southern San Francisco Bay, where total dissolved rare earth concentrations have increased relatively uniformly from 1993 (35 ng L–1) to 2013 (201 ng L–1) (Hatje et al. 2016). Since the late 1980s, gadolinium has been increasingly used as a contrast agent in medical imaging, with the result that temporal records of waters near high technology industrial and medical establishments have shown anomalously high and variable dissolved concentrations of gadolinium chelates (up to 1640 ng L–1) (Ebrahimi and Barbieri 2019)). A similar anthropogenic anomaly was seen for effluent waters from a wastewater treatment plant in waters in northern Australia (Lawrence et al. 2006). Such treatment plants are unable to remove the refractory forms of complexed gadolinium, such as gadolinium DTPA (diethylenetriamine pentaacetate) used in medical imaging (Hatje et al. 2016).
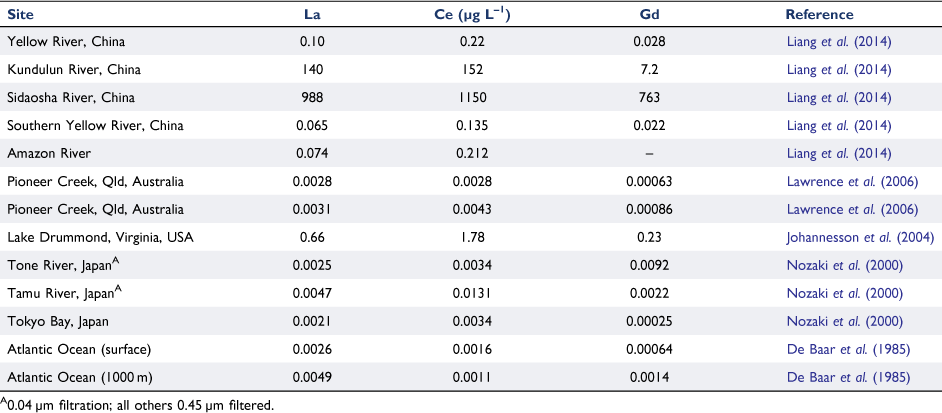
Mining activities can accelerate the release of dissolved rare earths to rivers by many orders of magnitude, as shown for rivers in China (Liang et al. 2014) (Table 3). As can be seen for sites removed from the mining activities, background concentrations are typically very low, < 0.2 ng L–1. Pristine rivers in Australia had values < 5 ng L–1, similar to those in Japan. The Japanese rivers which emptied into Tokyo Bay showed evidence of colloidal species, excluded using 0.04 µm filtration. Gadolinium concentrations decreased by a factor of up to six further upstream, where concentrations were extremely low. Lower concentrations were also found in Tokyo Bay, approaching those in surface waters of the Atlantic Ocean.
|
The speciation of rare earth elements has received limited consideration. Most (e.g. La and Gd) exist only in oxidation state (III), whereas elements such as cerium exist in both states (III) and (IV). In solution, in addition to the free ion, complexation takes place with carbonate, hydroxide and phosphate, but the low solubility product of LaPO4(s) limits the soluble concentrations of phosphate complexes (Khan et al. 2017). El-Akl et al. (2015) undertook useful laboratory investigations of cerium speciation and found that while at below pH 6, cerium(iii) is bound to carbonate and hydroxide, at pH 7, colloidal species, most likely as cerium(iv) are dominant.
In addition to these inorganic ligands, the rare earth elements also tend to bind to fulvic and humic acids. This complexation with natural organic matter has been studied in the laboratory, using capillary electrophoresis, fluorescence quenching, ion-exchange or partial ultrafiltration (Stern et al. 2007; Leguay et al. 2016; Nduwayezu et al. 2016). These data have been incorporated into the Windermere Humic Aqueous Model (WHAM VII: Tipping et al. 2011), however, there are relatively few actual measurements of REE speciation in natural waters and it is difficult to judge the accuracy of the model output. Marsac et al. (2021) provide a useful overview of the capacity of the WHAM VII model to predict REE speciation in synthetic solutions containing natural organic matter and other cations.
Aquatic toxicity
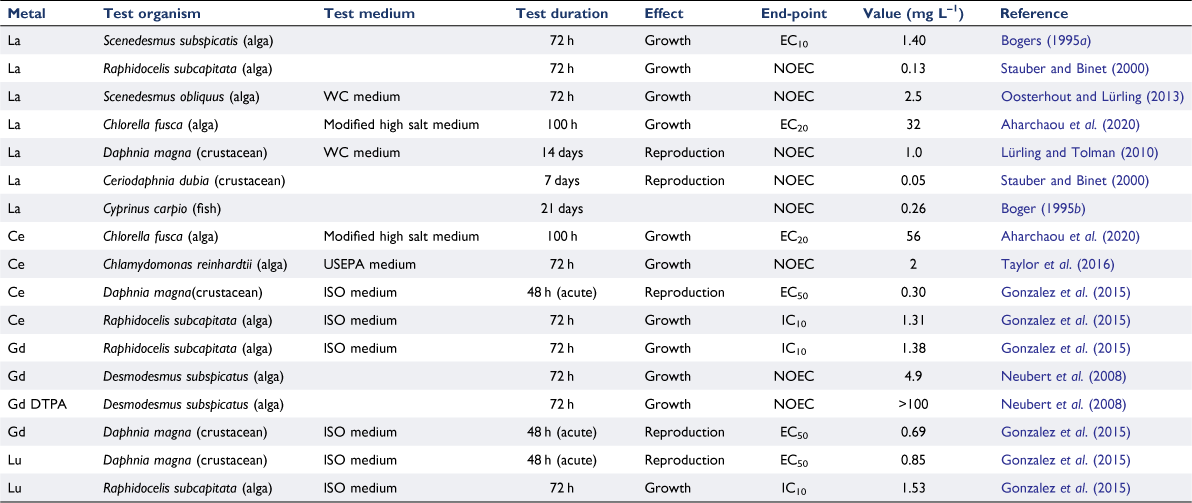
Several studies have reviewed the aquatic toxicity of rare earth elements (Gonzalez et al. 2014, 2015; Malhotra et al. 2020). While there are considerable data for impacts on biota in freshwaters, studies of marine species are limited. A selection of freshwater toxicity data is presented in Table 4.
|
The toxicity of lanthanum has been thoroughly reviewed by Herrmann et al. (2016) and Rogowska et al. (2018). A number of deficiencies in the published data were identified including a failure to adequately describe the test media and the use of nominal rather than measured concentrations. The importance of reporting measured concentrations was highlighted by Weltje et al. (2002, 2003), with losses due to adsorption to container surfaces and membrane filters being significant loss pathways.
The need to report measured concentrations is particularly important for toxicity studies with algae. Because lanthanide phosphates are relatively insoluble, the addition of a lanthanide to the test medium may lead to its precipitation as a phosphate with effects on algal growth that are due to nutrient limitation, not lanthanide toxicity (Herrmann et al. 2016). Such precipitation reactions will of course also reduce the true lanthanide concentration in the exposure medium. As a consequence, significant variations in endpoint concentrations are seen in reported algal tests. Aharchaou et al. (2020) discussed this point and suggested that a medium free of inorganic phosphates would be desirable to avoid both precipitation and loss of dissolved lanthanides and possible nutrient limitation. Some algae are able to utilise organic sources of phosphorus, and for such species an organophosphate such as glycerol-2-phosphate can be used as a phosphorus source (Nys et al. 2014). Alternatively, algae that are able to perform luxury uptake and store the excess phosphate intracellularly can be used for the toxicity test since they are able to grow for several days in the absence of an extracellular source of phosphorus.
Several laboratory studies using microalgae have identified calcium as a competitor with lanthanides that limits their internalisation by algal cells (Yang et al. 2014; El-Akl et al. 2015; Yang and Wilkinson 2018; Aharchaou et al. 2020). In simple test media, acidity also attenuates the uptake of lanthanides, likely due to the proton binding to the membrane transport site and inhibiting the binding and uptake of the lanthanide ion. Complexation of lanthanides by organic ligands would also be expected to limit their bioavailability, and indeed, in laboratory experiments with simple monomeric organic ligands such as malate, iminodiacetate and nitrilotriacetate, this has been demonstrated (Aharchaou et al. 2020). Similarly, the toxicity of the gadolinium-DPTA complex to algae was significantly lower than the toxicity of dissolved inorganic Gd (Gonzalez et al. 2015) (Table 4). However, with natural organic matter (NOM) as the complexing ligand, the limited experimental data are equivocal on this point. For example, uptake of cerium and samarium by C. reinhardtii in the presence of NOM was markedly reduced (El-Akl et al. 2015; Rowell et al. 2018). However, in similar work with lanthanum and a different alga, Chlorella fusca, uptake of the lanthanide was enhanced rather than attenuated in the presence of NOM and the EC50 value expressed as dissolved lanthanum was lower in the presence of the NOM (Rahal 2018).
Given the relative scarcity of toxicological data for the rare earth elements, as well as the experimental challenges of working with these elements in traditional toxicity testing media, no reliable PNEC or guideline values have yet been derived for lanthanum, or indeed any other rare earths. Herrmann et al. (2016) reported a PNEC value for lanthanum of 4 µg L–1, but this was based on an assessment factor approach rather than the preferred species sensitivity distribution method. More reliable estimates of PNEC values for cerium, gadolinium and lutetium were estimated by Gonzalez et al. (2015) as 54, 57 and 5.8 µg L–1 respectively, leading them to conclude that toxicity increases with atomic number. This certainly was not the case for the alga Raphidocelis subcapitata exposed to the same elements (Table 4).
Other elements
Lithium
The move to electric vehicles and associated lithium-ion batteries is creating greatly increased worldwide demands for lithium. As such, it is now considered as an emerging contaminant. Sources include lithium mining and associated tailings, lithium-rich brines and used lithium-containing products, in particular batteries (Aral and Vecchio-Sadus 2008; Bibienne et al. 2020).
Environmental concentrations and speciation
Surface water lithium concentrations are typically < 40 µg L–1, mostly in the range 1–10 µg L–1 while seawater has 170 µg L–1 (Aral and Vecchio-Sadus 2008). Given its status as a Class A or hard monovalent cation, the speciation of lithium in fresh waters is very simple, similar to that of Na+ and K+, with the free Li+ ion predominating. Similarly, because of its marked tendency to form ionic rather than covalent bonds and its weak affinity for reduced sulfur, its predicted toxicity is low.
Aquatic toxicity
Although relatively little work has been published on the ecotoxicity of lithium, there is no doubt that it is biologically active. Its effects on humans are well documented and it has been used for over 70 years as a mood stabiliser (Bibienne et al. 2020). Several comprehensive reviews have captured what little is known about the toxicity of lithium in natural water systems (Kszos and Stewart 2003; Aral and Vecchio-Sadus 2008) and the general conclusion is that the toxicity of lithium is low, similar to that of other hard cations (Na+, K+, Mg2+, Ca2+). An important finding was the antagonistic effect of sodium (Kszos et al. 2003). Chronic toxicity tests were conducted using Ceriodaphnia dubia (7-days reproduction), the snail Elimia clavaeformis (feeding rate) and fathead minnow, Pimephales promelas (larval growth). Kszos et al. (2003) reported an IC25 of 0.38 mg Li L–1 for C. dubia reproduction in a low sodium (2.85 mg L–1) control demineralised water, compared to 3.33 mg Li L–1 in a creek water with 17 mg Na L–1, and 100% survival in 40 mg Na L–1 water containing at least 4 mg Li L–1. Their tests of sodium antagonism on C. dubia showed that no toxicity was seen when the natural log of the molar ratio of sodium to lithium exceeded 1.63. These findings have important implications as in many natural waters the presence of sodium will be sufficient to prevent lithium toxicity.
