Effects of heading date and Epichloë endophyte on persistence of diploid perennial ryegrass (Lolium perenne). 2. Endophyte strain and interactions with heading date
Katherine N. Tozer A * , David E. Hume B , Catherine Cameron A , Rose Greenfield A , Tracy Dale A , Wade J. Mace
A * , David E. Hume B , Catherine Cameron A , Rose Greenfield A , Tracy Dale A , Wade J. Mace  B , Tony Craven A and Marty J. Faville B
B , Tony Craven A and Marty J. Faville B
A
B
Abstract
Data are lacking on the effects of selected endophytes of perennial ryegrass (Lolium perenne L.) on ryegrass persistence.
We aimed to determine the impact of Epichloë endophyte on the persistence of mid- and late-heading perennial ryegrass cultivars.
Two mid-heading (Samson, Bronsyn) and two late-heading (One50, Rohan) cultivars, infected with selected endophytes (AR37, nea2/6) or with standard toxic endophyte, were established in a replicated plot study grazed by cattle in a subtropical environment of the upper North Island of New Zealand. Persistence characteristics were quantified at least five times per annum, over 4 years.
Endophyte strain had an effect on persistence; infection with standard endophyte resulted in higher ryegrass ground cover percentage, ryegrass content in pasture dry matter and autumn yield than infection with nea2/6 on many occasions, and with AR37 on some occasions. There were negligible impacts on ryegrass tiller density or nutritive value. Trends were dominated by the main effect of endophyte; interactions with heading date were inconsistent.
Cultivars were more persistent when infected with standard endophyte than with selected endophytes, although persistence declined over 4 years for all cultivars and irrespective of heading date.
Reliance on selected endophyte is unlikely to prevent persistence decline of perennial ryegrass in a subtropical environment. Other strategies will be required to maintain the persistence of high-quality pastures based on perennial ryegrass.
Keywords: alkaloids, flowering date, herbage production, nutritive value, pasture persistence, perennial pastures, resilience, tiller populations.
Introduction
In temperate and subtropical pastures, multiple stresses, including soil-borne fungal diseases and invertebrate herbivory, contribute to the decline of productive pasture species (Chapman et al. 2011; Tozer et al. 2011; Zydenbos et al. 2011; Lee et al. 2017; Dignam et al. 2022). The ryegrass–Epichloë fungal endophyte association produces alkaloids that confer protection against invertebrate pest damage over and above equivalent endophyte-free ryegrass.
Commercially available cultivars of perennial ryegrass (Lolium perenne L.) sold in New Zealand are typically infected with selected strains of Epichloë. These confer protection from invertebrate pests, with fewer negative impacts on livestock performance than occur with standard toxic endophyte, which is endemic in naturalised perennial-ryegrass-based pastures (Caradus et al. 2021). Alkaloid profiles vary qualitatively amongst different endophyte strains and are affected quantitatively by environmental variables such as temperature (Caradus et al. 2022), and by host plant genetics (Faville et al. 2015). They also vary with the developmental stage of the ryegrass, with higher alkaloid levels typically occurring in reproductive than vegetative tillers.
We have found no published studies that investigate the combined effects of heading date and endophyte strain on perennial ryegrass persistence, with defoliation management based on reproductive stage rather than calendar date. In this study, we compare the persistence over 4 years of two mid-heading diploid cultivars and two late-heading diploid cultivars infected with a range of endophytes. The three endophytes used in the study were considered to provide adequate pest protection against a range of insects found in the upper North Island of New Zealand (Caradus et al. 2021). The hypothesis was that there would be no effect of endophyte strain on the persistence of the perennial ryegrass cultivars, irrespective of heading date, with similar perennial ryegrass tiller populations, ground cover and content, herbage production, and weed ingress over time. In the first of this two-part series, we focused on heading date; here we focus on endophyte effects and interactions with heading date.
Materials and methods
Site establishment
The study was undertaken between March 2018 and May 2022. Details on site establishment, experimental design and treatments, fertiliser and weed management, defoliation management, and measurements are provided in the companion paper (Tozer et al. 2024). A brief description is provided below.
The site was established on the Ruakura Research Station, Hamilton, upper North Island of New Zealand (37°46′15″S, 175°18′35″E; 20 m a.s.l.), on a Typic Orthic Gley (Hewitt 1998) of high fertility. Rainfall was below average for all four summer/summer–autumn periods and during spring 2018 and 2020. The average minimum monthly temperature was 5°C in winter and the average maximum monthly temperature was 25°C in summer (see fig. 1 of companion paper).
Herbicide was applied to remove the existing pasture, followed by ploughing and power harrowing in March 2018 to prepare the seedbed. Plots (3 m by 7 m) were drilled with insecticide-coated seed by using a plot seeder, and the buffer established by hand-broadcasting. Diammonium phosphate and slugbait were applied to the site after sowing. To control weeds, pre-emergent herbicide was applied in April and a post-emergent herbicide applied in June.
Experimental design and treatments
The design was a randomised complete block, with 11 treatments and four replicates of four perennial ryegrass cultivars, comprising two mid-season heading (‘mid-heading’) and two late-season heading (‘late-heading’) cultivars, infected with one of three strains of endophyte (Epichloë festucae var. lolii or Epichloë sp. LpTG-3) (Table 1). The two mid-heading cultivars were Bronsyn and Grasslands Samson (hereafter termed Samson), and the two late-heading cultivars were Rohan and One50. The endophytes were standard toxic endophyte (hereafter termed ‘SE’), and the selected endophytes AR37 and ‘nea2/6’. Endophyte nea2/6 is a 50:50 mix of nea2 and nea6, sold under the brand NEA2. These endophytes were chosen because they have alkaloids that protect ryegrass from some crucial invertebrate pests that are endemic in upper North Island pastures (Caradus et al. 2021). The combination One50–nea2/6 was not available.
| Endophyte strain | Heading date type and cultivar | ||||
|---|---|---|---|---|---|
| Mid-heading | Late-heading | ||||
| Bronsyn (day 0) | Samson (+3 days) | Rohan (+18 days) | One50 (+20 days) | ||
| SE (Epichloë festucae var. lolii) | ✓ | ✓ | ✓ | ✓ | |
| AR37 (Epichloë sp. LpTG-3) | ✓ | ✓ | ✓ | ✓ | |
| nea2/6 (E. festucae var. lolii) (50:50 nea2:nea6) | ✓ | ✓ | ✓ | – | |
Days relate to the number of days after the standard heading date of 22 October (Lee et al. 2012). –, not available.
Fertiliser and weed management
Fertiliser application was based on annual soil test results (see table 1 of companion paper). Lime was applied in 2019 and 2020; magnesium and potassium were applied after every grazing from May 2021; and 25 kg nitrogen ha−1 was applied after every grazing from September 2018. No herbicide was applied after site establishment.
