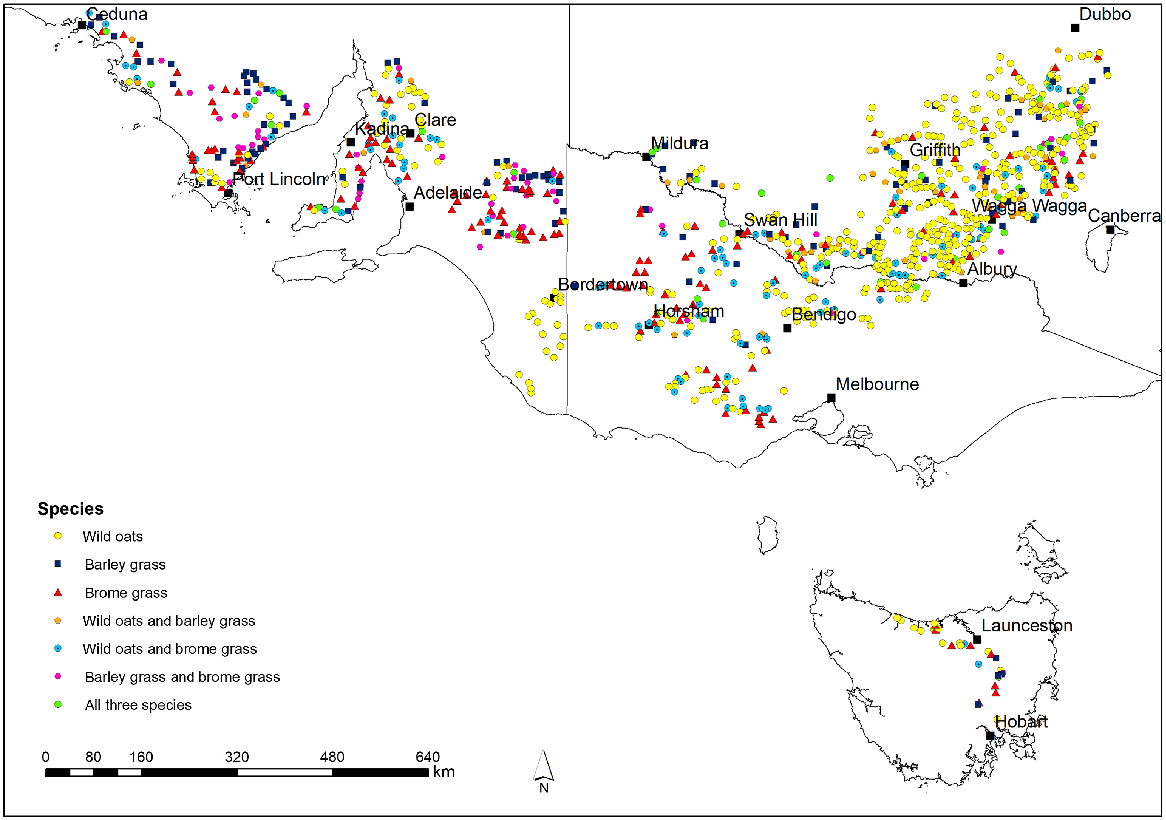
Frequency of herbicide resistance in wild oats (Avena spp.), brome grass (Bromus spp.) and barley grass (Hordeum spp.) as determined by random surveys across south-eastern Australia
John C. Broster A * , Peter Boutsalis B , Gurjeet S. Gill B and Christopher Preston
A * , Peter Boutsalis B , Gurjeet S. Gill B and Christopher Preston  B
B
A Gulbali Institute for Agriculture Water Environment, Charles Sturt University, Boorooma Street, Wagga Wagga, NSW 2678, Australia.
B School of Agriculture, Food and Wine, University of Adelaide, Glen Osmond, SA 5064, Australia.
Abstract
Wild oats (Avena spp.), brome grass (Bromus spp.) and barley grass (Hordeum spp.) are significant grass weeds of crop production in south-eastern Australia. The presence of herbicide resistance in these weed species is a major limiting factor on both productivity and profitability.
We aimed to determine the distribution of herbicide resistance in these weed species across south-eastern Australia.
Several surveys were conducted in randomly selected fields across four states in south-eastern Australia over a 5-year period, collecting 663 wild oats, 366 brome grass and 262 barley grass samples that were screened for resistance with up to five different herbicide groups or subgroups.
In wild oats, resistance was most common to clodinafop-propargyl (‘fop’ ACCase inhibitor), with 22% of samples resistant and resistance detected in all regions except Tasmania. Resistance to sulfonylurea herbicides (ALS inhibitors) was less common with 7% of samples resistant, but regionally more variable. For both brome grass and barley grass, a greater proportion of samples was resistant to the sulfonylurea mesosulfuron-methyl, at 24% and 19%, respectively, than to quizalofop-ethyl (‘fop’ ACCase inhibitor).
