Pre-germination treatment with hydrogen peroxide as a controlled elicitation strategy to improve chemical properties of hydroponic barley fodder
E. A. Delis-Hechavarría A , R. G. Guevara-González A , R. V. Ocampo-Velazquez A , J. G. Gómez-Soto B , E. G. Tovar-Pérez A , J. F. García-Trejo A and I. Torres-Pacheco A C
A C
A Engineering Faculty, Campus Amazcala, Autonomous University of Querétaro, Carretera Chichimequillas s/n Km 1, El Marqués, Querétaro, México CP 76265.
B Natural Science College, Autonomous University of Querétaro, Carretera Chichimequillas s/n Km 1, El Marqués, Querétaro, México CP 76265.
C Corresponding author. Email: irineo.torres@uaq.mx
Crop and Pasture Science 72(10) 815-822 https://doi.org/10.1071/CP21082
Submitted: 21 February 2021 Accepted: 24 June 2021 Published: 18 September 2021
Journal Compilation © CSIRO 2021 Open Access CC BY-NC-ND
Abstract
Barley (Hordeum vulgare L.) is one of the most used species for hydroponic green fodder. The chemical properties of fodders may be enhanced through use of various strategies during crop production, including stress applications. In this context, hydrogen peroxide (H2O2) is used as a stress factor in controlled elicitation, a technique used to increase secondary metabolites in food. The aim of this research was to evaluate the possibility of using controlled elicitation with H2O2 for enhancing the chemical properties of hydroponic barley fodder. H2O2 was applied to the seeds as a pre-germination treatment at three concentrations: 50, 100 and 150 mM. Morphological changes, enzymatic defence-related activities (superoxide dismutase, catalase, chalcone isomerase, phenylalanine ammonia-lyase), total phenolics content, and antioxidant capacity were evaluated. Significant increases in total phenolics content, phenylalanine ammonia-lyase activity and ABTS antioxidant capacity were obtained when seeds were treated with 50 mM H2O2. Plant growth was promoted with 100 mM and 150 mM H2O2 treatments, and germination of seeds with 100 mM H2O2. It was established that controlled elicitation using H2O2 increased some morphological and biochemical variables of hydroponic barley fodder related to food properties.
Keywords: controlled elicitation, enzymatic activities, phytochemicals, antioxidant capacity, chemical properties, fodder, secondary metabolites, nutritive value.
Introduction
Barley (Hordeum vulgare L.) is an important food and fodder resource globally, along with the other cereals maize, rice and wheat (Ghaffari et al. 2016). Barley is abundant in metabolites such as carbohydrates, protein and lipids, as well as minerals, dietary fibre, vitamins B and E and high levels of antioxidants (Hussain et al. 2019). This grain has important levels of dietary fibre and phytochemical compounds, among them flavonoids, folates, phenolic acids, lignans, tocols, phytosterols and folates (Idehen et al. 2017). Among these, the phenolic compounds, β-glucans, arabinoxylans are recognised for their positive role in human health (Sher et al. 2017).
Barley hydroponic green fodder is considered a sustainable feed option that could reduce feeding cost and increase product quality. With hydroponic technology, 8 kg of green fodder can be obtained from 1 kg of seed in a week (Abouelezz et al. 2019). The process used in hydroponic green fodder production increases protein content and availability owing to the sprouting grains converting complex proteins into amino acids and other compounds; the vitamin content is also enhanced as well as the availability of some minerals such as iron and phosphorus (Abouelezz et al. 2019).
Application of exogenous stress could influence plant growth, yield and stress tolerance; moreover, it has been proven to raise the production of specialised metabolites through secondary metabolism (Kaburagi et al. 2015; Ding et al. 2017). However, these effects depend on time of exposure and dose of stress application (Gorelick and Bernstein 2014; Vázquez-Hernández et al. 2019b). This phenomenon, called ‘hormesis’, refers to the application of a stress factor to an organism, generating beneficial effects (eustress) at low dose and toxic ones (distress) at high dose (Kacienė et al. 2015; Vargas-Hernandez et al. 2017). For this reason, it is desirable to test a broad spectrum of dosages of a stress factor, in order to place those dosages on the hormetic curve and thus determine whether a specified dosage is beneficial for our purposes (Vázquez-Hernández et al. 2019b).
This knowledge can be applied as a controlled elicitation technique to increase the production of specialised metabolites in crops (Garcia-Mier et al. 2014; Biczak et al. 2016). Controlled elicitation uses stress factors at specific dosages and application times to increase bioactive contents without negative effects on plant behaviour. For example, exogenous applications of salicylic acid to kidney beans improved seedling growth and total phenolics content (Limón et al. 2014). There are also examples of soybean seeds given pre-germination treatment with salicylic acid and broccoli seeds given chitosan, which improved seedling growth and the nutritional properties (Anaya et al. 2018). In the case of barley, exogenous applications of hydrogen peroxide (H2O2) at 0.25 M salt concentration had positive effects on seed germination index and vigour index but decreased biomass production (Kilic and Kahraman 2016). In addition, applications of ozone (O3) gas at 180 and 360 µg m−3 enhanced antioxidative enzymes and diminished dry biomass of shoots (Kacienė et al. 2015).
The controlled elicitation technique has been useful for increasing the content of secondary metabolites in several species including barley (Kacienė et al. 2015; Holub et al. 2019; Vazquez-Hernandez et al. 2019a; Parola-Contreras et al. 2020). In the case of the sprouts and fodders, this technique can be useful for enhancing the chemical properties, and so may be an appropriate option for human food and animal feed as was found for common beans (Mendoza-Sánchez et al. 2016), soybean (Anaya et al. 2018) and barley (Kacienė et al. 2015; Kilic and Kahraman 2016).