Defoliation management
The site was first grazed in August 2018 by 12-month-old heifers, and thereafter, by mixed-breed and mixed-age dairy heifers, with other stock classes used occasionally (e.g. dairy calves or beef heifers), of varying mob size. Treatments were grazed or mown on four occasions in 2018 between October and December; and on 10 occasions in 2019, 10 in 2020, 10 in 2021, and four in 2022 between January and May, when the experiment ended.
Between January and September, when perennial ryegrass was vegetative, the site was grazed when there were at least two new leaves since the last grazing (Fulkerson and Donaghy 2001) to a residual herbage mass of ~1500 kg DM ha−1.
Between October and December, when perennial ryegrass was undergoing reproductive development, livestock were excluded and treatments were mown to a height of 7 cm. Defoliation decision rules were based on perennial ryegrass phenology with a defoliation interval to allow anthesis. This entailed a long spring rotation of ~7 weeks for both treatments, to allow perennial ryegrass reproductive development. All treatments were generally defoliated in early October. The long spring rotation occurred between early October and mid-November for mid-heading cultivars, and late October and mid-December for late-heading cultivars, simulating a ‘split grazing’ (see fig. 2 of companion paper). All treatments were defoliated in mid-December.
Number and timing of measurements
Herbage mass was assessed in all treatments before and after grazing, or before and after mowing. Pre-grazing leaf stage, ground cover and herbage mass, and post-grazing residual herbage mass, were assessed for up to 11 grazings/mowings per annum (see table 5 of companion paper). In addition, tiller density, botanical composition, nutritive values and alkaloid content were assessed once before grazing in early and late spring, mid- to late summer, autumn and winter, for all treatments.
Pasture and soil measurements
Ground cover was visually assessed using a score of 1–10, with 1 equating to a perennial ryegrass ground cover of ≤10%, and 10 equating to ≥91%. Perennial ryegrass tiller density in each plot was determined in up to ten quadrats of 10 cm by 15 cm. Vegetation within each quadrat was cut to ground level, bulked for each plot and weighed. A subsample was removed and sorted into perennial ryegrass and ‘other vegetation’, the components were weighed, and the number of adult perennial ryegrass tillers was counted. Each component was oven-dried and weighed, and the data were used to estimate the tiller number per square metre.
Herbage mass was assessed before and after each grazing between sowing and August 2018, using a calibrated rising plate meter (RPM) randomly positioned in 20 locations per plot. Herbage mass was also measured in each plot by using a mower, six times per year for each cultivar, to estimate the yield on a per hectare basis and seasonal herbage production.
Nutritive values were determined from grab samples that were harvested to ground level, bulked, subsampled, frozen at −20°C, freeze-dried and ground to a fine powder with a 1-mm sieve for feed profile analyses by Hill Laboratories (Hamilton, New Zealand), using near-infrared spectroscopy (Corson et al. 1999). Values were estimated for metabolisable energy (ME), digestibility of organic dry matter, crude protein, soluble sugars, neutral detergent fibre (NDF) and ash.
For botanical composition assessment, a second subsample, using the same sampling method, was sorted into perennial ryegrass; ‘other grass’ – predominantly the temperate C3 species soft brome (Bromus hordeaceus L.), annual poa (Poa annua L.), browntop (Agrostis capillaris L.) and Yorkshire fog (Holcus lanatus L.), and the subtropical C4 species summer grass (Digitaria sanguinalis (L.) Scop.); white clover (Trifolium repens L.); other clovers; broadleaf weed; and dead vegetation. Reproductive development and leaf stage were assessed on the perennial ryegrass component from the botanical composition subsample.
Endophyte presence
The Epichloë endophyte tiller infection frequency was tested for sown seed and was tested in field plots in autumn 2018, spring 2018, and thereafter in autumn from 2019 to 2021, using the tissue print immunoblot assay of Simpson et al. (2012). Between 30 and 60 (depending on the year) randomly selected vegetative ryegrass tillers, each from a different plant, were cut at ground level from each plot. In the laboratory, tillers were recut and the moist tiller end was placed on nitrocellulose blotting paper. Subsequent antibody and colour development enabled presence/absence of endophyte to be determined and the frequency of endophyte infection estimated.
Using the tillers identified by immunoblotting as endophyte-infected, endophyte strain was determined in autumn (AR37 and SE plots only) and spring (nea2/6 plots only) of 2018, and for all endophyte treatments in the autumns of 2020 and 2021. Tillers from plots sown with nea2/6 were analysed using simple sequence repeat (SSR) markers B11 (Moon et al. 1999) and ans031 (Card et al. 2014), whereas those sown with AR37 or SE endophyte were analysed using a PCR method with high-resolution melt (HRM) analysis (Gagic et al. 2018) (Slipstream Automation, Palmerston North, New Zealand). Different methods were used because the HRM marker could not reliably detect and/or distinguish the ‘nea’ strains. Both methods give equivalent detection of AR37 and SE.
Alkaloid content
Endophyte alkaloid concentrations of perennial ryegrass were assessed before grazing, by excising to ground level three to five tillers from 24 randomly selected plants bulked for each plot. Each bulked sample was snap-frozen in liquid nitrogen, stored frozen at −80°C, freeze-dried (FD80 freeze dryer; Cuddon, Blenheim, New Zealand) and ground using a 1-mm sieve (as above). Samples were analysed at AgResearch, Palmerston North, New Zealand, where ergovaline, lolitrem B and peramine were extracted and quantified using a modification of the protocol described in Rasmussen et al. (2012). Peramine, ergovaline and lolitrem B were detected with a linear ion trap mass spectrometer (LTQxl; Thermo Fisher Scientific, Waltham, MA, USA) using the same parameters as described in Rasmussen et al. (2012). Lolitrem B was quantified using an external standard. Epoxyjanthitrems were extracted and analysed according to the method described by Hewitt et al. (2020).
Invertebrate populations
Invertebrate abundance across the site was ascertained on 19 March 2019 and 28 April 2021 for 10 spade squares (20 cm by 20 cm to 20 cm depth) randomly sampled from internal buffer areas. All visible soil invertebrates were identified and counted in the field, and the holes backfilled with the soil. Invertebrates assessed included: clover root weevil (Sitona obsoletus (Gmelin)), black beetle larvae and adults (Heteronychus arator (Fabricius)), whitefringed weevil (Naupactus leucoloma Boheman), grass grub larvae (Costelytra giveni Coca-Abia & Romero-Samper), wireworm (Coleoptera: Elateridae), greasy cutworm (Agrotis ipsilon (Hufnagel)), and earthworms (mainly Aporrectodea spp. and Lumbricus rubellus (Hoffmeister)).
