Photolysis and TiO2-catalysed degradation of diclofenac in surface and drinking water using circulating batch photoreactors
Devagi Kanakaraju A , Cherie A. Motti B , Beverley D. Glass A and Michael Oelgemöller A CA School of Pharmacy and Molecular Sciences, James Cook University Townsville, Qld 4811, Australia.
B Australian Institute of Marine Science (AIMS), Biomolecular Analysis Facility Townsville, Qld 4810, Australia.
C Corresponding author. Email: michael.oelgemoeller@jcu.edu.au
Environmental Chemistry 11(1) 51-62 https://doi.org/10.1071/EN13098
Submitted: 20 May 2013 Accepted: 17 November 2013 Published: 19 February 2014
Environmental context. Diclofenac, a common non-steroidal anti-inflammatory drug, is not completely removed from surface and drinking water by conventional treatment methods. Consequently, this drug is present in the aquatic environment and has been subsequently linked to toxic effects on organisms. We show that photolysis and TiO2-catalysed degradation in circulating batch reactors efficiently results in diclofenac removal under a variety of conditions. These photochemical methods thus may lead to more effective water treatment processes.
Abstract. The occurrence of diclofenac (DCF) as an emerging pollutant in surface waters and drinking water has been attributed to elevated global consumption and the inability of sewage treatment plants to remove DCF. In this study, DCF spiked drinking water and river water was subjected to photolysis and TiO2 photocatalytic treatments in a circulating laboratory-scale (immersion-well) and a demonstration-scale loop reactor (Laboclean). The operational parameters for the immersion-well reactor were optimised as follows: TiO2 P25 loading, 0.1 g L–1; natural pH, 6.2; initial concentration, 30 mg L–1; water type, distilled water. Complete DCF removal was realised within 15 min under the optimised conditions using the immersion-well reactor. Sunlight-mediated photochemical degradation required a prolonged exposure period of up to 360 min for complete DCF removal. DCF in distilled and drinking water was efficiently degraded in the larger Laboclean reactor. Differences were, however, observed based on their pseudo-first-order rate constants, which implies that the water matrix has an effect on the degradation rate. Six major photoproducts, 2-(8-chloro-9H-carbazol-1-yl)acetic acid, 2-(8-hydroxy-9H-carbazol-1-yl)acetic acid, 2,6-dichloro-N-o-tolylbenzenamine, 2-(phenylamino)benzaldehyde, 1-chloromethyl-9H-carbazole and 1-methyl-9H-carbazole, generated from TiO2 photocatalysis of DCF were identified by liquid chromatography–mass spectrometry (LCMS) and Fourier transform–ion cyclotron resonance–mass spectrometry (FT-ICR-MS). This work has shown that photocatalytic degradation kinetics of DCF are dependent on both the geometry of the photoreactor and the nature of the water matrices.
Additional keywords: advanced oxidation processes, pharmaceuticals, photocatalysis, titanium dioxide.
Introduction
Pharmaceuticals and personal care products have been frequently detected in waterways in trace levels ranging from parts per trillion (ppt, ng L–1) to parts per billion (ppb, µg L–1).[1,2] The presence of pharmaceuticals in the water cycle largely arises from their excretion either as metabolites or in their unmetabolised form. Pharmaceuticals and their active pharmaceutical ingredients (APIs) are generally designed to be both highly active and stable to efficiently execute a specific physiological action in humans and animals.[3] Their incomplete removal by wastewater treatment plants (WWTPs) thus represents an important urban source of these pharmaceuticals and their APIs in the aquatic environment.[4,5] In view of this, pharmaceutical abatement requires innovative technologies to combat their continued presence in the environment.[6] Advanced oxidation processes (AOPs) have been proposed as a superior technology over that of conventional water treatment methodologies for the removal of recalcitrant pharmaceuticals and other endocrine-disrupting chemicals.[7–9] All AOPs are based on the in situ generation of highly reactive and short-lived reactive oxygen species such as HO•, H2O2, O3 and O2•– for the mineralisation of organic compounds.[10] Among various AOPs, TiO2 photocatalysis has been extensively studied for the photodecomposition of a variety of pharmaceuticals used for different therapeutic effects.[11–14] One of the most widely consumed class of drugs globally are non-steroidal anti-inflammatory drugs (NSAIDs), which are primarily used to reduce inflammation.[15] DCF (2-[(2,6-dichlorophenyl)-amino]phenylacetic acid, diclofenac) a commonly used NSAID, is important environmentally because of its frequent detection in water bodies,[16,17] groundwater aquifers[18] and WWTP effluents[19] and its toxic effects on aquatic and terrestrial organisms.[20,21] In particular, DCF has displayed the highest acute toxicity in comparison to other NSAIDs.[22] A concentration of 11 454 µg L–1 was reported to cause 50 % of effect (EC50) on the Microtox system over 30 min.[23] Its removal efficiencies also greatly vary between 0 and 80 % depending on the operating conditions of WWTPs such as sunlight exposure or acidic conditions.[23,24] A recent study by Salgado et al. highlighted inefficient removal of DCF by WWTPs.[25] The prevalence of DCF in the environment has resulted in increased interest to combat its further accumulation in the environment.
The degradation of DCF has been investigated both under direct photolysis[26–29] and by TiO2 photocatalysis.[13,30–34] Existing studies describing photolysis either in the laboratory or in the natural environment using sunlight have reported direct photolysis as the main depletion mechanism for DCF. Discrepancies have, however, arisen between studies on the effect of artificial UV light on DCF phototransformations. For example, Méndez-Arriaga et al. observed up to 75 % DCF degradation,[13] whereas Calza et al. reported an insignificant photolysis of DCF.[34] Typical parameters, which control the photocatalytic behaviour of DCF, include TiO2 loading, initial concentrations, effect of oxidants and water matrices. Despite this, there is little known about the photocatalytic treatment of DCF in drinking water (tap water, TW) although monitoring studies have highlighted the presence of this API in this water source.[35] Furthermore, it is important to investigate surface water, especially river water (RW), which is one of the main sources of TW. Application of different photoreactor configurations for simultaneous pilot and laboratory scale investigations has largely led to non-systematic comparisons.[36] Also, there is no clear consensus on the superiority of direct photolysis over TiO2 photocatalysis or vice versa for DCF degradation, given that performance is greatly affected by the oxidation conditions used. Further studies are therefore necessary to examine the effects of these photochemical methods on DCF degradation.
The main objective of this study was to perform direct photolysis and TiO2-catalysed UV oxidation (UV/TiO2) of DCF, using two circulating photoreactors of different volumes equipped with medium pressure (MP) UV lamps. Illuminations with natural sunlight were furthermore conducted in the smaller circulating reactor. Degradation kinetics, extent of mineralisation with two indicators, dissolved organic carbon (DOC) and chemical oxygen demand (COD), and the identification of intermediates were also the focus of this study.
Experimental
Materials
Diclofenac sodium salt was purchased from Sigma–Aldrich (R&D grade) and was used without further purification. High-performance liquid chromatography (HPLC; Agilent Technologies Australia Pty Ltd, Melbourne) and Nuclear Magnetic Resonance (NMR; Varian Oxford 300 with the Varian Software VnmrJ Revision D, Agilent Technologies Australia) analyses showed no impurities. Titanium dioxide P25 Aeroxide (80 % anatase and 20 % rutile, Brunauer–Emmett–Teller (BET) surface area 50 m2 g–1) was supplied by Evonik industries whereas titanium(IV) oxide (99.8 % metal basis, anatase) was purchased from Aldrich and used for comparison. HPLC grade methanol and glacial acetic acid were obtained from RCI Labscan. Hydrogen peroxide (H2O2, 30 %) was obtained from Univar. Laboratory grade distilled water (DW) was used for all photodegradation studies, whereas Milli-Q water (Milli-Q system, Millipore) was used for the preparation of working standards and the mobile phase for HPLC analyses. RW was sampled from the Ross River (19°18′59″S, 146°45′7″E) in Townsville in October 2012 and was stored at 4 °C before irradiation and analysis. Unfiltered RW samples were used for the solar photodegradation studies in order to mimic environmentally relevant conditions. TW samples were freshly sampled from the laboratory at James Cook University. Table 1 displays the selected water quality properties of TW and RW.

