Benefits of oxygation of subsurface drip-irrigation water for cotton in a Vertosol
L. Pendergast A C , S. P. Bhattarai B and D. J. Midmore BA Department of Agriculture, Forestry and Fisheries (DAFF) Queensland, LMB 6, Emerald, Qld 4720, Australia.
B School of Medical and Applied Science (SMAS), Central Queensland University, Rockhampton, Qld 4702, Australia.
C Corresponding author. Email: lance.pendergast@daff.qld.gov.au
Crop and Pasture Science 64(12) 1171-1181 https://doi.org/10.1071/CP13348
Submitted: 9 October 2013 Accepted: 5 December 2013 Published: 18 December 2013
Journal Compilation © CSIRO Publishing 2013 Open Access CC BY-NC-ND
Abstract
Australian cotton (Gossypium hirsutum L.) is predominantly grown on heavy clay soils (Vertosols). Cotton grown on Vertosols often experiences episodes of low oxygen concentration in the root-zone, particularly after irrigation events. In subsurface drip-irrigation (SDI), cotton receives frequent irrigation and sustained wetting fronts are developed in the rhizosphere. This can lead to poor soil diffusion of oxygen, causing temporal and spatial hypoxia. As cotton is sensitive to waterlogging, exposure to this condition can result in a significant yield penalty. Use of aerated water for drip irrigation (‘oxygation’) can ameliorate hypoxia in the wetting front and, therefore, overcome the negative effects of poor soil aeration. The efficacy of oxygation, delivered via SDI to broadacre cotton, was evaluated over seven seasons (2005–06 to 2012–13). Oxygation of irrigation water by Mazzei air-injector produced significantly (P < 0.001) higher yields (200.3 v. 182.7 g m–2) and water-use efficiencies. Averaged over seven years, the yield and gross production water-use index of oxygated cotton exceeded that of the control by 10% and 7%, respectively. The improvements in yields and water-use efficiency in response to oxygation could be ascribed to greater root development and increased light interception by the crop canopies, contributing to enhanced crop physiological performance by ameliorating exposure to hypoxia. Oxygation of SDI contributed to improvements in both yields and water-use efficiency, which may contribute to greater economic feasibility of SDI for broadacre cotton production in Vertosols.
Additional keywords: drip irrigation, hypoxia, oxygation, root development, SDI, water productivity.
Introduction
The total value of the Australian cotton (Gossypium hirsutum L.) crop for 2010–11 was estimated at AU$2.87 billion (Cotton Australia 2013). The crop is predominately grown on heavy clay soils (Vertosols) (Thongbai et al. 2001) and irrigated via furrow irrigation, which often has inherent issues of poor water-use efficiencies including irrigation-induced runoff. Cotton is poorly adapted to waterlogging (Hodgson and Chan 1982; Hodgson et al. 1990), particularly if exposed to this condition during early squaring (Bange et al. 2004), leading to the possibility that cotton production performance in heavy clay soils suffers from exposure to hypoxia. Crop exposure to oxygen deficiency in the rhizosphere during and after an irrigation event in flood-irrigated cotton has been well documented (Milroy et al. 2009). Improving water-use efficiency in cotton production is a priority for research and development for sustainable cotton production in Australia.
Various alternative irrigation methodologies have been explored recently in the quest for improved water-use efficiencies by the cotton industry. Whereas subsurface drip irrigation (SDI) has been accepted in several other irrigated crop industries, this option has had limited uptake by Australian cotton irrigators, partly because the performance of SDI often failed to justify the capital investment required for installation of the SDI infrastructure (Raine and Foley 2002).
Subsurface drip irrigation is reportedly nearly 100% efficient (i.e. 100% of the water delivered is accounted for by crop evapotranspiration, ETc), compared with furrow irrigation, which typically averages 50% efficiency (Smith et al. 2005). However, Bhattarai et al. (2005) and McHugh et al. (2008) noted that yields of SDI cotton on a heavy clay soil did not respond to an irrigation rate exceeding 75% of the daily ETc. It was concluded that constrained performance of cotton irrigated at rates >75% ETc was most likely due to the temporal and spatial waterlogging in the rhizosphere, leading to hypoxic conditions characteristic of SDI in heavy clay soils. A similar phenomenon was noted by Payero et al. (2008) on corn in a Cozad silt loam (fine-silty, mixed, mesic Fluventic Haplustoll; Soil Survey Staff 2010) soil in North Platte, Nebraska. They attributed the lack of response at higher irrigation rate (>200 mm of seasonal irrigation) to low soil oxygen and possible leaching of nitrate.
The adverse effects of low soil oxygen availability on root performance have been extensively documented (Armstrong 1979; Vartapetian and Jackson 1997; Barrett-Lennard 2003; Shi et al. 2007) and are associated with a penalty on crop performance and yield. Various options, including the use of pressurised air for aerating the rhizosphere of the irrigated crops, have been evaluated in the past. However, injecting air alone produced dis-uniformities of distribution and was therefore not suitable for broadacre, irrigated production. A new approach for injecting aerated water containing bubbles utilising air-injection venturis (‘oxygation’) was tested to overcome the inherent problem associated with injecting air alone. Several studies including pot trials and small-scale plot trials (Goorahoo et al. 2002; Huber 2000; Bhattarai 2005; Bhattarai and Midmore 2009) attributed enhanced root performance and water-use efficiencies across a range of crop species in SDI systems to oxygation of irrigation water.