The overall verdict for lithium is that it is inherently highly available (negligible complexation by natural ligands) but also intrinsically not very toxic. Realistically, it should not be of environmental concern except in areas where it is being extracted from brines or mined, or possibly in areas where used batteries are being discarded. However, given an anticipated increase in the recycling of lithium from used batteries (Ambrose and Kendall 2020), this end-of-life source should decline with time.
Gallium
Gallium is finding extensive use as both gallium arsenide and gallium nitride semiconductors in microelectronics. It occurs as a by-product in the processing of bauxite, where its similar ionic radius leads to it replacing aluminium in the mineral. To a lesser degree, it replaces zinc in the zinc sulfide mineral, sphalerite (Foley et al. 2017).
Environmental concentrations and speciation
In uncontaminated fresh waters, gallium is present at extremely low concentrations. In their recent review of gallium geochemistry, Yuan et al. (2021) provided a range of 0.07–18 ng Ga L–1 for rivers in the USA; Foley et al. (2017) suggested a slightly broader range for world rivers (1–120 ng Ga L–1). Concentrations recorded for thermal waters are higher but still low (2–25 µg L–1). Groundwaters associated with semiconductor manufacturing areas were contaminated up to 40 µg L–1 (Chen 2006). In surface oceanic waters, concentrations are < 3 ng L–1, sourced largely from aeolian transport (Shiller 1998).
Unsurprisingly, the geochemistry of gallium is similar to that of aluminium. In aqueous solution, it forms a series of hydroxo-complexes (Ga(OH)n(3−n)+); as a function of pH, the speciation shifts from Ga3+ in very acidic solutions to Ga(OH)4− at pH values above 4.5 (Wood and Samson 2006). As the aqueous gallium concentration increases, metastable polynuclear species form, eventually leading to precipitation of amorphous Ga(OH)3(s). The influence of these speciation shifts on gallium bioavailability has not been investigated. However, it is known that, in some bacteria, GaIII can compete with FeIII for the same transmembrane transporter, this presumably being a reflection of their very similar ionic radii (0.62 Å for GaIII and 0.62 Å for FeIII); the known antimicrobial effects of gallium have been attributed to this Ga–Fe antagonism (Kaneko et al. 2007).
Aquatic toxicity
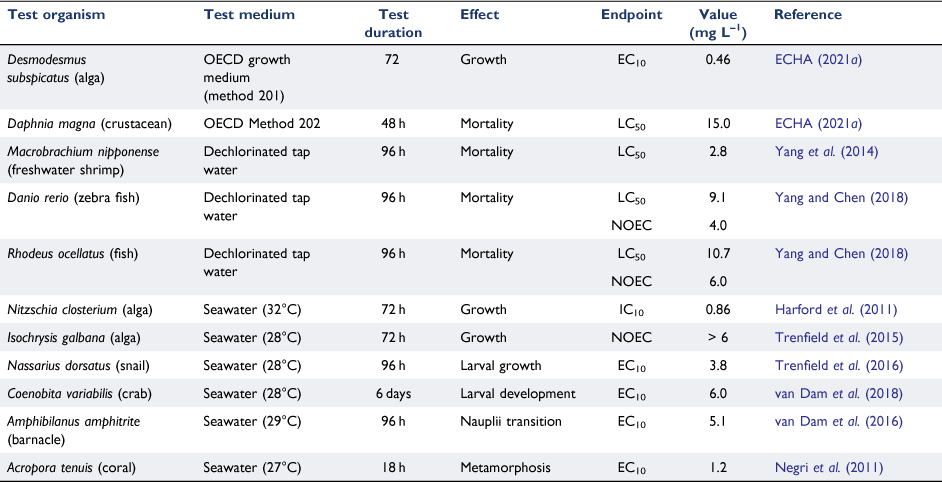
Selected data for the aquatic toxicity of gallium are shown in Table 5. Overall, gallium exhibits low toxicity in both fresh and marine waters. Of the species tested to date, algae were the most sensitive but with EC10 values above 460 µg L–1. Acute tests with Daphnia magna in freshwater gave an extremely insensitive LC50 of 15 mg L–1, surely approaching the solubility limit. Yang et al. (2014) noted that the order of acute toxicity to the zebrafish Danio rerio followed the order SbIII > GaIII > InIII.
|
Given that environmental concentrations of gallium in natural waters are in the ng L–1 range, it can be reliably concluded that the current risks from gallium to the health of aquatic biota are insignificant, although this may change if the predicted rapid growth in its use in photovoltaics and clean energy technologies occurs (Foley et al. 2017).
Germanium
Germanium is a metalloid that is mainly used in semiconductor applications, with additional applications in the fibre optics and telecommunications sectors. It occurs mainly in zinc sulfide ores (Holl et al. 2007) but because its ionic radius is similar to that of silicon, it is also commonly found dispersed in silicate minerals (Rosenberg 2009; Shanks et al. 2017). The overall geochemical behaviour of germanium closely resembles that of silicon, to the extent that it is taken up by siliceous organisms and incorporated into the cell wall of diatoms. Although germanium exists largely as GeIV in the natural environment, divalent forms are produced synthetically and used in industry.
Environmental concentrations and speciation
Germanium has been measured in the concentration range 0.05–0.1 µg L–1 in freshwaters, where it is predicted to be present as Ge(OH)40 at pH values below 8 (Rosenberg 2009). In surface oceanic waters, dissolved germanium concentrations have been measured as low as 0.3 ng L–1 (Ellwood and Maher 2003) but averaged over a range of depths values they lay between < 0.07 and 8.3 ng L–1 (Romero-Freire et al. 2019). Concentrations were up to 100 µg L–1 in coal mine waters, especially as a result of its enrichment in coal seams (Holl et al. 2007). Coal combustion has been identified as the largest industrial source of germanium emissions to the environment (Shanks et al. 2017).
As is the case for other elements in Group 14 (previously Group IVA) (e.g. Sn, Pb), germanium also forms strong covalent bonds to carbon. Provided the number of such covalent bonds does not exceed three, the resulting organo-germanium compounds tend to be soluble in natural waters, with hydroxide groups occupying the remaining coordination positions about the central germanium atom. Some of these organo-germanium compounds are synthesised industrially, but an additional source appears to be natural biomethylation. Methylgermanium and dimethylgermanium have been detected in natural waters, and since there is no known anthropogenic source of these methylated forms, it would appear that they must result from biomethylation (Thayer 2002). Their concentrations in freshwater are much lower than in seawater (where methylated forms account for 70% of dissolved germanium) (Romero-Freire et al. 2019). In the ocean, the methylated forms appear to be conservative, showing no change in concentration with depth, which has led biogeochemists to conclude that they do not participate actively in the germanium biogeochemical cycle.
Aquatic toxicity
Inorganic GeIV is known to enter or leave cells passively through protein-lined pores that allow the neutral solutes to traverse cell membranes (Nodulin 26-like intrinsic channel proteins or ‘NIPs’) (Pommerrenig et al. 2015). However, very few data exist on the aquatic toxicity of germanium. Especially given its limited solubility (Wood and Samson 2006; Rosenberg 2009), toxicity is expected to be low. A very early study by Lewin (1966) on 10 marine diatom species found on average an IC50 value of 0.69 mg Ge L–1 following exposure for 12 days. Testing used GeO2 dissolved in a NaOH solution and then neutralised. The formation of diatom frustules was inhibited in all but the most-weakly silicified diatom, Phaeodactylum tricornutum. The effect of germanium on silicate utilisation was reversed by the addition of more silica.
Given the low concentrations of germanium in natural waters, the risks posed to aquatic biota are likely to be extremely low.
Indium
The principal uses of indium are in electrically conducting thin film coatings on glass, solar cells and in semiconductor materials. It is a trace constituent in many minerals, especially those of tin, and is a major by-product of the refining of zinc ores (Schroll 1998; Jorgenson and George 2005; Shanks et al. 2017).
Environmental concentrations and speciation
In river waters, indium concentrations have been reported in the range from 0.003 to 0.05 ng L–1 in the Chao Praya River in Thailand compared to somewhat higher concentrations (0.11–1.7 ng L–1) in some Japanese rivers (Nozaki et al. 2000). The higher values were attributed to the presence of a medical waste product, In(DTPA)2−. The weathering of sulfidic tailings and the development of acid mine drainage would be expected to favour the mobilisation of indium; White et al. (2017) reviewed published values for indium in such drainage and suggested a concentration range from 0.02 to 500 μg L–1. In contaminated groundwaters, concentrations as high as 20 µg In L–1 have been reported (Chen 2006). In marine waters, concentrations ranged from 0.006 to 0.5 ng L–1 (Florence et al. 1974; Obata et al. 2007 and references therein), the highest values being found in surface waters.
Indium exists in both the (I) and (III) oxidation states, the latter being the more stable. Indium is somewhat softer than its congeners in the Periodic Table (Al, Ga) and it does show appreciable affinity for chloride and sulfide, but nevertheless In(OH)30 is predicted to be the dominant inorganic form in the pH range 5–9 (Wood and Samson 2006).
Aquatic toxicity
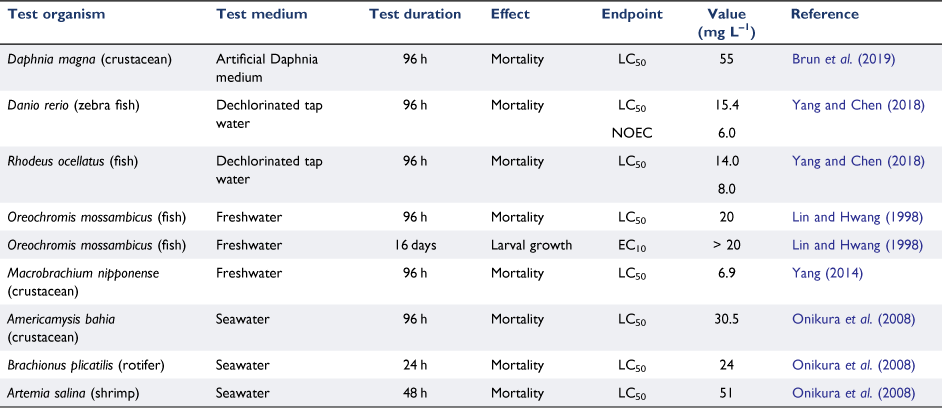
Very few data are available for indium toxicity to aquatic biota and these have been largely limited to acute testing in both fresh and marine waters (Table 6). The Daphnia test of Brun et al. (2019) was extended to 21 days to obtain a chronic endpoint, but specific values were not reported. These data suggest that indium is not particularly toxic, with all endpoints at high mg L–1 concentrations. Solubility is a potential limitation at such concentrations, as is the possible formation of transient polynuclear species. Although such species probably do not play an important role in the aqueous transport of indium (Wood and Samson 2006), their presence in toxicity test media may well have a bearing on our ability to determine the toxicity of the metal. Olivares et al. (2016) evaluated indium toxicity to the zebrafish Danio rerio in the presence of citrate (citrate/indium molar ratio of 3.75:1) in an attempt to overcome solubility problems; no mortality was observed at the highest tested concentration (103 mg In L–1). The researchers did not provide any speciation data, but citrate would be expected to complex indium and reduce its bioavailability. Indeed, these results are inconsistent with those of Yang and Chen (2018), who reported toxicity to two fish species at much lower concentrations (Table 6). In this work, indium was marginally more toxic than gallium to D. rerio and R. ocillatus.
|
In summary, it would appear that indium poses minimal risks to aquatic biota at its current usage rates.
