Developmental patterns of flowers and pods and the effect on seed number in French serradella (Ornithopus sativus) and yellow serradella (Ornithopus compressus) cultivars
Laura E. Goward A B * , Rebecca E. Haling
A B * , Rebecca E. Haling  A , Rowan W. Smith
A , Rowan W. Smith  B , Beth Penrose
B , Beth Penrose  B C and Richard J. Simpson
B C and Richard J. Simpson  A
A
A
B
C Present Address:
Abstract
Reliable seed production is a key requirement for successful year-on-year regeneration of annual pasture legumes.
The study aims were to investigate the developmental patterns of flowers and pods and the effect on seed number among cultivars of French (Ornithopus sativus Brot.) and yellow serradella (O. compressus L.); and to assess the effects of early flower loss.
Four cultivars of each species were grown in a glasshouse under non-limiting growth conditions. Date of flowering and numbers of flowers, pods and seeds were assessed for up to 20 reproductive nodes on two stem axes per plant (n = 5 plants). A flower removal treatment was imposed to assess whether early flower loss affected flower and/or pod production.
Flowering in the serradellas was indeterminate, but for all cultivars there was a peak period of flower and pod production, with the timing and duration of the peak period differing among cultivars. Peak flowering occurred primarily because the proportion of plants flowering began to decline, but the number of flowers per reproductive node and the number of pods formed per node also declined with time. Compensation for early flower loss was observed for most cultivars because of a longer duration of pod formation and/or greater numbers of pods developed on higher reproductive nodes.
This study demonstrated that there is diversity in the patterns of flowering and podding and number of seeds initiated among serradellas.
Diversity in flowering and podding patterns combined with a capacity to compensate for early flower loss may be used to develop serradellas better able to cope with environmental stressors (frost, drought, heat) experienced during the flowering window.
Keywords: adaptation, alternative legumes, annual pasture, Mediterranean, optimal flowering time, persistence, rate of reproductive node development, temperate.
Introduction
Reliable production of viable seeds is a key requirement for successful year-on-year regeneration and persistence of annual pasture legumes in temperate environments (Reed et al. 1989). This usually means that plants must flower soon after the risk of frost has subsided because frost damages flowers and developing seeds, and well before seed growth is affected by terminal drought, so that adequate numbers of mature seeds are produced (Rossiter 1966; Donald 1970; Aitken 1974). A primary management technique to minimise exposure to frost during flowering, and heat and drought stress during seed production, is selection of a cultivar with a ‘maturity type’ (e.g. early-flowering, mid-season-flowering and late-flowering) that ensures the time of flowering is appropriate for the environment in which it will be sown (e.g. Lattimore and McCormick 2013). The time of flowering must allow sufficient time for seed production while maximising the length of the vegetative period when legumes produce fodder with high nutritional value for animal production (Ru and Fortune 2000; Thomas et al. 2021).
The determinacy of flowering is expected to influence the rigidity of the optimum date for flowering. Plants with determinate flowering (i.e. the growing point is terminated by a flower and flowering occurs over a relatively short period: Rudall 2020; PlantNET (The NSW Plant Information Network System) 2023) must not begin to flower during frost periods because all, or a large proportion, of the seed production may be terminated by frost damage. By contrast, it is conceivable that legumes with indeterminate flowering (i.e. the apex maintains growth until it becomes exhausted, with flowers continuing to appear at reproductive nodes (RNs): Rudall 2020; PlantNET 2023) may be able to flower earlier, during periods with a higher risk of frost, without a substantive, adverse impact to the number of seeds produced.
Successful flower production, which dictates potential seed number, is also influenced by other factors such as the rate of flower appearance (i.e. how fast inflorescences are produced), the total number of RNs that a plant produces (e.g. subterranean clover (Trifolium subterraneum L.): Francis and Gladstones 1974; and yellow serradella (Ornithopus compressus L.): Revell et al. 1999), and whether or not flower shedding has occurred (Cocks 1990; Revell et al. 1999). Together, these parameters determine the duration of flower appearance (Francis and Gladstones 1974; Revell et al. 1999). Indeed, the rate of flower appearance has been recommended as a primary selection criterion for high seed yield among subterranean clover cultivars (Francis and Gladstones 1974).
In southern Australia, cultivars of French serradella (Ornithopus sativus Brot.) and yellow serradella are increasingly being recognised as viable alternatives or companion species for subterranean clover, which is the most widely grown annual pasture legume (Loi et al. 2005; Nichols et al. 2007). However, considerably less information is available concerning the patterns of flowering and pod and seed formation for the serradellas than for subterranean clover cultivars. Typically, an umbel of three to five flowers is produced at each RN for yellow serradellas, and three to six flowers per umbel for French serradella cultivars (Fu et al. 1994). The yellow serradella cultivars SerraMax (early maturity type, formerly known as GEH72-1A; Loi et al. 2021) and Avila (mid–late maturity type; Lattimore and McCormick 2013) have flower appearance rates of 3 days and 4 days per RN, respectively, when grown under irrigated field conditions in Perth, Western Australia (Revell et al. 1999). These are the only published data concerning rates at which flowers are produced by cultivars of serradella.
Potential for long durations of flowering in serradella cultivars may serve as a compensatory mechanism to mitigate the adverse consequences of variable flowering dates. This is important because a number of serradella cultivars exhibit less stable flowering dates than subterranean clovers of a comparable maturity type (Haling et al. 2023; Simpson et al., unpubl. data). This means that plants germinating on early rainfall in autumn may flower early during periods of high frost risk, with potentially adverse consequences for seed production. However, it is possible that, under these circumstances, the indeterminate flowering habit of the serradellas may enable seed formation to continue longer, if not indefinitely. At least some cultivars are known to continue growing in response to late summer rainfall (Hackney et al. 2021; Thomas et al. 2021). Revell et al. (1999) reported that the number of flowers per RN of cvv. SerraMax and Avila was sustained for at least 11 RNs (where RNs are counted from the node on which the first flower appears) when grown with late-season irrigation. However, they observed that the number of pods per node declined after seven and 10 RNs, and that pod development had ceased by RNs 11 and 13, respectively. These results suggest a finite period of seed production among serradellas and that observations of continuing flower production alone may not be a good indicator of their seed production potential.
The objective of this experiment was to gather essential data on flowering, including the rate of RN development, flower and pod numbers, and seed numbers per pod, for representative French serradella and yellow serradella cultivars. It was hypothesised that these reproductive characteristics were likely to be species- or cultivar-specific. The report by Revell et al. (1999) that duration of pod production was finite in two yellow serradella cultivars indicated that indefinite flowering and seed production may not occur in the serradellas, and therefore, that their indeterminate flowering patterns may not be sufficient to compensate fully for flower loss due to frost damage. A treatment in which flowers were removed (intended to mimic damage to flowers and immature pods from a frost event) was imposed to test whether seed production would be significantly impacted by a frost event soon after the first flowers had begun to appear. Data were used in scenarios to assess how adverse seasonal conditions during flowering may affect seed number.
Materials and methods
Experiment design
The experiment was conducted using a complete factorial combination of two species × four cultivars × two flower removal treatments (EFR, early flowers removed; and Control, no flowers removed) × five replicates (total pot number = 80). Flower removal treatments were included to test the capacity of cultivars to compensate for early flower loss.
Plant material
Four cultivars of French serradella (cvv. Margurita, Erica, Rosa and Serratas) and four cultivars of yellow serradella (cvv. SerraMax, King, Yellotas and Avila) ranging in maturity type from very early to late season (Lattimore and McCormick 2013; Boschma et al. 2019; Loi et al. 2021; Haling et al. 2023) were evaluated. Of these cultivars, Margurita and Erica are closely related, and were selected for hardseededness out of a population of cv. Cadiz (Nutt 2004a, 2004b).
Plant growth conditions and imposition of treatments
Seed was dehulled and scarified as required to ensure germination of hard seed (impermeable to water). The seed of Serratas were entirely soft (permeable to water) and did not require scarification. The seeds of all cultivars exhibited high germination rates (>90%) except for Erica (germination rate 50%).
