Diffusion limitation of zinc fluxes into wheat roots, PLM and DGT devices in the presence of organic ligands
A. Gramlich A D , S. Tandy A , E. Frossard B , J. Eikenberg C and R. Schulin AA Institute of Terrestrial Ecosystems, ETH Zurich, Universitätstraße 16, CH-8092 Zürich, Switzerland.
B Institute for Plant, Animal and Agroecosystems Sciences, ETH Zurich, Eschikon 33, CH-8315 Lindau, Switzerland.
C Radioanalytics Group, Paul Scherrer Institute, CH-5232 Villigen PSI, Switzerland.
D Corresponding author. Email: anja.gramlich@env.ethz.ch
Environmental Chemistry 11(1) 41-50 https://doi.org/10.1071/EN13106
Submitted: 7 June 2013 Accepted: 30 October 2013 Published: 13 February 2014
Environmental context. Zinc is an essential micronutrient for plants and many arid areas of the world have zinc-deficient soils. The bioavailability of Zn to plants is influenced by diffusion limitations and complex lability in the soil solution. To identify the relative importance of these two factors, we investigated the influence of diffusion layer thickness on Zn uptake by wheat and by two bio-mimetic devices in the presence of ethylenediaminetetraacetic acid and two natural ligands found in soil.
Abstract. Organic ligands can increase metal mobility in soils. The extent to which this can contribute to plant metal uptake depends among others, on complex lability and diffusion limitations in solute transfer from the soil solution to root uptake sites. We investigated the influence of diffusion layer thickness on zinc uptake by wheat seedlings in the presence of ethylenediaminetetraacetic acid (EDTA), citrate and histidine with similar free Zn by measuring 65Zn uptake from stirred, non-stirred and agar-containing solutions. Analogous experiments were performed using permeation liquid membranes (PLM) and ‘diffusive gradients in thin films’ (DGT) probes as bio-mimetic devices. In treatments with low EDTA concentrations (~2 µM) or ligand-free Zn solution, increasing diffusion layer thickness reduced Zn fluxes into roots to a similar extent as into PLM and DGT probes, indicating reduced uptake attributable to diffusion limitation. In the citrate treatments root Zn influx was similar to EDTA treatments under stirred conditions, but increasing diffusion layer thickness did not affect Zn uptake. This suggests complex dissociation compensated for reduced Zn2+ diffusion and that the entire complexes were not taken up. The Zn root influxes in the histidine treatments were found to be on average by a factor of 2.5 higher than in the citrate treatments and they also showed no decrease in non-stirred and agar treatments. Dissociation kinetics inferred from PLM measurements explained a large part, although not all, of the increased Zn uptake by the plants in the presence of histidine. The difference may be a result of the uptake of neutral or positive Zn–histidine complexes. The results of this study confirm that labile complexes can contribute to Zn uptake by wheat either through diffusion limitation and complex dissociation or through uptake of entire complexes, depending on the nature of the ligands.
Additional keywords: citrate, diffusive gradients in thin films, EDTA, ethylenediaminetetraacetic acid, histidine, permeation liquid membranes, Zn-bioavailability.
Introduction
Zinc is an essential trace metal for plant nutrition. From the total amount of Zn stored in soils usually only a small fraction is available to plants, making Zn a limiting factor for plant growth.[1] The bioavailability of trace elements in the soil solution depends on their chemical speciation, in particular the formation of complexes with dissolved organic ligands. If the supply of free metal to the roots limits the uptake rate, then the dissociation of complexes may add substantially to its uptake,[2] and this contribution is expected to increase with the lability of the complexes.[3,4] On the other hand, the uptake of intact complexes may also contribute to the uptake of metals by plants as found not only for liposoluble[5] but also for uncharged water-soluble complexes.[6] Apart from the chemical nature of the complexes, their physiological role also needs to be considered in this context, as the uptake of Fe and Zn phytosiderophore complexes by grasses illustrates.[7] Different factors may be rate limiting for different metals. Degryse et al.[8] found that metal diffusion in solution limited Zn and Cd uptake, whereas root internalisation was the limiting step in Ni uptake by spinach and tomato from solutions buffered with nitrilotriacetic acid (NTA). The same effects were also found for Thlaspi arvense in soils.[9]
Citrate and histidine are two ligands that play important physiological roles in Zn transport and storage in plants.[10,11] Both ligands are selectively taken up by higher plants[6] and were found to increase Zn uptake by wheat from hydroponic nutrient solutions compared to ethylenediaminetetraacetic acid (EDTA) treatments with the same free and the same total Zn concentrations.[12] EDTA is a very strong ligand for Zn, often used as a ligand to buffer Zn2+ concentrations in solution as its complexes are expected to contribute only a very small extent to Zn uptake.[12–14] Interestingly, histidine increased Zn uptake ~3 times more than citrate, although histidine has a higher binding strength for Zn than citrate. An important difference between Zn complexes with citrate and histidine is that citrate complexes are negatively charged at neutral pH, whereas histidine complexes are mainly neutral and to a minor extent even positively charged at pH 7, according to thermodynamic speciation calculations (using MINEQL). Although suggesting that uptake of intact neutral or positively charged Zn complexes may have contributed to the larger histidine effect, these experiments did not exclude a contribution of complex dissociation assuming that Zn uptake was limited by the rate of Zn supply to the uptake sites.[12] For the common mussel Mytilus edulis direct uptake of positively charged Zn–histidine complexes has already been suggested by Vercauteren and Blust.[15]
To mimic bioavailable fractions in solutions or soils and to study effects of diffusion limitation on metal uptake by plants, experimental methods such as ‘diffusive gradients in thin films’ (DGT) or the ‘permeation liquid membrane’ (PLM) technique may be helpful.[16–18] DGT has often been used to predict metal bioavailability in soils for microorganisms and plants.[2,18,19] However, as small organic complexes can enter the diffusive gel, depending on the pore size,[20] the bioavailable fraction may be overestimated if the complexes are not inert. In addition, if plant uptake mechanisms and not diffusion in the solution are limiting the metal influx, labile complexes are not expected to contribute to the root influx; in DGT measurements however, these complexes dissociate and may overestimate the root influx.[21]
PLM can be used as bio-mimetic membranes, as they are lipophilic and ion transfer requires binding to a carrier molecule (e.g. Kryptofix 22DD and lauric acid). The trans-membrane flux of a trace metal such as Zn is proportional to the concentration of the free ions plus a fraction of the metal complexes in the source solution that depends on the characteristics of the complexes and the specific conditions of the measurement.[22] Complexes passing through the membrane also contribute to PLM-measured metal concentrations. Apart from trans-membrane transport of lipophilic complexes,[23] transport of positively charged complexes is also likely.[24] The important feature of PLM measurements is that the limiting processes are in many respects analogous to those governing metal uptake by living cells.[5,12] PLM has been tested as a sensor for bioavailable cadmium, copper, nickel and lead to microalgae.[25–28] In these studies metal uptake depended on the free metal ion concentration. Recently, however, Aristilde et al.[29] and Xu et al.[30] found that complexes with selected ligands (cysteine, desferrioxamine-B, gluthation, histidine and phytochelatin 2) also contributed to Zn and Cd uptake by phytoplankton. They hypothesised that the complexes enhanced the uptake either by complex dissociation at the cell membranes as a consequence of diffusion limitations of the free metals or – more likely – through the formation of transient ternary complexes with bio-ligands at the uptake sites. Metal uptake by PLM has not yet been compared to metal uptake by roots, although this may give valuable insights into the role of diffusion limitations and the contribution of metal complexes in metal uptake by higher plants in a similar manner as seen for algae.
