Soil physicochemical characteristics and leaf nutrient contents on banana farms of North Queensland, Australia
Ryan Orr A * , Tobin D. Northfield
A * , Tobin D. Northfield  B , Anthony Pattison C and Paul N. Nelson A
B , Anthony Pattison C and Paul N. Nelson A
A James Cook University, College of Science and Engineering, Cairns, Qld 4878, Australia.
B Department of Entomology, Washington State University, Wenatchee, WA, USA.
C Qld Department of Agriculture and Fisheries, South Johnstone, Qld 4859, Australia.
Crop & Pasture Science 74(5) 483-493 https://doi.org/10.1071/CP22306
Submitted: 14 September 2022 Accepted: 29 November 2022 Published: 20 February 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Banana production in Australia is in three primary sub-regions within tropical North Queensland and the industry faces a variety of challenges including costs of production, disease and pests, and environmental impacts. The range of soil characteristics and banana leaf nutrient status on banana farms has not previously been systematically described. This knowledge gap makes it difficult to adapt research, management recommendations, and regulations to the needs of the three primary growing sub-regions.
Aims: In this work, we aimed to identify key soil factors that differentiate growing sub-regions, and provide context for future research and industry regulation.
Methods: We characterised soil and banana leaf samples from 28 banana farms on soil types accounting for >85% of Australia’s banana production.
Key results and conclusions: Variation in soil properties and leaf nutrient concentrations were driven largely by site- (principal component 1 in both cases) and management-related variables (principal component 2 in both cases). Management-related foliar nutrient concentrations did not differ between regions despite differences in the associated soil variables. The most important site characteristics appeared to be soil parent material and climate. The Mareeba sub-region has basaltic soils, low rainfall and temperature, whereas the other two sub-regions are hotter, wetter and have a variety of soil parent materials. Leaf nitrogen concentrations were mostly below the regulated limit for additional nitrogen fertiliser application.
Implications: Our findings can facilitate sub-region-specific site selection for research, extension, and monitoring and more targeted regulation of banana production- and environment-related issues.
Keywords: agronomy, climate, crop management, nitrogen allocation, phosphorus nutrition, soil parent material, tropical crops, tropical soils.
Introduction
Banana production in Australia is approximately 365 000 tonnes/year produced from 11 280 ha, of which greater than 90% is in North Queensland (ABGC 2017). The region is situated between approximately 15°50′S and 18°20′S latitude along the east coast of Queensland. Production is primarily on coastal plains and slopes, with annual rainfall ranging from 1800 to 4500 mm (Murtha 1986). Smaller growing areas exist on nearby elevated tablelands, up to 600 m elevation and with annual rainfall of approximately 1400 mm, supplemented by irrigation (Laffan 1988).
The variation of soils and plant nutrient status in the banana industry in North Queensland has previously been described in general terms. Soils of the region have been classified and mapped (Murtha 1986; Laffan 1988; Cannon et al. 1992; Murtha et al. 1996; Enderlin et al. 1997; Morrison et al. 2021), the location of banana farms is known (DSITI 2015; Biosecurity Queensland 2016; Clark and McKechnie 2020), and several reports on banana production and management mentioned soil types (Daniells 1984; Pattison et al. 2005; Harvey et al. 2018). Additionally, several surveys have sampled conventional banana farms in tandem with either organic or unfarmed areas (Pattison et al. 2008, 2018a; Geense et al. 2015). However, to our knowledge this is the most extensive survey of North Queensland banana farms, both geographically and in number of characteristics analysed, and is the first to sample based on maximising soil map unit diversity.
Understanding the characteristics of the soil used for banana production is important for applications such as nutrient and disease management, minimising off-site environmental impacts, and future expansion of the industry. For example, the severity of Panama disease, a current threat to the industry, is related to soil properties (Orr and Nelson 2018). Panama disease, or Fusarium wilt of banana, is caused by a soil-borne fungal pathogen (Fusarium oxysporum f. sp cubense) and the potential cost of a currently spreading strain, first detected in North Queensland in 2015, is greater than AU$138 million per year (Cook et al. 2015; O’Neill et al. 2016). Fusarium wilt severity differs among key soils of North Queensland (Bowen et al. 2019). Nutrient management, which is affected by soil type, has also been of particular concern in this region as it drains to the environmentally sensitive Great Barrier Reef (Rasiah et al. 2010; Armour et al. 2013; Armour 2018). Banana requires high inputs of potassium (K), moderate inputs of nitrogen (N) and relatively low inputs of phosphorus (Armour 2018). Regulations were recently introduced limiting the nitrogen and phosphorus (P) fertiliser application in banana production to a maximum of 400 kg N/ha.year, unless foliar nitrogen concentration is below 3.5% dry weight, and to 60 kg P/ha.year, unless foliar phosphorus concentration is below 0.22% dry weight (DES 2019). These rates consider neither the effect of soil characteristics on the availability and retention of nutrients nor the range of soil types currently under cultivation. The aim of this research was to characterise the soil characteristics and plant nutrient status across the banana farms of North Queensland, identify key differences between sub-regions and assess the sub-regional applicability of current regulations.