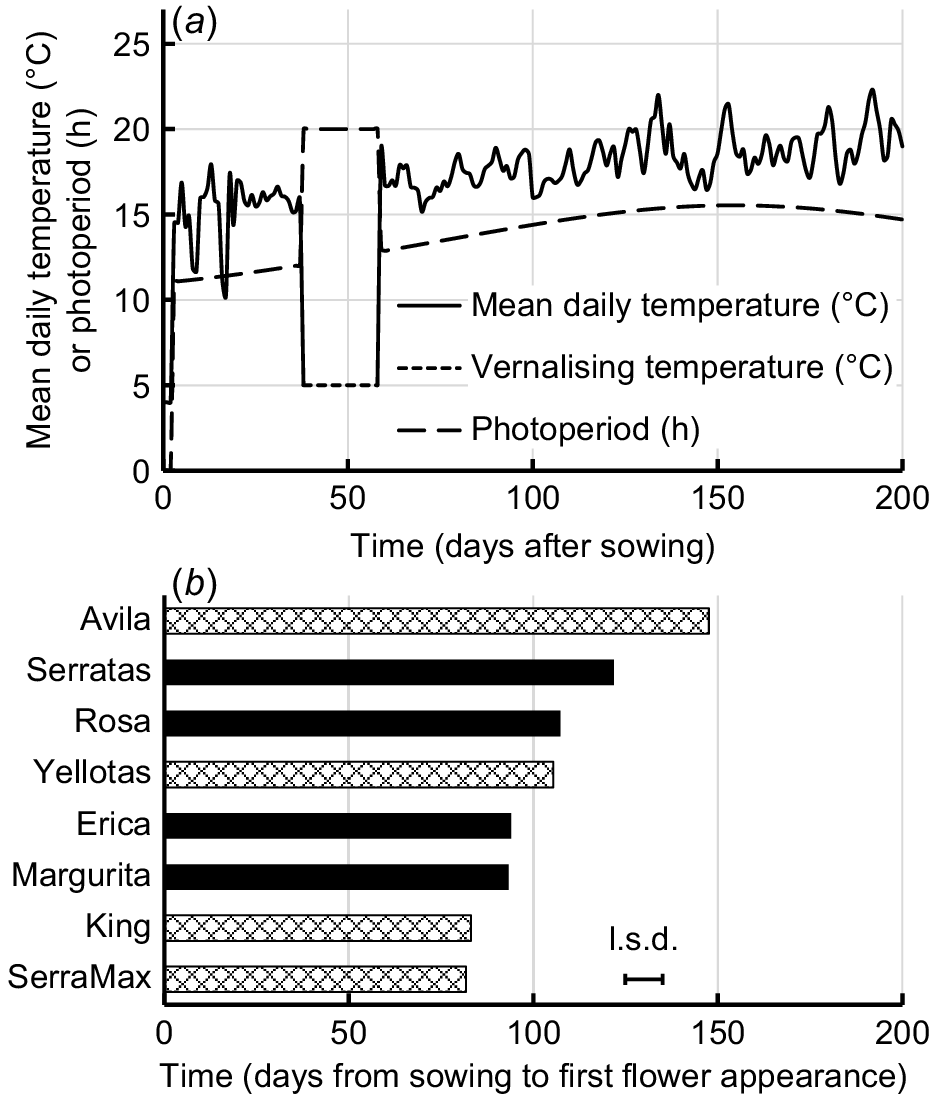
Ten seeds were sown in 23-cm-diameter pots containing 5.8 L of a nutrient-rich, pasteurised, potting soil known to be able to support the growth of the plants for the duration of the experiment. At sowing, the soil was moistened, and the pots were covered with black plastic and kept at 4°C for 3 days in a controlled environment to promote even germination. The plants were then transferred to a naturally lit glasshouse for 35 days with partial temperature control that permitted daytime temperatures to rise to, typically, ~22°C and night-time temperatures to decline no lower than ~5°C. In practice, mean daily temperatures ranged between 10°C and 18°C, with an average of 15°C for the 35-day period (Fig. 1a). By 38 days after sowing, the plants had developed five or six leaves and the pots were transferred to a controlled-environment plant growth chamber set to a constant vernalising temperature (5°C) with a long photoperiod (20 h). The vernalisation and long photoperiod treatments were intended to reduce the time that it would take for first flowers to appear on all of the cultivars (Goward et al. 2023). After 3 weeks under these conditions, plants were thinned to one uniform seedling per pot, each with six or seven leaves per plant (i.e. Stage 36–37, BBCH scale; Enriquez-Hidalgo et al. 2020). Each pot represented one replicate of each cultivar. Five replicate pots had been allocated previously, at random, to the Control treatment (i.e. no flowers removed) and five to the EFR treatment. Two pots of Erica seedlings designated for the EFR treatment did not survive the vernalising temperatures and were removed from the experiment. All plants were moved to a glasshouse on 14 September 2020 (59 days after sowing), when natural daylengths were lengthening (starting at 12.8 h, Fig. 1a) and temperatures were rising (average daily temperature starting at ~17°C and ending at ~19°C, Fig. 1a). Natural photoperiods were calculated using the civil twilight method (Goodspeed 1975).
(a) Mean daily temperatures and photoperiods in the glasshouse and in the controlled environments used during the experiment. (b) Mean number of days from sowing to appearance of first flower for yellow serradella (O. compressus) cultivars (diamond pattern bars) and French serradella (O. sativus) cultivars (solid bars). Bar indicates l.s.d. (P = 0.05).
Temperature data were collected for the duration of the experiment by placing a Thermocron iButton temperature sensor (model number DS1921G-F50; Maxim Integrated, San Jose, CA, USA), on the soil surface in two pots selected at random. Temperature data were recorded every 30 min (accuracy ±0.5°C, resolution 0.5°C) and mean daily temperatures were calculated from these data. Thermal time (degree-days) estimates were made by summing mean daily temperatures.
Plants were monitored regularly and were managed to avoid potential confounding effects of inadequate nutrient supply, insect pests or plant disease issues. Pots were hand-watered daily to ensure that plants were neither waterlogged nor water-stressed. The decision to cease the experiment was made based on the duration of flowering exceeding ~3 months after first flower appearance for most cultivars, a timeframe which is rarely exceeded, prior to the onset of hot and dry conditions that determine the end of a typical growing season for these plants. Nodulation was supported by inoculating the plants at 9 days after sowing with a peat slurry containing rhizobial strain Bradyrhizobium lupini (WSM471; New Edge Microbials, Albury, NSW, Australia), immediately prior to watering. No pesticides were required during the experiment.
Serradellas often have multiple reproductive axes. For this experiment, flowering dates, flower numbers and pod numbers were recorded along two reproductive axes (either a main and a primary axis, or along two primary axes; Goward et al. 2023; Supplementary Table S1) chosen as a consequence of being the first axis to bear a flower (denoted ‘axis A’) and the second stem axis on which a flower was born (‘axis B’). These axes were trained on vertical stakes to facilitate easy monitoring of each RN. Flower development was monitored daily and small, paper ‘jewellers’ tags were marked with the date of the appearance of the first flower (i.e. Stage 53, BBCH scale) and hung on the peduncle of each umbel when the first flower appeared at each node. Flower appearance was defined by petals being visible (~2–3 mm in length). At this time, the total number of flower buds that comprised the umbel developing at the RN was also counted and noted on the tag. The number of flowers and number of pods at each RN were recorded on both stem axes. The average of the two stem axes was used to represent the plant. However, it was impractical to count the number of seeds per pod on both stem axes owing to the very high numbers of pods. Therefore, seeds per pod at each RN was recorded only for axis A. Seeds were counted when the pod colour began to turn yellow because, at this stage in their development, it was possible to count seed numbers accurately without dissecting the pods. At this stage, there was visible ‘segmentation’ along each pod, indicating segments containing individual seeds. An unfortunate consequence of counting seed numbers late in pod development was that values are missing for the Serratas EFR treatment at RN 15–RN 17 due to early dehiscence of pods at these nodes. Observations of flowers and pods were collected from the monitored axes of each plant for 20 RNs, or until flowering ceased (if this occurred before RN 20).
Statistical analyses
Most measurements were collected by monitoring two stem axes on each replicate plant, and these measurements were treated as duplicates with the average being used to represent the replicate. Exceptions to this were: (1) seed number per pod, which was only counted on axis A; and (2) the split-line regressions used to estimate duration of flowering where all datapoints were used for the regression analysis. Except where indicated below, all statistical analyses were conducted using Genstat (22nd edition; VSN International, Hemel Hempstead, UK).
The time of appearance of first flower and the cultivar responses to flower removal treatments were evaluated using a two-way analysis of variance (ANOVA) that considered cultivar and flower removal treatments as factors. Following the ANOVA, Fischer’s least significant difference values were determined.
It was desirable to express rate of RN development in units of time per RN. Consequently, for each tracked stem axis, Excel (ver. 2208; Microsoft, Redmond, WA, USA) was used to form linear regressions between time of first flower appearance at each RN (the independent variable; y-axis) and RN number (RN 1, RN 2, etc., the dependent variable; x-axis). Time was measured in days or degree-days from the appearance of the first flower on RN 1. Rates of RN development were determined from these regressions. Values for duplicate axes were averaged to determine the replicate values used for ANOVA.