With the aim of finding a favourable strategy to provide animals with foods of high beneficial bioactive content and antioxidant properties, this research evaluated the effect of controlled elicitation, using H2O2, on the chemical properties of hydroponic barley fodder. To our knowledge, this is the first time that H2O2 has been applied as a pre-germination elicitor treatment for barley seeds.
Materials and methods
Plant material, growth conditions and stress treatments
Seeds of barley var. Cantabria were given pre-germination treatment for 24 h in pots that contained H2O2 (Golden Bell, India) at concentrations of 50 mM, 100 mM and 150 mM, with distilled water as the control. Seeds were drained and put in trays 54 cm by 35 cm by 7 cm, and these trays were placed in a fodder growth chamber for 11 days. At 24, 48 and 72 h after sowing, a sample was taken of 100 seeds per try and the germination percentage was determined. Conditions during the experiment were: temperature 25°C, photoperiod 14 h light/10 h dark, and relative humidity 80%. Seedlings were watered (no nutrients) six times per day for 2 min each time. On Day 11 after sowing, plant morphological variables were determined: stem height (SH, mm), measured with a graduated ruler; root length (RL, mm), measured with a graduated ruler; plant fresh weight (PW, g), determined with a balance (Etekcity, Anaheim, CA, USA). Fodder samples (leaves and roots) were collected on Day 11 and immediately frozen in liquid nitrogen and stored at −80°C for analysis.
Determination of antioxidant activity
Antioxidant activity in each treatment was evaluated through the 1,1-diphenyl-2-picrylhydrazyl (DPPH•) and 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS•+) tests.
DPPH• radical scavenging activity (DRSA)
The DRSA was determined according to the method of Chen et al. (2012), with some modifications. An aliquot (0.5 mL) of sample solution (600 µg mL−1) was added to 0.5 mL DPPH• radical (0.1 mM) in methanol solution. The mixture was shaken vigorously and incubated for 30 min in the dark at room temperature. The absorbance was measured at 515 nm. Methanol, instead of DPPH•, was used for the blank, and deionised water, instead of sample, was used for the control. DRSA was calculated by the following Eqn 1:

ABTS•+ radical scavenging activity (ARSA)
The ARSA was determined according to the method of Re et al. (1999), with some modifications. The ABTS•+ radical cation was generated by mixing ABTS stock solution (7 mM) with potassium persulfate (2.45 mM), and the resulting mixture was rested in the dark at room temperature for 12 h before use. The ABTS•+ radical solution was diluted in phosphate buffered saline (0.15 M, pH 7.4) at ~1:35 to obtain an absorbance of 0.70 ± 0.02 at 734 nm. This diluted solution (~3 mL) was mixed with an aliquot (150 µL) of sample solution (600 µg mL−1). The absorbance was read from 1 and up to 6 min after the initial mixing, under conditions of darkness. Phosphate buffered saline, instead of ABTS•+, was used for the blank, and deionised water, instead of the sample, was used for the control. ARSA was calculated by the following Eqn 2:

Enzyme activity assays
Sample preparation of enzymatic assays
Barley fodder dried samples (0.5 g) were homogenised with cold extraction buffer (1 mL) and the slurry centrifuged at 10188g for 20 min at 4°C. The supernatants were used to determine enzyme activities. Protein concentration in enzymatic extracts was determined according to the method reported by Bradford (1976), using bovine serum albumin as a standard for protein concentration (Sigma-Aldrich, St. Louis, MO, USA).
Superoxide dismutase (SOD) activity assay
The activity of SOD (EC 1.15.1.1) was assessed by the inhibition of photochemical reduction of nitro blue tetrazolium (NBT) according to the method described by Hayat et al. (2018). The reaction was performed by adding 1.5 mL 50 mM potassium phosphate buffer (pH 7.8), 0.3 mL 0.1 mM EDTA, 0.3 mL 0.13 M methionine, 0.3 mL 0.02 mM riboflavin, 0.05 mL enzymatic extract and 0.25 mL distilled water. The mixture was exposed to fluorescent light (86.86 μmol m–2 s–1) for 20 min. Subsequently, the solution absorbance was measured at 560 nm; 1 U SOD inhibits reduction of NBT by 50% at pH 7.8 and 25°C. Data are expressed as U mg–1 protein.
Catalase (CAT) activity assay
The activity of CAT (EC 1.11.1.6) was spectrophotometrically assessed by measuring the rate of H2O2 decrease at 240 nm according to the method described by Afiyanti and Chen (2014). The reaction was performed by adding 0.95 mL 50 mM potassium phosphate buffer (pH 8.0), 0.05 mL enzymatic extract and 0.1 mL 100 mM H2O2. The change in absorbance at 240 nm was measured for 1 min and used to determine the rate of decomposition of H2O2 by CAT; 1 U CAT decomposes 1 µmol H2O2 min–1 at pH 8.0 and 25°C. Data are expressed as U mg–1 protein (Afiyanti and Chen 2014).
Phenylalanine ammonia-lyase (PAL) activity
The activity of PAL (EC 4.3.1.5) was spectrophotometrically assessed according to the method described by Toscano et al. (2018). l-Phenylalanine was used as the substrate, and the released cinnamic acid was quantified by absorbance at 290 nm. The reaction was performed by adding 2.3 mL 0.1 M borate buffer (pH 8.8 containing 10 mM l-phenylalanine) and 0.2 mL enzymatic extract. The mixture was incubated at 40°C for 1 h and the reaction was stopped by the addition of 0.5 mL 5 N HCl. Subsequently, the solution absorbance was measured at 290 nm; 1 U PAL releases 1 µmol cinnamic acid min–1 at pH 8.8 and 40°C. Data are expressed as U mg–1 protein.