Statistical analyses
All data were analysed using Genstat 21st edition (VSN International, Hemel Hempstead, UK). Because grass cultivar and heading date were confounded, the data were analysed for grass cultivar and heading date separately. The design was unbalanced, and so data were analysed by residual maximum likelihood (Patterson and Thompson 1971) with grass cultivar or heading date, endophyte and their interaction as fixed effects, and replicate and row as random effects. Seasonal herbage production was calculated using the daily rate of growth between measurements, scaled to the number of days in the season. Post-grazing RPM and post-mowing RPM measurements were compared for the eight dates when both were recorded, using a split-plot ANOVA fitting plot and harvest within plot. Because all data met the normality assumptions of the analyses, no transformations of the data were performed.
Owing to the large number of statistical tests, interactions between endophyte and heading date are reported only for a variable where there were consistent effects (P < 0.05) on two or more occasions. Cultivar effects are also reported for alkaloids when significant (P ≤ 0.05) differences occurred between the two cultivars within each heading date category.
Leaf stage, ground cover, tiller density, botanical composition, nutritive values and alkaloid content data from the split-harvests are not reported in the Results. This is because mid- and late-heading cultivars were subjected to different defoliation regimes during the mid–late-spring period, which affects these persistence characteristics. Our focus was on measuring endophyte effects for the two mid-heading and late-heading cultivars, not defoliation effects.
Results
Establishment plant densities and endophyte infection
The density of perennial ryegrass plants was not affected (P = 0.174) by endophyte strain, averaging 126 plants m−2 in May 2018. Infection frequency of viable endophyte in sown seed averaged 95% and did not differ (P > 0.05) among endophyte treatments (Table 2). Endophyte tiller infection frequency for perennial ryegrass was high throughout the experiment, averaging ≥91% at each of the five measurements, with no change over time (October 2018–May 2021, P = 0.076) (Table 2).
| Endophyte infection frequency (%) | s.e.d. | P-value | ||||
|---|---|---|---|---|---|---|
| AR37 | nea2/6 | SE | ||||
| Sown seed | 95.0 | 94.0 | 96.0 | 1.76 | >0.05 | |
| 28 May 2018 | 91.3b | 86.9b | 96.0a | 2.27 | <0.001 | |
| 3 October 2018 | 93.3ab | 90.8b | 95.1a | 2.02 | 0.037 | |
| 13 May 2019 | 94.8 | 92.1 | 96.3 | 3.15 | 0.104 | |
| 6 May 2020 | 91.5b | 90.0b | 97.8a | 2.03 | <0.001 | |
| 6 May 2021 | 93.0b | 93.6b | 97.9a | 1.35 | <0.001 | |
For each measurement date, endophyte strain means followed by the same letter are not significantly different at P = 0.05.
s.e.d., standard error of difference.
Infection frequency was higher for SE-infected than nea2/6- and AR37-infected cultivars (97% vs an average of 92%, P = 0.001) based on a repeated measures analyses (October 2018–May 2021). In May of 2018, 2020 and 2021, endophyte infection frequency was higher (P < 0.001) for SE than AR37 and nea2/6 (Table 2). In October 2018, the infection frequency was also higher (P < 0.037) for SE than nea2/6, with AR37 intermediate and not different from either (Table 2).
In total, 4113 endophyte-infected tillers were analysed for endophyte strain (summed over all plots and all dates). Of these tillers, just nine (0.4%) were the non-sown AR1 endophyte strain. In the nea2/6- and SE-infected cultivars, 24 of 2784 endophyte-infected tillers (0.9%) were AR37. In the nea2/6- and AR37-infected cultivars, 123 of 2770 endophyte-infected tillers (4.4%) were SE. This SE contamination increased significantly (P < 0.001) over time (from 2.5% to 3.4% to 7.6% for 2018, 2020 and 2021) and was greater in nea2/6-infected cultivars than in AR37-infected cultivars (6% vs 3%, P < 0.037, averaged over all dates).
In the nea2/6-infected cultivars, the ratio of nea2 to nea6 showed a linear decline over time post-establishment (1.3 in May 2018, 1.0 November 2018, 0.7 April 2020, 0.5 May 2021; R2 = 0.96, P < 0.002).
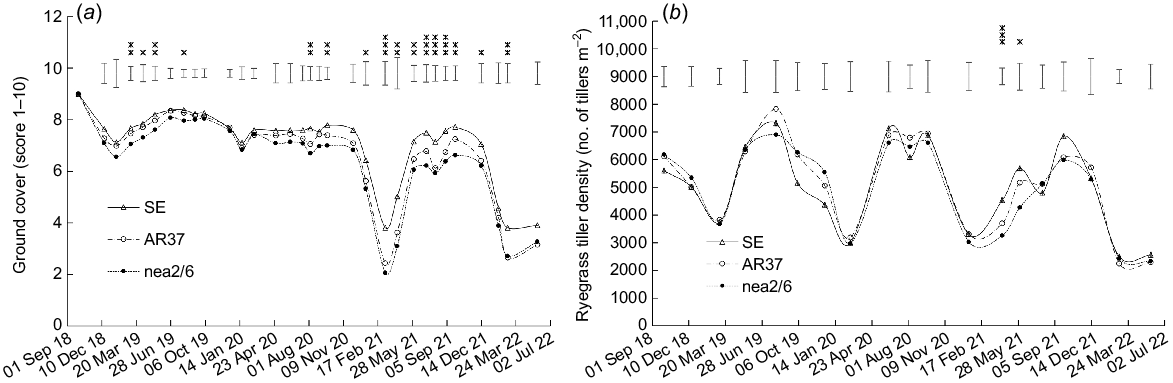
Perennial ryegrass ground cover
Ground cover was higher (P < 0.05) for SE-infected cultivars than nea2/6-infected cultivars on 16 occasions (Fig. 1a). Of those occasions, ground cover of AR37-infected cultivars was similar to SE-infected and higher than nea2/6-infected cultivars on two occasions, intermediate but not different from either SE- or nea2/6-infected cultivars on ten occasions, and lower than SE-infected and similar to nea2-infected cultivars on four occasions.
Tiller density and leaf stage
On one occasion, tiller densities were higher (P < 0.001) in the SE-infected than AR37- and nea2/6-infected cultivars (Fig. 1b). On another occasion, tiller densities were higher (P = 0.018) in SE-infected than nea2/6-infected cultivars, with AR37-infected cultivars intermediate but not different from either (Fig. 1b). There were no effects (P > 0.05) of endophyte strain on leaf stage (data not shown).
Herbage production
Herbage production was higher (P = 0.004) for SE-infected than nea2/6-infected cultivars in winter 2021, with AR37-infected cultivars intermediate but not different from either data not shown. Herbage production in autumn, when averaged over all 4 years, was higher in SE- and AR37-infected cultivars than in nea2/6-infected cultivars (average 770 kg vs 690 kg DM ha−1, P = 0.029).
