Changes in weed species since the introduction of glyphosate-resistant cotton
Jeff Werth A D , Luke Boucher A , David Thornby A , Steve Walker B and Graham Charles CA Agri-Science Queensland, Department of Agriculture, Fisheries and Forestry, Leslie Research Centre, 13 Holberton Street, Toowoomba, Qld 4350, Australia.
B Queensland Alliance for Agriculture and Food Innovation, The University of Queensland, 13 Holberton Street, Toowoomba, Qld 4350, Australia.
C NSW Department of Primary Industries, Cotton Catchment Communities Cooperative Research Centre, Locked Bag 1001, Narrabri, NSW 2390, Australia.
D Corresponding author. Email: jeff.werth@daff.qld.gov.au
Crop and Pasture Science 64(8) 791-798 https://doi.org/10.1071/CP13167
Submitted: 10 May 2013 Accepted: 21 August 2013 Published: 29 October 2013
Journal Compilation © CSIRO Publishing 2013 Open Access CC BY-NC-ND
Abstract
Weed management practices in cotton systems that were based on frequent cultivation, residual herbicides, and some post-emergent herbicides have changed. The ability to use glyphosate as a knockdown before planting, in shielded sprayers, and now over-the-top in glyphosate-tolerant cotton has seen a significant reduction in the use of residual herbicides and cultivation. Glyphosate is now the dominant herbicide in both crop and fallow. This reliance increases the risk of shifts to glyphosate-tolerant species and the evolution of glyphosate-resistant weeds.
Four surveys were undertaken in the 2008–09 and 2010–11 seasons. Surveys were conducted at the start of the summer cropping season (November–December) and at the end of the same season (March–April). Fifty fields previously surveyed in irrigated and non-irrigated cotton systems were re-surveyed.
A major species shift towards Conyza bonariensis was observed. There was also a minor increase in the prevalence of Sonchus oleraceus. Several species were still present at the end of the season, indicating either poor control and/or late-season germinations. These included C. bonariensis, S. oleraceus, Hibiscus verdcourtii and Hibiscus tridactylites, Echinochloa colona, Convolvulus sp., Ipomea lonchophylla, Chamaesyce drummondii, Cullen sp., Amaranthus macrocarpus, and Chloris virgata. These species, with the exception of E. colona, H. verdcourtii, and H. tridactylites, have tolerance to glyphosate and therefore are likely candidates to either remain or increase in dominance in a glyphosate-based system.
Additional keywords: weed survey, glyphosate resistance, species shift, glyphosate-resistant cotton.
Introduction
Weed management practices in cotton systems in the past decade have changed from using residual herbicides and cultivation to permanent beds, reduced cultivation, and a reliance on glyphosate (Charles et al. 2004). The introduction of glyphosate-resistant cotton in 2000 has created an even greater reliance on glyphosate. Glyphosate-resistant cotton allows up to four in-crop applications of glyphosate, with three of these being applied before the crop reaches 22 nodes. When combined with a glyphosate application pre-plant, up to five applications can occur in the cotton cropping season.
Field surveys conducted in 2001, when the reliance on glyphosate was becoming widespread, showed a shift in weed species to those favoured by glyphosate use and little or no cultivation. Reliance on glyphosate has continued, and as a result, populations of glyphosate-resistant Echinochloa colona (L.) Link, Urochloa panicoides P. Beauv., and Conyza bonariensis (L.) Cronquist are becoming evident in grain farming systems. These species have all been identified as high-risk candidates for evolution of glyphosate resistance (Werth et al. 2011), and they have glyphosate-resistant populations in cotton growing regions (Heap 2013). A population of glyphosate-resistant E. colona has now been identified in a glyphosate-resistant cotton farming system.
