The Chemistry of Cannabis and Cannabinoids
Peter J. Duggan A
A
A CSIRO Manufacturing, Research Way, Clayton, Vic. 3168, Australia; and College of Science and Engineering, Flinders University, Adelaide, SA 5042, Australia. Email: peter.duggan@csiro.au
Australian Journal of Chemistry 74(6) 369-387 https://doi.org/10.1071/CH21006
Submitted: 5 January 2021 Accepted: 17 February 2021 Published: 18 March 2021
Journal Compilation © CSIRO 2021 Open Access CC BY-NC-ND
Abstract
The science of cannabis and cannabinoids encompasses a wide variety of scientific disciplines and can appear daunting to newcomers to the field. The encroachment of folklore and ‘cannabis culture’ into scientific discussions can cloud the situation further. This Primer Review is designed to give a succinct overview of the chemistry of cannabis and cannabinoids. It is hoped that it will provide a useful resource for chemistry undergraduates, postgraduates and their instructors, and experienced chemists who require a comprehensive and up to date summary of the field. The Review begins with a brief overview of the history and botany of cannabis, then goes on to detail important aspects of the chemistry of phytocannabinoids, endocannabinoids and synthetic cannabinomimetics. Other natural constituents of the cannabis plant are then described including terpenes and terpenoids, polyphenolics, alkaloids, waxes and triglycerides, and important toxic contaminants. A discussion of key aspects of the pharmacology associated with cannabinoids and the endocannabinoid system then follows, with a focus on the cannabinoid receptors, CB1 and CB2. The medicinal chemistry of cannabis and cannabinoids is covered, highlighting the range of diseases targeted with cannabis and phytocannabinoids, as well as key aspects of phytocannabinoid metabolism, distribution, and delivery. The modulation of endocannabinoid levels through the inhibition of key endocannabinoid-degrading enzymes fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL) is then discussed. The Review concludes with an assessment of the much touted ‘entourage effect’. References to primary literature and more specialised reviews are provided throughout.
Introduction
With the recent relaxations in the legal restrictions on medicinal cannabis and hemp in many jurisdictions around the world, there has been a huge surge in interest in cannabis-related science, including all aspects of cannabis chemistry. There is still much to be learnt about the chemistry of the cannabis plant, its derived products, and the biological effects of its constituents. The lack of fundamental knowledge about some aspects of cannabis science stems from the fact that worldwide prohibition has greatly hindered scientific investigations in the field. The purpose of this primer review is to provide an overview of the current knowledge and future directions of cannabis chemistry research relevant to the commercial and medicinal applications of cannabis products.
History and Botany
Cannabis has been cultivated by humans since ancient times, with many researchers suggesting that it was one of the first crops to be domesticated.[1] There is debate about its natural geographic origin, with central and south-east Asia being most commonly suggested. However, there is strong evidence that its cultivation and use developed independently in Europe and east Asia.[2] From early times, the plant became a source of fibre, food, and herbal medicine, and was incorporated into religious practices. Cannabis became a ‘camp follower’, with seeds and perhaps a limited number of plants being carried between campsites. Cannabis plants readily emerged along tracks and roads, and thrived in nitrogen-rich, organic waste heaps close to settlements.[2]
Over many years, early farmers used selective breeding and other agricultural practices to produce taller varieties – optimised for fibre production – and cultivars that yielded larger seeds, more suitable as sources of food.[3] This led to the varieties collectively known today as hemp. Other phenotypes, selected for their ability to express high levels of resin and that often induced feelings of euphoria when consumed, are usually referred to today as marijuana or medicinal cannabis.
The taxonomy of cannabis is still subject to much debate. The forms rich in psychoactive secondary metabolites, i.e. phytocannabinoids, are commonly divided into three hypothetical species, Cannabis sativa L. (C. sativa), Cannabis indica Lam. (C. indica), and Cannabis ruderalis Janisch. (C. ruderalis). The latter variety tends to exist mainly as wild populations growing in central and eastern Europe and central Asia. By contrast, C. sativa, originally bred in India and south-east Asia, and C. indica, originating in Afghanistan, have been intensively cultivated. The taxonomic confusion stems from the fact that these three hypothetical species do not appear to possess any genetic or physiological features that limit cross-fertilisation,[4] and usually yield fully fertile hybrids.[3] This has been used to argue that marijuana-type cannabis should be considered to be one species, C. sativa, with variations classified in terms of subspecies and varieties.[2,4] Regardless, widespread interbreeding has meant that divisions between the ‘species’ are becoming increasingly blurred. Genetic analysis of representative samples of marijuana and hemp, however, revealed that these broad cultivar classes are genetically distinct.[4] European hemp and east Asian hemp are also thought to be genetically different.[2]
Cannabis is dioecious, meaning that it exists as separate male and female plants. Small glandular projections, known as trichomes, are expressed on its leaves, stems and especially bracts – small leaf-like structures associated with buds and flowers. The trichomes are where the majority of the interesting secondary metabolites are found, including phytocannabinoids and terpenes. The female buds tend to be the richest source of these compounds. It is believed that the plant produces the metabolites for defence and to limit competition from other plants. In particular, terpenes and phytocannabinoids can protect the plant from microbial infestations and deter predation by insects. Terpenes can also be used by the plant to attract insects that attack the larvae and eggs of predatory insects. The resinous nature of the mixtures expressed in the trichomes can also serve as a form of defence, ensnaring insects in a way similar to that used by some carnivorous plants to trap their prey. The more volatile terpenes produced by cannabis, responsible for its characteristic aroma, may also suppress the growth of proximal vegetation.[1] While it might seem surprising that humans have found uses for the secondary metabolites in cannabis that are totally unrelated to the purpose for which they originally evolved, this is not unique. There are actually many other examples of humans consuming defensive secondary metabolites produced by plants, including caffeine, the opiates, cocaine, and nicotine.
Cannabis is wind-pollinated and is highly susceptible to cross-breeding. Even in a controlled environment, genetic purity is difficult to maintain if sexual reproduction is used. For these reasons, commercial medicinal cannabis growers tend to propagate their production plants through cuttings from vegetative ‘mother plants’. The architecture of the female buds is influenced by day length. As the number of daylight hours begins to decrease, following a period of growth during longer days, buds undergo intensive branching,[5] ultimately leading to higher trichome yield. Phytocannabinoid content can also be influenced by growing conditions including temperature, humidity, and soil nutrients.[1] As a consequence of these attributes of cannabis, a lot of medicinal cannabis production occurs indoors where growing conditions, including light exposure, can be carefully controlled and incidental cross-pollination can be prevented. Many commercially grown cultivars have been bred to produce large, dense, and tightly packed female buds, giving higher phytocannabinoid yield per plant, but the moist conditions within the buds makes them highly susceptible to fungal infestations including grey mould (Botrytis cinerea Pers.). This is the same pathogen that causes ‘noble rot’ in grapes. Unfortunately, the natural resistance to fungal attack possessed by varieties originating in warm, humid climates such as south-east Asia has been inadvertently bred out by crossing with non-resistant plants emanating from arid zones like Afghanistan, where fungal attack poses little threat.[3]
Cannabinoid Chemistry
The term cannabinoid generally refers to any chemical substance that associates with mammalian cannabinoid receptors and elicits biological effects through this association. Cannabinoids are usually divided into three main groups: phytocannabinoids† – cannabinoids found in plants; endocannabinoids – endogenous compounds found in animals that modulate the same receptors as those affected by certain phytocannabinoids (the cannabinoid receptors); and synthetic cannabinomimetics – synthetic compounds that may or may not be structurally related to the phytocannabinoids that also produce agonistic effects in cannabinoid receptors.
Phytocannabinoids
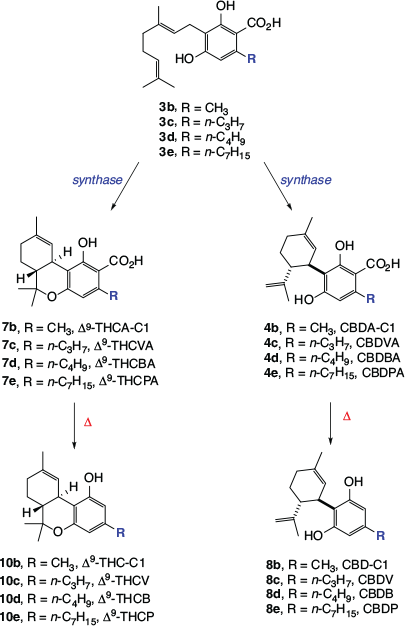
The chemical structure of the most well-known phytocannabinoid, Δ9-tetrahydrocannabinol, or Δ9-THC (10a), was reported by Gaoni and Mechoulam in 1964.[6] This is the compound, often simply referred to as ‘THC’, that is mainly responsible for the euphoric effects induced by marijuana-type cannabis. Allegedly, cannabis cultivars have now been bred to produce buds with up to 30 wt-% Δ9-THC equivalent when dried. Δ9-Tetrahydrocannabinol is just one of over 100 phytocannabinoids that have so far been found in cannabis. The most abundant ones bear an n-pentyl side chain, and their routes of formation are shown in Scheme 1.
Cannabigerolic acid (CBGA, 3a) is the common biosynthetic precursor for all of the most abundant phytocannabinoids. It is produced by linking two fragments, the geranyl portion of geranylpyrophosphate (1), which is generated via the terpene biosynthetic pathway, and olivetolic acid (2), which is formed via the polyketide biosynthetic pathway. The production of CBGA (3a) is catalysed by geranylpyrophosphate:olivetolic acid geranyltransferase (GOT).[7] Other plant enzymes catalyse three different types of cyclisation reactions of CBGA. Single ring closures produce cannabichromenic acid (CBCA, 6) and cannabidiolic acid (CBDA, 4a), and a double cyclisation leads to Δ9-tetrahydrocannabinolic acid (Δ9-THCA, 7a).[8] Technically, these three carboxylic acids, together with CBGA, are the only true cannabinoid natural products shown in Scheme 1. The other cannabinoids are effectively degradation products resulting from the exposure of the acids to heat, light, and oxidants. The four cannabinoid acids can all undergo thermally induced decarboxylation to the corresponding ‘neutral’ forms cannabigerol (CBG, 5), cannabichromene (CBC, 9), Δ9-THC (10a), and cannabidiol (CBD, 8a). This process begins spontaneously following harvest, but happens very slowly at room temperature. The loss of carbon dioxide occurs much more rapidly at high temperatures, for example when the dried crop is burnt or heated in other ways. Most of the biological effects resulting from cannabis administration are induced by the neutral cannabinoids, which explains why Δ9-THC is often delivered by smoking when cannabis is used recreationally. Δ9-Tetrahydrocannabinol (10a) can also undergo several chemical reactions, most notably isomerisation to Δ8-tetrahydrocannabinol (Δ8-THC, 11) through relocation of the Δ9-double bond, and oxidation to the fully aromatic cannabinol (CBN, 13). Cannabinol was actually the first cannabinoid to be fully characterised, following a series of investigations undertaken in Cahn’s laboratory in the 1930s.[9] An alternative degradation product, cannabicyclol (CBL, 12), is formed via a 2+2 photocyclisation of CBC, promoted by natural light.
The main cannabinoid produced in hemp-type cannabis is CBDA, whereas the marijuana-type cannabis cultivars were originally bred to produce high levels of Δ9-THCA. Since CBD and mixtures of CBD and Δ9-THC are of significant interest for their potential in the treatment of a spectrum of disorders and diseases in humans and companion animals (see below), medicinal cannabis manufacturers often cultivate a series of varieties that range from high CBDA producers through to cultivars that yield high levels of Δ9-THCA.