In Australia, most cotton is grown during summer, when most of the annual rainfall is received (Milroy et al. 2009). This, coupled with higher water temperature, contributes to low oxygen saturation in the irrigation water, and limits the availability of soil oxygen.
Improved root performance and increased yields and water-use efficiency of cotton grown with oxygated SDI in controlled-environment trials have been reported by Bhattarai and Midmore (2004). Although these results were encouraging, it is a significant leap from pot/small-scale plot trials to a broadacre context where the efficacy of oxygation for cotton had not been evaluated. In the present study, field trials of cotton were carried out over seven seasons to investigate whether the earlier promising results of oxygation in pot and small-plot experiments would translate to a commercial-scale, broadacre crop.
Materials and methods
Site and crop description
The experiment was conducted using an existing SDI system on a cotton farm, ‘Nyang’, Emerald, central Queensland, Australia (23°28′22.4″S, 148°19′49.8″E; elevation 190 m a.s.l.), on a Vertosol (Isbell 2002). Cotton variety Sicot 71BR was planted in the first year (2005–06) and Bollgard II Roundup Ready® in subsequent years (2007–2013). All were planted within the window of September–October to establish 10 plants m−2, using a tractor-driven seed dibber directly above the dripper lines. The field was managed uniformly across treatments and the farmer controlled all fertiliser, insecticide, growth regulators and defoliant applications as per standard industry practices across both treatments.
Experimental design and treatments
Annually the experiment was laid out in a randomised complete block design with 12 plots assigned to six replications of two irrigation treatments, both irrigated at 85% ETc: (i) aeration of irrigation water by mixing (Fig. 1) 12% air by volume of water (referred to as ‘oxygation’) using air-injectors (Model MI1583; Mazzei Corporation, Bakersfield, CA, USA; Fig. 2); and (ii) no aeration (the control). Oxygated water was thus delivered to the soil through the SDI tape. The SDI tape (Python 22135; Netafim Ltd, Tel Aviv, Israel), installed in 2001 and consisting of emitters spaced at 40 cm, each with a delivery capacity of 0.7 L h–1 (at 117 kPa), was buried at 40 cm depth and had a system capacity of 12 mm day–1. Irrigation was individually controlled to each of twelve 0.43-ha plots (i.e. 5.2 ha overall) by solenoid-operated in-line valves. An in-line water meter (Model HFS Flow Sensor; Hunter Industries, San Marcos, CA, USA) measured total applied water, and the computerised controller monitored volumes applied to individual plots (each 16 rows of 250 m in length), as outlined in Fig. 1.
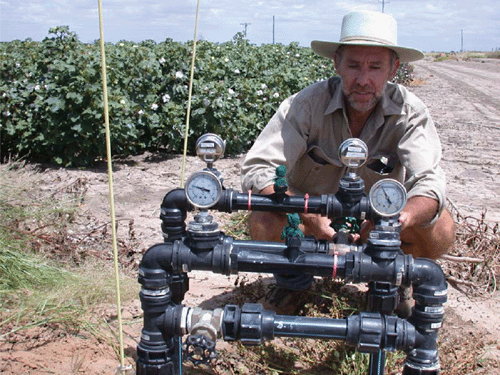
|
Irrigation scheduling
All irrigations were automated and rates adjusted daily on a rolling average of the ETc over the three previous days. Visual observation of leaf area and crop phenological development was used to determine the appropriate crop coefficients (Kc) (Allen et al. 1998), which were then applied to the computed reference crop ET (ETo) supplied by the on-site weather station (WM2000; Environdata Weather Stations Pty Ltd, Toowoomba, Qld), in order to calculate the daily ETc. The same station recorded rainfall, temperature, wind speed and direction, and humidity at 10 min-intervals and computed ETo using the modified Penman-Monteith equation described by Meyer et al. (1999). The relationship between cotton growth stages and heat units after planting (HUAP), documented by previous trials at the same site, provided a valuable guide to anticipated timing of the different stages of phenological development:
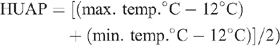
Soil moisture and oxygen monitoring
Soil moisture was monitored using capacitance probes calibrated on-site (Table 1) (EnviroSCAN; Sentek Technologies, Stepney, S. Aust.). A single probe with sensors recording data at 20, 40, 60, 80 and 110 cm depth was placed in each plot, positioned at 0.1 m from the tape and 0.1 m from an emitter.