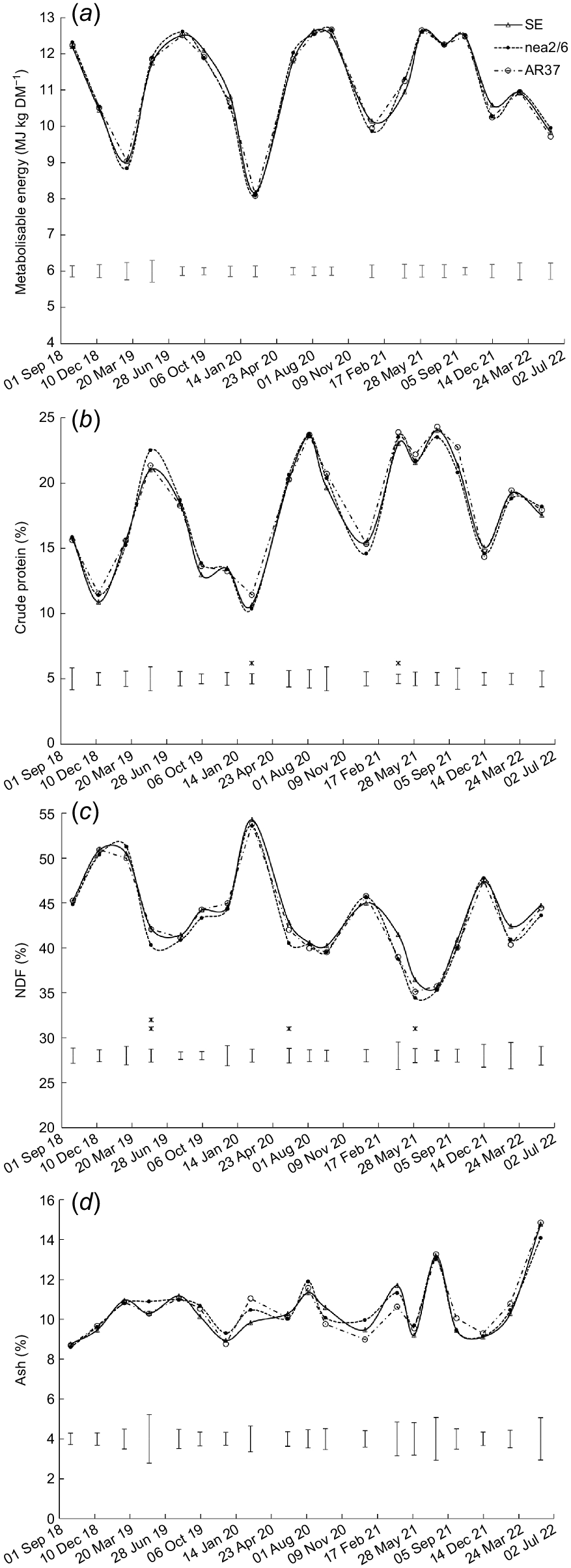
Botanical composition
There was a significant (P < 0.05) effect of endophyte on perennial ryegrass content on seven occasions (Fig. 2a). In May 2019, the ryegrass content was higher (P = 0.013) in swards sown with AR37-infected ryegrass than with SE- and nea2/6-infected ryegrass (Fig. 2a). This was followed by three occasions up to August 2020 when the ryegrass content was similar in swards sown with SE-infected and AR37-infected ryegrass, both of which had a higher (P < 0.05) ryegrass content than swards sown with nea2/6-infected ryegrass (Fig. 2a). Thereafter, on three occasions, the ryegrass content was higher (P < 0.05) in swards sown with SE-infected ryegrass than with AR37-infected and nea2/6-infected ryegrass (Fig. 2a).
Effect of endophyte strain (SE, AR37 and nea2/6) on the content of (a) perennial ryegrass, (b) other grass, (c) legumes, (d) broadleaf weed, and (e) dead vegetation. Data are the mean of two heading date treatments. Error bars represent the standard error of the mean. *P < 0.05; **P < 0.01; ***P < 0.001.
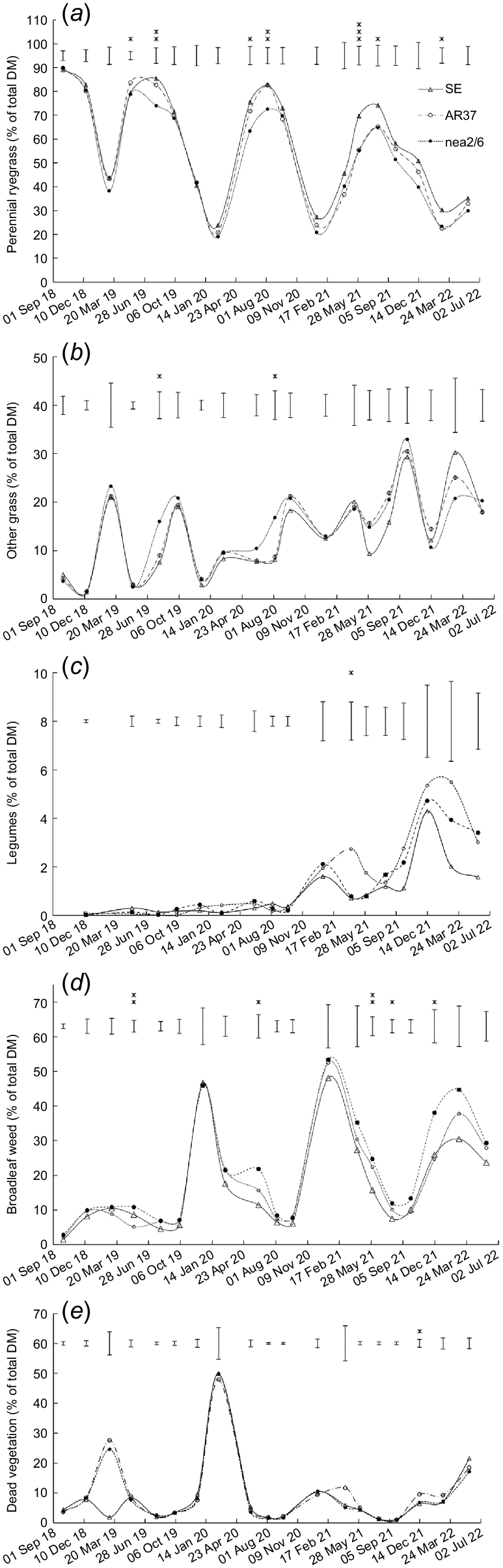
The content of other grass was higher (P < 0.05) in swards infected with nea2/6 than with AR37 or SE endophyte in August 2019 and 2020 (Fig. 2b). The legume content was higher (P = 0.033) in swards sown with AR37-infected ryegrass than with nea2/6- and SE-infected ryegrass on one occasion (Fig. 2c). Broadleaf weed content was also higher (P < 0.05) in swards sown with nea2/6-infected ryegrass than with AR37-infected and/or SE-infected ryegrass on five occasions (Fig. 2d). Content of dead vegetation was higher (P = 0.023) in swards sown with AR37-infected ryegrass than with SE-infected and nea2/6-infected ryegrass on one occasion (Fig. 2e).