Phytocannabinoids that have different numbers of carbons in their alkyl side chains have also been isolated from cannabis. In addition to the major phytocannabinoids that bear n-pentyl side chains (Scheme 1), Δ9-THC and CBD equivalents with methyl,[10] n-propyl,[10] n-butyl,[11] and n-heptyl[12] moieties have been found in cannabis (see Scheme 2). These metabolites occur at much lower levels than the major phytocannabinoids, with the ‘varinoids’ (tetrahydrocannabivarinic acid (Δ9-THCVA, 7c), tetrahydrocannabivarin (Δ9-THCV, 10c), cannabidivarinic acid (CBDVA, 4c), and cannabidivarin (CBDV, 8c)) being the only analogues that are currently routinely quantified in samples of medicinal cannabis. Interestingly, cis-perrottetinene and perrottetinenic acid, which are structurally closely related to Δ9-THC and Δ9-THCA respectively, have been isolated from liverworts of the Radula genus. They represent a different class of phytocannabinoid to that found in cannabis, having different stereochemistry and a side chain bearing a phenyl substituent.[13] Other types of cannabinoids, including CBG, CBGA, CBC, CBCA, and CBL analogues, have been isolated from a range of other flowering plants.[14]
|
Endocannabinoids
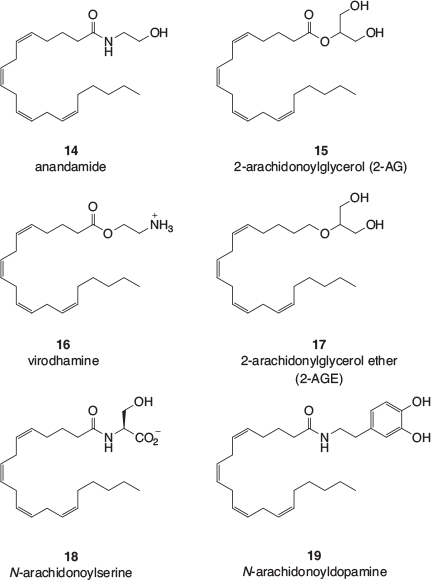
It was not until the early 1990s that the mammalian receptors for the phytocannabinoids, CB1 and CB2, were characterised (see below), and it was after those discoveries that the endogenous ligands for the receptors, termed the endocannabinoids, began to be identified. Examples are shown Fig. 1. Anandamide (14) and 2-arachidonoylglycerol (2-AG, 15) were the first to be identified and their biological roles are still the subject of intensive research. These arachidonic acid derivatives appear to be members of a large class of lipophilic amides, esters, and ethers that make up what has become known as the endocannabinoidome.[15] Virodhamine (16)[16] is the O-acyl analogue of anandamide and 2-arachidonylglycerol ether (2-AGE, 17) is a reduced form of 2-AG. Other endocannabinoids include N-acylamino acids (eg N-arachidonoylserine, 18) and N-acylated neurotransmitters (eg N-arachidonoyldopamine, 19).
|
The endocannabinoidome is also believed to include congeners of several of the endocannabinoids shown in Fig. 1.[15] For example, postulated congeners of anandamide are ethanolamides derived from other endogenous long chain fatty acids such as the highly unsaturated docosahexaenoic acid, the doubly unsaturated linoleic acid, the monounsaturated oleic acid, and the fully saturated palmitic acid. Similarly, congeners of 2-AG include 2-oleoylglycerol and 2-linoleoylglycerol. Participating N-acylated amino acid analogues of N-arachidonoylserine (18) include those derived from glycine and 2-aminoethanesulfonic acid (taurine). Together with the N-acyldopamine congeners, N-acylserotonins are also thought to be part of the endocannabinoidome.
Synthetic Cannabinomimetics
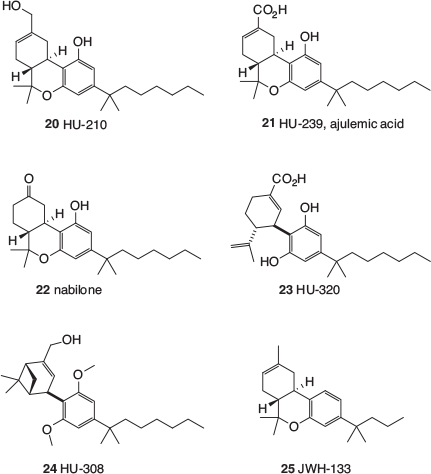
The earliest class of cannabinomimetics is sometimes referred to as ‘classical cannabinoids’, and largely emanated from the laboratory of Raphael Mechoulam at Hebrew University. Mechoulam is a pioneer in the field of cannabinoid chemistry and reported the first synthesis of Δ9-THC in 1972.[17] Using methods developed during the synthesis of such phytocannabinoids, Mechoulam’s group prepared a range of analogues and tested them for biological activity.[18] Most of these compounds are often referred to by their ‘HU’ codes, HU being short for Hebrew University. Results obtained from variation in the aliphatic side chain, inspired by much earlier work undertaken separately in the 1940s by Adams[19] and Todd,[20] reinforced the finding that stronger and longer-lasting activity in animals was obtained with THC analogues bearing heptyl side chains with attached methyl groups. The 1,1′-dimethylheptyl (DMH) substituent became prominent and can now be found in several important cannabinomimetics (Fig. 2) including HU-210 (20),[21] ajulemic acid (21),[22] and nabilone (22).[23] The former two (20 and 21) resemble metabolites of Δ8-THC and nabilone is a drug developed by Eli Lilly and used for the treatment of anorexia and chemotherapy-induced nausea and vomiting. Other cannabinomimetics that bear the DMH side chain are HU-320 (23),[24] which is analogous to a metabolite of CBD, and the more elaborate HU-308 (24).[25] A much simpler mimetic is JWH-133 (25),[26] which is closely related to Δ8-THC. JWH stands for John W. Huffman, whose laboratory at Clemsen University produced a large number of cannabinomimetics in the latter part of the 20th century.
|
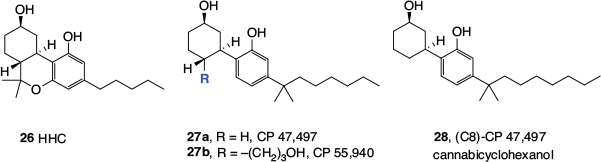
A series of cannabinomimetic cyclohexanols, often referred to as ‘non-classical cannabinoids’, have also been developed. They began with HHC (26, Fig. 3), which is a direct analogue of THC, and was reported by the US National Institutes of Health in 1976.[27] Ring-opened versions, CP 47,497 (27a) and CP 55,26 940 (27b), which also incorporated the DMH side chain, were later invented by Pfizer in the early 1980s.[28] The prefix ‘CP’ refers to Karl Pfizer, also known as Charles Pfizer, the founder of Pfizer Pharmaceuticals. The compound bearing the propyl alcohol substituent, 27b, is still commonly used as a pharmacological tool in cannabinoid receptor studies (see below).
|
Any discussion of synthetic cannabinomimetics inevitably leads to the subject of ‘synthetic cannabis’, herbal blends that are adulterated with cannabinomimetics and sold over the counter or the internet. These products have been of significant concern to health authorities, regulators, and law enforcement worldwide. The octyl analogue of 27a, (C8)-CP 47,497 (28), also regularly referred to as ‘cannabicyclohexanol’, was the first synthetic cannabinomimetic to be found in a commercial ‘synthetic cannabis’, along with a stereoisomer, CP 47,497 (27a) and JWH-018 (33c, Fig. 4).[29]
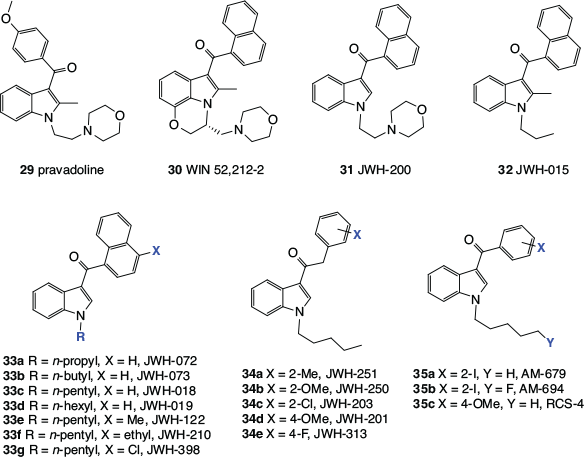
|
The discovery in the late 1980s that pravadoline (29), a non-steroidal anti-inflammatory drug (NSAID), and the related indole derivative, WIN 55,212-2 (30), produce pharmacological responses similar to those induced by phytocannabinoids like Δ9-THC,[30,31] opened up a whole new field of synthetic cannabinomimetics. These were the first cannabinomimetics with no obvious structural similarity to the phytocannabinoids and structure–activity relationships (SARs) for these compounds have been intensively investigated. Huffman’s laboratory has been a major source of new indole-based cannabinomimetics and hence many important compounds in this series bear the ‘JWH’ label.[32] The examples 29–35 shown in Fig. 4 demonstrate how the original ‘hits’, 29 and 30, were progressively simplified, leading to cannabinomimetics that synthetically are readily accessible. The benzoyl indoles (35a and 35b) were reported by Makriyannis and Deng, then at The University of Connecticut, in a patent,[33] whereas RCS-4 (35c) is an example of a ‘designer drug’. This exceedingly simple compound was first identified as an adulterant in illicit products available online by researchers at the Tokyo Metropolitan Institute of Public Health in 2011,[34] before any report in the scientific literature of its synthesis or biological activity.[35]
Many more synthetic cannabinomimetics have been reported but a majority of them are analogues of the structures described here, particularly the indoles. They have been varied by the replacement of all heteroatoms with carbon, the incorporation of additional heteroatoms, such as nitrogen and oxygen, into the indole core, or the replacement of the aromatic substituents with aliphatic sidechains.
The biological activity of synthetic cannabinomimetics is stereochemistry-dependent. For example, HU-211, which is the enantiomer of HU-210 (20), does not interact with cannabinoid receptors but instead blocks N-methyl-d-aspartate (NMDA) receptors,[36] and the R enantiomer of WIN 52,212-2 (30) is much more active than the S enantiomer.[37]
Terpenes and Terpenoids
Terpenes and terpenoids are largely responsible for the characteristic odour of cannabis and are thought by some to contribute to the potential therapeutic benefits of cannabis. They would undoubtedly play a prominent role in any possible placebo effects resulting from cannabis consumption. For these reasons, and their relative abundance in cannabis, terpenes and terpenoids are considered by consumers and medical scientists and practitioners to be the second-most important class of secondary metabolites produced by the cannabis plant. Terpenes identified to be present in cannabis consist of monoterpenes, 10-carbon hydrocarbons biosynthesised from geranylpyrophosphate (1), and sesquiterpenes. The latter are 15-carbon hydrocarbons biosynthesised from the 15-carbon analogue of geranylpyrophosphate, farnesylpyrophosphate. Several terpene synthases present in cannabis are multi-product enzymes, meaning that a single enzyme can catalyse the production of a range of different terpenes from the same precursor.[38]
The term terpenoid refers to terpenes that have been further modified by the incorporation of oxygen. This may occur as a result of enzyme action within the plant or by a range of non-enzymatic hydration or oxidation processes. These include thermal and photochemical rearrangements. Many of the non-enzymatic processes can occur within or on the crop following harvest. Terpene and terpenoid profiles can show considerable variation, even in plant material obtained from the same cannabis variety, and vary depending on the way the harvested material has been handled and stored. This is because monoterpenes can be quite volatile and hence easily lost after harvest, and the terpenoid content can continue to change over time as a result of the abovementioned non-enzymatic chemical processes.
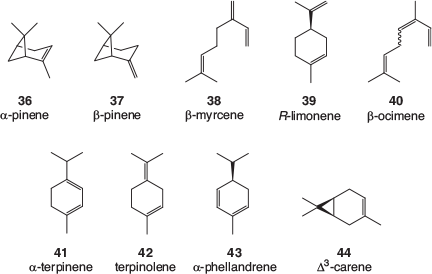
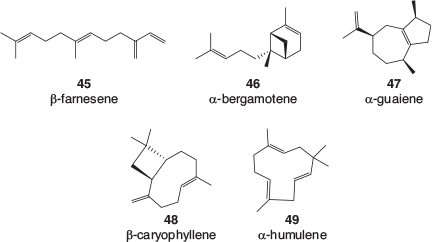
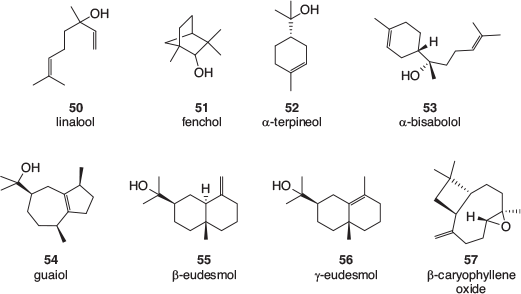
Many terpene profiles of cannabis varieties have been described, and while the identification of some of the common, major components is usually well founded, the reporting of some of the minor components can be less reliable. There is a lack of authentic samples with which to confirm identification, especially for sesquiterpenes and related terpenoids. Mass spectral library matching is often used, which is valid as long as a close match is found; however, the quality of the library matches is rarely reported. An examination of analytical results recently obtained by the CSIRO (data not published) as well as a selection of recently published cannabis terpene and terpenoid profiles[39–50] found that the following monoterpenes, 36–44 (Fig. 5), sesquiterpenes, 45–49 (Fig. 6), and terpenoids, 50–57 (Fig. 7) are most often found in cannabis varieties. Over 50 other cannabis terpenes and terpenoids have been described, but these were usually only reported in a single or small number of publications, and detected at low to very low levels.
|
|
|
Of the monoterpenes, the pinenes (α-pinene 36, and to a lesser extent β-pinene 37), β-myrcene 38, and R-limonene 39 are most often found in high levels in fresh cannabis, with β-ocimene 40 and terpinolene 42 also commonly present in significant amounts. These volatile compounds are primarily responsible for the characteristic odour of cannabis and variations in their expression is the most likely cause of different cannabis cultivars smelling slightly differently. Of the sesquiterpenes, β-caryophyllene 48 and α-humulene 49 are by far the most abundant in cannabis and the terpenoids linalool 50 and α-terpineol 52 can usually be easily detected.