|
The O2 concentration in the soil was measured over a single crop (2005–06) using PSt3 O2-sensitive fibre-optic mini-sensors (optodes) with Fibox-3 oxygen meters (PreSens GmbH, Regensburg, Germany) as described by Klimant et al. (1995). The optodes were placed at a uniform position with respect to an emitter (20 mm distant at right angles to the tape-line and 35 cm below the soil surface). Readings were conducted at a variety of locations to compare the various treatments, but were typically paired to compare the oxygation with the control at both proximal and distal locations within a drip-line, with respect to the location of the air-injector. Figure 3 shows the spike in soil O2 associated with a 2-h irrigation event, followed by a gradual decrease over 26 h.
Root sampling and measurement of root length density (RLD)
Manual hollow core (3.2-cm-diameter) root sampling was conducted to a depth of 100 cm to examine variation in spatial root length density. Soil cores were collected 35 m downfield from the irrigation mainline at the top end of the block, on a line perpendicular to the row and emitter at 1.5, 20 and 40 cm. A further core at the same distance was taken at 47 cm (the last a triangulation value, 40 cm perpendicular and 25 cm laterally). Sample cores were then subsampled to 2–4-cm sections to estimate RLD for 10, 30, 70 and 90 cm soil depths.
Roots were separated from the heavy black clay soil by soaking cores in a 1% solution of Groundbreaker (active constituent 10 g L–1 of buffered polylignosulfonate; Multicrop Pty Ltd, Scoresby, Vic.) for 2–3 h, before separation from the soil using a 45-µm sieve, following the floatation technique reported by Bhattarai (2005). Living roots were separated manually by discarding the dead ones based on visual observation of tissue colour as described by Caldwell and Virginia (1991). Root length and diameter were determined using a scanner (Hewlett Packard Australia Pty Ltd, Melbourne) and Delta-T software (Delta-T Devices Ltd, Cambridge, UK). Washed root samples were then oven-dried for 48 h at 70°C for determination of dry root mass. Total root mass and shoot : root ratio per plant reported on a treatment basis were derived from the root analysis.
Root sampling data were collected in the 2005–06 season, during the early stage of oxygation field trials, where oxygation treatments were evaluated at two different levels of irrigation regime (85 and 105% ETc). These data were pooled to compare oxygation and control treatment only. Coring undertaken at 91 days after planting (DAP) in 2005–06 allowed a comparative analysis of fibrous root characteristics and thus determination of treatment effect on RLD at various depths and distances from the plant and drip-tape row. These data, in association with taproot measurements collected from the whole plant sampling, permitted calculation of total root mass per plant and the shoot : root ratio.
Aboveground biomass and yield measurement
Cotton plants were sampled each year from trial plots before machine harvest was undertaken by the grower. Plot sampling consisted of two 2-m lengths at the top, middle and bottom of the rows (i.e. six samples per plot). Bolls were then separated into lint, husk and seed. In the 2005–06 season, plants harvested (at 118 DAP) were separated into bolls, stems and leaves to determine the partitioning of biomass. Data presented refer to lint yield and they are presented as g m–2. A cotton-ginning factor of 38% was applied across the treatments and years. The machine-harvested yield (bales ha–1, 1 bale = 227 kg) was also recorded in several years and the consistency between the machine harvest and sample plot harvest was evaluated.
Water-use efficiency parameters
Gross production water use index (GPWUI), based on total water inputs to the crop including rainfall, was calculated using measured lint weight and is reported as g dry weight (DW) m–3 water.
Canopy light interception
Canopy capture of photosynthetically active radiation (PAR) was measured at 118 DAP in 2005–06. Two averaged readings per plot were made, each consisting of one reading above and four readings at right angles to the row beneath the canopy (ground level), using a PAR ceptometer (Decagon Devices, Pullman, WA, USA). Per cent light interception was calculated as the difference between PAR above and below the canopy (% intercepted PAR = [(above – below)/above] × 100).
Statistical analyses
Twelve plots were assigned to six replications of two irrigation treatments (oxygation and control) in a randomised complete block design. The trial was repeated for seven years using the same plots and design. As such, repeated-measures were made on the plots. The effect of time (different years) and oxygation treatment on yield and predicted yield was analysed using residual maximum likelihood (REML) and modelling the variance–covariance component with an ante-dependence structure of order 1 using Genstat 16th Edition for Windows statistical software (VSN International Ltd, Hemel Hempstead, UK). Separation of means was conducted using least significant difference (l.s.d.) at P ≤ 0.05.
Pendergast (2011) evaluated effect of oxygation at two irrigation regimes (85 and 105% ETc), and there was no interaction effect between irrigation rate and oxygation treatment for lint yield of cotton. As the irrigation rate and oxygation treatment did not produce interaction effects, the data from 2005–06 were pooled into oxygation and control treatments.
Root data collected in the 2005–06 season were analysed separately. Since core sampling was done at fixed locations, it was analysed as a replicated split-split-plot with oxygation as the main plot, distance as subplot and depth as sub-subplot, since the variance–covariance structure could be adequately modelled using a uniform variance–covariance structure. All RLD data were also transformed (y = √(x + 1) following the methodology employed by Machado et al. (2003) to ensure that analysis of variance (ANOVA) was not distorted by zero values included in the original dataset.