Materials and methods
Site selection
Sampling locations were selected on the basis that they were used for banana production and to maximise the variability of soil characteristics as well as geographic distribution within the North Queensland banana growing region. Geology is primarily granite and other acid igneous rocks, metamorphosed sediments, alluvium and basalt (Table 1). The region was divided geographically into three sub-regions (Fig. 1). The sub-regions of Tully and Innisfail are on coastal plains and slopes, with summer dominant rainfall ranging from an annual average of 1800–4500 mm and mean monthly temperatures of 30.3–15.1°C (Murtha 1986). The smaller Mareeba sub-region is located on nearby elevated tablelands, up to 600 m elevation and with summer dominant rainfall with an annual average of approximately 1400 mm, supplemented by irrigation and mean monthly temperatures of 23–16°C (Laffan 1988).

|
|
|
A total of 28 sampling locations were selected on soil map units representing >94% of banana production in North Queensland, and >88% of that in Australia (Table 1). Geographic data sets were obtained from the Queensland Government spatial catalogue and analysed in ArcGIS ver. 10.3.1. The area under banana production was obtained from the ‘Commercial banana production areas for Panama disease tropical race 4 program – North Queensland’ data set and soil types were obtained from the ‘Soil and agricultural land suitability series’ data set. Maps of banana production were not available for the two northernmost sites (25 and 26) in the Lakeland agricultural region, but soil types chosen were representative of banana growing conditions in the region. For the purpose of analysis the Lakeland locations have been allocated to the ‘Mareeba’ sub-region, to which they are geographically and climatically most similar. Soil survey layers were clipped to banana growing areas, merged to a single layer and subdivided based on primary soil type. Soil types were ranked by total area used for banana cultivation in this region and those comprising >0.4% of the area were sampled. Three classifications were excluded; ‘Stream Channel’ as it is based on proximity to streams rather than soil characteristics, and ‘Jarra’ and ‘Dingo’ soil types due to their small area and restricted access due to presence of Fusarium wilt of banana Tropical Race 4. The soil series chosen have been described by Murtha (1986), Laffan (1988), Cannon et al. (1992), Grundy and Heiner (1994), Murtha et al. (1996), Enderlin et al. (1997), and Morrison et al. (2021).
Sampling
Composite soil samples, comprised of 12 samples, were taken at each location in February–April 2017. Each sampling area was 20 m long and four rows (approximately 35 m) wide. As plant species and crop duration have both been shown to affect the soil microbiome, sampling areas were restricted to fields in which Cavendish bananas (Musa AAA) had been grown continuously for at least 2 years; limiting the growing time further was not deemed practical (Smalla et al. 2001; Garbeva et al. 2004; Shen et al. 2018). At each site the samples were combined, homogenised and subsampled. Soil samples were taken 0.4 m from in front of the leading banana plant pseudostem, at 0.0–0.1 and 0.1–0.25 m depths. Plants sampled were mature, but not flowering or bunched, as development stage can influence soil and plant nutrition (Garbeva et al. 2004). Banana foliar samples were taken from the banana plant associated with each soil sample. Foliar samples were a 0.20 m-wide strip from the centre of the third completely emerged leaf, from each side to the midrib (Broadley et al. 2004). Samples were rinsed with deionised water, the 12 individual samples were combined and the sample was stored at 4°C until drying.
The fields sampled were managed in a variety of ways. Most had received applications of lime to neutralise soil pH prior to planting, with small doses annually. Most also had fertiliser blends applied regularly, with nitrogen primarily in the form of urea or ammonium, potassium as potassium chloride, phosphorus as either calcium or ammonium phosphate, and some calcium, magnesium and micronutrients. Sites 4, 14 and 15 (from Table 1) had nitrogen applied as calcium and potassium nitrate. In addition to inorganic fertiliser blends, sugarcane mill mud, a by-product from sugar mills, chicken manure or various ‘biological’ products were added to some fields. Site 5 was ‘Ecoganic’, applying less fertiliser, pesticides and nematicides than conventional, and site 21 was certified Organic, applying no synthetic nitrogen fertiliser, pesticides or herbicides.
Analysis
All analyses were performed on samples from both 0–0.1 m to 0.1–0.25 m depths, except for mineralogy, which was measured only on the 0.1–0.25 m samples. Chemical analyses were carried out by Nutrient Advantage Laboratory, Werribee, Victoria. Analysis included (with method codes from Rayment and Lyons (2011)): ammonium and nitrate nitrogen 7C2b; chloride (1:5 water) 5A2b; boron 12C2; electrical conductivity (1:5 water) 3A1; exchangeable cations (calcium, magnesium, potassium, sodium) (1 M ammonium acetate) 15D3; organic carbon (Walkley and Black) 6A1; pH (1:5 water) 4A1; phosphorus buffer index 9I2b; phosphorus (Colwell) 9B2; silicon (CaCl2) (Haysom and Chapman 1975); sulfur (mono-calcium phosphate method, MCP) 10B3; total nitrogen (combustion) 7A5; total carbon (combustion) 6B2b; total (acid digest) phosphorus, calcium, copper, iron, magnesium, manganese, potassium, sulfur, zinc 17B1 and sand, silt and clay (Gee and Or 2002). Water holding capacity of each soil was determined using a 1-bar ceramic pressure plate with −10 kPa pressure applied to a blended, dried sample. After equilibration on the pressure plate, soils were weighed, dried for 24 h at 105°C and reweighed, to determine water content.