Given the widespread confusion regarding the taxonomy of cannabis, originally based on the Cannabis sativa, C. indica, and C. ruderalis classifications that are largely related to growth habit, there has been a move to define varieties by their cannabinoid, and especially, terpene profiles.[40,51] As a result, cannabis scientists now tend to refer to cannabis varieties as ‘chemovars’ rather than using the traditional term, cultivar.
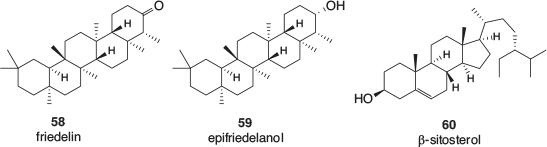
A small number of triterpenoids, specifically friedelin 58 and epifriedelanol 59, as well structurally related phytosterols such as β-sitosterol 60 (Fig. 8), have also been found in cannabis. These are more likely to be detected in the leaves, bark, and particularly roots of the plant.[52]
|
Polyphenolics
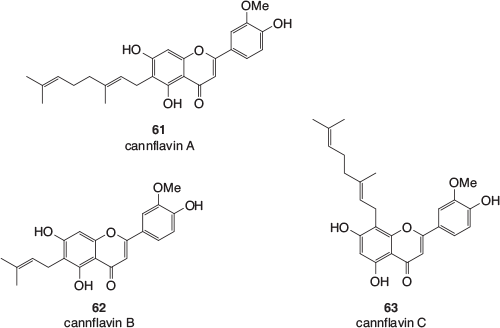
As with the terpenes and terpenoids, cannabis contains a wide range of polyphenolic compounds that are also common in other plants. However, there are some polyphenolics that appear to be mainly found in cannabis or can even be unique to the species.[53] Many have been given common names with the ‘cann’ prefix, pointing to their original discovery being in the extracts of cannabis. These polyphenolics do not tend to be found in the trichomes and usually occur in other parts of the plant. These include the geranylated and prenylated flavonoids cannflavin A, B, and C (61–63, Fig. 9), found in the leaves, twigs, flowers, and pollen of the cannabis plant.
|
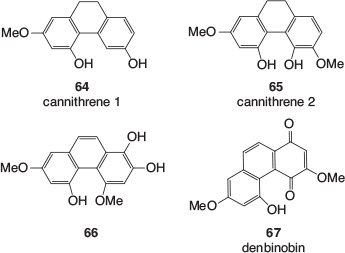
Another class of polyphenolics, which are found in cannabis leaves, stems, buds, flowers, and resin is the stilbenoids. These include phenanthrenes, dihydrostilbenes, and spiroindans. Examples of phenanthrene derivatives found in cannabis (64–67) are shown in Fig. 10.
|
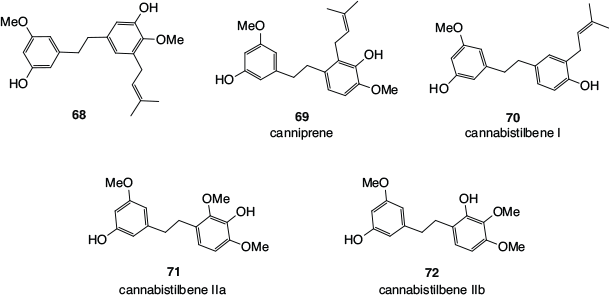
Some of the dihydrostilbenes found in cannabis (68–72), including the prenylated canniprene 69 and cannabistilbene I 70, are shown in Fig. 11. In the original investigation of the structure of cannabistilbene II,[54] the exact methylation pattern could not be determined, with both cannabistilbene IIa 71 and cannabistilbene IIb 72 being likely possibilities.
|
A range of spiroindans has been detected in cannabis, with cannabispirone 73, cannabispiradienone 74, cannabispirenone A 75, α-cannabispiranol 76, and β-cannabispiranol 77 (Fig. 12) being representative.
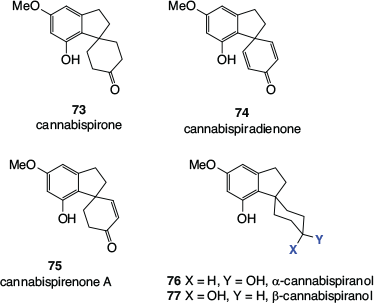
|
Finally, a large number of members of a class of polyphenolics known as lignanamides have been isolated from the roots, fruits, and seeds of cannabis. This has modern relevance, given the increased popularity of the consumption of hemp seeds in food. Representative examples are shown in Fig. 13 and include cannabisin A 78, cannabisin G 79, cannabisin E 80, cannabisin N 81, and cannabisin O 82, as well as N-trans-caffeoyltyramine 83 and N-trans-feruloyltyramine 84, the monomeric compounds from which most of the larger lignanamides are biosynthesised.
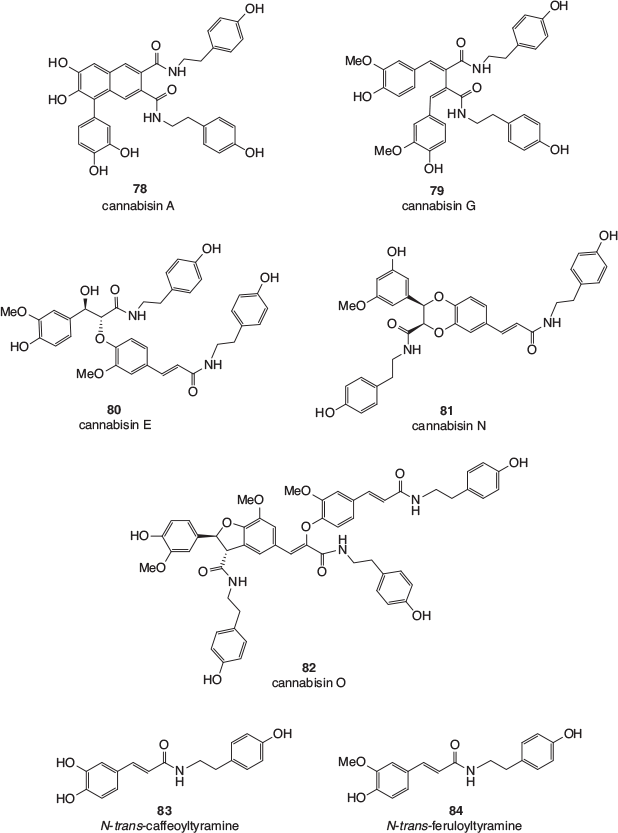
|
Alkaloids
Cannabis is a rare example of a plant with psychoactive properties in which the main compounds that cause the effect, the cannabinoids, are not alkaloids.[55] There have, however, been a small number of alkaloids isolated from cannabis (Fig. 14). These include the spermidine alkaloids cannabisativine[56] 85 and anhydrocannabisativine[57] 86, obtained from leaves and roots, and the diketopiperazine indole alkaloids neoechinulin A 87, (12S,22R)-dihydroxyisoechinulin A 88, and (12S,22S)-dihydroxyisoechinulin A 89, found in hemp seeds.[58] The latter compounds have previously been identified as fungal metabolites but, after a thorough investigation, Fan and coworkers concluded that neoechinulin A and the two stereoisomers of dihydroxyisoechinulin A were constituents of hemp seeds and did not result from fungal contamination of the crop.
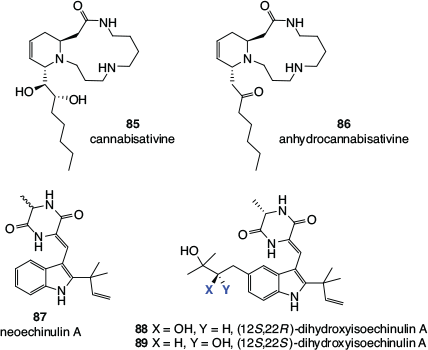
|
Waxes and Triglycerides
Involatile, higher molecular weight, hydrophobic compounds, collectively known as waxes, are produced by plants to protect their leaves and stems against moisture loss and pathogens. They are also expressed on plant inflorescence (flower heads) and potentially have the additional purpose of stabilising defensive compounds, which in the case of cannabis include the phytocannabinoids and terpenes. Cannabis inflorescences can have triple the levels of waxes compared with cannabis leaf materials.[59] Extraction of cannabis inflorescence with organic solvents or supercritical carbon dioxide is often the first step in the production of medicinal cannabis products and this usually results in ‘resin’ with a high wax content. Waxes are therefore an important class of phytochemical that typically need to be considered during such manufacture.[60] Cannabis waxes are mainly composed of straight-chain hydrocarbons, with n-pentacosane (C25H52), n-heptacosane (C27H56), n-nonacosane (C29H60), and n-hentriacontane (C31H64) being the most abundant. Of these, n-nonacosane and n-heptacosane appear to be the major contributors to cannabis wax.[59,60]
Hulled hemp seeds consist of almost 50 % fat (triglycerides) and the derived oil is unusual among food oils in that its triglycerides contain very low levels of saturated fatty acids (~9 %) and very high levels of polyunsaturated fatty acids (~80 %). The three main fatty acids present in hemp seed oil are linoleic acid (18:2ω6, 54–60 %), α-linolenic acid (18:3ω3, 18–23 %), and oleic acid (18:1ω9, 7–12 %).[61–64]
Important Contaminants
Medicinal cannabis producers are required to ensure that their products do not contain a range of dangerous contaminants. Apart from residual pesticide levels, the propensity for cannabis plants to become infested with an array of fungal pathogens[65] means that cannabis products are routinely tested for the presence of harmful mycotoxins, such as aflatoxins B1, B2, G1, G2, and ochratoxin A. The ingestion of these fungal metabolites can cause cancer, liver damage, and kidney damage. In addition, cannabis has long been known to be a heavy metal bio-accumulator, meaning that it can absorb heavy metals from the soil.[66] Its ability to tolerate and store heavy metals has led to interest in its use in soil bioremediation and bioprospecting.[67] The bio-accumulation properties of cannabis also mean that regulators routinely require cannabis products destined for human consumption to be tested for the presence of heavy metals. A less common but still concerning class of contaminants are the polyaromatic hydrocarbons, which can sometimes be found in crops exposed to diesel fumes.
Pharmacology and the Endocannabinoid System
In mammals, cannabinoids interact with two main receptors, cannabinoid receptor 1 (CB1) and cannabinoid receptor 2 (CB2). These were discovered in a period from the mid-1980s to the early 1990s when pharmacologists were searching for the receptors for the phytocannabinoids and synthetic cannabinomimetics.[68,69] The use of a tritium-labelled form of the cannabinomimetic 27b, [3H]-CP 55,940, was very important in these studies.[70] Both CB1 and CB2 are members of a large class of transmembrane proteins known as G-protein coupled receptors (GPCRs), that respond to extra-cellular signalling molecules by binding them, undergoing conformational changes, and eliciting intracellular responses through coupling with G-proteins (guanine nucleotide-binding proteins). Recently, X-ray crystal and cryo-electron microscopy investigations have more accurately defined the molecular structures of the cannabinoid receptors and how they associate with G-proteins.[71]
The CB1 receptors are found in a wide range of tissue types but are more highly concentrated in the central and peripheral nervous systems. By contrast, CB2 receptors tend to be found in tissues and organs associated with the immune system,[72] and can be strongly upregulated in response to inflammation or injury. As indicated above, the natural endogenous ligands for the CB receptors were discovered soon after the receptors themselves, beginning with anandamide (14) in 1992.[70] This relatively simple amide is now thought to be a member of a huge collection of endogenous signalling compounds – the endocannabinoidome. The levels of these important biological messengers are controlled by a complex system of biosynthetic and degrading enzymes, the latter being mainly amide hydrolases or lipases.[15] Together, the network of cannabinoid receptors, their endogenous ligands, and their associated metabolic enzymes is known as the endocannabinoid system or endocannabinoid signalling system.
It has become apparent that there are several other receptors and channels, apart from CB1 and CB2, that are modulated by cannabinoids. Prominent amongst these are the transient receptor potential cation channel subfamily V member 1 (TRPV1), a heat-sensing ion channel, the proliferator-activated receptor-α (PPARα), and the 5-hydroxytryptamine receptor 1A (5-HT1A) serotonin receptor. There are also two ‘orphan’ GPCRs, GPR55 and GPR18, that are being investigated as potential cannabinoid receptors.[15] If one considers all of these receptors, together with the abovementioned endocannabinoid system, it is clear that cannabinoids can influence a diverse array of physiological processes, and it becomes obvious why cannabinoids are associated with a highly diverse group of potential therapeutic interventions.