Resistance was recorded in all three species, with differences in the extent of resistance among herbicide groups and regions. Overall, a higher than average frequency of wild oats resistance to clodinafop-propargyl was found in regions of New South Wales and of brome grass and barley grass resistance to mesosulfuron-methyl in regions of South Australia. However, for all species some herbicides were still effective on all samples.
The presence of significant herbicide resistance in these weed species indicates that management decisions need to include consideration of resistance to enable successful control measures.
Keywords: ACCase-inhibiting herbicide, ALS-inhibiting herbicide, barley grass, brome grass, clodinafop-propargyl, glyphosate, pre-emergent herbicide, wild oats.
Introduction
Grass weeds are a major problem in grain cropping systems across south-eastern Australia. Annual ryegrass (Lolium rigidum Gaud.) is the major species of concern; however, other species such as wild oats (Avena spp.), brome grass (Bromus spp.) and barley grass (Hordeum spp.) are of high importance (Llewellyn et al. 2016). Changes in management practices for crop production combined with the development of non-herbicidal control systems used to control herbicide-resistant annual ryegrass (Walsh and Powles 2007; Llewellyn et al. 2016) may increase the importance these other grass weed species, if they are either more suited to the cropping systems or less suited for control under new weed management systems.
A survey of weed species remaining in fields ranked wild oats, brome grass and barley grass the third-, fifth- and ninth-most important weeds, respectively, across south-eastern Australia with regard to crop yield and revenue losses (Llewellyn et al. 2016). In parts of this area they are considered even more important; for example, in the Mallee region of South Australia (SA) and Victoria, brome grass is considered the most important weed of cereal crops and second-most important in canola and pulses (Llewellyn et al. 2016).
In 1993, a survey of southern New South Wales (NSW) found wild oats to be the second-most common weed of cereal crops, present in 72% of fields, behind only capeweed (Arctotheca calendula) and ahead of annual ryegrass (Lemerle et al. 1996). A subsequent survey of a portion of this area in 2010 recorded wild oats in 63% of fields, behind only annual ryegrass in prevalence (Broster et al. 2012a). Barley grass was recorded in 25% of the fields in 1993 and 17% in 2010, whereas in both surveys, brome grass was recorded in ~10% of the fields (Lemerle et al. 1996; Broster et al. 2012a).
Although annual ryegrass is the main herbicide-resistant weed in Australia, herbicide resistance has also been reported in other species of grass weeds. Resistance was first reported in wild oats (Avena fatua L.) in 1985 (Piper 1990) and Avena sterilis subsp. ludoviciana in 1989 (Mansooji et al. 1992); in two species of barley grass (Hordeum glaucum Steud. and H. leporinum Link) in 1982 and 1987, respectively (Powles 1986; Tucker and Powles 1991); and in two species of brome grass (Bromus diandrus Roth and B. rigidus Roth) in 1998 (Boutsalis and Preston 2006).
Many of the herbicides used for annual ryegrass control are also effective on these other grass species; therefore, these weed species have been subject to selection for resistance to these herbicides even if they were not the major targeted species in the fields. Previous surveys looking at herbicide resistance in wild oats have found resistance to several herbicides, albeit at lower levels than for annual ryegrass collected from the same regions (Owen and Powles 2009; Broster et al. 2011a, 2012b, 2013; Owen and Powles 2016). Similarly, the results for brome grass and barley grass samples collected in surveys show either very low levels of resistance (Owen et al. 2015) or no resistance (Broster et al. 2010).
The aim of this study was to test for herbicide resistance in wild oats, brome grass and barley grass samples and where present determine its distribution across south-eastern Australia. The samples collected were tested with a range of herbicides used for the control of these species in winter cropping in south-eastern Australia.
Materials and methods
Sample collection
From 2013 to 2017, surveys were conducted in the cropping regions of SA, Victoria, Tasmania and southern NSW (for survey sites and year of sampling, see Broster et al. 2022). Fields were selected by travelling for a predetermined distance (5 or 10 km depending upon the size of surveyed area). At each stop, a single field was surveyed with the aim to visit a minimum of 120 crop fields in each region and collect wild oats, brome grass and barley grass. Seeds were collected by either stripping the seed from the plant or removing the entire seed head. After collection, the samples were stored for after-ripening with all other seed samples collected in the survey. Full descriptions of the sampling methodology are provided in Boutsalis et al. (2012).
Plant growth and treatment
Samples collected from SA and Victoria were tested at the Waite Campus, University of Adelaide (UA), and those collected from Tasmania and NSW were tested at Charles Sturt University (CSU), Wagga Wagga. A standard susceptible sample was included in every test. At CSU, susceptible samples were wild oats BASF190143w, and for barley grass and brome grass a mix of samples previously found to be susceptible to each herbicide. At UA, susceptible populations of each species were collected from a field at Pallamana. A standard resistant sample was also included where one was available for that herbicide. Owing to limited seed availability for some samples, not all herbicides were applied to all samples.
