Current status of bacterial canker spread on kiwifruit in Italy
G. M. Balestra A B , A. Mazzaglia A , A. Quattrucci A , M. Renzi A and A. Rossetti AA Dipartimento di Protezione delle Piante, Università degli Studi della Tuscia, Via S. Camillo de Lellis 01100 Viterbo, Italy.
B Corresponding author. Email: balestra@unitus.it
Australasian Plant Disease Notes 4(1) 34-36 https://doi.org/10.1071/DN09014
Submitted: 17 February 2009 Accepted: 21 March 2009 Published: 6 April 2009
Abstract
A survey of kiwifruit orchards in central and northern Italy during 2007–2008 consistently detected serious damage caused by bacterial canker. The causal agent, Pseudomonas syringae pv. actinidiae, was repeatedly isolated from diseased plants, mainly Actinidia chinensis Pl. cultivars. Pseudomonas syringae pv. actinidiae was identified by morphological, physiological, and biochemical characteristics as well as molecular analyses. Disease severity, varietal susceptibility, trade implications and control strategies are discussed.
Worldwide, kiwifruit is very important economically with production reaching up to 1.6 million tons/year. Italy, with more than 470 000 t, is the largest kiwifruit producer ahead of China. Italy, together with New Zealand, is the world’s largest kiwifruit exporter. The main areas of kiwifruit production in Italy are: Lazio, Piedmont, Emilia Romagna and Veneto. The largest increases in area and production have been in the Lazio and Piedmont regions (Testolin and Ferguson 2009). Actinidia deliciosa Pl. (cv. Hayward) is the most widely grown cultivar in Italy. However, over the last 10 years there has been an increase in the cultivars Jin Tao (Kiwi Gold) and Hort 16 A (Zespri Gold) (Belrose Inc. 2008; Testolin and Ferguson 2009).
Three different bacterial diseases infect kiwifruit plants in Italy: Pseudomonas s. pv. actinidiae, P. s. pv. syringae (van Hall) and P. viridiflava (Burkholder) Dowson (Varvaro et al. 1990; Scortichini 1994; Balestra and Varvaro 1997). Pseudomonas s. pv. actinidiae was first recorded 15 years ago on a few A. deliciosa cv. Hayward kiwifruit plants, but there has been little mention of the disease since. Pseudomonas s. pv. actinidiae was first reported in Japan (Serizawa et al. 1989), then in Korea (Koh et al. 1994) and Iran (Mazarei and Mostofipour 1994) where it is considered one of the most damaging diseases on kiwifruit.
The aim of the present paper is to report on 2 years of disease surveys of kiwifruit orchards in central and northern Italy which focused on the current bacterial canker distribution, severity and varietal susceptibility. Trade implications and control strategies are also discussed.
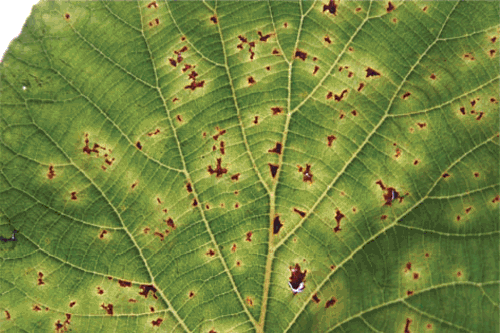
During 2007 and 2008 kiwifruit orchards were surveyed in central (Latina and Rome provinces) and in northern regions (Treviso province). Symptoms, which resembled those caused by P. s. pv. actinidiae (Serizawa et al. 1989), consisting of brown discolouration of the buds, dark brown spots surrounded by yellow haloes on leaves, cankers with reddish exudates on twigs, leaders and trunks, and collapse of fruits, were observed (Figs 1–5). Samples suspected of being infected with P. s. pv. actinidiae were collected and isolated to confirm bacterial infection. The identification of the bacterial isolates was carried out according to the morphological, cultural, physiological and biochemical properties as described by Takikawa et al. (1989). Genomic DNA extraction and sequencing of the 16S rDNA and 16S–23S rDNA intergenic spacer regions was used for molecular characterisation of 30 randomly chosen isolates. Sequences were compared with databases available at the National Centre for Biotechnology Information (NCBI) using their Blast search software (Altschul et al. 1990). Isolates were also tested with P. s. pv. actinidiae specific primers (Koh and Nou 2002).