The main compound derived from cannabis that induces euphoric effects in humans is Δ9-THC (10a). This phytocannabinoid is an agonist (activator) of both CB1 and CB2 receptors.[73] Stimulation of CB1 by 10a in animals is associated with the suppression of locomotor activity, hypothermia, catalepsy, pain desensitisation, and appetite enhancement, whereas activation of CB2 is associated with pain relief and anti-inflammatory effects. It should be noted that many synthetic cannabinomimetics are significantly more potent agonists of cannabinoid receptors than 10a or anandamide (14), but interestingly, the initial Δ9-THC metabolite produced in the liver, the 11-hydroxy derivative (90, Fig. 15) is also a more effective agonist than Δ9-THC itself. This alcohol (90) is readily metabolised to the corresponding carboxylic acid (91), which is non-psychoactive.[74]
The other most common cannabinoid derived from cannabis, CBD (8a), is not a cannabinoid receptor agonist and rather may be a CB1 antagonist, particularly in the presence of Δ9-THC (10a). It has, however, been found to be an agonist for TRPV1 and 5-HT1A receptors, and is described as having anticonvulsive, anti-inflammatory, and anti-psychotic effects. It has been shown to counteract some of the intoxicating and unwanted side effects of Δ9-THC, including anxiety, tachycardia (elevated pulse), increased appetite, and sedation,[73] and is widely believed to limit or even reverse the psychotic effects experienced by some heavy Δ9-THC users.
There have been many medical claims associated with terpenes and terpenoids, and particularly those present in cannabis,[73] and the most compelling relate to β-caryophyllene 48. This sesquiterpene, which is also found in cloves, is a potent CB2 agonist and has been shown to interact with a wide range of other mammalian receptors. It has been investigated for its anti-inflammatory effects, and as a possible treatment for depression and anxiety, among other indications. There are also suggestions that β-caryophyllene could act synergistically with both Δ9-THC and CBD.[73]
Medicinal Cannabis and Cannabinoids
Diseases and Conditions Targeted
The breadth of conditions that clinicians are currently considering, or have considered, as promising opportunities for the application of medicinal cannabis and cannabinoids is truly vast.[75] They include paediatric epilepsy, particularly Dravet and Lennox–Gastaut syndromes; chemotherapy-induced nausea and vomiting; cachexia (a wasting disorder associated with late stages of diseases like cancer and HIV/AIDS); loss of appetite, obesity, and eating disorders; chronic pain including neuropathic pain and fibromyalgia; insomnia, anxiety and depression, post-traumatic stress disorder (PTSD); multiple sclerosis and other autoimmune or autoinflammatory diseases like irritable bowel and Crohn’s disease, psoriasis and rheumatoid arthritis; autism, attention deficit hyperactivity disorder (ADHD), Tourette’s syndrome; cancer including neuroblastoma, glioblastoma, breast cancer, leukemia, osteosarcoma, and nephroblastoma; Parkinson’s disease; motor neurone disease; dementia and in palliative care. The inclusion of cannabis in Schedule IV of the 1961 United Nations Single Convention on Narcotic Drugs[76] has, however, severely limited the proper investigation of the efficacy and safety of cannabis and phytocannabinoids for the treatment of these diseases.[77] Until recently, legitimate placebo-controlled, random double-bind clinical trials, the gold standard required to prove drug safety and efficacy, have been virtually impossible with cannabis and phytocannabinoids in the signatory countries. The development of suitable placebo formulations is also particularly challenging for cannabis, especially when inhalation is the route of delivery, as the placebo effect can be quite prominent in such trials.
In 2017, the Australian Therapeutic Goods Administration (TGA) released guidance for the use of cannabis and cannabinoids in the treatment of several conditions for which cannabis or cannabinoid treatment was often sought.[78] These were: epilepsy in paediatric and young adult patients, the prevention or management of nausea and vomiting, multiple sclerosis, chronic non-cancer pain, and in palliative care. The development of the recommendations was hindered by a lack of evidence from rigorous clinical trials; however, patients with some of these conditions were thought likely to gain benefit from treatment with medicinal cannabis or cannabinoids. It was found that there was evidence for paediatric and young adult patients with epilepsy to have an improved chance of a reduction of seizure frequency, an increased likelihood of complete freedom from seizures and improved quality of life, when treated with oral CBD, relative to placebo. Dronabinol (synthetic Δ9-THC) prescribed for the treatment of nausea and vomiting was found to be as effective as traditional antiemetics, and with some conditions, more effective at improving appetite than placebo. At the time, no proper comparisons had been made with newer types of antiemetics. Medicinal cannabis treatments were also found to provide moderate relief from various types of chronic pain, including that associated with multiple sclerosis. Some multiple sclerosis patients also reported improvement in spasticity symptoms with nabiximols (oral spray containing ~1:1 Δ9-THC:CBD), and in fact this drug has been approved by the TGA, and in Canada and some European countries, for this indication. In no cases was medicinal cannabis recommended by the TGA as a first-line treatment, and concerns were expressed about potentially negative interactions between CBD and existing prescribed drugs, resulting from the inhibition of cytochrome P450 metabolic enzymes in the liver.[79] A review undertaken for the US National Academies of Sciences[80] conducted around the same time as the TGA reviews came to similar conclusions regarding the treatment of chemotherapy-induced nausea and vomiting, chronic pain, and multiple sclerosis-related spasticity, but could not find sufficient evidence to support or refute the use of cannabinoids for the treatment of epilepsy, or for any other conditions considered. Interestingly, in 2018, the US Food and Drug Administration (FDA) approved the use of Epidolex®, an oral liquid CBD formulation, for the treatment of Dravet and Lennox–Gastaut syndromes. Epidolex is the first FDA-approved drug that contains a purified drug substance derived from cannabis and has since been approved in Europe in 2019 and by the TGA in September 2020 for the treatment of the same epilepsy syndromes.
With laws allowing the medical use of cannabis having been introduced around the world, many careful clinical trials with a range of cannabis products have begun, albeit usually with quite small treatment groups. It is expected that a clearer picture of the efficacy of medicinal cannabis products will come to light in the coming years, as the results of these trials are analysed and released.
Metabolism and Delivery of Phytocannabinoids
As mentioned above, Δ9-THC (10a) undergoes oxidation in the liver in two stages, first to the 11-hydoxy derivative (90) and then on to the carboxylic acid (91). The latter can be glucuronidated, mainly to the glucuronide ester (92, Fig. 15).[81] Cannabidiol (8a) has been shown to be metabolised in similar ways to Δ9-THC.[74] The Δ9-THC-derived alcohol (90) can also be conjugated with long chain fatty acids and stored in adipose tissue,[81] which partially accounts for the fact that Δ9-THC metabolites can be present in the human system for a surprising length of time after administration.
The bioavailability of Δ9-THC from a cannabis cigarette typically ranges from ~10 to 35 %, with considerable variability between experienced and inexperienced smokers.[74] Approximately 30 % of the Δ9-THCA in the cigarette is thought to be destroyed through burning.[81] After inhalation, plasma Δ9-THC levels rise rapidly and peak within ~10 min,[81,82] after which the drug is rapidly distributed to many tissues around the body, eventually partitioning to the body fat, where it can be stored for extended periods.[81,83] This is consistent with Δ9-THC’s high lipophilicity and low total polar surface area.[84] The half-life of Δ9-THC in plasma is hard to estimate because it is released from adipose and other tissues back into the blood only slowly and at such low concentrations that it is difficult to quantify.[81] While levels of the initial Δ9-THC metabolite, the alcohol (90), never seem to reach high concentrations in plasma, the concentration of the corresponding acid (91) tends to rise over the first 30 min following inhalation and drops off very slowly. For example, it could be detected in the plasma of test subjects up to 7 days after smoking a Δ9-THCA-containing cigarette.[74] The observed latency of Δ9-THC and its metabolites in humans, apparently long after any observable impairment, presents significant challenges for law enforcement agencies when attempting to prosecute road users for driving under the influence of cannabis,[85] and consequently serves as a disincentive for patients to take up medicinal cannabis products that contain Δ9-THC. The bioavailability of smoked CBD is similar to Δ9-THC, ranging from 11 to 35 %,[81] with a plasma half-life of 27 to 35 h.[79] The metabolic profile and pattern of plasma distribution is also apparently similar to Δ9-THC, although unlike Δ9-THC, a high proportion of CBD is excreted unchanged.[81]
Cannabinoids can be delivered to the body in various ways. Historically, inhalation has been the most common method, through the pyrolysis of plant material, but there are now several alternative delivery techniques that use this route of delivery. These include devices that electrically heat plant material while avoiding complete combustion, e-cigarettes that either wick cannabinoid-containing liquids across a heating coil or allow the user to manually place the liquid on the hot coil, and dabbing in which a small amount of cannabis extract is placed on a preheated surface such as a nail.[86] All of these methods present concerns related to airway and lung damage caused by pyrolysis products of cannabis, and those of the carrier oils and their additives.[86] Devices that deliver a metered dose of inhaled cannabinoids that have been formulated into a powder are also under development.
Oral administration via oils, tablets or capsules has several advantages over inhalation, including well-controlled dosing, an uncomplicated route of administration, and wide patient acceptance. The oral delivery of cannabinoids in medium-chain triglyceride oil, derived for example from coconut oil, is now one of the most common ways by which medicinal cannabis products are administered. Dosing via the oral route has, however, a major disadvantage. It is subject to first-pass metabolism in the gut and liver before entry into the blood, and absorption can be slow, highly variable, and patient-dependent.[81,82] The oral bioavailability of Δ9-THC ranges from 2 to 14 %,[81] and 6 to 10 % for CBD.[87] The latter was found to have a plasma half-life of 2 to 5 days,[81,87] much longer than when it is delivered via inhalation. In the case of Δ9-THC, maximal plasma concentrations are commonly encountered 2–3 h after administration and tend to be only a fraction of those achieved through inhalation. At the same time, the levels of the Δ9-THC acid metabolite (91) in plasma rise steeply over the first 1–3 h and then trail away relatively slowly over many hours.[81] Plasma levels of the Δ9-THC alcohol metabolite (90) tend to be higher than those observed through inhalation.[81] For orally delivered CBD, the time to maximum plasma concentration was found to be 2.5 to 5 h,[87] only slightly longer than Δ9-THC.
Oral mucosal delivery via lozenges, patches placed on the gums, oral spray or sublingual (under the tongue) is thought to largely avoid many of the problems with first-pass metabolism, as drugs can be absorbed through the oral mucosa (the mucous membrane that lines the inside of the mouth) directly into the blood stream. Nabiximols is an example of a cannabinoid drug that uses the oral mucosal route and the development of other medicinal cannabis products of the types mentioned here is currently a very active area of commercial pharmaceutical research.
Transdermal delivery via creams and patches also avoids first-pass metabolism, and owing to its convenience, is also of great interest for the delivery of phytocannabinoids. Despite there being several cannabinoid patches available on the recreational market in North America, however, the potential of phytocannabinoids to penetrate skin has been the subject of only a small number of rigorous scientific investigations. The majority of this work has been with CBD (8a),[88] and promising results for the treatment of muscle tension and pain[89] and peripheral neuropathy[90] with topical CBD (8a) preparations have been recently described. An earlier report of an investigation into the transdermal delivery of Δ8-THC (11)[91] suggested that effective delivery of this compound and, by analogy, Δ9-THC (10a), is likely to be very difficult. This appears to be supported by findings from a recent study of the application of cannabis extract to beagles,[92] which revealed much better penetration of CBD (8a), CBDA (4a), and Δ9-THCA (7a) than Δ9-THC (10a). The high lipophilicity of Δ9-THC (10a) appears to be a major impediment to its penetration of the skin.
Prodrugs Derived from Phytocannabinoids
As mentioned above, key reasons for the low bioavailability of Δ9-THC and CBD are their relatively high hydrophobicity and low total polar surface area,[84] which results in them being rapidly distributed to adipose tissue, from which they are only slowly released. It can therefore be difficult to achieve therapeutic levels in plasma and at the required sites of action with these phytocannabinoids. One approach to this problem has involved the development of more hydrophilic prodrugs – covalently modified derivatives that are converted to the actual cannabinoid drug within the body. One of the first examples was the Δ9-THC-hemiglutarate (93, Fig. 16), which was investigated for systemic delivery of Δ9-THC via the oral transmucosal route, through incorporation into a polyethylene oxide matrix.[93] A slightly more elaborate modification involved the attachment of valine and succinic acid to the phenol functionality of Δ9-THC (94), for the purpose of developing topical treatments for glaucoma.[94] The CB1 and CB2 antagonistic activity of Δ9-THC is thought to decrease intraocular pressure. More recently, glycosylated versions of Δ9-THC, such as the Δ9-THC-glucoside (95), were developed by Vitality Biopharma for the treatment of drug-resistant inflammatory bowel disease.[82]
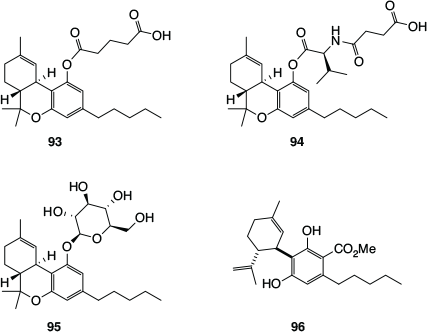
|
The natural phytocannabinoid acid CBDA (4a) has recently been investigated for its ability to suppress nausea and vomiting, including ‘anticipatory nausea’ experienced by chemotherapy patients when they return to the chemotherapy clinic, and anxiety under conditions of high stress. These effects are apparently mediated by CBDA’s activity at the 5-HT1A receptor, which appear to be superior to those of CBD. The spontaneous decarboxylation of CBDA (4a) to CBD (8a, Scheme 1) limits the suitability of CBDA as a mainstream drug, but this issue has been addressed by a simple modification, namely the formation of the methyl ester (96).[95] This methyl ester was found to be more stable than CBD and, fortuitously, was also found to be more effective at suppressing both acute and anticipatory nausea, and stress-induced anxiety in rats.