The duration of flowering for each cultivar was determined by the proportions of plants that continued to flower through time. Split-line regression analysis was used to assess each cultivar’s pattern of flowering by regressing the proportion of stems continuing to flower against RN number. Data beyond the RN at which a zero percentage of stems continuing to flower was recorded were excluded from the analysis to avoid biasing the regression. The break point of the split-line regression represented the RN beyond which there was a decline in the proportion of stems bearing new flowers. The standard errors associated with the break point were used to test for differences between flower removal treatments within cultivars (e.g. Dyson and Conyers 2013). Genstat did not recognise the very low RN at which the break point occurred in the Margurita EFR treatment, so the standard error for a predicted y-value was determined using the LINEST function in Excel to circumvent this issue.
Although the planned experiment was established with a fully balanced design (i.e. eight cultivars × two flower removal treatments × five replicates × 20 RNs), the flowering patterns observed in the serradellas resulted in a decreasing number of plants continuing to flower and set pods over time. As a result, the statistical design became unbalanced, with decreasing replication for ‘nodes bearing pods’ as the RN number increased. Separate ANOVAs were conducted for RN 1–RN 5 (2-way ANOVA (cultivar × RN) of the Control treatment), and for RN 6–RN 20 (3-way unbalanced ANOVA (cultivar × flower removal treatment × RN)) for flower number per RN, pod number per RN, and average number of seeds per pod at each RN. The use of separate ANOVAs was necessary because the removal of flowers and pods in the EFR treatment disrupted the development of pods and seeds on RN 1–RN 5.
The potential for seed production by each cultivar and the possible impacts of environmental stressors on seed production were explored by calculating the average total number of seeds initiated per stem axis:
where RNi is the first (Control treatment) or first ‘surviving’ (EFR treatment) RN, and RNj is the last node to produce a flower within a 500 or 700 degree-day window (as described below).
The total number of seeds per axis was determined for each replicate (i.e. plant) of each treatment (i.e. cultivar × flower removal treatment). Thus, the average value (and its standard error) derived for each treatment from these data also reflected the progressive declines in the proportion of plants that continued to flower.
Seed numbers produced in the Control treatment were assumed to represent a plant flowering at an optimal date with no loss of flowers or pods through frost. Seed numbers in the EFR treatment were intended to represent a plant that had flowered earlier than the optimal date, with loss of flowers and immature pods on the first five RNs through frost. The calculations explored two contrasting growing-season conditions for seed development: (1) a typical growing-season length in which the duration of the flowering window is largely unlimited (i.e. up to 700 degree-days); and (2) a shorter seed-development period (500 degree-days) to mimic a premature end to flowering and pod production due to heat stress or terminal drought. Time (degree-days) for development of seed numbers was counted from the appearance of the first flower (i.e. at RN 1) in the Control treatment, or from the appearance of the first ‘surviving’ flower (i.e. at RN 6) in the EFR treatment. For the purpose of these calculations, where 700 degree-days after the first surviving flower would have occurred at RNs >20 (i.e. after the flower and pod measurements had ceased), it was assumed that flowers and pods continued to be produced at RN 21 and above until the 700 degree-day window had elapsed. This extrapolation was necessary for cvv. Erica, Rosa and Margurita. For each of these cultivars, pods per RN and seed number per pod at RNs >20 were estimated using the average of pod and seed numbers recorded for RN 15–RN 20, and the number of nodes continuing to flower was estimated from the equations that describe the decline in podding duration and the rate of RN development for each cultivar, as reported in the Results. Missing data for seed number per pod for RN 15–RN 17 in the Serratas EFR treatment were substituted using the average seed number per pod derived from the other RNs in this treatment.
Standard errors associated with these estimates were calculated using all observations within the relevant period after the first or first-surviving flower had appeared (i.e. either 500 or 700 degree-days).
The total number of seeds initiated per stem in the Control treatment was compared with that in the EFR treatment under four ‘growing-season scenarios’ to test the ability of the cultivars to: (1) compensate for early flower loss (e.g. as may occur through frost), and/or (2) initiate seeds when the seed-production period was shortened prematurely.
Results
Time to first flower
The first flower was observed within a span of 6 weeks for all cultivars except Avila (Fig. 1b), during which the average daily temperatures ranged between 15°C and 22°C, and there was a gradual increase in photoperiod from 12.8 to 15.5 h (Fig. 1a). Five maturity groupings were evident among the cultivars: 1, Avila (late flowering); 2, Serratas; 3, Rosa and Yellotas; 4, Margurita and Erica; and 5, King and SerraMax (very early flowering). These rankings largely reflected maturity rankings observed in the field environments with cool winters, with the exception that Serratas usually starts to flower later than Avila (Boschma et al. 2019). However, this outcome can be explained by the quantitative responses to vernalising temperatures under a 20 h photoperiod by these particular cultivars (Goward et al. 2023) and was, therefore, a consequence of the artificial vernalisation and photoperiod treatment used to shorten the time from sowing to flowering for the convenience of the present experiment. Yellow serradella cultivars flowered predominantly on the primary axes, whereas French serradella cultivars flowered across both main and primary axes (Table S1).
Avila was relatively late to commence flowering (Fig. 1b), and first flowers on the replicate plants representing Avila appeared over a longer period than those of the other cultivars. Indeed, one replicate failed to flower before the end of the experiment (200 days after sowing). Very late flowering prevented collection of data at subsequent RNs of Avila and resulted in its removal from the analysis of most measures of flowering and pod development.
Rate of reproductive node development
Equations describing the relationship between time of first flower appearance at each RN (y) and the RN number at which each flower appeared (x) on each plant were highly linear. Regardless of whether y was expressed in either days or thermal timeafter first flower appearance at RN 1, coefficients of determination exceeded 0.91 (Table 1). Consequently, the rates of RN development along each stem axis were found to be constant. Removing flowers did not change the rate at which the first flowers appeared (days per RN, P = 0.90; degree-days per RN, P = 0.78). However, significant differences in rate of first flower appearance were observed among the serradella cultivars (P < 0.001 for both units of time) (Table 1). RN development (degree-days per RN) was in the order (fastest to slowest) Serratas > Rosa = Erica = Margurita > King = SerraMax > Yellotas, with rates of flower appearance ranging from 32 to 57 degree-days/RN (Table 1).
| Cultivar | Equation | r2 | Mean rates of first flower appearance at RNs | ||
|---|---|---|---|---|---|
| (days/RN) | (degree-days/RN) | ||||
| O. sativus | |||||
| Serratas | y = 1.63x − 2.04 | 0.95 | 1.6 | 32 | |
| Rosa | y = 2.27x − 3.10 | 0.94 | 2.0 | 38 | |
| Erica | y = 2.16x − 1.85 | 0.99 | 2.2 | 39 | |
| Margurita | y = 2.22x − 2.16 | 0.98 | 2.3 | 41 | |
| O. compressus | |||||
| King | y = 2.73x − 2.61 | 0.98 | 2.7 | 47 | |
| SerraMax | y = 2.77x − 3.41 | 0.96 | 2.9 | 50 | |
| Yellotas | y = 3.04x − 3.29 | 0.92 | 3.1 | 57 | |
| l.s.d. (P = 0.05) | 0.3 | 4 | |||
There were insufficient data for assessment of the rate of flower appearance for cv. Avila.
Equations describe the linear relationships observed between time of first flower appearance at each RN (y; days after first flower appearance at RN 1) and the RN number at which each inflorescence appeared (x).
High coefficients of variation (r2 > 0.91) within each treatment combination (flower removal × cultivar treatment, data not shown) indicated that the rate of first flower appearance along a stem axis was constant (i.e. linear with respect to time expressed either as days or degree-days). A two-way ANOVA was conducted to test the effects of flower removal and cultivar. Flower removal treatment had no significant effect on the rate at which the first flower on each RN appeared when assessed in units of days per RN (P = 0.90) or degree-days per RN (P = 0.78). Significant differences in rate of first flower appearance were observed among cultivars (P < 0.001).
