Stem, leaf and cotyledon resistance responses to a prevalent Sclerotinia sclerotiorum pathotype in Australia highlight new opportunities to improve white mould resistance in common bean
Muhammad Azam Khan A B , Dawid Brink Wentzel A , Ming Pei You A , Sally L. Norton C and Martin J. Barbetti A *
A *
A
B
C
Abstract
White mould (Sclerotinia sclerotiorum) inflicts major yield losses on common bean (Phaseolus vulgaris); yet, commercial cultivars known for their high yields and market-adapted grains lack physiological resistance to this disease.
This study aimed to test diverse common bean genotypes for resistance in stem, leaf and cotyledon tissues.
Thirty-four common bean genotypes with a wide range of agronomic traits and grain types, including genotypes noted previously for susceptible and resistant responses to white mould, were inoculated with the prevalent S. sclerotiorum isolate MBRS-1. Then they were assessed for resistance in stem, leaf and cotyledon tissues under controlled environment conditions, by inoculating plants with a 105 mL−1 hyphal fragment concentration.
There was significant (P < 0.001) variation in resistance responses in stem, leaf and cotyledon tissues across the genotypes. Contender, ICA Bunsi, XAN 280 and Taisho-Kintoki showed the highest resistance in stems, whereas Norvell 2558, Pico de Oro, Sanilac, Othelo and Negro Argel exhibited notable resistance in leaves. Metis, Canario 107, Pico de Oro, Pogonion and Jubilejnaja 287 displayed the most resistance in cotyledons.
This is the first reported attempt to determine the response of common bean germplasm to a prevalent pathotype of S. sclerotiorum in Australia. Bean genotypes exhibiting high-level resistance to white mould identified in this study can be used as parental lines for crosses in common bean breeding programs and/or directly as improved cultivars.
The study highlighted both the value of screening under controlled environmental conditions to reliably locate new stem, leaf and/or cotyledon resistances and the possibility of using rapid cotyledon screening to indicate stem resistances because the expression of resistances in cotyledons generally correlated strongly with those in stems.
Keywords: common bean, common bean diseases, disease screening, host resistance, Phaseolus vulgaris, physiological resistance, Sclerotinia rot, stem rot, white mould.
Introduction
Common bean, Phaseolus vulgaris, is one of the most widely grown pulse crops for direct consumption in both tropical and temperate climates worldwide. The total worldwide production of dry bean and area harvested in 2020 was 27.5 million metric tonnes and 34.8 million hectares, respectively (FAO 2022). Asia contributes approximately 43% to the global production, followed by North, Central and South America (29%), and then Africa (26%), whereas Europe and Oceania contribute approximately 2% of total production (Uebersax et al. 2023). In addition to the use of dry bean for human consumption or animal feed, the immature pods are widely consumed as green or string beans. In Australia, green bean production occupies more arable land than any vegetable product, except potatoes and maize (Australian Bureau of Statistics 2021), and is commonly rotated with other major vegetables such as potato and pea (Jones et al. 2011).
White mould (Sclerotinia sclerotiorum) is a major global disease of common bean (del Río et al. 2004; Singh et al. 2014; Kamvar et al. 2017) and causes significant yield losses to both irrigated and rain-fed bean production. For example, in the Americas, worst instances of yield losses of >90% occur under conducive weather conditions (Singh and Schwartz 2010; Schwartz and Singh 2013). Increased sowing density and irrigation, while necessary for high yields, increases the incidence of white mould (Mila et al. 2003; Abán et al. 2018; Robison et al. 2018). Because sclerotia of S. sclerotiorum can survive in soil for several years, crop rotation is often an ineffective form of management, making reliance on fungicidal sprays at flowering the most used means of disease control in many countries (McCaghey et al. 2019), including in Australia (Jones et al. 2012). However, owing to the cost with fungicide application, there has been increased use of wider row spacing and morphological characteristics that foster ‘disease escape’, such as enhanced standing ability as a means to lower canopy humidity and decrease contact among plants, and so reduce white mould infection (Miklas and Grafton 1992; Ando et al. 2007). However, although such traits are heritable and with associated quantitative trait loci (QTL) identified, they are generally also associated with reduced yield (Ender and Kelly 2005).
Developing cultivars with inherent physiological resistance is considered the most desirable avenue to manage this disease (Miklas et al. 2013; Singh et al. 2017). Despite ongoing efforts to pyramid high levels of physiological resistance from multiple other species in the Phaseolus secondary gene pool into common bean (Singh et al. 2014, 2017), commercial common bean cultivars with a combination of high yield, market-adapted size and colour, and high physiological resistance to white mould are not yet available (Robison et al. 2018; Campa et al. 2020). Screening the progeny of partially resistant genotypes crossed with commercially available cultivars of common bean has demonstrated that there is potential for breeding lines to contribute their resistance to new cultivars with favourable agronomic and seed characteristics (Miklas et al. 2004; Carvalho et al. 2013). However, regional variation in agronomic and market factors further complicates such efforts, as a successful commercial cultivar must be both adapted to the local climate in terms of high yield and meet the preference of local consumers in terms of grain attributes such as seed size and colour. Meeting consumer preferences for improved common bean cultivars can be an additional ongoing and large undertaking for breeding programs (Lehner et al. 2015; Lima et al. 2017). In addition, S. sclerotiorum has wide diversity, with isolates varying in their aggressiveness and interaction with host resistance in common bean. Hence, it is not surprising that resistant bean genotypes respond differently to different isolates at different stages of disease progression (Lehner et al. 2016; Abán et al. 2020).
Common bean germplasm is frequently screened using isolates specific to the region concerned (Singh et al. 2017), as is the case, for example, in Brazil (Lehner et al. 2016), Spain (Pascual et al. 2010) and Argentina (Abán et al. 2020). However, resistance in common beans to Australian isolates of S. sclerotiorum has not been investigated. Hence, taking 34 common bean genotypes from various countries, including those for partial resistance or susceptibility (Miklas et al. 2004; Viteri and Singh 2015), the objective was to screen these for resistance under controlled environmental conditions by comparing their varietal resistance responses on cotyledons, leaves and stems.