The objectives of this study were to investigate the role of diffusion limitation in Zn uptake by wheat from solutions containing organic ligands forming complexes with Zn of different binding strength and charge. For this purpose Zn influx into wheat roots was investigated in hydroponics in the presence of citrate, histidine or the strong synthetic ligand EDTA, under stirred and non-stirred conditions and from agar containing nutrient solutions (Fig. 1a). The results were compared to analogous experiments performed with DGT (Fig. 1b) and PLM (Fig. 1c) devices as metal receptors instead of roots. Hydroponics are simple systems with fewer factors influencing metal uptake by plants compared to conventional soil cultures. Although they may neglect some factors influencing metal uptake in soils, studies with hydroponics enable investigation of the different effects of single ligands and the effects of diffusion limitations on metal uptake in much more detail than it would be possible in a complex soil system. The knowledge about specific mechanisms influencing plant uptake contributes to a better understanding of processes also occurring in soil systems.
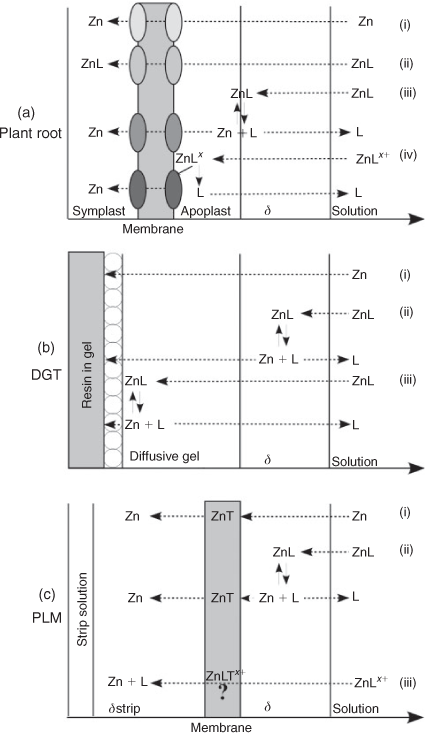
|
Experimental
Experimental treatments
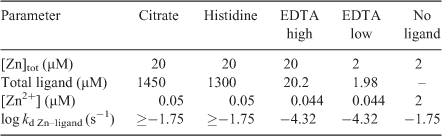
The same experimental solutions were used in the plant, DGT and PLM experiments (Table 1). Each experiment was carried out applying three different treatments that changed the diffusive layer thickness (δ): stirred (0.09 m s–1, always measured at 10 cm from the top, at the edge of the container with a radius of 4 cm and a total height of 20 cm (MiniAir 2, Schiltknecht, Gossau, Switzerland)), non-stirred and non-stirred after addition of extra pure agar (0.15 %) to the experimental solution (Merck). The agar solutions were heated to 90 °C before the other ingredients were added. The agar concentration was chosen such that the solution was at room temperature still liquid enough to immerse the roots without difficulty. For the experimental treatments, the plant roots, PLM and DGT devices were immersed in 1 L of the experimental solution in opaque dark plastic containers to prevent photodegradation of the ligands.
|
Plant experiment
Plant cultivation
Seeds of the wheat cultivar ‘Back Cross (BC) Rushan’ were used (Seed and Plant Improvement Institute (SPII), Karaj, Iran[31]). The plants were germinated on filter paper for 5 days and then hydroponically grown for three weeks in a greenhouse at a 24–14 °C day–night cycle with 16 h of light per day. A 20 % Hoagland nutrient solution (800 µM Ca(NO3)2, 1000 µM KNO3, 400 µM MgSO4, 200 µM KH2PO4, 40 µM NaCl, 20 µM Fe(NO3)3, 20 µM H3BO3, 4 µM MnSO4, 0.4 µM Cu(NO3)2, 0.2 µM Na2MoO4 and 0.2 µM ZnSO4) was used, buffered with 2.5 mM 3-(N-morpholino)propanesulfonic acid (MOPS) and adjusted with 1 M NaOH to a pH between 6.5 and 6.9. During the whole growth period, the solutions were well aerated. All treatments were carried out in three replicates, with four plants per replicate container.
Pre-experimental treatment
Prior to the experiment, the plants were washed for 10 min in ultrapure water and kept for 5 h in a pre-treatment solution consisting of 500 µM KNO3, 400 µM Ca(NO3)2 and 2.5 mM MOPS at a pH of 7.2 (adjusted with 1 M NaOH). MOPS buffer was used in all experimental solutions, because it has no influence on the chemical speciation in the solution.[32]
Experimental treatment
All experimental solutions consisted of a background solution of 500 µM KNO3, 400 µM Ca(NO3)2 and 2.5 mM MOPS at a pH of 7.2 (adjusted with 1 M NaOH) and total Zn concentrations ([Zn]tot) of either 2 or 20 µM. The concentrations of citrate, l-histidine and EDTA required to reach a free Zn concentration ([Zn2+]) of 50 nM with each of these ligands were calculated using MINEQL (Table 1). The solutions were prepared 12 h before the experiment was started. All treatment solutions were labelled with 5.4 µCi L–1 using 65ZnCl2 (Los Alamos National Laboratory, Los Alamos, NM, USA). The labelling was added 2 h before the start of the experiment. Plants were exposed to the treatments for 6 h.
Post-experimental treatment
After the experimental treatments, the plants were washed for 30 s with ice-cold ultrapure water, immersed for 15 min in an ice-cold desorption bath (background solution plus 100 µM ZnSO4) to replace 65Zn adsorbed at the root surface, and washed again for 30 s in ice-cold ultrapure water (adapted from Hart et al.[13] and Panfili et al.[4]). It can be assumed that Zn uptake stopped when the plants were immersed into the ice-cold water, since Hacisalihoglou et al.[14] found that Zn uptake by wheat was negligible from solutions at 2 °C compared to uptake from the same solutions at 23 °C. After the washing procedure, the plants were cut, dried for 5 days at 60 °C and weighed.
