Barley yellow dwarf virus infection affects physiology, morphology, grain yield and flour pasting properties of wheat
Shormin Choudhury A D , Philip Larkin B , Holger Meinke A C , M. D. Hasanuzzaman A E , Peter Johnson A and Meixue Zhou A FA TIA, University of Tasmania, Private Bag 1375, Prospect, Tas. 7250, Australia.
B CSIRO Agriculture and Food, PO Box 1700, Canberra, ACT 2601, Australia.
C TIA, University of Tasmania, Private Bag 54, Hobart, Tas. 7001, Australia.
D Department of Horticulture, Faculty of Agriculture, Sher-e-Bangla Agricultural University, Sher-e-Bangla Nagar, Dhaka 1207, Bangladesh.
E Department of Agronomy, Faculty of Agriculture, Sher-e-Bangla Agricultural University, Sher-e-Bangla Nagar, Dhaka 1207, Bangladesh.
F Corresponding author. Email: meixue.zhou@utas.edu.au
Crop and Pasture Science 70(1) 16-25 https://doi.org/10.1071/CP18364
Submitted: 29 July 2018 Accepted: 5 December 2018 Published: 8 January 2019
Abstract
Barley yellow dwarf virus (BYDV) is a phloem-limited virus that is persistently transmitted by aphids and causes significant yield losses in wheat (Triticum aestivum L.). The present study was conducted to investigate the effects of BYDV in wheat on physiological and morphological traits, yield attributes and pasting properties of flour, and to determine any differences for these traits between susceptible and resistant genotypes under BYDV infection. Significant impact on physiological and morphological traits and yield was observed in plants inoculated at the 2-leaf stage (Zadoks scale, Z12), with a greater impact in the three susceptible genotypes than in the resistant genotype. Yield reduction with inoculation at Z12 was 18–49%, and yield reduction with inoculation mid tillering (Z25) was 6–31%. There was a significant reduction in effective tiller number with both inoculation times, but 1000-kernel weight was affected only with early inoculation. Pasting properties were little affected by BYDV infection, with genotype having a larger effect than infection. Grain yield showed negative correlation with tissue-blot immunoassay and visual symptom score, and positive correlation with all gas-exchange parameters, chlorophyll fluorescence, leaf area and biomass weight. The results suggest that stomatal conductance, transpiration rate and chlorophyll fluorescence measurements are suitable for assessment of BYDV infection and for screening BYDV of susceptible and resistant wheat genotypes.
Additional keywords: phloem transport, resistance, TBIA, viruliferous aphids.
Introduction
Wheat (Triticum aestivum L.) is one of the most important cereal crops in Australia and the most widely cultivated cereal worldwide (Arzani and Ashraf 2017), with global annual production >700 Mt (WASDE 2018). Global wheat production is impacted by virus infections (Velandia et al. 2010), Barley yellow dwarf virus (BYDV) being one of the most harmful viruses. BYDV infection causes physiological disruption and serious economic losses (Trębicki et al. 2015) There are several strains of BYDV, among them BYDV-PAV, which belongs to the genus Luteovirus of the family Luteoviridae, and is the most common serotype (Griesbach et al. 1990). BYDV-PAV is persistently transmitted specifically by the aphids Rhopalosiphum padi and Sitobion avenae (Kaddachi et al. 2014).
Yellowing or reddening of the leaf tips, particularly of the flag leaf is the most obvious symptom of virus infection (Kosová et al. 2008). The severity of BYDV infection varies with crop species, genotype, and age and physiological conditions of host plant at the time of infection (Loebenstein and Thottappilly 2013). However, symptoms in BYDV-infected wheat are not always obvious and may be confused with those caused by other biotic and abiotic stresses. Some BYDV-infected plants show no symptoms (Irwin and Thresh 1990), even though the presence of virus is established by serological test (Osler et al. 1985). Wheat genotypes containing the Bdv2 gene showed less yellowing and lower viral titre than susceptible wheat genotypes when infected by BYDV (Kausar et al. 2015). Plants infected by BYDV may show a significant reduction in plant biomass, leaf chlorophyll content and grain yield (Jensen and D’Arcy 1995; McKirdy et al. 2002). Banks et al. (1995a) studied the yield effects of a range of levels of BYDV infection on susceptible wheat genotypes in the field and showed that yield loss increased with the level of infection in plots. Similar results have been reported by McKirdy et al. (2002), who found that yield loss due to BYDV infection can be up to 80%. Virus infection affects not only grain yield but also grain quality (Edwards et al. 2001; Trębicki et al. 2015). Among the quality traits, pasting properties of starch are closely linked to the texture of cereal-based processed products (Zhang et al. 2017). However, to the best of our knowledge, nobody has investigated the effect of BYDV infection on wheat flour pasting properties.
It is important to identify the most suitable physiological parameter to evaluate BYDV resistance. Although plant grain yield is the ultimate test, the entire life cycle in the field should be assessed, which is time-consuming and labour-intensive. Physiological traits such as photosynthesis, transpiration rate, stomatal conductance, CO2 assimilation, chlorophyll content, Fv/Fm (maximum quantum efficiency of light harvesting in PSII in dark-adapted leaves) and relative water content would be good measures. Variation in physiological processes due to viral diseases is one of the most important reasons for decreased crop productivity across the world (Agrios 1997). Yield reductions following virus infection might arise from decreased photosynthesis. The mechanisms through which viruses induce a reduction in photosynthesis and other physiological traits in host plants are not fully understood, nor are the mechanisms of BYDV resistance and tolerance. Photosynthesis might be impacted by reduction in chloroplast number and loss of chlorophyll content in various viral infections (Balachandran et al. 1997; Ryšlavá et al. 2003; Guo et al. 2005a), with gas-exchange parameters and chlorophyll fluorescence being possible suitable indicators (Bonfig et al. 2006; Berger et al. 2007). Chlorophyll fluorescence and, primarily, the Fv/Fm ratio have proved reliable indicators for abiotic and biotic stress tolerance (Durães et al. 2001). A significant decrease in Fv/Fm was observed in Nicotiana tabacum leaves infected by Potato virus A and Potato virus Y (Ryšlavá et al. 2003; Zhou et al. 2004). However, this is not always the case; Eupatorium makinoi plants infected by a geminivirus showed no significant changes in the Fv/Fm ratio (Funayama et al. 1997a).
Several methods are available for the control of BYDV. The aphid vector can be effectively controlled through insecticide treatments, but the timing is critical and multiple applications can make this approach expensive and environmentally damaging (Chain et al. 2005). The use of plant varieties carrying genetic resistances is one of the most effective, economical, sustainable and frequently employed strategies to control viral infections (Nicaise 2014), hence the importance of generating strategies for improving disease resistance in wheat. Uniform inoculation is essential for reliable selection of BYDV-tolerant varieties, and requires the controlled application of reared, viruliferous aphids; for effective breeding progress, natural infections in the field can be too unreliable. Controlled inoculation by infestation with viruliferous aphids also enables the assessment of particular virus isolates and impacts of inoculation at various developmental stages. A reliable method is needed to identify resistant/tolerant genotypes for large-scale selection in breeding programs. Despite the importance of BYDV, little attention has been given to physiological traits as potential selection criteria for resistance breeding.