Soil mineralogy was analysed by CSIRO Land and Water, Urrbrae, South Australia. Due to possible dehydration of the montmorillonite (smectite) interlayer samples were dispersed in 0.25 M calcium chloride, centrifuged at 5150g (Eppendorf Centrifuge 5810, Australia) for 10 min, calcium saturated again, washed with water then ethanol (centrifuging between each step) and oven dried at 60°C. X-ray diffraction patterns were recorded with a PANalytical X’Pert Pro Multi-purpose Diffractometer using iron filtered cobalt Kα radiation, automatic divergence slit, 2° anti-scatter slit and fast X’Celerator Si strip detector. The diffraction patterns were recorded from 3 to 80° in steps of 0.017° 2 theta with a 0.5 s counting time per step for an overall counting time of approximately 35 min.
Qualitative analysis was performed on the X-ray diffraction data using in-house XPLOT and HighScore Plus (from PANalytical) search/match software. Quantitative analysis was performed on the X-ray diffraction data using the commercial package SIROQUANT from Sietronics Pty Ltd. Results are presented as a percentage of soil, as opposed to a percentage of the clay fraction alone.
Foliar samples were dried at 70°C, ground to a fine powder and analysed by Nutrient Advantage Laboratory, Werribee, Victoria for calcium, magnesium, phosphorus, potassium, sodium, sulfur, boron, copper, iron, manganese and zinc using a nitric acid and hydrogen peroxide digest followed by analysis with inductively coupled plasma atomic emission spectroscopy (ICP-AES). Ammonia, nitrate and chloride were extracted in a 1:125 water extract and analysed by flow injection analysis (Kalra 1997). Total nitrogen was analysed by combustion (Kalra 1997). Two elemental ratios commonly used in diagnosis of nutrient deficiencies, N/P and N/K, were also included as variables.
To assess whether the samples collected were representative of North Queensland banana growing soils, the soil characteristics were compared to two anonymised soil data sets from North Queensland banana farms over the period 2012–2017 provided by Incitec Pivot (n = 738) and Total Grower Services (n = 1074). Soil characteristics chosen for comparison were those in which analysis methodology was consistent across all three data sets, i.e. pH (calcium chloride), electrical conductivity, cation exchange capacity, extractable phosphorus (Colwell), sulfur (MCP), calcium, magnesium and potassium (ammonium acetate), iron, manganese, copper and zinc (diethylenetriaminepentaacetic acid method, DTPA), and boron (hot calcium chloride).
Principal component analysis
There were 79 foliar nutrient and soil characteristics analysed, with a great deal of covariance between these. Thus, to reduce the number of predictor variables and collinearity between predictors, principal components analysis was performed, using the R platform (R Core Team 2017), on soil characteristics and foliar nutrients; to reduce the data set to fewer orthogonal variables. Soil characteristics were excluded from the principal components analysis if they were redundant analytical methods for the same characteristic to avoid overweighting certain components due to multiple inclusions of the same information. The reduced list of characteristics used for principal components analysis was: water holding capacity, pH (CaCl2), electrical conductivity, chloride, nitrate, ammonium (KCl), phosphorus (Colwell), phosphorus buffer index (Colwell), calcium (ammonium acetate), potassium (ammonium acetate), magnesium (ammonium acetate), sodium (ammonium acetate), cation exchange capacity (calculated from ammonium acetate), copper (DTPA), iron (DTPA), manganese (DTPA), zinc (DTPA), boron (Hot CaCl2), sulfur (MCP), organic carbon, silicon (CaCl2), total carbon, total nitrogen, carbon-to-nitrogen ratio, total cadmium, total calcium, total chromium, total copper, total iron, total lead, total magnesium, total manganese, total nickel, total phosphorus, total potassium, total sodium, total sulfur, total zinc, clay, sand, silt. The foliar nutrient concentration data were not reduced prior to principal components analysis.
Data were centred and scaled prior to running principal components analysis. Five principal components were retained for both soil and foliar analysis, based on their explained variance being greater than the average contribution of a single variable (Kaiser–Guttman Rule) (Kaiser 1991). Comparisons among sub-regions based on principal component values were calculated with a one-way analysis of variance followed by Tukey post hoc analysis to determine specific group differences at α = 0.05.
Principal components results were further analysed in relation to climatic variables. Rainfall and minimum temperature data were obtained from the Australian historical climate database SILO (Scientific Information for Land Owners) (Jeffrey et al. 2001). Minimum temperature was selected, as photosynthesis and growth are reduced at low temperature, whereas impacts of high temperature are related to water deficiency, which was overcome with irrigation in the plants sampled (Turner and Lahav 1983). Monthly rainfall and minimum temperature values were obtained for each location spanning from June 2016 to February 2017 and averaged. A 9-month period was chosen as it represented the likely growing period of the plants sampled, based on growth rates and plant development stage.