Seeds were sown, grown and treated with a range of pre-emergent and post-emergent herbicides as described by Broster et al. (2011a). The wild oats seeds were pre-germinated on either agar (0.6%) (at CSU) or moistened filter paper (at UA) and kept in a cold room at 10°C until emergence. They were then transplanted into plastic trays (CSU, 36 plants; UA, 24 plants). For the brome grass and barley grass samples, ~50 seeds were sown directly into pots or plastic trays and thinned to 25 seedlings per tray before treatment.
At CSU, plastic trays (150 mm by 100 mm by 60 mm) were used, containing either a 50:50 peat:sand mix (for testing clodinafop, quizalofop, clethodim, paraquat + diquat and glyphosate) or garden loam (for all other herbicides). At UA, 0.9-L pots containing a coco peat mix were used. Seeds treated with pre-emergent herbicides were sown on the soil surface, sprayed, and then covered with a 5-mm layer of either garden loam (CSU) or coco peat mix (UA). For post-emergent herbicides, seedlings were treated at the 1- or 2-leaf stage. At CSU the trials were conducted in a temperature-controlled glasshouse (10–25°C), whereas at UA they were conducted outdoors under natural autumn and winter growing conditions. At both locations the trials were watered daily and fertilised as required. After this, the plants were maintained as described by Broster et al. (2012b). The herbicides and rates used for the different species are listed in Table 1.
| Herbicide | Herbicide mode of action | Pre- or post-emergent treatment | Rate (g a.i. or a.e. ha−1) | Species treated | |
|---|---|---|---|---|---|
| Clodinafop | ACCase inhibitor (‘fop’) | Post | 20.4 | Wild oats | |
| Quizalofop | ACCase inhibitor (‘fop’) | Post | 30 | Brome grass, barley grass | |
| Clethodim | ACCase inhibitor (‘dim’) | Post | 96 | Wild oats (NSW, Tas., SA Mid North only), brome grass (NSW and Tas. only), barley grass (NSW, Tas., SA Mid North only) | |
| Iodosulfuron | ALS inhibitor (sulfonylurea) | Post | 20 | Wild oats (NSW, Tas. only) | |
| Mesosulfuron | ALS inhibitor (sulfonylurea) | Post | 10 | Wild oats (Vic., SA only), brome grass, barley grass | |
| Imazapyr + imazamox | ALS inhibitor (imidazolinones) | Post | 28.8 | Brome grass, barley grass | |
| Tri-allate | Fat synthesis inhibitor | Pre | 500 | Wild oats | |
| Paraquat + diquat | PSII inhibitor | Post | 300 | Barley grass | |
| Glyphosate | EPSPS inhibitor | Post | 540 | Wild oats (Southern NSW only), brome grass, barley grass |
ACCase, acetyl-CoA carboxylase; fop, aryloxyphenoxypropionate; dim, cyclohexanedione; ALS, acetolactate synthase; PSII, Photosystem II; EPSPS, 5-enolpyruvylshikimate-3-phospate; a.i., active ingredient; a.e., acid equivalent.
Plants were counted as survivors of the herbicide if they had emerged and developed to the 2-leaf stage at 28 days after treatment with pre-emergent herbicides or if they had new shoot growth at 21 days after treatment with post-emergent herbicides. Following Boutsalis et al. (2012), samples from each field were considered resistant if ≥20% of the individuals in that sample survived the herbicide application. In these self-pollinated species, samples with 1–19% survival were uncommon, so a category of developing resistance as used in some surveys for annual ryegrass (Owen et al. 2014) was not included.
Statistical analyses
In cases where (1) two different herbicide modes of action were used on a species, (2) two herbicides were used within a mode of action, or (3) the same mode of action was used on different weed species, a multinomial goodness of fit test (G-statistic) was used to test whether the frequency of resistance was similar (McDonald 2014). The null hypothesis was that resistance occurred equally between the two parameters.
The frequency of resistance to each herbicide in each region was compared with the average frequency across all regions, using an exact binomial test (McDonald 2014) with the null hypothesis being no difference between the regions. The same test was used to compare the presence of the three species across all regions with the null hypothesis that they were equally distributed across the regions.
Results
Samples collected and crop distribution
Over the period 2013–17, 996 of the 1760 fields visited contained one or more of wild oats, brome grass and barley grass. In total, 663 wild oats, 366 brome grass and 262 barley grass samples were collected from these fields (Table 2). Of these 996 fields, 741 contained only one of these species (458 wild oats, 169 brome grass, 114 barley grass), 215 contained two species (107 wild oats and brome grass, 58 wild oats and barley grass, 50 brome grass and barley grass), and 40 contained all three species (Fig. 1).
