Synthesis of a Novel Library of 1-Substituted Pyrido[1,2-a]benzimidazoles
Satyanarayana Gadde A B , Yun Cheuk Leung A , Mohan Bhadbade A , Belamy B. Cheung B C , David StC. Black A D and Naresh Kumar
A D and Naresh Kumar  A D
A D
A School of Chemistry, The University of New South Wales, Sydney, NSW 2052, Australia.
B Children’s Cancer Institute Australia for Medical Research, Lowy Cancer Research Centre, The University of New South Wales, Sydney, NSW 2052, Australia.
C School of Women’s and Children’s Health, The University of New South Wales, Sydney, Randwick, NSW 2031, Australia.
D Corresponding authors. Email: d.black@unsw.edu.au; n.kumar@unsw.edu.au
Australian Journal of Chemistry 73(12) 1208-1218 https://doi.org/10.1071/CH20173
Submitted: 30 May 2020 Accepted: 30 July 2020 Published: 28 August 2020
Journal Compilation © CSIRO 2020 Open Access CC BY-NC-ND
Abstract
The reactivity and synthesis of new analogues of pyrido[1,2-a]benzimidazoles have been explored. Twenty-three derivatives bearing phenoxy, thiophenoxy, aniline, and aryl groups at the 1-position were successfully synthesised in 25–91 % yield, via nucleophilic substitution, Buchwald–Hartwig amination, and Suzuki coupling type processes. Solvent free synthetic protocols were employed to achieve the nucleophilic substitution of anilines with electron donating groups or moderately electron withdrawing groups on a sterically demanding intermediate (7a). An unusual polycyclic heterocycle was identified as a side-product during this work: a dimeric bis(pyrido[1,2-a]benzimidazole).
Introduction
Pyrido[1,2-a]benzimidazoles are tricyclic derivatives of benzimidazole that contain a fused pyridyl ring (Fig. 1). First described by Morgan in 1937,[1] the highly conjugated aromatic system is of interest in materials science, and has found applications in fluorescence.[2] The pyrido[1,2-a]benzimidazole motif is also an important structure in medicinal chemistry: a diversity of biological applications are recognised, including the semi-synthetic antibiotic Rifaximin,[3] and a variety of antineoplastic, analgesic,[4] anti-bacterial,[5] anti-fungal,[6] anti-malarial,[7] and anticancer agents (e.g. 2–6).[8–10]

|
While the heteroarene cores of 1–6 are essential for achieving potent biological activity in each case, the peripheral substituents are also important.[7,8] For example, compounds 4 and 5 displayed quite different activity and selectivity patterns against leukemia cell lines in vitro.[1] It is particularly notable that replacing the chlorine atom in 6 with an aniline group in 5 gave a significant enhancement in anti-leukemia activity.[1,9] Thus, methods for systematically derivatising a particular position of the pyrido[1,2-a]benzimidazole core are of great value for providing compounds for biological screening and structure–activity relationship studies. In this context, we report herein the synthesis of a small library of pyrido[1,2-a]benzimidazole derivatives bearing a variety of substituents at position 1.
To investigate the potential anti-cancer activity of novel pyrido[1,2-a]benzimidazole derivatives, we desired to synthesise a series of highly substituted analogues 8 and 9 (Scheme 1), containing various phenoxy, thiophenoxy, aniline, and aryl groups at position 1. We aimed to access these compounds through aromatic nucleophilic substitution and Suzuki coupling of the corresponding 1-chloro derivatives 7 (Scheme 1). Compound 7 was previously shown to undergo aromatic nucleophilic substitution reactions, but with aliphatic amines as the nucleophiles.[6] At the outset of this study it was expected that the substitutions with less nucleophilic aromatic amines and coupling with aryl boronic acids would pose more of a challenge, and substitutions with oxygen or sulfur based nucleophiles may be more straightforward despite a lack of literature precedent for such transformations.