65Zn in nutrient solution and plant samples
Root, shoot, experimental solution (at the beginning and at the end of the experiment) and desorption solution samples were analysed for 65Zn using γ-spectrometry (high purity germanium detectors, ORTEC, Oak Ridge, TN, USA, with adjusted calibration for the geometry of the plant and liquid samples). The absolute Zn uptake into the plants during the exposure time was calculated based on the labelled fraction of the total Zn in the nutrient solution and the 65Zn concentration in the plant. The actual fluxes were then calculated by dividing the respective increases in whole-plant contents of Zn during exposure to the experimental solutions by dry root biomass and time of incubation. Fluxes into shoots were calculated by dividing the amount of Zn accumulated in the shoots during exposure by dry shoot biomass and time. The Zn adsorption to the root surface was calculated based on the amount of labelled Zn found in the desorption solution, divided by the root biomass.
Total Zn analysis
Root and shoot samples not exposed to radio-labelled solutions (samples grown under the same conditions as the experimental plants, but harvested just before exposure to the radio-labelled solutions) were ground and digested in 15 mL of 69 % HNO3 in a heating block at 120 °C. Total Zn concentrations of these samples and of the experimental solutions were measured using inductively coupled plasma–optical emission spectroscopy (ICP-OES, Vista-MPX, Varian, Melbourne, Vic., Australia).
DGT experiment
Loaded DGT deployment units (3.14-cm2 window, 0.4-mm Chelex gel, 0.78-mm open pore diffusive gel) were provided by DGT Research Ltd (Lancaster, UK). DGT experiments were carried out in a climate chamber at a constant temperature of 24 °C. DGT devices were exposed to the experimental solutions given in Table 1 for 24 h. After exposure, the binding gels were washed with ultrapure water, separated from the devices and soaked in 1 M ultrapure HNO3 for 24 h. The experimental solutions and the extracts were analysed for Zn using inductively coupled plasma–mass spectrometry (ICP-MS, ICP-MS-920, Varian). Each treatment was performed in three replicates. The DGT available concentration (CDGT) was calculated according to the DGT research manual.[33]
PLM experiment
Hollow fibre PLM (HF-PLM) experiments were set up in the same way as described by Gramlich et al.[24] using a carrier concentration of 0.05 M lauric acid (LA) and 0.1 M Kryptofix 22DD in the organic membrane (1/1, v/v, toluene/hexylbenzene mixture) and PLM membranes of 31-cm length. After 2-h exposure of the PLM membranes to the treatment solutions (Table 1), the Zn concentrations of the strip solutions were measured using ICP-OES (Vista-MPX, Varian). In the experiments with histidine, additional PLM experiments were performed in which the stirring speed was varied over 5 levels from 0 to 0.09 m s–1. Again, each treatment was performed at least in triplicate.
Speciation calculations
The program MINEQL (version 4.6, Environmental Research Software, Hallowell, Maine, US) was used for chemical speciation calculations. Values for Zn complex stability and organic ligand deprotonation constants were taken from the National Institute of Standards and Technology (NIST) database[34] and adjusted to zero ionic strength using the Davies equation.[35]
Statistical treatment of the data
The normality of data distributions was tested separately for each experiment using the Shapiro–Wilk Normality Test. If necessary the data were log-transformed. Treatment effects were analysed by one-way analysis of variance (ANOVA). Significant differences between treatments were determined by pairwise t-tests (two tailed, P-value adjustment method: holm). All statistical analyses were performed using the software package ‘R’, version 2.9.2.[36] If not otherwise stated, effects were considered significant for P ≤ 0.05.
Results
Plant experiments
The eight plant samples not exposed to radioactivity contained total Zn concentrations of 54 ± 4 mg kg–1 in the root biomass and 49 ± 4 mg kg–1 Zn in the shoot biomass, indicating a sufficient level of Zn nutrition. In the absence of organic ligands, Zn fluxes into the roots decreased by a factor of 1.6 from the stirred to the non-stirred and by a factor of 4.4 from the stirred to the agar treatment (Fig. 2a). Similar relative effects were found in the two EDTA treatments, except for a relatively high Zn root influx in the agar treatment with a high concentration of Zn–EDTA complexes. Despite the same nominal [Zn2+] in the two EDTA treatments, Zn fluxes into the roots were always larger (1.5–3 times) when Zn–EDTA complexes were supplied at the higher concentration, although the difference was statistically significant only in the agar treatment.
Systematically higher Zn fluxes into the roots were observed in the presence of the weaker ligands citrate and histidine compared to the two EDTA treatments (Martell et al.[34] estimated dissociation rate constants (kd) in Table S1 and Fig. S1 of the Supplementary material). For both citrate and histidine treatments no significant effects of stirred, non-stirred or agar on the Zn flux into the roots were observed (Fig. 2c). In the case of citrate, the increase was significant compared to both EDTA non-stirred treatments and to the EDTA low agar treatments. The Zn fluxes in the presence of histidine were significantly higher for all treatments than in the presence of EDTA and citrate.
Overall, the accumulation of Zn in the shoots was similarly affected by the treatments as that in the roots. (Fig. 2b,d). In agar, however, root-to-shoot translocation was reduced compared to the stirred and non-stirred solutions in all ligand treatments. This was also the case in the citrate and histidine treatments, in which we found no reduction in root influx. The flux reduction attributable to agar is already clearly evident in Fig. 2 for the citrate and histidine treatments and can be seen even more clearly in Table S3, Supplementary material, where root-to-shoot translocation factors (65Zn activity in shoots ÷ (65Zn activity in shoots + 65Zn activity in roots)) are shown. Fig. S2, however, shows shoot Zn accumulation was more reduced by agar than root influx in the treatments with EDTA and no ligands. The relationship between Zn fluxes into roots (Jroots) and shoots (Jshoots) was not linear, but rather followed a Michaelis–Menten type kinetics, as given in Eqn 1:
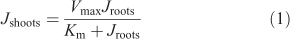
where Vmax is the maximal rate of shoot Zn accumulation and Km is the Zn flux into the roots at half of this maximum rate. Although Km was the same for treatments with and without agar (286 ± 62 nmol g–1 dry weight (DW) h–1), Vmax was twice as high in the stirred and non-stirred treatments (37 ± 3 nmol g–1 DW h–1) than in the agar treatment. In other words, there was a constant ratio between shoot Zn accumulation in the treatments with agar (Jshoots–agar) to those without agar for a given Zn flux into the roots, described by Jshoots–agar = Jshoots–water × 0.49 ± 0.03. Zn uptake (i.e. absorption), however, correlated overall linearly with Zn adsorption to the root surface, with the absorption rate being 4.7 times the adsorption rate (R2 = 0.73) (Fig. S3).
DGT experiment
The Zn fluxes into the DGT devices decreased with similar ratios from the stirred to the non-stirred to the agar treatment as the fluxes into the roots in the absence of ligands or presence of Zn–EDTA complexes (Figs 3a, 4a–c). In a similar manner as for root uptake, Zn fluxes into DGT were larger when more Zn–EDTA complexes were present (Fig. 3a), but the difference was larger than in the plant experiments and significant for stirred, non-stirred and agar treatments. The DGT-measured available Zn concentration (CDGT) in the presence of EDTA was on average 4 times higher than the nominal [Zn2+] in the EDTA low treatment and 16 times higher in the EDTA high treatment under stirred conditions (Table S2).