Results
The soils sampled were diverse, with a wide range of values for most properties measured (Fig. 2). The full dataset, including soil characteristics not reported here have been published separately (Orr and Nelson 2021). The range of values for soil characteristics in this study was close to that in the much larger data sets obtained from Incitec Pivot and Total Grower Services (Fig. 2).
Water holding capacity of the soils ranged from 12.3 to 36.6%, with a median value of 31.0%, and was principally determined by the clay content of the soil (R2 = 0.453, t26 = 4.832, P < 0.001). Kaolinite was the dominant clay mineral, accounting for >50% in most soils. Sampling locations (numbers from Table 1) with <50% kaolinite (with contents of other clay types >5% shown in brackets) were: five (K-Feldspar, Albite, Mica/Illite and Chlorite), nine (Gibbsite and Hematite), 23 and 27 (K-Feldspar) and 28 (Mica/Illite). Hematite was also common amongst soils of basaltic origin.
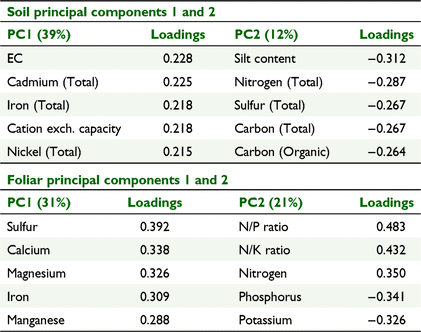
Due to the interrelated nature of soil characteristics many were highly correlated. Principal components analysis was useful in identifying those characteristics that represented the greatest variability within the dataset. For the soil characteristics, principal components 1 and 2 explained 39% and 12% of the variability, respectively (Table 2). Principal component 1 was most strongly associated with electrical conductivity, total cadmium, total iron, cation exchange capacity and total nickel concentration, which were largely related to clay content and mineralogy. Principal component 2 was most strongly associated with silt content, total nitrogen, sulfur and carbon, and organic carbon concentration, which were broadly related to organic matter content (Table 2).
|
Foliar nutrient concentrations, like soil characteristics, were highly intercorrelated, though less of the variance in the dataset was explained by the first principal component (Table 2). The foliar first principal component appears related to particular proteins (sulfur), chlorophyll and chloroplasts (magnesium and iron), cell walls (calcium) and enzyme cofactors (magnesium, iron and manganese). The second principal component was primarily dependent on the concentration of nitrogen, alone and relative to the other macronutrients phosphorus and potassium (Table 2).
The soil characteristics differed significantly between Mareeba and the other growing sub-regions, based on principal components 1 and 2 (Fig. 3). The Mareeba sub-region had a greater range in values for principal component 1 (clay content and mineralogy), whereas the Innisfail and Tully sub-regions had greater variation in principal component 2 (organic matter). Soil principal component 1 strongly differentiated between basalt soils and those of other parent material and is significantly affected by rainfall but not by temperature (Fig. 4). The foliar principal component 1 also showed significant separation of the Mareeba sub-region from the others (Fig. 3). Foliar principal component 1 was significantly related to average monthly rainfall and average minimum temperature for the 9 months preceeding sampling but did not differ between soil parent materials (Fig. 4). Foliar principal component 2, primarily indicative of the macronutrients nitrogen, potassium and phosphorus, does not differ significantly between sub-regions.
The range of foliar nutrients and soil characteristics measured here was important for several issues currently facing the banana industry. Suppressiveness of North Queensland soils to Panama disease, measured using a subset of the soils examined here, was positively correlated with clay and total iron content (Bowen et al. 2019). We did not identify reliable differences in clay content between the sub-regions (F2, 25 = 1.348, P = 0.278) but did in soil total iron content (F2, 25 = 4.493, P = 0.022) (Fig. 5), so risk of disease severity may differ between the sub-regions. Total foliar nitrogen and phosphorus content are important for determining if a grower may increase fertiliser use above the regulated maximum rate, which was established to prevent negative off-site environmental outcomes. Total foliar nitrogen content did not differ reliably between banana farms in the sub-regions of North Queensland (F2, 25 = 0.878, P = 0.428) and was below the regulated limit for nearly all sites tested (Fig. 5). Total foliar phosphorus content also did not differ reliably between sub-regions (F2, 25 = 0.219, P = 0.805) but many of the samples collected exceeded the regulated limit of 0.22% dry weight (Fig. 5).
|
|
Discussion
This is the first study of the north Queensland banana industry to systematically analyse soil and foliar properties based on their soil classification subunits. The importance of both intrinsic soil forming factors such as parent material and climate and managed characteristics such as fertiliser application is generally accepted (Singh and Schulze 2015; Armour 2018). We have determined that a farm’s soil and plant nutrient status, relative to the larger growing region, was predominantly determined by location and intrinsic characteristics (principal component 1), with management approaches such as fertiliser being of secondary importance (principal component 2). Despite this the importance of capturing soil type diversity for banana research within the Far North Queensland growing region has only occasionally been considered (Rasiah and Armour 2001; McKergow et al. 2004; Rasiah et al. 2009, 2010; Armour et al. 2013). Pattison et al. (2018a) sampled from a wide range of soil types throughout the region and concluded that farm management affected soil biological indices more than physical and chemical characteristics, but they did not consider the effect of soil type on management or biological indices. We have shown that variation in the Far North Queensland region is principally determined by intrinsic soil forming factors and secondarily by management, therefore future research should be structured to span key variables and parent materials.