Materials and methods
Pathogen isolate preparation and culturing
S. sclerotiorum isolate MBRS-1 was used to screen common bean breeding lines in this study. MBRS-1 was originally collected from canola (Brassica napus) in the Mount Barker region of Western Australia in 2004 (Li et al. 2006). This isolate was chosen because it is an aggressive strain belonging to Pathotype 76, the prevailing pathotype among broadleaf crops in Western Australia (Barbetti et al. 2014; Khan et al. 2020), particularly among lupin and Brassica crops (Ge et al. 2012). This isolate has been previously used to demonstrate differences in susceptible and resistance host responses across diverse Brassicaceae (Li et al. 2007; Garg et al. 2010). There has not been any previous characterisation of the pathogen associated with common bean.
Cultures were revived from dry-stored MBRS-1 sclerotia (Clarkson et al. 2003; Barbetti et al. 2014), briefly as follows. Dormant sclerotia were surface sterilised in 6% (v/v) sodium hypochlorite for 3 min and washed twice using sterile deionised water to ensure all hypochlorite was removed, then cut in half and placed face-down on a 2% potato dextrose agar (PDA) plate. Agar plugs 3 mm in diameter were cut from the original plate cultures when 7 days old and used to subculture the pathogen onto further freshly poured PDA plates, but containing 1% potato-derived peptone. Finally, 3-mm plugs from the growing edge of these colonies when 7 days old were used to produce inoculum for stem inoculations and/or for further subculturing as necessary. For cotyledon and leaf inoculations, hyphal inoculum was prepared in liquid culture by using the methodology of Garg et al. (2008). All cultures were incubated at 18°C.
Common bean cultivars
Thirty-three P. vulgaris genotypes were obtained from the international bean collection at the Australian Grains Genebank. Genotypes were chosen to represent the diversity of flowering time, seed weight and country of origin, and included available genotypes that had been used in previous studies involving S. sclerotiorum. Table 1 displays information about agronomic characteristics of bean genotypes used for this study. Some additional information was also extracted from Li et al. (2016). The selected genotypes included ICA Bunsi, a variety noted for moderate resistance to S. sclerotiorum (Tu and Beversdorf 1982; Miklas et al. 2004), and the Canadian cultivar Centralia descended from it (Park et al. 1988). Two genotypes known to exhibit susceptibility to the disease were included as susceptible checks A55 (Miklas et al. 2001; Singh et al. 2003) and Othello (Singh et al. 2014; Viteri and Singh 2015). Two separate batches of A55 germplasm were obtained from the Genebank and utilised but noted to have been supplied from different countries (India and Colombia). All selected genotypes had an upright growth habit (Type I or II) that is suitable for mechanical harvesting. Whereas this habit is considered to offer some reduction in the incidence of white mould under field conditions (Schwartz and Singh 2013), Othello growth habit was Type III (i.e. indeterminate prostate). An additional commercial cultivar, ‘Borlotti’, that is widely utilised in Australia (Yates Australia), was included as a comparison, totalling 34 test genotypes.
| Genotype | Country origin | Stem disease index (%) | Cotyledon disease index (%) A | Leaf disease index (%) | Days to flowering | Seed weight (g/100) B | Seed type | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Contender | United States | 9.7 | (1) | – | 52.1 | (33) | 34 | 54.2 | Shiny mottled cream | ||
| ICA Bunsi | Colombia | 16.0 | (2) | 47.9 | (21) | 13.9 | (19) | 42 | 15.8 | Dull white | |
| XAN 280 | Colombia | 21.5 | (3) | 44.9 | (18) | 17.4 | (26) | 49 | – | Black | |
| Taisho Kintoki | Japan | 23.6 | (4) | – | 12.9 | (16) | 36 | 58.7 | Dull mottled red | ||
| ARS-R93003 | United States | 32.6 | (5) | 48.1 | (22) | 12.9 | (16) | 38 | 41.2 | Shiny pink | |
| Radical | Colombia | 33.3 | (6) | 34.9 | (7) | 5.9 | (10) | 49 | 63.2 | Shiny red | |
| Centralia | Canada | 34.0 | (7) | 43.1 | (17) | 5.4 | (8) | 44 | – | White | |
| BAT 1217 | India | 34.0 | (7) | 37.7 | (9) | 39.5 | (32) | 42 | 21.4 | Shiny purple | |
| Royal Red | Colombia | 34.0 | (7) | 45.3 | (19) | 59.0 | (34) | 36 | – | Shiny red | |
| Burter’s Blight Proof | United States | 34.0 | (7) | 38.0 | (11) | 22.4 | (28) | 34 | 16.5 | Shiny white | |
| Light Red Kidney | United States | 35.4 | (11) | 32.5 | (6) | 30.0 | (30) | 40 | 44.5 | Shiny brown | |
| Teebus | South Africa | 40.3 | (12) | 50.3 | (23) | 10.0 | (12) | 39 | 23 | Shiny white | |
| Swedish Brown | Canada | 41.0 | (13) | 39.3 | (12) | 5.5 | (9) | 38 | 33 | Shiny yellow | |
| A55 (a) | Colombia | 42.2 | (14) | 41.2 | (14) | 20.0 | (27) | 46 | – | Dull black | |
| Othelo | Colombia | 44.4 | (15) | 41.4 | (16) | 2.7 | (4) | 36 | 43.4 | Shiny striped cream | |
| A55 (b) | India | 44.4 | (15) | 41.2 | (14) | 16.2 | (24) | 44 | 27.2 | Dull black | |
| A54 | India | 45.1 | (17) | – | 30.0 | (30) | 46 | 21.8 | Dull buff | ||
| Kingaroy 53 | Australia | 45.8 | (18) | 54.3 | (24) | 26.4 | (29) | 41 | 41.2 | White | |
| Negro Argel | Chile | 45.8 | (18) | 62.6 | (27) | 2.8 | (5) | 49 | 18.9 | Shiny black | |