In contrast to the Zn fluxes into the roots, the stirred, non-stirred and agar treatments had similar relative effects on DGT Zn fluxes in the presence of citrate and histidine complexes as in the absence of ligands or in the presence of EDTA (Fig. 3a,b). In further contrast to the plant experiment, the citrate and histidine treatments resulted in much larger DGT Zn fluxes under stirred conditions than the treatment with no ligands. Comparing respective CDGT and [Zn]tot in the citrate and histidine treatments shows 76 and 88 % of [Zn]tot were DGT-available under stirred conditions. Although the absolute fluxes into the DGT devices correlated closely with plant uptake when no ligands or just Zn–EDTA complexes were added, independent of stirred, non-stirred or agar treatment, no such correlation was found for the citrate and histidine treatments (Fig. 5a). Using the relationship determined from the other treatments, DGT fluxes would in general greatly over predict Zn uptake by the plants.
PLM experiment
The non-stirred and agar treatments in the absence of ligands or in the presence of Zn–EDTA complexes produced very similar effects as in the plant and DGT experiments (Figs 3c, 4a–c). Compared to the treatments with no ligands, however, PLM Zn fluxes in the EDTA treatments were more reduced than those into roots and DGT devices (Figs 2a, 3a,c). Again, Zn fluxes were significantly higher in the treatments with high rather than low Zn–EDTA complex concentrations, despite the same nominal free Zn concentration. As for the DGT Zn fluxes, there was a close common log–log relationship between the Zn fluxes into PLM and into the roots in the no ligand and EDTA treatments (Fig. 5b).
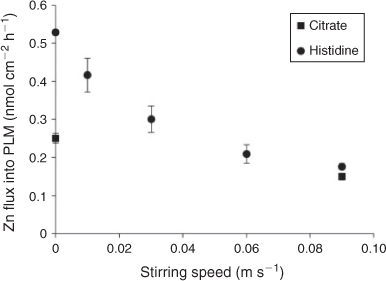
In the stirred treatments with citrate and histidine, the PLM Zn fluxes were significantly higher than in the EDTA low treatment and of similar magnitude as in the EDTA high treatment. In contrast to the no ligand and EDTA treatments, PLM Zn fluxes significantly increased going from stirred to non-stirred and agar conditions in the citrate and histidine treatments (Fig. 3d). This is also the opposite to what was observed for the DGT Zn fluxes (Fig. 3b), whereas the effects of non-stirred and agar application (relative to the stirred treatment) on root uptake of Zn in the presence of citrate or histidine were in between those of PLM and DGT fluxes (Fig. 4d). The additional PLM experiment with different stirring rates confirmed the finding of lower PLM Zn fluxes with stirring than with no stirring, giving a smooth inverse relationship between stirring speed and PLM Zn flux (Fig. 6).
|
The PLM Zn fluxes were higher under non-stirred and agar conditions in the histidine than in the citrate treatments, but not significantly different under stirred conditions. This means that the absence of stirring and the application of agar enhanced the PLM Zn flux much more in the presence of Zn histidine than in the presence of Zn–citrate complexes (Fig. 3d). The effects of these treatments were also much stronger on PLM Zn fluxes than on root Zn uptake in the presence of histidine (Fig. 4e). Comparing conditions yielding the same PLM fluxes, plant Zn uptake was 2 times higher in the presence of citrate and 3–4 times higher in the presence of histidine than predicted for the no ligand and EDTA treatments (Fig. 5b).
Discussion
Zn fluxes into plant roots, DGT and PLM in the absence of ligands and in the presence of EDTA
In the absence of organic ligands, Zn influx into roots was reduced to 63 % in the non-stirred and to 23 % in the agar treatment compared to the stirred treatment. This reduction suggests that the flux was limited by Zn diffusion in the solution and not by plant uptake kinetics under the conditions of our experiments, in agreement with the findings of Degryse et al.[8] for Zn uptake by spinach and tomato. In a previous study, Degryse et al.[18] estimated the diffusion layer thickness (δstirred) at the root surface of spinach and wheat to be 0.35 mm in stirred solutions. Assuming that [Zn2+] at the root surface was negligible compared to that of the bulk solution and assuming furthermore that the difference in [Zn2+] between the bulk soil solution and root surfaces was the same in all treatments, δ would be expected to be inversely proportional to the diffusive root influx according to Fick’s law.[18] δnon-stirred would therefore equal δstirred ÷ 0.63 = 0.56 mm (calculations of Degryse et al.[18] resulted in a δnon-stirred of 0.6 mm). δagar is consequently estimated to be 1.5 mm.
The hypothesis that Zn uptake by the roots was limited by reduced Zn diffusion in the solution because of increased diffusion layer thickness in the non-stirred and agar treatments is further supported by the fact that DGT and PLM fluxes were reduced to a similar degree as plant influx by the same treatments (Fig. 4a). The much lower Zn fluxes in the EDTA treatments are in line with the common notion that Zn uptake by plants is dominated by [Zn2+] and that Zn–EDTA complexes were too stable to make a substantial contribution to it. However, the fact that higher Zn fluxes were observed in all three systems for the higher concentration of Zn–EDTA complexes at the same nominal [Zn2+] indicates that some complex dissociation may have occurred, contributing a measurable, although small flux to the uptake (or binding) sites of the experimental receptors. As the results of the high EDTA concentration treatments were in all three systems greater than in the low EDTA concentration treatment, it is unlikely that an experimental error caused the differences we found. It is also unlikely that it was attributable to cell membrane disruption by EDTA, as the EDTA concentrations applied here were too low to cause such an artefact according to the work of other authors.[37,38]
The results are in agreement with the findings of Degryse et al.,[21] who found that DGT fluxes positively correlated with plant uptake and, although not being proportional to the predicted free Cd concentration, were inversely related to complex stability in the presence of the synthetic ligands trans-1,2-cyclohexandiaminetetraacetic acid (CDTA), ethylene glycol tetraacetic acid (EGTA), (hydroxyethyl)ethylenediaminetriacetic acid (HEDTA) and NTA. This means that DGT can be used to give estimations of the bioavailable metal fractions in chelate-buffered solutions as long as the complexes are stable enough.[21] Our results show that the same is also valid for the PLM method. The fact that log–log regression gave better descriptions of the relationships between root Zn uptake and the fluxes into the two bio-mimetic devices than ordinary linear regression indicates that plant uptake was governed by kinetics with influx saturation at high Zn concentrations in solution.
