First report of corm rot disease caused by Sclerotium rolfsii in banana
R. Thangavelu A B and M. M. Mustaffa AA National Research Centre for Banana, Thayanur (Post), Tiruchirapalli – 620102, Tamil Nadu, India.
B Corresponding author. Email: bananathanga@rediffmail.com
Australasian Plant Disease Notes 5(1) 30-33 https://doi.org/10.1071/DN10012
Submitted: 11 November 2009 Accepted: 10 March 2010 Published: 7 April 2010
Abstract
A corm rot disease was observed for the first time in banana. The disease was found to occur in the majority of commercial cultivars grown in different banana-growing states of India. The incidence of the disease was up to 50% and found to occur at an altitude up to 3000 m asl. The pathogen was identified as Sclerotium rolfsii based on morphological characters and rDNA internal transcribed spacer sequence data.
Bananas (Musa spp.) are the world’s fourth most important global food crop grown in more than 120 countries (Molina and Valmayor 1999) and are the staple food for more than 400 million people (Swennen et al. 1995). India is the world’s largest banana producer and 26 million tonnes are produced annually from 709 000 ha. However, various pests and disease problems, particularly Fusarium wilt, cause significant losses (Ploetz 2005).
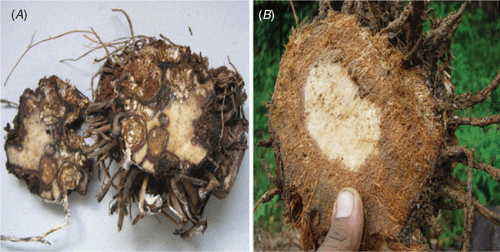
During a disease survey in different banana-growing regions of India in March 2005, a new wilt-like disease was observed in cv. Rasthali (Silk-AAB) in the Tirukattupalli area of Thanjavur district in Tamil Nadu, India. The important symptoms observed were yellowing of leaves from base to apex, extensive pseudostem sheath rot with profuse mycelial growth on the pseudostem (Fig. 1A, B). The colour of the rotted portion was yellowish-red to reddish-brown (Fig. 1B, C), with numerous brown sclerotia present. Splitting of the basal portion or the pseudostem sheath was observed, and the bottom leaves had desiccated. The infected plant emanated a mushroom odour. The inner tissues of the corm were spongy and colonised extensively with white mycelium (Fig. 2A). At higher altitudes of 2000–3000 m asl, the cortical portion of the corm was completely converted into a mass of fibre (like coconut fibre) with profuse growth of fungal mycelium (Fig. 2B). In some plants, the infection extended up to half of the medulla region. The emerging peeper was necrotic and colonised extensively with white mycelium. In some cases the mycelial growth was seen on the growing tip of the young bud and resulted in reddish brown necrosis. The symptoms were observed in all stages of plant growth.

|
|
Samples of the diseased plant parts were collected and brought to the laboratory and cultured on potato dextrose agar (PDA). White mycelial growth was observed within a week. Subsequently, small round dark-brown sclerotia developed in the culture. The fungus was grown for 15 days in banana pseudostem pieces which had been shade-dried to 60% moisture and autoclaved, to provide inoculum for pathogenicity tests. Three-month old tissue-cultured banana plants of cv. Rasthali were potted in sterile standard potting mixture (Thangavelu and Jayanthi 2009). Two to three colonised pseudostem pieces were applied 5 cm below the soil surface around each plant and covered with the same top soil. All the 10 plants inoculated expressed similar symptoms to those observed in the field (Fig. 3A, B) and all inoculated plants died within 15 days. The control plants maintained without inoculation did not show any symptoms. The same fungus was re-isolated from the infected plants and confirmed by cultural and morphological characters. The placement of single sclerotia in the pseudostem sheath of tissue-cultured plants wounded with pinpricks also caused rotting of tissues in 3 days.

|
Microscopic examination of the fungus revealed that in the profuse growth of the compact, septate, hyaline mycelium, there were protuberances that later developed into sclerotia. The number of sclerotia produced in culture on PDA (Fig. 4) ranged from 47 to 199 per 9-cm plate, and the sclerotial diameter ranged from 0.8 to 1.0 mm. Clamp connections were also observed on the hyphae. These morphological characters are very similar to those of Sclerotium rolfsii (Sarma et al. 2002). From the purified cultures, the rDNA internal transcribed spacer region (ITS) was amplified and sequenced using primers ITS1 and ITS4 (White et al. 1990). The nucleotide Basic Local Alignment Search Tool (BLAST) performed on the National Center for Biotechnology Information (NCBI) website (http://blast.ncbi.nlm.nih.gov/) indicated that the sequence is 100% homologous to Athelia rolfsii isolated from the southern blight plants of Zamioculcas zamiifolia in China (GQ121442). The sequence was deposited in GenBank as accession number GQ 215695. The culture has been deposited in the Microbial Type Culture Collection (MTCC), Institute of Microbial Technology (IMTECH), Chandigarh, India.