This research investigated the effect of BYDV infection on physiological parameters, biomass, yield traits and dough pasting properties of wheat inoculated at different plant growth stages, and their correlations in BYDV-susceptible and -resistant genotypes.
Materials and methods
Plant materials, growing conditions, and experimental design
The field experiment was conducted at Tasmanian Institute of Agriculture, Launceston, Tasmania, from September 2017 to January 2018. Four wheat genotypes (cvv. Mace, Preston, Wallup and BC Preston) were used. BC Preston (Preston*4/Pontin13) is the only resistant genotype, being homozygous for the Bdv2 resistant gene and is a BC3 derivative in the background of cv. Preston. The Bdv2 gene is carried on the Pontin13 recombinant alien translocation, originally derived from Thinopyrum intermedium (Ayala-Navarrete et al. 2013). A molecular marker (ByAgi) was used at every backcross and in the F3 families to confirm homozygosity; and the effectiveness of the resistance in this and other backcross derivatives was confirmed in preliminary experiments (P. Larkin, unpubl. data) and this study (Fig. 1).
All genotypes were grown in outside tanks (1.2 m by 1.0 m by 0.6 m) filled with a mixture of sandy loam soil and with plant spacing of 10 cm by 10 cm. Fertiliser (N : P : K : S, 5 : 10 : 10 : 5) was applied at sowing at the rate of 250 kg ha–1. An additional 75 kg N ha–1 was topdressed at the stem elongation stage (Z31; Zadoks et al. 1974). A water tray was used to supply water to the bottom of each tank. The bottom of each tank contained 50 mm coarse gravel overlaid with drainage matting, and the soil was placed on top to a depth of 400 mm. The water level of each container was maintained at 75 mm depth by fitting a float valve to the water tray. Excess water from rainfall flowed back to the water tray and out of an overflow. Any water lost from the plant containers through evapotranspiration caused a local drop in the 750 mm water level, which was resupplied by the water tray. The lowest soil level remained fully saturated while the surface was dry. There was a gradient of moisture by depth between the top and bottom.
The experiment was conducted as a randomised complete block design with three replications, with four genotypes and three virus inoculation treatments (see below). Fifteen plants from each genotype–inoculation treatment combination were randomly selected for measurements of all of the physiological parameters, visual symptom score, and virus detection by tissue-blot immunoassay (TBIA). All of the genotypes were assessed on the same day when most of the lines reached flowering stage (Z65, 50% of plants flowering). The plants that were assessed for TBIA were the same plants assessed for symptom severity and all physiological and morphological parameters.
Aphid colony and virus inoculations
A viruliferous aphid colony (Rhopalosiphum padi) was multiplied for 6 weeks in a growth chamber at 20 ± 2°C under a photoperiod of 16 h light, 8 h dark on a sensitive wheat genotype (cv. Revenue) infected with BYDV-PAV. Colony infection status was regularly tested by using a TBIA (see below) to ensure that aphid colonies were viruliferous. There were three treatments: inoculation with BYDV-PAV at 2-leaf stage (Z12, 3 weeks after sowing); inoculation with BYDV-PAV at mid-tillering stage (Z25, 5 weeks after sowing); and control (protected from aphid infection). All genotypes showed a similar phenology; thus, the inoculation was conducted on the same dates for all genotypes. Inoculation was done by placing 5–10 aphids on the second leaf from the top of each plant of each treatment. To prevent the transmission of the aphids and to maintain similar growing conditions, all plots, inoculated and control, were shielded with transparent aphid-proof mesh. One week after inoculation, the mesh was removed and the plants were sprayed with the insecticide Astound (alpha-cypermethrin) to kill all aphids.
Tiller sampling for virus detection
Plants harvested at flowering were tested for the presence of BYDV-PAV using TBIA. One tiller from each of 15 plants per treatment replicate was assessed according to the procedure outlined in Schwinghamer et al. (2014). Each tiller was blotted onto nitrocellulose membranes, examined with polyclonal (BYDV-PAV) or monoclonal (BYDV-PAV) antisera (Agdia, Elkhart, IN, USA), and visually evaluated under a dissecting microscope.
Assessment of BYDV infection
The severity of BYDV infection tested by TBIA was scored on a 0–4 scale, taking into account the number of vascular bundles infected by the virus: 0, no virus; 1, very low level of virus, <10% of vascular bundles infected and very low intensity of staining of the vascular bundle; 2, low number of vascular bundles (>10–25%) infected and low intensity staining of vascular bundle; 3, moderate number of vascular bundles (>25–50%) infected and moderate intensity staining of vascular bundle; 4, higher number of vascular bundles (>50%) infected and intensely stained vascular bundles.
Visual assessment of infection
The severity of symptom development on BYDV-infected plants at flowering was scored on a 0–5 scale. The scale assessed the proportion of leaves showing red–yellow discoloration on the inoculated plants: 0, whole plant without symptoms; 1, a few (<20%) discoloured leaves; 2, ~20% of leaves affected; 3, 40% of leaves affected; 4, 60% of leaves affected; 5, almost all (>60%) leaves affected. Average visual symptom scores were calculated.
Measurement of physiological parameters
Photosynthetic gas exchange
Gas exchange at flowering was measured between 10 : 00 and 13 : 00 local time on a sunny and cloudless day. Net photosynthetic rate (Pn), stomatal conductance (Gs), intercellular CO2 concentration (Ci) and transpiration rate (E) were measured from the middle portion of fully expanded flag leaves by using a Li-Cor 6400 portable photosynthesis system (Li-Cor, Lincoln, NE, USA). Temperature was set at 20°C with Tleaf reading. Photosynthetically active radiation (PAR) was set at 1500 µmol m–2 s–1 inside the chamber; CO2 was supplied artificially and was kept at concentration 400 µmol mol–1 inside the chamber with a stable flow rate of 500 µmol m–2 s–1 (Singh et al. 2014). Chamber fan speed was set to high as default. Chamber relative humidity was maintained in the range 40–50% by adjusting the H2O scrub. The sample and reference infrared gas analyser were matched between every five measurements.
SPAD chlorophyll meter reading and chlorophyll fluorescence (Fv/Fm ratio)
Leaf chlorophyll content at flowering was measured for 15 plants from each treatment replicate by using a SPAD-502 chlorophyll meter (Konica Minolta, Osaka, Japan). Measurements were recorded from the middle of the flag leaves.
The maximum quantum yield of PSII photochemistry (Fv/Fm ratio) of the plants was measured by using a modulated chlorophyll fluorometer OS1-FL (Opti-Sciences, Tyngsboro, MA, USA). Leaves were dark-adapted for 30 min before measurements. Measurements were done on the upper surface of the flag leaves.