Chalcone isomerase (CHI)
The activity of CHI was measured by using the methods proposed by Dixon et al. (1982) and Mol et al. (1985). The reaction was performed by adding 2.5 µL substrate solution (1.0 mg naringenin chalcone mL–1 ethanol), 2.5 mL sulfate potassium buffer and 2.5 µL enzymatic extract (300 g sample and 2.0 mL extraction buffer). The extraction buffer consisted of 0.1 M sodium phosphate, pH 8, and 1.4 mM 2-mercaptonol. The absorbance was determined on the spectrophotometer at 400 nm during six time intervals (15 s, 30 s, 60 s, 120 s, 240 s and 280 s).
Total phenolic compounds
The hydroponic green fodder from each treatment was sampled and ground in liquid nitrogen. Ground samples of fodder were macerated with methanol in a 1:10 ratio (w/v). The suspensions were collocated in a sonicator (Bransonic M2800-CPX-HE; Emerson, Ferguson, MO, USA) for 30 min and centrifuged at 5590g for 15 min at 4°C. The resultant supernatants were frozen at 4°C in vials in dark conditions until use.
Total phenols were determined spectrophotometrically by the Folin–Ciocalteu method as reported by Vergara-Castañeda et al. (2010), using gallic acid as a standard. Briefly, 140 µL extract was mixed with 460 µL distilled water and 250 µL Folin–Ciocalteu (1 N) reagent. After 5 min, 1250 µL 20% (w/v) sodium carbonate was added. The mixture was shaken in the vortex and incubated for 2 h in darkness. Absorbance was measured at 760 nm. Results are expressed as mg equivalent gallic acid (EGA) g–1 fresh weight.
Statistical analyses
A randomised completely block experimental design was used to evaluate the effect of the pre-germination treatment of seed on morphological changes, antioxidant activity, phenolic compounds and enzymatic activity. The arrangement of the experiment was four treatments with three blocks and three experimental units per treatment. Data was subjected to analysis of variance (ANOVA) and Tukey test (α = 0.05), using the Statgraphics Centurion XVI statistical software (StatPoint Technologies, Bedford, MA, USA).
Results
Germination percentage
Germination percentages on trays at 24, 48 and 72 h after sowing are presented in Fig. 1. After 24 h, the 100 mM H2O2 treatment showed significantly higher (P < 0.05) germination percentage than the other treatments. After 48 h, the 100 mM H2O2 treatment gave the highest germination percentage, although not significantly different (P > 0.05) from the 50 mM H2O2 treatment. Finally, at 72 h, the 100 mM H2O2 treatment again gave the highest germination percentage, although not significantly different from 150 mM H2O2.
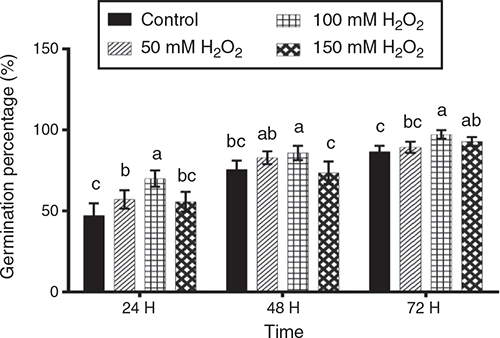
|
Morphological responses
Leaf height was significantly greater (P < 0.05) in the 150 mM H2O2 treatment than the control and 50 mM H2O2 treatments; the 100 mM H2O2 treatment was intermediate and not significantly different (P > 0.05) from any other treatment (Fig. 2a). Plant weight was significantly greater (P < 0.05) in the 150 mM H2O2 treatment than in the remaining treatments (Fig. 2b). Pre-germination treatment with H2O2 had no significant effect (P > 0.05) on root length (data not presented).
Antioxidant activity
Natural antioxidants act as free radical scavengers and reduction agents. These compounds express a wide variety of biological effects in barley (Madhujith et al. 2006), and the DPPH and ABTS antioxidant activity can be associated with phenols content. With regard to ARSA (Fig. 3), the 50 mM H2O2 treatment had the highest antioxidant capacity, although not significantly different (P > 0.05) from the 100 mM treatment. The 150 mM H2O2 treatment showed significantly lower (P < 0.05) antioxidant capacity than the other two H2O2 treatments. The control was intermediate between 100 mM and 150 mM treatments. Pre-germination treatment with H2O2 had no significant effect (P > 0.05) on DRSA.
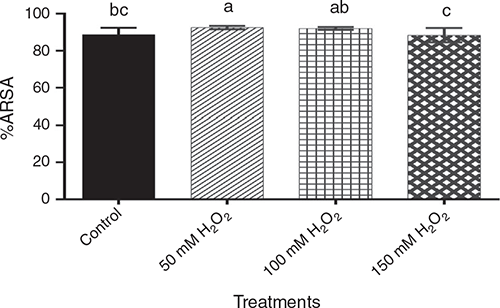
|
Enzymatic activity assays
Enzymatic activity is associated with the activation of defence response to diverse stresses in plants. Some enzymes such as POD (peroxidase), SOD, CAT, PAL and CHI could increase their activity as a response to application of an elicitor (Mejía-Teniente et al. 2013). Pre-germination treatment of barley seed with H2O2 induced variation in SOD, CAT and PAL activities (Fig. 4), whereas CHI activity was not affected. Stress application triggers the activation of NADPH oxidase and O2–, which is quickly converted by SOD into H2O2, followed by the activation of another reactive oxygen species (ROS)-scavenging enzyme such CAT and POD (Tamás et al. 2010). SOD activity was significantly lower (P < 0.05) in the 50 mM H2O2 treatment than the 100 mM H2O2 and control treatments; the 150 mM H2O2 treatment was intermediate. CAT activity was significantly higher (P < 0.05) in the 100 mM H2O2 treatment than all other treatments, which did not differ (P > 0.05) from each other. Finally, PAL activity was highest (P < 0.05) in the 50 mM H2O2 treatment, followed by 100 mM H2O2 treatment, then the 150 mM H2O2 and control treatments.