| Tweed Wonder | Australia | 45.8 | (18) | – | 14.9 | (21) | 40 | 52.7 | Shiny red | ||
| Sanilac | United States | 46.5 | (21) | 55.3 | (25) | 1.4 | (3) | 36 | 22 | Shiny white | |
| SEA 2 | Colombia | 49.3 | (22) | 37.9 | (10) | 10.2 | (14) | 42 | – | Dotted buff | |
| Metis | Colombia | 50.0 | (23) | 19.7 | (1) | 14.9 | (21) | 34 | 14 | Shiny white | |
| Norvell 2558 | Guatemala | 50.0 | (23) | 46.7 | (20) | 0 | (1) | 46 | 28.1 | Dull black | |
| Pogonion | Colombia | 52.1 | (25) | 26.8 | (4) | 5.3 | (7) | 36 | 61.8 | Shiny yellow | |
| PP 1088 | Turkey | 56.9 | (26) | 40.8 | (13) | 9.4 | (11) | 38 | 41.8 | Shiny yellow | |
| A99 | India | 59.0 | (27) | 56.2 | (26) | 15.9 | (23) | 38 | 27.4 | Dull buff | |
| Canario 107 | Colombia | 60.4 | (28) | 24.0 | (2) | 10.9 | (15) | 36 | – | Shiny yellow | |
| Jubilejnaja 287 | Russian Federation | 64.6 | (29) | 29.2 | (5) | 9.9 | (12) | 38 | 24.5 | Shiny white | |
| Borlotti | Colombia | 64.6 | (29) | – | 16.4 | (25) | 40 | 41.2 | Mottled brown | ||
| Pico de Oro | Brazil | 65.3 | (31) | 24.3 | (3) | 0 | (1) | 42 | – | Dull buff | |
| XPB 155 | India | 66.0 | (32) | 35.8 | (8) | 13.8 | (18) | 38 | 22.4 | Dull white | |
| Seaway | United States | 66.0 | (32) | 64.3 | (28) | 14.4 | (20) | 36 | 17.4 | Shiny white | |
| Hebar | Bulgaria | 70.1 | (34) | – | 3.9 | (6) | 36 | 26.4 | White | ||
| Significance | <0.001 | <0.001 | <0.001 | ||||||||
| l.s.d. (P < 0.05) | – | 26.9 | 18.6 | 4.21 | |||||||
Plant establishment
Four seeds per each of the 34 selected genotypes were sown into each pot (pots 10 × 10 × 20 cm depth) at a depth of 3 cm. Pots were filled with modified University of Western Australia potting mix consisting of 50% composted pine bark, 20% coco peat and 30% fine river sand that together had been pasteurised at 65°C for 90 min. Throughout experiments, all plants were kept in a controlled environment room maintained at 24°C by day, 18°C by night, constant 70% humidity and with a 12-h photoperiod. This temperature range is considered optimal for Australian common bean production. Plants were fertilised with Thrive All-Purpose fertiliser (N:P:K ratio of 25:5:8.8, Yates Australia) according to manufacturer recommendations.
Inoculation of cotyledons and disease assessment
Seven 3-mm plugs of PDA were cut from the growing edge of each S. sclerotiorum colony. These were used to inoculate 75-mL batches of liquid potato dextrose medium (24 g L−1 potato dextrose) containing 10 g L−1 potato peptone. Cultures were then incubated in 250 mL flasks at room temperature for 72 h on a rotary shaker at 120 rpm at 25°C. The resulting mycelial mat was removed and washed twice with sterile deionised water to remove any residual fungal metabolites from the liquid medium, then resuspended in 125 mL of liquid medium. Mycelium was macerated for 5 min using a sterilised stick-blender and then filtered using four layers of muslin cloth. Using a haemocytometer counting chamber (Superior), liquid medium was used to dilute samples until a hyphal fragment concentration of 105 mL−1 was achieved.
Cotyledons were inoculated, with a single 10-μL droplet applied by micropipette to the surface of each fully opened cotyledon 8 days after sowing. During inoculation, the mycelial suspension was regularly mixed by handshaking to prevent clumping of hyphae. Plants were maintained for 72 h post-inoculation (hpi) in translucent sealed plastic boxes containing water to a depth of 2 cm, so as to maintain high humidity. This ensured both reduced lighting and high humidity, which together favour S. sclerotiorum infection (Garg et al. 2008).
Cotyledons were assessed 72 hpi. Lesions were rated according to their length (mm) as a proportion of cotyledon size, from 0 (0% lesion coverage) to 5 (100% lesion coverage) and the mean lesion score across all plants in each pot was taken to be a single replicate score. Ratings were converted into a mean percentage cotyledon disease index (%CDI) on the basis of the method of McKinney and Davis (1923), as follows:
where a, b, c, d, e and f are the number of plants with leaf-disease scores of 0, 1, 2, 3, 4 and 5, respectively, as widely used for cotyledon infection studies (Murtza et al. 2021).
Leaf and stem inoculations and disease assessments in adult plants
To screen common bean breeding lines, for leaves, liquid inoculum was prepared using the method described above for cotyledons. Forty days after sowing, when visible flower buds were present on >50% of plants and flowers had begun to open on each plant, the uppermost fully opened and expanded leaf was inoculated in two locations (both leaf lobes) with a 10-μL droplet applied using a micropipette and allowed to dry slightly to ensure adhesion to the leaf surface. High humidity was maintained post-inoculation by hand-misting inoculated plants with deionised water and covering them with a translucent plastic cover for 72 h. At 72 hpi, leaf lesion diameter was measured on all inoculated leaves using calipers and a mean leaf lesion diameter computed on a 0–9 scale, where 0 = no disease symptoms; 1 = <1 mm; 2 = 1–<3; 3 = 3–<6; 4 = 6–<9; 5 = 9–<12; 6 = 12–<15; 7 = 15–<18; 8 = 18–<21; and 9 = >21 mm. Ratings were converted into a mean percentage leaf disease index, as described for cotyledon disease above, but modified to include the greater number of score categories.
