First report of an Oidium sp. [neolycopersici] on Solanum betaceum in India
P. Baiswar A C , U. Braun B , S. Chandra A and S. V. Ngachan AA ICAR Research Complex for NEH Region, Umiam-793103, Meghalaya, India.
B Martin-Luther-Universität, Institut für Biologie, Bereich Geobotanik, Herbarium, Neuwerk 21, D-06099 Halle/S, Germany.
C Corresponding author. Email: pbaiswar@yahoo.com
Australasian Plant Disease Notes 4(1) 32-33 https://doi.org/10.1071/DN09013
Submitted: 18 July 2008 Accepted: 12 March 2009 Published: 6 April 2009
Abstract
In February 2008, a severe outbreak of powdery mildew disease was observed on Solanum betaceum in India. Based on the morphological characters, the pathogen was identified as an Oidium [neolycopersici] sp. morphologically similar to O. neolycopersici. This is the first report of this fungus causing powdery mildew on S. betaceum in India.
Tamarillo or tree tomato (Solanum betaceum, syn. Cyphomandra betacea) belongs to the family Solanaceae. Based on molecular phylogenetic studies, tree tomato as well as tomato (Solanum lycopersicum, syn.: Lycopersicon esculentum), the type host of Oidium neolycopersici, are allied species that have to be considered members of the genus Solanum (Peralta and Spooner 2001; Bohs 2005). Solanum betaceum is a small, tender 2–3-m tall tree which prolifically bears egg-shaped berries with pointed ends in clusters near the young shoots and it is grown as a backyard venture for its edible berries in North-east and South India. Tree tomato is consumed as a chutney when raw or after roasting and peeling off the skin. People desire it due to its unique flavour (Thakur et al. 1988; Rai et al. 2004).
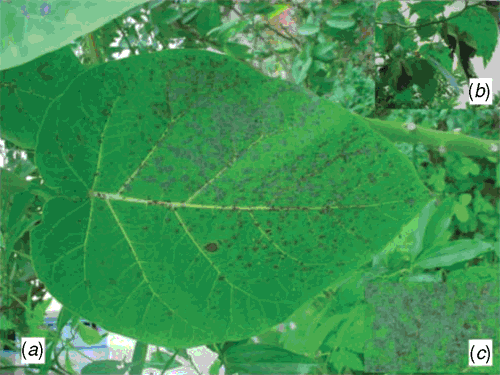
During February 2008, in Barapani, Meghalaya, leaves of S. betaceum were found to be heavily infected by powdery mildew. Almost all the plants in Barapani and nearby areas were found to be infected. Disease symptoms included grayish-white patches (initially circular but later on irregular) consisting of epiphytic mycelia and conidia mainly on the upper surface of the leaves. Spots later turned necrotic (Fig. 1). Older leaves were found to be highly susceptible. Symptoms were not present on berries. Voucher specimens have been deposited in Martin-Luther-Universität, Institut für Biologie, Bereich Geobotanik, Herbarium, Germany (HAL 2244 F.) and in the Institute Herbarium of ICAR Research Complex for NEH Region, Umiam, Meghalaya, India (ICARHNEN 24).
|
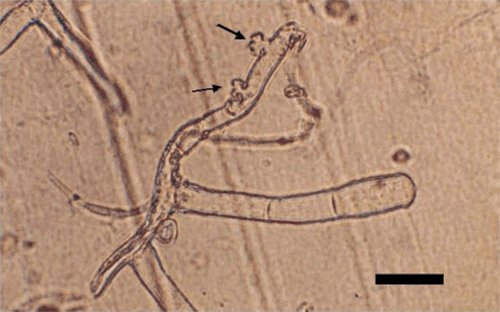
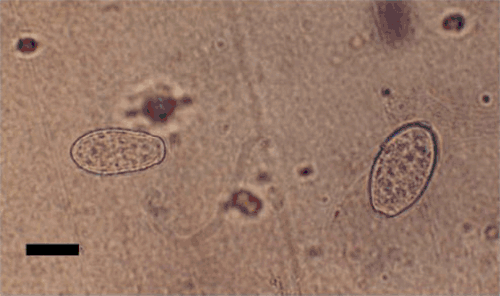
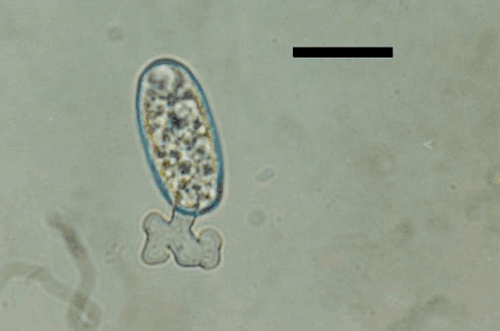