Thallium
Thallium is found in the environment associated with sulfide minerals (Karbowska 2016; Belzile and Chen 2017; Zhuang and Song 2021), entering the environment as a product of mining operations, mineral smelting and coal burning. It has a range of uses, including in electronics and specialty glasses.
Environmental concentrations and speciation
The concentrations of thallium in both marine and freshwaters have been summarised in several publications (Couture et al. 2011; Karbowska 2016; Belzile and Chen 2017). In seawater, concentrations range from 1.3 to 20 ng L–1 (Belzile and Chen 2017), while in river waters concentrations cover a similar range. Zhuang and Song (2021) also compiled data for contaminated waters, with freshwaters in the 20–50 ng L–1 range and coastal waters from 80 to 3500 ng L–1. Concentrations as high as 1000 µg L–1 have been reported for rivers near mining areas (Belzile and Chen 2017).
Thallium occurs in the environment in two oxidation states: TlI and TlIII. Batley and Florence (1975) were among the first to show that in seawater and some fresh waters, thallium was present as TlIII, contrary to thermodynamic predictions. This dominance was later attributed to a combination of photo-oxidation and microbial oxidation (Twining et al. 2003; Li et al. 2005).
Chemical equilibrium calculations suggest that thallium(i) is largely present as the free ion in fresh waters, but its affinity for chloride is such that in seawater the mono-chloro TlCl0 complex is predicted to be of similar importance. Thallium(iii) would be expected to form hydroxo complexes in aqueous solutions, but there is some disagreement in the literature regarding the relevant thermodynamic constants (Couture et al. 2011). Similarly, the trivalent state would be expected to bind to humic and fulvic acids, but the extent of this complexation is also uncertain. Finally, TlI is subject to microbial oxidation and biomethylation, yielding dimethylthallium, (CH3)2Tl+, which has been detected in seawater where it accounted for 3–48% of the total dissolved thallium (Belzile and Chen 2017).
Aquatic toxicity
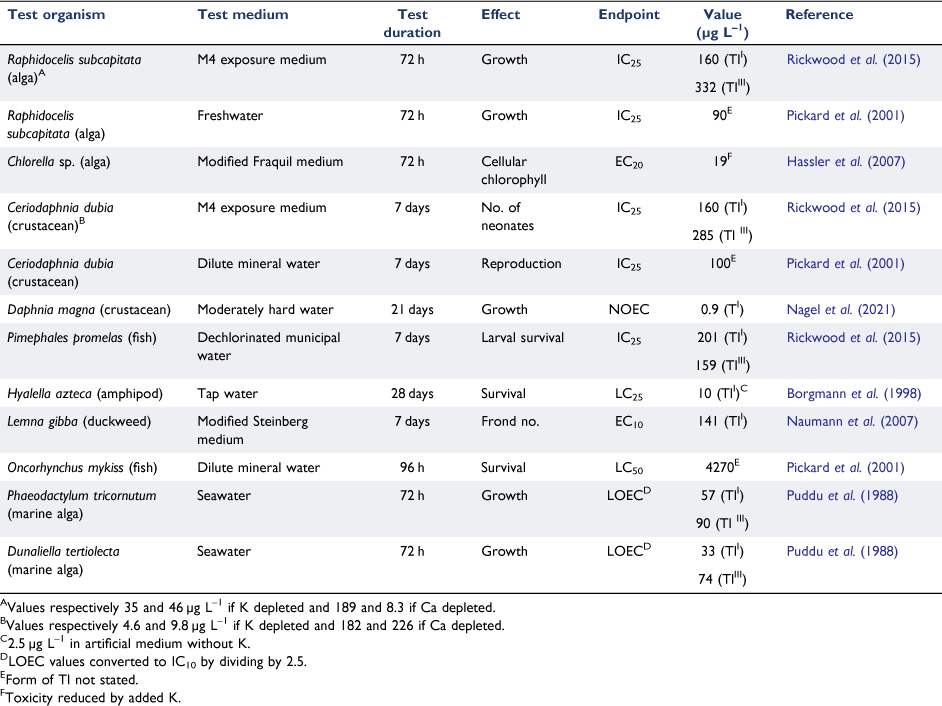
Toxicity data evaluations are complicated by the differing toxicities of TlI and TlIII. Early studies by Puddu et al. (1988) suggested that TlI exhibited greater toxicity to marine algae than TlIII. This is supported by other more recent toxicity data in Table 7. Ralph and Twiss (2002) showed that based on the modelled free ion concentration, Tl3+ was 50 000 times more toxic than Tl+ to the freshwater alga Chlorella sp., although no toxicity values (ECx) were reported. However, because the TlIII in their toxicity tests was predicted to be almost entirely in the form of Tl(OH)30, supposedly of low bioavailability, the total dissolved thallium concentrations that resulted in comparable reductions in algal growth rates were similar for both oxidation states. They could not, however, eliminate the possibility of TlIII reduction and they recommended that water quality guidelines be based on TlI.
|
Toxicity assessment is further complicated by the confounding effects of potassium. There is now considerable evidence that TlI, having a similar ionic radius to K+, is taken up by organisms via potassium transport channels in the cell membrane (Hassler et al. 2007; Couture et al. 2011). An elegant study by Zhang and Rickaby (2020) demonstrated that differences in phytoplankton responses to thallium could be related to differences in potassium transporters. Cyanobacteria and haptophytes were the least affected by thallium while chlorophytes were the most affected. None were impacted at environmental concentrations < 100 ng Tl L–1. Potassium influences both the uptake and toxicity of thallium. This was demonstrated for Hyalella azteca by Borgmann et al. (1998) and for other biota by Rickwood et al. (2015), who showed that both TlI and TlIII toxicities were affected by potassium (Table 7) and also noted an inhibitory effect of Ca2+.
The test species that is most sensitive to thallium appears to be the crustacean, Daphnia magna. Nagel et al. (2021) used a moderately hard water (containing 3.1 mg K L–1) and derived a NOEC of 0.9 µg L–1 for growth. Reproduction was a less sensitive endpoint and the authors were unable to calculate a 21-days LC50, although it lay between 424 (no mortality) and 702 µg L–1 (100% mortality). We note that there was an order of magnitude gap in concentrations from 0.9 to the next highest concentration of 9 µg L–1. The authors concluded that there are differences in mechanisms of toxicity for different crustacean species (cf. Ceriodaphnia dubia), and between acute and chronic exposures.
In their algal work, Zhang and Rickaby (2020) also determined the subcellular distribution of thallium in the different algal species and noted that it was consistently found largely in the cytosolic fraction. Similar work on juvenile yellow perch (Perca flavescens) collected along a metal contamination gradient in the field, also indicated that hepatic thallium tended to concentrate in the cytosol and that it remained there even after a heat-denaturation step (Caron et al. 2018). However, unlike silver and cadmium, which as expected were bound to metallothionein in the heat-stable fraction, thallium was associated with an unidentified cytosolic ligand of much lower molecular weight (< 0.12 kDa).
In deriving water quality guideline values, the confounding effects of both potassium and calcium need to be taken into consideration, as well as the differing toxicities of the two oxidation states. Based on the results in Table 7 and the above discussion, depending on the solution chemistry, the toxicity of thallium could be considered comparable to common metal toxicants such as copper and cadmium. There is hence the possibility that waste streams contaminated with thallium could reach concentrations that could affect sensitive biota such as algae. More data are required to better define the behaviour of thallium in natural waters and to permit derivation of water quality guideline values of higher reliability for both oxidation states.
Niobium and Tantalum
Niobium and tantalum are both members of Group 5 (previously Group VA) of the periodic table (along with vanadium) and as such have very similar physical and chemical properties. In the minerals where they occur (typically oxide and hydroxide minerals), they are found together (Schulz et al. 2017). Niobium is mainly used in microalloyed steel but is finding increasing use in superconducting magnets (for magnetic resonance imaging) (Schulz et al. 2017). Tantalum is largely used by the electronics industry in capacitors for energy storage and release. Brazil is the largest source of both niobium and tantalum.
Environmental concentrations and speciation
In the environment, both elements exist in the (V) oxidation state. In seawater, niobium is suggested to exist as Nb(OH)6− and to a lesser extent Nb(OH)5, while for tantalum, Ta(OH)5 is said to dominate over Ta(OH)6− (Koschinsky and Hein 2003). Measured concentrations of niobium and tantalum in natural waters have been summarised by Firdaus et al. (2008). In river waters, world average concentrations have been reported as 1.7 and 1.1 ng L–1 respectively, with the Uji River in Japan having 7.0 and 0.7 ng L–1 respectively. Reported values for coastal seawater were respectively 0.7 and 0.05 ng L–1. A more recent study by Poehle and Koschinsky (2017) examined niobium concentrations in Atlantic and Pacific Ocean waters finding values near 0.3 ng L–1 in surface waters, increasing only slightly with depth. There was no evidence of a significant colloidal fraction.
Nb/Ta concentration ratios have been shown to exist in a narrow range in most rocks but have been shown to differ in the order: open ocean seawater > coastal seawater > river water > crustal abundance (Firdaus et al. 2008). The authors indicated how these can be used to understand the fate of these elements in the aquatic environment.
Aquatic toxicity
A thorough literature search revealed only one study of the ecotoxicology of niobium and tantalum. This was a study of 7-day mortality of 63 metals to Hyalella azteca in both hard and soft waters (Borgmann et al. 2005). LC50 values for niobium were 26 and 1640 µg L–1 in soft and hard waters respectively, while for tantalum, the respective values were 2 and 1980 µg L–1. Based on this single report, the toxicity of niobium appears significant, at least in soft waters. However, as niobium and tantalum were of minor interest in the Borgmann et al. study and in the absence of other data, it is presently difficult to draw conclusions as to the risks posed by these metals, although given their chemical inertness and extremely low aqueous solubilities (Schulz et al. 2017), risks could be expected to be low.
Rhenium
Rhenium is an extremely rare metal, being found as a by-product of copper mining, largely associated with molybdenite (John et al. 2017). Chile is the source of more than 50% of rhenium worldwide. Rhenium is mainly used in high-temperature superalloys and in catalysts for the petrochemical industry (John et al. 2017).
Environmental concentrations and speciation
Rhenium exists in the (VII) oxidation state as the perrhenate ion ReO4− in natural waters. Tagami et al. (2006) used an anion exchange resin separation to demonstrate that, in seawater, rhenium was 100% in that form and completely dissolved. They measured concentrations ranging from 6.1 to 7.4 ng L–1 in Japanese coastal waters, similar to the 7.6 ng L–1 reported by Dickson et al. (2020) for Atlantic Ocean surface waters. Tagami and Uchida (2008) measured rhenium in 45 Japanese rivers finding concentrations in the range from 0.12 to 8.7 ng L–1 (mean of 0.81 ng L–1). A strong correlation with sulfate concentrations suggested an origin as sedimentary sulfides, such as ReS2.