Forty days after sowing, when visible flower buds were present on >50% of plants and flowers had begun to open on each plant, stems were inoculated using the method described for canola screening by Barbetti et al. (2015). This involved taking S. sclerotiorum-colonised 5-mm plugs from freshly cultured peptone–PDA. Petri dishes of S. sclerotiorum and attaching a single colonised agar plug to each stem directly below the 1st node, by using Parafilm. This method roughly approximates the ‘straw test’ commonly used to test P. vulgaris disease response in the field (Singh and Terán 2008). High humidity post-inoculation was provided as for leaf studies, and stem infection was recorded at 72 hpi. The nine-point scale described by Terán et al. (2006) and modified by Singh et al. (2014) for cut-stem and straw test screenings of common bean infection was adapted to compute stem disease severity scores as follows: Scores 1–3 represented a resistant response in which the fungus failed to progress past any node; Scores 4–6 represented moderate infection proceeding past the first post-inoculation node; and Scores 7–<9 represented infection past the second post-inoculation node. Score 0 indicated no infection, whereas any plant destroyed by stem collapse because of infection was assigned a score of 9. These scores were converted to mean percentage stem disease index, as described above for cotyledons, but modified to include the greater number of score categories.
Data analysis
The experiment was fully repeated once. Each experiment had six single pot replications per genotype, with a total of 204 pots arranged as a ‘fully randomised design’, generated by using the ‘Generate a Standard Design’ function of GenStat 18.1 (18th edition, Lawes Agricultural Trust, Rothamsted Research, UK). Normality of data and homogeneity of the original and repeat experiments were tested before conducting analyses. Data from the original and the repeat experiments were not significantly (P > 0.05) different by using a Student’s t-test nor when comparing cultivar responses across the duplicate experiments when compared using an F-test for equality of two variances. Therefore, data from the original and repeat experiments were combined, and analysed together as a single data set, using completely randomised ANOVA function in GenStat. Fisher’s least significant difference (l.s.d. at P ≤ 0.05) test was used to highlight differences among genotypes in relation to the three different disease assessment parameters (percentage cotyledon, leaf or stem disease indices). Where appropriate, correlation gradients were plotted and R2 values calculated using Microsoft Excel.
Results
Cotyledon inoculations
Cotyledons developed characteristic lesions and significant differences in %CDI were observed (P < 0.001) (Table 1, Fig. 1a–c). The disease index of the cotyledon ranged between 19.7% (Metis) and 64.3% (Seaway). Other than Metis, genotypes also showing high level of cotyledon resistance included Canario 107, Pico de Oro, Pogonion and Jubilejnaja 287, with index values of 24.0, 24.3, 26.8 and 29.2, respectively. Whereas no significant difference was observed in %CDI between ICA Bunsi (47.9), Othello (41.4) and A55a,b (both 41.2), these three genotypes had significantly greater %CDI values than did the most resistant genotype Metis (19.7).
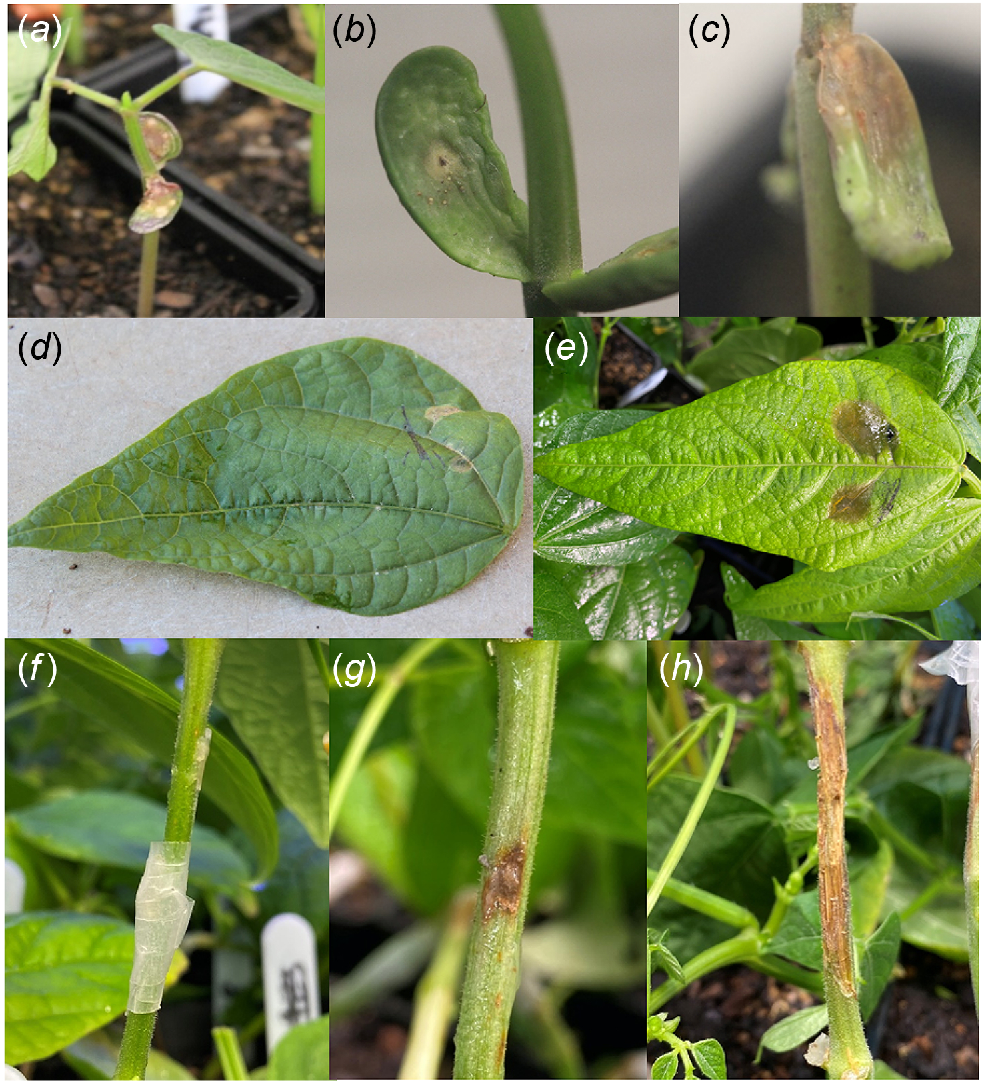
Symptoms of S. sclerotiorum infection in common bean cotyledon, stem and leaf tissue. (a) Typical seedling, with the growing tip removed to maintain cotyledon attachment and cotyledons with typical white mould infection; (b, c) cotyledon of resistant and susceptible genotypes, respectively; (d, e) leaf of resistant and susceptible genotypes, respectively; (f) how Sclerotinia sclerotiorum colonised agar plug is attached using Parafilm to inoculate stems; (g, h) stem lesions on resistant and susceptible genotypes, respectively.