Previous field surveys conducted in southern Queensland and northern New South Wales cropping areas have identified >100 weed species present (Charles et al. 2004; Rew et al. 2005; Walker et al. 2005). This variability in species results in high variability in biological characteristics. Using a variety of weed-control tactics helps to account for the biological characteristics that allow species to survive and reproduce. A reliance on one tactic is likely to leave the system exposed to species that have inherent characteristics enabling them to reproduce when that tactic is used on its own. A weed-management system reliant on one tactic is therefore the main driver for species shift and herbicide resistance. A recent survey of grower practices in glyphosate-resistant cotton systems showed that an average of three glyphosate applications were common in-crop, with the number of applications ranging from two to six (Werth et al. 2011). This therefore contributes to greater reliance on glyphosate in the system, increasing the risk for species shift and glyphosate resistance (Werth et al. 2011).
Glyphosate is a broad-spectrum herbicide; however, it is not effective on species such as Ipomea lonchophylla J. M. Black, Rhynchosia minima (I.) DC, Convolvulus sp., Neptunia gracilis Benth., and Cyperus rotundus L. (Charles et al. 2004). These species have already increased in or maintained importance since the reliance on glyphosate increased in cotton systems (Charles et al. 2004). The reduction in cultivation favours small-seeded species that prefer surface germination, such as C. bonariensis, Sonchus oleraceus L., and to a lesser extent E. colona. As a result, we would expect to see an increase in these species.
The purpose of this study was to revisit fields previously surveyed to identify any further changes in the weed spectrum that have resulted from a decade of glyphosate-resistant cotton use and continued reliance on glyphosate in fallow situations.
Method
Fifty fields were selected that had been previously surveyed by Walker et al. (2005) in non-irrigated cotton systems in 2001, and by Charles et al. (2004) in irrigated cotton systems in 1992, 1996, and 2001. Twenty-six of the same non-irrigated and 24 of the same irrigated fields were revisited. The fields were located in the Darling Downs (22 fields), and the McIntyre (4 fields), Gwydir (15 fields), and Lower Namoi (9 fields) Valleys in southern Queensland and northern New South Wales.
Four surveys were undertaken in the 2008–09 and 2010–11 seasons. Surveys were conducted at the start of the summer cropping season (November–December; just after planting) and at the end of the same season (March–April; just before harvest). Surveys were done at these times to determine which weed species were present at the time early-season herbicides are applied, and to identify weeds that either germinated later in the season or were poorly controlled throughout the season. The surveys in 2010–11 were conducted in order to ascertain changes in weed species since 2001, not to identify changes with respect to the 2008–09 season.
Surveys were done in a similar manner to Walker et al. (2005). Transects with quadrats ~50 m apart were surveyed in each section so that 20 quadrats per field were surveyed; quadrats were 10 m by 1 m. The presence and density of each weed species were noted in each quadrat. Species density was rated using a 0–3 scale: 0, no weeds/10 m2; 1, 1–9 weeds/10 m2; 2, 10–100 weeds/10 m2; 3, >100 weeds/10 m2.
Results
2008–09 survey
In the 2008–2009 survey, 12 fields were in sorghum; four in irrigated, glyphosate-resistant cotton; two in maize; one in sunflower; and the remaining 31 in fallow. Over 70 species were observed in the surveys with the majority present in low densities.