Aquatic toxicity
Only one study (Stolte et al. 2015) could be found that had investigated the aquatic toxicity of rhenium. Results were reported for four species using ammonium perrhenate as the test compound. A chronic IC50 of > 280 mg L–1 was measured after only 24 h for growth of the alga Raphidocelis subcapitata in a synthetic soft water culture medium. For the crustacean, Daphnia magna, the acute 48-h LC50 value was > 466 mg L–1. The duckweed Lemna minor grown in Steinberg medium for 7 days had an EC50 of 78 mg L–1, whereas inhibition of bioluminescence by the marine bacterium Vibrio fischeri gave an EC50 of > 2.0 g L–1. Little detail was provided on QA/QC for these tests, but the data as presented indicate that rhenium has extremely low toxicity. Coupled with the extremely low environmental concentrations, this low toxicity suggests that risks to aquatic biota are very low.
Tellurium
Tellurium is another extremely rare metalloid. It is frequently found in association with gold, as well as in porphyry copper and sulfide minerals. Volcanogenic sulfides on the sea floor are a further source of tellurium (Goldfarb et al. 2017). Its major uses are in photovoltaic solar cells and in alloys for thermoelectric cooling products (Goldfarb et al. 2017; Filella et al. 2019).
Environmental concentrations and speciation
Tellurium has a very low crustal abundance and consequently occurs at low environmental concentrations. Its chemistry parallels that of selenium, a fellow member of Group 16 (previously Group VIA) in the periodic table. Thus, it exists in four oxidation states (−II, 0, IV and VI) (Filella et al. 2019). Although it is not a biologically essential element, tellurium does form a variety of organo-tellurium compounds where it has replaced sulfur or selenium in the normal biochemical cycle of these essential elements (Ogra 2017).
The concentrations of tellurium in natural waters have been comprehensively reviewed by Belzile and Chen (2015) and by Filella et al. (2019). A number of studies used techniques to separately determine values for TeIV and TeVI in addition to total dissolved tellurium. In surface seawater samples from the South Atlantic Ocean, Lee and Edmond (1985) found TeVI to comprise some 70% of 0.15 ng L–1 of total dissolved tellurium. Similar total concentrations have been found by several studies, but there are a few that quote seawater concentrations as high as 29 ng L–1 (e.g. Huang and Hu 2008; Najafi et al. 2010), again with over 70% TeVI. River water concentrations were typically below 50 ng L–1 total dissolved tellurium, but speciation analyses were rarely carried out (Filella et al. 2019). These authors expressed concern about the reliability of results where pre-concentration was required, especially in the absence of certified reference materials.
Aquatic toxicity
Very few references were found to tellurium toxicity to either freshwater or marine biota. ECHA (2021b) reported wonly three results. For the freshwater alga Raphidocelis subcapitata, a chronic EC10 value of 2.7 mg Te L–1 was quoted, based on testing of the water-accommodated fraction of tellurium dioxide based on its limited solubility, i.e. a saturated solution of TeO2 was diluted to provide a range of exposure concentrations. For the crustacean Daphnia magna, a 24-h acute immobilisation EC50 was 5.8 mg Te L–1. For the rainbow trout (Oncorhynchus mykiss), a 96-h LC50 was > 37 mg Te L–1. These results indicate a low risk of toxicity given that chronic effects are seen at concentrations some six orders of magnitude above natural environmental concentrations.
Conclusions
In the foregoing text, where we have considered individual metals or groups of metals, the recurring themes have included: the limited solubility of many of the higher valence metals; the regrettable use of nominal rather than measured concentrations; the absence of speciation measurements in the toxicity test media; the focus on waterborne metals; and the scarcity of chronic toxicity data covering a full range of taxonomic groups. Clearly, there is a need for better communication between aquatic geochemists and toxicologists, particularly with respect to the design and implementation of toxicity tests for TCEs.
In our review, we have catalogued the concentrations observed in pristine environments and in severely contaminated waters and have compared these concentrations with those that have been shown to cause adverse effects in test organisms. In many cases, the severely contaminated waters have been collected downstream from mines that are extracting metals from sulfidic ores and have mismanaged their pyrite-rich tailings. This leads us to the general observation that for many of the TCEs, the major impact will probably not be from the individual metal finding its way into the receiving environment at the point of use, but rather from the mining activities that are needed to unearth and process the ores from which the TCEs are extracted.
A second general observation is that the previous generation of environmental chemists, who were largely working on divalent cations (Cd2+, Cu2+, Ni2+, Pb2+, Zn2+), benefitted greatly from the careful work that had been carried out by analytical chemists around the world, establishing stability constants for the complexation reactions of these metals with model ligands. Unfortunately, this type of work has fallen out of favour and very few new data are being generated. For example, the US National Institute of Standards and Technology database of ‘Critically Selected Stability Constants of Metal Complexes’ was unceremoniously discontinued in 2004. As a result, the thermodynamic data needed to predict the speciation of many of the TCEs are incomplete.
Sediments are an obvious repository for many of the elements being studied here and sediment pore waters may well represent an ecologically significant exposure medium. Further investigation of this pathway is recommended. We also suggest that, as a complement to the monitoring of TCE concentrations in receiving waters, the use of biomonitor organisms should be explored. We are using the term biomonitor in a limited sense, meaning the analysis of sessile, long-lived and widespread organisms to determine whether bioavailable TCE concentrations are in fact increasing in the aquatic environment.
Data availability
This paper contained no new or unpublished data.
Conflicts of interest
Graeme Batley is an Editor for Environmental Chemistry and was blinded from the peer review process for this manuscript.
Declaration of funding
This research did not receive any specific funding.
References
Adams W, Blust R, Dwyer R, Mount D, Nordheim E, Rodriguez PH, Spry D (2020). Bioavailability assessment of metals in freshwater environments: a historical review. Environmental Toxicology and Chemistry 39, 48–59.| Bioavailability assessment of metals in freshwater environments: a historical review.Crossref | GoogleScholarGoogle Scholar | 31880839PubMed |
Aharchaou I, Beaubien C, Campbell PGC, Fortin C (2020). Lanthanum and cerium toxicity to the freshwater green alga Chlorella fusca: applicability of the biotic ligand model. Environmental Toxicology and Chemistry 39, 996–1005.
| Lanthanum and cerium toxicity to the freshwater green alga Chlorella fusca: applicability of the biotic ligand model.Crossref | GoogleScholarGoogle Scholar | 32135577PubMed |
Ambrose H, Kendall A (2020). Understanding the future of lithium: Part 2, temporally and spatially resolved life-cycle assessment modeling. Journal of Industrial Ecology 24, 90–100.
| Understanding the future of lithium: Part 2, temporally and spatially resolved life-cycle assessment modeling.Crossref | GoogleScholarGoogle Scholar |
Amyot M, Clayden MG, MacMillan GA, Perron T, Arscott-Gauvin A (2017). Fate and trophic transfer of rare earth elements in temperate lake food webs. Environmental Science & Technology 51, 6009–6017.
| Fate and trophic transfer of rare earth elements in temperate lake food webs.Crossref | GoogleScholarGoogle Scholar |
Aral H, Vecchio-Sadus A (2008). Toxicity of lithium to humans and the environment–a literature review. Ecotoxicology and Environmental Safety 70, 349–356.
| Toxicity of lithium to humans and the environment–a literature review.Crossref | GoogleScholarGoogle Scholar | 18456327PubMed |
Batley GE, Florence TM (1975). Determination of thallium in natural waters by anodic stripping voltammetry. Journal of Electroanalytical Chemistry 61, 205–211.
| Determination of thallium in natural waters by anodic stripping voltammetry.Crossref | GoogleScholarGoogle Scholar |
Belzile N, Chen Y-W (2015). Tellurium in the environment: A critical review focused on natural waters, soils, sediments and airborne particles. Applied Geochemistry 63, 83–92.
| Tellurium in the environment: A critical review focused on natural waters, soils, sediments and airborne particles.Crossref | GoogleScholarGoogle Scholar |
Belzile N, Chen Y-W (2017). Thallium in the environment: A critical review focused on natural waters, soils, sediments and airborne particles. Applied Geochemistry 84, 218–243.
| Thallium in the environment: A critical review focused on natural waters, soils, sediments and airborne particles.Crossref | GoogleScholarGoogle Scholar |
Bibienne T, Magnan JF, Rupp A, Laroche N (2020). From mine to mind and mobiles: Society’s increasing dependence on lithium. Elements 16, 265–270.
| From mine to mind and mobiles: Society’s increasing dependence on lithium.Crossref | GoogleScholarGoogle Scholar |
Biesinger KE, Christensen GM (1972). Effects of various metals on survival, growth, reproduction, and metabolism of Daphnia magna. Journal of the Fisheries Research Board of Canada 29, 1691–1700.
| Effects of various metals on survival, growth, reproduction, and metabolism of Daphnia magna.Crossref | GoogleScholarGoogle Scholar |
Bogers M (1995a). ‘Scenedesmus subspicatus, fresh water algal growth inhibition test with Lanthanum (La), Report no.: 134358. Testing laboratory: NOTOX B. V.‘s-Hertogenbosch.’ (Owner company: Kemira Pernis B. V. Rotterdam: The Netherlands)
Bogers M (1995b). ‘Lanthanum (La): Prolonged toxicity study with carp in a semi-static system, Report no.: 139488. Testing laboratory: NOTOX B. V. ‘s-Hertogenbosch, The Netherlands.’ (Owner company: Kemira Pernis B. V. Rotterdam: The Netherlands)
Borgmann U, Cheam V, Norwood WP, Lechner J (1998). Toxicity and bioaccumulation of thallium in Hyalella azteca, with comparison to other metals and prediction of environmental impact. Environmental Pollution 99, 105–114.
| Toxicity and bioaccumulation of thallium in Hyalella azteca, with comparison to other metals and prediction of environmental impact.Crossref | GoogleScholarGoogle Scholar | 15093335PubMed |
Borgmann U, Couillard Y, Doyle P, Dixon DG (2005). Toxicity of sixty-three metals and metalloids to Hyalella azteca at two levels of water hardness. Environmental Toxicology and Chemistry 24, 641–652.
| Toxicity of sixty-three metals and metalloids to Hyalella azteca at two levels of water hardness.Crossref | GoogleScholarGoogle Scholar | 15779765PubMed |
Bridges CC, Zalups RK (2005). Molecular and ionic mimicry and the transport of toxic metals. Toxicology and Applied Pharmacology 204, 274–308.
| Molecular and ionic mimicry and the transport of toxic metals.Crossref | GoogleScholarGoogle Scholar | 15845419PubMed |
Brun NR, Fields PD, Horsfield S, Mirbahai L, Ebert D, Colbourne JK, Fent K (2019). Mixtures of aluminum and indium induce more than additive phenotypic and toxicogenomic responses in Daphnia magna. Environmental Science & Technology 53, 1639–1649.