Results
Weather and rainfall during crop period
Rainfall, daily evapotranspiration and accumulated heat units for all 7 years (2005–06 to 2012–13) at the trial site are presented in Fig. 4. Heat units for crop maturity ranged from 1500 to 2400 degree-days, and the reference crop evapotranspiration (ETo) reached as high as 10 mm day–1. Of 7 years, 2 years (2007–08 and 2010–11) were wetter (>500 mm), 2 years (2008–09 and 2009–10) were moderately wet (300–400 mm) and the other 3 years (2005–06, 2011–12, 2012–13) were reasonably low in rainfall (100–200 mm) (Fig. 4).
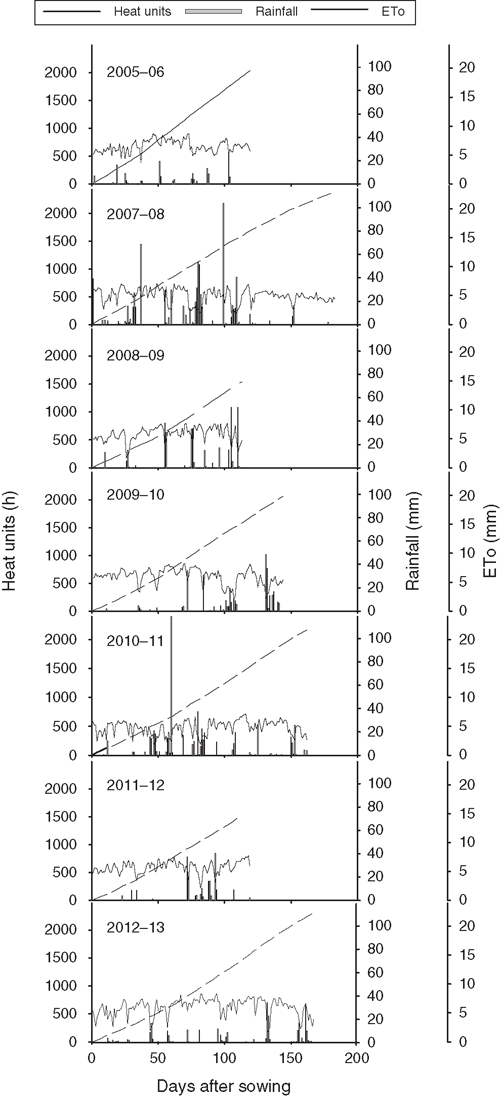
|
Water input and soil water balance
Irrigation input to the crop was matched for oxygation and control treatments. Irrigation inputs ranged across years from 2.48 to 7.14 ML ha–1 for the crop period, and the large volumes were applied particularly when the in-season rainfall was low. Average SDI input across years was 4.13 ML ha–1, whereas total crop water use was 7.77 ML ha–1 (Table 2), the difference representing rainfall and carry-over residual soil moisture.
Change in soil moisture in the profile
The season-long soil-moisture status reflected the difference in soil moisture supply and extraction patterns between the two treatments (oxygation and control). Comparison of soil-moisture profile over the full crop-growing season, measured at four sensor depths (20, 40, 80 and 100 cm) for the 2004–05 season cotton crop (Fig. 5), shows that the soil-moisture profile of the control treatment remained wetter at all depths throughout the crop season compared with the oxygated treatment at similar volumes of irrigation and rainfall input. That is, with oxygation the soil was less likely to be saturated even at shallower depths. Soil-moisture extraction from depth of 80 and 100 cm was very low irrespective of the treatment. Measured yield and predicted yield following REML procedures were consistent for treatment effects over years.
Differences in soil-moisture profiles over the season between the two treatments were noticeable, and there was a distinct difference during the initial stage of crop development, i.e. from emergence to initial flowering (1 November–18 December), when the soil moisture depletion was more pronounced than in the later part of the crop growth season.
Yield and yield components
There was no interaction (P > 0.01) between time and treatment. Oxygation increased yield by 10% (P < 0.001; 182.7 v. 200.3). Yield was greatest in 2009–10 and 2011–12, least in 2008–09 and 2012–13, and intermediate in 2005–06, 2007–08 and 2010–11 (Table 2).
The total plant biomass sampled in the 2005–06 season at 118 DAP for the oxygation treatment was significantly greater (by 12%) than the control. Oxygated plants produced 15% more bolls, resulting in 13% higher boll weights per plant than those in the control (Table 3). The greater yield with oxygation can be attributed to the contribution made by the combined effect of number of bolls and the resultant total boll weight per plant (Table 3).
Root properties
Data collected in the 2005–06 season showed significantly greater (by 17%) total root mass per plant (Table 4) with oxygation than with the control. Oxygation also resulted in higher (53%) RLD, greater (2%) fibrous root mass, and significantly larger (26%) taproots per plant than the control (Table 4).