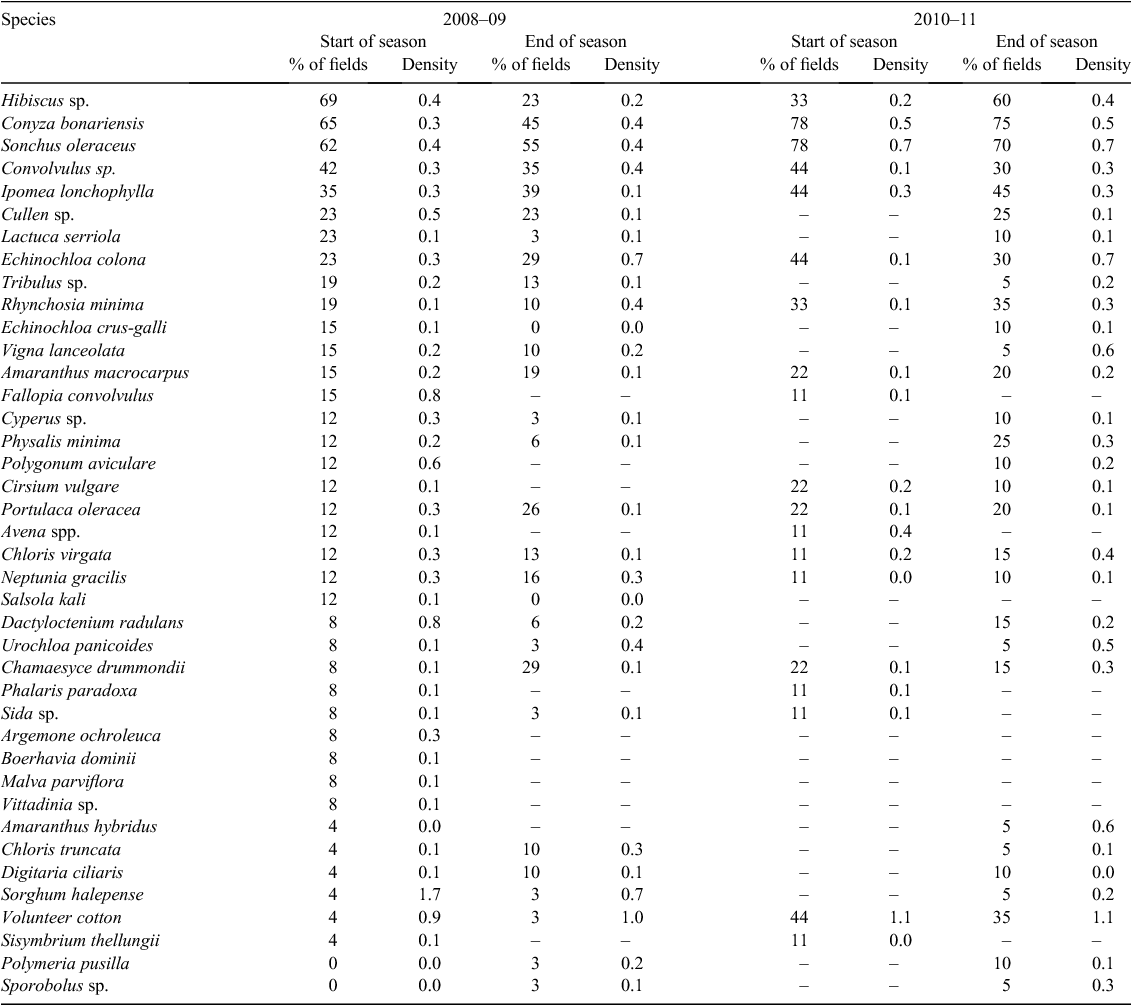
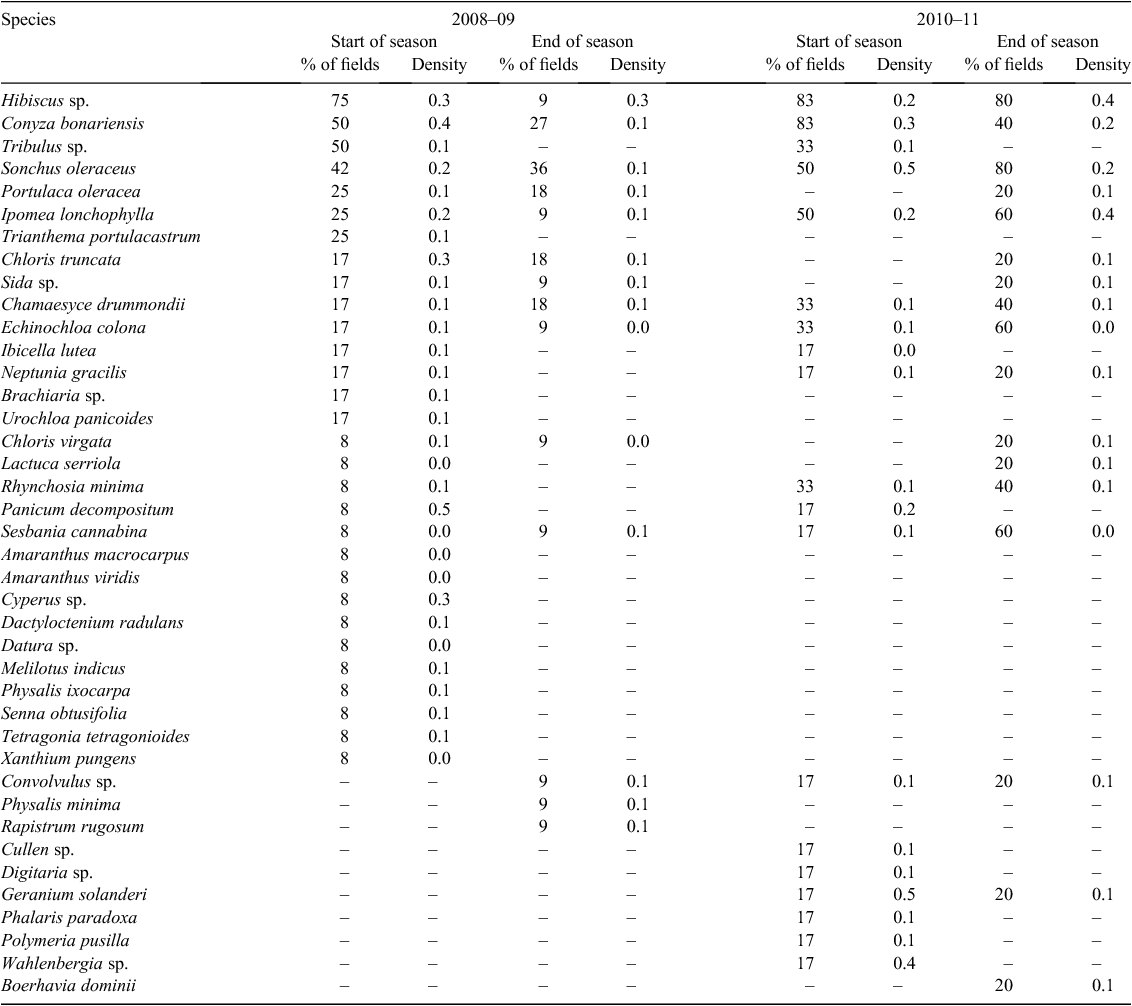
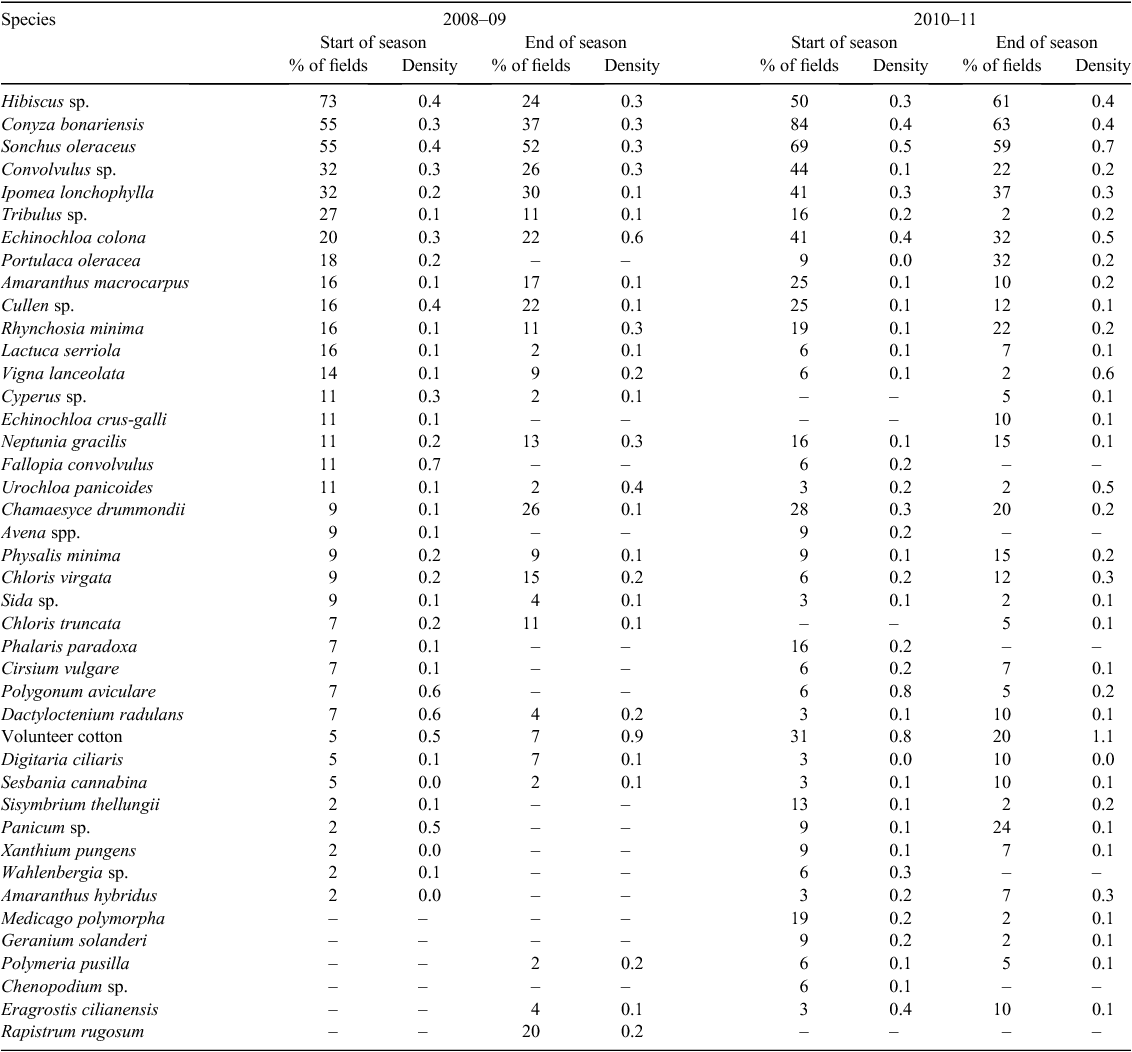
The most common weeds present across all fields at the start of the season were the two Hibiscus species (Hibiscus tridactylites Lindley and H. verdcourtii Craven), which were present in 73% of fields (Table 1). These two species look very similar in early growth stages and, as a result, were grouped together. Conyza bonariensis and S. oleraceus were present in 55% of fields. The next most common weeds were Convolvulus sp. and I. lonchophylla, both of which were present in 32% of fields. These were followed by