First report of anthracnose (Colletotrichum trifolii) on Cullen australasicum (syn. Psoralea australasica)
R. M. Nair A B D , C. Wilmshurst A , M. H. Russ A , A. Williams A B and M. Priest CA South Australian Research & Development Institute (SARDI), GPO Box 397, Adelaide, SA 5001, Australia.
B Future Farm Industries Cooperative Research Centre, The University of Western Australia, 35 Stirling Highway, Crawley, WA 6009, Australia.
C Industry and Investment NSW, Orange Agricultural Institute, Orange NSW 2800, Australia.
D Corresponding author. Email: ram.madhavannair@sa.gov.au
Australasian Plant Disease Notes 5(1) 34-36 https://doi.org/10.1071/DN10013
Submitted: 11 November 2009 Accepted: 17 March 2010 Published: 7 April 2010
Abstract
We report the incidence of anthracnose caused by Colletotrichum trifolii on Cullen australasicum, a native Australian legume. Natural infection was observed on plants grown in a genetic resources characterisation experiment at Waite Research Precinct, Adelaide. The affected plants showed wilting of the branches and light brown lesions on the leaves. The characteristic symptom was a bluish black discolouration on the infected leaves and stems. In some plants, the infection spread from the leaves and stems across the entire plant leading to the death of the plant.
Introduction
In Australia, perennial-based farming systems are needed for farmers to remain competitive and keep ahead of natural resource degradation, climate change and variability. There is an urgent need for resilient perennial pasture species in the low to medium rainfall (250–500 mm) regions of southern and northern Australia. Cullen australasicum (Schltdl.) J.W. Grimes (syn. Psoralea australasica Schltdl.) (syn. cullen, native scurfpea, native verbine) has been identified as a promising native perennial legume (Bennett et al. 2006; Dear et al. 2007; Hughes et al. 2008) and is currently a high priority species under development within the Future Farm Industries Cooperative Research Centre (FFI CRC).
Anthracnose is a common disease affecting a wide range of plant species. Colletotrichum trifolii has been well documented as the causal agent responsible for anthracnose in perennial pasture legumes such as lucerne (Medicago sativa L.) (Irwin 1974) and is one of the factors responsible for the poor persistence of lucerne in Australia (Irwin 1977).
Disease symptoms were observed in the second year of a genetic resource characterisation experiment conducted at the Waite Research Precinct, Adelaide, SA, Australia. The trial included 70 accessions of C. australasicum, each accession being represented by 30 plants across three replicates. In order to determine the differences in re-growth during winter among the accessions, the plants had been trimmed at ~50 cm from the ground level in late autumn in the second year of growth (6 months after planting; planting was done in spring 2008).
The affected plants showed wilting of branches (Fig. 1). The leaves had light brown lesions, which varied in size. The characteristic symptom was the appearance of bluish black discolouration on the leaves (Fig. 2) and stems. The infection from the stems spread onto the other parts of the plants and in some instances lead to the death of the whole plant. The level of disease on each of the accessions was assessed on a plot basis using a rating scale 0 to 10 (0 – no disease; 10 – all plants dead). Significant variation (P < 0.001) was observed among the accessions for the level of disease. The mean disease scores in the experiment ranged from 0.11 to 9.98.