Relative water content (RWC)
The RWC was determined according to Smart and Bingham (1974). For each treatment replicate, five flag leaves were pooled, and their fresh weight (FW) determined. The leaves were then immersed in water for 12 h at room temperature to regain turgidity. The turgid tissue was then quickly blotted to remove excess water, then turgid weight (TW) was measured. The samples were then dried in an oven at 56°C for 24 h to determine dry weight (DW). RWC was calculated by using the following formula:
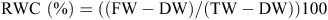
Leaf area
Flag leaves were randomly collected at flowering from five plants from each treatment replicate, and leaf area was measured by using the Paton Electronic Planimeter (Paton Industries P/L, Geelong, Vic, Australia). The area of the leaf is measured as it is drawn through the scanning head. The scanning head was combined with a transparent belt conveyer with constant speed in order to measure the area of detached leaves.
Plant biomass and yield traits
Three of the plants randomly sampled from each treatment replicate at flowering were dried for 2 days at 65°C for 72 h before taking biomass.
Later, at maturity, a further10 plants were harvested from the centre of each treatment replicate (i.e. 30 plants in total) for determining number of effective tillers. Grain yield and 1000-kernel weight were determined after threshing the mature spikes.
Pasting properties
The Rapid Visco-Analyser (RVA-4D; Newport Scientific, Sydney) was used to measure pasting properties via the method of Zhou and Mendham (2005). After harvesting and threshing, a 10.0-g sample of cleaned grains from each genotype in each treatment replicate was ground on a Cyclotech 1903 Mill (Foss, Hillerød, Denmark). Then, a 4.0-g sample of the flour was dissolved in 25.0 g 0.1 m silver nitrate (AgNO3) solution in an aluminium canister and mixed well before placing into the RVA. To ensure the dispersion of the grist, the RVA was used for 10 s at 960 rpm then reduced to 160 rpm for the test run. The temperature was initially 50°C for 1.0 min, then elevated to 95°C for 3.7 min, held for 2.5 min and cooled to 50°C over 3.8 min, then held for 2.0 min.
Statistical analyses
Statistical analyses were performed with Statistical Analysis System (SAS) version 9.4 (SAS Institute, Cary NC, USA), using ANOVA followed by the Duncan’s multiple range test (DMRT) to evaluate the genotype and treatment effects. The significance of correlations between different parameters was determined by bivariate correlations based on Pearson’s correlation (2-tailed).
Results
Validation of inoculation
Infections with BYDV were confirmed by TBIA, with susceptible genotypes showing higher levels of infection than the resistant genotype (Fig. 1a). When inoculated at the 2-leaf stage, the highest TBIA score was observed in susceptible wheat genotypes Mace and Wallup (2.8) and Preston (2.6). Mace also showed the highest TBIA score in plants inoculated at tillering stage (2.3), followed by Wallup (2.1) and Preston (1.8). BC Preston had the lowest infection scores at both inoculation stages: 1.8 and 1.3 for 2-leaf and mid-tillering stages, respectively.
Development of leaf symptoms
Symptom expression was recorded at the flowering stage. All genotypes except BC Preston developed strong leaf-yellowing symptoms, indicating the lower field infection of this genotype. Disease severity was greater when plants were infected at the 2-stage, causing the greatest leaf discoloration of susceptible genotypes (Fig. 1b). The wheat genotype containing the Bdv2 gene, BC Preston, showed a significantly lower disease symptom score (1.8) than Mace (3.8), Wallup (3.6) and Preston (3.2).
Photosynthetic gas exchange, chlorophyll content and chlorophyll fluorescence
Consistent with leaf-symptom-development data, BC Preston showed less reduction in photosynthesis than the three susceptible genotypes under BYDV stress. Mace showed higher photosynthetic rate (Pn) than the other three genotypes when grown under non-inoculated conditions (Fig. 2a). BC Preston showed a reduction in Pn by only 12% and 6% (compared with control) for early (Z12) and late (Z25) infection, respectively. Much stronger reduction was found in the three susceptible genotypes (~44% in Wallup; 56% in Mace; 40% in Preston; under early infection).
Chlorophyll content (measured by SPAD meter) was significantly reduced in BYDV-infected wheat across the genotypes, and more reduction was observed in plants inoculated at the 2-leaf stage than at mid tillering (Fig. 2b). The average reduction of leaf chlorophyll content was 35–38% (P < 0.01) for early and 24–33% (P < 0.01) for late inoculation, whereas the resistant genotype BC Preston exhibited only ~16% and 10% (P < 0.05) reduction, respectively.
Similar to Pn, stomatal conductance (Gs) and transpiration rate (E) were significantly reduced by BYDV infection across the four genotypes (Fig. 2c, d). In BC Preston, reduction in Gs was only 10% and –20% (relative to control) when infected at tillering and the 2-leaf stage, respectively (Fig. 2c). When inoculated at the 2-leaf stage, Wallup and Mace showed a ~70% reduction in Gs, and inoculation at tillering resulted in a reduction of ~60%. Reduction in E was similar in Wallup and Mace (45%) at early infection, whereas the reduction was ~32% and 7% in Preston and BC Preston, respectively (Fig. 2d). Among all of the gas-exchange parameters, intercellular CO2 concentration (Ci) was least affected, with only 4–12% decrease when inoculated at the 2-leaf stage and 1–6% decrease, when inoculated at mid-tillering (Fig. 2e).
Inoculation with BYDV also reduced chlorophyll fluorescence (Fv/Fm ratio). Fv/Fm values ranged from 0.81 to 0.82 among the genotypes under control conditions; however, a significant variation in Fv/Fm values was found in BYDV-infected plants (P < 0.01; Fig. 2f). Reduction in Fv/Fm was ~20% in both Wallup and Mace, and ~14% in Preston, at the early infection stage. Furthermore, lower reduction of Fv/Fm values was observed in BC Preston, which was as low as 2% and 5%, respectively, under late and early infection.