Tribulus sp. and E. colona, present in 27% and 20% of fields, respectively. Hibiscus sp. was the most common weed found in all crops, with 69% in fallow (Table 2), 75% in sorghum (Table 3), and 50% in cotton (Table 4). Conyza bonariensis was next with 65% in fallow, 50% in sorghum, and 25% in cotton. Sonchus oleraceus was present in 62% of fields in fallow, 42% in sorghum, and 25% in cotton.
|
The most common species at the end of the season across all fields was S. oleraceus, which was present in 52% of fields. This was followed by C. bonariensis (37%), I. lonchophylla (30%), and Convolvulus sp. and Chamaesyce drummondii (Boiss.) D.C. Hassall (both present in 26% of fields). In fallow, the most common species were S. oleraceus (55%), C. bonariensis (45%), I. lonchophylla (39%), Convolvulus sp. (35%), and E. colona and C. drummondii (29%). Species found at the end of the season in sorghum were S. oleraceus (36%), C. bonariensis (27%), and Portulaca oleracea L., Chloris truncata R.Br., and C. drummondii (all present in 18% of fields). The cotton fields surveyed in 2008–09 were all irrigated fields, and at the time the surveys were conducted, the cotton had reached canopy closure; in fields that were able to be entered, no visible weeds could be identified.