Modulation of Endocannabinoid Levels
An alternative to the control of the endocannabinoid system through the activation and inhibition of cannabinoid receptors with phytocannabinoids and their derivatives is the modulation of endocannabinoid levels through the inhibition of endocannabinoid-degrading and biosynthetic enzymes. Some medical conditions are thought to result from imbalances in some aspect of the patients’ endocannabinoid systems and adjusting the endogenous concentrations of certain endocannabinoids by slowing their degradation or limiting their formation has hence been a very active area of research. Cannabinoid dependence, Tourette syndrome, schizophrenia, and fear conditioning such as that associated with PTSD have been considered for such pharmaceutical interventions. Much of the work on endocannabinoid modulators in the pharmaceutical industry has, however, been focussed on the development of new pain medications.
By far the most attention has been given to the development of inhibitors of the hydrolases that catalyse the degradation endocannabinoids like anandamide (14), particularly fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL), an enzyme that catalyses the degradation of glycerol derivatives like 2-AG (15). The inhibition of these enzymes allows the corresponding endocannabinoids to accumulate, increasing their agonistic effects on cannabinoid receptors. This appears to be supported by the interesting case of a woman with reduced expression and activity of FAAH in her central nervous system (CNS), and consequent high circulating levels of anandamide and other endocannabinoids, who was found to have remarkable pain insensitivity, a highly optimistic outlook, and very low anxiety and depression scores.[96]
An enormous number of inhibitors have been developed for FAAH, and these have been thoroughly reviewed elsewhere.[97–99] The enzyme employs an amidase serine–serine–lysine catalytic triad and early inhibitors were electrophilic analogues of anandamide, either being competitive reversible or irreversible inhibitors. After extensive investigations, particularly in Boger’s laboratories, α-ketoheterocycles like OL-135 (97, Fig. 17) were developed. This class of inhibitor has been shown to reversibly form a covalent hemiketal intermediate with an active site serine residue in FAAH. The flexible OL-135 (97) was found to inhibit FAAH with a half maximal inhibitory concentration (IC50) value of 4.7 nM, and briefly induced analgesia in several in vivo preclinical pain models. The apparent rapid metabolism of 97 in rats was not seen with the analogue 98, a compound with similar potency, which blocked pain in rats over more prolonged periods.
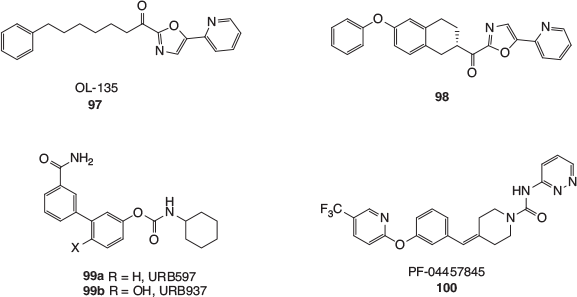
|
Another class of FAAH inhibitors are the carbamates, one of the earliest and most thoroughly studied being URB597 (99a). This simple and quite compact compound was found to inhibit FAAH with an IC50 value of 4.6 nM and has demonstrated analgesic and anti-inflammatory effects in animal models. There is strong evidence to show that URB597 acts as a covalent inhibitor of FAAH, with the phenol part of the carbamate displaced by an active site serine. Interestingly, the closely related phenol URB937 (99b) is excluded from the CNS through the action of an active membrane transporter,[100] and has been investigated for its modulation of the peripheral endocannabinoid system in animal models. The discovery of these ‘URB’ compounds has stimulated wide-ranging searches in academia and the pharmaceutical industry for even more therapeutically effective carbamates that target FAAH, which have continued unabated for over a decade.
Related to the carbamates are the arylurea FAAH inhibitors. Somewhat surprisingly, given the hydrolytic stability of the urea functional group, these inhibitors have also been shown to attach covalently to an active site serine, with the (hetero)aryl amine fragment becoming the leaving group. The first FAAH inhibitor to reach Phase II clinical trials was Pfizer’s urea, PF-04457845 (100), which has been assessed for its suitability to treat inflammatory and non-inflammatory pain. It was unfortunately found to be ineffective against osteoarthritis pain. Many other classes of FAAH inhibitors have been developed in recent years, including reversible covalent inhibitors based on boronic acids and propanone derivatives, and a range of non-covalent heterocyclic compounds. It has also been speculated that some anaesthetics and NSAIDs may in part derive some of their efficacy from FAAH inhibition. For example, propofol, a very common anaesthetic, has been shown to competitively inhibit rat FAAH with an IC50 of 14 μM. The best evidence that FAAH inhibition may contribute to its activity comes from the study of plasma levels of anandamide in patients undergoing orthopaedic surgery. Anandamide levels in the patients who had been anesthetised with propofol were found to be up to 1.5 to 2 times higher than in the control group.
Compared with FAAH, the development of inhibitors of MAGL has received much less attention,[101,102] presumably because it was not until 2002 that the natural degradation of 2-AG (15) was discovered to occur primarily through the action of this enzyme. Monoacylglycerol lipase employs a serine–histidine–aspartate catalytic triad similar to that employed by serine proteases and, as with FAAH, covalent inhibitors have played a key role in gaining an understanding of the enzyme’s role and in the development of potential therapeutics. MAGL inhibitors have been pursued as potential treatments for a wide range of CNS disorders including Alzheimer’s disease, Parkinson’s disease, motor neurone disease, multiple sclerosis, stroke, traumatic brain injury, chronic traumatic encephalopathy, seizures, anxiety, and pain.
One MAGL inhibitor that has been extensively investigated both in vitro and in vivo is JZL184 (101a, Fig. 18). This p-nitrophenylcarbamate irreversibly inhibits MAGL with an IC50 of 8 nM by forming a covalent adduct with the enzyme’s active site serine, and was found to be highly selective for MAGL over FAAH and other hydrolases. Concerns have since surfaced regarding JZL184’s inhibition of carboxylesterases in peripheral tissues and undesirable outcomes on repeated administration in animal models. The O-hexafluoroisopropyl analogue of JZL184, KML29 (101b) was found to be similarly potent, while presenting few issues on repeated administration. Several other active site carbamoylating MAGL inhibitors have also been developed, some of which have provided valuable structural information about the enzyme’s active site through protein crystal structure determinations.
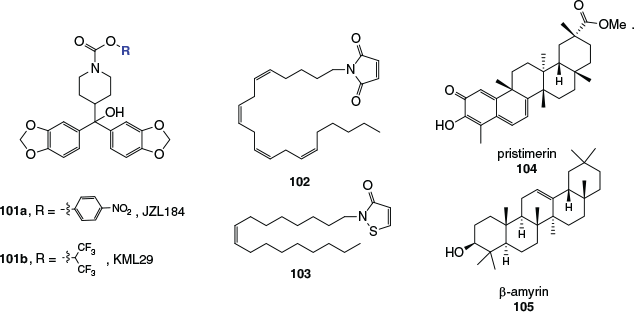
|
Monoacylglycerol lipase possesses three cysteine residues in the vicinity of its catalytic site that, while not themselves involved in the catalytic mechanism of the enzyme, have been successfully targeted with inhibitory compounds. Inhibitors like N-arachidonylmaleimide (102) are Michael acceptors and form covalent carbon–sulfur links with cysteine thiol functionalities, whereas isothiazolinines like N-oleoylisothiazolinine (103) are thought to deactivate the enzyme through the formation of a disulfide link, which can be reversed by the action of dithiothreitol.
Interestingly, the triterpenoid natural products pristimerin (104) and β-amyrin (105) have been shown to increase intracellular 2-AG levels through the inhibition of MAGL and other 2-AG degrading enzymes. They inhibit MAGL through a reversible, non-competitive mechanism. The structural similarity between β-amyrin, a known anti-inflammatory agent and analgesic, and triterpenoids found in cannabis such as epifriedelanol (59) is noteworthy. The search for new reversible MAGL inhibitors of various structural classes continues to be an active area of research and new synthetic inhibitors have recently shown in vitro antiproliferative effects on a range of cancer cell lines.[103]
Cannabinoid-Related Drug Failures
Since the endocannabinoid system in humans is so complex and apparently ubiquitous, it is perhaps not surprising that drugs that target this network are prone to unwanted side effects. Complicating the drug development process further is, for example, the finding that chemical modulators of endocannabinoid levels can have quite disparate inter-species activity,[101] sometimes making the translation of results obtained in animal models to humans challenging. Potentially negative psychological effects of agents that target receptors in the CNS can also be difficult to assess in animal models.
A prominent example is the case of rimonabant (106, Fig. 19), the first selective and orally active CB1 antagonist, developed by Sanofi–Synthélabo (now Sanofi–Aventis) in 1994,[104] which was approved in Europe for the treatment of obesity in 2006, only to be suspended and withdrawn from sale in 2008. Cannabinoid receptor antagonists, as opposed to agonists, were and continue to be developed with the aim of eliciting the opposite effects of cannabinoids. A well-known effect of cannabis consumption is the enhancement of appetite, which is modulated through the CB1 receptor, and is the reason CB1 agonistic cannabinoid drugs have been developed to treat anorexia and cachexia. It follows that CB1 antagonists should suppress appetite and hence have application in the treatment of obesity and metabolic syndrome. Rimonabant, technically a CB1 inverse agonist, while showing promise as an effective treatment for obesity and also as an aid to smoking cessation, was unfortunately found to be associated with a range of undesirable side-effects. The most concerning of these were adverse psychiatric effects such as an increased incidence of depression, anxiety, and suicide ideation, with a combined 2-fold increase in suicide risk across obesity and smoking cessation trials.[105] These side effects were serious enough for rimonabant to be withdrawn from sale as an obesity treatment, and it was never marketed as an aid to assist in smoking cessation. More recently, scientists have been pursuing CB1 antagonists that cannot cross the blood–brain barrier, so as to target the CB1 receptors in peripheral organs such as the liver. An example is Jenrin Discovery’s JD-5037 (107).[106]
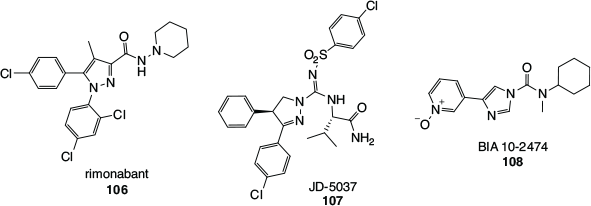
|
An alarming situation occurred in 2016, in which a volunteer participating in a Phase I clinical trial of the FAAH inhibitor BIA 10-2474 (108) died and four others required hospitalisation. All four suffered from mild to severe neurological effects and two experienced long-term neurological damage. The drug was developed by the pharmaceutical company Bial for the treatment of anxiety, Parkinson’s disease, and cancer-related pain. While criticisms of the preclinical animal studies and the dosing regimen used in the Phase I trial have surfaced, it has also been established that BIA 10-2474 has significant off-target effects.[107] Unlike PF-04457845 (100), which is highly selective for FAAH and a related hydrolase, FAAH2, BIA 10-2474 was found to be a promiscuous inhibitor of lipid hydrolases and drug-metabolising enzymes. Many of the off-target hydrolases are expressed in human brain tissue and prolonged exposure of human cortical neurons to BIA 10-2474 was shown to have a marked effect on lipid metabolism within this donor tissue. In addition, some off-target inhibitory effects shown by BIA 10-2474 were more pronounced with the human enzymes than the corresponding mouse enzymes, highlighting risks associated with the interpretation of pre-clinical animal data. It was speculated that the disruption of neuronal lipid networks contributed to BIA 10-2474’s neurotoxicity.
The ‘Entourage Effect’
It has become a widely held view in the medicinal cannabis community that therapeutic effects that stem from the use of cannabis are not entirely due to the main decarboxylated phytocannabinoid constituents, Δ9-THC and CBD, alone. Instead it is believed by many that improved therapeutic outcomes can be achieved through the administration of ‘whole plant’ or ‘full spectrum’ cannabis extracts. The term ‘entourage effect’ has become ubiquitous in the cannabis field and invokes the concept that the pharmacological effects of the major phytocannabinoids Δ9-THC and CBD (the ‘main players’) are modulated in important ways by other cannabis constituents (the accompanying ‘entourage’). These secondary components are commonly thought to consist of the minor phytocannabinoids, terpenes and terpenoids, and flavonoids, but the concept is not restricted to these chemical classes alone.