Zn fluxes into plant roots, DGT and PLM in the presence of citrate and histidine
In contrast to the experiments with EDTA or no ligands, there were large differences between the plant and the DGT system in their responses to the experimental treatments when citrate or histidine was added. The findings that CDGT was close to [Zn]tot in the bulk solution under stirred conditions in the presence of citrate and histidine and that the DGT Zn fluxes were affected in the same way by the change in diffusive layer thickness as in the absence of ligands, indicates that diffusion was the limiting process. It also indicates that there was little kinetic limitation in complex dissociation in the diffusive layer at the resin surface because of the high binding strength of the resin (Fig. 1b(iii)). Apart from the kinetic limitation in complex dissociation, a reason for the small differences between CDGT and [Zn]tot may also have been that diffusion of the Zn–ligand complexes was slower than the diffusion of the free Zn ions within the diffusive gel.
In contrast to the DGT experiments, increasing the diffusion layer thickness by omission of stirring or adding agar did not decrease Zn fluxes into the roots in the plant experiment in the presence of citrate and even increased them in the PLM experiment. An important difference between these systems is that for Zn the DGT resin represents an essentially infinite and instantaneous sink, where virtually all citrate and histidine complexes dissociate, whereas there is a membrane-transfer step in the plant and PLM systems, which partially limits the Zn uptake rate. This partial limitation of trans-membrane transport provides an explanation as to why the slower diffusion in the less mixed systems did not result in reduced Zn fluxes into PLM and into roots (Figs 2c, 3d). It shows that in plant and PLM systems complex dissociation kinetics were more relevant than diffusion limitations of [Zn]tot. Such partial membrane transfer limitations had already been observed in a previous study, where Zn fluxes into wheat roots from solutions containing citrate were found to be much lower than the fluxes from solutions with the same [Zn]tot, but no ligands, under otherwise similar conditions.[12]
The fact that the differences in Zn fluxes between EDTA and citrate treatments increased when the solutions were less mixed indicates that dissociation of Zn–citrate complexes contributed substantially to plant Zn uptake under conditions with no mixing, but much less so when the diffusion layer was reduced (Figs 2a,c, 3c,d). It implies that trans-membrane transfer was more limiting than free Zn diffusion under stirred conditions, however, the less the systems were mixed, the more diffusion and complex dissociation became rate limiting. Trans-membrane transfer of negatively charged complexes can be excluded in the PLM system.[25,39,40] The similarity of the stirred, non-stirred and agar treatment effects on the Zn fluxes in the presence of Zn–citrate complexes in the plant and the PLM systems thus suggests that for these effects to occur there was no requirement of uptake of undissociated complexes (Fig. 4d).
Although the relative effects of the stirred, non-stirred and agar treatments were similar on Zn influx into roots and PLM Zn flux, the relationship between absolute root Zn and PLM Zn flux in the presence of citrate was enhanced compared to the respective relationship in the presence of EDTA or no ligands (Fig. 5b(ii)). A possible explanation for this difference may be that the root apoplast represents a diffusional barrier that comes in addition to the stagnant boundary layer between the bulk solution and root surface, which cannot be reduced by stirring, similar to the diffusive gel layer in the DGT system. As discussed before, reduced diffusion would have made the contribution of complex dissociation to Zn fluxes more important and increased the contribution of labile complexes more than that of stabile complexes. Another difference between PLM and plant roots is that additional ligands exuded by the roots may have been present in the plant experiments. Their effect, however, was probably negligible in this short-term experiment in comparison to the high concentrations of externally applied ligands.
Similar reasoning as for the influence of citrate on the Zn fluxes into plant and PLM systems should also apply for histidine. However, in the presence of histidine the Zn fluxes into plant roots and PLM were even larger than in the presence of citrate (Fig. 2c), and PLM Zn fluxes showed a much stronger increase with reduced stirring than in the respective citrate treatments (Fig. 6), even though Zn–histidine complexes have higher stability constants than Zn–citrate complexes.[22,34] The higher Zn fluxes could still be explained by complex dissociation of Zn–histidine complexes, if we assume that the dissociation rate of Zn–histidine complexes was higher than that of Zn–citrate complexes despite the higher stability constant.[3] Unfortunately, we found no published values of the dissociation rate constants for Zn–histidine and Zn–citrate complexes under similar conditions, and attempts to test this assumption experimentally failed because both dissociation reactions were too fast (Table S1 and Fig. S1 of the Supplementary material).
A high kd of Zn–histidine complexes alone would not be sufficient, however, to explain the observed increase in PLM Zn fluxes with reduced stirring. In addition a change in chemical equilibrium in the diffusion layer at the PLM surface would be required to actually increase Zn fluxes with reduced mixing and not just to buffer them by compensatory complex dissociation. For a decrease in total Zn flux from stirred to non-stirred treatments by 36 % (average decrease in Zn PLM fluxes in EDTA and no ligand treatments) we calculated a free Zn concentration at the PLM surface that is higher by ~40 % than the one in the bulk solution, assuming that the concentration of histidine would decrease by the same degree. This is a reasonable assumption as the diffusion coefficients of histidine and Zn are very similar.[41] Although such a change in equilibrium conditions may explain a substantial fraction of the increase in PLM Zn fluxes with reduced stirring rate, it is not sufficient to explain it completely.
Another possibility to explain the high Zn fluxes in the presence of histidine is that the increased fluxes into plant roots in comparison to the citrate treatments were attributable to uptake of intact neutral or positively charged Zn–histidine complexes (80 % of the complexes are neutral and 20 % were positively charged). Neutral complexes may enter plant cells through aquaporines or specific transporters for the free ligands.[6,42,43] Positively charged complexes have been found to enhance metal uptake by the formation of ternary complexes with bio-ligands at the membrane uptake sites.[29] Also in the PLM system it may be possible that direct contribution of positively charged complexes occurs, as they may form ternary complexes with the carrier molecules (Fig. 1c(iii)). These ternary complexes may also partially explain the increased Zn flux we found in the agar solution (Fig. 6), as their formation may be hindered to some extent under stirred conditions.
In a previous study, over a large concentration range, a systematically enhanced flux was measured compared to the calculated [Zn2+] in the presence of histidine and based on that it was assumed that a fraction of the positively charged Zn–histidine complexes influenced the result.[24] Uptake of intact complexes by plants may also explain why histidine caused a larger deviation than citrate between measured Zn fluxes into plants and predictions based on the relationship between root and PLM Zn fluxes in the EDTA and no ligand treatments (Fig. 5b(i)), whereas it cannot be excluded on the other hand that faster complex dissociation was at least partially responsible also for this difference between histidine and citrate treatments.
The overall ligand concentrations applied in this study were higher than the concentrations normally observed in soil solutions.[44,45] However, the idea of this study was to test the role of diffusion limitation, complex dissociation and intact complex uptake on Zn uptake by plants and to use citrate and histidine as examples of many other carboxylic and amino acids. For this purpose a large part of the Zn needed to be complexed, so rather high ligand concentrations were needed.