|

|
Isolation
Infected leaves were placed in a humid chamber for 2 days, resulting in extensive sporulation of conidia. In a laminar flow chamber, conidia were removed from leaves using a sterile needle and placed on potato dextrose agar (PDA) plates. After 7 days incubation at 20–25°C under 12 : 12 h light/dark cycle, large numbers of conidia were produced. After a further week of incubation, single spore isolation was performed to establish a pure culture. This culture is now stored under water in a refrigerator at 4°C.
Fungal morphology
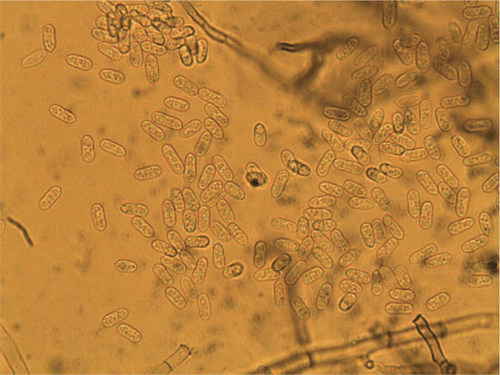
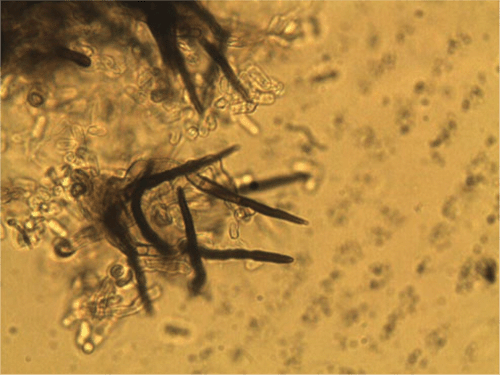
On PDA the conidia are salmon-apricot in colour, hyaline, non-septate, mostly oblong, straight, rounded at the ends, occasionally narrowing to a base that is truncate and protuberant, and measured (9–) 11–13 (–15) µm in length and 3–4 µm in width (Fig. 3). Dark brown setae are formed abundantly on the conidiomata and measured (60–) 80–112 × 3–5 µm, narrowing gradually to the apex, and 1 (–2) septate (Fig. 4) and sclerotia were abundant. Based on the morphology, the isolate is considered to be Colletotrichum trifolii Bain & Essary. Descriptions of C. trifolii given by various authors show some variation in conidial length and width, with our isolate being close to or identical with the original description of Bain and Essary (1906) and Massenot and Raynal (1973). Recent DNA analysis of Colletotrichum isolates by Liu et al. (2007) and O’Neill et al. (1997) suggest that variation in isolates from lucerne (alfalfa) and other legumes are normal and that C. trifolii and C. orbiculare are molecularly conspecific but are host-specific on various hosts including C. trifolii on Medicago and Trifolium. Certainly the narrowing of some conidia resembles those of C. orbiculare as figured by Walker et al. (1991). Although acknowledging that there are pending taxonomic issues for this fungus group, it was decided that the name C. trifolii was more adequate for the fungus that causes anthracnose on C. australasicum. A culture of C. trifolii has been deposited in the Plant Pathology Herbarium, NSW as DAR 80444.
|
|
Pathogenicity
Pathogenicity of the fungus isolate was tested on C. australasicum seedlings in a glasshouse experiment. Seedlings were inoculated by spraying a suspension of 2 × 106 spores/mL. Controls were inoculated using sterilised water. Plants were placed in a misting tent at 28–30°C for a week and then moved to a glasshouse maintained at 24–25°C. Disease symptoms were observed 7 days post-inoculation and 14/16 inoculated plants showed the disease symptoms, including 6/16 that were dead. Koch’s postulates were fulfilled by re-isolation of C. trifolii. Pathogenicity of the fungus isolate was also tested on lucerne in a glasshouse experiment. Eight cultivars (50 seedlings each) were inoculated by spraying a suspension of 2 × 106 spores/mL as described above. Four weeks after inoculation, the cultivars showed significant variation (P < 0.001) for the level of resistance to the pathogen (0–56.67%).
It is possible that the infection might have originated from a 3-year-old lucerne experiment which was adjacent to the cullen characterisation trial. In addition the trimming of the plants in late autumn could have provided a physical injury through which the pathogen may have entered the plants to cause anthracnose disease.
We believe this is the first report of infection of C. australasicum by C. trifolii and that the disease could be limiting to this potential pasture species. Some of the accessions seemed to show good level of resistance and hence selection for resistance (Clements et al. 1984) could be a practical strategy to combat this disease.
Acknowledgements
The authors thank the FFI CRC for supporting this work which is part of the project on ‘Low rainfall perennial legumes’. The authors thank Jenny Davidson, SARDI, for constructive comments on the manuscript.
Bain SM, Essary SH
(1906) A new anthracnose of alfalfa and red clover. Journal of Mycology 12, 192–193.
| Crossref | GoogleScholarGoogle Scholar |

Clements RJ,
Turner JW,
Irwin JAG,
Langdon PW, Bray RA
(1984) Breeding disease resistant, aphid resistant lucerne for subtropical Queensland. Australian Journal of Experimental Agriculture and Animal Husbandry 24, 178–188.
| Crossref | GoogleScholarGoogle Scholar |

Dear BS,
Li GD,
Hayes RC,
Hughes SJ,
Charman N, Ballard RA
(2007) Cullen australasicum (syn. Psoralea australasica): a review and some preliminary studies related to its potential as a low rainfall perennial pasture legume. The Rangeland Journal 29, 121–132.
| Crossref | GoogleScholarGoogle Scholar |

Hughes SJ,
Snowball R,
Reed KFM,
Cohen B,
Gajda K,
Williams AR, Groeneweg SL
(2008) The systematic collection and characterisation of herbaceous forage species for recharge and discharge environments in southern Australia. Australian Journal of Experimental Agriculture 48, 397–408.
| Crossref | GoogleScholarGoogle Scholar |

Irwin JAG
(1974) Crown rot of lucerne in Queensland caused by Colletotrichum trifolii. Australian Journal of Experimental Agriculture and Animal Husbandry 14, 197–200.
| Crossref |

Irwin JAG
(1977) Factors contributing to poor lucerne persistence in southern Queensland. Australian Journal of Experimental Agriculture and Animal Husbandry 17, 998–1003.
| Crossref | GoogleScholarGoogle Scholar |

Liu B,
Wasilwa LA,
Morelock TE,
O’Neill NR, Correll JC
(2007) Comparison of Colletotrichum orbiculare and several allied Colletotrichum spp. for mtDNA RFLPs, intron RFLP and sequence variation, vegetative compatibility, and host specificity. Phytopathology 97, 1305–1314.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Massenot M, Raynal G
(1973) Les maladies des legumineues fourageres 1. les anthracnoses provoquees par les melanconiales. Annales Phytopathologique 5, 83–100.

O’Neill NR,
van Berkum P,
Lin JJ,
Kuo J,
Ude GN,
Kenworthy W, Saunders JA
(1997) Application of amplified restriction fragment length polymorphism for genetic characterization of Colletotrichum pathogens of alfalfa. Phytopathology 87, 745–750.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Walker J,
Nikandrow A, Millar GD
(1991) Species of Colletotrichum on Xanthium (Asteraceae) with comments on some taxonomic and nomenclatural problems in Colletotrichum. Mycological Research 95, 1175–1193.
| Crossref | GoogleScholarGoogle Scholar |