|
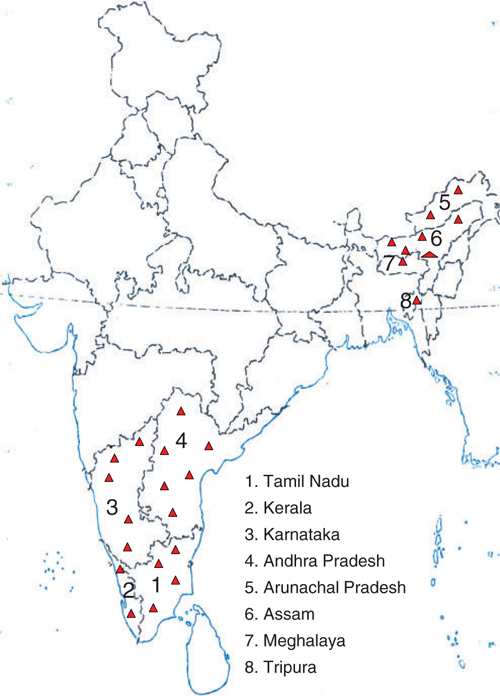
During a subsequent survey conducted in different parts of banana-growing states of India in 2005, the disease was observed in many commercial varieties such as Rasthali (Silk-AAB), Virupakshi (Hill banana-AAB) and Karpuravalli (Pisang Awak-ABB) in Tamil Nadu, Amritapani and Mortamon (an ecotype of Rasthali) in Andhra Pradesh, Neypoovan (AB) in Karnataka, Chinali (AAB), Matti (AB), Boothibale (Monthan-ABB), Kijinan (AB) in Kerala, Athiakol (BB) in Assam, Malbhog (Silk-AAB) in Meghalaya and Sabri (Silk-AAB) in Tripura. In most of the areas surveyed, the disease was observed in only few plants but in two areas, west Godavari district of Andhra Pradesh and A. Pudupatti village of Madurai district in Tamil Nadu, the disease incidence was more than 50% in cv. Rasthali (AAB). Thus the disease is widespread in India (Fig. 5).
|
A root and corm rot of banana, caused by Sclerotium sp., was reported in Enset at Areka Experimental Station in Welayita, Ethiopia where it caused disease in young seedlings and transplants (Tessera and Quimio 1994). Mohan and Lakshmanan (1989) reported pseudostem rot disease caused by Corticium rolfsii in 3–5-month-old banana plants in Tamil Nadu, India but they did not observe any corm rot disease due to this pathogen. Moreover, the symptoms on the pseudostem described are not exactly similar to the symptoms described in this report. Besides, in all cases, the corm rot disease reported here was observed only in older plants, at least 7 months after planting and not in the young plants as stated by Mohan and Lakshmanan (1989). The disease described in this paper was initially suspected to be Marasmiellus pseudostem and root rot disease (Stover 1972) as the symptoms of the two diseases in the pseudostem are very similar. However, our studies have led to the identification of the pathogen as Sclerotium rolfsii, the first report of this pathogen causing corm rot disease in banana.
Acknowledgements
The authors wish to thank Mr. R. Pitchaimuthu and P. Gobi for technical assistance, and Mr. T. Sekar for assistance in the preparation of manuscript.
Mohan S, Lakshmanan P
(1989) Pseudostem rot caused by Corticium rolfsii, a new disease of Musa sp. in Tamil Nadu, India. Pytoparasitica 17, 127–130.
| Crossref |

Sarma BK,
Singh UP, Singh KP
(2002) Variability in Indian isolates of Sclerotium rolfsii. Mycologia 94, 1051–1058.
| Crossref | GoogleScholarGoogle Scholar |

Swennen R,
Vuylsteke D, Ortiz R
(1995) Phenotypic diversity and patterns of variation in West and Central African Plantains (Musa spp., AAB Group Musaceae). Economic Botany 49, 320–327.

Thangavelu R, Jayanthi A
(2009) RFLP analysis of rDNA-ITS regions of native non-pathogenic Fusarium oxysporum isolates and their field evaluation for the suppression of Fusarium wilt disease of banana. Australasian Plant Pathology 38, 13–21.
| Crossref | GoogleScholarGoogle Scholar |
CAS |