Leaf inoculations
There were significant (P < 0.05) differences in %LDI across the test genotypes (Table 1, Fig. 1d, e). Percentage leaf disease index values ranged from 0 (Norvell 2558, Pico de Oro) to 59.0 (Royal Red). Other genotypes showing a high level of leaf resistance included Sanilac (1.4), Othelo (2.7) and Negro Argel (2.8). The %LDI for Othelo (2.7) was significantly lower than that for ICA Bunsi (13.9), the latter, in turn, being significantly lower than that for A55a (20.0) but not for A55b (16.2). The latter three genotypes had significantly greater %LDI values than the most resistant genotypes Norvell 2558 and Pico de Oro (both 0), Sanilac (1.4), Othelo (2.7) and Negro Argel (2.8).
Stem inoculations
There were significant (P < 0.001) differences in %SDI (Table 1, Fig. 1f–h), ranging from 9.7 (Contender) to 70.1 (Hebar). Genotypes other than Contender showing a high level of stem resistance included ICA Bunsi (16.0), XAN 280 (21.5) and Taisho Kintoki (23.6). Contender had a significantly lower %SDI than did both Othello (44.4) and A55a,b from both origins (44.4, 42.2), but ICA Bunsi (16.0) was not significantly different from Othello or the A55a,b genotypes.
Discussion
This is the first reported attempt to determine the response of common bean germplasm to a prevalent pathotype of S. sclerotiorum in Australia. The most resistant responses in stems were Contender, ICA Bunsi, XAN 280 and Taisho-Kintoki, in leaves were Norvell 2558, Pico de Oro, Sanilac, Othelo and Negro Argel, and in cotyledons were Metis, Canario 107, Pico de Oro, Pogonion and Jubilejnaja 287. Bean genotypes exhibiting partial resistance to white mould identified in this study can be used as parental lines for crosses in common bean breeding programs and/or directly as improved cultivars where no resistance currently exists. Of the three plant components assessed, resistance to white mould in terms of stem resistance is considered the most critical towards effective management of white mould. The level of stem resistance found in Contender was similar to that of the moderately resistant variety ICA Bunsi, which in turn significantly outperformed A55a,b and Othello. Contender (also marketed as Buff Valentine) was developed in South Carolina, released in 1950, and has been well studied in the context of its genetic diversity within P. vulgaris and as a worldwide reference variety for production studies (Gepts et al. 1986; Nienhuis and Sass 2016; Meena et al. 2018). However, for this variety, there has not been any previous expression of physiological or morphological resistance on any plant component to white mould.
In previous studies, ICA Bunsi has consistently outperformed Othello, despite not being considered highly resistant (Viteri and Singh 2015). Although the difference in response between these two cultivars is not always large (Viteri and Singh 2015), in the current study ICA Bunsi showed much greater stem resistance to white mould. Resistance to white mould is known to be highly variable across common bean cultivars (Schwartz and Singh 2013), being similar to a wide range of host resistances/susceptibilities noted in previous studies across diverse Brassicaceae screened against this same isolate of S. sclerotiorum (Uloth et al. 2013, 2015; Barbetti et al. 2014; You et al. 2016). Interestingly, in previous studies, individual Othello plants have been identified as resistant to some isolates of S. sclerotiorum, even when the mean response is overall susceptible (Singh et al. 2014). Furthermore, Othello displays a Type III growth habit (indeterminate prostrate), a growth habit that is known to generally be more susceptible to white mould in the field than the more upright types of common bean (Ando et al. 2007; Schwartz and Singh 2013).
The four genotypes that showed significantly smaller stem lesions than did A55a or A55b in the current study also included ICA Bunsi, but not its derived cultivar, Centralia. The other three, being Contender, breeding line XAN 280 and the Japanese landrace Taisho-Kintoki, have not previously been noted for resistance to S. sclerotiorum. However, XAN 280 has been previously noted for its high resistance to bacterial blight (Xanthomonas campestris) in the field (Rodriguez et al. 1999). Hence, XAN 280 may be particularly useful for locating combined disease resistance where both white mould and bacterial blight co-occur. Whereas Taisho-Kintoki has been noted for low yield, its seed quality and early maturation have led to the development of higher-yielding cultivars of Kintoki bean such as Fukura Kintoki, which may be a worthwhile inclusion in future resistance-screening studies (Narikawa 1972; Ebe et al. 2005).
S. sclerotiorum is capable of damaging beans in the field at all stages of growth (Schwartz and Singh 2013). However, yield loss from white mould primarily occurs once a canopy has developed, encouraged by both the rising humidity around the stems and the senescent leaf and flower tissues that act as additional inoculum. In addition, airborne ascospores of S. sclerotiorum frequently land on and directly infect leaves in addition to flowers, as found in Brassicaceae even when not flowering (Uloth et al. 2013; M. J. Barbetti, unpubl. data). Such infested leaves collapse around stems and this leads to additional severe stem disease (Uloth et al. 2013; M. J. Barbetti, unpubl. data). Ideally, combining stem and leaf resistances into new commercial cultivars would significantly improve overall management of white mould.
Percentage stem disease index correlated strongly and positively with percentage cotyledon disease index and with seed weight. This suggests that there is scope for using a rapid cotyledon test as a preliminary screen for stem resistance to white mould. Conversely, it appears that resistance expressed in leaves is under separate genetic control to that in stems, such that separate searches are required for determining stem and leaf resistances. The correlation between %SDI and seed weight is interesting, with large-seeded beans being seemingly more susceptible to severe white mould. Perhaps this could relate to subsequent stem diameter, because Li et al. (2006) showed that stem diameter was an important determinate of the severity of Sclerotinia stem rot in Brassicaceae.