|
Photoreactors
Two types of circulating batch photoreactors, an advanced laboratory-scale immersion-well reactor and a novel demonstration-scale loop reactor were used in this study. The smaller immersion-well photoreactor (Heraeus UV-RS-1, Hanau, Germany, length of 38.4 cm with an optical path <2 cm) was made of Pyrex glass (cut-off wavelength ≤290 nm) and is shown in Fig. 1. The reactor was filled with 400 mL of DCF solution (C0 = 30 mg L–1) or suspensions of TiO2/DCF and irradiated with a MP Hg vapour lamp (TQ 150 Heraeus, Germany, 150 W). The reactor was equipped with a powerful magnetic circulation system, which pumped the reaction mixture turbulently through the attached side-arm. This feature provided very effective circulation and mixing, even under heterogeneous conditions. In contrast, most common batch reactors experience poor mixing, especially in the narrow space between the sides of the outer reactor vessel and the inner immersion-well. Chilled water was used to prevent the lamp from overheating. The reaction set-up was placed behind a UV-shield and maintained inside a light-tight fume cabinet.

|
The larger loop reactor (Laboclean, Tandem UV, a.c.k. aqua concept GmbH, Karlsruhe, Germany) was fitted with both low pressure (LP, 40 W) and MP (500 W) mercury lamps in separate horizontal chambers (Fig. 2). The lamps were housed in Quartz mantles, whereas the outer chambers were made of Pyrex glass. In this study, only the top MP mercury lamp was used. A 6-L solution was filled into the external reservoir tank and continuously pumped through the system for 10 min. The liquid streamed tangentially through the UV chambers creating a screw-like flow. This pattern enabled high mass transfer and high turbulence, which avoided sedimentation of photocatalyst. A cooling jacket, which was cooled with chilled water, was placed at the end of the irradiation chambers on top of the reservoir. Measured temperatures of solution in the reservoir did not exceed 35 °C. The circulating reaction medium provided cooling for the lamps. The UV modules were placed behind a large UV-shield and the reactor loop behind a light tight curtain. Samples were withdrawn before commencing irradiation and at selected times throughout the irradiation period. The MP mercury lamp in both reactors emits polychromatic light in a variety of wavelengths including 254, 313 and 366 nm in the UV region and 405, 436, 546 and 578 nm in the visible region.[37]

|
Dark adsorption study
An adsorption study was conducted to evaluate the extent of DCF adsorption on the TiO2 surface in the dark. Adsorption experiments were conducted using 100 mL of DCF solution of different concentrations (10–60 mg L–1) and 0.1 g L–1 of TiO2 P25. The suspensions were mixed using an orbital shaker (Stuart Model SSL1) under continuous agitation at 230 rpm and exclusion of light for 24 h. Adsorption experiments were also conducted at different pH values (3–11). The equilibrium concentrations were determined by HPLC after filtration through a 0.22-µm syringe filter. Details on the HPLC method applied are provided below.
Photocatalysis and photolysis using an immersion-well reactor equipped with a MP mercury lamp
In a typical irradiation experiment, a suspension of DCF (30 mg L–1) and TiO2 (0.1 g L–1) was stirred at 1000 rpm in the dark for 30 min to establish an adsorption–desorption equilibrium. The temperature in the reaction solution did not exceed 29 °C and all reactions took place at pH ~6.2, with the exception of the pH studies. Samples were withdrawn at specified time intervals and filtered through a 0.22-µm syringe filter before the concentration of DCF was determined by HPLC. Direct photolysis experiments were similarly performed, but excluding the photocatalyst. To optimise the process, initial DCF concentrations (10–60 mg L–1), amounts of TiO2 (0.01–2.0 g L–1) and pH (4.9–10.8) were varied. For the pH study, the pH of each DCF solution was adjusted with dilute NaOH or HCl and measured using an UB-10 model pH-meter (Denver Instrument) before addition of TiO2. The pH of each suspension was determined again after TiO2 addition. All analyses were carried out in duplicate to confirm reproducibility.
Photocatalysis and photolysis using an immersion-well reactor under sunlight
Solar DCF degradation studies were performed on sunny days outside the laboratory in October 2012 using the same immersion-well reactor, but without the immersed MP lamp. This strategy allowed for a direct comparison with studies conducted under laboratory conditions. All solar photochemical experiments were carried out at James Cook University (latitude 19°19′42″S and longitude 146°45′36″E) in Townsville, Australia. Reaction conditions optimised for the indoor experiments with artificial light were applied. The adsorption–desorption process was allowed to equilibrate in the dark for 15 min before the first sample was withdrawn in the laboratory. This process allowed determination of the amount of DCF adsorbed before solar exposure. Cooling water was circulated from a reservoir using a pump driven by a solar panel to avoid the necessity of electricity. Samples were withdrawn at regular intervals during the 6 h of solar exposure. The intensity of light was recorded using an auto digital Luxmeter (Model 1010A) and the data were converted into watts per square metre. Table 2 summarises the experimental details for each solar study conducted.
Photocatalysis and photolysis using a loop reactor equipped with a MP mercury lamp
DCF concentrations (30 mg L–1) and TiO2 amount (0.1 g L–1) were used for experiments in the larger Laboclean reactor. In addition to photolytic and photocatalytic studies with TW, DW spiked with DCF was also investigated to determine the effect of the water matrix. In addition, the effect of adding H2O2 was evaluated for the photocatalytic degradation of DCF in DW and TW.
Analytical methods
The degradation kinetics of DCF was monitored using HPLC. A Varian 940-LC instrument equipped with a photodiode array (PDA) detector, Galaxie Chromatography data system software and an autosampler was employed. Millipore membrane type FH filters with a pore diameter of 0.45 μm (Millipore, Ireland) were used to filter the mobile phase, a mixture of 80 % (v/v) methanol and 20 % water (pH 3 adjusted with glacial acetic acid). A 20-µL aliquot of sample from the photoreactor was injected onto a C18 Phenomenex column (150 × 4.6 mm, 2.6 µm) with a flow rate of 0.5 mL min–1. The absorbance was monitored on a UV-Vis spectrophotometer (Model Varian 50 Bio) with Cary WIN UV Scan software at 274 nm, corresponding to the maximum DCF absorbance. A total organic carbon (TOC) analyser (Model Shimadzu 5000 A) equipped with an ASI-5000A autosampler was used to quantify DOC in the filtered samples (0.45-µm GF/F filter). COD analysis was carried out using the open reflux titrimetric method, according to the Standard Methods for the Examination of Water and Wastewater.[38] The concentration of chloride ion was measured using ion chromatography (ICS 2100 Dionex). An anion column (AS 19; 4 × 250 mm) and conductivity detector with suppression was used. Identification of photoproducts was achieved using an Agilent 1100 HPLC system comprising a degasser, auto injector, binary pump and PDA connected to a Bruker Esquire3000 ion trap mass spectrometer with an Apollo electrospray ionisation source (ESI) ion source operating in either positive or negative mode (ESI+/ESI–). All liquid chromatography–mass spectrometry (LCMS) data were collected using Bruker Daltonics Esquire Control v5.3 and Hystar v3.1 (Bruker Daltonics, Melbourne) operating on Windows XP Professional. The DCF standard (0.5 mg mL–1; 10 μL) and UV/TiO2 irradiated sample (0.5 mg mL–1; 100 μL) were injected onto a C18 Phenomenex column (150 × 4.6 mm, 2.6 μm) and eluted using the same conditions as described above and monitored at a wavelength of 220 nm. MS analysis was performed using the following conditions: nebuliser gas 138 kPa (20 psi), drying gas 6.0 L min–1 and drying temperature 350 °C. The presence of the parent and product ions was determined by monitoring the base peak chromatogram (BPC). High resolution MS data were acquired on a Bruker BioApex 47 Fourier transform ion cyclotron resonance mass spectrometer with an ESI Analytica of Branford source (Bruker Daltonics, Billerica, CA, USA). Both the DCF standard and irradiated samples were analysed in either ESI+/ESI– mode within a mass range of m/z 50–2000 by direct infusion of the diluted LCMS sample (1 : 100 methanol) at a flow rate of 1.7 µL min–1.
Results and discussion
Dark adsorption study
The effect of different initial concentrations of DCF (10–60 mg L–1) in the presence of 0.1 g L–1 TiO2 P25 was investigated. A maximum extent of 2.2 ± 0.8 to 10.8 ± 1.6 % was observed for DCF at the end of the adsorption period. This low adsorption is in accordance to dark adsorption studies of DCF on TiO2 as reported by Rizzo et al.[32]
Photophysical properties of DCF
A comparison of the absorption spectrum of DCF and the radiant flux distribution of the TQ-150 lamp is shown in Fig. 3. Light below 290 nm is filtered when Pyrex glass is used in the immersion-well photoreactor. In water, the maximum absorbance of DCF was found to be at 274 nm, with a shoulder absorbance up to ~320 nm, the latter allowing for direct sunlight photolysis. The extinction coefficient for DCF at 274 nm (ϵ274nm) in water was calculated to be 13 340 M–l cm–l.[39]