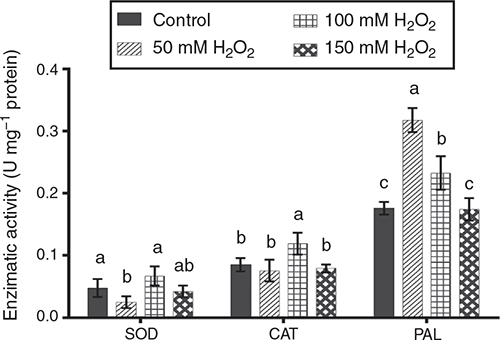
|
Total phenolic compounds
For total phenols, the data showed significant (P < 0.05) differences among pre-germination treatments (Fig. 5), being highest in the 50 mM H2O2 treatment, followed by 100 mM H2O2 treatment, then the 150 mM H2O2 treatment, which was statistically similar to the control.
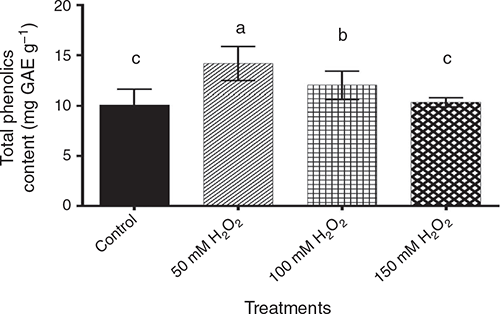
|
Discussion
Hydrogen peroxide can enhance environmental stress tolerance; it reduces the negative effects of stress over germination and improves growth parameters, activating the plant immune system in barley (Hussain et al. 2019). Therefore, barley could respond positively to pre-germination treatment with H2O2. Furthermore, H2O2 has a growth promoting effect because the pretreatment of the seeds relieves latency (Bahin et al. 2011). Significant difference in the length of barley acrospires is found with of exogenous application of H2O2, which is highly correlated with the regulation of growth (Baldus et al. 2020). According to those authors, it can be inferred that H2O2 is naturally accumulated during the process of germination, inhibiting gibberellic acid (GA) catabolism and simultaneously promoting its biosynthesis. The germination process seems to be a consequence of growth and elongation of cells overcoming dormancy.
Likewise, H2O2 is a powerful molecule signalling transduction related to external stimuli (Petrov and Van Breusegem 2012). Fodder, as feed for cattle, produced after pre-germination treatment with H2O2 can help to improve the quality of the products such as meat and milk (Chen et al. 2017). For example, the use of H2O2 increases the shelf life of cow milk (Ivanova et al. 2019). In the same sense, H2O2 is added to activate the natural enzyme lactoperoxidase, which has antimicrobial activity and increases the shelf life of pasteurised milk; this treatment is allowed in only a few countries, but it can be used in the case of cheese production (Lima et al. 2020). Moreover, the antioxidant properties in the milk protein reduce ROS such as hydroxyl radicals, peroxide radicals and superoxide radicals (Khan et al. 2019).
Our results show that the antioxidant capacity that featured in the 50 mM H2O2 treatment is dependent on the production of phenolic compounds, as reinforced by PAL activity; the 50 mM H2O2 treatment showed significantly higher PAL activity and total phenols than other treatments. The ABTS antioxidant capacity and total phenols were the result of PAL enzymatic activity. Besides, it is known that the total phenolic compound activity in plants is responsible for the antioxidant activity (Ren and Sun 2014).
On the other hand, the antioxidant activity induced with the 50 mM H2O2 treatment in general caused a reduction in plant growth promotion relative to 100 mM and 150 mM H2O2 treatments and in germination of seeds relative to the 100 mM H2O2 treatment. The induction of phenolic production in some way redirected metabolism to this activity, to the detriment of growth. Greater antioxidant plant capacity related to secondary metabolite production, in this case phenolic compounds, has certain effects on plant growth (Jamwal et al. 2018).
Natural forages with high antioxidant content reduce oxidative stress mechanisms that are behind pathologies such as cardiac, digestive and respiratory diseases, bringing benefits to health and meat production of livestock (Vizzari et al. 2021).
Activity of SOD was highest in the 100 mM H2O2 treatment, although it was probably due to the stress action; there was not a high induction of phenol synthesis, and so, no adverse effect on plant growth, as also seen with 150 mM H2O2. SOD and CAT are enzymes that respectively increase and decrease the quantity of H2O2 in plants, and they are related to antioxidant capacity (Gupta et al. 2018). CAT is the key enzyme in the catalytic scavenging of H2O2 to oxygen and water, and usually, an increase of the CAT activity improves the antioxidant defence in plants; however, plant growth can be negatively affected (Gupta et al. 2018).
The treatment with 100 mM H2O2 significantly increased the germination rate of barley seeds. H2O2 in low concentrations promotes an improvement in the germination process through the breaking of seed dormancy (Çavuşoğlu and Kabar 2010). Those authors found in barley that treatment with 30 mM H2O2 accelerated germination at 20°C to 84% on the second day, and that it maintained growth parameters at 25°C, such as radicle length (1.03-fold) and fresh weight (1.04-fold). In that study, the same dose of H2O2 ameliorated effects of 0.30 M NaCl at 20°C, improving germination 7.11-fold on the second day, radicle length 1.34-fold and fresh weight 1.15-fold.