2010–11 survey
In total, 46 fields were surveyed in 2010–11 due to a change of ownership on two farms that contained four fields. Of these, 15 fields contained glyphosate-resistant cotton (two non-irrigated), six contained sorghum, two contained maize, with the remaining 23 in fallow. At the start of the season, nine fields in the Lower Namoi Valley were unable to be reached to due to an extremely wet season. These fields were surveyed at the end of the season in February 2011.
Conyza bonariensis was by far the most common species present across all fields at the start of the season, found in 84% of fields. Sonchus oleraceus was next, followed by Hibiscus sp., present in 69% and 50% of fields, respectively. Convolvulus sp. (44%) and I. lonchophylla and E. colona (both at 41%) were also common. Volunteer cotton was also found in 31% of fields. Both C. bonariensis and S. oleraceus were widespread in the fallow, and were found in 78% of fields surveyed. Ipomea lonchophylla, Convolvulus sp., E. colona, and volunteer cotton were all found in 44% of fields in fallow. Hibiscus sp. and C. bonariensis dominated sorghum fields and were both found in 83% of fields, with S. oleraceus and I. lonchophylla present in 50% of fields. In cotton, C. bonariensis was found in 10 of the 11 fields (91%) surveyed at the start of the season, while S. oleraceus was found in 64% and Cullen sp. (emu-foot) and I. lonchophylla were both found in 55% of fields.
At the end of the season, C. bonariensis was present in 63% of fields, followed by Hibiscus sp. (61%) and S. oleraceus (59%). Fallow fields were dominated by the same species: C. bonariensis (75%), S. oleraceus (70%), and Hibiscus sp. (60%). Ipomea lonchophylla was present in 45% of fallow fields, and volunteer cotton and Rhynchosia minima were both present in 35% of fields. Sorghum fields were also dominated by C. bonariensis and S. oleraceus (80%), followed by I. lonchophylla, E. colona, and Sesbania cannabina (Retz.) Pers. (60%). In the 15 cotton fields surveyed at the end of the season (surveyed slightly earlier, before total canopy closure), 53% of fields contained C. bonariensis and Hibiscus sp., 47% contained P. oleracea, and 40% S. oleraceus. Echinochloa colona was present in 27% of fields surveyed.
Discussion
The major change since the earlier surveys has been the increase in C. bonariensis. In non-irrigated fields, in the surveys conducted by Walker et al. (2005) it was ranked fourteenth compared with second in 2008 and first in 2010 (Table 5). In irrigated fields, C. bonariensis did not rank in the top 20 in surveys conducted by Charles et al. (2004) and in 2010 was also ranked first (Table 6). Conyza bonariensis is particularly adapted to no-till systems based on glyphosate. It has long been considered tolerant, and more recently has been confirmed resistant to glyphosate (Heap 2013). It only emerges from the top 0.5 cm of soil and thrives in a no-till system due to its ability to survive glyphosate-based herbicide applications, and its capacity to produce >100 000 seeds per plant (Wu et al. 2007). Even now, with the advent of the ‘double-knock’ as a control tactic (Werth et al. 2010), cropping systems without tillage are struggling to provide effective long-term control, particularly in cotton systems where the variety of herbicides available is restricted due to residual effects on the following cotton crop.

|

|
The prevalence of C. bonariensis has been slower to develop in irrigated cotton systems than grain systems and, to a lesser extent, non-irrigated cotton–grain systems. This is most likely due to a wider reduced reliance of glyphosate, particularly with the first generation glyphosate-resistant cotton varieties only allowing two over-the-top applications of glyphosate before four nodes of cotton growth (Charles and Taylor 2006). However, the increase in and continued prevalence of C. bonariensis has forced some growers to incorporate other herbicides and tillage, even if it is short-term. One grower, in particular, applied diuron before planting cotton. As a result, his fleabane (C. bonariensis) density reduced from 0.8/10 m2 in 2008 to 0.2/10 m2 in 2010; this was also in a wet/cool season, which favoured fleabane emergence. This practice has now been adopted widely throughout the cotton industry. Other growers have been incorporating tillage into their program to improve C. bonariensis management.