|
Direct photolysis using an immersion-well equipped with a MP mercury lamp
Photolysis of DCF solutions at initial concentrations ranging between 10 and 60 mg L–1 was investigated. The levels chosen for the photolysis study were higher than the typical background concentration of DCF present in the environment, however, this strategy allowed for the detection and identification of photodegradants without pre-concentration. As expected, UV photolysis resulted in a more rapid DCF degradation at lower concentrations (10 and 30 mg L–1) compared to that at higher concentrations (50 and 60 mg L–1) (Fig. 4). Degradation was accompanied by a significant decrease in pH because of the dehydrochlorination of DCF leading to the formation of hydrochloric acid and, after further degradation, other organic acids such as formic and oxalic acid.[40] In contrast to the rapid disappearance of DCF, DOC was removed by less than 10 % over the chosen concentration range indicating the presence of stable degradation products, which are not further oxidised under direct photolysis. The photolysis of DCF has been previously studied by Martínez et al. who found a rapid degradation upon UV irradiation with a first-order rate constant of 0.40 ± 0.02 min–1.[41] A recent study also reported a high degradation rate for DCF on UV irradiation, emitted from a MP mercury lamp.[39] Another study, however, reported an insignificant degradation efficiency upon direct photolysis.[34] These somewhat contradictory results may be attributed to differences in the radiation intensity from the chosen light sources and variations in the water matrices.[1] In particular, natural organic matter present in the water can compete for light absorption. Direct photolysis is thus not a suitable technology for the complete degradation of DCF. TiO2 photocatalysis may offer a more appropriate alternative for water treatment instead.

|
Photocatalytic degradation using an immersion-well reactor equipped with a MP mercury lamp
Effect of TiO2 loading
TiO2 concentrations ranging between 0.01 and 2 g L–1 were examined for a 30-mg L–1 DCF model solution. Photocatalytic degradation rates were determined after 30 min of pre-adsorption in the dark. In the absence of light, no noticeable degradation took place indicating the requirement of external light to initiate the degradation process. Concentrations of 0.01 and 0.1 g L–1 of TiO2 led to complete degradation of DCF within 30 min of irradiation, whereas higher loadings of 1 and 2 g L–1 resulted in almost complete degradation only after 60 min of irradiation (Fig. 5). Coefficients of determination (R2) ranging from 0.96 to 0.99 confirmed that the experimental data fit pseudo-first-order kinetics (Table 3).

|

|
In the present study, the addition of 0.01 g L–1 of TiO2 P25 produced the lowest remaining DCF concentration followed by a concentration of 0.1 g L–1 TiO2, which resulted in the same degradation within 15 min of irradiation. Higher doses of TiO2 clearly retarded the degradation rate because of the scattering of light and agglomeration of TiO2. Above the optimal loading, degradation rates decreased with higher catalyst concentrations.[42] Similar findings were reported for the antibiotic oxolinic acid, where an increase above 1 g L–1 led to a decrease in the API removal.[43]
Although the highest photocatalytic efficiency was achieved with 0.01 g L–1, further experiments were carried out with the mid-level concentration of 0.1 g L–1. This concentration is commonly applied with TiO2 P25, although loadings can vary from 0.1 to 5.0 g L–1.[42] A loading above 1.0 g L–1 was not considered for further studies because of the lower degradation rate caused by increased sedimentation, which could not be avoided despite stirring at 1000 rpm. The optimum dose of photocatalyst represents a critical parameter in the photocatalytic degradation process. Inconsistencies in optimum TiO2 loadings reported have been linked to the geometry of the chosen photoreactor and the nature of the compound under investigation.[44,45] Thus far, the optimal TiO2 loading for DCF photocatalysis reported in related studies was 1,[41] 0.624 [34] and 0.25 g L–1.[31] The much lower TiO2 loading determined in this study can be attributed to the very effective circulation in the immersion-well setup chosen, which ensured optimal distribution of photocatalyst throughout the entire reaction mixture (Fig. 1).
Effect of DCF concentrations
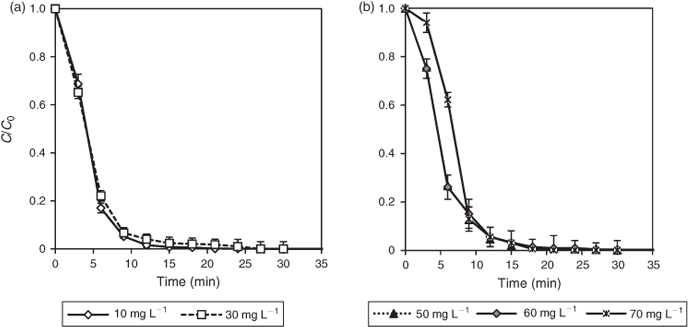
Fig. 6 shows the time-course of DCF degradation at different concentrations. The reaction followed pseudo-first-order kinetics.[46] Complete degradation was achieved for low range initial concentrations, 10 and 30 mg L–1 (Fig. 6a), within 30 min, whereas for higher ranges, 50 to 70 mg L–1 (Fig. 6b), comparable irradiation times resulted in the degradation of ~98–99 % of initial DCF.
At a low DCF concentration, interactions between DCF and the TiO2 surface decrease, which causes a decrease in the degradation rate (Table 4). The initial degradation rate (r0) increases until 60 mg L–1 and the values decrease thereafter becoming independent of initial concentrations. Higher concentrations of DCF resulted in occupancies of more TiO2 active sites, which retarded the generation of HO• or other oxidants. DCF molecules also absorbed more photons directly and thus led to a decrease in the available photons to activate the TiO2 surface.[47] The results are similar to a study on atenolol, where the same trend was observed.[48]

|
Effect of solution pH
The complex electrostatic interaction between semiconductor, solvent, substrate and charged radicals formed during the treatment make the pH an important reaction parameter. pH studies were thus conducted to determine the extent of degradation based on the ionisation state of the photocatalysts and DCF. The adsorption of compounds is determined by the electric charge of both the catalysts and substrate. The surface of TiO2 is amphoteric resulting in adsorption being affected by pH. The zero point charge (pHzpc) of TiO2 has been determined as 6.25 resulting in a positive surface charge below pHpzc (pH < pHzpc) and negative surface charge above pHpzc (pH > pHzpc).[49] The pKa value of DCF (carboxyl group) is known to be 4.15 (at 25 °C).[50] At a pH < pKa, DCF is present in its neutral or protonated form, whereas at a pH > pKa it predominantly exists in deprotonated form, i.e. as a negatively charged carboxylate. The initial pH values of the solutions were adjusted to 3.0, 4.9, 5.6, 7.8 and 10.8 to represent three conditions: strongly acidic, neutral and strongly basic. The degradation rate at different initial pH values as a function of irradiation time is illustrated in Fig. 7. The DCF experiment performed at pH 3 did not result in any degradation (result not shown). This may be attributed to the insolubility of DCF in water at pH 3, as DCF is known to become practically insoluble below a pH of 4.[50] DCF degradation studies conducted with other AOPs such as photo-Fenton and ozonation have also been conducted under slightly acidic conditions (pH 5.0–6.0).[28,50,51] The adsorption decreased to 20.2 % at pH 5 and then to 6.5 % at pH 11, because of the electrostatic repulsion effects of the negatively charged DCF and the TiO2 surface.