Flowering and pod formation, and effects of removing flowers
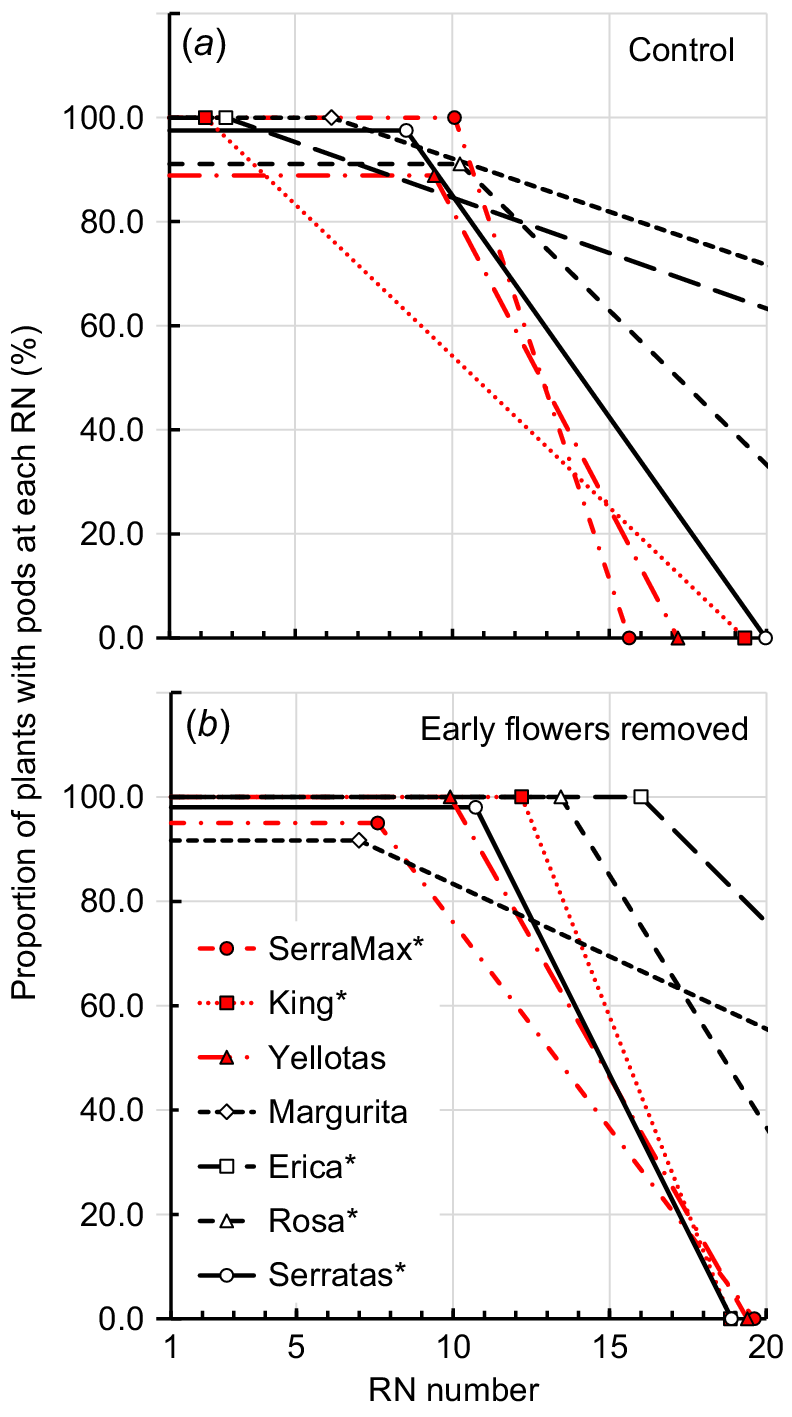
The physiology of flowering and pod formation was examined in relation to RN number. All serradella cultivars followed a similar pattern of first flower appearance whereby there was an initial period over which all plants representing the cultivar were flowering on successive RNs (hereafter referred to as ‘full flowering’; Table 2, Fig. 2). At each node, all flowers in the umbel opened in quick succession after the first flower had emerged (usually over a 1–2-day period). Thereafter, the proportion of plants that continued to produce flowers at a higher RN declined, and ultimately each cultivar ceased flowering or was projected to do so. The RN number at which full flowering ceased differed among cultivars (P < 0.05; Table 2, Fig. 2). For example, in the Control treatment, Rosa was in full flower until RN 14, and at the other extreme, Margurita and Erica remained in full flower until only RN 7–9. Most cultivars exhibited a similar rate of decline in the proportion of plants that remained in flower after their full flowering period and were observed or projected to cease flowering by between six and 12 RNs after the end of their full-flowering period. Margurita and Erica were exceptions to this, with very similar and substantially slower declines in proportion of plants continuing to flower at successive RNs (Fig. 2, Table 2). Although Margurita and Erica completed their full-flowering periods at a relatively low RN, their slower decline in plants continuing to flower meant that they were expected to continue flowering on a substantially higher RN than occurred for any of the other cultivars (Table 2).
| Cultivar | Flower removal treatment | Flowering | Pod formation | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Highest RN at which full flowering occurredA | s.e. of RN at which full flowering occurred | Eqn describing decline in proportion of plants flowering (x = RN; y = % of plants continuing to flower) | s.e. of gradient describing decline in proportion of plants flowering (% flowering/RN) | r 2 | Predicted RN at which all flowering by cultivar had ceasedB | Highest RN at which all plants in population were forming podsC | s.e. of RN at which all plants were forming pods | |||
| O. compressus | ||||||||||
| SerraMax | Control | 10 | 0.2 | y = −16.4x + 256.4 | 0.7 | 0.98 | 16 | 10 | 0.2 | |
| EFR | 9 | 0.5 | y = −8.2x + 170.6 | 0.5 | 0.95 | 21 | 8 | 0.6 | ||
| King | Control | 12 | 0.2 | y = −27.5x + 436.2 | 2.1 | 0.93 | 16 | 2 | 1.7 | |
| EFR | 14 | 0.1 | y = −19.1x + 360.9 | 0.7 | 0.98 | 19 | 12 | 0.1 | ||
| Yellotas | Control | 11 | 0.2 | y = −20.0x + 320.0 | 1.4 | 0.96 | 16 | 9 | 0.3 | |
| EFR | 10 | 0.5 | y = −10.9x + 211.2 | 0.8 | 0.92 | 19 | 10 | 0.6 | ||
| O. sativus | ||||||||||
| Erica | Control | 9 | 0.6 | y = −3.6x + 131.8 | 0.3 | 0.91 | 37 | 3 | 2.1 | |
| EFR | 16 | 0.4 | y = −6.0x + 196.0 | 1.0 | 0.75 | 33 | 16 | 0.5 | ||
| Margurita | Control | 7 | 1.3 | y = −2.2x + 114.8 | 0.3 | 0.79 | 53 | 6 | 1.6 | |
| EFR | 16 | 0.4 | y = −4.4x + 168.9 | 0.5 | 0.80 | 38 | 7 | 2.7 | ||
| Rosa | Control | 14 | 0.3 | y = −11.4x + 254.4 | 0.9 | 0.95 | 22 | 10 | 0.7 | |
| EFR | 16 | 0.2 | y = −12.2x + 295.6 | 0.8 | 0.95 | 24 | 13 | 0.3 | ||
| Serratas | Control | 12 | 0.4 | y = −10.6x + 231.1 | 0.8 | 0.91 | 22 | 9 | 0.8 | |
| EFR | 14 | 0.5 | y = −11.7x + 266.6 | 1.5 | 0.83 | 23 | 11 | 0.4 | ||
Split-line regressions describing the proportion of plants that represented each serradella cultivar that continued to produce flowers at each reproductive node (RN) in the: (a) Control treatment (i.e. no flowers removed), and (b) EFR treatment (i.e. all flowers removed from plant when the first flower appeared at RN 5). There were significant differences (P < 0.05) in the highest RN number at which full flowering occurred between the Control and EFR treatments for all cultivars, as reported in Table 2. Solid symbols and red lines, data for yellow serradella (O. compressus) cultivars; open symbols and black lines, French serradella (O. sativus).

Removing flowers lengthened the duration of the full flowering period (P = 0.05) for five of the seven cultivars assessed (Fig. 2, Table 2). SerraMax and Yellotas were the exceptions with no significant change to their full flowering periods. For King, Rosa and Serratas, the response to removal of flowers was to extend full flowering by two RNs. However, Erica and Margurita over-compensated for the removal of inflorescences and young pods on the first five RNs and extended full flowering by seven and nine RNs, respectively.
The pattern and, consequently, duration of pod formation largely reflected the pattern and duration of flowering among the cultivars (Fig. 3, Table 2). The duration of full flowering largely determined the highest RN at which all plants in each cultivar population formed pods. With only a few exceptions, the last RN at which pods were developed on all plants in a cultivar population was typically within ~2 nodes of the last RN for full flowering. The rate of decline in the proportion of plants forming pods for each cultivar was also similar to the rate of decline in the proportion of plants flowering after the period of full flowering.