Spatial analysis of root length density
No significant interaction between irrigation treatment and distance from the row was identified for RLD in 2005–06. The RLD was greater closer to the row; RLD at 1.5 cm from the row was at least twice that at ≥20 cm distance from the row. Overall, RLD diminished with depth (Fig. 6) and distance from the row, although the major decline was between the row and 20 cm distance. However, there was a significant interaction between distance from the row and depth for RLD (Fig. 6). With the exception of the shallowest sample interval at both 1.5 and 20 cm from the drip line, RLD of the 2005–06 cotton at 36–40 cm at each distance was significantly higher (P ≤ 0.05; s.e.d. (d.f. 14.71) = 0.146) than at all other points (Fig. 6).
Canopy characteristics and light interception
Oxygated plants, as measured at 118 DAP in 2005–06, produced more extensive canopies, characterised by greater light interception, than their control counterparts (Table 3). Oxygated plants produced marginally larger leaves and greater leaf area than the control. Canopy light interception of oxygated plants was significantly greater than that of plants in the control treatment (by 3%). The greater light interception of the oxygation treatment was consistent with the effect of treatment on the leaf area.
Irrigation and water use efficiencies
Averaged over the 7 years, the GPWUI of oxygated cotton exceeded that of the control by 7% (Table 2). The effects of oxygation treatment on GPWUI were only marginally significant (P < 0.13), whereas the effects due to year for this parameter were significant (P < 0.001).
Discussion
Higher soil moisture extraction rates (i.e. drier soil at similar irrigation rate) in the wetting fronts in the oxygated treatments suggests greater root activity (i.e. drier soil at similar irrigation rate) due to oxygation, which is likely to contribute to increased crop growth. This observation is corroborated by the fact that the plants in the oxygated treatment recorded greater light interception (75% v. 72% for oxygation v. control) (Table 3). Results of root sampling conducted in 2005–06 show that oxygation favoured development of higher root density and heavier root mass per plant. The enhanced root development of oxygated cotton, as expressed by total weights (~17%) or RLD (52%) (Table 4), suggests that the hypoxic rhizosphere conditions associated with SDI cotton grown in heavy clay soils could have been alleviated by oxygation (Bhattarai et.al. 2004). The development of greater RLD, and consequently a root system capable of supporting more vigorous crop growth, resulted in significantly greater biomass and lint yields of oxygated compared with control cotton.
These results are consistent with earlier results by Goorahoo et al. (2002), who reported enhanced root performance as the primary mediator of oxygation response in terms of improved yield and water-use efficiencies in several crops including cotton (Bhattarai et al. 2004). The development of more extensive root systems by oxygated plants, and maintenance of soil moisture levels at below field capacity, reduced the tendency for water to move down the profile, and thus reduced the potential for deep drainage, which impacts negatively on water-use efficiencies. This was evident in the increased GPWUI with oxygation (Table 2).
Increased root activities in the oxygated treatment are implied, with greater access to oxygen driving a higher rate of root respiration in the oxygated treatment (Bhattarai et al. 2005). We present some basic calculations to demonstrate the volume of oxygen available in the wetting front. For example, in an irrigation event delivering 8 mm water, a venturi introducing 12% air by volume of water into the irrigation stream would, compared with control irrigation, deliver an additional 1150 L O2 ha–1 to the rhizosphere as air bubbles:
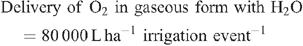
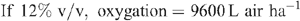
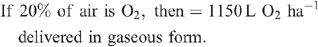
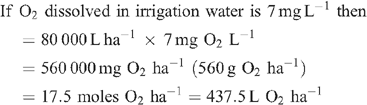
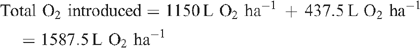
At a consumption rate of 3500 L O2 ha–1 h–1 (Pendergast 2011), this additional 1587.5 L O2 ha–1 is sufficient to support root respiration for the whole soil mass to 1 m depth for 20 min. However, the O2 delivered in irrigation water is not available to the whole soil mass. Therefore, we should consider only the saturated zone around the emitter in this calculation. An irrigation event of 8 mm involves delivery of 3.2 L of water per dripper, and 199 mL O2 (in both gaseous and dissolved forms). If the difference in water content of the soil before irrigation and saturation is 30% v/v, then 3.2 L of water will saturate a volume of ~10 L (0.01 m3). Assuming a respiration rate of 0.35 L O2 m–2 h–1, the O2 delivered in the irrigation water is sufficient to support root respiration within the saturated zone for 5.7 h.
As the irrigation water input to the crop during the season is directly influenced by the amount of rainfall received, it is expected that seasonal variation will have a strong influence on GPWUI. Despite the significant variation in climatic conditions across the 7 years, it was notable that oxygated plants returned a 7% improvement in GPWUI (Table 2). Increased oxygen availability in the rhizosphere enables development of a more extensive and effective root system, which increases the capability of the plant to extract water from the soil profile, particularly from the zone of wetting fronts. The comparison of extraction rates (Fig. 5) indicated that oxygated plants were more capable of extracting soil moisture than their control counterparts. In addition to more vigorous plant growth and the capacity for increased yields, enhanced root function contributes to a reduction in potential loss of water through deep drainage and, conversely, to utilising the water in transpiration, which contributed to higher GPWUI.