RWC and leaf area
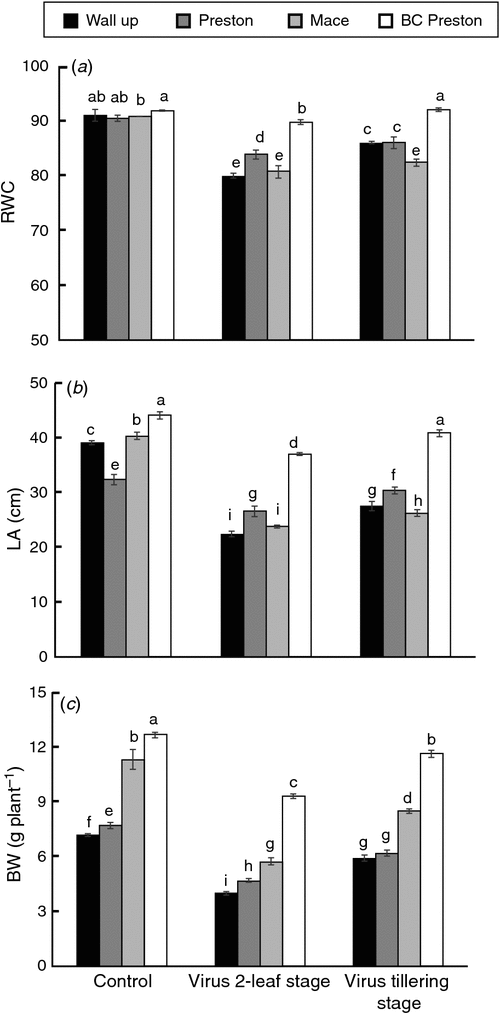
Infection with BYDV caused significant reductions in RWC in flag leaves of all susceptible wheat genotypes, ranging from 7% for Preston to 12% for Wallup when inoculated at the 2-leaf stage and from 4% for Preston to 6% for Mace when inoculated at mid-tillering. No significant change was found in the resistant genotype with late-stage inoculation (Fig. 3a).
|
Flag leaf area was significantly (P < 0.01) reduced in all wheat genotypes when virus was inoculated at the 2-leaf stage (Fig. 3b). Wallup showed the greatest reduction (42%), whereas BC Preston showed the lowest reduction (15%). Late infection (at mid-tillering) caused less reduction in flag leaf area, being 18–29% for sensitive genotypes and only 6% for BC Preston.
Plant biomass
Early BYDV infection resulted in a significant reduction in plant biomass across the genotypes (Fig. 3c). BC Preston showed the smallest reduction in shoot biomass, whereas Preston, Wallup and Mace were much more sensitive to BYDV treatment. Even with early infection, BC Preston was able to maintain its biomass weight at ~75% of control. For Preston, Wallup and Mace, biomass weight was reduced to 40%, 45% and 50%, respectively, of that in the control. The same trends were shown when inoculated at mid-tillering (Fig. 3c).
Grain yield, effective tiller number and 1000-kernel weight
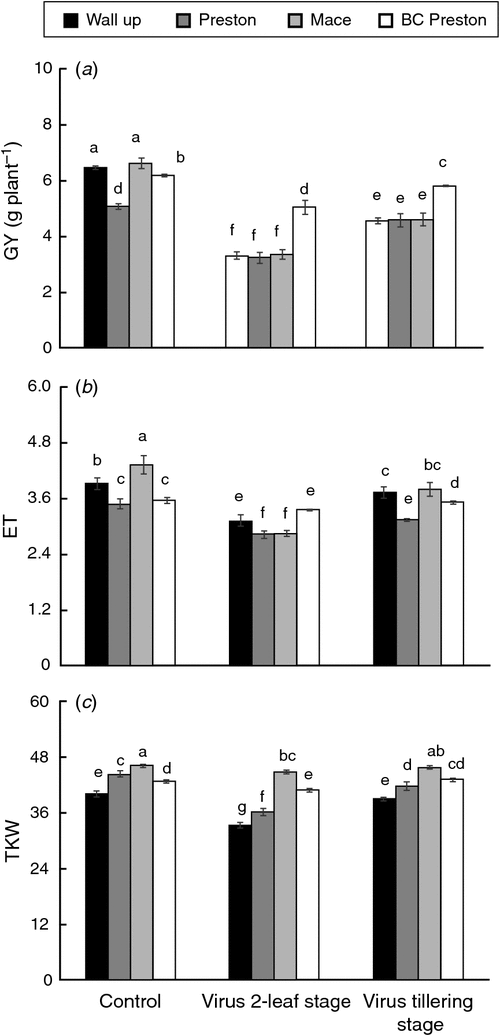
Grain yield was significantly reduced in BYDV-infected wheat across the genotypes, especially when inoculated at the 2-leaf stage (Fig. 4a). Grain-yield loss due to BYDV infection ranged from 14% in the resistant genotype BC Preston to 49% in the sensitive genotypes Mace and Wallup when inoculated at the 2-leaf stage. Late BYDV infection had much less effect on grain yield, with only 10% (Preston) to 31% (Wallup) reduction in sensitive genotypes and no significant reduction in the resistant genotypes (Fig. 4a).
|
The number of effective tillers was significantly (P ≤ 0.01) reduced by BYDV infection in all wheat genotypes inoculated at the 2-leaf stage compared with control, with much greater reduction in susceptible genotypes (Fig. 4b). Reduction in number of tillers was greatest in susceptible wheat genotype Mace (34%) followed by Wallup (21%) and Preston (19%), and smallest in BC Preston (5%). There was a 4–12% (P < 0.05) decrease in number of effective tillers per plant in susceptible wheat genotypes inoculated at tillering (Fig. 4b); however, the resistant genotype (BC Preston) showed no significant change. 1000-kernel weight was also significantly (P < 0.05) affected by virus infection in all genotypes inoculated at the 2-leaf stage, ranging from 3% to 18% (Fig. 4c). BYDV inoculation at mid-tillering showed no significant effects on 1000-kernel weight except in Preston.
Effects of BYDV on pasting properties
Pasting parameters including peak viscosity (PV), breakdown viscosity (BV), final viscosity (FV) and setback viscosity (SV) of the different wheat genotypes showed different responses under BYDV infection (Fig. 5). In Wallup, PV (5%) and BV (15%) were significantly decreased but there was no effect on FV and SV compared with the control. However, in Mace, FV (9%) and SV (11%) were significantly decreased, with no effect on PV and BV. Preston showed significantly decreased PV (11%), BV (23%) and FV (4%) in BYDV-inoculated plants compared with control. However, in BC Preston, the reduction of different pasting properties ranged from 2% to 10%.
Correlation analysis
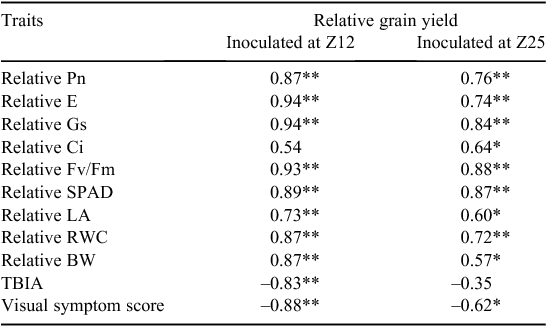
Pearson’s correlation coefficients among BYDV treatments are listed in Table 1. Grain yield was positively correlated with all the physiological and morphological parameters and negatively correlated with TBIA and symptom score measured following two different inoculation stages. With early inoculation (at Z12), stomatal conductance and transpiration rate showed the highest correlation with grain yield, whereas with late inoculation, chlorophyll fluorescence showed the highest correlation with grain yield.
|
Discussion
We investigated the effect of BYDV infection on different physiological parameters and yield traits following inoculation at two different developmental stages. TBIA results indicated successful viral infection following both early and late inoculation. The study showed that the proportion of infected plants depended on plant genetic background. The experiment showed that earlier virus infection led to a greater impact on physiological parameters, and subsequently on plant biomass and yield. The experiment showed that heavy yield losses could occur in susceptible genotypes following both early and late infection. The resistant genotype was less affected by virus infection than susceptible genotypes, including its recurrent parental background.