|
DCF degradation is not significantly affected by pH as the degradation reached 100 % after 30 min under all conditions. A pseudo-first order degradation rate was also observed for initial pH values and the k values obtained are shown in Table 5. Comparison of the initial degradation rate at the pHs investigated showed that an increase in pH from acidic to strongly alkaline caused a decrease in the degradation rate. An initial pH beyond 5.6 inhibited the degradation rate, whereas the highest initial rates of 0.27 and 0.26 min–1 resulted at pH values of 5.6 and 7.8. At an inherent pH (~6.2), a slightly higher rate of 0.30 min–1 was obtained, compared to that at other pH values. These results demonstrate that a pH adjustment is not necessary prior to the photocatalytic degradation of DCF. The photocatalytic degradation of oxytetracycline was also reported to be removed in solution without pH modification.[52]

|
The change in pH was monitored throughout the course of the irradiation reaction. The pH values after 2 h of irradiation changed to pH < 4 for the acidic and neutral conditions tested, whereas for the basic conditions a pH < 6 was recorded. These reductions in pH were observed as a result of the formation of hydrochloric acid and other mineral or carboxylic acids.
Effect of cooling jacket material and TiO2 type
The effect of a quartz immersion-well as well as another commercially available titania, TiIV oxide (Aldrich) was compared to that of a Pyrex cooling jacket and TiO2 P25 respectively. As expected, rapid DCF degradation occurred with the quartz immersion-well with both TiO2 P25 and TiIV oxide photocatalysts (Fig. 8). Because the cut-off wavelength of quartz glass is as low as λ < 200 nm, the intensive 254-nm emission from the MP lamp is likely to provide more energy-rich photons available for direct photolysis (Fig. 3).[37] A comparable degradation rate was observed for TiIV oxide (k = 0.29 ± 0.12, R2 = 0.96) and the standard TiO2 P25 (k = 0.27 ± 0.06, R2 = 0.97). Initially, 3 min of photolysis with 0.1 g L–1 TiIV oxide and TiO2 P25 led to 50 and 35 % degradation. Complete degradation was achieved after 30 min of irradiation with both TiO2 materials. The efficiency of both TiO2 materials can be attributed to their catalytic activity. Pure anatase has a higher density of superficial hydroxyl groups compared to the pure rutile form.[53] In contrast, TiO2 P25, a combination of anatase and rutile, promotes charge pair separation and inhibits electron–hole recombinations.[31]

|
Solar degradation of DCF with immersion-well reactor
The efficiency of TiO2 photocatalysis for DCF removal was more apparent under solar irradiation. The results confirm our previous findings for DCF degradation under natural and artificial sunlight where TiO2 photocatalysis also accelerated the degradation process of DCF.[30] For experiments conducted with DW, greater DCF degradation resulted from the TiO2-mediated process, compared to direct photolysis (Fig. 9). Degradation of more than 90 % was observed over the first 180 min in the presence of TiO2, but slowed thereafter as a result of interference or inner filter effects of intermediates generated during the course of irradiation.[51] At least 6 h of exposure was required to achieve maximum DCF removal under natural sunlight for both conditions. In contrast, only 30 min was needed for complete removal under laboratory conditions. These results clearly indicate differences between artificial UV irradiation and natural sunlight. The lamp provided a constant photon supply in the UV range, whereas for sunlight only 5 % of the total solar flux is in the required UV or near-UV region (300 to 400 nm) for TiO2 photocatalysis.[54] In addition, radiation in both direct and diffuse forms is discontinuous for sunlight and depends on weather and operation time (Table 2). In terms of COD removal, the photocatalytic process conducted with DW achieved 40 and 55 % for DCF initial concentrations of 10 and 60 mg L–1 with artificial UV light. In contrast, measured COD for DW under natural sunlight showed 39 and 44 % removal for direct photolysis and photocatalysis. As with the laboratory experiments, the pH values during photolysis and photocatalysis with sunlight decreased constantly (Table 2). Similar observations were reported for the photolytic degradation of DCF in demineralised water as a result of hydrochloric acid release.[50]

|
Water matrix effect
In order to assess the effect of the water matrix, solutions of DCF in TW and RW were exposed to sunlight. Interestingly, solar photolysis produced higher degradation rates compared to solar TiO2 photocatalysis for these water matrices (Fig. 9). A comparison of degradation kinetics for TW and RW is shown in Fig. 10. RW containing DCF was only degraded up to 66 % upon photocatalysis (k = 0.0028 ± 0.0012 min–1, R2 = 0.96), whereas 82 % degradation was achieved under direct sunlight (k = 0.0048 ± 0.0014 min–1, R2 = 0.99). Likewise, a total of 84 and 53 % of removal was accomplished with TW under direct photolysis (k = 0.0051 ± 0.0009 min–1, R2 = 0.99) and photocatalysis (k = 0.015 ± 0.006 min–1, R2 = 0.97). A deficiency of HO• radicals and other oxidising species caused by the presence of naturally occurring organic matter, anions and bicarbonates in RW and TW can be attributed to the retarded photocatalytic process.[55] The hardness of the sampled RW and TW measured as CaCO3 was 151.6 and 41.8 mg L–1, respectively classified as hard and soft. Inorganic anions such as Cl– and SO42– were also observed (Table 1) as they are common in real waters. Radical scavenging as a result of the presence of such compounds has been linked to a decrease in various photocatalytic degradation studies, in particular when raw water samples were used.[11,56] However, COD was removed up to 46.7 % upon photocatalysis compared to only 23.3 % under direct photolysis, indicating the presence of considerable amounts of carbonaceous materials, which had not been completely oxidised and mineralised to CO2 and H2O. The pH of the RW samples was not significantly affected throughout the course of the irradiation (Table 2), which suggests a buffering capacity of natural freshwater as already proposed by Agüera and co-workers.[57] In contrast to previous findings, a higher magnitude of degradation was attained with our experimental set-up. The composition of the chosen freshwater samples, irradiance levels of sunlight and the type of photoreactor all contributed to these differences. Solar photodegradation is known to be dependent on factors such as light intensity, water matrix, season and latitude.[58]

|
Photocatalysis and photolysis of DCF using a loop reactor
The laboratory-scale Laboclean reactor was used for the photochemical and photocatalytic degradation of DCF in DW and TW (Fig. 2). The degradation reasonably fitted pseudo-first-order kinetics (R2 > 0.98) (Table 6). DCF spiked in DW was degraded at a higher rate in the presence of 0.1 g L–1 TiO2, compared to direct photolysis (Fig. 11). DCF was removed within 60 min under TiO2 photocatalysis, whereas direct photolysis took up to 120 min for 90 % removal. The addition of 250 mg L–1 H2O2 significantly enhanced the degradation of DCF, which was completed within 30 min of irradiation.