Split-line regressions describing the proportion of plants representing each serradella cultivar that continued to produce pods at each reproductive node (RN) in the: (a) Control treatment (i.e. no flowers removed), and (b) EFR treatment (i.e. all flowers removed from plant when the first flower appeared at RN 5). Significant differences (P < 0.05) in the highest RN at which full podding occurred between the Control and EFR treatments as reported in Table 2 and shown on the figure by asterisks. Solid symbols and red lines, data for yellow serradella (O. compressus) cultivars; open symbols and black lines, French serradella (O. sativus) data.
Four patterns in the numbers of flowers produced at each RN were evident among the cultivars (Fig. 4). First, for Erica and Margurita, there were four flowers at RN 1 but flower number increased to a maximum of six per RN by RN 5 or RN 6 and this was maintained until about RN 10. Thereafter, flower numbers per RN declined gradually. Second, cvv. Rosa and Serratas exemplified a pattern in which flowering commenced with the maximum number of flowers (five or six flowers per RN) and this presentation was maintained up to ~RN 15. Flower number per RN then declined gradually with successive RNs. Third, King and SerraMax started with two flowers at RN 1 but increased to a maximum of four flowers per RN by RN 5 of RN 6 and this was maintained until ~RN 10. Thereafter, flower numbers per RN declined rapidly with no flowers or only one flower per RN from RN 15 onwards. Finally, Yellotas commenced flowering with four flowers per RN, slowly increased flower number to five per RN by RN 5, and then experienced an immediate decline in flower number until there was only one flower per RN at RN 17 and above.
Mean number of flowers (left panels) and pods (right panels) per reproductive node (RN) for each cultivar. Solid symbols, Control treatment (i.e. no flowers removed); open symbols, EFR treatment (i.e. all flowers and immature pods on RN 1–RN 5 removed from plants when first flowers appeared at RN 5). The dashed line at RN 5 indicates the point at which flower removal occurred. Bars associated with each symbol show 2 × s.e. The l.s.d. bars (P = 0.05) shown to the left of the dashed lines represent the results from 2-way ANOVAs (cultivar × RN) of flower numbers and of pod numbers on RN 1–RN 5. The l.s.d. bars (P = 0.05) to the right of the dashed line represent the results from 3-way ANOVAs (cultivar × flower removal × RN) of flower numbers and of pod numbers at RN 6–RN 20. French serradella (O. sativus) cultivar names are presented as standard text, whereas yellow serradella (O. compressus) cultivar names are in bold text.
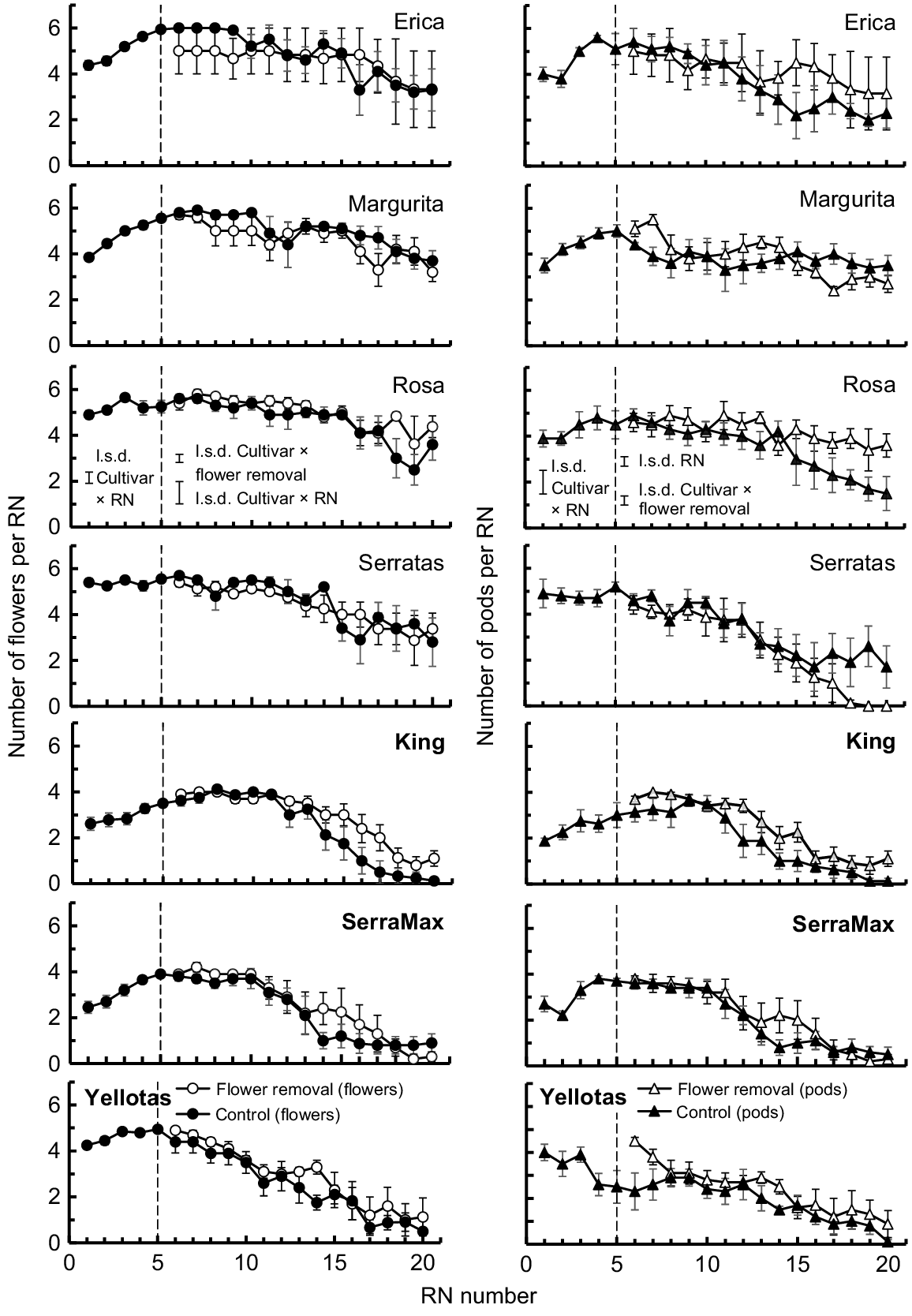
In most cases, the EFR treatment did not change subsequent flower numbers per RN compared with the Control. However, a significant cultivar × flower removal treatment interaction (P < 0.05) existed because King maintained up to two more flowers per RN at RNs >14 in the EFR treatment. Likewise, there were a few other instances where there was one or two extra flowers per RN for cultivars (i.e. Rosa, SerraMax and Yellotas) in the EFR treatment compared with the Control.
The patterns of pod number per RN produced by the cultivars usually reflected the patterns of flower number per RN. The highest flower and pod numbers were observed by about RN 5 or RN 6. Thereafter, the numbers of pods produced were either similar to, or marginally lower than (typically by one or two pods per RN), the number of flowers per RN (Fig. 4).
A significant (P < 0.05) cultivar × flower removal treatment interaction was observed for pod numbers. Following a peak in pod numbers per RN, they typically declined in the Control. Removing flowers led to a delay in this decline in pod numbers for cvv. Erica, Rosa’ and King. Compensatory production of pods by this means was only clearly associated with production of additional flowers per node by one cultivar (King). For Erica and Rosa, the additional pods in the EFR treatment were due to more flowers (at RN 15–RN 20) developing into a pod. For most other cultivars, there was no consistent delay in the decline in pod number per RN due to removal of flower. Serratas was an anomaly in that, at RN 17–RN 20, pod number declined more rapidly and then ceased prematurely in the EFR treatment compared with the Control.