| State/region | Year surveyed | No. of fields visited | No. of samples collected | |||
|---|---|---|---|---|---|---|
| Wild oats | Brome grass | Barley grass | ||||
| New South Wales | ||||||
| Slopes | 2013 | 199 | 100 | 39 | 43 | |
| Eastern | 2014 | 75 | 35 | 11 | 13 | |
| Western | 2015 | 164 | 96 | 30 | 50 | |
| Plains | 2016 | 152 | 71 | 9 | 7 | |
| Southern | 2017 | 162 | 108 | 23 | 10 | |
| Tasmania | 2014 | 75 | 17 | 12 | 6 | |
| South Australia | ||||||
| Mid North | 2013 | 150 | 47 | 45 | 19 | |
| Eyre Peninsula | 2014 | 167 | 31 | 52 | 56 | |
| Mallee | 2017 | 164 | 11 | 56 | 39 | |
| South East | 2017 | 74 | 20 | 0 | 0 | |
| Victoria | ||||||
| Southern | 2014 | 116 | 26 | 22 | 0 | |
| Wimmera–Mallee | 2015 | 140 | 42 | 50 | 14 | |
| North East | 2016 | 122 | 59 | 17 | 5 | |
| Total | 1760 | 663 | 366 | 262 | ||
Extent of herbicide resistance
Wild oats samples were screened to three herbicide modes of action, except for samples from Southern Victoria on which tri-allate was not used. No resistance to clethodim (ACCase inhibitor (‘fop’)), glyphosate (EPSPS inhibitor) or tri-allate (fat synthesis inhibitor) was identified. Resistance to the ACCase-inhibitor clodinafop-propargyl was highest in NSW, with four of the five regions having more resistance than the average (Slopes, Eastern, Plains and Southern regions) (Table 3). Resistance in SA and Victoria ranged from 2% to 10% of samples, whereas none of the samples from Tasmania were resistant (Table 3).
| State/region | Clodinafop-propargyl | Iodosulfuron-methyl-Na or mesosulfuron-methyl | |||
|---|---|---|---|---|---|
| Samples resistant (%) | P-value | Samples resistant (%) | P-value | ||
| New South Wales | |||||
| Slopes | 49 | <0.0001 | 1 | 0.014 | |
| Eastern | 43 | 0.0034 | 47 | <0.0001 | |
| Western | 12 | 0.017 | 2 | 0.066 | |
| Plains | 31 | 0.058 | 0 | 0.014 | |
| Southern | 30 | 0.056 | 0 | 0.0063 | |
| Tasmania | 0 | 0.033 | 6 | 1 | |
| South Australia | |||||
| Mid North | 2 | 0.0004 | 28 | <0.0001 | |
| Eyre Peninsula | 3 | 0.0079 | 3 | 0.727 | |
| Mallee | 9 | 0.475 | 0 | 1 | |
| South East | 5 | 0.099 | 0 | 0.396 | |
| Victoria | |||||
| Southern | 4 | 0.029 | 0 | 0.258 | |
| Wimmera–Mallee | 10 | 0.06 | 29 | <0.0001 | |
| North East | 9 | 0.015 | 0 | 0.021 | |
| Mean | 22 | 7 | |||
The exact binomial test compared the extent of resistance for each region with the average distribution across south-eastern Australia.
Number of samples collected from each region provided in Table 2; not all samples were tested to all herbicides when seed supplies were limited.
Resistance to the ALS-inhibiting sulfonylurea herbicides screened (iodosulfuron-methyl-Na in NSW and Tasmania, and mesosulfuron-methyl in SA and Victoria) was more localised (Table 3). In each of NSW, SA and Victoria, there was one region with a high frequency of resistance (>25%), but no other regions in those states had >3% resistant samples, whereas 6% of the Tasmanian samples were resistant (Table 3). There was more resistance to ACCase-inhibiting herbicides in wild oats than to ALS-inhibiting herbicides (G-statistic 98.7; P < 0.0001) (Table 4).
| Comparison | G-statistic | P-value | ||
|---|---|---|---|---|
| ACCase inhibitors vs ALS inhibitors | Avena spp. | 98.7 | <0.0001 | |
| Bromus spp. | 395 | <0.0001 | ||
| Hordeum spp. | 132 | <0.0001 | ||
| Sulfonylurea vs imidazolinones | Bromus spp. | 154 | <0.0001 | |
| Avena spp. vs Bromus spp. | ACCase inhibitors | 157 | <0.0001 | |
| ALS inhibitors | 86.8 | <0.0001 | ||
| Avena spp. vs Hordeum spp. | ACCase inhibitors | 92.7 | <0.001 | |
| ALS-inhibitors | 33.8 | <0.0001 | ||
| Bromus spp. vs Hordeum spp. | ACCase inhibitors | 2.87 | 0.091 | |
| ALS inhibitors | 3.49 | 0.06 |
Unlike wild oats where resistance to the ACCase-inhibitor was more common than to the ALS-inhibiting sulfonylurea herbicides, brome grass had higher resistance to the sulfonylurea herbicide than the ACCase-inhibiting herbicide (Tables 4 and 5). Less than 1% of samples showed resistance to the ACCase inhibitor quizalofop-ethyl, and all of these were from the Wimmera–Mallee region in Victoria, compared with 24% of samples resistant to a sulfonylurea herbicide, mesosulfuron-methyl (Table 5). Resistance to sulfonylurea herbicide was most common in SA, with three regions in that state having 40–45% of samples resistant, all higher than the mean (Mallee region, P = 0.0011; Eyre Peninsula region P = 0.0027; Mid North region P = 0.013). Sulfonylurea herbicide resistance was next most common in Tasmania (21% of samples). Only a single region in both NSW (Western region) and Victoria (Wimmera–Mallee region) had samples resistant to the sulfonylurea herbicide, but in both of these regions >10% of samples showed resistance (Table 5). Resistance was less common to the ALS-inhibiting imidazolinone herbicide mix (Table 4), recorded only in NSW Western region and the SA Eyre Peninsula, and no samples tested were resistant to clethodim or glyphosate (Table 5).