The addition of oxygen to the soil is considered not only to satisfy demand by roots, but also to influence the soil microbial community, also evidenced by greater soil respiration rate. This aspect has been evaluated for recent crops in several oxygation trials over time (Dhungel et al. 2012). An effect on soil microbial populations may also influence nutrient cycling. Plant nutrient-use efficiencies and various soil characteristics, including hydraulic parameters, should also be considered in relation to long-term SDI, and oxygation of SDI.
In general, the positive effect of the oxygation treatment on lint yield was significant (10%), with no interaction between years and oxygated treatments. The year with moderate rain and reasonable irrigation inputs (2011–12) recorded the highest yields. However, in 3 years (2007–08, 2009–10 and 2011–12), yields exceeded the district average; in 2 years (2005–06 and 2010–11) the yields were similar; and in two years (2008–09 and 2012–13) the yields were appreciably lower. In the 2008–09 season the crop received total water of only 6 ML ha–1, suggesting that crop growth was related to the low water input, whereas the low yield in the 2012–13 season is attributed to poor performance of the SDI system and its controllers.
In 2005–06 when a more extensive physiological examination was undertaken, cotton grown on the oxygated treatment produced significantly higher lint yields (Table 2). Higher lint yield was strongly correlated with both total biomass (r2 = 0.93) and aboveground biomass (r2 = 0.95) (Pendergast 2011). This result is consistent with the pot trials of Bhattarai et al. (2004) and Bhattarai and Midmore (2009). The main contributor to the increased yield with oxygation was the number of bolls per plant (correlation with yield r2 = 0.91) and the resultant total boll weight per plant (Table 3). Total boll weight per plant for the oxygation treatment was heavier than in the control counterparts (12.8%). There was no response of individual weight per boll to the treatments. These outcomes are in agreement with the conclusions of Bange et al. (2004), who reported reduction in final boll number as the principal driver of waterlogging-induced yield depression, with boll size and percentage lint unaffected. However, the results are at variance with those of Bhattarai et al. (2004) and Bhattarai and Midmore (2009) who, in each case, attributed yield enhancement of oxygation not only to increased number (~20% and 17%, respectively) but also to individual weight per boll (7% and 7%, respectively).
Results of oxygation trials conducted in pots under a controlled environment (Bhattarai et al. 2004) reported yield increases of ~27%; however, the long-term yield gain with oxygation from the field trials was only 10%. This shows a clear yield gap of 17% for the increase with oxygated treatment between controlled conditions and in the field. In pot trials, the root system is confined to the area that is irrigated (and therefore liable to suffer hypoxia) and is not subject to the soil heterogeneity associated with black cracking clays (Améglio et al. 1999); in the field, roots can forage beyond the irrigation zone and, especially under rainfed conditions, this can affect root supply of oxygen, diluting the benefit of oxygation. Further investigation, with a focus on uniformity of air distribution and optimised application rates, may prove beneficial.
Cost of aerating the SDI water and payback period for system installation
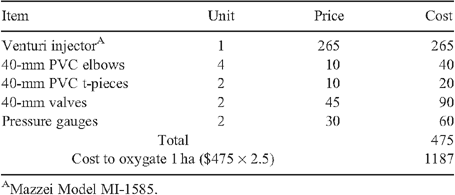
Cost of material for retro-fitting the injectors to the existing SDI system was AU$475 per 0.4-ha plot (Table 5), which equates to $1187 ha–1. This cost would be reduced if installing larger injector units capable of supplying oxygated water to a larger area than the 0.4-ha individual replicate plots. The long-term average yield for control and oxygation was 8.09 and 8.70 bales ha–1. The difference of 0.61 bales ha–1 at the price of $500 bale–1 will bring an additional return of $305 ha–1 season–1. The payback period for the system installation that cost $1187 will be 3.9 years. The current system of oxygation at the site has been running for 9 years and shows no signs of deterioration. Hence, with a continuation of attention to its annual maintenance program, the expected life of the system is considered to be 20 years (Zoldoske 2013).
|
Conclusions
This study involved a scale-up from previous pot trials to a field-scale trial. The results clearly demonstrate the potential to increase yield and water-use efficiencies of cotton on a Vertosol soil through oxygation of irrigation water. Oxygation of SDI broadacre cotton over seven crop seasons resulted in significantly greater yields and enhanced water-use efficiencies, associated with more extensive root systems and increased light interception by the canopy. Averaged over the 7 years, SDI oxygation significantly increased cotton lint yields by 10%, while the GPWUI increased by 7%. The yield gap between results from controlled environment trials and the field trials reported here can potentially be narrowed and gains made to further increase the yield of SDI field cotton.
The installation of SDI requires a high level of confidence by potential investors that anticipated benefits would justify the capital investment. An increase in yield and water-use efficiency with oxygation of SDI will be a factor of interest to those considering the installation of drip irrigation.