Kilic and Kahraman (2016) reported the highest vigour index, germination index, coleoptile length and fresh weight when barley received pre-germination treatment with 0.3 µM H2O2. In addition, H2O2 pre-germination treatment had positive effects on sizes and count of photosynthetic apertures of leaves and on plant growth. Exogenous H2O2 applications can enhance root growth, aiding development of a vigorous root system, as well as reducing germination time and plant development under stress conditions (Cesur and Tabur 2011).
In the present study, the reduction of ABTS•+ and DPPH• radicals occurred through methanolic extract of H2O2 treatments that presented antioxidant activity. The transfer of electrons existing in the phenolic compounds counteracts the free radicals. From the results, ARSA could be regulated by the composition of the phenols present in the extracts. The degree of antioxidant activity is correlated with the number of hydroxyl groups; this also is connected with biological activity such as avoidance of a few types of cancer, cardiovascular diseases and antioxidative injuries in the organism (Holub et al. 2019). The stress treatments affected the enzymatic activities, which in turn caused a chain reaction of the phenylpropanoid pathway (Fig. 4). The increase in PAL can directly affect the number of phenolic compounds throughout the development of barley.
The antioxidant enzyme system is involved in ROS scavenging. Plant development has a balance of antioxidants and pro-oxidants; a minimum change in this balance triggers the cellular response, including SOD, CAT, POD and POX (guaiacol peroxidase) (Caverzan et al. 2016). SOD is the most important enzyme against ROS, the main function being to eliminate the superoxide radical (O2–) by conversion into H2O2 and O2 (Kaya et al. 2015). Elevated ROS synthesis is a component of stresses including oxidative stress (H2O2) as a result of biotic and abiotic stress factors (Tamás et al. 2010).
Tamás et al. (2010) showed that the response of barley root in vitro to 10 mM H2O2 was an increased synthesis of O2–, activation of SOD and elevated H2O2 formation. Triggering O2– occurred possibly through the activation of NADPH oxidase. By contrast, our experiment resulted in decreased SOD activity in barley with pre-germination treatment; this response could be due increased CAT activity, ameliorating excessive H2O2 in the fodder, and avoiding SOD activity.
Furthermore, increased CAT activity could positively influence seed germination and seedling growth stages (Kilic and Kahraman 2016). A direct relationship was found between morphological growth and enzyme activity. PAL activity in Capsicum annuum L. showed significant difference 5 min post-application of 18 mM H2O2, after 1 day at 14 mM H2O2; however, at the fifth day, major PAL activity was registered with the 6 mM H2O2 treatment (Mejía-Teniente et al. 2013).
Conclusions
Based on the above, it can be concluded that the pre-germination treatment of barley significantly increased morphological growth and triggered the adaptive process. In addition, barley seeds treated at 50 mM H2O2 showed enhanced biochemical mechanisms such as PAL activity, total phenol content and ABTS antioxidant activity as seedlings. Higher activities of antioxidant defence processes correlated with the phenolic compounds and antioxidative activity under stress conditions. Pre-germination application in barley hydroponic green fodder may be an option to enhance the food and feed properties for humans and livestock. Finally, it was established that controlled elicitation using H2O2 increased some morphological and biochemical variables of hydroponic barley fodder related to food properties.
Conflict of interest
The authors declare that they have no conflict of interests.
Data availability statement
The data that support this study are available online as supplementary material.
Acknowledgement
We acknowledge CONACYT for the scholarship granted to Emilio A. Delis Hechavarría (Grant no. 807820). CONACYT was not involved in the preparation of the manuscript, or in the decision to submit the article for publication.
References
Abouelezz KFM, Sayed MAM, Abdelnabi MA (2019) Evaluation of hydroponic barley sprouts as a feed supplement for laying Japanese quail: effects on egg production, egg quality, fertility, blood constituents, and internal organs. Animal Feed Science and Technology 252, 126–135.| Evaluation of hydroponic barley sprouts as a feed supplement for laying Japanese quail: effects on egg production, egg quality, fertility, blood constituents, and internal organs.Crossref | GoogleScholarGoogle Scholar |
Afiyanti M, Chen HJ (2014) Catalase activity is modulated by calcium and calmodulin in detached mature leaves of sweet potato. Journal of Plant Physiology 171, 35–47.
| Catalase activity is modulated by calcium and calmodulin in detached mature leaves of sweet potato.Crossref | GoogleScholarGoogle Scholar | 24331417PubMed |
Anaya F, Fghire R, Wahbi S, Loutfi K (2018) Influence of salicylic acid on seed germination of Vicia faba L. under salt stress. Journal of the Saudi Society of Agricultural Sciences 17, 1–8.
| Influence of salicylic acid on seed germination of Vicia faba L. under salt stress.Crossref | GoogleScholarGoogle Scholar |
Bahin E, Bailly C, Sotta B, Kranner I, Corbineau F, Leymarie J (2011) Crosstalk between reactive oxygen species and hormonal signalling pathways regulates grain dormancy in barley. Plant, Cell & Environment 34, 980–993.
| Crosstalk between reactive oxygen species and hormonal signalling pathways regulates grain dormancy in barley.Crossref | GoogleScholarGoogle Scholar |
Baldus M, Heukäufer F, Großpietsch C, Methner FJ (2020) Accumulation of hydrogen peroxide in barley seeds: a key factor for malt quality? Journal of the American Society of Brewing Chemists 79, 1–15.