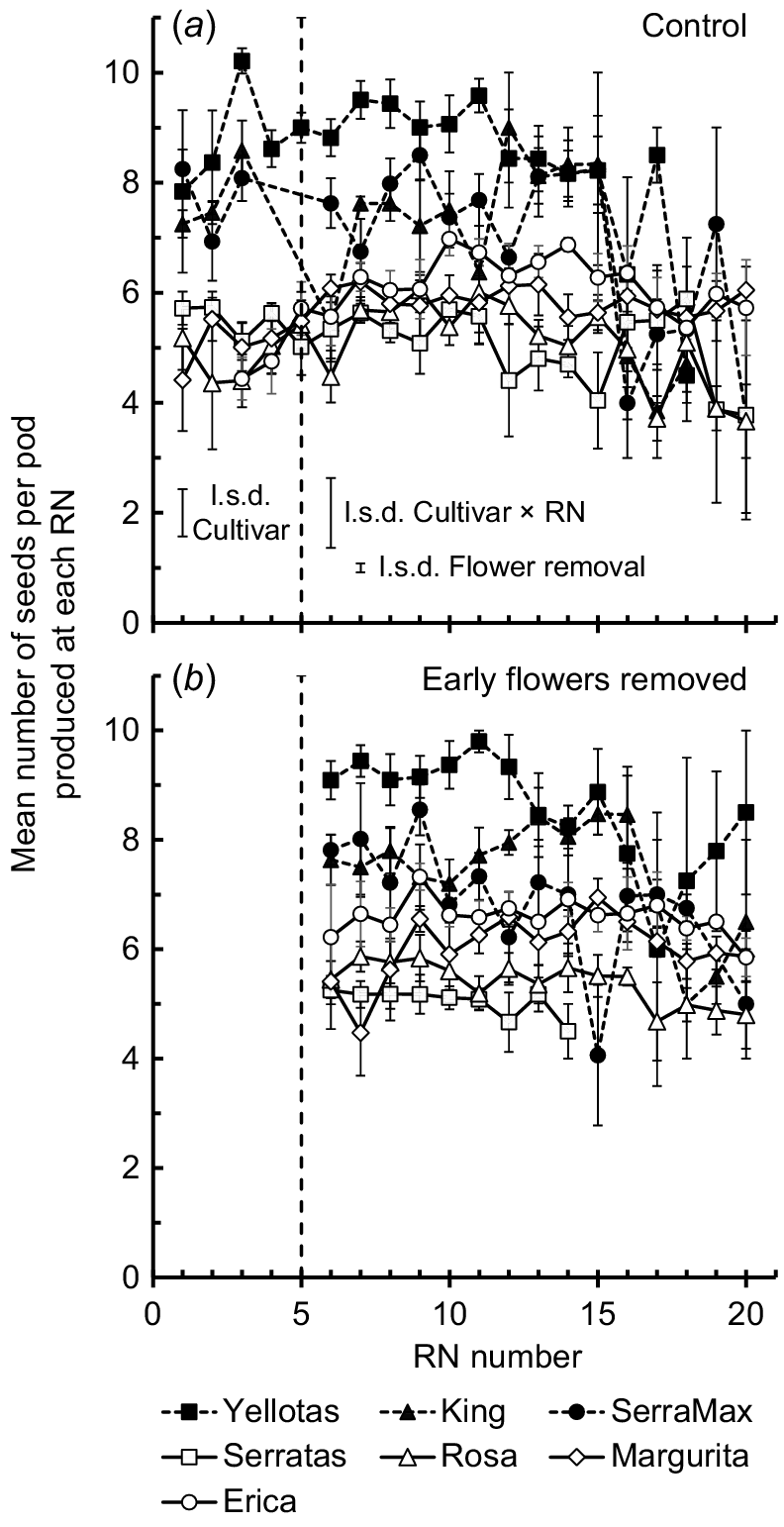
For the Control treatment, the average number of seeds per pod produced by each cultivar was stable for the first five RNs (no significant effect of RN, P = 0.47) but there was a main effect of cultivar (P < 0.001) (Fig. 5). The yellow serradella cultivars tended to produce more seeds per pod (average 8 seeds/pod, range 5–10 seeds/pod) than the French serradella cultivars (average 6 seeds/pod, range 5–7 seeds/pod). For both the Control and EFR treatments, stability of seed numbers per pod was maintained until at least RN 10, after which, marginal declines in the number of seeds per pod occurred for a few cultivars (i.e. one or two fewer seeds per pod). Although there was a significant main effect of flower removal (P = 0.03) on the average number of seeds per pod, the difference was marginal, with an average increase (over all cultivars) of only 0.24 seeds/pod in the EFR treatment compared with the Control.
Mean number of seeds per pod at each reproductive node (RN) for each cultivar. Bars associated with each symbol show 2 × s.e. Solid symbols with dashed lines, yellow serradella cultivars; open symbols with solid lines, French serradella cultivars. l.s.d. bars (P = 0.05) resulting from 2-way ANOVA (cultivar × RN) of seed numbers per pod on RN 1–RN 5 are shown to the left of the dashed line, which indicates the RN at which EFR treatments were imposed. l.s.d. bars (P = 0.05) resulting from a separate 3-way ANOVA (cultivar × flower removal × RN) of seed number per pod on RN 6–RN 20 are shown to the right side of the dashed line. Missed observations for Serratas EFR treatment: RN 15, RN 16 and RN 17. Solid symbols, data for yellow serradella (O. compressus) cultivars; open symbols, French serradella (O. sativus) data.
Impact of environmental stressors on seed number for different growing-season scenarios
Four growing-season scenarios were evaluated:
Total number of seeds initiated per stem in the Control and EFR treatments were compared at 700 degree-days after the appearance of the first flower on RN 1 (Fig. 6a). This tested whether the cultivars were able to compensate for removal of early flowers and pods by initiating additional seeds within the same time frame. Cvv. King, Rosa and Erica initiated a similar total number of seeds per stem in both treatments, indicating that additional seeds had been produced at RNs >RN 5 in response to removal of early flowers and immature pods (RN 1–RN 5). For cv. King, compensation for early loss of flowers occurred as a result of an increase in the production of flowers in the EFR treatment. However, for Rosa and Erica, the compensation occurred because more of the existing flowers (at higher RNs) produced a pod. Margurita, Yellotas and SerraMax did not compensate adequately for the early flower loss by producing enough additional pods per RN (Fig. 4). Of these cultivars, only Margurita mitigated against removal of flowers by extending its period of full flowering (Fig. 2). However, because pod numbers per RN were still increasing between RN 1 and RN 5 in all three cultivars (Fig. 4), the impact of losing flowers and immature pods at these nodes was comparatively modest (i.e. 24–33% fewer seeds initiated). Removing flowers had greater impact on Serratas (i.e. 51% fewer seeds initiated) than on Margurita, Yellotas and SerraMax, and Serratas exhibited little capacity to compensate for early flower loss: (1) it produced its highest numbers of flowers and pods per RN between RN 1 and RN 5 (which were then removed) (Fig. 4); (2) it did not extend its full flowering period substantially (Fig. 2); and (3) it appeared to cease pod formation prematurely (at RN 18) in the EFR treatment (Fig. 4).
Seed numbers initiated by each cultivar on main and/or primary stems under different growing-season scenarios. (a) Scenario 1: no flowers or pods removed (Control treatment) compared with all flowers and pods removed from the plant at appearance of the first flower on RN 5 (EFR treatment) to simulate the effect of early flower loss such as due to frost, up to 700 degree-days from first flower appearance at RN 1. (b) Scenario 2: Control compared with EFR treatment, with seed number initiation measured from the first surviving flower, i.e. RN 1 for the Control and RN 6 for EFR, to simulate the effect of purposefully flowering early/in a high-frost-risk window. *For cvv. Erica, Rosa and Margurita, extrapolated values were used for the EFR treatment because data collection ended prior to 700 degree-days after first flowers appeared at RN 6. (c) Scenario 3: seed number initiated in the Control up to 700 degree-days compared with the same treatment but under a more restricted time frame of 500 degree-days (e.g. to simulate the effect of a drought season). (d) Scenario 4: seed number initiation restricted to 500 degree-days (e.g. drought), with (EFR) or without (Control) flower loss (e.g. frost and drought).

Total seed numbers per stem were assessed at 700 degree-days from the first flower (i.e. from RN 1 for the Control) or the first surviving flower (i.e. RN 6 for the EFR treatment) (Fig. 6b). This was intended to mimic a situation where the cultivar had flowered early and been frosted, losing all flowers and young pods on RN 1–RN 5. This scenario differs from Scenario 1 in that the time available for seed initiation from appearance of the first or first surviving flowers was the same (700 degree-days). This permitted similar numbers of seeds to be initiated in the EFR treatment compared with the Control for most cultivars; the exceptions were Serratas and SerraMax (Fig. 6b). The routes by which compensation for flower loss occurred among the cultivars differed. Margurita and Erica extended their period of full flowering substantially in response to removal of flowers (Fig. 2). King, Rosa and Erica produced additional pods at nodes above ~RN 12, (Fig. 4). Yellotas appeared to benefit from a marginally slower decline in the proportion of plants continuing to flower and set pods in the EFR treatment. By contrast, seed initiation by Serratas was limited by its poor capacity for any compensatory pod production (see above). SerraMax was one of the few cultivars that did not extend full flowering in response to early flower loss, and although it did slow the decline in plants producing flowers and pods, this was not sufficient to compensate fully for the early loss of flowers and immature pods.