An entourage effect was first proposed in connection with cannabinoids by Mechoulam, initially to explain the potentiation of the binding of the endocannabinoid 2-AG to CB1 and CB2 receptors by otherwise inactive 2-AG analogues,[108] and then by making the connection to plant-derived medicines. It was stated that it was a ‘widely held (but not experimentally based) view that in some cases plants are better drugs than the natural products isolated from them’. In relation to cannabis extracts, the entourage effect has been described as a form of ‘herbal synergy’ that operates by several potential mechanisms including the enhancement of phytocannabinoid activity by active and inactive constituents, antagonism, additive effects, multi-target effects, and pharmokinetic and metabolic interactions.[109]
It does not take a huge leap of imagination to accept that minor phytocannabinoids present in cannabis products could contribute to their pharmacological effects, since the structural similarities between the minor and major phytocannabinoids would suggest that the minor components should be active. Indeed, the biological activities of many of these minor phytocannabinoids have been confirmed in vitro and in vivo.[11,12,14] However, despite the fairly low levels of terpenes and terpenoids in dried cannabis plant matter and concentrated cannabis extracts, putative additive or even synergic effects exerted by cannabis terpenes and terpenoids have also been widely promoted.[109] As indicated above, the case for β-caryophyllene (48) acting in this way is compelling,[110] and it is reasonable to assume that other sesquiterpenes and sesquiterpenoids might also contribute to biological responses to cannabis consumption.[47] The potential for synergic effects from the more volatile and easily metabolised monoterpenes and monoterpenoids, however, is affected by their very low levels in many cannabis preparations and the mode of administration of the drug. When taken orally, the process of first-pass metabolism means that there is little chance for them to reach their targets at therapeutic levels. Even via inhalation, which would be expected to give the best opportunity for these terpenes to exert their effects in vivo, the high rates of clearance and short half-lives for elimination observed for some monoterpenes suggest that accumulation to therapeutic levels is improbable.[111] Polyphenolics such as the flavonoids have also been shown to have poor oral bioavailability, leading to very low in vivo plasma levels following ingestion.[112] The opportunity for polyphenolic constituents of cannabis to contribute to any putative entourage effect thus appear to be very limited.
A further, significant complication that hinders attempts to take clinical advantage of the entourage effect is one that is common to many herbal remedies. If the important active agents are not known, it is very difficult to produce reliable products that have consistent levels of these constituents. With cannabis, even if a product is produced from the same chemovar, cultivation conditions and processing methods can cause variability in its secondary metabolite profile. The use of a combination of HPLC and GC ‘fingerprints’ might assist with product reproducibility, but this assumes that all the important ‘entourage compounds’ can be detected by these methods. Further, to the author’s knowledge, there are no clinical trials aimed at validating the existence of the entourage effect with medicinal cannabis. In addition, recent in vitro studies failed to uncover evidence for ‘entourage-like’ effects of cannabis terpenes on CB1 and CB2,[113] or TRPV1 and the related transient receptor potential cation channel subfamily A member 1 (TRPA1)[114] channels.
Conclusion
Cannabis was one of the first plants to be actively cultivated by humans and since ancient times, has been used as a source of food, fibre, and herbal medicine. The taxonomy of cannabis is still subject to debate, with plants bred for fibre or food production generally referred to as hemp, whereas those rich in psychoactive secondary metabolites are usually labelled marijuana or medicinal cannabis. The majority of the interesting secondary metabolites produced by cannabis are expressed in small glandular projections known as trichomes, which tend to be concentrated around flower buds. The plant is believed to produce secondary metabolites such as phytocannabinoids and terpenes as a form of defence against insects and microbial pathogens.
There are three main classes of cannabinoids, the phytocannabinoids produced by plants, the endocannabinoids – endogenous compounds present in mammals that activate the same receptors as phytocannabinoids, and synthetic cannabinomimetics – synthetic compounds that activate the same receptors. A large number of phytocannabinoids have been detected in cannabis, but the dominant ones tend to be Δ9-THCA (7a) and CBDA (4a). These spontaneously undergo thermally induced decarboxylation to the more biologically active forms, Δ9-THC (10a) and CBD (8a). There are a large number of endocannabinoids, with the two most intensively investigated being anandamide (14) and 2-AG (15). The development of synthetic cannabinomimetics was initially facilitated by successful total syntheses of phytocannabinoids and then later by the finding that NSAIDs like pravadoline (29) and WIN 52,212-2 (30) produce pharmacological responses similar to those induced by phytocannabinoids like Δ9-THC (10a). Synthetic cannabinomimetics began appearing in ‘synthetic cannabis’ herbal blends just over a decade ago and have become a major concern for health authorities, regulators, and law enforcement worldwide.
Like many plants, cannabis is rich in a variety of terpenes and terpenoids, with the pinenes (36 and 37), β-myrcene (38), R-limonene (39), β-caryophyllene (48), and α-humulene (49) usually being most abundant. Polyphenolics are also found in various parts of the plant, of which the cannflavins (61–63) have attracted the most attention. One unusual aspect of cannabis is that its main psychoactive constituents, the phytocannabinoids, are not alkaloids. Nonetheless, a small number of alkaloids have been found in cannabis.
In mammals, cannabinoids interact with a complex signalling and control network known as the endocannabinoid system. Central to this are two GPCRs, the cannabinoid receptors CB1 and CB2. The CB1 receptors are more highly concentrated in the central and peripheral nervous systems, whereas CB2 receptors tend to be found in tissues and organs associated with the immune system and are associated with inflammatory responses. Cannabinoids have also been shown to modulate other receptors including TRPV1, PPARα, the 5-HT1A serotonin receptor, and some ‘orphan’ GPCRs. The main compound derived from cannabis that induces euphoric effects in humans, Δ9-THC (10a), is an agonist of CB1 and CB2 receptors. Cannabidiol (8a) is not a cannabinoid receptor agonist and rather may be a CB1 antagonist, particularly in the presence of Δ9-THC (10a), but has been found to be an agonist for TRPV1 and 5-HT1A receptors. Interestingly, β-caryophyllene (48), also found in cannabis, is a potent CB2 agonist.
Medicinal cannabis and cannabinoids have been considered for the treatment of a vast range of medical conditions, but thorough clinical trials aimed at evaluating their effectiveness have been challenging to perform and are still rare. Promising results have been obtained with the treatment of epilepsy in paediatric and young adult patients, the prevention or management of nausea and vomiting, multiple sclerosis, and chronic non-cancer pain.
Smoked cannabinoids like Δ9-THC (10a) and CBD (8a) have a bioavailability of 10–35 %, and even lower when taken orally. Several delivery mechanisms and formulation strategies are being investigated with the hope of improving cannabinoid bioavailability. Once they reach the circulatory system, however, both Δ9-THC (10a) and CBD (8a) tend to be rapidly distributed to a range of tissues around the body, eventually partitioning to the body fat, being released back into the blood only slowly. Consequently, drugs like Δ9-THC (10a) and its metabolites can be detected in some subjects long after initial consumption.
A very active area of research involves the modulation of endocannabinoid levels through inhibition of their degrading enzymes, FAAH and MAGL. Irreversible covalent inhibitors, reversible covalent inhibitors, and reversible non-covalent inhibitors have all been explored. Off-target effects with some of these bioactives can be a concern and in one case resulted in an extreme outcome in a Phase I clinical trial of an FAAH inhibitor. A CB1 antagonist developed for the treatment of obesity also had to be withdrawn from sale owing to the severe adverse psychological effects experienced by some patients.
The ‘entourage effect’ is a widely used term in the medicinal cannabis field and has been described as a form of ‘herbal synergy’ in which the action of the main cannabinoids, Δ9-THC (10a) and CBD (8a), is enhanced or modulated by other components present in the plant and its extracts. While this is a reasonable concept, particularly when considering the minor phytocannabinoids, sesquiterpenes, and sesquiterpenoids, it is confounded by the variability in the levels of minor secondary metabolites in cannabis preparations, the limited nature of the analyses usually performed on such products and the limited bioavailabilities of many of the components of interest.
Abbreviations
-
2-AG, 2-arachidonoylglycerol
-
2-AGE, 2-arachidonoylglycerol ether
-
5-HT1A, 5-hydroxytryptamine receptor 1A
-
ADHD, attention deficit hyperactivity disorder
-
CB1, cannabinoid receptor 1
-
CB2, cannabinoid receptor 2
-
CBC, cannabichromene
-
CBCA, cannabichromenic acid
-
CBD, cannabidiol
-
CBDV, cannabidivarin
-
CBDA, cannabidiolic acid
-
CBDVA, cannabidivarinic acid
-
CBG, cannabigerol
-
CBGA, cannabichromenic acid
-
CBL, cannabicyclol
-
CBN, cannabinol
-
CNS, central nervous system
-
DMH, 1,1′-dimethylheptyl
-
FAAH, fatty acid amide hydrolase
-
FDA, US Food and Drug Administration
-
GOT, geranylpyrophosphate:olivetolic acid geranyltransferase
-
GPCR, G-protein coupled receptor
-
IC50, half maximal inhibitory concentration
-
MAGL, monoacylglycerol lipase
-
NMDA, N-methyl-D-aspartate
-
NSAID, non-steroidal anti-inflammatory drug
-
PPARα, proliferator-activated receptor-α
-
PTSD, post-traumatic stress disorder
-
SAR, structure–activity relationship
-
TGA, Australian Therapeutic Goods Administration
-
Δ8-THC, Δ8-tetrahydrocannabinol
-
Δ9-THC, Δ9-tetrahydrocannabinol
-
Δ9-THCB, Δ9-tetrahydrocannabutol
-
Δ9-THCV, Δ9-tetrahydrocannabivarin
-
Δ9-THCA, Δ9-tetrahydrocannabinolic acid
-
Δ9-THCVA, Δ9-tetrahydrocannabivarinic acid
-
TRPA1, transient receptor potential cation channel subfamily A member 1
-
TRPV1, transient receptor potential cation channel subfamily V member 1
Conflicts of Interest
The CSIRO has undertaken commercial research with several Australian medicinal cannabis companies including Cann Group Ltd, CannPal, and CannaPacific, and in some cases the research is ongoing.
Acknowledgements
The author acknowledges other CSIRO staff including Jamie Freemont, Wioleta Kowalczyk, Marc McEwan, Stephanie Logan, Pete Cass, Peter Mansour, Henry Sabarez, and Pablo Juliano, as well as staff of the CSIRO’s commercial clients, particularly Filippa Brugliera of Cann Group Ltd, for their contributions to our collective understanding of the fascinating and multi-faceted field of medicinal cannabis. No specific funding was used in the preparation of this manuscript.
References
[1] S. A. Bonini, M. Premoli, S. Tambaro, A. Kumar, G. Maccarinelli, M. Memo, A. Mastinu, J. Ethnopharmacol. 2018, 227, 300.| Crossref | GoogleScholarGoogle Scholar | 30205181PubMed |
[2] T. Long, M. Wagner, D. Demske, C. Leipe, P. E. Tarasov, Veg. Hist. Archaeobot. 2017, 26, 245.
| Crossref | GoogleScholarGoogle Scholar |
[3] R. C. Clarke, M. D. Merlin, Crit. Rev. Plant Sci. 2016, 35, 293.
| Crossref | GoogleScholarGoogle Scholar |
[4] C. L. P. Grof, Br. J. Clin. Pharmacol. 2018, 84, 2463.
| Crossref | GoogleScholarGoogle Scholar |
[5] B. Spitzer-Rimon, S. Duchin, N. Bernstein, R. Kamenetsky, Front. Plant Sci. 2019, 10, 350.
| Crossref | GoogleScholarGoogle Scholar | 31001293PubMed |
[6] Y. Gaoni, R. Mechoulam, J. Am. Chem. Soc. 1964, 86, 1646.
| Crossref | GoogleScholarGoogle Scholar |
[7] M. Fellermeier, M. H. Zenk, FEBS Lett. 1998, 427, 283.
| Crossref | GoogleScholarGoogle Scholar | 9607329PubMed |
[8] Y. Shoyama, M. Yagi, I. Nishioka, T. Yamauchi, Phytochemistry 1975, 14, 2189.
| Crossref | GoogleScholarGoogle Scholar |
[9] R. S. Cahn, J. Chem. Soc. 1933, 1400.
| Crossref | GoogleScholarGoogle Scholar |
[10] L. O. Hanuš, S. M. Meyer, E. Muñoz, O. Taglialatela-Scafati, G. Appendino, Nat. Prod. Rep. 2016, 33, 1357.
| Crossref | GoogleScholarGoogle Scholar | 27722705PubMed |
[11] P. Linciano, C. Citti, L. Luongo, C. Belardo, S. Maione, M. A. Vandelli, F. Forni, G. Gigli, A. Laganà, C. M. Montone, G. Cannazza, J. Nat. Prod. 2020, 83, 88.