Nutritive values
There was no significant effect (P > 0.05) of endophyte on ME content (Fig. 3a). However, significant (P < 0.05) endophyte × heading date interactions were observed for ME content on two occasions (Supplementary Table S1); the ME content was lower in swards sown with late- than mid-heading cultivars in August 2019 and in February 2022, but only for those infected with AR37 (Table S1). Significant (P < 0.05) endophyte effects were found for crude protein content on two occasions, although differences were not consistent (Fig. 3b). NDF content was higher (P < 0.05) in swards infected with SE than with nea2/6 on three occasions, with AR37-infected swards intermediate and not different from either (Fig. 3c). There was no effect (P > 0.05) of endophyte on ash content (Fig. 3d).
Alkaloid concentrations
There were strong seasonal patterns in endophyte alkaloid concentrations (Fig. 4). For most years, the concentrations of total epoxyjanthitrems and lolitrem B peaked over summer and fell to negligible levels in early spring. Seasonal patterns were less consistent for ergovaline and peramine, although there were several peaks in early autumn.
Effect of heading date (mid, mid-season heading cultivars; late, late-season heading cultivars) and endophyte strain (SE, AR37 and nea2/6) on alkaloid concentrations: (a, b) total epoxyjanthitrems; (c, d) lolitrem B; (e, f) ergovaline; and (g, h) peramine. Error bars represent the standard error of the mean. *P < 0.05; **P < 0.01; ***P < 0.001.
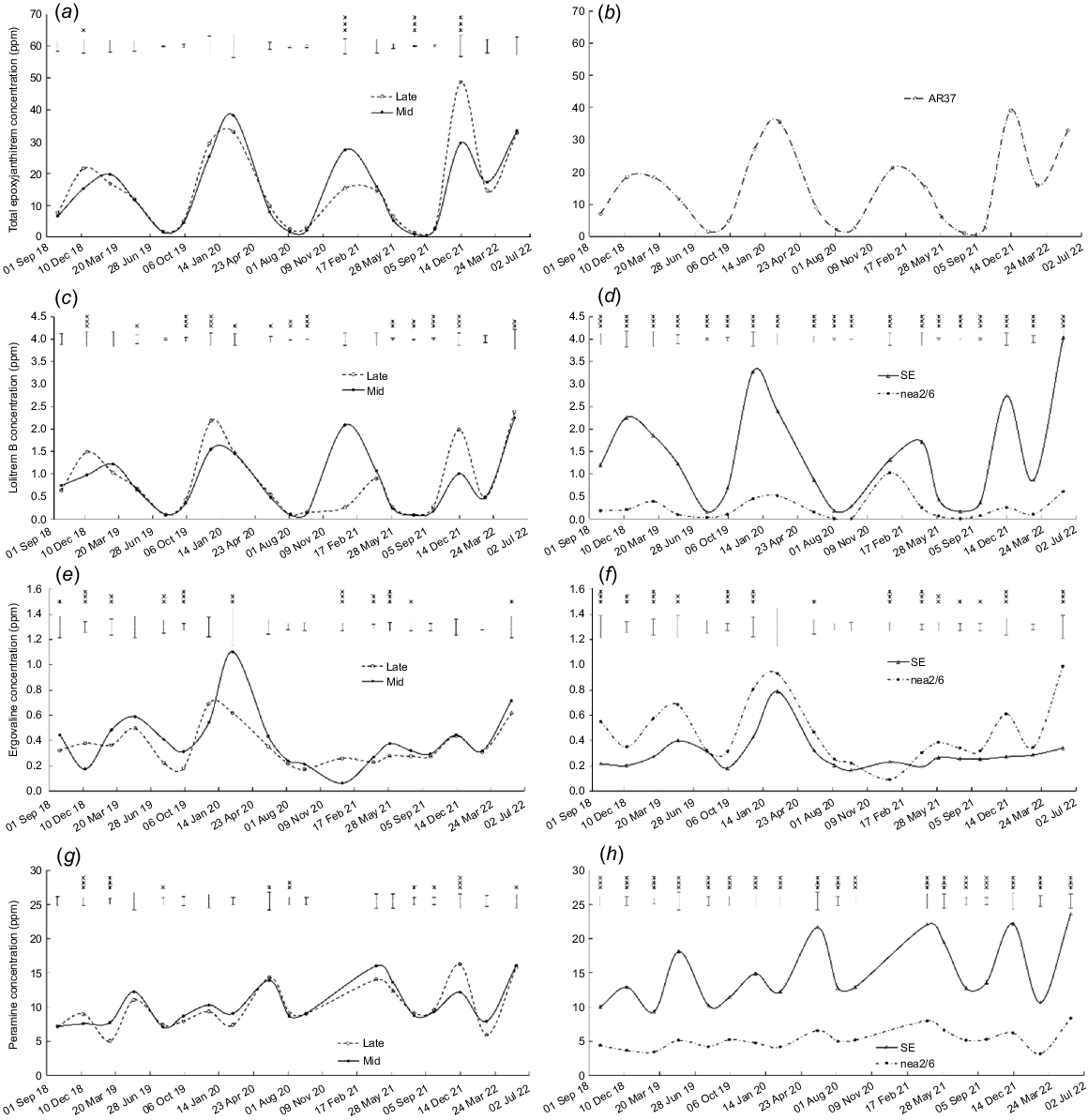
The concentration of total epoxyjanthitrems was higher (P < 0.05) in late-heading than mid-heading cultivars infected with AR37 on three occasions, and lower (P < 0.05) on one occasion (Fig. 4a). It was also lower in Bronsyn AR37 than Samson AR37 on two occasions, higher in Bronsyn–AR37 than Samson–AR37 on one occasion, and higher in One50–AR37 than Rohan–AR37 on eight occasions (all P < 0.05, Table S2).
The concentration of lolitrem B was higher (P < 0.05) in late-heading than mid-heading cultivars on 13 occasions for cultivars infected with SE or nea2/6 endophytes (Fig. 4c) and higher (P < 0.001) in SE-infected than nea2/6-infected cultivars on all 19 occasions (Fig. 4d). Five endophyte × heading date interactions (P < 0.05) were found for content of lolitrem B, which was higher in swards sown with late-heading than mid-heading cultivars infected with SE on three occasions, lower in swards sown with late-heading than mid-heading cultivars infected with SE on two occasions, and similar for late- and mid-heading cultivars infected with nea2/6 for all five occasions (Table S1). The concentration of lolitrem B was greater (P < 0.05) in swards sown with One50 than with Rohan on 18 occasions (Table S2).