| Region | Quizalofop-ethyl | Mesosulfuron-methyl | Imazapyr + imazamox | Glyphosate | |||||
|---|---|---|---|---|---|---|---|---|---|
| Samples resistant (%) | P-value | Samples resistant (%) | P-value | Samples resistant (%) | P-value | Samples resistant (%) | P-value | ||
| New South Wales | |||||||||
| Slopes | 0 | 1 | 0 | <0.0001 | 0 | 1 | 0 | 1 | |
| Eastern | 0 | 1 | 0 | 0.082 | 0 | 1 | 0 | 1 | |
| Western | 0 | 1 | 17 | 0.781 | 7 | 0.0078 | 0 | 1 | |
| Plains | 0 | 1 | 0 | 0.347 | 0 | 1 | 0 | 1 | |
| Southern | 0 | 1 | – | 0 | 1 | 0 | 1 | ||
| Tasmania | 0 | 1 | 21 | 0.777 | 0 | 1 | 0 | 1 | |
| South Australia | |||||||||
| Mid North | 0 | 1 | 40 | 0.013 | 0 | 1 | 0 | 1 | |
| Eyre Peninsula | 0 | 1 | 44 | 0.0027 | 2 | 0.013 | 0 | 1 | |
| Mallee | 0 | 1 | 44 | 0.0011 | 0 | 1 | 0 | 1 | |
| South East | n.d. | n.d. | n.d. | n.d. | |||||
| Victoria | |||||||||
| Southern | 0 | 1 | 0 | 0.0044 | 0 | 1 | 0 | 1 | |
| Wimmera–Mallee | 6 | 0.0082 | 12 | 0.065 | 0 | 1 | 0 | 1 | |
| North East | 0 | 1 | 0 | 0.019 | 0 | 1 | 0 | 1 | |
| Mean | 1 | 24 | 1 | 0 | |||||
–, not tested; n.d., species not detected in surveyed fields for that region.
The exact binomial test compared the extent of resistance for each region with the average distribution across south-eastern Australia.
Number of samples collected from each region provided in Table 2; not all samples were tested to all herbicides when seed numbers were limited.
For barley grass, resistance was more common to the sulfonylurea herbicide than to the ACCase inhibitor (Table 4), with only 2% of samples resistant to quizalofop-ethyl compared with 19% resistant to mesosulfuron-methyl (Table 6). All samples resistant to quizalofop-ethyl were from SA. The SA Mid North and SA Mallee regions had higher than the mean frequency of barley grass resistant to mesosulfuron-methyl (Table 6).
| Region | Quizalofop-ethyl | Mesosulfuron-methyl | Clethodim | Paraquat + diquat | |||||
|---|---|---|---|---|---|---|---|---|---|
| Samples resistant (%) | P-value | Samples resistant (%) | P-value | Samples resistant (%) | P-value | Samples resistant (%) | P-value | ||
| New South Wales | |||||||||
| Slopes | 0 | 1 | 7 | 0.05 | 0 | 1 | 3 | 0.517 | |
| Eastern | 0 | 1 | 8 | 0.49 | 0 | 1 | 0 | 1 | |
| Western | 0 | 1 | 9 | 0.09 | 0 | 1 | – | ||
| Plains | 0 | 1 | 20 | 1 | 0 | 1 | – | ||
| Southern | 0 | 1 | – | 0 | 1 | 11 | 0.42 | ||
| Tasmania | 0 | 1 | 17 | 1 | 0 | 1 | 33 | 0.044 | |
| South Australia | |||||||||
| Mid North | 11 | 0.05 | 53 | 0.0009 | 5 | 0.122 | – | ||
| Eyre Peninsula | 4 | 0.274 | 16 | 0.862 | – | – | |||
| Mallee | 3 | 0.536 | 42 | 0.0066 | – | – | |||
| South East | n.d. | n.d. | n.d. | n.d. | |||||
| Victoria | |||||||||
| Southern | n.d. | n.d. | n.d. | n.d. | |||||
| Wimmera–Mallee | 0 | 1 | 7 | 0.49 | – | – | |||
| North East | 0 | 1 | 0 | 0.592 | – | – | |||
| Mean | 2 | 19 | 0.7 | 6 | |||||
–, not tested; n.d., species not detected in surveyed fields for that region.