The soil characteristics that most contributed to variation in principal component 1 were largely related to parent material and weathering (Table 2, Fig. 4). Electrical conductivity can be related to the amount of rainfall a location received or how recently fertiliser was applied, as the soluble salts that cause conductivity are flushed away in high rainfall areas like Innisfail and Tully, but accumulate in low rainfall irrigated areas such as Mareeba (Fig. 4) (Brady and Weil 2000). Clay and total metal content were highly correlated and were largely determined by the parent material of the soil. Most of the soils from the area surrounding Mareeba had basalt parent material whereas the soils of the Innisfail and Tully regions had a variety of parent materials (Table 1) (Murtha 1986; Cannon et al. 1992; Enderlin et al. 1997). Soil principal component 2 is largely based on managed characteristics such as nitrogen and organic matter-related properties. While some variation exists due to inherent site variability, many of these characteristics were controlled by growers through the application of agri-chemicals and management practices such as irrigation and cover cropping.
The foliar first principal component appears related to the secondary nutrients such as magnesium and calcium, those that are added either as balance ions in fertilisers, such as sulfur, or those not extensively managed with fertilisers, such as iron and manganese. Values were generally higher on banana farms in Mareeba than the other sub-regions, presumably due to the same variables explaining the difference in soil characteristics, i.e. soil parent material and rainfall (Anderson 1988). The second foliar principal component, which explained a relatively large proportion of the variation, covers the major nutrients applied in fertiliser; nitrogen, phosphorus, potassium and their ratios. It is perhaps not surprising that there is little separation of the sub-regions based on these characteristics, as the application of nitrogen, phosphorus and potassium is actively managed on most farms, typically based on foliar content, to within a narrow range (Reuter and Robinson 1997; Armour 2018).
The results of the principal component analysis could be used to guide future sampling and experimentation to ensure results are representative of banana farms. Differences exist between banana farms in the sub-regions, largely attributable to parent material, with basalt soil separating from the other materials, and climate variables such as temperature and rainfall (Fig. 4). Therefore, if time and resources permit, it would be beneficial to incorporate at least one location in the Mareeba sub-region and one in either the Innisfail or Tully region. Additionally, at least one site should be on basalt soils and one on another parent material, as conclusions drawn from one sub-region, or parent material, may not be applicable to another.
Our findings here can provide context to research at particular sites or on particular soil types. Bowen et al. (2019), determined the disease severity of Fusarium wilt of banana in a pot trial using soils from six sites that were a subset of the samples collected in this work. Disease severity was negatively correlated with clay content, field capacity water content, extractable boron and the concentration of total iron, copper and cadmium (Bowen et al. 2019) in agreeance with previous work both in Australia (Peng et al. 1999) and internationally (Stotzky et al. 1961; Stotzky and Torrence Martin 1963; Domínguez et al. 2001). Although only six soils were studied, they covered most of the range of clay and total iron concentration on North Queensland banana farms (Fig. 5). Additionally, we can see that the soil types chosen represent 61.7% of the growing area, including the five most prominent soil types (Table 1).
Our findings demonstrate the relationship between the banana industry and recently introduced fertiliser regulations for nitrogen and phosphorus (DES 2019). All but one location had a foliar total nitrogen concentration below the regulated value of 3.5% (Fig. 5) and farms would be able to increase nitrogen fertiliser use, even if they were already at the regulated maximum rate. Conversely, a large number of locations have a higher foliar phosphorus concentration than the regulated value of 0.22%. This means that farmers would be unable to apply phosphorus fertiliser at a rate exceeding the regulated limit of 60 kg/ha.year. Also, it is interesting to note that there is little difference in leaf nitrogen or phosphorus concentrations between the sub-regions (Fig. 5). This is likely due to the extent to which phosphorus and nitrogen content are optimised for yield by the farmer, irrespective of location and soil type (Reuter and Robinson 1997; Armour 2018). This then raises the question whether different management is required for farms with different soil types (basalt vs other) and different climates (Mareeba vs other) to achieve the same plant nutritional and production outcomes.
Experiments to determine optimal nitrogen rates in banana have been undertaken in the Innisfail or Tully sub-region as that is representative of the largest proportion of production (Prasertsak et al. 2001; Armour et al. 2013). One nitrogen rate trial has been performed on a basaltic soil (Pattison et al. 2018b) and the optimal application rate differed from a trial performed on non-basalt soils (Armour et al. 2013) though the trials occurred over different years and using different forms of nitrogen. Masters (2019) is the only one, to our knowledge, to compare nitrogen fluxes on basalt and non-basalt soils under banana production in Far North Queensland. They found that nitrous oxide emission differed considerably between the two locations based on rainfall and soil permeability (Masters 2019). Presumably, other nitrogen pathways would also differ. The results here suggest that it would be beneficial for future nutrient research and regulations to take in to account sub-regional variability to ensure farmers are not disadvantaged by uniform regulations that do not take account of location.