The photosynthetic system is the physiological basis of crop growth and yield (Sun et al. 2009). Reduction in photosynthetic rate due to virus infection is associated with physical damage to the chloroplast structure and deterioration of its membranes (Fraser and Fraser 1987). BYDV infection caused significant reductions in Pn of susceptible wheat genotypes, which showed greater negative correlation with symptom score likely because virus infection can reduce green leaf area or cause transport blockage and accumulation of photosynthate in infected leaves. This abnormal accumulation may inhibit further photosynthesis by disrupting chloroplast structure, reducing CO2 diffusion or light availability, and may be accentuated by phloem necrosis (Esau et al. 1967). Jensen (1969, 1968) found that BYDV-infected plants had greater accumulation of carbohydrate in leaves, possibly through disruption of normal phloem transport and corresponding reduced chlorophyll content and rate of photosynthesis. Reduction of Pn was observed in Eupatorium makinoi leaves infected by a geminivirus, and it was suggested that the reduction may be due to the decline of chlorophyll per unit leaf area in infected leaves (Funayama et al. 1997b). In our results, chlorophyll content of plants infected with BYDV was reduced significantly more in susceptible genotypes than in the resistant genotype. Similar results were found in a study of Rice tungro virus on rice plants, in which virus-infected resistant genotypes showed minimal loss of chlorophyll (Jabeen et al. 2017). Reduction in chlorophyll contents with virus infection might be a result of chlorophyll degradation (Liu et al. 2014) or chlorophyll synthesis inhibition (Shimura et al. 2011).
During photosynthesis, stomata play a critical role in CO2 assimilation and carbon fixation, which ultimately contribute to increased plant biomass and grain yield (Hetherington and Woodward 2003). A decrease in stomatal conductance (Gs) and transpiration rate (E) was observed in grapevine leaves affected by Grapevine leafroll virus (Bertamini et al. 2004), in radish leaves infected by Turnip mosaic virus (Guo et al. 2005b), and in tobacco leaves affected by Potato virus Y (Spoustová et al. 2013). Stomata were less opened in sugarcane leaves inoculated with Sugarcane yellow leaf virus (Lehrer and Komor 2008). In this study, stomatal closure was evident as a drastic reduction in stomatal conductance, which presumably acted as a major factor in reducing net photosynthesis (Pn) in BYDV-susceptible genotypes. BC Preston showed minimal decrease in Gs, as well as transpiration rate, even when inoculated early. Our results also showed a positive and significant correlation between E and Gs in all wheat genotypes tested.
Virus infection in susceptible plants induced reduction in photosynthetic traits including chlorophyll fluorescence (Fv/Fm ratio) and CO2 assimilation (Ci) (Rys et al. 2014). The Fv/Fm ratio provides basic information regarding photosynthetic apparatus (Rapacz and Hura 2004). In our result, light interception by PSII measured by the Fv/Fm ratio was significantly reduced in susceptible wheat genotypes, suggesting that virus infection destroys functional photosynthetic reaction centres, leading to chlorophyll degradation (lower SPAD values). In addition, BYDV infection also led to Ci reduction in susceptible wheat. The reduction of Pn in wheat leaves infected with Wheat streak mosaic virus was associated with reduced Ci (Pradhan et al. 2015).
Infection with BYDV severely reduced leaf area of susceptible wheat plants compared with the control. Cauliflower mosaic virus caused significant reduction of the leaf area of Brassica rapa and Arabidopsis thaliana plants (Doumayrou et al. 2013). Banana plants infected with Banana bunchy top virus showed an apparent significant decrease in leaf area at 50 days after infection (Hooks et al. 2008), leading to reductions in light interception (Kumar et al. 2012). In our study, susceptible genotypes infected with BYDV had significantly reduced leaf area, photosynthetic efficiency and biomass weight, whereas the resistant genotype infected with BYDV showed little effect on plant biomass.
The gene Bdv2 confers resistance in the sense of reducing but not eliminating viral load (Banks et al. 1995b); in addition, it seems to reduce the efficiency of transmission to plants (Ayala-Navarrete et al. 2013; Jahier et al. 2009). The reduced virus load in the present study resulted in reduced effects on most of the physiological and morphological parameters measured. In our results, two susceptible genotypes, Wallup and Mace, showed greater yield reduction (>30%) at both early and late infection. However, the reduction of yield was more pronounced with earlier inoculation, as has been frequently observed with BYDV (Smith and Sward 1982; Thackray et al. 2009; Finlay and Luck 2011; GRDC 2013). BYDV infection at a later developmental stage has less time to disrupt plant physiological parameters, thus causing less yield reduction. Grain yield reduction in wheat plants infected with BYDV was mainly expressed in reduced number of effective tillers per plant (El-Yamani and Hill 1990) rather than 1000-kernel weight, especially when BYDV infection happened at a later stage. BYDV-resistant genotype BC Preston, which carries the resistance gene Bdv2, showed good performance in physiological and yield-contributing parameters with low levels of infection rates.
Infection with BYDV had minimum effects on grain flour pasting properties. The difference between genotypes was greater than the effects of the infection, confirming that pasting properties are largely influenced by genotype (Zhou et al. 2008).
In conclusion, the present study suggests that gas-exchange parameters (Pn, E and Gs), chlorophyll content, Fv/Fm, leaf area, RWC and plant biomass under BYDV stress could all be used as reference indicators for selecting BYDV-resistant genotypes. However, Fv/Fm is relatively simple and rapid to measure and, thus. is likely to be more efficient for screening a large number of genotypes. Both TBIA and visual symptom score showed significant (negative) correlation with grain yield. However, neither can be scored at early growth stage and TBIA requires more time.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
This project was supported by funds from the Grains Research and Development Corporation of Australia.