| Accumulation of hydrogen peroxide in barley seeds: a key factor for malt quality?Crossref | GoogleScholarGoogle Scholar |
Biczak R, Pawłowska B, Telesiński A, Ciesielski W (2016) The effect of the number of alkyl substituents on imidazolium ionic liquids phytotoxicity and oxidative stress in spring barley and common radish seedlings. Chemosphere 165, 519–528.
| The effect of the number of alkyl substituents on imidazolium ionic liquids phytotoxicity and oxidative stress in spring barley and common radish seedlings.Crossref | GoogleScholarGoogle Scholar | 27681108PubMed |
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72, 248–254.
| A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding.Crossref | GoogleScholarGoogle Scholar | 942051PubMed |
Caverzan A, Casassola A, Brammer SP (2016) Antioxidant responses of wheat plants under stress. Genetics and Molecular Biology 39, 1–6.
| Antioxidant responses of wheat plants under stress.Crossref | GoogleScholarGoogle Scholar | 27007891PubMed |
Çavuşoğlu K, Kabar K (2010) Effects of hydrogen peroxide on the germination and early seedling growth of barley under NaCl and high temperature stresses. EurAsian Journal of Biosciences 79, 70–79.
| Effects of hydrogen peroxide on the germination and early seedling growth of barley under NaCl and high temperature stresses.Crossref | GoogleScholarGoogle Scholar |
Cesur A, Tabur S (2011) Chromotoxic effects of exogenous hydrogen peroxide (H2O2) in barley seeds exposed to salt stress. Acta Physiologiae Plantarum 33, 705–709.
| Chromotoxic effects of exogenous hydrogen peroxide (H2O2) in barley seeds exposed to salt stress.Crossref | GoogleScholarGoogle Scholar |
Chen N, Yang H, Sun Y, Niu J, Liu S (2012) Purification and identification of antioxidant peptides from walnut (Juglans regia L.) protein hydrolysates. Peptides 38, 344–349.
| Purification and identification of antioxidant peptides from walnut (Juglans regia L.) protein hydrolysates.Crossref | GoogleScholarGoogle Scholar | 23022588PubMed |
Chen X, Zhang L, Li J, Gao F, Zhou G (2017) Hydrogen peroxide-induced change in meat quality of the breast muscle of broilers is mediated by ROS generation, apoptosis, and autophagy in the NF-κB signal pathway. Journal of Agricultural and Food Chemistry 65, 3986–3994.
| Hydrogen peroxide-induced change in meat quality of the breast muscle of broilers is mediated by ROS generation, apoptosis, and autophagy in the NF-κB signal pathway.Crossref | GoogleScholarGoogle Scholar | 28447793PubMed |
Ding Z, Jia S, Wang Y, Xiao J, Zhang Y (2017) Phosphate stresses affect ionome and metabolome in tea plants. Plant Physiology and Biochemistry 120, 30–39.
| Phosphate stresses affect ionome and metabolome in tea plants.Crossref | GoogleScholarGoogle Scholar | 28982053PubMed |
Dixon RA, Dey PM, Whitehead IM (1982) Purification and properties of chalcone isomerase from cell suspension cultures of Phaseolus vulgaris. Biochimica et Biophysica Acta - General Subjects 715, 25–33.
| Purification and properties of chalcone isomerase from cell suspension cultures of Phaseolus vulgaris.Crossref | GoogleScholarGoogle Scholar |
Garcia-Mier L, Jimenez-Garcia SN, Chapa-Oliver AM, Mejia-Teniente L, Ocampo-Velazquez RV, Rico-García E, Feregrino-Pérez AA, Guevara-Gonzalez R, Torres-Pacheco I (2014) Strategies for sustainable plant food production: facing the current agricultural challenges. Agriculture for today and tomorrow. In ‘Biosystems engineering: biofactories for food production in the Century XXI’. (Eds R Guevara-Gonzalez, I Torres-Pacheco) pp. 1–50. (Springer International Publishing: Cham, Switzerland)
Ghaffari MR, Shahinnia F, Usadel B, Junker B, Schreiber F, Sreenivasulu N, Hajirezaei MR (2016) The metabolic signature of biomass formation in barley. Plant & Cell Physiology 57, 1943–1960.
| The metabolic signature of biomass formation in barley.Crossref | GoogleScholarGoogle Scholar |
Gorelick J, Bernstein N (2014) Elicitation: an underutilized tool in the development of medicinal plants as a source of therapeutic secondary metabolites. In ‘Advances in agronomy’. pp. 201–230. (Elsevier)
Gupta DK, Palma JM, Corpas FJ (2018) ‘Antioxidants and antioxidant enzymes in higher plants.’ (Springer)
Hayat S, Ahmad H, Ali M, Ren K, Cheng Z (2018) Aqueous garlic extract stimulates growth and antioxidant enzymes activity of tomato (Solanum lycopersicum). Scientia Horticulturae 240, 139–146.
| Aqueous garlic extract stimulates growth and antioxidant enzymes activity of tomato (Solanum lycopersicum).Crossref | GoogleScholarGoogle Scholar |
Holub P, Nezval J, Štroch M, Špunda V, Urban O, Jansen MAK, Klem K (2019) Induction of phenolic compounds by UV and PAR is modulated by leaf ontogeny and barley genotype. Plant Physiology and Biochemistry 134, 81–93.
| Induction of phenolic compounds by UV and PAR is modulated by leaf ontogeny and barley genotype.Crossref | GoogleScholarGoogle Scholar | 30143263PubMed |
Hussain I, Parveen A, Rasheed R, Ashraf MA, Ibrahim M, Riaz S, Afzaal Z, Iqbal M (2019) Exogenous silicon modulates growth, physio-chemicals and antioxidants in barley (Hordeum vulgare L.) exposed to different temperature regimes. Silicon 11, 2753–2762.