Data availability
The data associated with this manuscript can be found in its entirety at https://doi.org/10.25903/mvp4-2759.
Conflicts of interest
The authors declare that there are no conflicts of interest relating to this work.
Declaration of funding
This work was funded by Hort Innovation, using the Hort Innovation banana research and development levy, co-investment from the Queensland Government and contributions from the Australian Government under the BA14014 research program.
Acknowledgements
We would like to thank all of the banana growers for access to their properties, information regarding their management practices and general assistance. We would also like to thank Rob Dwyer from Incitec Pivot Fertilisers and Shane Fitzgerald from Total Grower Services for advice and provision of the large data sets. Finally, thank you to Tegan Kukulies, Stewart Lindsay and Shanara Veivers at the Queensland Department of Agriculture for help with organising site visits. This project was funded by Hort Innovation, using the Hort Innovation banana research and development levy, co-investment from Queensland Government and contributions from the Australian Government. Hort Innovation is the grower-owned, not-for-profit research and development corporation for Australian horticulture.
References
ABGC (2017) The Australian banana industry. Available at https://abgc.org.au/our-industry/key-facts [Accessed 31 January 2021]Anderson DW (1988) The effect of parent material and soil development on nutrient cycling in temperate ecosystems. Biogeochemistry 5, 71–97.
| The effect of parent material and soil development on nutrient cycling in temperate ecosystems.Crossref | GoogleScholarGoogle Scholar |
Armour J (2018) Nutrient management plan for the banana industry (of north Queensland). Available at https://abgc.org.au/wp-content/uploads/2018/04/Ban-NMP_submit-R1.pdf
Armour JD, Nelson PN, Daniells JW, Rasiah V, Inman-Bamber NG (2013) Nitrogen leaching from the root zone of sugarcane and bananas in the humid tropics of Australia. Agriculture, Ecosystems & Environment 180, 68–78.
| Nitrogen leaching from the root zone of sugarcane and bananas in the humid tropics of Australia.Crossref | GoogleScholarGoogle Scholar |
Biosecurity Queensland (2016) Commercial banana production areas for Panama disease tropical race 4 program – North Queensland. (Queensland Department of Agriculture and Fisheries: Brisbane, Australia)
Bowen A, Orr R, McBeath AV, Pattison A, Nelson PN (2019) Suppressiveness or conduciveness to Fusarium wilt of bananas differs between key Australian soils. Soil Research 57, 158–165.
| Suppressiveness or conduciveness to Fusarium wilt of bananas differs between key Australian soils.Crossref | GoogleScholarGoogle Scholar |
Brady NC, Weil RR (2000) ‘Elements of the nature and properties of soils.’ (Prentice Hall: Upper Saddle River, NJ, USA)
Broadley R, Rigden P, Chay-Prove P, Daniells J (2004) ‘Subtropical banana grower’s handbook.’ pp. 1–206. (Queensland Department of Primary Industries: Brisbane, Qld)
Cannon MG, Smith CD, Murtha GG (1992) Soils of the Cardwell–Tully area, North Queensland. (CSIRO: Glen Osmond, SA, Australia)
Clark A, McKechnie J (2020) Detecting banana plantations in the wet tropics, Australia, using aerial photography and U-Net. Applied Sciences 10, 2017
| Detecting banana plantations in the wet tropics, Australia, using aerial photography and U-Net.Crossref | GoogleScholarGoogle Scholar |
Cook DC, Taylor AS, Meldrum RA, Drenth A (2015) Potential economic impact of panama disease (Tropical race 4) on the Australian banana industry. Journal of Plant Diseases and Protection 122, 229–237.
| Potential economic impact of panama disease (Tropical race 4) on the Australian banana industry.Crossref | GoogleScholarGoogle Scholar |
Daniells JW (1984) The banana industry in north Queensland. Queensland Agricultural Journal 110, 282–290.
DES (2019) ‘Agricultural ERA standard for banana cultivation – version 1.’ (Queensland Government: Queensland)
Domínguez J, Negrín MA, Rodríguez CM (2001) Aggregate water-stability, particle-size and soil solution properties in conducive and suppressive soils to Fusarium wilt of banana from Canary Islands (Spain). Soil Biology and Biochemistry 33, 449–455.
| Aggregate water-stability, particle-size and soil solution properties in conducive and suppressive soils to Fusarium wilt of banana from Canary Islands (Spain).Crossref | GoogleScholarGoogle Scholar |
DSITI (2015) Land use mapping – 1999 to 2015 – wet tropics NRM region. (Queensland Department of Science, Information Technology and Innovation: Brisbane) Available at http://qldspatial.information.qld.gov.au/catalogue/custom/detail.page?fid={0082B3DB-A37F-44A1-B4CD-FC9C70644CA7}
Enderlin NG, Barry EV, Philip SR (1997) ‘Soils of the Mareeba-Dimbulah irrigation area (MDIA).’ (Queensland Department of Natural Resources: Mareeba, Qld)
Garbeva P, van Veen JA, van Elsas JD (2004) Microbial Diversity in Soil: selection of microbial populations by plant and soil type and implications for disease suppressiveness. Annual Review of Phytopathology 42, 243–270.