The reduction in tillage and subsequent reliance on glyphosate in irrigated cotton systems was noted when comparing species present with surveys conducted by Charles et al. (2004). Conyza bonariensis prevalence increased dramatically, particularly in the non-cotton phases of the rotation when virtually no tillage is done. Sonchus oleraceus was ranked thirteenth in the fields surveyed by Charles et al. (2004), and increased to third in 2008 and second in 2010. It was also ranked highly in the Walker et al. (2005) survey. Like C. bonariensis, S. oleraceus is a small-seeded asteraceae that is favoured by no-till cropping systems as it only germinates from the soil surface. It has the ability to germinate year-round, making it a difficult weed for which to achieve season-long control.
In both seasons, C. bonariensis all but maintained its prevalence in and density throughout the season in fallow, and slightly decreased in sorghum and cotton. The use of atrazine in sorghum and diuron in cotton facilitated this decline. The cool wet season in 2010–11 enabled more C. bonariensis germination than is expected in a warmer season, resulting in greater prevalence. Sonchus oleraceus prevailed in much the same way as C. bonariensis, and in many fields was present in higher numbers resulting in higher density ratings. At one farm, both species had set seed in the non-crop area between fields; this is a ready source on re-infestation that can undermine attempts to manage these species in the fields, particularly with their high seed production.
Hibiscus sp. continues to be one of the major weeds in all fields and years. Hibiscus sp. seed is persistent, with previous studies showing that after 24 months of burial almost half of the seeds were still viable (Walker et al. 2010). It can also emerge from the surface down to 5 cm deep. Therefore, it stays in the seed bank for long periods. Hibiscus sp. is capable of producing >5000 seeds per plant (Johnson et al. 2003). It also has the ability to germinate throughout the season, as reflected in its prevalence in the surveys conducted at the end of the season. Plants surveyed at this time were a combination of surviving plants and new germinations, and in the relatively wet 2010–11 season, prevalence of Hibiscus sp. was higher across all fields at the end of season compared with the beginning (Tables 1–4). These factors all combine to replenish the Hibiscus sp. seed bank continually.
Ipomea lonchophylla has also maintained prevalence since 2001. Seeds have strong dormancy and can remain viable in the soil for many years. Ipomea lonchophylla can also emerge year-round with available soil moisture from rainfall or irrigation. In addition, glyphosate provides little control (Charles and Taylor 2006). The prevalence of Amaranthus macrocarpus, a small-seeded species favoured by reduced tillage, also increased from sixteenth to fifth in fields previously surveyed by Charles et al. (2004) (Table 6).
Other weeds that remained at the end of the season were Convolvulus sp., E. colona, P. oleracea, C. drummondii, R. minima, and N. gracilis. These species all have the ability to emerge throughout the season.
In the 2010–11 survey, a slight increase in prevalence in some annual summer grasses was observed. These include Dactyloctenium radulans (R.Br.) Beauv., Panicum decompositum R.Br., Digitaria ciliaris (Retz.) Koel., and Chloris virgata Sw. Prevalence of C. virgata was similar to that in the 2008–09 survey. It is a problem weed in central Queensland (Osten 2008), and appears to be on the increase in the survey region as well.
The incidence of volunteer cotton increased substantially from 5% of fields in 2008 to 31% in 2010. This was largely due to more cotton being grown in the survey region in the previous season (26 600 ha in 2007–08 compared with 146 000 ha in 2009–10). These are predominately glyphosate-resistant cotton volunteers surviving fallow glyphosate sprays. Volunteer cotton is becoming an increasing issue across the region, particularly because of negative impacts on insects and diseases (Taylor and Maas 2013).
This study has shown that the reliance on one weed-control method, in this case glyphosate, has led to changes in the weed spectrum to glyphosate-tolerant and -resistant species. Irrigated cotton systems, which used to employ a wide range of control tactics such as residual herbicides and tillage, are also seeing changes in weed species associated with an increased reliance on glyphosate. The recent study by Werth et al. (2011) has indicated that glyphosate use, and herbicide use in general, in irrigated crops is becoming very similar to use in non-irrigated crops. Growers are being forced to implement additional tactics such as the double-knock and residual herbicides to control these species, in particular C. bonariensis. However, to be effective in the long term, these additional tactics need to be planned rather than reactive.