| Mixtures of aluminum and indium induce more than additive phenotypic and toxicogenomic responses in Daphnia magna.Crossref | GoogleScholarGoogle Scholar |
Byrne RH, Sholkovitz ER (1996). Marine chemistry and geochemistry of the lanthanides, Chapter 158. In ‘Handbook on the Physics and Chemistry of Rare Earths’. (Eds KA Gschneidner, L Eyring) Vol. 23, pp. 497–593. (Elsevier: Amsterdam)
Cardon P-Y, Roques O, Caron A, Rosabal M, Fortin C, Amyot M (2019). Role of prey subcellular distribution on the bioaccumulation of yttrium (Y) in the rainbow trout. Environmental Pollution 258, e113804
| Role of prey subcellular distribution on the bioaccumulation of yttrium (Y) in the rainbow trout.Crossref | GoogleScholarGoogle Scholar |
Caron A, Rosabal M, Drevet O, Couture P, Campbell PGC (2018). Binding of trace elements (Ag, Cd, Co, Cu, Ni and Tl) to cytosolic biomolecules in livers of juvenile yellow perch (Perca flavescens) collected from lakes representing metal-contamination gradients. Environmental Toxicology and Chemistry 37, 576–586.
| Binding of trace elements (Ag, Cd, Co, Cu, Ni and Tl) to cytosolic biomolecules in livers of juvenile yellow perch (Perca flavescens) collected from lakes representing metal-contamination gradients.Crossref | GoogleScholarGoogle Scholar | 28984389PubMed |
Chen H-W (2006). Gallium, indium, and arsenic pollution of groundwater from a semiconductor manufacturing area of Taiwan. Bulletin of Environmental Contamination and Toxicology 77, 289–296.
| Gallium, indium, and arsenic pollution of groundwater from a semiconductor manufacturing area of Taiwan.Crossref | GoogleScholarGoogle Scholar | 16977532PubMed |
Cobelo-Garcia A, Turner A, Millward GE, Couceiro F (2007). Behaviour of palladium(II), platinum(IV), and rhodium(III) in artificial and natural waters: influence of reactor surface and geochemistry on metal recovery. Analytica Chimica Acta 585, 202–210.
| Behaviour of palladium(II), platinum(IV), and rhodium(III) in artificial and natural waters: influence of reactor surface and geochemistry on metal recovery.Crossref | GoogleScholarGoogle Scholar | 17386666PubMed |
Cobelo-García A, López-Sánchez DE, Almécija C, Santos-Echeandía J (2013). Behavior of platinum during estuarine mixing (Pontevedra Ria, NW Iberian Peninsula). Marine Chemistry 150, 11–18.
| Behavior of platinum during estuarine mixing (Pontevedra Ria, NW Iberian Peninsula).Crossref | GoogleScholarGoogle Scholar |
Cobelo-García A, Filella M, Croot P, Frazzoli C, Du Laing G, Ospina-Alvarez N, Rauch S, Salaun P, Schäfer J, Zimmermann S (2015). COST action TD1407: network on technology-critical elements (NOTICE)-from environmental processes to human health threats. Environmental Science and Pollution Research 22, 15188–15194.
| COST action TD1407: network on technology-critical elements (NOTICE)-from environmental processes to human health threats.Crossref | GoogleScholarGoogle Scholar | 26286804PubMed |
Couture P, Fortin C, Hare L, Lapointe D, Pitre D (2011) Critical review of thallium in aquatic ecosystems. Institut national de la recherche scientifique, Centre Eau Terre Environnement, Research Report No R-1272, Québec, QC, Canada, 36 pp.
De Baar HJW, Bacon MP, Brewer PG, Bruland KW (1985). Rare earth elements in the Pacific and Atlantic Oceans. Geochimica Cosmochimica Acta 49, 1943–1959.
| Rare earth elements in the Pacific and Atlantic Oceans.Crossref | GoogleScholarGoogle Scholar |
Dickson AJ, Hsieh Y-T, Bryan A (2020). The rhenium isotope composition of Atlantic Ocean seawater. Geochimica Cosmochimica Acta 287, 221–228.
| The rhenium isotope composition of Atlantic Ocean seawater.Crossref | GoogleScholarGoogle Scholar |
Ebrahimi P, Barbieri M (2019). Gadolinium as an emerging microcontaminant in water resources: threats and opportunities. Geosciences 9, 93
| Gadolinium as an emerging microcontaminant in water resources: threats and opportunities.Crossref | GoogleScholarGoogle Scholar |
ECHA (2021a) Gallium. In European Chemicals Agency (ECHA) registration dossier. Available at https://echa.europa.eu/fr/registration-dossier/-/registered-dossier/23228/6/2/6
ECHA (2021b) Tellurium. In European Chemicals Agency (ECHA) registration dossier. Available at https://echa.europa.eu/fr/registration-dossier/-/registered-dossier/14525/6/2/2
El-Akl P, Smith S, Wilkinson KJ (2015). Linking the chemical speciation of Ce to its bioavailability in water for a freshwater alga. Environmental Toxicology and Chemistry 34, 1711–1719.
| Linking the chemical speciation of Ce to its bioavailability in water for a freshwater alga.Crossref | GoogleScholarGoogle Scholar | 25772589PubMed |
Ellwood MJ, Maher WA (2003). Germanium cycling in the waters across a frontal zone: the Chatham Rise, New Zealand. Marine Chemistry 80, 145–159.
| Germanium cycling in the waters across a frontal zone: the Chatham Rise, New Zealand.Crossref | GoogleScholarGoogle Scholar |
Filella M, Reimann C, Biver M, Rodushkin I, Rodushkina K (2019). Tellurium in the environment: current knowledge and identification of gaps. Environmental Chemistry 16, 215–228.
| Tellurium in the environment: current knowledge and identification of gaps.Crossref | GoogleScholarGoogle Scholar |
Firdaus ML, Norisuye K, Nakagawa Y, Nakatsuka S, Sohrin Y (2008). Dissolved and labile particulate Zr, Hf, Nb, Ta, Mo and W in the Western North Pacific Ocean. Journal of Oceanography 64, 247–257.
| Dissolved and labile particulate Zr, Hf, Nb, Ta, Mo and W in the Western North Pacific Ocean.Crossref | GoogleScholarGoogle Scholar |
Florence TM, Batley GE, Farrar YJ (1974). The determination of indium by anodic stripping voltammetry; application to natural waters. Journal of Electroanalytical Chemistry 56, 301–309.
| The determination of indium by anodic stripping voltammetry; application to natural waters.Crossref | GoogleScholarGoogle Scholar |
Foley NK, Jaskula BW, Kimball BE, Schulte RF (2017) Gallium. In ‘Critical Mineral Resources of the United States—Economic and Environmental Geology and Prospects for Future Supply’. (Eds KJ Schulz, JH DeYoung, RR Seal, DC Bradley) p. 35. (U.S. Geological Survey: Reston, Virginia)
Fortin C, Wang F, Pitre D (2011) Critical review of platinum group elements (Pd, Pt, Rh) in aquatic ecosystems. Institut National de la Recherche Scientifique, Centre Eau Terre Environnement Research Report No R-1269, Quebec, Canada, for Environment Canada, 39 pp. Available at http://espace.inrs.ca/id/eprint/879/1/R001269.pdf
Frank S, Singer C, Sures B (2008). Metallothionein (MT) response after chronic palladium exposure in the zebra mussel, Dreissena polymorpha. Environmental Research 108, 309–314.
| Metallothionein (MT) response after chronic palladium exposure in the zebra mussel, Dreissena polymorpha.Crossref | GoogleScholarGoogle Scholar | 18762294PubMed |
Fujiwara K, Matsumoto Y, Kawakami H, Aoki M, Tuzuki M (2008). Evaluation of metal toxicity in Chlorella kessleri from the perspective of the periodic table. Bulletin of the Chemical Society of Japan 81, 478–488.
| Evaluation of metal toxicity in Chlorella kessleri from the perspective of the periodic table.Crossref | GoogleScholarGoogle Scholar |
Goldfarb RJ, Berger BR, George MW, Seal RR (2017) Tellurium. In ‘Critical Mineral Resources of the United States—Economic and Environmental Geology and Prospects for Future Supply’. (Eds KJ Schulz, JH DeYoung, RR Seal, DC Bradley) p. 50. (U.S. Geological Survey: Reston, Virginia)
Gonzalez V, Vignati DA, Leyval C, Giamberini L (2014). Environmental fate and ecotoxicity of lanthanides: are they a uniform group beyond chemistry?. Environment International 71, 148–157.
| Environmental fate and ecotoxicity of lanthanides: are they a uniform group beyond chemistry?.Crossref | GoogleScholarGoogle Scholar | 25036616PubMed |
Gonzalez V, Vignati DAL, Pons MN, Montarges-Pelletier E, Bojic C, Giamberini L (2015). Lanthanide ecotoxicity: First attempt to measure environmental risk for aquatic organisms. Environmental Pollution 199, 139–147.
| Lanthanide ecotoxicity: First attempt to measure environmental risk for aquatic organisms.Crossref | GoogleScholarGoogle Scholar | 25645063PubMed |
Gulley AL, Nassar NT, Xun S (2018). China, the United States, and competition for resources that enable emerging technologies. Proceedings of the National Academy of Sciences 115, 4111–4115.
| China, the United States, and competition for resources that enable emerging technologies.Crossref | GoogleScholarGoogle Scholar |
Harford AJ, Hogan AC, Tsang JJ, Parry DL, Negri AP, Adams MS, Stauber JL, van Dam RA (2011). Effects of alumina refinery wastewater and signature metal constituents at the upper thermal tolerance of: 1. The tropical diatom Nitzschia closterium. Marine Pollution Bulletin 62, 466–473.
| Effects of alumina refinery wastewater and signature metal constituents at the upper thermal tolerance of: 1. The tropical diatom Nitzschia closterium.Crossref | GoogleScholarGoogle Scholar | 21310438PubMed |
Hassler CS, Chafin RD, Klinger MB, Twiss MR (2007). Application of the biotic ligand model to explain potassium interaction with thallium uptake and toxicity to plankton. Environmental Toxicology and Chemistry 26, 1139–1145.
| Application of the biotic ligand model to explain potassium interaction with thallium uptake and toxicity to plankton.Crossref | GoogleScholarGoogle Scholar | 17571678PubMed |
Hatje V, Bruland KW, Flegal RA (2016). Increases in anthropogenic gadolinium anomalies and rare earth element concentrations in San Francisco Bay over a 20 year record. Environmental Science & Technology 50, 4159–4168.
| Increases in anthropogenic gadolinium anomalies and rare earth element concentrations in San Francisco Bay over a 20 year record.Crossref | GoogleScholarGoogle Scholar |
Herrmann H, Nolde J, Berger S, Heise S (2016). Aquatic ecotoxicity of lanthanum – A review and an attempt to derive water and sediment quality criteria. Ecotoxicology and Environmental Safety 124, 213–238.
| Aquatic ecotoxicity of lanthanum – A review and an attempt to derive water and sediment quality criteria.Crossref | GoogleScholarGoogle Scholar | 26528910PubMed |
Holl R, Kling M, Schroll E (2007). Metallogenesis of germanium—a review. Ore Geology Reviews 30, 145–180.
| Metallogenesis of germanium—a review.Crossref | GoogleScholarGoogle Scholar |
Hu Q, Yang X, Huang Z, Chen J, Yang G (2005). Simultaneous determination of palladium, platinum, rhodium and gold by on-line solid phase extraction and high performance liquid chromatography with 5-(2-hydroxy-5-nitrophenylazo)thiorhodanine as pre-column derivatization regents. Journal of Chromatography A 1094, 77–82.