Acknowledgements
The authors are grateful to CQUniversity, Australia, for assistance (financial and other) provided throughout the conduct of the trials, National Program for Sustainable Irrigation (NPSI) for the funding support provided for continuation of trials in 2008–10, Cotton CRC for the scholarship support provided (2008–10) to Jay Dhungel, and Department of Agriculture, Forestry and Fisheries Qld (DAFF) for its ongoing commitment to the conduct of the trials. Special thanks are extended to cotton grower, Tony Ronnfeldt, for providing the site. His continuous, unwavering support was crucial for the conduct of trials over the extended period. Appreciation is also extended to Jay Dhungel and Deepak Sharma Paudel for their contribution and assistance in data collection, sample processing and analysis from 2009–13.
References
Allen RG, Pereira LS, Raes D, Smith M (1998) ‘Crop evapotranspiration: Guidelines for computing crop requirements.’ Irrigation and Drainage Paper No. 56. (FAO: Rome)Améglio T, Archer P, Cohen M, Valancogne C, Daudet F, Dayau S, Cruiziat P (1999) Significance and limits in the use of predawn leaf water potential for tree irrigation. Plant and Soil 207, 155–167.
| Significance and limits in the use of predawn leaf water potential for tree irrigation.Crossref | GoogleScholarGoogle Scholar |
Armstrong W (1979) Aeration in higher plants. Advances in Botanical Research 7, 225–332.
Bange MP, Milroy SP, Thongbai P (2004) Growth and yield of cotton in response to waterlogging. Field Crops Research 88, 129–142.
| Growth and yield of cotton in response to waterlogging.Crossref | GoogleScholarGoogle Scholar |
Barrett-Lennard EG (2003) ‘Saltland pastures in Australia—A practical guide.’ 2nd edn (Department of Agriculture of Western Australia: South Perth, W. Aust.)
Bhattarai SP (2005) The physiology of water use efficiency of crops subjected to subsurface drip irrigation, aeration and salinity in a heavy clay soil. PhD Thesis, Faculty of Arts, Health and Sciences, Central Queensland University, Qld, Australia.
Bhattarai SP, Midmore DJ (2004) Oxygation of rhizosphere with subsurface aerated irrigation water improves lint yield and performance of cotton on saline heavy clay soil. In ‘New directives for a diverse planet. Proceedings 4th International Crop Science Congress’. 26 Sept.–1 Oct. 2004. Brisbane, Qld. (The Regional Institute Ltd: Gosford, NSW) Available at: www.cropscience.org.au/icsc2004/poster/3/6/2/665_bhattarrai.htm
Bhattarai SP, Midmore DJ (2009) Aeration enhances growth, gas exchange and salt tolerance of vegetable soybean and cotton in a saline vertisol. Journal of Integrative Plant Biology 51, 675–688.
| Aeration enhances growth, gas exchange and salt tolerance of vegetable soybean and cotton in a saline vertisol.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXptlOisbs%3D&md5=9005a4d43ff48a811b411838baecd165CAS | 19566646PubMed |
Bhattarai SP, Huber S, Midmore DJ (2004) Aerated subsurface irrigation water gives growth and yield benefits to zucchini, vegetable soybean and cotton in heavy clay soils. Annals of Applied Biology 144, 285–298.
| Aerated subsurface irrigation water gives growth and yield benefits to zucchini, vegetable soybean and cotton in heavy clay soils.Crossref | GoogleScholarGoogle Scholar |
Bhattarai SP, Su N, Midmore DJ (2005) Oxygation unlocks yield potentials of crops in oxygen-limited soil environments. Advances in Agronomy 88, 313–377.
| Oxygation unlocks yield potentials of crops in oxygen-limited soil environments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXitlKisro%3D&md5=febdf057e3a123101e423594106191f3CAS |
Caldwell MM, Virginia RA (1991) Root systems. In ‘Plant physiological ecology: Field methods and instrumentation’. (Eds RW Pearcy, J Ehleringer, HA Mooney, PW Rundel) pp. 367–398. (Chapman and Hall: London)
Cotton Australia (2013) Statistics. Cotton Australia, Sydney, NSW. Available at: http://cottonaustralia.com.au/cotton-library/statistics
Dhungel J, Bhattarai SP, Midmore DJ (2012) Aerated water irrigation (oxygation) benefits to pineapple yield, water use efficiency and crop health. Advances in Horticultural Science 26, 3–16.
Goorahoo D, Carstensen G, Zoldoske DF, Norum E, Mazzei A (2002) Using air in subsurface drip irrigation (SDI) to increase yields in bell peppers. International Water and Irrigation 22, 39–42.