The exact binomial test compared the extent of resistance for each region with the average distribution across south-eastern Australia.
Number of samples collected from each region provided in Table 2; not all samples were tested to all herbicides when seed numbers were limited.
In addition, barley grass samples from NSW, Tasmania and SA Mid North were tested to the dim ACCase inhibitor clethodim, with one sample from SA Mid North resistant (142 tested). Samples from NSW (Slopes, Eastern and Southern) and Tasmania were tested for resistance to paraquat, with 6% of these resistant (64 tested) including two of the six Tasmanian samples, resulting in a higher frequency of resistance to paraquat in this region than the mean (Table 6).
Discussion
For each of the grass species, resistance to at least one herbicide was identified in each of the states where samples were collected. The extent of resistance in these three grass species is lower than observed for annual ryegrass in Australia not only for the equivalent surveying period and region (Broster et al. 2022), but also in previous annual ryegrass surveys (Broster et al. 2011b, 2012b, 2013; Boutsalis et al. 2012; Owen et al. 2014). However, more than 20% of samples of wild oats collected were resistant to the ACCase-inhibiting herbicide clodinafop-propargyl and brome grass to the ALS-inhibiting herbicide mesosulfuron-methyl.
In the case of wild oats, there was considerable variation among regions sampled, with 32% of samples resistant to clodinafop-propargyl in NSW, but only 8% in Victoria and 4% in SA (Table 3). This higher frequency of resistance in NSW is also higher than reported by Owen and Powles (2016), who found only 7% of samples from Western Australia (WA) resistant to diclofop-methyl, another ACCase-inhibiting herbicide. These frequencies are lower than those reported from Canada, where Beckie et al. (2013) found 44% of wild oats samples from the Canadian Prairies to be resistant to one or more fop herbicides. However, Travlos et al. (2011) found that 28% of wild oats samples from wheat fields in Greece were resistant to clodinafop-propargyl. By contrast, a low level of resistance to fop herbicides (9%) was identified in the Canterbury region of New Zealand (Buddenhagen et al. 2021).
Previous surveys have shown differences in the extent of resistance between the fop herbicides. Owen and Powles (2009) reported that the number of samples resistant to diclofop-methyl (16%) was higher than to clodinafop-propargyl (3%) in WA fields. Travlos et al. (2011) reported similar findings, with resistance to clodinafop-propargyl lower than to either fenoxaprop-P-ethyl (41%) or diclofop-methyl (64%). Beckie et al. (2013) screened wild oats to three fop herbicides (fenoxaprop-P-ethyl, clodinafop-propargyl and quizalofop-ethyl) but did not report results for the different herbicides.
The frequency of resistance to the fop herbicides in NSW Slopes and Plains has increased over the 6-year period since previously surveyed. Previously, 30% of samples from NSW Slopes (2007) (Broster et al. 2011a) and 19% from the NSW Plains (2010) (Broster et al. 2013) were resistant to diclofop-methyl compared with 49% of samples from the NSW Slopes resistant to clodinafop-propargyl in 2013 (P < 0.0001) and 31% from the NSW Plains in 2016 (P = 0.009) (Table 3).
Resistance to the ALS-inhibiting herbicides was lower than to ACCase-inhibiting herbicides in wild oats (Table 5). However, resistance was more common in SA (12% of samples) and Victoria (10% of samples) than in NSW (5% of samples; Table 3). These differences between states likely reflect different crop rotations and herbicides used. Owen and Powles (2016) identified one sample with resistance to sulfonylurea herbicides in 118 samples of wild oats tested from WA. Beckie et al. (2013), on the other hand, identified resistance to ALS-inhibiting herbicides in 12% of samples collected from the Canadian Prairies.
In contrast to wild oats, resistance to ACCase-inhibiting herbicides was less common in barley grass and brome grass (Table 4). Only three samples of brome grass were resistant to quizalofop-ethyl, all from Victoria Wimmera–Mallee (Table 5), and five samples of barley grass, all from SA (Table 6). There was no difference in the frequency of ACCase-inhibitor resistance between brome grass and barley grass (Table 4). Previous surveys in NSW and Tasmania identified no resistance to the ACCase-inhibiting herbicides in these two weed species (Broster et al. 2010, 2012b; Owen et al. 2015). Boutsalis et al. (2014) reported resistance to ACCase-inhibiting herbicides in brome grass from the Wimmera–Mallee at a similar frequency to the present survey and in 14% of samples from South East SA. A smaller survey of barley grass in the Eyre Peninsula and Mid North of SA identified resistance (>20% survival) to quizalofop-ethyl in 6 of the 90 barley grass samples screened (Shergill et al. 2015), similar to the frequency of resistance identified in this survey (Table 6). In a survey of brome grass and barley grass in WA, 1% of brome grass samples had survivors when treated with fluazifop-butyl and clethodim; however, all of the barley grass samples were completely controlled by ACCase-inhibiting herbicides (Owen et al. 2015).