|
Results and Discussion
Preparation of Starting Materials
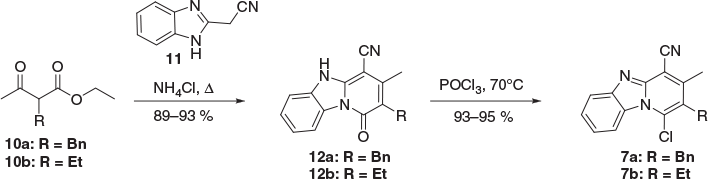
The synthesis of pyrido[1,2-a]benzimidazoles is well documented. The most common route involves [3 + 3] cyclocondensation of C2 methylene-containing benzimidazoles with appropriate bielectrophiles.[11] More recently, other strategies including multicomponent reactions and copper-catalysed reactions[12] have also been developed, contributing to a flourishing body of research in this area. The requisite starting materials for this study 7a,b were readily obtained through a two-step sequence (Scheme 2).[2,13,14] Thus, [3 + 3] cyclocondensation of benzimidazoleacetonitrile 11 with substituted ethyl acetoacetate derivatives 10a,b afforded the 1-oxo compounds 12a,b in high yield, and subsequent chlorination with POCl3 delivered the desired compounds 7a,b in multigram quantities.[2]
Aromatic Nucleophilic Substitution Reactions
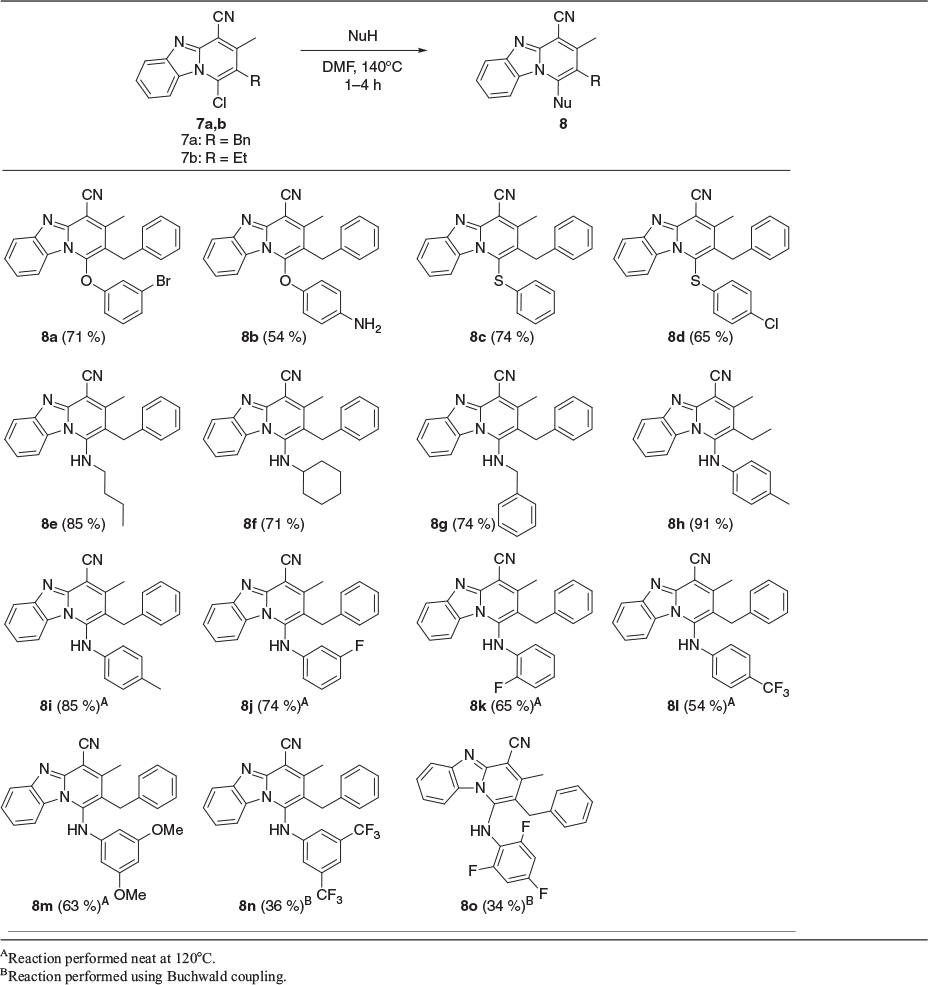
With the precursors 7a,b in hand, their reactivity towards nucleophiles was then investigated (Table 1). A variety of phenols, thiophenols, and aliphatic amines were found to successfully react with 7a, giving the desired products 8a–g in moderate to good yields under conditions identical to those previously reported for aliphatic amine nucleophiles.[6] Attention was next turned towards the use of aromatic amines 8h–o (Table 1). It was found that p-toluidine reacted efficiently with 7b to give the desired product 8h in excellent yield, despite the lower nucleophilicity. To investigate whether the reduced steric demand of the ethyl substituent of 7b was contributing to the efficiency of this reaction, substrate 7a was trialled in the same process. However, in this case no reaction was observed under the standard conditions, or even under a variety of other conditions including the use of a higher-boiling solvent, addition of strong base, microwave assisted heating,[15,16] or ultrasonic irradiation.