| Simultaneous determination of palladium, platinum, rhodium and gold by on-line solid phase extraction and high performance liquid chromatography with 5-(2-hydroxy-5-nitrophenylazo)thiorhodanine as pre-column derivatization regents.Crossref | GoogleScholarGoogle Scholar | 16257292PubMed |
Huang C, Hu B (2008). Speciation of inorganic tellurium from seawater by ICP-MS following magnetic SPE separation and preconcentration. Separation Science 31, 760–767.
| Speciation of inorganic tellurium from seawater by ICP-MS following magnetic SPE separation and preconcentration.Crossref | GoogleScholarGoogle Scholar |
Johannesson KH, Tang J, Daniels JM, Bounds WJ, Burdige DJ (2004). Rare earth element concentrations and speciation in organic-rich blackwaters of the Great Dismal Swamp, Virginia, USA. Chemical Geology 209, 271–294.
| Rare earth element concentrations and speciation in organic-rich blackwaters of the Great Dismal Swamp, Virginia, USA.Crossref | GoogleScholarGoogle Scholar |
John DA, Seal RR, Polyak DE (2017) Rhenium. In ‘Critical Mineral Resources of the United States—Economic and Environmental Geology and Prospects for Future Supply’. (Eds KJ Schulz, JH DeYoung, RR Seal, DC Bradley). p. 50. (U.S. Geological Survey: Reston, Virginia)
Jorgenson JD, George MW (2005) ‘Indium. Mineral Commodity Profile’. p. 20. (U.S. Geological Survey: Reston, Virginia, USA)
Kaneko Y, Thoendel M, Olakanmi O, Britigan BE, Singh PK (2007). The transition metal gallium disrupts Pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity. The. Journal of Clinical Investigation 117, 877–888.
| The transition metal gallium disrupts Pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity. The.Crossref | GoogleScholarGoogle Scholar | 17364024PubMed |
Karbowska B (2016). Presence of thallium in the environment: sources of contaminations, distribution and monitoring methods. Environmental Monitoring and Assessment 188, e640
| Presence of thallium in the environment: sources of contaminations, distribution and monitoring methods.Crossref | GoogleScholarGoogle Scholar |
Khan AM, Bakar NKA, Bakar AFA, Ashraf MA (2017). Chemical speciation and bioavailability of rare earth elements (REEs) in the ecosystem: a review. Environmental Science and Pollution Research 24, 22764–22789.
| Chemical speciation and bioavailability of rare earth elements (REEs) in the ecosystem: a review.Crossref | GoogleScholarGoogle Scholar | 27722986PubMed |
Khangarot BS, Das S (2009). Acute toxicity of metals and reference toxicants to a freshwater ostracod, Cypris subglobosa Sowerby, 1840 and correlation to EC50 values of other test models. Journal of Hazardous Materials 172, 641–649.
| Acute toxicity of metals and reference toxicants to a freshwater ostracod, Cypris subglobosa Sowerby, 1840 and correlation to EC50 values of other test models.Crossref | GoogleScholarGoogle Scholar | 19683870PubMed |
Koschinsky A, Hein JR (2003). Uptake of elements from seawater by ferromanganese crusts: solid-phase associations and seawater speciation. Marine Geology 198, 331–351.
| Uptake of elements from seawater by ferromanganese crusts: solid-phase associations and seawater speciation.Crossref | GoogleScholarGoogle Scholar |
Kszos LA, Stewart AJ (2003). Review of lithium in the aquatic environment: distribution in the United States, toxicity and case example of groundwater contamination. Ecotoxicology 12, 439–447.
| Review of lithium in the aquatic environment: distribution in the United States, toxicity and case example of groundwater contamination.Crossref | GoogleScholarGoogle Scholar | 14649426PubMed |
Kszos LA, Beauchamp JJ, Stewart AJ (2003). Toxicity of lithium to three freshwater organisms and the antagonistic effect of sodium. Ecotoxicology 12, 427–437.
| Toxicity of lithium to three freshwater organisms and the antagonistic effect of sodium.Crossref | GoogleScholarGoogle Scholar | 14649425PubMed |
Lawrence MG, Jupiter SD, Kamber BS (2006). Aquatic geochemistry of the rare earth elements and yttrium in the Pioneer River catchment, Australia. Marine and Freshwater Research 57, 725–736.
| Aquatic geochemistry of the rare earth elements and yttrium in the Pioneer River catchment, Australia.Crossref | GoogleScholarGoogle Scholar |
Le Faucheur S, Mertens J, Van Genderen E, Boullemant A, Fortin C, Campbell PGC (2021). Development of QICAR models to address the lack of toxicological data for Technology-Critical Metals. Environmental Toxicology and Chemistry 40, 1139–1148.
| Development of QICAR models to address the lack of toxicological data for Technology-Critical Metals.Crossref | GoogleScholarGoogle Scholar | 33315280PubMed |
Lee DS, Edmond JM (1985). Tellurium species in seawater. Nature 313, 782–785.
| Tellurium species in seawater.Crossref | GoogleScholarGoogle Scholar |
Leguay S, Campbell PGC, Fortin C (2016). Determination of the free-ion concentration of rare earth elements by an ion-exchange technique: implementation, evaluation and limits. Environmental Chemistry 13, 478–488.
| Determination of the free-ion concentration of rare earth elements by an ion-exchange technique: implementation, evaluation and limits.Crossref | GoogleScholarGoogle Scholar |
Lewin J (1966). Silicon metabolism in diatoms. V. Germanium dioxide, a specific inhibitor of diatom growth. Phycologia 6, 1–12.
| Silicon metabolism in diatoms. V. Germanium dioxide, a specific inhibitor of diatom growth.Crossref | GoogleScholarGoogle Scholar |
Li DX, Gao ZM, Zhu YX, Yu YM, Wang H (2005). Photochemical reaction of Tl in aqueous solution and its environmental significance. Geochemical Journal 39, 113–119.
| Photochemical reaction of Tl in aqueous solution and its environmental significance.Crossref | GoogleScholarGoogle Scholar |
Liang T, Li K, Wang L (2014). State of rare earth elements in different environmental components in mining areas of China. Environmental Monitoring and Assessment 186, 1499–1513.
| State of rare earth elements in different environmental components in mining areas of China.Crossref | GoogleScholarGoogle Scholar | 24135922PubMed |
Lin HC, Hwang PP (1998). Acute and chronic effects of indium chloride (InCl3) on tilapia (Oreochromis mossambicus) larvae. Bulletin of Environmental Contamination and Toxicology 61, 123–128.
| Acute and chronic effects of indium chloride (InCl3) on tilapia (Oreochromis mossambicus) larvae.Crossref | GoogleScholarGoogle Scholar | 9657840PubMed |
Liu Y, Ding F, Zhang L, Wang L, Liu H, Wu D, Ji C, Fu B (2021). Transport of palladium in urban water environments and its simulation: a case study of Haikou, Hainan Province, China. Urban Water Journal 18, 25–32.
| Transport of palladium in urban water environments and its simulation: a case study of Haikou, Hainan Province, China.Crossref | GoogleScholarGoogle Scholar |
Lürling M, Tolman Y (2010). Effects of lanthanum and lanthanum-modified clay on growth, survival and reproduction of Daphnia magna. Water Research 44, 309–319.
| Effects of lanthanum and lanthanum-modified clay on growth, survival and reproduction of Daphnia magna.Crossref | GoogleScholarGoogle Scholar | 19801159PubMed |
Malhotra N, Hsu H-S, Liang S-T, Jmelou M, Roldan M, Lee J-S, Ger T-R, Hsiao C-D (2020). An updated review of toxicity effect of the rare earth elements (REEs) on aquatic organisms. Animals 10, e1663
| An updated review of toxicity effect of the rare earth elements (REEs) on aquatic organisms.Crossref | GoogleScholarGoogle Scholar | 32947815PubMed |
Marsac R, Catrouillet C, Davranche M, Bouhnik-Le Coz M, Briant N, Janot N, Otero-Fariña A, Groenenberg JE, Pédrot M, Dia A (2021). Modeling rare earth elements binding to humic acids with model VII. Chemical Geology 567, e120099
| Modeling rare earth elements binding to humic acids with model VII.Crossref | GoogleScholarGoogle Scholar |
Nagel AH, Cuss CW, Goss GG, Shotyk W, Glover CN (2021). Chronic toxicity of waterborne thallium to Daphnia magna. Environmental Pollution 268, e115776
| Chronic toxicity of waterborne thallium to Daphnia magna.Crossref | GoogleScholarGoogle Scholar |
Najafi NM, Tavakoli H, Alizadeh R, Seidi S (2010). Speciation and determination of ultra trace amounts of inorganic tellurium in environmental water samples by dispersive liquid–liquid microextraction and electrothermal atomic absorption spectrometry. Analytica Chimica Acta 670, 18–23.
| Speciation and determination of ultra trace amounts of inorganic tellurium in environmental water samples by dispersive liquid–liquid microextraction and electrothermal atomic absorption spectrometry.Crossref | GoogleScholarGoogle Scholar | 20685411PubMed |
Naumann B, Eberius M, Appenroth K-J (2007). Growth rate based dose-response relationships and EC-values of ten heavy metals using the duckweed growth inhibition test (ISO 20079) with Lemna minor L. clone St. Journal of Plant Physiology 164, 1656–1664.
| Growth rate based dose-response relationships and EC-values of ten heavy metals using the duckweed growth inhibition test (ISO 20079) with Lemna minor L. clone St.Crossref | GoogleScholarGoogle Scholar | 17296247PubMed |
Nduwayezu I, Mostafavirad F, Hadioui M, Wilkinson KJ (2016). Speciation of a lanthanide (Sm) using an ion exchange resin. Analytical Methods 8, 6774–6781.
| Speciation of a lanthanide (Sm) using an ion exchange resin.Crossref | GoogleScholarGoogle Scholar |
Negri AP, Harford AJ, Parry DL, van Dam RA (2011). Effects of alumina refinery wastewater and signature metal constituents at the upper thermal tolerance of: 2. The early life stages of the coral Acropora tenuis. Marine Pollution Bulletin 6, 474–482.
| Effects of alumina refinery wastewater and signature metal constituents at the upper thermal tolerance of: 2. The early life stages of the coral Acropora tenuis.Crossref | GoogleScholarGoogle Scholar |
Neubert C, Lange R, Steger-Hartmann T (2008). Gadolinium containing contrast agents for magnetic resonance imaging (MRI) investigations on the environmental fate and effects. In ‘Fate of Pharmaceuticals in the Environment and in Water Treatment Systems’. (Ed. DS Aga) pp. 101–120. (CRC: Boca Raton, FL, USA)
Nozaki Y, Lerche D, Alibo DS, Tsutsumi M (2000). Dissolved indium and rare earth elements in three Japanese rivers and Tokyo Bay: evidence for anthropogenic Gd and. Geochimica Cosmochimica Acta 64, 3975–3982.
| Dissolved indium and rare earth elements in three Japanese rivers and Tokyo Bay: evidence for anthropogenic Gd and.Crossref | GoogleScholarGoogle Scholar |
Nys C, Janssen CR, Mager EM, Esbaugh AJ, Brix KV, Grosell M, Stubblefield WA, Holtze K, De Schamphelaere KA (2014). Development and validation of a biotic ligand model for predicting chronic toxicity of lead to Ceriodaphnia dubia. Environmental Toxicology and Chemistry 33, 394–403.