| Exogenous silicon modulates growth, physio-chemicals and antioxidants in barley (Hordeum vulgare L.) exposed to different temperature regimes.Crossref | GoogleScholarGoogle Scholar |
Idehen E, Tang Y, Sang S (2017) Bioactive phytochemicals in barley. Journal of Food and Drug Analysis 25, 148–161.
| Bioactive phytochemicals in barley.Crossref | GoogleScholarGoogle Scholar | 28911532PubMed |
Ivanova AS, Merkuleva AD, Andreev SV, Sakharov KA (2019) Method for determination of hydrogen peroxide in adulterated milk using high performance liquid chromatography. Food Chemistry 283, 431–436.
| Method for determination of hydrogen peroxide in adulterated milk using high performance liquid chromatography.Crossref | GoogleScholarGoogle Scholar | 30722894PubMed |
Jamwal K, Bhattacharya S, Puri S (2018) Plant growth regulator mediated consequences of secondary metabolites in medicinal plants. Journal of Applied Research on Medicinal and Aromatic Plants
| Plant growth regulator mediated consequences of secondary metabolites in medicinal plants.Crossref | GoogleScholarGoogle Scholar |
Kaburagi E, Yamada M, Fujiyama H (2015) Sodium, but not potassium, enhances root to leaf nitrate translocation in Swiss chard (Beta vulgaris var. cicla L.). Environmental and Experimental Botany 112, 27–32.
| Sodium, but not potassium, enhances root to leaf nitrate translocation in Swiss chard (Beta vulgaris var. cicla L.).Crossref | GoogleScholarGoogle Scholar |
Kacienė G, Milčė JŽE, Juknys R (2015) Role of oxidative stress on growth responses of spring barley exposed to different environmental stressors. Journal of Plant Ecology 8, rtv026
| Role of oxidative stress on growth responses of spring barley exposed to different environmental stressors.Crossref | GoogleScholarGoogle Scholar |
Kaya C, Ashraf M, Sönmez O, Tuna AL, Aydemir S (2015) Exogenously applied nitric oxide confers tolerance to salinity-induced oxidative stress in two maize (Zea mays L.) cultivars differing in salinity tolerance. Turkish Journal of Agriculture and Forestry 39, 909–919.
| Exogenously applied nitric oxide confers tolerance to salinity-induced oxidative stress in two maize (Zea mays L.) cultivars differing in salinity tolerance.Crossref | GoogleScholarGoogle Scholar |
Khan IT, Nadeem M, Imran M, Ullah R, Ajmal M, Jaspal MH (2019) Antioxidant properties of milk and dairy products: a comprehensive review of the current knowledge. Lipids in Health and Disease 18, 41
| Antioxidant properties of milk and dairy products: a comprehensive review of the current knowledge.Crossref | GoogleScholarGoogle Scholar | 30717735PubMed |
Kilic S, Kahraman A (2016) The mitigation effects of exogenous hydrogen peroxide when alleviating seed germination and seedling growth inhibition on salinity-induced stress in barley. Polish Journal of Environmental Studies 25, 1053–1059.
| The mitigation effects of exogenous hydrogen peroxide when alleviating seed germination and seedling growth inhibition on salinity-induced stress in barley.Crossref | GoogleScholarGoogle Scholar |
Lima MJA, Sasaki MK, Marinho OR, Freitas TA, Faria RC, Reis BF, Rocha FRP (2020) Spot test for fast determination of hydrogen peroxide as a milk adulterant by smartphone-based digital image colorimetry. Microchemical Journal 157, 105042
| Spot test for fast determination of hydrogen peroxide as a milk adulterant by smartphone-based digital image colorimetry.Crossref | GoogleScholarGoogle Scholar |
Limón RI, Peñas E, Martínez-Villaluenga C, Frias J (2014) Role of elicitation on the health-promoting properties of kidney bean sprouts. Lebensmittel-Wissenschaft + Technologie 56, 328–334.
| Role of elicitation on the health-promoting properties of kidney bean sprouts.Crossref | GoogleScholarGoogle Scholar |
Madhujith T, Izydorczyk M, Shahidi F (2006) Antioxidant properties of pearled barley fractions. Journal of Agricultural and Food Chemistry 54, 3283–3289.
| Antioxidant properties of pearled barley fractions.Crossref | GoogleScholarGoogle Scholar | 16637686PubMed |
Mejía-Teniente L, Duran-Flores F. de D., Chapa-Oliver AM, Torres-Pacheco I, Cruz-Hernández A, González-Chavira MM, Ocampo-Velázquez RV, Guevara-González RG (2013) Oxidative and molecular responses in Capsicum annuum L. after hydrogen peroxide, salicylic acid and chitosan foliar applications. International Journal of Molecular Sciences 14, 10178–10196.
| Oxidative and molecular responses in Capsicum annuum L. after hydrogen peroxide, salicylic acid and chitosan foliar applications.Crossref | GoogleScholarGoogle Scholar | 23676352PubMed |
Mendoza-Sánchez M, Guevara-González RG, Castaño-Tostado E, Mercado-Silva EM, Acosta-Gallegos JA, Rocha-Guzmán NE, Reynoso-Camacho R (2016) Effect of chemical stress on germination of cv. Dalia bean (Phaseolus vularis L.) as an alternative to increase antioxidant and nutraceutical compounds in sprouts. Food Chemistry 212, 128–137.
| Effect of chemical stress on germination of cv. Dalia bean (Phaseolus vularis L.) as an alternative to increase antioxidant and nutraceutical compounds in sprouts.Crossref | GoogleScholarGoogle Scholar | 27374516PubMed |
Mol JNM, Robbins MP, Dixon RA, Veltkamp E (1985) Spontaneous and enzymic rearrangement of naringenin chalcone to flavanone. Phytochemistry 24, 2267–2269.