|
|

|

|

|
Twenty plants each of 2-year-old pot grown healthy A. chinensis and A. deliciosa kiwifruit cultivars Hort 16 A, Jin Tao, and Hayward were used for pathogenicity testing. Inoculations were performed according to the methods of Serizawa and Ichikawa (1993). Control kiwifruit plants were similarly treated with SDW. Pathogenicity experiments were scored daily and reisolations made from all plants showing typical symptoms of bacterial canker. Known strains of P. viridiflava (NCPPB 1252), P. s. pv. syringae (NCPPB 1087) and P. s. pv. actinidiae (NCPPB 3739) were used as controls during these tests.
Isolations from kiwifruit plants suspected of being infected with bacterial canker collected during the field surveys yielded round bacterial colonies, with entire to ondulate margins that were convex and white coloured. They were positive for levan, hydrolysed Tween 80, produced acid from sorbitol, utilised sucrose, and produced a positive tobacco hypersensitivity reaction. They were negative for production of fluorescent pigments, oxidase and arginine dihydrolase reactions, potato rot, 2-Keto-gluconate production, gelatine liquefaction, trehalose utilization, Gram staining, nitrate reduction, growth at 40°C, hydrolysis of arbutin, esculin, casein, tyrosinase and urease.
The 16S rDNA and 16S–23S ITS rDNA sequences of the strains chosen for molecular characterisation shared 100% identity with the analogous sequences of P. s. pv. actinidiae available in the international databases. Furthermore, their provisional identity was confirmed by polymerase chain reaction amplification with pathovar-specific primers (Koh and Nou 2002) that correctly lead to the amplification of a single specific DNA fragment of the expected size.
The pathogenicity tests were confirmed on all the artificially inoculated plants. Symptoms on the leaves, twigs and branches were observed within 3–4 weeks after artificial inoculation with the pathogen. No symptoms were observed on the control plants inoculated with SDW.
The results of this testing confirmed that isolates collected during the field surveys were P. syringae pv. actinidae. Disease symptoms in the field were more common on A. chinensis plants, but occasionally A. deliciosa plants showed symptoms, especially Hort 16 A and Jin Tao cultivars aged 2–8 years. Disease incidence in the field ranged from 50% to 80%, and in some cases, due to the extremely high percentage of disease, eradication of the whole orchard was required.
The highest disease incidence was associated with the A. chinensis cultivars, especially in orchards of cv. Hort 16 A (always up to 70%) in Latina and Rome provinces (Lazio region). Disease symptoms on this cultivar were characterised by cankers with copious reddish exudates on the leaders and trunks. Kiwifruit plants cv. Jin Tao in the Treviso province (Veneto region) showed a lower disease incidence and severity and were characterised by symptoms affecting mainly the leaves, buds and flowers. In contrast, A. deliciosa cv. Hayward, from samples collected in areas surrounding A. chinensis orchards only showed 10% disease incidence.
Actinidia chinensis (cvv. Hort 16 A and Jin Tao) plants have an irregular distribution inside Italian kiwifruit orchards and the epidemiology and spread of this disease poses a risk to the kiwifruit industry in Italy. A wider study on its epidemiology and appropriate control strategies needs to be developed.
Economic and trade-related losses due to bacterial canker on kiwifruit in Italy have been estimated at around €2 million. However, with the increased planting of the new A. chinensis cultivars, it will be important to understand the real extent of disease and evaluate possible negative effects of bacterial canker on the A. chinensis industry in Italy. The introduction of a certification scheme to produce high-health plants by in vitro tissue culture would reduce potential disease problems on kiwifruit plants in Italy. Past control strategies against P. s. pv. actinidiae, have not been particularly successful (Serizawa et al. 1989).
At present, appropriate cultural practices and preventive copper treatments are important. In addition, new pesticides based on biocontrol agents and recent cupric formulations could provide new control opportunities. However, these will need careful testing to determine their efficacy. Also, it will be important to develop a robust monitoring program to determine the location of infected kiwifruit orchards so they can be isolated to protect disease-free areas.
Acknowledgements
The study was partially supported by Regione Lazio, PRAL 118/2003 and MIPAAF 893/2006, projects. Moreover the authors are particularly grateful to M. Baretta and S. Graziani (Cooperativa Agrintesa), Dr R. Spinelli (Zespri Fresh Produce Italy), and to Dr S. Moulducci (Consorzio Kiwi Gold) for their fundamental technical assistance.
Altschul SF,
Gish W,
Miller W,
Myers EW, Lipman DJ
(1990) Basic local alignment search tool. Journal of Molecular Biology 215, 403–410.
|
CAS |
PubMed |

Balestra GM, Varvaro L
(1997)
Pseudomonas syringae pv. syringae Causal Agent of Disease on Floral Buds of Actinidia deliciosa (A. Chev) Liang et Ferguson in Italy. Journal of Phytopathology 145, 375–378.
| Crossref | GoogleScholarGoogle Scholar |

Koh JK, Nou IS
(2002) DNA Markers for Identification of Pseudomonas syringae pv. actinidiae. Molecules and Cells 13, 309–314.
|
CAS |
PubMed |

Koh JK,
Cha BJ,
Chung HJ, Lee DH
(1994) Outbreak and spread of bacterial canker in kiwifruit. Korean Journal of Plant Pathology 10, 68–72.

Mazarei M, Mostofipour P
(1994) First report of bacterial canker of kiwifruit in Iran. Plant Pathology 43, 1055–1056.
| Crossref | GoogleScholarGoogle Scholar |

Serizawa S, Ichikawa T
(1993) Epidemiology of bacterial canker of kiwifruit. 1. Infection and bacterial movement in tissue of new canes. Annals of the Phytopathological Society of Japan 59, 452–459.

Serizawa S,
Ichikawa T,
Takikawa Y,
Tsuyumu S, Goto M
(1989) Occurrence of bacterial canker of kiwifruit in Japan: description of symptoms, isolation of the pathogen and screening of bactericides. Annals of the Phytopathological Society of Japan 55, 427–436.

Scortichini M
(1994) Occurrence of Pseudomonas syringe pv. actinidiae on kiwifruit in Italy. Plant Pathology 43, 1035–1038.
| Crossref | GoogleScholarGoogle Scholar |

Takikawa Y,
Serizawa S, Ichikawa T
(1989)
Pseudomonas syringae pv. actinidiae pv. nov.: the causal bacterium of canker of kiwifruit in Japan. Annals of the Phytopathological Society of Japan 55, 437–444.

Testolin R, Ferguson AR
(2009) Kiwifruit (Actinidia spp.) production and marketing in Italy. New Zealand Journal of Crop and Horticultural Science 37, 1–32.

Varvaro L,
Magro P, Mainolfi P
(1990) Comparsa di Pseudomonas viridiflava su Actinidia deliciosa in Italia. Informatore Fitopatologico 6, 49–53.