| Crossref | GoogleScholarGoogle Scholar | 31891265PubMed |
[12] C. Citti, P. Linciano, F. Russo, L. Luongo, M. Iannotta, S. Maione, A. Laganà, A. L. Capriotti, F. Forni, M. A. Vandelli, G. Gigli, G. Cannazza, Sci. Rep. 2019, 9, 20335.
| Crossref | GoogleScholarGoogle Scholar | 31889124PubMed |
[13] Y. Asakawa, F. Nagashima, A. Ludwiczuk, J. Nat. Prod. 2020, 83, 756.
| Crossref | GoogleScholarGoogle Scholar | 32142276PubMed |
[14] T. Gülck, B. L. Møller, Trends Plant Sci. 2020, 25, 985.
| Crossref | GoogleScholarGoogle Scholar | 32646718PubMed |
[15] L. Cristino, T. Bisogno, V. Di Marzo, Nat. Rev. Neurol. 2020, 16, 9.
| Crossref | GoogleScholarGoogle Scholar | 31831863PubMed |
[16] A. C. Porter, J.-M. Sauer, M. D. Knierman, G. W. Becker, M. J. Berna, J. Bao, G. G. Nomikos, P. Carter, F. P. Bymaster, A. B. Leese, C. C. Felder, J. Pharmacol. Exp. Ther. 2002, 301, 1020.
| Crossref | GoogleScholarGoogle Scholar | 12023533PubMed |
[17] R. Mechoulam, P. Braun, Y. Gaoni, J. Am. Chem. Soc. 1972, 94, 6159.
| Crossref | GoogleScholarGoogle Scholar | 5054408PubMed |
[18] H. Edery, Y. Grunfeld, Z. Ben-Zvi, R. Mechoulam, Ann. N. Y. Acad. Sci. 1971, 191, 40.
| Crossref | GoogleScholarGoogle Scholar |
[19] R. Adams, S. Loewe, C. M. Smith, W. D. McPhee, J. Am. Chem. Soc. 1942, 64, 694.
| Crossref | GoogleScholarGoogle Scholar |
[20] A. R. Todd, Experientia 1946, 2, 55.
| Crossref | GoogleScholarGoogle Scholar | 21016843PubMed |
[21] R. Mechoulam, N. Lander, A. Breuer, J. Zahalka, Tetrahedron Asymmetry 1990, 1, 315.
| Crossref | GoogleScholarGoogle Scholar |
[22] S. Burstein, C. A. Audette, A. Breuer, W. A. Devane, S. Colodner, S. A. Doyle, R. Mechoulam, J. Med. Chem. 1992, 35, 3135.
| Crossref | GoogleScholarGoogle Scholar | 1507202PubMed |
[23] R. Archer, W. B. Blanchard, W. A. Day, D. W. Johnson, E. R. Lavagnino, C. W. Ryan, J. E. Baldwin, J. Org. Chem. 1977, 42, 2277.
| Crossref | GoogleScholarGoogle Scholar | 874609PubMed |
[24] L. O. Hanuš, S. Tchilibon, D. E. Ponde, A. Breuer, E. Frideb, R. Mechoulama, Org. Biomol. Chem. 2005, 3, 1116.
| Crossref | GoogleScholarGoogle Scholar | 15750656PubMed |
[25] L. O. Hanuš, A. Breuer, S. Tchilibon, S. Shiloah, D. Goldenberg, M. Horowitz, R. G. Pertwee, R. A. Ross, R. Mechoulam, E. Fride, Proc. Natl. Acad. Sci. USA 1999, 96, 14228.
| Crossref | GoogleScholarGoogle Scholar |
[26] J. W. Huffman, J. Liddle, S. Yu, M. M. Aung, M. E. Abood, J. L. Wiley, B. R. Martin, Bioorg. Med. Chem. 1999, 7, 2905.
| Crossref | GoogleScholarGoogle Scholar | 10658595PubMed |
[27] R. S. Wilson, E. L. May, B. R. Martin, W. L. Dewey, J. Med. Chem. 1976, 19, 1165.
| Crossref | GoogleScholarGoogle Scholar | 978681PubMed |
[28] L. S. Melvin, M. R. Johnson, C. A. Harbert, G. M. Milne, A. Weissman, J. Med. Chem. 1984, 27, 67.
| Crossref | GoogleScholarGoogle Scholar | 6690685PubMed |
[29] V. Auwärter, S. Dresen, W. Weinmann, M. Müller, M. Pütz, N. Ferreirós, J. Mass Spectrom. 2009, 44, 832.
| Crossref | GoogleScholarGoogle Scholar | 19189348PubMed |
[30] M. R. Bell, T. E. D’Ambra, V. Kumar, M. A. Eissenstat, J. L. Herrmann, J. R. Wetzel, D. Rosi, R. E. Philion, S. J. Daum, D. J. Hlasta, R. K. Kullnig, J. A. Ackerman, D. R. Haubrich, D. A. Luttinger, E. R. Baizman, M. S. Miller, S. J. Ward, J. Med. Chem. 1991, 34, 1099.
| Crossref | GoogleScholarGoogle Scholar | 1900533PubMed |
[31] G. P. Moloney, J. A. Angus, A. D. Robertson, M. J. Stoermer, M. Robinson, C. E. Wright, K. McRae, A. Christopoulos, Eur. J. Med. Chem. 2008, 43, 513.
| Crossref | GoogleScholarGoogle Scholar | 17582659PubMed |
[32] J. W. Huffman, K.-S. C. Marriott, Curr. Top. Med. Chem. 2008, 8, 187.
| Crossref | GoogleScholarGoogle Scholar | 18289088PubMed |
[33] A. Makriyannis, H. Deng, World Patent WO2001028557A1 2001.
[34] J. Nakajima, M. Takahashi, T. Seto, C. Kanai, J. Suzuki, M. Yoshida, T. Hamano, Forensic Toxicol. 2011, 29, 95.
| Crossref | GoogleScholarGoogle Scholar |
[35] For further reading regarding the clandestine design of synthetic cannabinomimetics, see: S. M. Wilkinson, S. D. Banister, M. Kassiou, Aust. J. Chem. 2015, 68, 4.
| Crossref | GoogleScholarGoogle Scholar |
[36] J. J. Feigenbaum, F. Bergmann, S. A. Richmond, R. Mechoulam, V. Nadler, Y. Kloog, M. Sokolovsky, Proc. Natl. Acad. Sci. USA 1989, 86, 9584.
| Crossref | GoogleScholarGoogle Scholar | 2556719PubMed |
[37] T. E. D’Ambra, K. G. Estep, M. R. Bell, M. A. Eissenstat, K. A. Josef, S. J. Ward, D. A. Haylock, E. R. Baizman, F. M. Casiano, N. C. Beglin, S. M. Chippari, J. D. Grego, R. K. Kullnig, G. T. Daley, J. Med. Chem. 1992, 35, 124.
| Crossref | GoogleScholarGoogle Scholar | 1732519PubMed |
[38] J. K. Booth, J. E. Page, J. Bohlmann, PLoS One 2017, 12, e0173911.
| Crossref | GoogleScholarGoogle Scholar | 28355238PubMed |
[39] J. T. Fischedick, A. Hazekamp, T. Erkelens, Y. H. Choi, R. Verpoorte, Phytochemistry 2010, 71, 2058.
| Crossref | GoogleScholarGoogle Scholar | 21040939PubMed |
[40] A. Hazekamp, J. T. Fischedick, Drug Test. Anal. 2012, 4, 660.
| Crossref | GoogleScholarGoogle Scholar | 22362625PubMed |
[41] M. W. Giese, M. A. Lewis, L. Geise, K. M. Smith, J. AOAC Int. 2015, 98, 1503.
| Crossref | GoogleScholarGoogle Scholar | 26651562PubMed |
[42] O. Aizpurua-Olaizola, U. Soydaner, E. Öztürk, D. Schibano, Y. Simsir, P. Navarro, N. Etxebarria, A. Usobiaga, J. Nat. Prod. 2016, 79, 324.
| Crossref | GoogleScholarGoogle Scholar | 26836472PubMed |
[43] J. T. Fischedick, Cannabis Cannabinoid Res. 2017, 2, 34.
| Crossref | GoogleScholarGoogle Scholar | 28861503PubMed |
[44] D. Jin, S. Jin, Y. Yu, C. Lee, J. Chen, J. Anal. Bioanal. Tech. 2017, 8, 349.
| Crossref | GoogleScholarGoogle Scholar |
[45] R. Gallily, Z. Yekhtin, L. O. Hanuš, Cannabis Cannabinoid Res. 2018, 3, 282.
| Crossref | GoogleScholarGoogle Scholar | 30596146PubMed |
[46] M. Sexton, K. Shelton, P. Haley, M. West, Planta Med. 2018, 84, 234.
| Crossref | GoogleScholarGoogle Scholar | 28926863PubMed |
[47] T. Nuutinen, Eur. J. Med. Chem. 2018, 157, 198.
| Crossref | GoogleScholarGoogle Scholar | 30096653PubMed |
[48] D. Namdar, M. Mazuz, A. Ion, H. Koltai, Ind. Crops Prod. 2018, 113, 376.
| Crossref | GoogleScholarGoogle Scholar |
[49] J. K. Booth, J. Bohlmann, Plant Sci. 2019, 284, 67.
| Crossref | GoogleScholarGoogle Scholar | 31084880PubMed |
[50] E. A. Ibrahim, M. Wang, M. M. Radwan, A. S. Wanas, C. G. Majumdar, B. Avula, Y.-H. Wang, I. A. Khan, S. Chandra, H. Lata, G. M. Hadad, R. A. Abdel Salam, A. K. Ibrahim, S. A. Ahmed, M. A. ElSohly, Planta Med. 2019, 85, 431.
| Crossref | GoogleScholarGoogle Scholar | 30646402PubMed |
[51] S. Elzinga, J. Fischedick, R. Podkolinski, J. C. Raber, Nat. Prod. Chem. Res. 2015, 3, 181.
| Crossref | GoogleScholarGoogle Scholar |
[52] D. Jin, K. Dai, Z. Xie, J. Chen, Sci. Rep. 2020, 10, 3309.
| Crossref | GoogleScholarGoogle Scholar | 32094454PubMed |
[53] F. Pollastro, A. Minassi, L. G. Fresu, Curr. Med. Chem. 2018, 25, 1160.
| Crossref | GoogleScholarGoogle Scholar | 28799497PubMed |
[54] H. N. ElSohly, G.-E. Ma, C. E. Turner, M. A. ElSohly, J. Nat. Prod. 1984, 47, 445.
| Crossref | GoogleScholarGoogle Scholar | 6481358PubMed |
[55] G. Bar-Sela, A. Avisar, R. Batash, M. Schaffer, Curr. Med. Chem. 2014, 21, 1923.
| Crossref | GoogleScholarGoogle Scholar | 24606496PubMed |
[56] C. Turner, M.-F. H. Hsu, J. E. Knapp, P. L. Schiff, D. J. Slatkin, J. Pharm. Sci. 1976, 65, 1084.
| Crossref | GoogleScholarGoogle Scholar | 957120PubMed |
[57] M. A. ElSohly, C. Turner, C. H. Phoebe, J. E. Knapp, P. L. Schiff, D. J. Slatkin, J. Pharm. Sci. 1978, 67, 124.
| Crossref | GoogleScholarGoogle Scholar | 619102PubMed |
[58] X. Yan, Y. Zhou, J. Tang, M. Ji, H. Lou, P. Fan, Phytochem. Lett. 2016, 18, 77.
| Crossref | GoogleScholarGoogle Scholar |
[59] B. J. Tipple, B. Hambach, J. E. Barnette, L. A. Chesson, J. R. Ehleringer, Forensic Sci. Int. 2016, 262, 233.
| Crossref | GoogleScholarGoogle Scholar | 27045381PubMed |
[60] F. M. A. Leyva-Gutierrez, J. P. Munafo, T. Wang, J. Agric. Food Chem. 2020, 68, 7648.
| Crossref | GoogleScholarGoogle Scholar |
[61] C. R. Vogl, H. Mölleken, G. Lissek-Wolf, A. Surböck, J. Kobert, J. Ind. Hemp 2004, 9, 51.
| Crossref | GoogleScholarGoogle Scholar |
[62] J. Callaway, U. Schwab, I. Harvima, P. Halonen, O. Mykkänen, P. Hyvönen, T. Järvinen, J. Dermatolog. Treat. 2005, 16, 87.
| Crossref | GoogleScholarGoogle Scholar | 16019622PubMed |
[63] T. Chen, J. He, J. Zhang, H. Zhang, P. Qian, J. Hao, L. Li, J. Diet. Suppl. 2010, 7, 117.