References
Agrios GN (1997) ‘Plant pathology.’ 4th edn (Academic Press: San Diego, CA, USA)Arzani A, Ashraf M (2017) Cultivated ancient wheats (Triticum spp.): a potential source of health‐beneficial food products. Comprehensive Reviews in Food Science and Food Safety 16, 477–488.
| Cultivated ancient wheats (Triticum spp.): a potential source of health‐beneficial food products.Crossref | GoogleScholarGoogle Scholar |
Ayala-Navarrete L, Mechanicos A, Gibson J, Singh D, Bariana H, Fletcher J, Shorter S, Larkin PJ (2013) The Pontin series of recombinant alien translocations in bread wheat: single translocations integrating combinations of Bdv2, Lr19 and Sr25 disease-resistance genes from Thinopyrum intermedium and Th. ponticum. Theoretical and Applied Genetics 126, 2467–2475.
| The Pontin series of recombinant alien translocations in bread wheat: single translocations integrating combinations of Bdv2, Lr19 and Sr25 disease-resistance genes from Thinopyrum intermedium and Th. ponticum.Crossref | GoogleScholarGoogle Scholar | 23807636PubMed |
Balachandran S, Hurry V, Kelley S, Osmond C, Robinson S, Rohozinski J, Seaton G, Sims D (1997) Concepts of plant biotic stress. Some insights into the stress physiology of virus‐infected plants, from the perspective of photosynthesis. Physiologia Plantarum 100, 203–213.
| Concepts of plant biotic stress. Some insights into the stress physiology of virus‐infected plants, from the perspective of photosynthesis.Crossref | GoogleScholarGoogle Scholar |
Banks PM, Davidson JL, Bariana H, Larkin PJ (1995a) Effects of barley yellow dwarf virus on the yield of winter wheat. Australian Journal of Agricultural Research 46, 935–946.
| Effects of barley yellow dwarf virus on the yield of winter wheat.Crossref | GoogleScholarGoogle Scholar |
Banks PM, Larkin PJ, Bariana HS, Lagudah ES, Appels R, Waterhouse PM, Brettell RIS, Chen X, Xu HJ, Xin ZY, Qian YT, Zhou XM, Cheng ZM, Zhou GH (1995b) The use of cell culture for sub-chromosomal introgressions of barley yellow dwarf virus resistance from Thinopyrum intermedium to wheat. Genome 38, 395–405.
| The use of cell culture for sub-chromosomal introgressions of barley yellow dwarf virus resistance from Thinopyrum intermedium to wheat.Crossref | GoogleScholarGoogle Scholar | 18470178PubMed |
Berger S, Sinha AK, Roitsch T (2007) Plant physiology meets phytopathology: plant primary metabolism and plant–pathogen interactions. Journal of Experimental Botany 58, 4019–4026.
| Plant physiology meets phytopathology: plant primary metabolism and plant–pathogen interactions.Crossref | GoogleScholarGoogle Scholar | 18182420PubMed |
Bertamini M, Muthuchelian K, Nedunchezhian N (2004) Effect of grapevine leafroll on the photosynthesis of field grown grapevine plants (Vitis vinifera L. cv. Lagrein). Journal of Phytopathology 152, 145–152.
| Effect of grapevine leafroll on the photosynthesis of field grown grapevine plants (Vitis vinifera L. cv. Lagrein).Crossref | GoogleScholarGoogle Scholar |
Bonfig KB, Schreiber U, Gabler A, Roitsch T, Berger S (2006) Infection with virulent and avirulent P. syringae strains differentially affects photosynthesis and sink metabolism in Arabidopsis leaves. Planta 225, 1–12.
| Infection with virulent and avirulent P. syringae strains differentially affects photosynthesis and sink metabolism in Arabidopsis leaves.Crossref | GoogleScholarGoogle Scholar | 16807755PubMed |
Chain F, Riault G, Trottet M, Jacquot E (2005) Analysis of accumulation patterns of Barley yellow dwarf virus-PAV (BYDV-PAV) in two resistant wheat lines. European Journal of Plant Pathology 113, 343–355.
| Analysis of accumulation patterns of Barley yellow dwarf virus-PAV (BYDV-PAV) in two resistant wheat lines.Crossref | GoogleScholarGoogle Scholar |
Doumayrou J, Leblaye S, Froissart R, Michalakis Y (2013) Reduction of leaf area and symptom severity as proxies of disease-induced plant mortality: the example of the Cauliflower mosaic virus infecting two Brassicaceae hosts. Virus Research 176, 91–100.
| Reduction of leaf area and symptom severity as proxies of disease-induced plant mortality: the example of the Cauliflower mosaic virus infecting two Brassicaceae hosts.Crossref | GoogleScholarGoogle Scholar | 23742852PubMed |
Durães FOM, Gama EEG, Magalhães PC, Marriel IE, Casela CR, Oliveira AC, Luchiari A Jr, Shanahan JF (2001) The usefulness of chlorophyll fluorescence in screening for disease resistance, water stress tolerance, aluminium toxicity tolerance, and N use efficiency in maize. In ‘Integrated approaches to higher maize productivity in the new millennium. Proceedings Seventh Eastern and Southern Africa Regional Maize Conference’. Nairobi, Kenya. (Eds DK Friesen, AFE Palmer) pp. 356–360.
Edwards MC, Fetch TG, Schwarz PB, Steffenson BJ (2001) Effect of Barley yellow dwarf virus infection on yield and malting quality of barley. Plant Disease 85, 202–207.
| Effect of Barley yellow dwarf virus infection on yield and malting quality of barley.Crossref | GoogleScholarGoogle Scholar |
El-Yamani M, Hill J (1990) Identification and importance of barley yellow dwarf virus in Morocco. Plant Disease 74, 291–294.
| Identification and importance of barley yellow dwarf virus in Morocco.Crossref | GoogleScholarGoogle Scholar |
Esau K, Cronshaw J, Hoefert LL (1967) Relation of beet yellows virus to the phloem and to movement in the sieve tube. The Journal of Cell Biology 32, 71–87.
| Relation of beet yellows virus to the phloem and to movement in the sieve tube.Crossref | GoogleScholarGoogle Scholar | 10976202PubMed |
Finlay K, Luck J (2011) Response of the bird cherry-oat aphid (Rhopalosiphum padi) to climate change in relation to its pest status, vectoring potential and function in a crop–vector–virus pathosystem. Agriculture, Ecosystems & Environment 144, 405–421.
| Response of the bird cherry-oat aphid (Rhopalosiphum padi) to climate change in relation to its pest status, vectoring potential and function in a crop–vector–virus pathosystem.Crossref | GoogleScholarGoogle Scholar |
Fraser RSS, Fraser RSS (1987) ‘Biochemistry of virus-infected plants.’ Vol. 259. (Research Studies Press: Letchworth, UK)
Funayama S, Hikosaka K, Yahara T (1997a) Effects of virus infection and growth irradiance on fitness components and photosynthetic properties of Eupatorium makinoi (Compositae). American Journal of Botany 84, 823–829.
| Effects of virus infection and growth irradiance on fitness components and photosynthetic properties of Eupatorium makinoi (Compositae).Crossref | GoogleScholarGoogle Scholar | 21708634PubMed |
Funayama S, Sonoike K, Terashima I (1997b) Photosynthetic properties of leaves of Eupatorium makinoi infected by a geminivirus. Photosynthesis Research 53, 253–261.
| Photosynthetic properties of leaves of Eupatorium makinoi infected by a geminivirus.Crossref | GoogleScholarGoogle Scholar |
GRDC (2013) Barley yellow dwarf virus (BYDV) [Online]. Grains Research and Development Corporation, Canberra, ACT. Available at: https://grdc.com.au/__data/assets/pdf_file/0029/75809/grdc-fs-bydv-pdf.pdf.pdf (accessed 2018)
Griesbach J, Falk B, Valverde R (1990) Incidence of barley yellow dwarf viruses in California cereals. Plant Disease 74, 111–114.