Over 40% of samples from all three regions in SA where brome grass was collected showed resistance to sulfonylurea herbicides (Table 5) with resistance also occurring in >10% of the samples from Western NSW, Tasmania and the Wimmera–Mallee in Victoria. For barley grass, all regions except for the Victorian North East had samples resistant to mesosulfuron-methyl (Table 6), with two of the three regions in which it was collected in SA having >40% of samples resistant (Table 6). In both brome grass and barley grass, there was more resistance to sulfonylurea herbicides than to ACCase inhibitors, and there was more resistance to sulfonylurea herbicides than occurred in wild oats (Table 4). Previous surveys of brome grass (2008–12) in SA and Victoria reported that 2–45% of samples collected were resistant to mesosulfuron-methyl (Boutsalis et al. 2014). There was less resistance found to mesosulfuron-methyl in Victoria and more in SA in this survey. Previous surveys in Tasmania (Broster et al. 2012b) and SA (Shergill et al. 2015) identified no resistance to ALS-inhibiting herbicides in barley grass. However, Owen et al. (2015) reported 8% of barley grass samples collected in WA were resistant to sulfonylurea herbicides. There has been a rapid increase in resistance to ALS-inhibiting herbicides in barley grass in south-eastern Australia in recent years.
Across the surveyed regions, annual ryegrass was the most commonly collected grass weed species, collected from 81% of fields, and in no region was it collected from <60% of fields (Broster et al. 2022). By comparison, wild oats was collected from 38% of fields, brome grass 21% and barley grass 15%. There were differences among regions in the incidence of the different weed species, likely resulting from different environments and soil types present. Wild oats was most common in NSW, collected from 55% of fields, and less common in SA and Victoria, collected from 20% and 34% of fields, respectively (Table 2). On the other hand, brome grass was most common in SA, collected from 28% of fields, compared with only 15% of fields in NSW (Table 2).
Resistance in wild oats was more common in NSW than in the other states (Table 3), and likewise, resistance in brome grass was more common in SA than in NSW (Table 5). This suggests that resistance is being selected more often where there are higher populations of these grass weeds. This agrees with studies from Canada and Greece. In Canada, wild oats was the most common weed species, found in 67% of paddocks, with 44% of these resistant to a fop herbicide, whereas in Greece, wild oats was collected from ~20% of fields and the resistance level was lower at 28% of samples (Travlos et al. 2011; Beckie et al. 2013). If the initial frequency of resistance to herbicides is similar among the weed species, then lower populations make it less likely that resistance will evolve (Preston and Powles 2002; Kreiner et al. 2018).
This group of surveys of three major grass weed species across south-eastern Australia shows that these species are found across this region and that resistance occurs in all species. The extent of resistance differs among species, herbicides and regions, reflecting the importance of the weed in a region and/or the herbicides used for weed control in that region as has been shown in annual ryegrass (Broster et al. 2019). Despite the high levels of resistance in some regions, there are still herbicides that control all populations, allowing alternatives for control.
Data availability
The data that support this study are available in the article and upon request to the corresponding author.
Acknowledgements
The authors thank Allison Chambers, Alicia Merriam, David Brunton, Geetha Velappan and Ruwan Lenorage and casual staff and students at Charles Sturt University for technical assistance.
References
Beckie HJ, Lozinski C, Shirriff S, Brenzil CA (2013) Herbicide-resistant weeds in the Canadian prairies: 2007 to 2011. Weed Technology 27, 171-183.
| Crossref | Google Scholar |
Boutsalis P, Preston C (2006) Resistance to acetyl-Coenzyme A carboxylase (ACCase)-inhibiting herbicides in Bromus spp. in Australia. In ‘Proceedings of the 14th Australian weeds conference’. 24–28 September, Adelaide, SA, Australia. (Eds C Preston, JH Watts, ND Crossman) pp. 538–540. (Weed Management Society of South Australia)
Boutsalis P, Gill GS, Preston C (2012) Incidence of herbicide resistance in rigid ryegrass (Lolium rigidum) across southeastern Australia. Weed Technology 26, 391-398.
| Crossref | Google Scholar |
Boutsalis P, Kleemann S, Gill GS, Preston C (2014) A hidden threat: widespread Group B herbicide resistance in brome across south-eastern Australia. In ‘Science, community and food security: the weed challenge. Proceedings of the 19th Australasian weeds conference’. Hobart, Tas., Australia. (Ed. M Baker) pp. 202–205. (Tasmanian Weed Society)
Broster JC, Koetz EA, Wu H (2010) A survey of southern New South Wales to determine the level of herbicide resistance in brome grass and barley grass populations. In ‘Proceedings of the 17th Australasian weeds conference’. Christchurch, New Zealand’. (Ed. SM Zydenbos) pp. 274–277. (New Zealand Plant Protection Society)
Broster JC, Koetz EA, Wu H (2011a) Herbicide resistance in wild oats (Avena spp.) in southern New South Wales. Plant Protection Quarterly 26, 106-110.