| Crossref | GoogleScholarGoogle Scholar | 22435611PubMed |
[64] US Department of Agriculture, Agricultural Research Service, ‘Food Data Central – Seeds, hemp seed, hulled’. Available at: https://fdc.nal.usda.gov/fdc-app.html#/food-details/170148/nutrients (accessed 12 October 2020)
[65] Z. K. Punja, D. Collyer, C. Scott, S. Lung, J. Holmes, D. Sutton, Front. Plant Sci. 2019, 10, 1120.
| Crossref | GoogleScholarGoogle Scholar | 31681341PubMed |
[66] M. Galić, A. Perčin, Ž. Zgorelec, I. Kisić, J. Cent. Eur. Agric. 2019, 20, 1099.
| Crossref | GoogleScholarGoogle Scholar |
[67] M. T. Shah, S. Begum, S. Khan, Environ. Earth Sci. 2010, 60, 1091.
| Crossref | GoogleScholarGoogle Scholar |
[68] L. A. Matsuda, S. J. Lolait, M. J. Brownstein, A. C. Young, T. T. Bonner, Nature 1990, 346, 561.
| Crossref | GoogleScholarGoogle Scholar | 2165569PubMed |
[69] S. Munro, K. L. Thomas, M. Abu-Shaar, Nature 1993, 365, 61.
| Crossref | GoogleScholarGoogle Scholar | 7689702PubMed |
[70] R. G. Pertwee, Br. J. Pharmacol. 2006, 147, S163.
| Crossref | GoogleScholarGoogle Scholar | 16402100PubMed |
[71] T. Hua, X. Li, L. Wu, C. Iliopoulos-Tsoutsouvas, Y. Wang, M. Wu, L. Shen, C. A. Johnston, S. P. Nikas, F. Song, X. Song, S. Yuan, Q. Sun, Y. Wu, S. Jiang, T. W. Grim, O. Benchama, E. L. Stahl, N. Zvonok, S. Zhao, L. M. Bohn, A. Makriyannis, Z.-J. Liu, Cell 2020, 180, 655.
| Crossref | GoogleScholarGoogle Scholar | 32004463PubMed |
[72] I. Svíženská, P. Dubový, A. Šulcová, Pharmacol. Biochem. Behav. 2008, 90, 501.
| Crossref | GoogleScholarGoogle Scholar | 18584858PubMed |
[73] E. Russo, J. Marcu, in Advances in Pharmacology (Eds D. Kendall, S. Alexander) 2017, Vol 80, Ch. 3, pp. 67–134 (Academic Press: Cambridge, MA).
[74] J. Gonçalves, T. Rosado, S. Soares, A. Y. Simão, D. Caramelo, Â. Luís, N. Fernández, M. Barroso, E. Gallardo, A. P. Duarte, Medicines 2019, 6, 31.
| Crossref | GoogleScholarGoogle Scholar |
[75] (a) The International Congress on Clinical Trials in Cannabis, 24 October 2019, London, United Kingdom.
(b) Cannabinoid 2019 Conference: IACM 10th Conference on Cannabinoids in Medicine, 31 October 2019, Berlin, Germany.
[76] https://www.unodc.org/pdf/convention_1961_en.pdf
[77] In December 2020, the UN Commission on Narcotic Drugs took the decision to remove cannabis from Schedule IV of the 1961 Single Convention on Narcotic Drugs, where the most dangerous category of drugs are listed. See https://news.un.org/en/story/2020/12/1079132
[78] https://www.tga.gov.au/medicinal-cannabis-guidance-documents#review-bibliographies
[79] S. Zhornitsky, S. Potvin, Pharmaceuticals 2012, 5, 529.
| Crossref | GoogleScholarGoogle Scholar | 24281562PubMed |
[80] National Academies of Sciences, Engineering, and Medicine, The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research 2017 (The National Academies Press: Washington, DC).
[81] F. Grotenhermen, Clin. Pharmacokinet. 2003, 42, 327.
| Crossref | GoogleScholarGoogle Scholar | 12648025PubMed |
[82] N. Bruni, C. Della Pepa, S. Oliaro-Bosso, E. Pessione, D. Gastaldi, F. Dosio, Molecules 2018, 23, 2478.
| Crossref | GoogleScholarGoogle Scholar |
[83] J. P. Goulle, E. Saussereau, C. Lacroix, Ann. Pharm. Fr. 2008, 66, 232.
| Crossref | GoogleScholarGoogle Scholar | 18847571PubMed |
[84] L. L. Anderson, I. K. Low, S. D. Banister, I. S. McGregor, J. C. Arnold, J. Nat. Prod. 2019, 82, 3047.
| Crossref | GoogleScholarGoogle Scholar | 31686510PubMed |
[85] (a) N. Duggan, ‘Mobile drug testing: high behind the wheel’, Honi Soit, published by Students’ Representative Council, 2019, University of Sydney. Available at: https://honisoit.com/2019/05/mobile-drug-testing-high-behind-the-wheel/
(b) L. Ferri, ‘Controversial magistrate QUITS because he’s fed up with making people lose their licences, jobs and homes under ‘grossly unfair’ drug-driving laws’, Daily Mail Australia, 2020, Associated Newspapers Ltd. Available at: https://www.dailymail.co.uk/news/article-8435311/Controversial-magistrate-loathes-drug-driving-laws-QUITS-fed-licences-people.html
[86] J. Meehan-Atrash, W. Luo, K. J. McWhirter, R. M. Strongin, ACS Omega 2019, 4, 16111.
| Crossref | GoogleScholarGoogle Scholar | 31592479PubMed |
[87] S. Lattanzi, G. Zaccara, E. Russo, A. La Neve, M. A. M. Lodi, P. Striano, Expert Rev. Neurother. 2021, 21, 99.
| Crossref | GoogleScholarGoogle Scholar | 33026899PubMed |
[88] A. Casiraghi, U. M. Musazzi, G. Centin, S. Franzè, P. Minghetti, Pharmaceuticals 2020, 13, 337.
| Crossref | GoogleScholarGoogle Scholar |
[89] A. Nitecka-Buchta, A. Nowak-Wachol, K. Wachol, K. Walczyńska-Dragon, P. Olczyk, O. Batoryna, W. Kempa, S. Baron, J. Clin. Med. 2019, 8, 1886.
| Crossref | GoogleScholarGoogle Scholar |
[90] D. H. Xu, B. D. Cullen, M. Tang, Y. Fang, Curr. Pharm. Biotechnol. 2020, 21, 390.
| Crossref | GoogleScholarGoogle Scholar | 31793418PubMed |
[91] A. L. Stinchcomb, S. Valiveti, D. C. Hammell, D. R. Ramsey, J. Pharm. Pharmacol. 2004, 56, 291.
| Crossref | GoogleScholarGoogle Scholar | 15025853PubMed |
[92] M. B. Hannon, K. A. Deabold, B. N. Talsma, A. Lyubimov, A. Iqbal, A. Zakharov, L. J. Gamble, J. J. Wakshlag, J. Vet. Pharmacol. Ther. 2020, 43, 508.
| Crossref | GoogleScholarGoogle Scholar | 32735381PubMed |
[93] S. Thumma, S. Majumdar, M. A. El Sohly, W. Gul, M. A. Repka, Int. J. Pharm. 2008, 362, 126.
| Crossref | GoogleScholarGoogle Scholar | 18652884PubMed |
[94] G. R. Adelli, P. Bhagav, P. Taskar, T. Hingorani, S. Pettaway, W. Gul, M. A. ElSohly, M. A. Repka, S. Majumdar, Invest. Ophthalmol. Vis. Sci. 2017, 58, 2167.
| Crossref | GoogleScholarGoogle Scholar | 28399267PubMed |
[95] R. G. Pertwee, E. M. Rock, K. Guenther, C. L. Limebeer, L. A. Stevenson, C. Haj, R. Smoum, L. A. Parker, R. Mechoulam, Br. J. Pharmacol. 2018, 175, 100.
| Crossref | GoogleScholarGoogle Scholar | 29057454PubMed |
[96] A. M. Habib, A. L. Okorokov, M. N. Hill, J. T. Bras, M.-C. Lee, S. Li, S. J. Gossage, M. van Drimmelen, M. Morena, H. Houlden, J. D. Ramirez, D. L. H. Bennett, D. Srivastava, J. J. Cox, Br. J. Anaesth. 2019, 123, e249.
| Crossref | GoogleScholarGoogle Scholar | 30929760PubMed |
[97] M. Seierstad, J. G. Breitenbucher, J. Med. Chem. 2008, 51, 7327.
| Crossref | GoogleScholarGoogle Scholar | 18983142PubMed |
[98] A. Lodola, R. Castelli, M. Mor, S. Rivara, Expert Opin. Ther. Pat. 2015, 25, 1247.
| 26413912PubMed |
[99] R. K. P. Tripathi, Eur. J. Med. Chem. 2020, 188, 111953.
| Crossref | GoogleScholarGoogle Scholar |
[100] G. Moreno-Sanz, B. Barrera, A. Guijarro, I. d’Elia, J. A. Otero, A. I. Alvarez, T. Bandiera, G. Merino, D. Piomelli, Pharmacol. Res. 2011, 64, 359.
| Crossref | GoogleScholarGoogle Scholar | 21767647PubMed |
[101] L. Scalvini, D. Piomelli, M. Mor, Chem. Phys. Lipids 2016, 197, 13.
| Crossref | GoogleScholarGoogle Scholar | 26216043PubMed |
[102] N. Grimsey, J. R. Savinainen, B. Attili, M. Ahamed, Drug Discov. Today 2020, 25, 330.
| Crossref | GoogleScholarGoogle Scholar | 31622747PubMed |
[103] C. Granchi, M. Lapillo, S. Glasmacher, G. Bononi, C. Licari, G. Poli, M. el Boustani, I. Caligiuri, F. Rizzolio, J. Gertsch, M. Macchia, F. Minutolo, T. Tuccinardi, A. Chicca, J. Med. Chem. 2019, 62, 1932.
| Crossref | GoogleScholarGoogle Scholar | 30715876PubMed |
[104] J. H. M. Lange, C. G. Kruse, Drug Discov. Today 2005, 10, 693.
| Crossref | GoogleScholarGoogle Scholar |
[105] J. A. Stapleton, Addiction 2009, 104, 277.
| Crossref | GoogleScholarGoogle Scholar | 19149824PubMed |
[106] R. J. Chorvat, J. Berbaum, K. Seriacki, J. F. McElroy, Bioorg. Med. Chem. Lett. 2012, 22, 6173.
| Crossref | GoogleScholarGoogle Scholar | 22959249PubMed |
[107] A. C. M. van Esbroeck, A. P. A. Janssen, A. B. Cognetta, D. Ogasawara, G. Shpak, M. van der Kroeg, V. Kantae, M. P. Baggelaar, F. M. S. de Vrij, H. Deng, M. Allarà, F. Fezza, Z. Lin, T. van der Wel, M. Soethoudt, E. D. Mock, H. den Dulk, I. L. Baak, B. I. Florea, G. Hendriks, L. De Petrocellis, H. S. Overkleeft, T. Hankemeier, C. I. De Zeeuw, V. Di Marzo, M. Maccarrone, B. F. Cravatt, S. A. Kushner, M. van der Stelt, Science 2017, 356, 1084.
| Crossref | GoogleScholarGoogle Scholar |
[108] R. Mechoulam, S. Ben-Shabat, Nat. Prod. Rep. 1999, 16, 131.
| Crossref | GoogleScholarGoogle Scholar | 10331283PubMed |
[109] E. B. Russo, Br. J. Pharmacol. 2011, 163, 1344.
| Crossref | GoogleScholarGoogle Scholar | 21749363PubMed |
[110] H. M. Hashiesh, M. F. N. Meeran, C. Sharma, B. Sadek, J. Al Kaabi, S. K. Ojha, Nutrients 2020, 12, 2963.
| Crossref | GoogleScholarGoogle Scholar |
[111] C. Kohlert, I. van Rensen, R. März, G. Schindler, E. U. Graefe, M. Veit, Planta Med. 2000, 66, 495.
| Crossref | GoogleScholarGoogle Scholar | 10985073PubMed |
[112] B. Wu, K. Kulkarni, S. Basu, S. Zhang, M. Hu, J. Pharm. Sci. 2011, 100, 3655.
| Crossref | GoogleScholarGoogle Scholar | 21484808PubMed |
[113] M. Santiago, S. Sachdev, J. C. Arnold, I. S. McGregor, M. Connor, Cannabis Cannabinoid Res. 2019, 4, 165.
| Crossref | GoogleScholarGoogle Scholar | 31559333PubMed |
[114] M. Heblinski, M. Santiago, C. Fletcher, J. Stuart, M. Connor, I. S. McGregor, J. C. Arnold, Cannabis Cannabinoid Res. 2020, 5, 305.
| Crossref | GoogleScholarGoogle Scholar | 33376801PubMed |
† A more accurate definition of a phytocannabinoid is: a chemical found in a plant that is structurally related to Δ9-THC and its biosynthetic precursors (back to CBGA), and their derivatives. Note that not all of these compounds interact with cannabinoid receptors.