|

|
In the experiments with TW, both direct photolysis and TiO2 photocatalysis were also found to be efficient in completely degrading DCF (Fig. 12). A comparison of rate constants, however, showed that DCF degradation was slightly faster by direct photolysis (k = 0.10 ± 0.03 min–1) than TiO2 photocatalysis (k = 0.09 ± 0.02 min–1). The other advanced oxidation method tested used a combination of UV, TiO2 and H2O2, and increased the degradation rate significantly to 0.19 min–1.

|
Enhancement of degradation in the presence of H2O2 during the TiO2 photocatalytic process in both water matrices was attributed to the generation of more HO• radicals (Eqn 1). In addition, the reactor material (quartz mantle) does allow direct cleavage of H2O2 (UV–H2O2). H2O2 can also act as an electron acceptor inhibiting the recombination of electron–hole pairs.[59]

In addition to focussing on the disappearance of the DCF parent molecule, the determination of the degree of mineralisation in terms of DOC is crucial to evaluate the effectiveness of the photochemical and TiO2 photocatalytic treatments employed. TiO2 photocatalysis led to a slightly higher DOC removal than direct photolysis both in TW and DW (Fig. 13). The DOC removal profiles of direct photolysis in these water matrices were rather similar and both proceeded at slow rates yielding a maximum removal of 27 %. Although direct photolysis was capable of degrading the parent DCF, this oxidation method was less efficient in oxidising the intermediates and degradation products formed. Within the experimental time frame of 3 h, TiO2 photocatalysis contributed to a slightly higher DOC removal both in DW and TW by increasing the removal by 6–7 %. Stable carboxylic acid-derived photoproducts generated might prevent a higher DOC removal. In contrast, TiO2 photocatalytic oxidation in the presence of H2O2 corresponded to the highest DOC removal in TW with 70 %. The removal profile showed that DOC removal took place constantly over the entire irradiation time of 3 h. Thus, in addition to H2O2 efficiently degrading DCF, it also considerably increased the DOC removal.

|
Photocatalytic transformation products
A DCF sample taken after 2 h of irradiation was analysed for photoproducts by LCMS. LCMS analysis was performed by applying a simple isocratic mode, which resulted in better resolution of DCF and its photoproducts than the gradient modes commonly applied in other studies.[34,41] Table 7 summarises the six major photoproducts identified. Subsequent Fourier-transform–ion cyclotron resonance–mass spectrometry (FT-ICR-MS) analysis (characteristic isotope distributions and the accurate mass determination) confirmed the structures of the photoproducts as shown in the proposed degradation pathway (Fig. 14 and Table 7).[60] All photoproducts identified appeared at shorter retention times (tR) than the parent DCF peak suggesting the formation of more hydrophilic photoproducts. Loss of HCl and subsequent photocyclisation of DCF resulted in carbazole C1 (tR = 6.1 min), 2-(8-chloro-9H-carbazol-1-yl)acetic acid. The degradation proceeded with subsequent photosubstitution of the remaining Cl group in C1 with a hydroxy group to C2 (tR = 4.4 min), another carbazole, 2-(8-hydroxy-9H-carbazol-1-yl)acetic acid. The presence of intermediates C1 and C2 were confirmed in both negative and positive ESI modes. These carbazole intermediates have also been identified as major products in a photolytic degradation of DCF in methanol[40] and have been linked to an increase in phototoxicity.[41] Decarboxylation of DCF led to the formation of C3, 2,6-dichloro-N-o-tolylbenzenamine (tR = 6.8 min), which further underwent dechlorination and oxidation to generate intermediate C4, 2-(phenylamino) benzaldehyde (tR = 4.4 min). Intermediate C3 also underwent loss of HCl to form C5, 1-chloromethyl-9H-carbazole (tR = 6.1 min). Intermediate C5 could be alternatively formed as a result of decarboxylation from C1. The loss of the second Cl from C5 and photoreduction resulted in the generation of intermediate C6, 1-methyl-9H-carbazole (tR = 4.9 min). Intermediates C3–C6 were identified in ESI negative mode. All intermediates found in this study were identical to those reported by Martínez et al. except for [2-(2,6-dichlorophenylamino)phenyl]methanol.[41] This compound, proposed as an intermediate in the transformation from C3 to C4, was undetectable, possibly because of its rapid oxidation under the conditions investigated. These intermediates, including the release of HCl and CO2, also explain the observed decrease in pH: formation of hydrochloric acid (Fig. 14, conversion of DCF into C1 and into C5 by way of C3) and other organic acids (Fig. 14, conversion of DCF into C1 and C2). Chloride evolution indeed increased with prolonged irradiation time as depicted in Fig. 15.