Phaseolus is a genetically diverse genus and has two major gene pools, the Andean and Mesoamerican, which reflect its multiple centres of origin and subsequent hybridisation (Gepts 1998; Bitocchi et al. 2017). ICA Bunsi and its derivatives are derived from the Mesoamerican gene pool (Miklas et al. 2004), as are A55a,b and Othello (Seo 2003; Singh et al. 2003). However, Contender is derived from the Andean gene pool, and is further assigned to a subgroup of the pool because of its uncommon phaseolin banding pattern (de la Fuente et al. 2012). Further study into stem, leaf and cotyledon resistance to white mould displayed by Andean and hybrid beans by using more wide-ranging Australian Sclerotinia isolates of varying aggressiveness and across different pathotypes would be useful.
Common bean canopy architecture and growth habit can be important determinants of S. sclerotiorum disease severity and their influence could be different under controlled environment versus field conditions (Schwartz and Singh 2013). However, stem inoculations under controlled conditions are known to provide more consistent results than are field trials (Kull et al. 2003). Despite this, additional field studies would allow assessment of the impact of any field environment and morphological ‘disease avoidance’ mechanisms on partial physiological resistances highlighted in the current study.
Conclusions
These present studies have reported the first response of common bean genotypes to a prevalent pathotype of S. sclerotiorum in Australia. Genotypes identified with high-level stem or leaf resistance are of particularly significant value for developing new white mould-resistant cultivars of common bean. Even genotypes identified with moderate levels of resistance in this study can be used as parental lines aimed at increasing resistance levels in common bean breeding programs. If deployed commercially, these resistances offer significant prospects for improving current integrated disease management strategies, as compared with current reliance on cultural and/or chemical controls utilised with cultivars generally lacking ‘useful’ resistance. Finally, as resistance was identified for the first time across some of these genotypes, it is likely that they could constitute new sources and/or types of host resistance not previously identified.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of interest
All authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Declaration of funding
This study was supported by the School of Agriculture and Environment at the University of Western Australia.
Acknowledgements
The authors thank Robert Creasy and Bill Piasini of the UWA Plant Growth Facilities for their technical assistance in plant growth facilities.
References
Abán CL, Taboada G, Spedaletti Y, Aparicio M, Curti RN, Casalderrey NB, Maggio ME, Chocobar MO, Salgado M, Galván MZ (2018) Molecular, morphological and pathogenic diversity of Sclerotinia sclerotiorum isolates from common bean (Phaseolus vulgaris) fields in Argentina. Plant Pathology 67, 1740-1748.
| Crossref | Google Scholar |
Abán CL, Taboada GM, Casalderrey NB, Maggio ME, Chocobar MO, Spedaletti YA, Gonzalez MAA, Vizgarra OV, Galván MZ (2020) Screening common bean germplasm for resistance to genetically diverse Sclerotinia sclerotiorum isolates from Argentina. Acta Scientarum Agronomy 42, e42786.
| Crossref | Google Scholar |
Ando K, Grumet R, Terpstra K, Kelly JD (2007) Manipulation of plant architecture to enhance crop disease control. CABI Reviews 2(026), 1-8.
| Google Scholar |
Australian Bureau of Statistics (2021) Agricultural commodities, Australia and state/territory and ASGS (Statistical Area 4) regions – 2019–20. Available at https://www.abs.gov.au/statistics/industry/agriculture/agricultural-commodities-australia/2019-20
Barbetti MJ, Banga SK, Fu TD, Li YC, Singh D, Liu SY, Ge XT, Banga SS (2014) Comparative genotype reactions to Sclerotinia sclerotiorum within breeding populations of Brassica napus and B. juncea from India and China. Euphytica 197, 47-59.
| Crossref | Google Scholar |
Barbetti MJ, Li CX, Banga SS, Banga SK, Singh D, Sandhu PS, Singh R, Liu SY, You MP (2015) New host resistances in Brassica napus and Brassica juncea from Australia, China and India: key to managing Sclerotinia stem rot (Sclerotinia sclerotiorum) without fungicides. Crop Protection 78, 127-130.
| Crossref | Google Scholar |
Bitocchi E, Rau D, Bellucci E, Rodriguez M, Murgia ML, Gioia T, Santo D, Nanni L, Attene G, Papa R (2017) Beans (Phaseolus ssp.) as a model for understanding crop evolution. Frontiers in Plant Science 8, 722.
| Crossref | Google Scholar | PubMed |
Campa A, Garcia-Fernandez C, Ferreira JJ (2020) Genome-wide association study (GWAS) for resistance to Sclerotinia sclerotiorum in common bean. Genes 11, 1496.
| Crossref | Google Scholar | PubMed |
Carvalho RSB, Lima IA, Alves FC, dos Santos JB (2013) Selection of carioca common bean progenies resistant to white mold. Crop Breeding and Applied Biotechnology 13, 172-177.
| Crossref | Google Scholar |
Clarkson JP, Staveley J, Phelps K, Young CS, Whipps JM (2003) Ascospore release and survival in Sclerotinia sclerotiorum. Mycological Research 107, 213-222.
| Crossref | Google Scholar | PubMed |
de la Fuente M, López-Pedrouso M, Alonso J, Santalla M, De Ron AM, Álvarez G, Zapata C (2012) In-depth characterization of the phaseolin protein diversity of common bean (Phaseolus vulgaris L.) based on two-dimensional electrophoresis and mass spectrometry. Food Technology and Biotechnology 50, 315-325.
| Google Scholar |
del Río LE, Venette JR, Lamey HA (2004) Impact of white mold incidence on dry bean yield under nonirrigated conditions. Plant Disease 88, 1352-1356.