Concentrations of ergovaline were higher (P < 0.001) in late-heading than mid-heading cultivars on two occasions and lower (P < 0.05) on nine occasions (Fig. 4e). The concentration of ergovaline was higher (P < 0.05) for nea2/6-infected than SE-infected cultivars on 13 occasions and lower (P < 0.05) on one occasion (Fig. 4f). The concentration of ergovaline was higher (P < 0.05) for Bronsyn than Samson on five occasions and for Rohan than One50 on five occasions (Table S2).
The concentration of peramine followed a similar trend to lolitrem B. It was higher (P < 0.05) in late-heading than mid-heading cultivars on seven occasions and in mid-heading than late-heading cultivars on two occasions (Fig. 4g), and it was higher (P < 0.001) in SE-infected than nea2/6-infected cultivars on 18 occasions (Fig. 4h). There were endophyte × heading date interactions (P < 0.05) for peramine content on nine occasions, which generally followed a consistent pattern. On nearly all of those occasions, the concentration of peramine was higher in mid-heading than late-heading cultivars infected with SE endophyte but similar when infected with nea2/6 endophyte (Table S1). The concentration of peramine was higher (P < 0.05) for Bronsyn than Samson on seven occasions and for One50 than Rohan on five occasions (Table S2).
Invertebrate abundance
No clover root weevil or black beetle adults were present in 2019 but they were present in 2021 (Table S3). No black beetle larvae, whitefringed weevil adults, grass grub larvae or greasy cutworm were present. Whitefringed weevil larvae were present in both years, as were wireworm and earthworm species.
Discussion
Establishment of treatments
Endophyte infection frequency for all cultivar–endophyte combinations was well above the 70% threshold considered necessary for ‘high endophyte’ seed lines (Eady 2021), and contamination with non-sown endophytes was low. Given this, the experimental swards enabled a robust test of the hypothesis that there would be no effect of endophyte strain on persistence.
Endophyte effect
Contrary to our hypothesis, there were effects of endophyte strain on pasture persistence. These effects were smaller than those of heading date, and regardless of endophyte strain (or heading date), there was a decline in persistence for all four cultivars over the study (see companion paper).
Endophyte strains differed in their effects on some plant characteristics such as ryegrass content and, particularly, ryegrass ground cover, with SE-infected cultivars demonstrating greater persistence than cultivars infected with nea2/6, and with AR37 to a lesser extent. For nea2/6-infected cultivars, the poorer persistence was also evident in the higher contents of other plant species, and lower autumn yields when meaned over the 4 years. There were few effects of endophyte for other characteristics such as perennial ryegrass tiller density and nutritive values, with differences between treatments being much smaller than those that occurred for heading date (see companion paper - Tozer et al. 2024).
The three endophytes used in the study provide varying levels of pest protection to a range of insects, which largely determines agronomic performance (Caradus et al. 2021). AR37 has the best overall pest protection profile, followed by SE, and then nea2/6. Effects in any particular experiment are dependent on which pest species are present and their densities, and this can vary between years. In our study, the key pasture pests, black beetle and grass grub, were absent or at levels below pasture damage thresholds (Zydenbos et al. 2011). More frequent sampling, and for a greater range of invertebrates that are known to be controlled by Epichloë endophytes, would be required to determine whether invertebrate pests were a likely contributor to persistence decline in this study.
In other perennial ryegrass studies from the upper North Island of New Zealand, effects of endophyte have been variable in magnitude. For example, under sheep grazing at two sites, Hume et al. (2007) reported that AR37-infected Samson ryegrass, over 3–4 years of yield measurements, outyielded SE- and AR1-infected Samson lines by 17–36%. At one of these sites, SE- and AR37-infected Samson lines had similar tiller densities by the fifth year, whereas AR1-infected Samson had down to half the tiller densities of the other endophyte-infected lines. Under dairy grazing, Thom et al. (2014) reported that AR37-infected swards of ryegrass cv. Grasslands Commando had higher tiller densities than those infected with SE, AR1 or Nil endophyte during a summer drought in the third year, with no differences in annual yields over all years. AR1 or Nil endophyte were not tested in our study, because they do not provide the required protection against invertebrate herbivory present in the upper North Island of New Zealand, particularly that of black beetle, which compromises persistence (Popay and Hume 2011).
There were few (i.e. one, so not reported) or no interactions between endophyte and heading date for herbage production, ryegrass tiller density and leaf stage, botanical composition and most nutritive values over the duration of the study. Interactions occurred for ME and alkaloid levels. Further, there were no consistent patterns regarding the time of year when the interactions occurred (i.e. seasonal trends). The lack of interactions detected occurred despite the large number of statistical tests (on more than 20 occasions for most measurements) and suggests that heading date and endophyte effects are mainly independent of each other for agronomic, morphological and biochemical characteristics (i.e. alkaloid content).
In the nea2/6-infected cultivars, the change in the nea2:nea6 ratio over the experimental period (from 1.3 to 0.5) indicates that the nea6 strain had an agronomic advantage over the nea2 strain within this mix. Although nea6 lacks lolitrem B, it does have considerably higher concentrations of ergovaline, which may be imparting a greater degree of pest protection than nea2, particularly against black beetle, an important pest in this region (Popay et al. 2021; Caradus et al. 2022). Pest pressure may have reduced the abundance of the nea2 strain. Detailed observations would be required to determine the causes of tiller mortality, plant mortality and persistence decline; our measurements focused on measuring surviving tiller populations and vegetation.
Contamination of pastures with non-sown toxic endophytes such as SE is of concern if the animal health and productivity advantages of selected endophytes are to be realised and maintained over time (Hume and Barker 2005; Hume et al. 2024). SE was the major contaminating endophyte in the nea2/6-infected and AR37-infected plots, and although low at just 4%, this level of contamination increased over the experimental period (from 2.5% to 7.6%), similar to that reported for commercial dairy farms in the upper North Island (Hume et al. 2024).
Endophyte alkaloids
Endophyte alkaloids play a key role in pasture persistence and productivity, along with negatively impacting animal health and performance (Caradus et al. 2021). Endophyte strains can differ with regard to which alkaloids are produced (e.g. only AR37 produces epoxyjanthitrems) and the concentrations that are expressed. In addition, concentrations vary with plant genetics and season. Within this study, season had a large influence on concentrations throughout the year, particularly for the indole diterpenes alkaloids, that is, epoxyjanthitrems and lolitrem B (Fig. 4). Similar seasonal effects, with large differences between years, have been reported by Thom et al. (2014) and Bluett et al. (2005) in dairy-grazed pastures close to the Ruakura Research Station.