| Incidence of barley yellow dwarf viruses in California cereals.Crossref | GoogleScholarGoogle Scholar |
Guo DP, Guo YP, Zhao JP, Liu H, Peng Y, Wang QM, Chen JS, Rao GZ (2005a) Photosynthetic rate and chlorophyll fluorescence in leaves of stem mustard (Brassica juncea var. tsatsai) after turnip mosaic virus infection. Plant Science 168, 57–63.
| Photosynthetic rate and chlorophyll fluorescence in leaves of stem mustard (Brassica juncea var. tsatsai) after turnip mosaic virus infection.Crossref | GoogleScholarGoogle Scholar |
Guo YP, Guo DP, Peng Y, Chen JS (2005b) Photosynthetic responses of radish (Raphanus sativus var. longipinnatus) plants to infection by turnip mosaic virus. Photosynthetica 43, 457–462.
| Photosynthetic responses of radish (Raphanus sativus var. longipinnatus) plants to infection by turnip mosaic virus.Crossref | GoogleScholarGoogle Scholar |
Hetherington AM, Woodward FI (2003) The role of stomata in sensing and driving environmental change. Nature 424, 901–908.
| The role of stomata in sensing and driving environmental change.Crossref | GoogleScholarGoogle Scholar | 12931178PubMed |
Hooks C, Wright M, Kabasawa D, Manandhar R, Almeida R (2008) Effect of banana bunchy top virus infection on morphology and growth characteristics of banana. Annals of Applied Biology 153, 1–9.
| Effect of banana bunchy top virus infection on morphology and growth characteristics of banana.Crossref | GoogleScholarGoogle Scholar |
Irwin M, Thresh J (1990) Epidemiology of barley yellow dwarf: a study in ecological complexity. Annual Review of Phytopathology 28, 393–424.
| Epidemiology of barley yellow dwarf: a study in ecological complexity.Crossref | GoogleScholarGoogle Scholar |
Jabeen A, Kiran T, Subrahmanyam D, Lakshmi D, Bhagyanarayana G, Krishnaveni D (2017) Variations in chlorophyll and carotenoid contents in Tungro infected rice plants. Journal of Research and Development 5, 1–7.
Jahier J, Chain F, Barloy D, Tanguy AM, Lemoine J, Riault G, Margale E, Trottet M, Jacquot E (2009) Effect of combining two genes for partial resistance to Barley yellow dwarf virus-PAV (BYDV-PAV) derived from Thinopyrum intermedium in wheat. Plant Pathology 58, 807–814.
| Effect of combining two genes for partial resistance to Barley yellow dwarf virus-PAV (BYDV-PAV) derived from Thinopyrum intermedium in wheat.Crossref | GoogleScholarGoogle Scholar |
Jensen SG (1968) Photosynthesis, respiration and other physiological relationships in barley infected with barley yellow dwarf virus. Phytopathology 58, 204–208.
Jensen SG (1969) Composition and metabolism of barley leaves infected with barley yellow dwarf virus. Phytopathology 59, 1694–1698.
Jensen SG, D’Arcy CJ (1995) Effects of barley yellow dwarf virus on host plants. In ‘Barley yellow dwarf: 40 years of progress’. (Eds CJ D’Arcy, PA Burnett) pp. 55–74. (American Phytopathological Society Press: St. Paul, MN, USA)
Kaddachi I, Souiden Y, Achouri D, Chéour F (2014) Barley yellow dwarf virus (BYDV): characteristics, hosts, vectors, disease symptoms and diagnosis. International Journal of Phytopathology 3, 155–160.
Kausar S, Hameed S, Saleem K, Ul Haque I, Zamurrad M, Ashfaq M (2015) Molecular confirmation of Bdv2 gene in wheat germplasm and its field based assessment for resistance against barely yellow dwarf viruses. Advancements in Life Sciences 3, 16–22.
Kosová K, Chrpova J, Sip V (2008) Recent advances in breeding of cereals for resistance to barley yellow dwarf virus. Czech Journal of Genetics and Plant Breeding 44, 1–10.
| Recent advances in breeding of cereals for resistance to barley yellow dwarf virus.Crossref | GoogleScholarGoogle Scholar |
Kumar U, Singh P, Boote K (2012) Effect of climate change factors on processes of crop growth and development and yield of groundnut (Arachis hypogaea L.). Advances in Agronomy 116, 41–69.
| Effect of climate change factors on processes of crop growth and development and yield of groundnut (Arachis hypogaea L.).Crossref | GoogleScholarGoogle Scholar |
Lehrer AT, Komor E (2008) Carbon dioxide assimilation by virus-free sugarcane plants and by plants which were infected by Sugarcane Yellow Leaf Virus. Physiological and Molecular Plant Pathology 73, 147–153.
| Carbon dioxide assimilation by virus-free sugarcane plants and by plants which were infected by Sugarcane Yellow Leaf Virus.Crossref | GoogleScholarGoogle Scholar |
Liu J, Yang J, Bi H, Zhang P (2014) Why mosaic? Gene expression profiling of African cassava mosaic virus‐infected cassava reveals the effect of chlorophyll degradation on symptom development. Journal of Integrative Plant Biology 56, 122–132.
| Why mosaic? Gene expression profiling of African cassava mosaic virus‐infected cassava reveals the effect of chlorophyll degradation on symptom development.Crossref | GoogleScholarGoogle Scholar | 24237761PubMed |
Loebenstein G, Thottappilly G (2013) ‘Virus and virus-like diseases of major crops in developing countries.’ (Springer Science and Business Media: Dordrecht, The Netherlands)
McKirdy S, Jones R, Nutter JRF (2002) Quantification of yield losses caused by Barley yellow dwarf virus in wheat and oats. Plant Disease 86, 769–773.
| Quantification of yield losses caused by Barley yellow dwarf virus in wheat and oats.Crossref | GoogleScholarGoogle Scholar |
Nicaise V (2014) Crop immunity against viruses: outcomes and future challenges. Frontiers of Plant Science 5, 660
| Crop immunity against viruses: outcomes and future challenges.Crossref | GoogleScholarGoogle Scholar |
Osler R, Loi N, Refatti E, Lorenzoni C, Snidaro M (1985) Barley yellow dwarf virus infections in maize (Zea mays L.) inbreds and hybrids in Northern Italy. Maydica 30, 285–290.