|
Finally, it was discovered that the desired compound 8i could be obtained in high yield through heating a mixture of 7a and p-toluidine in the absence of solvent (Table 1).[17] Solvent-free synthetic protocols are popular in modern organic synthesis for two reasons: first, there are environmental benefits from avoiding the use of organic solvents; and second, the rates of reactions can be significantly increased.[18] The substitution reaction to give 8i (Table 1) proved that solvent-free conditions are ideal for the synthesis of 1-anilino-pyrido[1,2-a]benzimidazoles. The reaction gave moderate to high yields with anilines bearing electron-donating or moderately electron-withdrawing groups 8i–m. An additional benefit was the ease of work-up: the reaction mixtures were simply triturated with hexane, filtered, and the solid residue subjected to chromatography on silica gel.
However, the strongly electron deficient[19] substrates 3,5-bis(trifluoromethyl)aniline 8n and 2,4,6-trifluoroaniline 8o were found to be unreactive even in the absence of solvent. Thus, other methods were needed to be explored for such substrates.
Buchwald–Hartwig Amination Reactions
Over the past two decades, palladium-catalysed C–N bond formation (the Buchwald–Hartwig amination reaction)[19–21] has become the method of choice for synthesising targets that contain aromatic moieties linked by a nitrogen atom. The palladium-catalysed approach is highly versatile, allowing most aryl halides (including chlorides, bromides, and iodides) to be successfully coupled with a wide range of amines (primary, secondary, electron-deficient, and heterocyclic amines).[22] Recent investigations have shown that the use of tris(dibenzylideneacetone)dipalladium(0) (Pd2dba3) and [2-dicyclohexylphosphino-2′,4′,6′-triisopropylbiphenyl] (XPhos)[23] provides an excellent catalytic system for the coupling of aryl chlorides with aniline derivatives,[24–26] sometimes even at room temperature.[27,28] Hence, this catalyst system was chosen for this research.
When compound 7a was treated with strongly electron deficient aniline derivatives in the presence of Pd2dba3, XPhos, and KOtBu at room temperature, the desired products 8n and 8o were isolated in 34–36 % yield (Scheme 3). Higher temperatures did not increase the yields, but instead led to complex product mixtures. Nevertheless the Buchwald–Hartwig approach was found to provide a convenient route to otherwise inaccessible pyrido[1,2-a]benzimidazoles.

|
In an attempt to rationalise the modest yields of the Buchwald–Hartwig amination, one major side product 13, was carefully isolated in 30 % yield (Scheme 3). It seems that under the strongly basic reaction conditions (KOtBu, 3 equiv.), deprotonation of the methyl group of 7a and subsequent nucleophilic attack onto another molecule of 7a affords the dimeric structure 13. In addition, besides 13, further oligomers and/or coupling products are possible, and this can explain the modest yields of the desired products 8n and 8o.