| Development and validation of a biotic ligand model for predicting chronic toxicity of lead to Ceriodaphnia dubia.Crossref | GoogleScholarGoogle Scholar | 24142571PubMed |
Obata H, Yoshida T, Ogawa H (2006). Determination of picomolar levels of platinum in estuarine waters: A comparison of cathodic stripping voltammetry and isotope dilution-inductively coupled plasma mass spectrometry. Analytica Chimica Acta 580, 32–38.
| Determination of picomolar levels of platinum in estuarine waters: A comparison of cathodic stripping voltammetry and isotope dilution-inductively coupled plasma mass spectrometry.Crossref | GoogleScholarGoogle Scholar | 17723753PubMed |
Obata H, Alibo DS, Nozaki Y (2007). Dissolved aluminium, indium, and cerium in the Sea of Japan and the Sea of Okhotsk: Comparison to the marginal seas of the western North Pacific. Journal of Geophysical Research 112, C12003
| Dissolved aluminium, indium, and cerium in the Sea of Japan and the Sea of Okhotsk: Comparison to the marginal seas of the western North Pacific.Crossref | GoogleScholarGoogle Scholar |
Ogra Y (2017). Biology and toxicology of tellurium explored by speciation analysis. Metallomics 9, 435–441.
| Biology and toxicology of tellurium explored by speciation analysis.Crossref | GoogleScholarGoogle Scholar | 28345104PubMed |
Olivares CI, Field JA, Simonich M, Tanguay RL, Sierra-Alvarez R (2016). Arsenic (III, V), indium (III), and gallium (III) toxicity to zebrafish embryos using a high-throughput multi-endpoint in vivo developmental and behavioral assay. Chemosphere 148, 361–368.
| Arsenic (III, V), indium (III), and gallium (III) toxicity to zebrafish embryos using a high-throughput multi-endpoint in vivo developmental and behavioral assay.Crossref | GoogleScholarGoogle Scholar | 26824274PubMed |
Onikura N, Nakamura A, Kishi K (2008). Acute toxicity of thallium and indium toward brackish–water and marine organisms. Journal of the Faculty of Agriculture Kyushu University 53, 467–469.
| Acute toxicity of thallium and indium toward brackish–water and marine organisms.Crossref | GoogleScholarGoogle Scholar |
Oosterhout F, Lürling M (2013). The effect of phosphorus binding clay (Phoslock) in mitigating cyanobacterial nuisance: a laboratory study on the effects on water quality variables and plankton. Hydrobiologia 710, 265–277.
| The effect of phosphorus binding clay (Phoslock) in mitigating cyanobacterial nuisance: a laboratory study on the effects on water quality variables and plankton.Crossref | GoogleScholarGoogle Scholar |
Padan J, Marcinek S, Cindric A-M, Layglon N, Garnier C, Salaun P, Cobelo-Garcıa A, Omanovic D (2020). Determination of sub-picomolar levels of platinum in the pristine Krka River estuary (Croatia) using improved voltammetric methodology. Environmental Chemistry 17, 77–84.
| Determination of sub-picomolar levels of platinum in the pristine Krka River estuary (Croatia) using improved voltammetric methodology.Crossref | GoogleScholarGoogle Scholar |
Pickard J, Yang R, Duncan B, McDevitt CA, Eickhoff C (2001). Acute and sublethal toxicity of thallium to aquatic organisms. Bulletin of Environmental Contamination and Toxicology 66, 94–101.
| Acute and sublethal toxicity of thallium to aquatic organisms.Crossref | GoogleScholarGoogle Scholar | 11080342PubMed |
Poehle S, Koschinsky A (2017). Depth distribution of Zr and Nb in seawater: The potential role of colloids or organic complexation to explain non-scavenging-type behavior. Marine Chemistry 188, 18–32.
| Depth distribution of Zr and Nb in seawater: The potential role of colloids or organic complexation to explain non-scavenging-type behavior.Crossref | GoogleScholarGoogle Scholar |
Pommerrenig B, Diehn TA, Bienert GP (2015). Metalloido-porins: Essentiality of Nodulin 26-like intrinsic proteins in metalloid transport. Plant Science 238, 212–227.
| Metalloido-porins: Essentiality of Nodulin 26-like intrinsic proteins in metalloid transport.Crossref | GoogleScholarGoogle Scholar | 26259189PubMed |
Puddu A, Pettine M, La Noce T, Pagnotta R, Bacciu F (1988). Factors affecting thallium and chromium toxicity to marine algae. Science of the Total Environment 71, 572
| Factors affecting thallium and chromium toxicity to marine algae.Crossref | GoogleScholarGoogle Scholar |
Rahal R (2018) Toxicité du lanthane chez l’algue verte Chlorella fusca en présence de matière organique naturelle. MSc Thesis, Université du Quebec Institut national de la recherche scientifique, Centre Eau Terre Environnement, Quebec, Canada. 75 pp.
Ralph L, Twiss MR (2002). Comparative toxicity of thallium(I), thallium(III), and cadmium(II) to the unicellular alga Chlorella isolated from Lake Erie. Bulletin of Environmental Contamination and Toxicology 68, 261–268.
| Comparative toxicity of thallium(I), thallium(III), and cadmium(II) to the unicellular alga Chlorella isolated from Lake Erie.Crossref | GoogleScholarGoogle Scholar | 11815797PubMed |
Rauch S, Fatoki OS (2015) Impact of platinum group element emissions from mining and production activities. In ‘Platinum Metals in the Environment’. (Eds F Zereini, CLS Wiseman) pp. 19–30. (Springer: Heidelberg Germany)
Rauch S, Morrison GM (1999). Platinum uptake by the freshwater isopod Asellus aquaticus in urban rivers. Science of the Total Environment 235, 261–268.
| Platinum uptake by the freshwater isopod Asellus aquaticus in urban rivers.Crossref | GoogleScholarGoogle Scholar | 10535125PubMed |
Rauch S, Morrison GM (2000) Routes for bioaccumulation and transformation of platinum in the urban environment. In ‘Anthropogenic Platinum-Group Element Emissions: Their Impact on Man and Environment’. (Eds F Zereini, F Alt) pp. 85–93. (Springer-Verlag: Berlin)
Rauch S, Morrison GM (2008). Environmental relevance of the platinum-group elements. Elements 4, 259–263.
| Environmental relevance of the platinum-group elements.Crossref | GoogleScholarGoogle Scholar |
Rauch S, Peucker-Ehrenbrink B (2015) Sources of platinum group elements in the environment. In ‘Platinum Metals in the Environment’. (Eds F Zereini, CLS Wiseman). pp. 3–18. (Springer: Heidelberg Germany)
Rauch S, Paulsson M, Wilewska M, Blanck H, Morrison GM (2004). Short-term toxicity and binding of platinum to freshwater periphyton communities. Archives of Environmental Contamination and Toxicology 47, 290–296.
| Short-term toxicity and binding of platinum to freshwater periphyton communities.Crossref | GoogleScholarGoogle Scholar | 15386122PubMed |
Rauch S, Hemond H, Barbante C, Owari M, Morrison GM, Peucker-Ehrenbrink B, Wass U (2005). Importance of automobile exhaust catalyst emissions for the deposition of platinum, palladium and rhodium in the northern hemisphere. Environmental Science & Technology 39, 8156–8162.
| Importance of automobile exhaust catalyst emissions for the deposition of platinum, palladium and rhodium in the northern hemisphere.Crossref | GoogleScholarGoogle Scholar |
Reith F, Campbell SG, Ball AS, Pring A, Southam G (2014). Platinum in Earth surface environments. Earth-Science Reviews 131, 1–21.
| Platinum in Earth surface environments.Crossref | GoogleScholarGoogle Scholar |
Rickwood CJ, King M, Huntsman-Mapila P (2015). Assessing the fate and toxicity of thallium I and thallium III to three aquatic organisms. Ecotoxicology and Environmental Safety 115, 300–308.
| Assessing the fate and toxicity of thallium I and thallium III to three aquatic organisms.Crossref | GoogleScholarGoogle Scholar | 25659481PubMed |
Rogowska J, Olkowska E, Ratajczyk W, Wolska L (2018). Gadolinium as a new emerging contaminant of aquatic environments. Environmental Toxicology and Chemistry 37, 1523–1534.
| Gadolinium as a new emerging contaminant of aquatic environments.Crossref | GoogleScholarGoogle Scholar | 29473658PubMed |
Romero-Freire A, Santos-Echeandía J, Neira P, Cobelo-García A (2019). Less-studied technology-critical elements (Nb, Ta, Ga, In, Ge, Te) in the marine environment: Review on their concentrations in water and organisms. Frontiers in Marine Science 6, e532
| Less-studied technology-critical elements (Nb, Ta, Ga, In, Ge, Te) in the marine environment: Review on their concentrations in water and organisms.Crossref | GoogleScholarGoogle Scholar |
Rosenberg E (2009). Germanium: environmental occurrence, importance and speciation. Reviews of Environmental Science and Biotechnology 8, 29–57.
| Germanium: environmental occurrence, importance and speciation.Crossref | GoogleScholarGoogle Scholar |
Rowell J-A, Fillion M-A, Smith S, Wilkinson KJ (2018). Determination of the speciation and bioavailability of Sm to Chlamydomonas reinhardtii in the presence of natural organic matter. Environmental Toxicology and Chemistry 37, 1623–1631.
| Determination of the speciation and bioavailability of Sm to Chlamydomonas reinhardtii in the presence of natural organic matter.Crossref | GoogleScholarGoogle Scholar | 29396990PubMed |
Roy G (2009) Les éléments du groupe platine (Pd, Pt et Rh) dans les eaux de surface et leur toxicité chez l’algue verte Chlamydomonas reinhardtii. MSc Thesis, Université du Québec, Institut national de la recherche scientifique, Centre Eau Terre Environnement, Quebec, Canada. 145 pp.
Schertzinger G, Zimmermann S, Grabner D, Sures B (2017). Assessment of sublethal endpoints after chronic exposure of the nematode Caenorhabditis elegans to palladium, platinum and rhodium. Environmental Pollution 230, 31–39.
| Assessment of sublethal endpoints after chronic exposure of the nematode Caenorhabditis elegans to palladium, platinum and rhodium.Crossref | GoogleScholarGoogle Scholar | 28644982PubMed |
Schroll E (1999) Indium: Element and geochemistry. In ‘Geochemistry. Encyclopedia of Earth Science’. (Eds CP Marshall, RW Fairbridge) (Springer: Dordrecht)
| Crossref |
Schulz KJ, Piatak NM, Papp JF (2017) Niobium and tantalum. In ‘Critical Mineral Resources of the United States—Economic and Environmental Geology and Prospects for Future Supply’. (Eds KJ Schulz, JH DeYoung, RR Seal, DC Bradley) p. 34. (U.S. Geological Survey: Reston, Virginia)
Shanks WCP, Kimball BE, Tolcin AC, Guberman DE (2017) Germanium and indium. In ‘Critical Mineral Resources of the United States—Economic and Environmental Geology and Prospects for Future Supply’. (Eds KJ Schulz, JH DeYoung, RR Seal, DC Bradley) p. 127. (U.S. Geological Survey: Reston, Virginia)
Shiller AM (1998). Dissolved gallium in the Atlantic Ocean. Marine Chemistry 61, 87–99.
| Dissolved gallium in the Atlantic Ocean.Crossref | GoogleScholarGoogle Scholar |
Soyol-Erdene T-O, Huh Y (2012). Dissolved platinum in major rivers of East Asia: Implications for the oceanic budget. Geochemistry Geophysics Geosystems 13, Q06009
| Dissolved platinum in major rivers of East Asia: Implications for the oceanic budget.Crossref | GoogleScholarGoogle Scholar |
Stauber JL, Binet MT (2000) Canning River Phoslock field trials—Ecotoxicity testing final report. ET 317R. CSIRO Land and Water, Lucas Heights, NSW, Australia; WA Waters and Rivers Commission, East Perth, Australia.