Hodgson AS, Chan KY (1982) The effect of short-term waterlogging during furrow irrigation of cotton in a cracking grey clay. Australian Journal of Agricultural Research 33, 109–116.
| The effect of short-term waterlogging during furrow irrigation of cotton in a cracking grey clay.Crossref | GoogleScholarGoogle Scholar |
Hodgson AS, Constable GA, Duddy GR, Daniells IG (1990) A comparison of drip and furrow irrigated cotton on a cracking clay soil: Water use efficiency, waterlogging, root distribution and soil structure. Irrigation Science 11, 143–148.
| A comparison of drip and furrow irrigated cotton on a cracking clay soil: Water use efficiency, waterlogging, root distribution and soil structure.Crossref | GoogleScholarGoogle Scholar |
Huber S (2000) New uses for drip irrigation—partial root zone drying and forced aeration. MSc thesis, Technische Universitat Munchen, Germany.
Isbell RF (2002) ‘The Australian Soil Classification.’ Rev edn (CSIRO Publishing: Melbourne)
Klimant L, Meyer V, Kuhl M (1995) Fibre-optic oxygen micro-sensors, a new tool in aquatic biology. Limnology and Oceanography 40, 1159–1165.
| Fibre-optic oxygen micro-sensors, a new tool in aquatic biology.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXpsVCrur8%3D&md5=2b38f2784277928a1f6a96d41c4f5df6CAS |
Machado RMA, Oliveira G, Rosario MD, Portas CAM (2003) Tomato root distribution, yield and fruit quality under subsurface drip irrigation. Plant and Soil 255, 333–341.
| Tomato root distribution, yield and fruit quality under subsurface drip irrigation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXotV2jsr8%3D&md5=a6f75b8c6732d4496ce20f98e89cf31dCAS |
McHugh AD, Bhattarai SP, Lotz G, Midmore DJ (2008) Effects of subsurface drip irrigation rates and furrow irrigation for cotton grown on a vertisol on off-site movements of sediments, nutrients and pesticides. Agronomy for Sustainable Development 28, 507–519.
| Effects of subsurface drip irrigation rates and furrow irrigation for cotton grown on a vertisol on off-site movements of sediments, nutrients and pesticides.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsFekurbI&md5=11bff34cc24b1d10edf4f0c7a0f255f8CAS |
Meyer WS, Smith DJ, Shell GE (1999) Estimating reference evaporation and crop evapotranspiration from weather data and crop coefficients. CSIRO Land and Water Technical Report 34/98.
Milroy SP, Bange MP, Thongbai P (2009) Cotton leaf nutrient concentrations in response to waterlogging under field conditions. Field Crops Research 113, 246–255.
| Cotton leaf nutrient concentrations in response to waterlogging under field conditions.Crossref | GoogleScholarGoogle Scholar |
Payero JO, Tarkalson DD, Irmak S, Davison D, Petersen JL (2008) Effect of irrigation amounts applied with subsurface drip irrigation on corn evapotranspiration, yield, water use efficiency, and dry matter production in a semiarid climate. Agricultural Water Management 95, 895–908.
| Effect of irrigation amounts applied with subsurface drip irrigation on corn evapotranspiration, yield, water use efficiency, and dry matter production in a semiarid climate.Crossref | GoogleScholarGoogle Scholar |
Pendergast L (2011) Benefits of aeration of subsurface drip irrigation water: field evidence on CQ highlands vertosols. PhD Thesis, Central Queensland University Australia, Rockhampton, Qld, Australia.
Raine S, Foley J (2002) Comparing systems for cotton irrigation. The Australian Cottongrower 23, 30–35.
Shi K, Hu WH, Dong DK, Zhou YH, Yu JQ (2007) Low O2 supply is involved in the poor growth. Environmental and Experimental Botany 61, 181–189.
| Low O2 supply is involved in the poor growth.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXpvF2qtbg%3D&md5=1e04c7baf2085a7a4734e2fac7295161CAS |
Smith RJ, Raine SR, Minkovich J (2005) Irrigation application efficiency and deep drainage potential under surface irrigated cotton. Agricultural Water Management 71, 117–130.
| Irrigation application efficiency and deep drainage potential under surface irrigated cotton.Crossref | GoogleScholarGoogle Scholar |
Soil Survey Staff (2010) ‘Keys to Soil Taxonomy.’ 11th edn (USDA-Natural Resources Conservation Service: Washington, DC)
Thongbai P, Milroy S, Bange M, Rapp G, Smith T (2001) Agronomic responses of cotton to low soil oxygen during water logging. In ‘Proceedings of the 10th Australian Agronomy Conference’. January, Hobart, Tas. (Australian Society of Agronomy/The Regional Institute Ltd: Gosford, NSW) Available at: http://regional.org.au/au/asa/2001/2/b/thongbai.htm
Vartapetian BB, Jackson MB (1997) Plant adaptations to anaerobic stress. Annals of Botany 79, 3–20.
| Plant adaptations to anaerobic stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXhtlWrs78%3D&md5=715584cf1fd70e06622c74203a2b9a54CAS |
Zoldoske DF (2013) Subsurface drip irrigation: The future of irrigation is underground. Geoflow, Corte Madera, CA, USA. Available at: www.geoflow.com/agriculture/zoldoske.htm