Root-to-shoot translocation of Zn in stirred, non-stirred and agar treatments
Even though no significant differences were found in root uptake of Zn between the stirred, non-stirred and agar treatments when citrate and histidine were present, root-to-shoot translocation in all ligand treatments was reduced by the agar (Figs 2d, S2). Although the agar probably reduced the transpirational water stream to and through the plants due to increased resistance to flow, this should not have substantially affected root-to-shoot translocation of Zn if the limiting step in Zn uptake was diffusion to or cellular uptake in the roots. The fact that Zn root-to-shoot translocation followed Michaelis–Menten-type kinetics suggests that it was governed by an enzymatic reaction. Similar saturation effects in Zn root-to-shoot translocation have also been found by Kalis et al.[46] The agar treatment did not change the type of this relationship but just decreased the maximum rate by half, which would be expected if small units of agarose polymers would somehow block access to half of the uptake sites and thus not only reduce diffusion in the rhizosphere but also the rate-limiting uptake capacity in the roots.
Conclusions
In treatments with no ligands or EDTA, diffusion of [Zn2+] was mainly limiting for Zn uptake by wheat and the fluxes could be well described by DGT and PLM measurements. The lack of a decrease in Zn root influx in the non-stirred and agar as compared to the stirred treatments with citrate and histidine suggests that reduced diffusion was compensated for by increased Zn supply resulting from complex dissociation. This hypothesis is supported by the fact that analogous effects were found with the PLM system, where uptake of entire negatively charged complexes can be excluded. It is therefore likely that complex dissociation also played a role for Zn uptake in the presence of histidine. However, as Zn influx into plants and PLM was higher in the presence of histidine than of citrate it is likely that here the uptake of neutral or positively charged Zn–histidine complexes also contributed to Zn uptake.
Acknowledgements
This work was funded by the Swiss National Science Foundation (project number: IZ70Z0_123920/1).
References
[1] I. Cakmak, Enrichment of cereal grains with zinc: agronomic or genetic biofortification? Plant Soil 2008, 302, 1.| Enrichment of cereal grains with zinc: agronomic or genetic biofortification?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXltVKn&md5=27be8ffa8cd92ee7f20ab68e76c43a87CAS |
[2] P. Wang, D. M. Zhou, X. S. Luo, L. Z. Li, Effects of Zn-complexes on zinc uptake by wheat (Triticum aestivum) roots: a comprehensive consideration of physical, chemical and biological processes on biouptake. Plant Soil 2009, 316, 177.
| Effects of Zn-complexes on zinc uptake by wheat (Triticum aestivum) roots: a comprehensive consideration of physical, chemical and biological processes on biouptake.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFOksb0%3D&md5=3fa4c254762261ff3d70885450238803CAS |
[3] F. Degryse, E. Smolders, D. R. Parker, Metal complexes increase uptake of Zn and Cu by plants: implications for uptake and deficiency studies in chelator-buffered solutions. Plant Soil 2006, 289, 171.
| Metal complexes increase uptake of Zn and Cu by plants: implications for uptake and deficiency studies in chelator-buffered solutions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1WnsrrJ&md5=2ab1bee8951b5045e7b800cde62b21c2CAS |
[4] F. Panfili, A. Schneider, A. Vives, F. Perrot, P. Hubert, S. Pellerin, Cadmium uptake by durum wheat in presence of citrate. Plant Soil 2009, 316, 299.
| Cadmium uptake by durum wheat in presence of citrate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFOks70%3D&md5=8961b24154691787d851300e1f48d0bbCAS |
[5] S. P. Stacey, M. J. Mclaughlin, I. Cakmak, G. M. Hetitiarachchi, K. G. Scheckel, M. Karkkainen, Root uptake of lipophilic zinc–rhamnolipid complexes. J. Agric. Food Chem. 2008, 56, 2112.
| Root uptake of lipophilic zinc–rhamnolipid complexes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXisFWkt74%3D&md5=b7cf034895786ea6c38976da615f93f4CAS | 18303840PubMed |
[6] P. F. Bell, M. J. Mclaughlin, G. Cozens, D. P. Stevens, G. Owens, H. South, Plant uptake of 14C-EDTA, 14C-Citrate, and 14C-Histidine from chelator-buffered and conventional hydroponic solutions. Plant Soil 2003, 253, 311.
| Plant uptake of 14C-EDTA, 14C-Citrate, and 14C-Histidine from chelator-buffered and conventional hydroponic solutions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXlsFKru7c%3D&md5=6d1ed1052c3560c7bbfd9cd522376cdeCAS |
[7] N. von Wiren, H. Marschner, V. Römheld, Roots of iron-efficient maize also absorb phytosiderophore-chelated zinc. Plant Physiol. 1996, 111, 1119.
| 1:CAS:528:DyaK28XltVGgt7k%3D&md5=6b488d3f655812300305965104cfd0b5CAS | 12226351PubMed |
[8] F. Degryse, A. Shahbazi, L. Verheyen, E. Smolders, Diffusion limitations in root uptake of cadmium and zinc, but not nickel, and resulting bias in the Michaelis constant. Plant Physiol. 2012, 160, 1097.
| Diffusion limitations in root uptake of cadmium and zinc, but not nickel, and resulting bias in the Michaelis constant.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsFaktr7M&md5=ef43a3dc4de3a88407da18013d0bd94dCAS | 22864584PubMed |
[9] J. Luo, H. Zhang, F. J. Zhao, W. Davison, Distinguishing diffusional and plant control of Cd and Ni uptake by hyperaccumulator and nonhyperaccumulator plants. Environ. Sci. Technol. 2010, 44, 6636.
| Distinguishing diffusional and plant control of Cd and Ni uptake by hyperaccumulator and nonhyperaccumulator plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXps1Wht7s%3D&md5=f9a1d47d8eee55c473405168993b070dCAS | 20681510PubMed |
[10] M. J. Haydon, C. S. Cobbett, Transporters of ligands for essential metal ions in plants. New Phytol. 2007, 174, 499.
| Transporters of ligands for essential metal ions in plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXmsFOktL0%3D&md5=107c82aaf766b12b82f77bc74a3296e6CAS | 17447906PubMed |
[11] P. J. White, M. R. Broadley, Biofortification of crops with seven mineral elements often lacking in human diets – iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49.
| Biofortification of crops with seven mineral elements often lacking in human diets – iron, zinc, copper, calcium, magnesium, selenium and iodine.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXksVKhtbw%3D&md5=3537142b40d8f44313dfa2eb85e747ddCAS | 19192191PubMed |
[12] A. Gramlich, S. Tandy, E. Frossard, J. Eikgenberg, R. Schulin, Availability of zinc and the ligands citrate and histidine to wheat – does uptake of entire complexes play a role? J. Agric. Food Chem. 2013, 61, 10 409.
| Availability of zinc and the ligands citrate and histidine to wheat – does uptake of entire complexes play a role?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsFGhu7%2FI&md5=3dc86e0d52189e9dc93caac8e0867f1aCAS |
[13] J. J. Hart, W. A. Norvell, R. M. Welch, L. A. Sullivan, L. V. Kochian, Characterization of zinc uptake, binding, and translocation in intact seedlings of bread and durum wheat cultivars. Plant Physiol. 1998, 118, 219.