Suzuki Coupling Reactions
Having developed methods for aromatic nucleophilic substitution of 7a with diverse nucleophiles (Schemes 2 and 3), efforts were next directed towards the formation of a carbon–carbon bond with 7a. The Suzuki coupling reaction was employed to achieve the formation of the C–C bond. This palladium catalysed cross-coupling reaction between the organic halides or triflates and organoboron compounds is a powerful and general methodology for C–C bond formation. It offers several advantages, such as being largely unaffected by the presence of water and tolerates a broad range of functional groups.
Compound 7a was treated with a variety of aromatic boronic acids or boronic acid esters in the presence of Pd(PPh3)4 and Na2CO3 at 90°C to afford the desired products 14a–h in moderate to good yields (25–76 %) after column chromatography and crystallisation (Table 2). One major side product formed was the dimer of 7a (Compound 13). The structures of the compounds 14a (Fig. 2), 14d (Fig. 3), and 13 (Fig. 4) were confirmed by X-ray crystallography.[29]

|

|

|

|
Conclusion
The chemistry of pyrido[1,2-a]benzimidazoles has been explored. Various 1-phenoxy, 1-thiophenoxy, 1-anilino, and 1-aryl derivatives have been synthesised from the 1-chloro precursors via either aromatic nucleophilic substitution processes or, for strongly electron deficient 1-anilino derivatives, Buchwald–Hartwig amination and Suzuki coupling conditions. The synthetic methods developed herein will underpin the ongoing development of this important class of heterocycles for future applications in medicine and materials science.
Experimental
General
The solvents and reagents were purchased from commercial sources (Combi-Blocks, Sigma Aldrich) and used without purification. The reactions were monitored by TLC (thin-layer chromatography) using Merck SilicaGel 60 254 plates, and UV light (254 nm) was used for visualisation. Grace Davison LC60A 40–63 µm silica gel columns were used as the stationary phase for column chromatography and the mobile phase was varied depending upon the polarity of the compounds. The melting points were measured using a Mel-Temp melting point apparatus and a Thermo Nicolet 370 FT-IR spectrometer was used to record the infrared spectra. High-resolution mass spectrometry (HRMS) was performed at the Bioanalytical Mass Spectrometry Facility, UNSW using the electrospray ionisation (ESI) method. 1H and 13C NMR spectra were recorded at room temperature in deuterated solvents using Bruker Avance 300, 400, or 600 MHz instruments. Tetramethylsilane was used as the internal standard and the chemical shifts (δ) are reported in parts per million (ppm).
2-Benzyl-1-(3-bromophenoxy)-3-methylbenzo[4,5]imidazo[1,2-a]pyridine-4-carbonitrile (8a)
A mixture of compound 7a (100 mg, 0.30 mmol) and m-bromophenol (85.2 mg, 0.45 mmol) in DMF (10 mL) was heated at reflux for 4 h. The crude product was purified by flash chromatography on silica gel using 2 : 3 ethyl acetate/hexane to afford compound 8a as a yellow solid (100.0 mg, 71 %). Mp 207–209°C. δH (300 MHz, DMSO-d6) 7.92 (dt, J 8.3, 1.0, 1H), 7.83 (dt, J 8.4, 1.0, 1H), 7.59–7.46 (m, 3H), 7.29 (ddd, J 8.3, 7.2, 1.1, 1H), 7.26–7.10 (m, 7H), 3.98 (s, 2H), 2.52 (d, J 2.3, 3H). δC (75 MHz, DMSO-d6) 154.3, 152.0, 146.7, 146.2, 144.1, 137.8, 133.3, 128.5, 127.9, 127.4, 126.4, 122.1, 121.8, 119.2, 117.6, 116.3, 115.3, 114.7, 112.6, 97.65, 30.5, 19.2. νmax (KBr)/cm−1 3063, 3022, 2229, 1635, 1596, 1499, 1385, 1369, 1306, 1272, 1249, 1230, 1198, 1171, 1115, 1103, 954, 935, 849, 775, 745, 698. HRMS (+ESI) m/z 468.0703, [M + H]+; calcd for [C26H18BrN3O + H] 468.0711.