| Microbial Diversity in Soil: selection of microbial populations by plant and soil type and implications for disease suppressiveness.Crossref | GoogleScholarGoogle Scholar |
Gee GW, Or D (2002) Particle-size analysis. In ‘Methods of soil analysis: part 4 physical methods’. (Eds JH Dane, GC Topp) pp. 255–293. (Soil Science Society of America: Madison, WI, USA)
Geense P, Pattison AB, Kukulies TL, Scholberg JMS, Molina AB (2015) Can changes in soil properties in organic banana production suppress Fusarium wilt? Natural Resources 6, 181–195.
| Can changes in soil properties in organic banana production suppress Fusarium wilt?Crossref | GoogleScholarGoogle Scholar |
Grundy MJ, Heiner IJ (1994) Soils of Lakeland Downs. (Queensland Department of Primary Industries: Brisbane, Qld, Australia)
Harvey S, Cook S, Poggio M (2018) Economic assessment of best management practices for banana growing. (Department of Agriculture and Fisheries Queensland: Queensland, Australia). Available at https://www.publications.qld.gov.au/dataset/banana-economics/resource/498cb877-4dad-4773-8e17-14b8cab3a525
Haysom MBC, Chapman LS (1975) Some aspects of the calcium silicate trials at Mackay. Proceedings Queensland Society of Sugar Cane Technologists 42, 117–122.
Jeffrey SJ, Carter JO, Moodie KB, Beswick AR (2001) Using spatial interpolation to construct a comprehensive archive of Australian climate data. Environmental Modelling & Software 16, 309–330.
| Using spatial interpolation to construct a comprehensive archive of Australian climate data.Crossref | GoogleScholarGoogle Scholar |
Kaiser HF (1991) Coefficient alpha for a principal component and the Kaiser-Guttman rule. Psychological Reports 68, 855–858.
| Coefficient alpha for a principal component and the Kaiser-Guttman rule.Crossref | GoogleScholarGoogle Scholar |
Kalra Y (1997) ‘Handbook of reference methods for plant analysis.’ (CRC Press)
Laffan MD (1988) ‘Soils and land use on the Atherton Tableland, North Queensland.’ (CSIRO Australia, Division of Soils: Adelaide, SA, Australia)
Masters BL (2019) Nitrous oxide emissions from soil in mango and banana fields: effects of nitrogen rate, fertiliser type, and ground cover practices. Masters Thesis, James Cook University, Cairns, Qld, Australia.
McKergow LA, Prosser IP, Grayson RB, Heiner D (2004) Performance of grass and rainforest riparian buffers in the wet tropics, Far North Queensland. 2. Water quality. Soil Research 42, 485–498.
| Performance of grass and rainforest riparian buffers in the wet tropics, Far North Queensland. 2. Water quality.Crossref | GoogleScholarGoogle Scholar |
Morrison D, Enderlin N, Barker M, Whiteing T, Kolkert S (2021) Soils and agricultural land suitability of the Lakeland District, North Queensland. (Queensland Department of Resources: Brisbane, Qld, Australia)
Murtha GG (1986) Soils of the Tully-Innisfail area North Queensland. (CSIRO Division of Soils: Canberra, ACT, Australia)
Murtha GG, Cannon MG, Smith CD (1996) Soils of the Babinda-Cairns area, North Queensland. (CSIRO Division of Soils: Glen Osmond, SA, Australia)
Orr R, Nelson PN (2018) Impacts of soil abiotic attributes on Fusarium wilt, focusing on bananas. Applied Soil Ecology 132, 20–33.
| Impacts of soil abiotic attributes on Fusarium wilt, focusing on bananas.Crossref | GoogleScholarGoogle Scholar |
Orr R, Nelson P (2021) North Queensland banana farm survey 2017. (James Cook University) https://doi.org/10.25903/mvp4-2759
O’Neill WT, Henderson J, Pattemore JA, O’Dwyer C, Perry S, Beasley DR, Tan YP, Smyth AL, Goosem CH, Thomson KM, Hobbs RL, Grice KRE, Trevorrow P, Vawdrey LL, Pathania N, Shivas RG (2016) Detection of Fusarium oxysporum f. sp. cubense tropical race 4 strain in northern Queensland. Australasian Plant Disease Notes 11, 33
| Detection of Fusarium oxysporum f. sp. cubense tropical race 4 strain in northern Queensland.Crossref | GoogleScholarGoogle Scholar |
Pattison T, Smith L, Moody P, Armour J, Badcock K, Cobon J, Rasiah V, Lindsay S, Gulino L (2005) ‘Banana root and soil health project – Australia.’ pp. 149–165. (International Network for the Improvement of Banana and Plantain (INIBAP): Montpellier, France)
Pattison AB, Moody PW, Badcock KA, Smith LJ, Armour JA, Rasiah V, Cobon JA, Gulino L-M, Mayer R (2008) Development of key soil health indicators for the Australian banana industry. Applied Soil Ecology 40, 155–164.