| Characterization of zinc uptake, binding, and translocation in intact seedlings of bread and durum wheat cultivars.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXmtV2mu78%3D&md5=2411dc9f25b8233dc00a8d5df2a429aaCAS | 9733541PubMed |
[14] G. Hacisalihoglu, J. J. Hart, L. V. Kochian, High- and low-affinity zinc transport systems and their possible role in zinc efficiency in bread wheat. Plant Physiol. 2001, 125, 456.
| High- and low-affinity zinc transport systems and their possible role in zinc efficiency in bread wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXjslyls7Y%3D&md5=b6a93958483064f4c8202a58e8359f19CAS | 11154353PubMed |
[15] K. Vercauteren, R. Blust, Bioavailability of dissolved zinc to the common mussel Mytilus edulis in complexing environments. Mar. Ecol. Prog. Ser. 1996, 137, 123.
| Bioavailability of dissolved zinc to the common mussel Mytilus edulis in complexing environments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XlsVSnu7c%3D&md5=1008b4a982c98c9dd071d72830e16ed0CAS |
[16] H. P. van Leeuwen, Metal speciation dynamics and bioavailability: Inert and labile complexes. Environ. Sci. Technol. 1999, 33, 3743.
| Metal speciation dynamics and bioavailability: Inert and labile complexes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXlvV2jtLc%3D&md5=01ba596cdb30a5d9cfe36b128bff313cCAS |
[17] A. L. Nolan, H. Zhang, M. J. Mclaughlin, Prediction of zinc, cadmium, lead, and copper availability to wheat in contaminated soils using chemical speciation, diffusive gradients in thin films, extraction, and isotopic dilution techniques. J. Environ. Qual. 2005, 34, 496.
| Prediction of zinc, cadmium, lead, and copper availability to wheat in contaminated soils using chemical speciation, diffusive gradients in thin films, extraction, and isotopic dilution techniques.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXislOlt7k%3D&md5=9bc9af38a7305afc565f526799d99660CAS | 15758102PubMed |
[18] F. Degryse, E. Smolders, D. R. Parker, An agar gel technique demonstrates diffusion limitations to cadmium uptake by higher plants. Environ. Chem. 2006, 3, 419.
| An agar gel technique demonstrates diffusion limitations to cadmium uptake by higher plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtlWnur7P&md5=eb10b7e8ea83523a9cf93ec67258e2adCAS |
[19] H. Zhang, W. Davison, Direct in situ measurements of labile inorganic and organically bound metal species in synthetic solutions and natural waters using diffusive gradients in thin films. Anal. Chem. 2000, 72, 4447.
| Direct in situ measurements of labile inorganic and organically bound metal species in synthetic solutions and natural waters using diffusive gradients in thin films.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXls1Oku70%3D&md5=19d8be12ffc8f144ae87bb282d8200d2CAS | 11008782PubMed |
[20] S. Scally, W. Davison, H. Zhang, Diffusion coefficients of metals and metal complexes in hydrogels used in diffusive gradients in thin films. Anal. Chim. Acta 2006, 558, 222.
| Diffusion coefficients of metals and metal complexes in hydrogels used in diffusive gradients in thin films.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XotFygtw%3D%3D&md5=d4fec5b95bb959613edea7d541b63742CAS |
[21] F. Degryse, E. Smolders, H. Zhang, W. Davison, Predicting availability of mineral elements to plants with the DGT technique: a review of experimental data and interpretation by modelling. Environ. Chem. 2009, 6, 198.
| Predicting availability of mineral elements to plants with the DGT technique: a review of experimental data and interpretation by modelling.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXht1CjurfI&md5=cafd8c0632f34b93501644322053cd5cCAS |
[22] H. P. van Leeuwen, R. M. Town, J. Buffle, R. F. M. Cleven, W. Davison, J. Puy, W. H. Van Riemsdijk, L. Sigg, Dynamic speciation analysis and bioavailability of metals in aquatic systems. Environ. Sci. Technol. 2005, 39, 8545.
| Dynamic speciation analysis and bioavailability of metals in aquatic systems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtVOis73M&md5=784d2d8f64142e8b9e859ade0577a2a7CAS | 16323747PubMed |
[23] N. Parthasarathy, M. Pelletier, J. Buffle, Transport of lipophilic ligands through permeation liquid membrane in relation to natural water analysis. J. Membr. Sci. 2008, 309, 182.
| Transport of lipophilic ligands through permeation liquid membrane in relation to natural water analysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXitVan&md5=bc651bdd77f28995430a6e3632d88e59CAS |
[24] A. Gramlich, S. Tandy, V. I. Slaveykova, A. Duffner, R. Schulin, The use of permeation liquid membranes for free zinc measurements in aqueous solution. Environ. Chem. 2012, 9, 429.
| The use of permeation liquid membranes for free zinc measurements in aqueous solution.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhslSmtrrJ&md5=58493c35db9c4e0ba6d87bfb331750edCAS |
[25] V. I. Slaveykova, N. Parthasarathy, J. Buffle, K. J. Wilkinson, Permeation liquid membrane as a tool for monitoring bioavailable Pb in natural waters. Sci. Total Environ. 2004, 328, 55.
| Permeation liquid membrane as a tool for monitoring bioavailable Pb in natural waters.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXltVWjt7g%3D&md5=d0dcf2ae211dfe466fd8df23922c1e53CAS | 15207573PubMed |
[26] S. Bayen, I. Worms, N. Parthasarathy, K. Wilkinson, J. Buffle, Cadmium bioavailability and speciation using the permeation liquid membrane. Anal. Chim. Acta 2006, 575, 267.
| Cadmium bioavailability and speciation using the permeation liquid membrane.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XnvVSku7o%3D&md5=c43506c704171eb3f6547351cd12a1dbCAS | 17723601PubMed |
[27] S. Bayen, K. J. Wilkinson, J. Buffle, The permeation liquid membrane as a sensor for free nickel in aqueous samples. Analyst (Lond.) 2007, 132, 262.
| The permeation liquid membrane as a sensor for free nickel in aqueous samples.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXitVOksrk%3D&md5=a01410b6bef1e00da51b9c5a910c93bbCAS |
[28] V. I. Slaveykova, I. B. Karadjova, M. Karadjov, D. L. Tsalev, Trace metal speciation and bioavailability in surface waters of the Black Sea coastal area evaluated by HF-PLM and DGT. Environ. Sci. Technol. 2009, 43, 1798.
| Trace metal speciation and bioavailability in surface waters of the Black Sea coastal area evaluated by HF-PLM and DGT.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhvVylurk%3D&md5=7354b21bab99fc5a2bce6e172adc080aCAS | 19368174PubMed |
[29] L. Aristilde, Y. Xu, F. M. M. Morel, Weak organic ligands enhance zinc uptake in marine phytoplankton. Environ. Sci. Technol. 2012, 46, 5438.