Seed initiation was then examined in the Control treatment over a seed production period that was restricted to 500 degree-days from first flower, in order to mimic the potential effect of a shorter ‘spring’. Fewer seeds (~15–26% less seed) were initiated by most cultivars (i.e. Margurita, Rosa, Yellotas and King) compared with the achievement within the longer, 700 degree-day seed initiation window (Fig. 6c). However, SerraMax and Serratas, which are characterised by having short periods of flowering and podding (Figs 2 and 3), largely completed these activities within the 500 degree-day window.
The cultivars were finally compared by assessing the potential effects of early flowering that had resulted in flower loss due to frost in a short spring (500 degree-days from first surviving flower; Fig. 6d). Within this very restricted timeframe, the number of seeds initiated was relatively low in both the Control and EFR treatments. For the cultivars that had previously exhibited capacity to compensate for removal of flowers, there was no further reduction in the number of seeds initiated as a result of this ‘early flowering with flower loss’ scenario. The exceptions were again SerraMax and Serratas, which had previously exhibited little or no ability to compensate for flower removal.
Discussion
Patterns and duration of flowering among serradellas
Flowering by the serradellas continued over successive nodes but the duration of flowering was finite for most plants (Fig. 2, Table 2). All cultivars exhibited a ‘peak period’ of flowering followed by a decline in flower appearance due to plants progressively ceasing to flower. Most commonly, full flowering occurred for about 8–12 RNs. However, this varied depending on the cultivar, as did the subsequent rate of decline in plants still flowering. Consequently, there was substantial variation in the total duration of flowering evident among the cultivars. Despite the focus of the present experiment only on the first two axes of each plant to flower, our conclusion that flowering was delimited by a peak period of flowering aligns with field observations that peak flower numbers occur before the midpoint of the flowering window in French (Grasslands Koha) and yellow (Elgara) serradella cultivars (Dodd and Orr 1995). The data in Table 2 indicate that the peak period of flowering on the first few axes of a serradella plant can be expected to occur over about a 3–4-week period, assuming typical early spring (i.e. September–October) temperatures in southern Australia.
The existence of a peak flowering period is important for decisions concerning the interactions of genotype, environment and management. For example, strategically avoiding grazing during peak flowering is commonly recommended to boost the soil seedbank to underpin persistence by serradella in a permanent pasture (Gladstones and Devitt 1971; Edward et al. 1998; Hackney et al. 2021). Likewise, it is clear that indeterminate flowering alone will not protect seed production by serradellas if the peak period of flowering does not coincide with the optimum environmental conditions for seed production.
For this study, it proved feasible to compare the flowering of serradella cultivars using RN number as a surrogate measure of flowering duration because the rate of RN development (consequently flower appearance) along stems was constant within each cultivar (Table 1). This was consistent with observations of RN development in other annual pasture legumes (e.g. barrel medic (Medicago truncatula Gaertn.); Moreau et al. 2007). However, the cultivars differed substantially (up to ~2-fold) in the rate at which RNs (and consequently flowers) appeared. The rate of first flower appearance by French serradella cultivars was consistently faster than that observed among the yellow serradella cultivars examined in this experiment, and faster rates of flowering were typically associated with higher numbers of seeds initiated in all of the exploratory scenarios (Fig. 6), provided that early loss of flowers had not occurred.
It is potentially significant that Margurita and Erica exhibited very similar flowering times (Fig. 1b), similar rates of RN formation (Table 1), similar patterns of flowering and pod production with an early end to full flowering (Figs 2 and 3), and a uniquely slow rate of decline in the proportion of plants flowering. They were projected to have the longest flowering duration among the cultivars studied (Fig. 2). Given that these cultivars were derived from the same parent (Cadiz) and are effectively sister lines (Nutt 2004a, 2004b), it is likely they are genetically similar. This suggests that it may be feasible to breed for differing patterns of flowering among the serradellas. Although the duration of the peak flowering varied between species and among cultivars, there were no indications that it was linked with cultivar maturity type.
Flower number did not always predict pod number
Patterns of pod formation often reflected the patterns of flowering, and therefore, a peak period in pod formation was also indicated. For about half of the cultivars, the end to full flowering occurred within one RN of the decline in the proportion of plants forming pods (Table 2), indicating that, in these cases, flower production was a reasonable predictor of pod production. However, for the other half, the proportion of plants forming pods (Fig. 3) began to decline at an earlier RN number than that delineating the end to full flowering (Fig. 2). For these genotypes, the RN number at which pod formation declined was between three and 14 earlier than that delineating the decline in flowering. These observations were similar to those reported for two cultivars of yellow serradella (Avila and SerraMax) in which pod number per RN declined well before a decrease in flower number per RN (Revell et al. 1999), and this is also known to occur in other pasture legumes (Medicago spp.; Cocks 1990). Under field conditions, differences between the numbers of flowers and pods that are attributed to flower shedding (Cocks 1990; Revell et al. 1999) could be exacerbated by interplant competition, and heat and drought stresses, which were not permitted in the present experiment. We conclude that appearance of flowers in a serradella pasture, particularly late in the flowering widow, may not always be a good predictor of the potential for pod formation.
Potential impacts of seasonal conditions and flowering date instability on seed number
Artificial scenarios intended to mimic the effects of stressors occurring early (e.g. frost causing flower loss) or late (e.g. heat/drought terminating seed growth) in the flowering window were explored to assess whether serradella flowering and pod formation patterns were modified by early flower loss, and/or impacted by shortening of the seed production period. We estimated, using the rates of RN formation (Table 1), that the treatment may mimic a frost event occurring up to ~2 weeks (e.g. assuming an average daily temperature of 15°C during early spring in southern Australia) before a nominal ‘optimum’ date for flowering (i.e. the date after which no frost occurs). In reality, frost events are unpredictable and optimum flowering dates are defined by having a statistically acceptable, low risk of frost (e.g. Flohr et al. 2017). Therefore, a cultivar’s ability to protect seed production by compensating for early flower loss may be important irrespective of the date on which the cultivar flowers (i.e. to protect seed yield against unseasonably late frost events). We found that most of the serradella cultivars were able to compensate for the early loss of flowers and immature pods through modified patterns of flowering and pod formation. This may also have implications for loss of flowers and pods due to grazing early in the flowering window. However, it should be noted that grazing would remove herbage as well as flowers, and so it is unclear whether the plants would be capable of compensating for grazing losses in the manner observed in treatments that mimicked loss due to an environmental stressor such as frost.
The scenarios exploring impacts of environmental stress on seed number (Fig. 6) were also intended to assess the extent to which some flowering date instability among serradella cultivars may be acceptable, such as flowering too early in years with early germination events. This was investigated because the flowering date of a number of serradella cultivars is known to vary substantially depending on germination date (Haling et al. 2023; R. J. Simpson et al., unpubl. data). Flowering date instability is considered not desirable because flowering too early in spring may risk damage to seed production by frost, and flowering too late reduces the time available for seed growth before terminal drought occurs (Donald 1970). The scenarios indicated that most serradella cultivars exposed to complete flower loss at ~2 weeks after flowering had commenced (i.e. flower removal when first flowers occurred on RN 5) were able to compensate for the loss of flowers and immature pods by subsequent adjustments to flowering and podset. The timing of the EFR treatment proved to be conveniently similar to the degree to which flowering dates of many yellow serradella cultivars (and a few French serradella cultivars) vary after germinating early versus late in the autumn in southern Australia (i.e. ~2 weeks; Boschma et al. 2019; Haling et al. 2023). This suggested that many current yellow serradella cultivars may display a tolerable amount of flowering date instability. However, this is only true for cultivars able to respond to early flower loss by compensating with additional flower and pod production. Seed production by cultivars with limited capacity (e.g. Serratas, SerraMax) remains at risk if flowering dates are unstable. Likewise, a relatively large number of current French serradella cultivars display higher levels of flowering date instability (flowering dates that vary by >2 weeks; Boschma et al. 2019; Haling et al. 2023), consequently, and it is likely that they are exposed to an unacceptably high environmental risk for seed production in the variable climate of southern Australia. This is because there is an increased chance of peak flowering periods coinciding with periods of higher frost risk.
Effect of a droughted ‘spring’
The effectiveness of compensatory seed production will also be influenced by the timing of terminal drought (Donald 1970) because premature closure of the flowering window will limit the expression of compensatory flowering and podset. It was found that cultivars expressing patterns that led to rapid or early seed production (e.g. Serratas) were more resilient and able to maintain seed number in the face of a premature end to flowering (i.e. a ‘short spring’). This was consistent with recommendations for selecting cultivars with fast rates of inflorescence production in Mediterranean environments (Francis and Gladstones 1974), which are often subject to early onset of terminal drought. The scenario combining early flower loss (e.g. due to frost) and a short spring (Fig. 6d) predicted that most cultivars would not be further disadvantaged in a short spring, provided they exhibited some form of compensatory flower/pod production.