Stern JC, Sonke JE, Salters VJM (2007). A capillary electrophoresis-ICP-MS study of rare earth element complexation by humic acids. Chemical Geology 246, 170–180.
| A capillary electrophoresis-ICP-MS study of rare earth element complexation by humic acids.Crossref | GoogleScholarGoogle Scholar |
Stolte S, Bui HTT, Steudte S, Korinth V, Arning J, Białk-Bielińska A, Bottin-Weber U, Cokoja M, Hahlbrock A, Fetz V, Stauber R, Jastorff B, Hartmann C, Fischerg RW, Kühn FE (2015). Preliminary toxicity and ecotoxicity assessment of methyltrioxorhenium and its derivatives. Green Chemistry 17, 1136–1144.
| Preliminary toxicity and ecotoxicity assessment of methyltrioxorhenium and its derivatives.Crossref | GoogleScholarGoogle Scholar |
Sures B, Ruchter N, Zimmermann S (2015). Biological effects of PGE on aquatic organisms. In ‘Platinum Metals in the Environment’. (Eds F Zereini, C Wiseman). pp. 383–399. (Springer: Heidelberg, Germany)
Tagami K, Uchida S (2008). Rhenium contents in Japanese river waters measured by isotope dilution ICP-MS and the relationship of Re with some chemical components. Journal of Nuclear Science and Technology 45, 128–132.
| Rhenium contents in Japanese river waters measured by isotope dilution ICP-MS and the relationship of Re with some chemical components.Crossref | GoogleScholarGoogle Scholar |
Tagami K, Uchida S, Tsukada H (2006). Vertical distribution of rhenium in seawater samples collected at three locations off the coast of Aomori, Japan. Journal of Radioanalytical and Nuclear Chemistry 267, 631–635.
| Vertical distribution of rhenium in seawater samples collected at three locations off the coast of Aomori, Japan.Crossref | GoogleScholarGoogle Scholar |
Taylor NS, Merrifield R, Williams TD, Chipman JK, Lead JR, Viant MR (2016). Molecular toxicity of cerium oxidenanoparticles to the freshwater alga Chlamydomonas reinhardtii isassociated with supra-environmental exposure concentrations. Nanotoxicology 10, 32–41.
| Molecular toxicity of cerium oxidenanoparticles to the freshwater alga Chlamydomonas reinhardtii isassociated with supra-environmental exposure concentrations.Crossref | GoogleScholarGoogle Scholar | 25740379PubMed |
Thayer JS (2002). Biological methylation of less-studied elements. Applied Organometallic Chemistry 16, 677–691.
| Biological methylation of less-studied elements.Crossref | GoogleScholarGoogle Scholar |
Tipping E, Lofts S, Sonke JE (2011). Humic Ion-Binding Model VII: a revised parameterisation of cation-binding by humic substances. Environmental Chemistry 8, 225–235.
| Humic Ion-Binding Model VII: a revised parameterisation of cation-binding by humic substances.Crossref | GoogleScholarGoogle Scholar |
Trenfield M, van Dam J, Harford A, Parry D, Streten-Joyce C, Gibb K, van Dam R (2015). Aluminium, gallium, and molybdenum toxicity to the tropical marine microalga Isochrysis galbana. Environmental Toxicology and Chemistry 34, 1833–1840.
| Aluminium, gallium, and molybdenum toxicity to the tropical marine microalga Isochrysis galbana.Crossref | GoogleScholarGoogle Scholar | 25809393PubMed |
Trenfield MA, van Dam JW, Harford AJ, Parry D, Streten C, Gibb K, van Dam RA (2016). A chronic toxicity test for the tropical marine snail Nassarius dorsatus to assess the toxicity of copper, aluminium, gallium, and molybdenum. Environmental Toxicology and Chemistry 35, 1788–1795.
| A chronic toxicity test for the tropical marine snail Nassarius dorsatus to assess the toxicity of copper, aluminium, gallium, and molybdenum.Crossref | GoogleScholarGoogle Scholar | 26643415PubMed |
Twining BS, Twiss MR, Fisher NS (2003). Oxidation of thallium by freshwater plankton communities. Environmental Science and Technology 37, 2720–2726.
| Oxidation of thallium by freshwater plankton communities.Crossref | GoogleScholarGoogle Scholar | 12854711PubMed |
van Dam JW, Trenfield MA, Harries SJ, Streten C, Harford AJ, Parry D, van Dam RA (2016). A novel bioassay using the barnacle Amphibalanus amphitrite to evaluate chronic effects of aluminium, gallium and molybdenum in tropical marine receiving environments. Marine Pollution Bulletin 112, 427–435.
| A novel bioassay using the barnacle Amphibalanus amphitrite to evaluate chronic effects of aluminium, gallium and molybdenum in tropical marine receiving environments.Crossref | GoogleScholarGoogle Scholar | 27423445PubMed |
van Dam JW, Trenfield MA, Streten C, Harford AJ, Parry D, van Dam RA (2018). Assessing chronic toxicity of aluminium, gallium and molybdenum in tropical marine waters using a novel bioassay for larvae of the hermit crab Coenobita variabilis. Ecotoxicology and Environmental Safety 165, 349–356.
| Assessing chronic toxicity of aluminium, gallium and molybdenum in tropical marine waters using a novel bioassay for larvae of the hermit crab Coenobita variabilis.Crossref | GoogleScholarGoogle Scholar | 30216893PubMed |
Vannini C, Domingo G, Marsoni M, Fumagalli A, Terzaghi R, Labra M, De Mattia F, Onelli E, Bracale M (2011). Physiological and molecular effects associated with palladium treatment in Pseudokirchneriella subcapitata. Aquatic Toxicology 102, 104–113.
| Physiological and molecular effects associated with palladium treatment in Pseudokirchneriella subcapitata.Crossref | GoogleScholarGoogle Scholar | 21371618PubMed |
Weltje L, Brouwer AH, Verburg TG, Wolterbeek HT, de Goeij JJM (2002). Accumulation and elimination of lanthanum by duckweed (Lemna minor L.) as influenced by organism growth and lanthanum sorption to glass. Environmental Toxicology and Chemistry 21, 1483–1489.
| Accumulation and elimination of lanthanum by duckweed (Lemna minor L.) as influenced by organism growth and lanthanum sorption to glass.Crossref | GoogleScholarGoogle Scholar | 12109750PubMed |
Weltje L, den Hollander W, Wolterbeek HT (2003). Adsorption of metals to membrane filters in view of their speciation in nutrient solution. Environmental Toxicology and Chemistry 22, 265–271.
| Adsorption of metals to membrane filters in view of their speciation in nutrient solution.Crossref | GoogleScholarGoogle Scholar | 12558156PubMed |
White SO, Hussain FA, Hemond HF, Sacco SA, Shine JP, Runkel RL, Walton-Day K, Kimball BA (2017). The precipitation of indium at elevated pH in a stream influenced by acid mine drainage. Science of the Total Environment 574, 1484–1491.
| The precipitation of indium at elevated pH in a stream influenced by acid mine drainage.Crossref | GoogleScholarGoogle Scholar |
Wood SA, Samson IM (2006). The aqueous geochemistry of gallium, germanium, indium, and scandium. Ore Geology Reviews 28, 57–102.
| The aqueous geochemistry of gallium, germanium, indium, and scandium.Crossref | GoogleScholarGoogle Scholar |
Yang JL (2014). Comparative acute toxicity of gallium(III), antimony(III), indium(III), cadmium(II), and copper(II) on freshwater swamp shrimp (Macrobrachium nipponense). Biological Research 47, 13
| Comparative acute toxicity of gallium(III), antimony(III), indium(III), cadmium(II), and copper(II) on freshwater swamp shrimp (Macrobrachium nipponense).Crossref | GoogleScholarGoogle Scholar | 25027256PubMed |
Yang JL, Chen LH (2018). Toxicity of antimony, gallium, and indium toward a teleost model and a native fish species of semiconductor manufacturing districts of Taiwan. Journal of Elementology 23, 191–199.
| Toxicity of antimony, gallium, and indium toward a teleost model and a native fish species of semiconductor manufacturing districts of Taiwan.Crossref | GoogleScholarGoogle Scholar |
Yang G, Wilkinson KJ (2018). Biouptake of a rare earth metal (Nd) by Chlamydomonas reinhardtii – Bioavailability of small organic complexes and role of hardness ions. Environmental Pollution 243, 263–269.
| Biouptake of a rare earth metal (Nd) by Chlamydomonas reinhardtii – Bioavailability of small organic complexes and role of hardness ions.Crossref | GoogleScholarGoogle Scholar | 30189390PubMed |
Yang G, Tan Q-G, Zhu L, Wilkinson KJ (2014). The role of complexation and competition in the biouptake of europium by a unicellular alga. Environmental Toxicology and Chemistry 33, 2609–2615.
| The role of complexation and competition in the biouptake of europium by a unicellular alga.Crossref | GoogleScholarGoogle Scholar | 25132226PubMed |
Yin X, Martineau C, Demers I, Basiliko N, Fenton NJ (2021). The potential environmental risks associated with the development of rare earth element production in Canada. Environmental Reviews 29, 354–377.
| The potential environmental risks associated with the development of rare earth element production in Canada.Crossref | GoogleScholarGoogle Scholar |
Yuan W, Chen J, Teng H, Chetelat B, Cai H, Liu J, Wang Z, Bouchez J, Moynier F, Gaillardet J, Schott J, Liu C (2021). A review on the elemental and isotopic geochemistry of gallium. Global Biogeochemical Cycles 35, e2021GB007033
| A review on the elemental and isotopic geochemistry of gallium.Crossref | GoogleScholarGoogle Scholar |
Zereini F, Wiseman CLS (2015) ‘Platinum Metals in the Environment’. p. 492. (Springer: Heidelberg Germany)
Zhang Q, Rickaby REM (2020). Interactions of thallium with marine phytoplankton. Geochimica Cosmochimica Acta 276, 1–13.
| Interactions of thallium with marine phytoplankton.Crossref | GoogleScholarGoogle Scholar |
Zhuang W, Song J (2021). Thallium in aquatic environments and the factors controlling Tl behavior. Environmental Science and Pollution Research 28, 35472–35487.
| Thallium in aquatic environments and the factors controlling Tl behavior.Crossref | GoogleScholarGoogle Scholar | 34021893PubMed |
Zimmermann S, Sures B, Ruchter N (2015). Laboratory studies on the uptake and bioaccumulation of PGE by aquatic plants and animals. In ‘Platinum Metals in the Environment’. (Eds F Zereini, C Wiseman) pp. 361–381. (Springer: Heidelberg, Germany)
Zimmermann S, Wolff C, Sures B (2017). Toxicity of platinum, palladium and rhodium to Daphnia magna in single and binary metal exposure experiments. Environmental Pollution 224, 368–376.
| Toxicity of platinum, palladium and rhodium to Daphnia magna in single and binary metal exposure experiments.Crossref | GoogleScholarGoogle Scholar | 28222978PubMed |