Of the treatments in the study, endophyte strain had the largest and most consistent effect on alkaloid concentrations (Fig. 4). Differences between SE and nea2/6 were clearly evident, with nea2/6-infected cultivars repeatedly having substantially lower concentrations of lolitrem B and peramine, and on numerous occasions higher ergovaline, than SE-infected cultivars. This is consistent with previous reports for lolitrem B and peramine, whereas results for ergovaline are more variable (Fletcher et al. 2017; Caradus et al. 2021).
Differences in alkaloid expression between mid- and late-heading cultivars were less frequent and of lower magnitude than the effects of endophyte strain. The late-heading cultivars tended to have higher concentrations of lolitrem B and peramine, particularly so for lolitrem B. Conversely, ergovaline concentrations were generally lower in late-heading than mid-heading cultivars. There were few and inconsistent heading date effects on epoxyjanthitrems. There were also differences in alkaloid concentrations between the two late-heading cultivars; Rohan had higher concentrations of ergovaline, lower concentrations of peramine, and, in particular, lower concentrations of lolitrem B and total epoxyjanthitrems, on numerous occasions.
In terms of pasture persistence, high expression levels of these alkaloids can provide a protective advantage to the plant, which is particularly important when pastures are under drought and insect pest pressure over summer and early autumn, as well as protection from overgrazing by livestock (Hewitt et al. 2021; Caradus 2023). Despite quite large contrasts between endophyte strains, and differences between heading date groups and cultivars, there appeared to be no clear, direct link between alkaloid expression and persistence/productivity in our study. Persistence is likely to be a result of a combination of both endophyte strain and plant genetics, and a greater range of cultivars and endophytes would need to be studied to understand their relative importance.
Alkaloids provide protective properties to the host plant; however, consideration must also be given to the known mammalian toxic alkaloids (all except peramine) and how these might negatively impact animal health and welfare through disorders such as ryegrass staggers (caused by epoxyjanthitrems and lolitrem B; Fletcher et al. 2017) and by exacerbating heat stress (ergovaline) (Caradus and Johnson 2020), and how they might be detrimental to overall animal productivity. In this study, lolitrem B concentrations in SE-infected cultivars were at, or exceeded, ryegrass staggers thresholds (range 1.8–2.5 mg kg−1), and there would also have been a risk of ryegrass staggers with AR37-infected cultivars (Hume et al. 2016). Elevated concentrations of ergovaline in nea2/6 may also be a concern if grazing intensely in autumn with no non-toxic supplementation (Caradus and Johnson 2020).
Limitations
This study compared three endophyte types for two mid-heading and two late-heading cultivars of perennial ryegrass in the upper North Island of New Zealand. To be confident of the validity of these results, further studies in different years would be required, given the interacting effects of climate, soils, pest pressure, management and germplasm (Thom et al. 1996; Hume et al. 2010; Tozer et al. 2014; Cosgrove et al. 2020; Caradus et al. 2021). Therefore, although results are suggestive of a moderate endophyte effect, further research, using multiple cultivar–endophyte combinations in different regions, would be of value in validating our results.
Conclusion
Results were contrary to the hypothesis that there would be no effect of endophyte strain on persistence. Endophyte strain had less effect than heading date on persistence, based on the number and magnitude of significant results reported in this two-part series. For ryegrass ground cover, ryegrass content and autumn yield, and to some extent contents of other species, cultivars infected with SE demonstrated greater persistence than those infected with nea2/6 and, to a lesser extent, AR37, particularly by the end of the study. The greatest variation in ryegrass productivity was due to seasonal effects, with large declines in productivity over summer and subsequent recovery during late autumn and into spring.
Conflicts of interest
D. E. Hume is among the inventors of AR37. The authors have no other conflicts of interest to declare.
Declaration of funding
This project was funded from the AgResearch Strategic Science Investment Fund.
Acknowledgements
We thank Libby North, Bridget Wise, Jan Sprosen, Won Hong and Yulia Morozova of AgResearch for technical assistance; Tim Hale and Lee Falini of AgResearch and Shane Hill of Tainui Holdings for stock management; Agricom and Barenbrug for supplying seed; and Agricom for sowing the experiment. We also thank John Caradus (Grasslanz Technology), Jim Crush (AgResearch), Alan Stewart and Michael Norriss (PGG Wrightson Seeds), Graham Kerr (Barenbrug) and David Chapman (DairyNZ) for their useful comments that helped improve this manuscript.
References
Bluett SJ, Thom ER, Clark DA, Macdonald KA, Minneé EMK (2005) Effects of perennial ryegrass infected with either AR1 or wild endophyte on dairy production in the Waikato. New Zealand Journal of Agricultural Research 48, 197-212.
| Crossref | Google Scholar |
Caradus JR (2023) Epichloë fungal endophytes – a vital component for perennial ryegrass survival in New Zealand. New Zealand Journal of Agricultural Research 1-18.
| Crossref | Google Scholar |
Caradus JR, Johnson LJ (2020) Epichloë fungal endophytes – from a biological curiosity in wild grasses to an essential component of resilient high performing ryegrass and fescue pastures. Journal of Fungi 6, 322.
| Crossref | Google Scholar | PubMed |
Caradus J, Chapman D, Cookson T, Cotching B, Deighton M, Donnelly L, Ferguson J, Finch S, Gard S, Hume D, Johnson L, Kerr G, Norriss M, Peddie K, Popay A (2021) Epichloë endophytes – new perspectives on a key ingredient for resilient perennial grass pastures. NZGA: Research and Practice Series 17, 347-360.
| Crossref | Google Scholar |
Caradus JR, Card SD, Finch SC, Hume DE, Johnson LJ, Mace WJ, Popay AJ (2022) Ergot alkaloids in New Zealand pastures and their impact. New Zealand Journal of Agricultural Research 65, 1-41.
| Crossref | Google Scholar |
Card SD, Faville MJ, Simpson WR, Johnson RD, Voisey CR, de Bonth ACM, Hume DE (2014) Mutualistic fungal endophytes in the Triticeae – survey and description. FEMS Microbiology Ecology 88, 94-106.
| Crossref | Google Scholar | PubMed |
Chapman D, Edwards G, Nie Z (2011) Plant responses to climate and relationships with pasture persistence. NZGA: Research and Practice Series 15, 99-107.
| Crossref | Google Scholar |
Corson DC, Waghorn GC, Ulyatt MJ, Lee J (1999) NIRS: forage analysis and livestock feeding. Proceedings of the New Zealand Grassland Association 61, 127-132.
| Crossref | Google Scholar |
Cosgrove GP, Trolove MR, Staincliffe MR, Tozer KN (2020) Persistence of perennial ryegrass, tall fescue and cocksfoot following sequential annual sowings: pasture yield, composition and density in three establishment years under cattle grazing in Waikato. Journal of New Zealand Grasslands 82, 149-159.
| Crossref | Google Scholar |
Dignam BEA, Marshall SDG, Wall AJ, Mtandavari YF, Gerard EM, Hicks E, Cameron C, Aalders LT, Shi S, Bell NL (2022) Impacts of soil-borne disease on plant yield and farm profit in dairying soils. Journal of Sustainable Agriculture and Environment 1, 16-29.