Conclusion
Serradellas displayed indeterminate flowering but there was substantial variation in the patterns of flowering and seed production between species and among cultivars. All cultivars of yellow and French serradella displayed a peak flowering period as observed by a reduction in the proportion of plants producing pods at higher reproductive node numbers. Despite the appearance of long flowering durations under non-limiting conditions (e.g. 2–3 months), there is a narrower period during which seed number is largely determined for these species (~3–4 weeks). This knowledge will inform genotype × environment × management decisions about the timing of grazing to maximise seed production, and the selection of cultivars to match environmental constraints (frost, heat stress, drought) that can adversely impact seed number. Scenarios that considered losses to seed number from stressors occurring early (e.g. due to frost) or late (e.g. due to drought) in the flowering window indicate that many cultivars can compensate for early flower loss by extending flowering duration, increasing flower numbers and/or developing more pods from existing flowers. However, some cultivars lack this capacity and will be at greater risk of reduced seed production if subjected to frost during flowering. Many cultivars of serradella are known to display unstable flowering dates. This experiment indicated that the levels of flowering date instability observed in many yellow serradella cultivars (e.g. SerraMax) may be tolerable provided the cultivar also exhibits some capacity for compensatory flowering and pod production. However, the higher levels of flowering date instability reported for some French serradella cultivars (e.g. Margurita) are likely to prove risky for seed production and persistence in a permanent pasture under temperate or Mediterranean climatic conditions.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Declaration of funding
AW Howard Memorial Trust Research Fellowship and Australian Sustainable Agriculture Scholarships (University of Tasmania and CSIRO) have provided financial support to the corresponding author (Laura Goward).
References
Cocks PS (1990) Dynamics of flower and pod production in annual medics (Medicago spp.). I. Spaced plants. Australian Journal of Agricultural Research 41, 911-921.
| Crossref | Google Scholar |
Dodd MB, Orr SJ (1995) Seasonal growth, flowering patterns, and phosphate response of 18 annual legume species grown in a hill-country soil. New Zealand Journal of Agricultural Research 38, 21-32.
| Crossref | Google Scholar |
Dyson CB, Conyers MK (2013) Methodology for online biometric analysis of soil test–crop response datasets. Crop & Pasture Science 64, 435-441.
| Crossref | Google Scholar |
Edward AY, Ewing MA, Revell CK (1998) Fate of serradella, medic and biserrula seeds in pods ingested by sheep. In ‘Proceedings of the 9th Australian Agronomy Conference’. pp. 199–202. (Australian Society of Agronomy) Available at http://agronomyaustraliaproceedings.org/images/sampledata/1998/1/289edward.pdf
Enriquez-Hidalgo D, Cruz T, Teixeira DL, Steinfort U (2020) Phenological stages of Mediterranean forage legumes, based on the BBCH scale. Annals of Applied Biology 176, 357-368.
| Crossref | Google Scholar |
Flohr BM, Hunt JR, Kirkegaard JA, Evans JR (2017) Water and temperature stress define the optimal flowering period for wheat in south-eastern Australia. Field Crops Research 209, 108-119.
| Crossref | Google Scholar |
Francis CM, Gladstones JS (1974) Relationships among rate and duration of flowering and seed yield components in subterranean clover (Trifolium subterraneum). Australian Journal of Agricultural Research 25, 435-442.
| Crossref | Google Scholar |
Fu SM, Hampton JG, Williams WM (1994) Description and evaluation of serradella (Ornithopus L.) accessions. New Zealand Journal of Agricultural Research 37, 471-479.
| Crossref | Google Scholar |
Gladstones JS, Devitt AC (1971) Breeding and testing early-flowering strains of yellow-flowered serradella (Ornithopus compressus). Australian Journal of Experimental Agriculture and Animal Husbandry 11(51), 431-439.
| Crossref | Google Scholar |
Goodspeed MJ (1975) Computer routines for solar position, daylength and related qualities. Technical Memorandum No. 75/11. (CSIRO Division of Water and Land Resources) Available at https://scholar.google.com/scholar_lookup?title=Computer%20Routines%20for%20Solar%20Position%2C%20Daylength%20and%20Related%20Qualities.%20Technical%20Memorandum%20No.%2075%2F11&author=M.J.%20Goodspeed&publication_year=1975
Goward LE, Haling RE, Smith RW, Penrose B, Simpson RJ (2023) Flowering responses of serradella (Ornithopus spp.) and subterranean clover (Trifolium subterraneum L.) to vernalisation and photoperiod and their role in maturity type determination and flowering date stability. Crop & Pasture Science 74, 769-782.
| Crossref | Google Scholar |
Hackney B, Rodham C, Dyce G, Piltz J (2021) Pasture legumes differ in herbage production and quality throughout spring, impacting their potential role in fodder conservation and animal production. Grass and Forage Science 76, 116-133.
| Crossref | Google Scholar |
Haling RE, Goward L, Stefanski A, Simpson RJ (2023) Variation in flowering time and flowering date stability within a cultivar of French serradella. Crop & Pasture Science 74, 756-768.
| Crossref | Google Scholar |
Loi A, Howieson JG, Nutt BJ, Carr SJ (2005) A second generation of annual pasture legumes and their potential for inclusion in Mediterranean-type farming systems. Australian Journal of Experimental Agriculture 45, 289-299.
| Crossref | Google Scholar |
Loi A, Revell C, Nutt B (2021) Technical dossier for SerraMax Yellow Serradella (Ornithopus compressus L.). Available at https://www.agric.wa.gov.au/sites/gateway/files/Technical%20dossier%20for%20SerraMax%20-%20Final%20version%207%20Dec%20%28A5516500%29.pdf
Moreau D, Salon C, Munier-Jolain N (2007) A model-based framework for the phenotypic characterization of the flowering of Medicago truncatula. Plant, Cell & Environment 30, 213-224.
| Crossref | Google Scholar | PubMed |
Nichols PGH, Loi A, Nutt BJ, Evans PM, Craig AD, Pengelly BC, Dear BS, Lloyd DL, Revell CK, Nair RM, Ewing MA, Howieson JG, Auricht GA, Howie JH, Sandral GA, Carr SJ, de Koning CT, Hackney BF, Crocker GJ, Snowball R, Hughes SJ, Hall EJ, Foster KJ, Skinner PW, Barbetti MJ, You MP (2007) New annual and short-lived perennial pasture legumes for Australian agriculture – 15 years of revolution. Field Crops Research 104, 10-23.
| Crossref | Google Scholar |
Nutt B (2004a) French serradella (Ornithopus sativus) ‘Margurita’. Plant Varieties Journal 17, 311-312.
| Google Scholar |
Nutt B (2004b) French serradella (Ornithopus sativus) ‘Erica’. Plant Varieties Journal 17, 313-315.
| Google Scholar |
PlantNET (The NSW Plant Information Network System) (2023) Royal Botanic Gardens and Domain Trust Sydney. Available at https://plantnet.rbgsyd.nsw.gov.au [Accessed 9 May 2023]
Reed KFM, Mathison MJ, Crawford EJ (1989) The adaptation, regeneration, and persistence of annual legumes in temperate pasture. In ‘Persistence of forage legumes’. (Eds GC Marten, AG Matches, RF Barnes, RW Brougham, RJ Clements, GW Sheath) pp. 69–89. (John Wiley & Sons, Ltd) doi:10.2134/1989.persistenceofforagelegumes.c6
Revell CK, Taylor GB, Cocks PS (1999) Effect of length of growing season on development of hard seeds in yellow serradella and their subsequent softening at various depths of burial. Australian Journal of Agricultural Research 50, 1211-1223.
| Crossref | Google Scholar |
Rossiter RC (1966) The success or failure of strains of Trifolium subterraneum L. in a Mediterranean environment. Australian Journal of Agricultural Research 17, 425-446.
| Crossref | Google Scholar |
Ru YJ, Fortune JA (2000) Effect of grazing intensity and cultivar on morphology, phenology, and nutritive value of subterranean clover II. Nutritive value during the growing season. Australian Journal of Agricultural Research 51(8), 1047-1055.
| Crossref | Google Scholar |
Rudall PJ (2020) ‘Anatomy of flowering plants: an introduction to plant structure and development.’ (Cambridge University Press: Cambridge, UK) doi:10.1017/9781108782104
Thomas DT, Flohr BM, Monjardino M, Loi A, Llewellyn RS, Lawes RA, Norman HC (2021) Selecting higher nutritive value annual pasture legumes increases the profitability of sheep production. Agricultural Systems 194, 103272.
| Crossref | Google Scholar |