|
Conclusion
Photochemical and TiO2 photocatalytic degradation of DCF, using two circulating batch reactors resulted in different rates of DCF degradation for both the photolysis and TiO2 photocatalysis experiments, which is attributed to whether TW or surface water was used. This shows the importance of the water quality in influencing the degradation rate of DCF. In addition, the differences between studies performed using artificial light and sunlight in the immersion-well reactor highlights the effect of the different light source on the degradation of DCF. Optimisation studies for TiO2 photocatalysis demonstrated that although TiO2 concentrations, DCF concentrations and the nature of the glass (Pyrex or quartz) affect the degradation rate of DCF, pH had no major effect. Maximum degradation of DCF was obtained in the presence of 0.1 g L–1 TiO2 P25 and in suspension, without pH adjustment. The larger loop-reactor completely degraded DCF from solution whereas addition of H2O2 enhanced the degradation rate of DCF both in DW and TW. Higher DOC removal was also obtained in TW in the presence of H2O2 compared to TiO2 photocatalytic oxidation. However, incomplete mineralisation, as evident from incomplete removal of DOC and COD, suggests that DCF and its photoproducts were not completely transformed to CO2 and H2O within the time frame of the irradiations. Six photoproducts of DCF photocatalysis were identified and a mechanism for their formation is proposed. This study has demonstrated that both direct photolysis and TiO2-catalysed oxidation have great potential to degrade DCF in both TW and surface water. In conclusion, although AOP processes offer an attractive option for wastewater treatment, the complex nature of real effluents with co-existing pollutants and higher levels of organic matter call for combinations with other AOP methods such as ozonation or photo-Fenton to enhance the degradation.
Acknowledgements
This work was supported by research grants from James Cook University (FAIG award 2009 and GRS awards 2011 and 2012). D. Kanakaraju also thanks the Malaysian Government for a University Doctorate Training Award. The authors thank Evonik industries for the donation of Titanium dioxide P25 Aeroxide and Mr Stephen Boyle (AIMS) for TOC analyses.
References
[1] K. Kümmerer, The presence of pharmaceuticals in the environment due to human use – present knowledge and future challenges. J. Environ. Manage. 2009, 90, 2354.| The presence of pharmaceuticals in the environment due to human use – present knowledge and future challenges.Crossref | GoogleScholarGoogle Scholar | 19261375PubMed |
[2] I. Sirés, E. Brillas, Remediation of water pollution caused by pharmaceutical residues based on electrochemical separation and degradation technologies: a review. Environ. Int. 2012, 40, 212.
| Remediation of water pollution caused by pharmaceutical residues based on electrochemical separation and degradation technologies: a review.Crossref | GoogleScholarGoogle Scholar | 21862133PubMed |
[3] B. Halling-Sørensen, S. N. Nielsen, P. F. Lanzky, F. Ingerslev, H. C. H. Lützhøft, S. E. Jørgensen, Occurrence, fate and effects of pharmaceutical substances in the environment – a review. Chemosphere 1998, 36, 357.
| Occurrence, fate and effects of pharmaceutical substances in the environment – a review.Crossref | GoogleScholarGoogle Scholar | 9569937PubMed |
[4] P. Verlicchi, M. Al Aukidy, E. Zambello, Occurrence of pharmaceutical compounds in urban wastewater: removal, mass load and environmental risk after a secondary treatment – a review. Sci. Total Environ. 2012, 429, 123.
| Occurrence of pharmaceutical compounds in urban wastewater: removal, mass load and environmental risk after a secondary treatment – a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xos1Cntbo%3D&md5=c5333dabb60ace66f22dd2fb29a5bc7eCAS | 22583809PubMed |
[5] S. Mompelat, B. Le Bot, O. Thomas, Occurrence and fate of pharmaceutical products and by-products, from resource to drinking water. Environ. Int. 2009, 35, 803.
| Occurrence and fate of pharmaceutical products and by-products, from resource to drinking water.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXlsl2gt7c%3D&md5=e4f4fe6161d419df5f20d9fc94098ca3CAS | 19101037PubMed |
[6] A. M. Deegan, B. Shaik, K. Nolan, K. Urell, M. Oelgemöller, J. Tobin, A. Morrissey, Treatment options for wastewater effluents from pharmaceutical companies. Int. J. Environ. Sci. Technol. 2011, 8, 649.
| Treatment options for wastewater effluents from pharmaceutical companies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtVSqt77M&md5=d6a9040453622eb3dd5c2b584b987d07CAS |
[7] A. Y. C. Tong, R. Braund, D. S. Warren, B. M. Peake, TiO2-assisted photodegradation of pharmaceuticals – a review. Cent. Eur. J. Chem. 2012, 10, 989.
| TiO2-assisted photodegradation of pharmaceuticals – a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XotVajs74%3D&md5=69ad501dfa9096427870b19d81cb2bc5CAS |
[8] O. K. Dalrymple, D. H. Yeh, M. A. Trotz, Removing pharmaceuticals and endocrine-disrupting compounds from wastewater by photocatalysis. J. Chem. Technol. Biotechnol. 2007, 82, 121.
| Removing pharmaceuticals and endocrine-disrupting compounds from wastewater by photocatalysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXis1yrtr0%3D&md5=7ca0e047b5aa5cedc293b7677f411ad2CAS |
[9] M. Klavarioti, D. Mantzavinos, D. Kassinos, Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ. Int. 2009, 35, 402.
| Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXpvVWltQ%3D%3D&md5=45a4f5e75673284d4d132c6b5854efc2CAS | 18760478PubMed |
[10] K. Ikehata, N. J. Naghashkar, M. G. Ei-Din, Degradation of pharmaceuticals by ozonation and advanced oxidation processes: a review. Ozone Sci. Eng. 2006, 28, 353.
| Degradation of pharmaceuticals by ozonation and advanced oxidation processes: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXkt1Gk&md5=b2124126acf68c21be75f115343661a5CAS |
[11] D. Dimitrakopoulou, I. Rethemiotaki, Z. Frontistis, N. P. Xekoukoulotakis, D. Venieri, D. Mantzavinos, Degradation, mineralization and antibiotic inactivation of amoxicillin by UV-A/TiO2 photocatalysis. J. Environ. Manage. 2012, 98, 168.
| Degradation, mineralization and antibiotic inactivation of amoxicillin by UV-A/TiO2 photocatalysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xit1artbo%3D&md5=5feed6829ceddf92973a3b838cf87405CAS | 22277347PubMed |
[12] V. Romero, N. De la Cruz, R. F. Dantas, P. M. J. Giménez, S. Esplugas, Photocatalytic treatment of metoprolol and propranolol. Catal. Today 2011, 161, 115.
| Photocatalytic treatment of metoprolol and propranolol.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXis1emt7o%3D&md5=9f63220fc163f09033c3643dfcf1a0f0CAS |
[13] F. Méndez-Arriaga, S. Esplugas, J. Gimenéz, Photocatalytic degradation of non-steroidal anti-inflammatory drugs with TiO2 and simulated solar irradiation. Water Res. 2008, 42, 585.
| Photocatalytic degradation of non-steroidal anti-inflammatory drugs with TiO2 and simulated solar irradiation.Crossref | GoogleScholarGoogle Scholar | 17761209PubMed |
[14] T. E. Doll, F. H. Frimmel, Removal of selected persistent organic pollutants by heterogeneous photocatalysis in water. Catal. Today 2005, 101, 195.
| Removal of selected persistent organic pollutants by heterogeneous photocatalysis in water.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXktFajtbs%3D&md5=7bee5f361e2d03396900338a24e8f918CAS |
[15] A. Ziylan, N. H. Ince, The occurrence and fate of anti-inflammatory and analgesic pharmaceuticals in sewage and fresh water: treatability by conventional and non-conventional processes. J. Hazard. Mater. 2011, 187, 24.
| The occurrence and fate of anti-inflammatory and analgesic pharmaceuticals in sewage and fresh water: treatability by conventional and non-conventional processes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXisVKhurY%3D&md5=611554d9994fee0f016559044b9efb24CAS | 21315511PubMed |
[16] R. Rodil, J. B. Quintana, E. Concha-Grana, P. Lopez-Mahia, S. Muniategui-Lorenzo, D. Prada-Rodriguez, Emerging pollutants in sewage, surface and drinking water in Galicia (NW Spain). Chemosphere 2012, 86, 1040.
| Emerging pollutants in sewage, surface and drinking water in Galicia (NW Spain).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvVyisrs%3D&md5=75d4be92095ce4fcc9fc9e6c43315493CAS | 22189380PubMed |