| Spontaneous and enzymic rearrangement of naringenin chalcone to flavanone.Crossref | GoogleScholarGoogle Scholar |
Parola-Contreras I, Tovar-Perez EG, Rojas-Molina A, Luna-Vazquez FJ, Torres-Pacheco I, Ocampo-Velazquez RV, Guevara-González RG (2020) Changes in affinin contents in Heliopsis longipes (chilcuague) after a controlled elicitation strategy under greenhouse conditions. Industrial Crops and Products 148, 112314
| Changes in affinin contents in Heliopsis longipes (chilcuague) after a controlled elicitation strategy under greenhouse conditions.Crossref | GoogleScholarGoogle Scholar |
Petrov VD, Van Breusegem F (2012) Hydrogen peroxide: a central hub for information flow in plant cells. AoB Plants 2012, pls014
| Hydrogen peroxide: a central hub for information flow in plant cells.Crossref | GoogleScholarGoogle Scholar | 22708052PubMed |
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology & Medicine 26, 1231–1237.
| Antioxidant activity applying an improved ABTS radical cation decolorization assay.Crossref | GoogleScholarGoogle Scholar |
Ren SC, Sun JT (2014) Changes in phenolic content, phenylalanine ammonia-lyase (PAL) activity, and antioxidant capacity of two buckwheat sprouts in relation to germination. Journal of Functional Foods 7, 298–304.
| Changes in phenolic content, phenylalanine ammonia-lyase (PAL) activity, and antioxidant capacity of two buckwheat sprouts in relation to germination.Crossref | GoogleScholarGoogle Scholar |
Sher H, Barkworth ME, de Boer HJ (2017) Medicinal and aromatic plant cultivation in the Swat valley, north-western Pakistan, for economic development and biodiversity conservation. Genetic Resources and Crop Evolution 64, 237–245.
| Medicinal and aromatic plant cultivation in the Swat valley, north-western Pakistan, for economic development and biodiversity conservation.Crossref | GoogleScholarGoogle Scholar |
Tamás L, Mistrík I, Huttová J, Halušková L, Valentovičová K, Zelinová V (2010) Role of reactive oxygen species-generating enzymes and hydrogen peroxide during cadmium, mercury and osmotic stresses in barley root tip. Planta 231, 221–231.
| Role of reactive oxygen species-generating enzymes and hydrogen peroxide during cadmium, mercury and osmotic stresses in barley root tip.Crossref | GoogleScholarGoogle Scholar | 19898864PubMed |
Toscano S, Ferrante A, Leonardi C, Romano D (2018) PAL activities in asparagus spears during storage after ammonium sulfate treatments. Postharvest Biology and Technology 140, 34–41.
| PAL activities in asparagus spears during storage after ammonium sulfate treatments.Crossref | GoogleScholarGoogle Scholar |
Vargas-Hernandez M, Macias-Bobadilla I, Guevara-Gonzalez RG, Romero-Gomez S. de J., Rico-Garcia E, Ocampo-Velazquez RV, Alvarez-Arquieta L. de L., Torres-Pacheco I (2017) Plant hormesis management with biostimulants of biotic origin in agriculture. Frontiers in Plant Science 8, 1762
| Plant hormesis management with biostimulants of biotic origin in agriculture.Crossref | GoogleScholarGoogle Scholar | 29081787PubMed |
Vazquez-Hernandez C, Feregrino-Perez AA, Perez-Ramirez I, Ocampo-Velazquez RV, Rico-García E, Torres-Pacheco I, Guevara-Gonzalez RG (2019a) Controlled elicitation increases steviol glycosides (SGs) content and gene expression-associated to biosynthesis of SGs in Stevia rebaudiana B. cv. Morita II. Industrial Crops and Products 139, 111479
| Controlled elicitation increases steviol glycosides (SGs) content and gene expression-associated to biosynthesis of SGs in Stevia rebaudiana B. cv. Morita II.Crossref | GoogleScholarGoogle Scholar |
Vázquez-Hernández MC, Parola-Contreras I, Montoya-Gómez LM, Torres-Pacheco I, Schwarz D, Guevara-González RG (2019b) Eustressors: chemical and physical stress factors used to enhance vegetables production. Scientia Horticulturae 250, 223–229.
| Eustressors: chemical and physical stress factors used to enhance vegetables production.Crossref | GoogleScholarGoogle Scholar |
Vergara-Castañeda HA, Guevara-González RG, Ramos-Gómez M, Reynoso-Camacho R, Guzmán-Maldonado H, Feregrino-Pérez AA, Oomah BD, Loarca-Piña G (2010) Non-digestible fraction of cooked bean (Phaseolus vulgaris L.) cultivar Bayo Madero suppresses colonic aberrant crypt foci in azoxymethane-induced rats. Food & Function 1, 294–300.
| Non-digestible fraction of cooked bean (Phaseolus vulgaris L.) cultivar Bayo Madero suppresses colonic aberrant crypt foci in azoxymethane-induced rats.Crossref | GoogleScholarGoogle Scholar |
Vizzari F, Massányi M, Knížatová N, Corino C, Rossi R, Ondruška Ľ, Massányi P (2021) Effects of dietary plant polyphenols and seaweed extract mixture on male-rabbit semen: quality traits and antioxidant markers. Saudi Journal of Biological Sciences 28, 1017–1025.
| Effects of dietary plant polyphenols and seaweed extract mixture on male-rabbit semen: quality traits and antioxidant markers.Crossref | GoogleScholarGoogle Scholar | 33424395PubMed |