Conidia were harvested by dislodging them from infected tissue onto a strip of clear tape, using a camelhair brush. The tapes were mounted on microscopic slides as described in Correll et al. (1987). Morphological characteristics of the pathogen such as location of mycelia on the host, shape of appressoria, presence or absence of dimorphic conidia, size and shape of conidia, and branching of conidiophores were recorded. Hyphae were up to 3–8 μm wide with lobed appressoria (4–10 μm diam.), solitary (Fig. 2) or in pairs, opposite to each other. Conidiophores were mostly erect, consisting of a foot cell (24–45 × 7–11 μm) followed by two or three short cells (Fig. 2). Conidia were ellipsoid-doliiform (-cylindrical), (24-) 28–42 × 13–20 (-25) µm and produced singly (Fig. 3). Fibrosin bodies were absent in conidia. Germ tubes with lobed appressoria arose sub-terminally from conidia (Fig. 4). Based on these morphological characters the pathogen was identified as Oidium neolycopersici (Kiss et al. 2001). No perfect stage (chasmothecium) was found to be associated with this fungus. Pathogenicity was confirmed by dusting conidia on healthy leaves of S. betaceum, non-inoculated plants served as control. Inoculated plants developed symptoms after 8–10 days whereas control plants remained healthy.
|
|
|
An Oidium sp. and ‘Erysiphe communis’, which might refer to the same fungus, have been recorded on this host from the USA (Amano 1986; Farr et al. 1989). Golovinomyces cichoracearum has been recorded on this host from Australia and New Zealand, Leveillula taurica from Guinea, and Oidium sp. from Argentina, Australia, New Zealand and Sri Lanka (Amano 1986). Oidium sp. on Cyphomandra sp. is also reported from Nepal (Amano 1986). Oidium sp. is also reported from tamarillo in India (Gupta and Srivastava 1994). However, to our knowledge, this is the first record of a morphologically well-characterised powdery mildew fungus on Solanum betaceum in India.
Correll JC,
Gordon TR, Elliott VJ
(1987) Host range, specificity and biometrical measurements of Leveillula taurica in California. Plant Disease 71, 248–251.
| Crossref | GoogleScholarGoogle Scholar |

Gupta DK, Srivastava LS
(1994) Powdery Mildew Flora of Sikkim. Indian Journal of Hill Farming 7, 207–209.

Kiss L,
Cook RTA,
Saenz GS,
Cunnington JH,
Takamatsu S,
Pascoe I,
Bardin M,
Nicot PC,
Sato Y, Rossman AY
(2001) Identification of two powdery mildew fungi, Oidium neolycopersici sp. nov. and an Oidium subgenus Reticuloidium, infecting tomato in different parts of the world. Mycological Research 105, 684–697.
| Crossref | GoogleScholarGoogle Scholar |

Peralta IE, Spooner DM
(2001) Granule-bound starch synthase (Gbssi) gene phylogeny of wild tomatoes (Solanum L. section Lycopersicon [Mill.] Wettst. subsection Lycopersicon). American Journal of Botany 88, 1888–1902.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Rai N,
Asati BS,
Patel RK,
Patel KK, Yadav DS
(2004) Underutilized horticultural crops in north eastern region. ENVIS Bulletin Himalayan Ecology 13, 46–52.

Thakur NSA,
Sharma YP, Barwal RN
(1988) Tree tomato cultivation in Meghalaya. Indian Farming 37, 3.