[17] H. R. Buser, T. Poiger, M. D. Müller, Occurrence and fate of the pharmaceutical drug diclofenac in surface waters: rapid photodegradation in a lake. Environ. Sci. Technol. 1998, 32, 3449.
| Occurrence and fate of the pharmaceutical drug diclofenac in surface waters: rapid photodegradation in a lake.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXlvFKmtL0%3D&md5=3c4afd2e783046263132e40b5edb2a88CAS |
[18] T. Heberer, A. Mechlinski, B. Fanck, A. Knappe, G. Massmann, A. Pekdeger, B. Fritz, Field studies on the fate and transport of pharmaceutical residues in bank filtration. Ground Water Monit. Remediat. 2004, 24, 70.
| Field studies on the fate and transport of pharmaceutical residues in bank filtration.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXltFWktbY%3D&md5=0f0027a6659179db84709eedf83187c5CAS |
[19] N. Paxéus, Removal of selected non-steroidal anti-inflammatory drugs (NSAIDs), gemfibrozil, carbamazepine, beta-blockers, trimethoprim and triclosan in conventional wastewater treatment plants in five EU countries and their discharge to the aquatic environment. Water Sci. Technol. 2004, 50, 253.
| 15497855PubMed |
[20] B. Hoeger, B. Köllner, D. R. Dietrich, B. Hitzfeld, Water-borne diclofenac affects kidney and gill integrity and selected immune parameters in brown trout (Salmo trutta f. fario). Aquat. Toxicol. 2005, 75, 53.
| Water-borne diclofenac affects kidney and gill integrity and selected immune parameters in brown trout (Salmo trutta f. fario).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtVWisbrE&md5=33916a718d256975a95ce042b208d51bCAS | 16139376PubMed |
[21] J. L. Oaks, M. Gilbert, M. Z. Virani, R. T. Watson, C. U. Meteyer, B. A. Rideout, H. L. Shivaprasad, S. Ahmed, M. J. I. Chaudhry, M. Arshad, S. Mahmood, A. Ali, A. A. Khan, Diclofenac residues as the cause of vulture population decline in Pakistan. Nature 2004, 427, 630.
| Diclofenac residues as the cause of vulture population decline in Pakistan.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhtFCnu7o%3D&md5=0a92b54a65877965b653c90053e69bfcCAS | 14745453PubMed |
[22] K. Fent, A. A. Weston, D. Caminada, Ecotoxicology of human pharmaceuticals. Aquat. Toxicol. 2006, 76, 122.
| Ecotoxicology of human pharmaceuticals.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xjsl2qtQ%3D%3D&md5=5c96664f4b973725ca03d03ea1ab44d2CAS | 16257063PubMed |
[23] Y. J. Zhang, S. U. Geißen, C. Gal, Carbamazepine and diclofenac: removal in wastewater treatment plants and occurrence in water bodies. Chemosphere 2008, 73, 1151.
| Carbamazepine and diclofenac: removal in wastewater treatment plants and occurrence in water bodies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtlSgs7fI&md5=13d6833952e96bab40245e9997c4f4faCAS |
[24] T. A. Ternes, Occurrence of drugs in German sewage treatment plants and rivers. Water Res. 1998, 32, 3245.
| Occurrence of drugs in German sewage treatment plants and rivers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXmslaqt7k%3D&md5=35ff56b6dd4ca7457217753628af0b65CAS |
[25] R. Salgado, R. Marques, J. P. Noronha, G. Carvalho, A. Oehmen, M. A. M. Reis, Assessing the removal of pharmaceuticals and personal care products in a full-scale activated sludge plant. Environ. Sci. Pollut. Res. 2012, 19, 1818.
| Assessing the removal of pharmaceuticals and personal care products in a full-scale activated sludge plant.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xpt12htL4%3D&md5=9f110a451ec86c42fbcaf6b07e54d745CAS |
[26] C. Baeza, D. R. U. Knappe, Transformation kinetics of biochemically active compounds in low-pressure UV photolysis and UV/H2O2 advanced oxidation processes. Water Res. 2011, 45, 4531.
| Transformation kinetics of biochemically active compounds in low-pressure UV photolysis and UV/H2O2 advanced oxidation processes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXpt1altbs%3D&md5=f5469fa14ef9ac6c2a202cffed8af15fCAS | 21714983PubMed |
[27] P. Bartels, W. von Tümpling, Solar radiation influence on the decomposition process of diclofenac in surface waters. Sci. Total Environ. 2007, 374, 143.
| Solar radiation influence on the decomposition process of diclofenac in surface waters.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhvV2qt7g%3D&md5=9ff0747f5f9bc04e7a81fee75c01951aCAS | 17258294PubMed |
[28] D. Vogna, R. Marotta, A. Napolitano, R. Andreozzi, M. d'Ischia, Advanced oxidation of the pharmaceutical drug diclofenac with UV/H2O2 and ozone. Water Res. 2004, 38, 414.
| Advanced oxidation of the pharmaceutical drug diclofenac with UV/H2O2 and ozone.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXpsFelsL8%3D&md5=fc2d4c61213d4ab57d6b444adfe0e209CAS | 14675653PubMed |
[29] J. L. Packer, J. J. Werner, D. E. Latch, K. McNeill, W. A. Arnold, Photochemical fate of pharmaceuticals in the environment: naproxen, diclofenac, clofibric acid and ibuprofen. Aquat. Sci. 2003, 65, 342.
| Photochemical fate of pharmaceuticals in the environment: naproxen, diclofenac, clofibric acid and ibuprofen.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXht1ehurw%3D&md5=6c61665c3645dfe71d3c59ae1ba937a7CAS |
[30] J. Kockler, D. Kanakaraju, B. D. Glass, M. Oelgemöller, Photochemical and photocatalytic degradation of diclofenac and amoxicillin using natural and simulated sunlight. J. Sustain. Sci. Manage. 2012, 7, 23.
| 1:CAS:528:DC%2BC38XhtVagtLzP&md5=557cfa3037daa05dde7471a046b15594CAS |
[31] A. Achilleos, E. Hapeshi, N. P. Xekoukoulotakis, D. Mantzavinos, D. Fatta-Kassinos, Factors affecting diclofenac decomposition in water by UV-A/TiO2 photocatalysis. Chem. Eng. J. 2010, 161, 53.
| Factors affecting diclofenac decomposition in water by UV-A/TiO2 photocatalysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXntVyjsb4%3D&md5=94be7635f5877321b37f7c5fab23fb3eCAS |
[32] L. Rizzo, S. Meric, D. Kassinos, M. Guida, F. Russo, V. Belgiorno, Degradation of diclofenac by TiO2 photocatalysis: UV absorbance kinetics and process evaluation through a set of toxicity bioassays. Water Res. 2009, 43, 979.
| Degradation of diclofenac by TiO2 photocatalysis: UV absorbance kinetics and process evaluation through a set of toxicity bioassays.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXisVGjuro%3D&md5=48750a9c195f62f88be3458a2a652e80CAS | 19081596PubMed |
[33] L. Rizzo, S. Meric, M. Guida, D. Kassinos, V. Belgiorno, Heterogeneous photocatalytic degradation kinetics and detoxification of an urban wastewater treatment plant effluent contaminated with pharmaceuticals. Water Res. 2009, 43, 4070.
| Heterogeneous photocatalytic degradation kinetics and detoxification of an urban wastewater treatment plant effluent contaminated with pharmaceuticals.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtFWlt7nK&md5=25b720523bee2a88b875c8f7a5946edcCAS | 19596131PubMed |
[34] P. Calza, V. A. Sakkas, C. Medana, C. Baiocchi, A. Dimou, E. Pelizzetti, T. Albanis, Photocatalytic degradation study of diclofenac over aqueous TiO2 suspensions. Appl. Catal. B 2006, 67, 197.
| Photocatalytic degradation study of diclofenac over aqueous TiO2 suspensions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XptlSrsbs%3D&md5=dc4e2583e91c9472e588fd77902211faCAS |
[35] T. Heberer, Tracking persistent pharmaceutical residues from municipal sewage to drinking water. J. Hydrol. 2002, 266, 175.
| Tracking persistent pharmaceutical residues from municipal sewage to drinking water.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XnvFyjur0%3D&md5=6ba934c364dfe957a1fbebd3dd7d3985CAS |
[36] B. Ohtani, Photocatalysis A to Z – what we know and what we do not know in a scientific sense. J. Photochem. Photobiol. Photochem. Rev. 2010, 11, 157.
| Photocatalysis A to Z – what we know and what we do not know in a scientific sense.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXkvVKjtLk%3D&md5=385e5d0619095b3299944854fad82aadCAS |
[37] A. M. Braun, M. T. Maurette, E. Oliveros, Photochemical Technology 1991 (Wiley-VCH: Weinheim).
[38] APHA, Standard Methods for the Examination of the Water and Wastewater, 18th edn 1992 (American Public Health Association: Washington DC).
[39] R. Salgado, V. J. Pereira, G. Carvalho, R. Soeiro, V. Gaffney, C. Almeida, V. V. Cardoso, E. Ferreira, M. J. Benoliel, T. A. Ternes, A. Oehmen, M. A. M. Reis, J. P. Noronha, Photodegradation kinetics and transformation products of ketoprofen, diclofenac and atenolol in pure water and treated wastewater. J. Hazard. Mater. 2013, 244–245, 516.