| Weak organic ligands enhance zinc uptake in marine phytoplankton.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xltl2ht7c%3D&md5=7368eb9754a3c109609836c5be8ec301CAS | 22494184PubMed |
[30] Y. Xu, D. Shi, L. Aristilde, F. M. M. Morel, The effect of pH on the uptake of zinc and cadmium in marine phytoplankton: possible role of weak complexes. Limnol. Oceanogr. 2012, 57, 293.
| The effect of pH on the uptake of zinc and cadmium in marine phytoplankton: possible role of weak complexes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XivVarsLk%3D&md5=c3a9aef48cac08b4d4e2b2457a8fbbd5CAS |
[31] A. H. Khoshgoftarmanesh, A. Sadrarhami, H. R. Sharifi, D. Afiuni, R. Schulin, Selecting zinc-efficient wheat genotypes with high grain yield using a stress tolerance index. Agron. J. 2009, 101, 1409.
| Selecting zinc-efficient wheat genotypes with high grain yield using a stress tolerance index.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsV2jtLrI&md5=7f20607f76e1f2fa23461b7abe32f0e4CAS |
[32] A. Kandegedara, D. B. Rorabacher, Noncomplexing tertiary amines as “better” buffers covering the range of pH 3–11. Temperature dependence of their acid dissociation constants. Anal. Chem. 1999, 71, 3140.
| Noncomplexing tertiary amines as “better” buffers covering the range of pH 3–11. Temperature dependence of their acid dissociation constants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXjvFams7o%3D&md5=7d9b389eade8d690f822a92bfbad391aCAS | 21662904PubMed |
[33] DGT Research Manual DGT for measurement in waters, soils and sediments 2003 (DGT Research Ltd: Quernmore, UK). Available at http://www.dgtresearch.com/WebProducts.aspx?CATID=TEC [Verified 4 December 2013].
[34] A. E. Martell, R. M. Smith, R. J. Motekaitis, NIST critically selected stability constants of metal complexes, NIST Standard Reference Database 46 2001 (US Department of Commerce: Gaithersburg, MD).
[35] M. R. Twiss, O. Errecalde, C. Fortin, P. G. C. Campbell, C. Jumarie, F. Denizeau, E. Berkelaar, B. Hale, K. Van Rees, Coupling the use of computer chemical speciation models and culture techniques in laboratory investigations of trace metal toxicity. Chem. Spec. Bioavail. 2001, 13, 9.
| Coupling the use of computer chemical speciation models and culture techniques in laboratory investigations of trace metal toxicity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXotFGqsb8%3D&md5=55da72c4ea93b5f1bf1dd65be0b0904bCAS |
[36] R Development Core Team, R: a language and environment for statistical computing 2009 (R Institute for Statistical Computing: Vienna, Austria). Available at http://www.R-project.org [Verified 17 December 2012].
[37] M. Häussling, C. A. Jorns, G. Lehmbecker, C. Hechtbuchholz, H. Marschner, Ion and water-uptake in relation to root development in norway spruce (Picea abies (L.) Karst.). J. Plant Physiol. 1988, 133, 486.
| Ion and water-uptake in relation to root development in norway spruce (Picea abies (L.) Karst.).Crossref | GoogleScholarGoogle Scholar |
[38] A. D. Vassil, Y. Kapulnik, I. Raskin, D. E. Salt, The role of EDTA in lead transport and accumulation by Indian mustard. Plant Physiol. 1998, 117, 447.
| The role of EDTA in lead transport and accumulation by Indian mustard.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXjvFGks7w%3D&md5=699a3158f631e7d5e7a5803ede597b44CAS | 9625697PubMed |
[39] N. Parthasarathy, M. Pelletier, J. Buffle, Hollow fiber based supported liquid membrane: a novel analytical system for trace metal analysis. Anal. Chim. Acta 1997, 350, 183.
| Hollow fiber based supported liquid membrane: a novel analytical system for trace metal analysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXlsVWgt70%3D&md5=3ddce79b138fac208b384d5830daab79CAS |
[40] N. Parthasarathy, M. Pelletier, J. Buffle, Permeation liquid membrane for trace metal speciation in natural waters – transport of liposoluble CuII complexes. J. Chromatogr. A 2004, 1025, 33.
| Permeation liquid membrane for trace metal speciation in natural waters – transport of liposoluble CuII complexes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXpvVWrtrw%3D&md5=1992381afff275c0acbeeb94c7a45ec8CAS | 14753668PubMed |
[41] J. Buffle, Z. Zhang, K. Startchev, Metal flux and dynamic speciation at (Biol.)interfaces. part 1: critical evaluation and compilation of physicochemical parameters for complexes with simple ligands and fulvic/humic substances. Environ. Sci. Technol. 2007, 41, 7609.
| Metal flux and dynamic speciation at (Biol.)interfaces. part 1: critical evaluation and compilation of physicochemical parameters for complexes with simple ligands and fulvic/humic substances.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXht1ymtr3E&md5=bb9e478acf30e32ed4514934dc0fe993CAS | 18075065PubMed |
[42] C. Maurel, M. J. Chrispeels, Aquaporins. A molecular entry into plant water relations. Plant Physiol. 2001, 125, 135.
| Aquaporins. A molecular entry into plant water relations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXjslymur4%3D&md5=be27252c3467007e51e797185da1e88aCAS | 11154316PubMed |
[43] H. Svennerstam, S. Jamtgard, I. Ahmad, K. Huss-Danell, T. Nasholm, U. Ganeteg, Transporters in Arabidopsis roots mediating uptake of amino acids at naturally occurring concentrations. New Phytol. 2011, 191, 459.
| Transporters in Arabidopsis roots mediating uptake of amino acids at naturally occurring concentrations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtFSqtr%2FF&md5=900a2876c8cc9bb436cc160fa0ec8627CAS | 21453345PubMed |
[44] D. L. Jones, Organic acids in the rhizosphere – a critical review. Plant Soil 1998, 205, 25.
| Organic acids in the rhizosphere – a critical review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXhtlGjs78%3D&md5=c4c36a5b71f655c2908e55f121734685CAS |
[45] D. L. Jones, D. Shannon, T. Junvee-Fortune, J. F. Farrarc, Plant capture of free amino acids is maximized under high soil amino acid concentrations. Soil Biol. Biochem. 2005, 37, 179.
| Plant capture of free amino acids is maximized under high soil amino acid concentrations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXovVKntLc%3D&md5=8591b8d95d05f4411d442f8a46318441CAS |
[46] E. Kalis, E. Temminghoff, L. Weng, W. Van Riemsdijk, Effects of humic acid and competing cations on metal uptake by Lolium perenne. Environ. Toxicol. Chem. 2006, 25, 702.
| Effects of humic acid and competing cations on metal uptake by Lolium perenne.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XitVaju7s%3D&md5=45cf472b4c3218394952ef3e10203956CAS | 16566154PubMed |