| Development of key soil health indicators for the Australian banana industry.Crossref | GoogleScholarGoogle Scholar |
Pattison AB, Kukulies T, Smith MK, Sciacca F (2018a) Soil indicators respond to changes in banana plantation management. Acta Horticulturae 1196, 147–154.
| Soil indicators respond to changes in banana plantation management.Crossref | GoogleScholarGoogle Scholar |
Pattison AB, East D, Ferro K, Dickinson G (2018b) Agronomic consequences of vegetative groundcovers and reduced nitrogen applications for banana production systems. Acta Horticulturae 1196, 155–162.
| Agronomic consequences of vegetative groundcovers and reduced nitrogen applications for banana production systems.Crossref | GoogleScholarGoogle Scholar |
Peng HX, Sivasithamparam K, Turner DW (1999) Chlamydospore germination and Fusarium wilt of banana plantlets in suppressive and conducive soils are affected by physical and chemical factors. Soil Biology and Biochemistry 31, 1363–1374.
| Chlamydospore germination and Fusarium wilt of banana plantlets in suppressive and conducive soils are affected by physical and chemical factors.Crossref | GoogleScholarGoogle Scholar |
Prasertsak P, Freney JR, Saffigna PG, Denmead OT, Prove BG (2001) Fate of urea nitrogen applied to a banana crop in the wet tropics of Queensland. Nutrient Cycling in Agroecosystems 59, 65–73.
| Fate of urea nitrogen applied to a banana crop in the wet tropics of Queensland.Crossref | GoogleScholarGoogle Scholar |
R Core Team (2017) ‘R: a language and environment for statistical computing.’ (R Foundation for Statistical Computing: Vienna, Austria)
Rasiah V, Armour JD (2001) Nitrate accumulation under cropping in the Ferrosols of Far North Queensland wet tropics. Soil Research 39, 329–341.
| Nitrate accumulation under cropping in the Ferrosols of Far North Queensland wet tropics.Crossref | GoogleScholarGoogle Scholar |
Rasiah V, Armour JD, Moody PW, Pattison AB, Lindsay S, Florentine S (2009) Characterising and improving the deteriorating trends in soil physical quality under banana. Soil Research 47, 574–584.
| Characterising and improving the deteriorating trends in soil physical quality under banana.Crossref | GoogleScholarGoogle Scholar |
Rasiah V, Armour JD, Cogle AL, Florentine SK (2010) Nitrate import–export dynamics in groundwater interacting with surface-water in a wet-tropical environment. Soil Research 48, 361–370.
| Nitrate import–export dynamics in groundwater interacting with surface-water in a wet-tropical environment.Crossref | GoogleScholarGoogle Scholar |
Rayment GE, Lyons DJ (2011) ‘Soil chemical methods: Australasia.’ (CSIRO Publishing: Melbourne, Vic., Australia)
Reuter D, Robinson JB (1997) ‘Plant analysis: an interpretation manual.’ (CSIRO Publishing: Melbourne, Vic., Australia)
Shen Z, Penton CR, Lv N, Xue C, Yuan X, Ruan Y, Li R, Shen Q (2018) Banana Fusarium wilt disease incidence is influenced by shifts of soil microbial communities under different monoculture spans. Microbial Ecology 75, 739–750.
| Banana Fusarium wilt disease incidence is influenced by shifts of soil microbial communities under different monoculture spans.Crossref | GoogleScholarGoogle Scholar |
Singh B, Schulze DG (2015) Soil minerals and plant nutrition. Nature Education Knowledge 6, 1
Smalla K, Wieland G, Buchner A, Zock A, Parzy J, Kaiser S, Roskot N, Heuer H, Berg G (2001) Bulk and rhizosphere soil bacterial communities studied by denaturing gradient gel electrophoresis: plant-dependent enrichment and seasonal shifts revealed. Applied and Environmental Microbiology 67, 4742–4751.
| Bulk and rhizosphere soil bacterial communities studied by denaturing gradient gel electrophoresis: plant-dependent enrichment and seasonal shifts revealed.Crossref | GoogleScholarGoogle Scholar |
Stotzky G, Torrence Martin R (1963) Soil mineralogy in relation to the spread of Fusarium wilt of banana in central America. Plant and Soil 18, 317–337.
| Soil mineralogy in relation to the spread of Fusarium wilt of banana in central America.Crossref | GoogleScholarGoogle Scholar |
Stotzky G, Dawson JE, Martin RT, Ter Kuile CHH (1961) Soil mineralogy as factor in spread of Fusarium wilt of banana. Science 133, 1483–1485.
| Soil mineralogy as factor in spread of Fusarium wilt of banana.Crossref | GoogleScholarGoogle Scholar |
Turner DW, Lahav E (1983) The growth of banana plants in relation to temperature. Functional Plant Biology 10, 43–53.
| The growth of banana plants in relation to temperature.Crossref | GoogleScholarGoogle Scholar |