| Crossref | Google Scholar | PubMed |
Ebe S, Sato H, Mikami K, Murata K, Chiba I, Shinada Y, Shimada H (2005) A new common bean [Phaseolus vulgaris] variety ‘Fukura-kintoki’ with early maturity, large seed size and high yield. Bulletin of Hokkaido Prefectural Agricultural Experiment Stations 10, 89.
| Google Scholar |
Ender M, Kelly JD (2005) Identification of QTL associated with white mold resistance in common bean. Crop Science 45, 2482-2490.
| Crossref | Google Scholar |
FAO (2022) ‘World food and agriculture – statistical yearbook 2022.’ (FAO: Rome, Italy) doi:10.4060/cc2211en
Garg H, Sivasithamparam K, Banga SS, Barbetti MJ (2008) Cotyledon assay as a rapid and reliable method of screening for resistance against Sclerotinia sclerotiorum in Brassica napus genotypes. Australasian Plant Pathology 37, 106-111.
| Crossref | Google Scholar |
Garg H, Li H, Sivasithamparam K, Kuo J, Barbetti MJ (2010) The infection processes of Sclerotinia sclerotiorum in cotyledon tissue of a resistant and a susceptible genotype of Brassica napus. Annals of Botany 106, 897-908.
| Crossref | Google Scholar | PubMed |
Ge XT, Li YP, Wan ZJ, You MP, Finnegan PM, Banga SS, Sandhu PS, Garg H, Salisbury PA, Barbetti MJ (2012) Delineation of Sclerotinia sclerotiorum pathotypes using differential resistance responses on Brassica napus and B. juncea genotypes enables identification of resistance to prevailing pathotypes. Field Crops Research 127, 248-258.
| Crossref | Google Scholar |
Gepts P (1998) Origin and evolution of common bean: past events and recent trends. HortScience 33, 1124-1130.
| Crossref | Google Scholar |
Gepts P, Osborn TC, Rashka K, Bliss FA (1986) Phaseolin-protein variability in wild forms and landraces of the common bean (Phaseolus vulgaris): evidence for multiple centers of domestication. Economic Botany 40, 451-468.
| Crossref | Google Scholar |
Jones SJ, Gent DH, Pethybridge SJ, Hay FS (2011) Spatial characteristics of white mould epidemics and the development of sequential sampling plans in Australian bean fields. Plant Pathology 60, 1169-1182.
| Crossref | Google Scholar |
Jones SJ, Gent DH, Pethybridge SJ, Hay FS (2012) Site-specific risk factors of white mould epidemics in bean (Phaseolus vulgaris) in Tasmania, Australia. New Zealand Journal of Crop and Horticultural Science 40, 147-159.
| Crossref | Google Scholar |
Kamvar ZN, Amaradasa BS, Jhala R, McCoy S, Steadman JR, Everhart SE (2017) Population structure and phenotypic variation of Sclerotinia sclerotiorum from dry bean (Phaseolus vulgaris) in the United States. PeerJ 5, e4152.
| Crossref | Google Scholar | PubMed |
Khan MA, Cowling W, Banga SS, You MP, Tyagi V, Bharti B, Barbetti MJ (2020) Inheritance of leaf resistance to Sclerotinia sclerotiorum in Brassica napus and its genetic correlation with cotyledon resistance. Euphytica 216, 188.
| Crossref | Google Scholar |
Kull LS, Vuong TD, Powers KS, Eskridge KM, Steadman JR, Hartman GL (2003) Evaluation of resistance screening methods for Sclerotinia stem rot of soybean and dry bean. Plant Disease 87, 1471-1476.
| Crossref | Google Scholar | PubMed |
Lehner MS, Teixeira H, Paula Junior TJ, Vieira RF, Lima RC, Carneiro JES (2015) Adaptation and resistance to diseases in Brazil of putative sources of common bean resistance to white mold. Plant Disease 99, 1098-1103.
| Crossref | Google Scholar | PubMed |
Lehner MdS, Paula Junior TJd, Vieira RF, Lima RC, Soares BdA, Silva RA (2016) Reaction of sources of resistance to white mold to microsatellite haplotypes of Sclerotinia sclerotiorum. Scientia Agricola (Piracicaba, Braz.) 73, 184-188.
| Crossref | Google Scholar |
Li CX, Li H, Sivasithamparam K, Fu TD, Li YC, Liu SY, Barbetti MJ (2006) Expression of field resistance under Western Australian conditions to Sclerotinia sclerotiorum in Chinese and Australian Brassica napus and Brassica juncea germplasm and its relation with stem diameter. Australian Journal of Agricultural Research 57, 1131-1135.
| Crossref | Google Scholar |
Li CX, Li H, Siddique AB, Sivasithamparam K, Salisbury P, Banga SS, Banga S, Chattopadhyay C, Kumar A, Singh R, Singh D, Agnihotri A, Liu SY, Li YC, Tu J, Fu TD, Wang YF, Barbetti MJ (2007) The importance of the type and time of inoculation and assessment in the determination of resistance in Brassia napus and B. juncea to Sclerotinia sclerotiorum. Australian Journal of Agricultural Research 58, 1198-1203.
| Crossref | Google Scholar |
Li YP, You MP, Norton S, Barbetti MJ (2016) Resistance to Pythium irregulare root and hypocotyl disease in diverse common bean (Phaseolus vulgaris) varieties from 37 countries and relationships to waterlogging tolerance and other plant and seed traits. European Journal of Plant Pathology 146, 147-176.
| Crossref | Google Scholar |
McCaghey M, Willbur J, Smith DL, Kabbage M (2019) The complexity of the Sclerotinia sclerotiorum pathosystem in soybean: virulence factors, resistance mechanisms, and their exploitation to control Sclerotinia stem rot. Tropical Plant Pathology 44, 12-22.
| Crossref | Google Scholar |
McKinney HH, Davis RJ (1923) Influence of soil temperature and moisture on infection of wheat seedlings by Helminthosporium sativum. Journal of Agricultural Research 26, 195-218.
| Google Scholar |
Meena M, Chamola BP, Rana DK, Singh KK (2018) Studies on performance of French Bean (Phaseolus vulgaris L.) cv. Contender for seed production under Garhwal Himalayas. International Journal of Current Microbiology and Applied Sciences 7, 676-681.