| Photodegradation kinetics and transformation products of ketoprofen, diclofenac and atenolol in pure water and treated wastewater.Crossref | GoogleScholarGoogle Scholar | 23177274PubMed |
[40] D. E. Moore, S. Robertsthomson, D. Zhen, C. C. Duke, photochemical studies on the antiinflammatory drug diclofenac. Photochem. Photobiol. 1990, 52, 685.
| photochemical studies on the antiinflammatory drug diclofenac.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXht1Slug%3D%3D&md5=a97f4d49283a694be7e25305ee897389CAS | 2089417PubMed |
[41] C. Martínez, M. Canle, M. I. Fernandez, J. A. Santaballa, J. Faria, Aqueous degradation of diclofenac by heterogeneous photocatalysis using nanostructured materials. Appl. Catal. B 2011, 107, 110.
| Aqueous degradation of diclofenac by heterogeneous photocatalysis using nanostructured materials.Crossref | GoogleScholarGoogle Scholar |
[42] X. Van Doorslaer, P. M. Heynderickx, K. Demeestere, K. Debevere, H. Van Langenhove, J. Dewulf, TiO2 mediated heterogeneous photocatalytic degradation of moxifloxacin: operational variables and scavenger study. Appl. Catal. B 2012, 111–112, 150.
| TiO2 mediated heterogeneous photocatalytic degradation of moxifloxacin: operational variables and scavenger study.Crossref | GoogleScholarGoogle Scholar |
[43] A. L. Giraldo, G. A. Peneula, R. A. Torres-Palna, N. J. Pino, R. A. Palominos, H. D. Mansilla, Degradation of the antibiotic oxolinic acid by photocatalysis with TiO2 in suspension. Water Res. 2010, 44, 5158.
| Degradation of the antibiotic oxolinic acid by photocatalysis with TiO2 in suspension.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXht1KhtbbJ&md5=0180daabcdd0d4fbcea970e1ce3b318dCAS | 20633918PubMed |
[44] J. Blanco-Galvez, P. Fernández-Ibáñez, S. Malato-Rodríguez, Solar photocatalytic detoxification and disinfection of water: recent overview. J. Sol. Energy Eng. 2007, 129, 4.
| Solar photocatalytic detoxification and disinfection of water: recent overview.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXivVaqsw%3D%3D&md5=16fae7583f50b5045bd659e048ce6513CAS |
[45] D. Friedmann, C. Mendive, D. Bahnemann, TiO2 for water treatment: parameters affecting the kinetics and mechanisms of photocatalysis. Appl. Catal. B 2010, 99, 398.
| TiO2 for water treatment: parameters affecting the kinetics and mechanisms of photocatalysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtFOnt7fK&md5=2e2b8275429ce364cbab85d153e9fd68CAS |
[46] S. Basha, C. Barr, D. Keane, K. Nolan, A. Morrissey, M. Oelgemöller, J. M. Tobin, On the adsorption/photodegradation of amoxicillin in aqueous solutions by an integrated photocatalytic adsorbent (IPCA): experimental studies and kinetics analysis. Photochem. Photobiol. Sci. 2011, 10, 1014.
| On the adsorption/photodegradation of amoxicillin in aqueous solutions by an integrated photocatalytic adsorbent (IPCA): experimental studies and kinetics analysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXmvVaqtrg%3D&md5=c52c59e0b25f46f4c90f65de8476c7feCAS | 21380442PubMed |
[47] L. Yang, L. E. Yu, M. B. Ray, Degradation of paracetamol in aqueous solutions by TiO2 photocatalysis. Water Res. 2008, 42, 3480.
| Degradation of paracetamol in aqueous solutions by TiO2 photocatalysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXnsFagurY%3D&md5=b61ea6fe8647c843cb5100d7c3543a07CAS | 18519147PubMed |
[48] E. Hapeshi, A. Achilleos, M. I. Vasquez, C. Michael, N. P. Xekoukoulotakis, D. Mantzavinos, D. Kassinos, Drugs degrading photocatalytically: kinetics and mechanisms of ofloxacin and atenolol removal on titania suspensions. Water Res. 2010, 44, 1737.
| Drugs degrading photocatalytically: kinetics and mechanisms of ofloxacin and atenolol removal on titania suspensions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXisFSht74%3D&md5=eeeffb6f324a6d94b66e01eb4d3e09caCAS | 20031189PubMed |
[49] M. R. Hoffmann, S. T. Martin, W. Choi, D. W. Bahnemann, Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69.
| Environmental applications of semiconductor photocatalysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXjtF2qur4%3D&md5=d2efbe5fe187d364d407052cb0160d06CAS |
[50] L. A. Pérez-Estrada, M. I. Maldonado, W. Gernjak, A. Agüera, A. R. Fernández-Alba, M. M. Ballesteros, S. Malato, Decomposition of diclofenac by solar driven photocatalysis at pilot plant scale. Catal. Today 2005, 101, 219.
| Decomposition of diclofenac by solar driven photocatalysis at pilot plant scale.Crossref | GoogleScholarGoogle Scholar |
[51] L. A. Pérez-Estrada, S. Malato, W. Gernjak, A. Agüera, E. M. Thurman, I. Ferrer, A. R. Fernández-Alba, Photo-Fenton degradation of diclofenac: identification of main intermediates and degradation pathway. Environ. Sci. Technol. 2005, 39, 8300.
| Photo-Fenton degradation of diclofenac: identification of main intermediates and degradation pathway.Crossref | GoogleScholarGoogle Scholar | 16294867PubMed |
[52] J. H. O. S. Pereira, V. J. P. Villar, M. T. Borges, O. González, S. Esplugas, R. A. R. Boaventura, Photocatalytic degradation of oxytetracycline using TiO2 under natural and simulated solar radiation. Sol. Energy 2011, 85, 2732.
| Photocatalytic degradation of oxytetracycline using TiO2 under natural and simulated solar radiation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXht12itL3K&md5=b6505a94fc6a7e9355a03d4afd1a22c8CAS |
[53] U. I. Gaya, A. H. Abdullah, Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: a review of fundamentals, progress and problems. J. Photochem. Photobiol. Photochem. Rev. 2008, 9, 1.
| Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: a review of fundamentals, progress and problems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXlvVSgtLY%3D&md5=509b1c16e7828a4eb6e01afac2f90ea3CAS |
[54] D. Bahnemann, Photocatalytic water treatment: solar energy applications. Sol. Energy 2004, 77, 445.
| Photocatalytic water treatment: solar energy applications.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXovFGiurs%3D&md5=d81f499c617400d014e6791ad29ab5d8CAS |
[55] S. Loiselle, D. Vione, C. Minero, V. Maurino, A. Tognazzi, A. M. Dattilo, C. Rossi, L. Bracchini, Chemical and optical phototransformation of dissolved organic matter. Water Res. 2012, 46, 3197.
| Chemical and optical phototransformation of dissolved organic matter.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XlsVajs7s%3D&md5=5003f67995b766a80911f493a1923321CAS | 22503589PubMed |
[56] P. Calza, E. Pelizzetti, Photocatalytic transformation of organic compounds in the presence of inorganic ions. Pure Appl. Chem. 2001, 73, 1839.
| Photocatalytic transformation of organic compounds in the presence of inorganic ions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XjtVWntLY%3D&md5=3bc2a609bbbacd2e87f1a1f9a4337dabCAS |
[57] A. Agüera, L. A. P. Estrada, I. Ferrer, E. M. Thurman, S. Malato, A. R. Fernández-Alba, Application of time-of-flight mass spectrometry to the analysis of phototransformation products of diclofenac in water under natural sunlight. J. Mass Spectrom. 2005, 40, 908.
| Application of time-of-flight mass spectrometry to the analysis of phototransformation products of diclofenac in water under natural sunlight.Crossref | GoogleScholarGoogle Scholar | 15934037PubMed |
[58] C. Sirtori, A. Agüera, W. Gernjak, S. Malato, Effect of water-matrix composition on Trimethoprim solar photodegradation kinetics and pathways. Water Res. 2010, 44, 2735.
| Effect of water-matrix composition on Trimethoprim solar photodegradation kinetics and pathways.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXltFGrtbc%3D&md5=54d3a54f060cf84c830625b2f36849adCAS | 20206373PubMed |
[59] C. Rodrigues-Silva, M. G. Maniero, S. Rath, J. R. Guimarães, Degradation of flumequine by photocatalysis and evaluation of microbial activity. Chem. Eng. J. 2013, 224, 46.
| Degradation of flumequine by photocatalysis and evaluation of microbial activity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhslyksLzJ&md5=b245c9db633cc17d1a52b2841dde0694CAS |
[60] V. Augugliaro, M. Bellardita, V. Loddo, G. Palmisano, L. Palmisano, S. Yurdakal, Overview on oxidation mechanisms of organic compounds by TiO2 in heterogeneous photocatalysis. J. Photochem. Photobiol. Photochem. Rev. 2012, 13, 224.
| Overview on oxidation mechanisms of organic compounds by TiO2 in heterogeneous photocatalysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XntVyrs7c%3D&md5=b5ac0286ec57e89e9ebedcc7fbfaf456CAS |