Pradhan GP, Xue Q, Jessup KE, Hao B, Price JA, Rush CM (2015) Physiological responses of hard red winter wheat to infection by Wheat streak mosaic virus. Phytopathology 105, 621–627.
| Physiological responses of hard red winter wheat to infection by Wheat streak mosaic virus.Crossref | GoogleScholarGoogle Scholar | 25901871PubMed |
Rapacz M, Hura K (2004) Relationship between quantum efficiency of PSII and cold-induced plant resistance to fungal pathogens. Acta Physiologiae Plantarum 26, 141–148.
| Relationship between quantum efficiency of PSII and cold-induced plant resistance to fungal pathogens.Crossref | GoogleScholarGoogle Scholar |
Rys M, Juhász C, Surówka E, Janeczko A, Saja D, Tóbiás I, Skoczowski A, Barna B, Gullner G (2014) Comparison of a compatible and an incompatible pepper–tobamovirus interaction by biochemical and non-invasive techniques: chlorophyll a fluorescence, isothermal calorimetry and FT-Raman spectroscopy. Plant Physiology and Biochemistry 83, 267–278.
| Comparison of a compatible and an incompatible pepper–tobamovirus interaction by biochemical and non-invasive techniques: chlorophyll a fluorescence, isothermal calorimetry and FT-Raman spectroscopy.Crossref | GoogleScholarGoogle Scholar | 25194777PubMed |
Ryšlavá H, Müller K, Semorádová Š, Synková H, Čeřovská N (2003) Photosynthesis and activity of phosphoenolpyruvate carboxylase in Nicotiana tabacum L. leaves infected by Potato virus A and Potato virus Y. Photosynthetica 41, 357–363.
| Photosynthesis and activity of phosphoenolpyruvate carboxylase in Nicotiana tabacum L. leaves infected by Potato virus A and Potato virus Y.Crossref | GoogleScholarGoogle Scholar |
Schwinghamer MW, Schilg MA, Walsh JA, Bambach RW, Cossu RM, Bambridge JM, Hind-Lanoiselet TL, Mccorkell BE, Cross P (2014) Turnip mosaic virus: potential for crop losses in the grain belt of New South Wales, Australia. Australasian Plant Pathology 43, 663–678.
| Turnip mosaic virus: potential for crop losses in the grain belt of New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Shimura H, Pantaleo V, Ishihara T, Myojo N, Inaba JI, Sueda K, Burgyán J, Masuta C (2011) A viral satellite RNA induces yellow symptoms on tobacco by targeting a gene involved in chlorophyll biosynthesis using the RNA silencing machinery. PLoS Pathogens 7, e1002021
| A viral satellite RNA induces yellow symptoms on tobacco by targeting a gene involved in chlorophyll biosynthesis using the RNA silencing machinery.Crossref | GoogleScholarGoogle Scholar | 21573143PubMed |
Singh A, Nakar R, Chakraborty K, Kalariya K (2014) Physiological efficiencies in mini-core peanut germplasm accessions during summer season. Photosynthetica 52, 627–635.
| Physiological efficiencies in mini-core peanut germplasm accessions during summer season.Crossref | GoogleScholarGoogle Scholar |
Smart RE, Bingham GE (1974) Rapid estimates of relative water content. Plant Physiology 53, 258–260.
| Rapid estimates of relative water content.Crossref | GoogleScholarGoogle Scholar | 16658686PubMed |
Smith P, Sward R (1982) Crop loss assessment studies on the effects of barley yellow dwarf virus in wheat in Victoria. Australian Journal of Agricultural Research 33, 179–185.
| Crop loss assessment studies on the effects of barley yellow dwarf virus in wheat in Victoria.Crossref | GoogleScholarGoogle Scholar |
Spoustová P, Synková H, Valcke R, Čeřovská N (2013) Chlorophyll a fluorescence as a tool for a study of the Potato virus Y effects on photosynthesis of nontransgenic and transgenic Pssu-ipt tobacco. Photosynthetica 51, 191–201.
| Chlorophyll a fluorescence as a tool for a study of the Potato virus Y effects on photosynthesis of nontransgenic and transgenic Pssu-ipt tobacco.Crossref | GoogleScholarGoogle Scholar |
Sun C, Qi H, Hao J, Miao L, Wang J, Wang Y, Liu M, Chen L (2009) Single leaves photosynthetic characteristics of two insect-resistant transgenic cotton (Gossypium hirsutum L.) varieties in response to light. Photosynthetica 47, 399–408.
| Single leaves photosynthetic characteristics of two insect-resistant transgenic cotton (Gossypium hirsutum L.) varieties in response to light.Crossref | GoogleScholarGoogle Scholar |
Thackray D, Diggle A, Jones R (2009) BYDV PREDICTOR: a simulation model to predict aphid arrival, epidemics of Barley yellow dwarf virus and yield losses in wheat crops in a Mediterranean‐type environment. Plant Pathology 58, 186–202.
| BYDV PREDICTOR: a simulation model to predict aphid arrival, epidemics of Barley yellow dwarf virus and yield losses in wheat crops in a Mediterranean‐type environment.Crossref | GoogleScholarGoogle Scholar |
Trębicki P, Nancarrow N, Cole E, Bosque‐pérez NA, Constable FE, Freeman AJ, Rodoni B, Yen AL, Luck JE, Fitzgerald GJ (2015) Virus disease in wheat predicted to increase with a changing climate. Global Change Biology 21, 3511–3519.
| Virus disease in wheat predicted to increase with a changing climate.Crossref | GoogleScholarGoogle Scholar | 25846559PubMed |
Velandia M, Rejesus RM, Jones DC, Price JA, Workneh F, Rush CM (2010) Economic impact of Wheat streak mosaic virus in the Texas High Plains. Crop Protection 29, 699–703.
| Economic impact of Wheat streak mosaic virus in the Texas High Plains.Crossref | GoogleScholarGoogle Scholar |
WASDE (2018) WASDE report. https://www.usda.gov/oce/commodity/wasde/latest.pdf
Zadoks JC, Chang TT, Konzak CF (1974) A decimal code for the growth stages of cereals. Weed Research 14, 415–421.
| A decimal code for the growth stages of cereals.Crossref | GoogleScholarGoogle Scholar |
Zhang F, Liu M, Mo F, Zhang M, Zheng J (2017) Effects of acid and salt solutions on the pasting, rheology and texture of lotus root starch–konjac glucomannan mixture. Polymers 9, 695
| Effects of acid and salt solutions on the pasting, rheology and texture of lotus root starch–konjac glucomannan mixture.Crossref | GoogleScholarGoogle Scholar |
Zhou M, Mendham N (2005) Predicting barley malt extract with a rapid viscoanalyser. Journal of Cereal Science 41, 31–36.
| Predicting barley malt extract with a rapid viscoanalyser.Crossref | GoogleScholarGoogle Scholar |
Zhou Y, Peng Y, Lei J, Zou L, Zheng J, Yu J (2004) Effects of potato virus YNTN infection on gas exchange and photosystem 2 function in leaves of Solanum tuberosum L. Photosynthetica 42, 417–423.
| Effects of potato virus YNTN infection on gas exchange and photosystem 2 function in leaves of Solanum tuberosum L.Crossref | GoogleScholarGoogle Scholar |
Zhou M, Li H, Chen Z, Mendham NJ (2008) Combining ability of barley flour pasting properties. Journal of Cereal Science 48, 789–793.
| Combining ability of barley flour pasting properties.Crossref | GoogleScholarGoogle Scholar |