| Crossref | Google Scholar |
Miklas PN, Grafton KF (1992) Inheritance of partial resistance to white mold in inbred populations of dry bean. Crop Science 32, 943-948.
| Crossref | Google Scholar |
Miklas PN, Johnson WC, Delorme R, Gepts P (2001) QTL conditioning physiological resistance and avoidance to white mold in dry bean. Crop Science 41, 309-315.
| Crossref | Google Scholar |
Miklas PN, Hauf DC, Henson RA, Grafton KF (2004) Inheritance of ICA Bunsi-derived resistance to white mold in a navy × pinto bean cross. Crop Science 44, 1584-1588.
| Crossref | Google Scholar |
Miklas PN, Porter LD, Kelly JD, Myers JR (2013) Characterization of white mold disease avoidance in common bean. European Journal of Plant Pathology 135, 525-543.
| Crossref | Google Scholar |
Mila AL, Carriquiry AL, Zhao J, Yang XB (2003) Impact of management practices on prevalence of soybean Sclerotinia stem rot in the north-central United States and on farmers’ decisions under uncertainty. Plant Disease 87, 1048-1058.
| Crossref | Google Scholar | PubMed |
Murtza T, You MP, Barbetti MJ (2021) Temperature and relative humidity shape white leaf spot (Neopseudocercosporella capsellae) epidemic development in rapeseed (Brassica napus). Plant Pathology 70, 1936-1944.
| Crossref | Google Scholar |
Nienhuis J, Sass ME (2016) Vegetable Cultivar Descriptions for North America – Bean, Green. NC State University, Department of Horticultural Science. Available at https://cucurbitbreeding.wordpress.ncsu.edu/2016/05/24/bean-green-m-z/
Park SJ, Tu JC, Aylesworth JW, Buzzell RI (1988) Centralia field bean. Canadian Journal of Plant Science 68, 1149-1151.
| Crossref | Google Scholar |
Pascual A, Campa A, Perez-Vega E, Giraldez R, Miklas PN, Ferreira JJ (2010) Screening common bean for resistance to four Sclerotinia sclerotiorum isolates collected in northern Spain. Plant Disease 94, 885-890.
| Crossref | Google Scholar | PubMed |
Robison FM, Turner M, Jahn CE, Schwartz HF, Prenni JE, Brick MA, Heuberger AL (2018) Common bean varieties demonstrate differential physiological and metabolic responses to the pathogenic fungus Sclerotinia sclerotiorum. Plant Cell Environment 41, 2141-2154.
| Crossref | Google Scholar | PubMed |
Rodriguez O, Faure B, Benitez R, Carballo RM, Capote J (1999) Avances en el estudio de la resistencia a bacteriosis comun del frijol en Cuba. Agronomia Mesoamericana 10, 55-58.
| Crossref | Google Scholar |
Schwartz HF, Singh SP (2013) Breeding common bean for resistance to white mold: a review. Crop Science 53, 1832-1844.
| Crossref | Google Scholar |
Singh SP, Schwartz HF (2010) Breeding common bean for resistance to diseases: a review. Crop Science 50, 2199-2223.
| Crossref | Google Scholar |
Singh SP, Terán H (2008) Evolution of screening methods for identification of physiological resistance to white mold in dry bean. Annual Report of the Bean Improvement Cooperative 51, 40-41.
| Google Scholar |
Singh SP, Gutierrez JA, Teran H (2003) Registration of indeterminate tall upright small black-seeded common bean germplasm A 55. Crop Science 43, 1887-1888.
| Crossref | Google Scholar |
Singh SP, Schwartz HF, Viteri D, Teran H, Otto K (2014) Introgressing white mold resistance from Phaseolus coccineus PI 439534 to common pinto bean. Crop Science 54, 1026-1032.
| Crossref | Google Scholar |
Singh SP, Schwartz HF, Teran H, Centeno C, Otto K (2017) Large-seeded common bean PRA152, PRA154, and PRA155 with high levels of broad-spectrum white mold resistance. Journal of Plant Registrations 11, 305-310.
| Crossref | Google Scholar |
Terán H, Lema M, Schwartz HF, Duncan R, Gilbertson R, Singh SP (2006) Modified Petzoldt and Dickson scale for white mold rating of common bean. Annual Report of the Bean Improvement Cooperative 49, 115-116.
| Google Scholar |
Tu JC, Beversdorf WD (1982) Tolerance to white mold (Sclerotinia sclerotiorum (Lib.) De Bary) in Ex Rico 23, a cultivar of white bean (Phaseolus vulgaris L.). Canadian Journal of Plant Science 62, 65-69.
| Crossref | Google Scholar |
Uebersax MA, Cichy KA, Gomez FE, Porch TG, Heitholt J, Osorno JM, Kamfwa K, Snapp SS, Bales S (2023) Dry beans (Phaseolus vulgaris L.) as a vital component of sustainable agriculture and food security – a review. Legume Science 5, e155.
| Crossref | Google Scholar |
Uloth MB, You MP, Finnegan PM, Banga SS, Banga SK, Sandhu PS, Yi H, Salisbury PA, Barbetti MJ (2013) New sources of resistance to Sclerotinia sclerotiorum for crucifer crops. Field Crops Research 154, 40-52.
| Crossref | Google Scholar |
Uloth MB, You MP, Barbetti MJ (2015) Host resistance to Sclerotinia stem rot in historic and current Brassica napus and B. juncea varieties: critical management implications. Crop & Pasture Science 66, 841-848.
| Crossref | Google Scholar |
Viteri DM, Singh SP (2015) Inheritance of white mold resistance in an Andean common bean A 195 and its relationship with Andean G 122. Crop Science 55, 44-49.
| Crossref | Google Scholar |
You MP, Uloth MB, Li XX, Banga SS, Banga SK, Barbetti MJ (2016) Valuable new resistances ensure improved management of sclerotinia stem rot (Sclerotinia sclerotiorum) in horticultural and oilseed Brassica species. Journal of Phytopathology 164, 291-299.
| Crossref | Google Scholar |