| Google Scholar |
Broster JC, Koetz EA, Wu H (2011b) Herbicide resistance levels in annual ryegrass (Lolium rigidum Gaud.) in southern New South Wales. Plant Protection Quarterly 26, 22-28.
| Google Scholar |
Broster JC, Koetz EA, Wu H (2012b) Herbicide resistance frequencies in ryegrass (Lolium spp.) and other grass species in Tasmania. Plant Protection Quarterly 27, 36-42.
| Google Scholar |
Broster JC, Koetz EA, Wu H (2013) Herbicide resistance levels in annual ryegrass (Lolium rigidum Gaud.) and wild oats (Avena spp.) in south-western New South Wales. Plant Protection Quarterly 28, 126-132.
| Google Scholar |
Broster JC, Pratley JE, Ip RHL, Ang L, Seng KP (2019) Cropping practices influence incidence of herbicide resistance in annual ryegrass (Lolium rigidum) in Australia. Crop & Pasture Science 70, 77-84.
| Crossref | Google Scholar |
Broster J, Boutsalis P, Gill GS, Preston C (2022) The extent of herbicide resistance in Lolium rigidum Gaud. (annual ryegrass) across south-eastern Australia as determined from random surveys. Crop & Pasture Science 73, 1308-1317.
| Crossref | Google Scholar |
Buddenhagen CE, James TK, Ngow Z, Hackell DL, Rolston MP, Chynoweth RJ, Gunnarsson M, Li F, Harrington KC, Ghanizadeh H (2021) Resistance to post-emergent herbicides is becoming common for grass weeds on New Zealand wheat and barley farms. PLoS ONE 16, e0258685.
| Crossref | Google Scholar |
Kreiner JM, Stinchcombe JR, Wright SI (2018) Population genomics of herbicide resistance: Adaptation via evolutionary rescue. Annual Review of Plant Biology 69, 611-635.
| Crossref | Google Scholar |
Lemerle D, Tang H, Murray GM, Morris S, Tang HY (1996) Survey of weeds and diseases in cereal crops in the southern wheat belt of New South Wales. Australian Journal of Experimental Agriculture 36, 545-554.
| Crossref | Google Scholar |
Mansooji AM, Holtum JA, Boutsalis P, Matthews JM, Powles SB (1992) Resistance to aryloxyphenoxypropionate herbicides in two wild oat species (Avena fatua and Avena sterilis ssp. ludoviciana). Weed Science 40, 599-605.
| Crossref | Google Scholar |
Owen MJ, Powles SB (2009) Distribution and frequency of herbicide-resistant wild oat (Avena spp.) across the Western Australian grain belt. Crop & Pasture Science 60, 25-31.
| Crossref | Google Scholar |
Owen MJ, Powles SB (2016) The frequency of herbicide-resistant wild oat (Avena spp.) populations remains stable in Western Australian cropping fields. Crop & Pasture Science 67, 520-527.
| Crossref | Google Scholar |
Owen MJ, Martinez NJ, Powles SB (2014) Multiple herbicide-resistant Lolium rigidum (annual ryegrass) now dominates across the Western Australian grain belt. Weed Research 54, 314-324.
| Crossref | Google Scholar |
Owen MJ, Martinez NJ, Powles SB (2015) Herbicide resistance in Bromus and Hordeum spp. in the Western Australian grain belt. Crop & Pasture Science 66, 466-473.
| Crossref | Google Scholar |
Powles SB (1986) Appearance of a biotype of the weed, Hordeum glaucum Steud., resistant to the herbicide paraquat. Weed Research 26, 167-172.
| Crossref | Google Scholar |
Preston C, Powles SB (2002) Evolution of herbicide resistance in weeds: initial frequency of target site-based resistance to acetolactate synthase-inhibiting herbicides in Lolium rigidum. Heredity 88, 8-13.
| Crossref | Google Scholar |
Shergill LS, Fleet B, Preston C, Gill G (2015) Incidence of herbicide resistance, seedling emergence, and seed persistence of smooth barley (Hordeum glaucum) in South Australia. Weed Technology 29, 782-792.
| Crossref | Google Scholar |
Travlos IS, Giannopolitis CN, Economou G (2011) Diclofop resistance in sterile wild oat (Avena sterilis L.) in wheat fields in Greece and its management by other post-emergence herbicides. Crop Protection 30, 1449-1454.
| Crossref | Google Scholar |
Tucker ES, Powles SB (1991) A biotype of hare barley (Hordeum leporinum) resistant to paraquat and diquat. Weed Science 39, 159-162.
| Crossref | Google Scholar |
Walsh MJ, Powles SB (2007) Management strategies for herbicide-resistant weed populations in Australian dryland crop production systems. Weed Technology 21, 332-338.
| Crossref | Google Scholar |