Acknowledgements
This research was done in partnership with the Cotton Catchment Communities Cooperative Research Centre, the Cotton Research and Development Corporation and Monsanto Australia Ltd.
References
Charles G, Taylor I (2006) Positioning the second generation of herbicide tolerant cotton varieties—Roundup Ready Flex® and Liberty Link® cottons—into Australian cotton farming systems: opportunities and threats. In ‘15th Australian Weeds Conference’. Adelaide, S. Aust. (Eds C Preston, JH Watts, ND Crossman) pp. 359–362. (Weed Management Society of South Australia Inc.: Adelaide, S. Aust.)Charles G, Taylor I, Roberts G (2004) The impact of the cotton farming system on weed succession: implications for herbicide resistance and adoption of an integrated weed management approach. In ‘14th Australian Weeds Conference’. Wagga Wagga, NSW. (Eds BM Sindel, SB Johnson) pp. 410–413. (Weed Society of New South Wales Inc.: Sydney, NSW)
Heap I (2013) The International Survey of Herbicide Resistant Weeds. Available at: www.weedscience.org.
Johnson SB, Sindel BM, Charles G (2003) What bladder ketmia have you got? In ‘The Australian Cottongrower’. Vol. 24(5). pp. 50–54. (Greenmount Press: Toowoomba, Qld)
Osten V (2008) ‘Managing feathertop Rhodes grass: A best weed management guide.’ (Department of Employment, Economic Development and Innovation: Brisbane, Qld)
Rew LJ, Medd RW, Van de Ven R, Gavin JJ, Robinson GR, Tuitee M, Barnes J, Walker S (2005) Weed species richness, density and relative abundance on farms in the subtropical grain region of Australia. Australian Journal of Experimental Agriculture 45, 711–723.
| Weed species richness, density and relative abundance on farms in the subtropical grain region of Australia.Crossref | GoogleScholarGoogle Scholar |
Taylor F, Maas S (2013) Volunteer and ratoon cotton. In ‘Cotton Pest Management Guide 2012–2013’. (Cotton Research and Development Corporation: Narrabri, NSW)
Walker SR, Taylor IN, Milne G, Osten VA, Hoque Z, Farquharson RJ (2005) A survey of management and economic impact of weeds in dryland cotton cropping systems of subtropical Australia. Australian Journal of Experimental Agriculture 45, 79–91.
| A survey of management and economic impact of weeds in dryland cotton cropping systems of subtropical Australia.Crossref | GoogleScholarGoogle Scholar |
Walker S, Wu H, Bell K (2010) Emergence and seed persistence of Echinochloa colona, Urochloa panicoides and Hibiscus trionum in the sub-tropical environment of north-eastern Australia. Plant Protection Quarterly 25, 127–132.
Werth J, Walker S, Boucher L, Robinson G (2010) Applying the double knock technique to control Conyza bonariensis. Weed Biology and Management 10, 1–8.
| Applying the double knock technique to control Conyza bonariensis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXltVanurk%3D&md5=02ee41ab5f7e6bd5c5939012dd1d2cf3CAS |
Werth J, Thornby D, Walker S (2011) Assessing weeds at risk of evolving glyphosate resistance in Australian sub-tropical glyphosate-resistant cotton systems. Crop & Pasture Science 62, 1002–1009.
| Assessing weeds at risk of evolving glyphosate resistance in Australian sub-tropical glyphosate-resistant cotton systems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhs1Gru7jJ&md5=c6dca04913fc19f4651f9a0340eb5bf2CAS |
Wu H, Walker S, Rollin MJ, Tan DKY, Robinson G, Werth J (2007) Germination, persistence, and emergence of flaxleaf fleabane (Conyza bonariensis [L.] Cronquist). Weed Biology and Management 7, 192–199.
| Germination, persistence, and emergence of flaxleaf fleabane (Conyza bonariensis [L.] Cronquist).Crossref | GoogleScholarGoogle Scholar |