| Crossref | Google Scholar |
Eady C (2021) The impact of alkaloid-producing Epichloë endophyte on forage ryegrass breeding: a New Zealand perspective. Toxins 13, 158.
| Crossref | Google Scholar | PubMed |
Faville MJ, Briggs L, Cao M, Koulman A, Jahufer MZZ, Koolaard J, Hume DE (2015) A QTL analysis of host plant effects on fungal endophyte biomass and alkaloid expression in perennial ryegrass. Molecular Breeding 35, 161.
| Crossref | Google Scholar |
Fletcher LR, Finch SC, Sutherland BL, deNicolo G, Mace WJ, van Koten C, Hume DE (2017) The occurrence of ryegrass staggers and heat stress in sheep grazing ryegrass-endophyte associations with diverse alkaloid profiles. New Zealand Veterinary Journal 65, 232-241.
| Crossref | Google Scholar | PubMed |
Fulkerson WJ, Donaghy DJ (2001) Plant-soluble carbohydrate reserves and senescence – key criteria for developing an effective grazing management system for ryegrass-based pastures: a review. Australian Journal of Experimental Agriculture 41, 261-275.
| Crossref | Google Scholar |
Gagic M, Faville MJ, Zhang W, Forester NT, Rolston MP, Johnson RD, Ganesh S, Koolaard JP, Easton HS, Hudson D, Johnson LJ, Moon CD, Voisey CR (2018) Seed transmission of Epichloë endophytes in Lolium perenne is heavily influenced by host genetics. Frontiers in Plant Science 9, 1580.
| Crossref | Google Scholar |
Hewitt KG, Mace WJ, McKenzie CM, Matthew C, Popay AJ (2020) Fungal alkaloid occurrence in endophyte-infected perennial ryegrass during seedling establishment. Journal of Chemical Ecology 46, 410-421.
| Crossref | Google Scholar | PubMed |
Hewitt KG, Popay AJ, Hofmann RW, Caradus JR (2021) Epichloë − a lifeline for temperate grasses under combined drought and insect pressure. Grass Research 1, 7.
| Crossref | Google Scholar |
Hume DE, Ryan DL, Cooper BM, Popay AJ (2007) Agronomic performance of AR37-infected ryegrass in northern New Zealand. Proceedings of the New Zealand Grassland Association 69, 201-205.
| Crossref | Google Scholar |
Hume DE, Hickey MJ, Lyons TB, Baird DB (2010) Agronomic performance and water-soluble carbohydrate expression of selected ryegrasses at two locations in New Zealand. New Zealand Journal of Agricultural Research 53, 37-57.
| Crossref | Google Scholar |
Hume DE, Luo D, Rennie GM, King WM, Taylor AL, Faville MJ, Tozer KN (2024) Stability and purity of selected ryegrass Epichloë endophytes in New Zealand dairy pastures. Grassland Research 1-10.
| Crossref | Google Scholar |
Lee JM, Matthew C, Thom ER, Chapman DF (2012) Perennial ryegrass breeding in New Zealand: a dairy industry perspective. Crop & Pasture Science 63, 107-127.
| Crossref | Google Scholar |
Lee JM, Thom ER, Waugh CD, Bell NL, McNeill MR, Wilson DJ, Chapman DF (2017) Trajectory and causes of decline in the botanical composition of dairy-grazed pasture in the Waikato. Journal of New Zealand Grasslands 79, 89-96.
| Crossref | Google Scholar |
Moon CD, Tapper BA, Scott B (1999) Identification of Epichloë endophytes in planta by a microsatellite-based PCR fingerprinting assay with automated analysis. Applied and Environmental Microbiology 65, 1268-1279.
| Crossref | Google Scholar | PubMed |
Patterson HD, Thompson R (1971) Recovery of inter-block information when block sizes are unequal. Biometrika 58, 545-554.
| Crossref | Google Scholar |
Popay A, Hume D (2011) Endophytes improve ryegrass persistence by controlling insects. NZGA: Research and Practice Series 15, 149-156.
| Crossref | Google Scholar |
Popay AJ, Hume DE, Mace WJ, Faville MJ, Finch SC, Cave V (2021) A root aphid Aploneura lentisci is affected by Epichloë endophyte strain and impacts perennial ryegrass growth in the field. Crop & Pasture Science 72, 155-164.
| Crossref | Google Scholar |
Rasmussen S, Lane GA, Mace W, Parsons AJ, Fraser K, Xue H (2012) The use of genomics and metabolomics methods to quantify fungal endosymbionts and alkaloids in grasses. Methods in Molecular Biology 860, 213-226.
| Crossref | Google Scholar |
Simpson WR, Schmid J, Singh J, Faville MJ, Johnson RD (2012) A morphological change in the fungal symbiont Neotyphodium lolii induces dwarfing in its host plant Lolium perenne. Fungal Biology 116, 234-240.
| Crossref | Google Scholar | PubMed |
Thom ER, van Vught VT, Mccabe RJ (1996) Growth and persistence of perennial ryegrass lines with different tolerances to “pulling” during grazing. Proceedings of the New Zealand Grassland Association 58, 67-72.
| Crossref | Google Scholar |
Thom ER, Popay AJ, Waugh CD, Minneé EMK (2014) Impact of novel endophytes in perennial ryegrass on herbage production and insect pests from pastures under dairy cow grazing in northern New Zealand. Grass and Forage Science 69, 191-204.
| Crossref | Google Scholar |
Tozer KN, Cameron CA, Thom ER (2011) Pasture persistence: farmer observations and field measurements. NZGA: Research and Practice Series 15, 25-30.
| Crossref | Google Scholar |
Tozer KN, Chapman DF, Bell NL, Crush JR, King WM, Rennie GM, Wilson DJ, Mapp NR, Rossi L, Aalders LT, Cameron CA (2014) Botanical survey of perennial ryegrass-based dairy pastures in three regions of New Zealand: implications for ryegrass persistence. New Zealand Journal of Agricultural Research 57, 14-29.
| Crossref | Google Scholar |
Tozer KN, Hume DE, Cameron C, Greenfield R, Dale T, Mace WJ, Craven T, Faville MJ (2024) Effects of heading date and Epichloë endophyte on persistence of diploid perennial ryegrass (Lolium perenne). 1. Heading date. Crop & Pasture Science 75, CP23266.
| Crossref | Google Scholar |
Zydenbos S, Barratt B, Bell N, Ferguson C, Gerard P, McNeill M, Phillips C, Townsend R, Jackson T (2011) The impact of invertebrate pests on pasture persistence and their interrelationship with biotic and abiotic factors. NZGA: Research and Practice Series 15, 109-117.
| Crossref | Google Scholar |