1-(4-Aminophenoxy)-2-benzyl-3-methylbenzo[4,5]imidazo[1,2-a]pyridine-4-carbonitrile (8b)
A mixture of compound 7a (100 mg, 0.30 mmol) and 4-amino phenol (49.3 mg, 0.45 mmol) in DMF (4 mL) was heated at reflux for 4 h. The crude product was purified by flash chromatography on silica gel using ethyl acetate/hexane (10–90 %) to afford compound 8b as a yellow solid (54.1 mg, 54 %). Mp 227–229°C. δH (600 MHz, CD2Cl2) 7.99 (dt, J 8.4, 1.0, 1H), 7.94 (dt, J 8.3, 1.0, 1H), 7.53 (ddd, J 8.3, 7.2, 1.1, 1H), 7.29–7.21 (m, 3H), 7.23–7.16 (m, 1H), 7.07 (ddt, J 7.5, 1.3, 0.7, 2H), 6.75–6.69 (m, 2H), 6.62–6.56 (m, 2H), 3.99 (s, 2H), 3.76–3.54 (br s, 2H), 2.57 (s, 3H). δC (150 MHz, CD2Cl2) 152.09, 148.48, 148.19, 147.42, 145.11, 144.10, 138.39, 129.02, 128.47, 128.29, 126.91, 126.71, 122.44, 120.06, 116.61, 116.28, 115.73, 115.45, 112.99, 98.73, 31.43, 19.73. νmax (KBr)/cm−1 3452, 3357, 3030, 2229, 1626, 1481, 1443, 1382, 1307, 1231, 1180, 1021, 921, 819, 726. HRMS (+ESI) m/z 405.1710, [M + H]+; calcd for [C26H20N4O + H] 405.1709.
2-Benzyl-3-methyl-1-(phenylthio)benzo[4,5]imidazo[1,2-a]pyridine-4-carbonitrile (8c)
A mixture of compound 7a (100 mg, 0.30 mmol) and thiophenol (56.9 mg, 0.45 mmol) in DMF (10 mL) was heated at reflux for 4 h. The crude product was purified by flash chromatography on silica gel using 2 : 3 ethyl acetate/hexane to afford compound 8c as a yellow solid (90.5 mg, 74 %). Mp 242–244°C. δH (300 MHz, DMSO-d6) 8.71 (dt, J 8.7, 0.9, 1H), 7.90 (ddd, J 8.3, 1.2, 0.7, 1H), 7.52 (ddd, J 8.2, 7.1, 1.1, 1H), 7.34–7.11 (m, 11H), 4.55 (s, 2H), 2.56 (s, 3H). δC (75 MHz, DMSO-d6) 149.2, 146.2, 143.8, 138.2, 135.0, 132.7, 131.2, 130.2, 130.0, 128.6, 127.9, 127.0, 126.4, 126.3, 126.2, 121.9, 119.2, 116.9, 115.0, 105.0, 102.8, 100.1, 35.6, 19.9. νmax (KBr)/cm−1 3410, 3072, 3021, 2226, 1602, 1585, 1454, 1442, 1393, 1387, 1298, 1190, 1076, 1004, 822, 760, 742. HRMS (+ESI) m/z 428.1187, [M + Na]+; calcd for [C26H19N3S + Na] 428.1197.
2-Benzyl-1-((4-chlorophenyl)thio)-3-methylbenzo[4,5]imidazo[1,2-a]pyridine-4-carbonitrile (8d)
A mixture of compound 7a (100 mg, 0.30 mmol) and p-chlorothiophenol (72.3 mg, 0.45 mmol) in DMF (10 mL) was heated at reflux for 4 h. The crude product was purified by flash chromatography using 2 : 3 ethyl acetate/hexane to afford compound 8d as a yellow solid (86.2 mg, 65 %). Mp 244–246°C. δH (300 MHz, DMSO-d6) 8.66 (dt, J 8.7, 1.0, 1H), 7.91 (dt, J 8.3, 1.0, 1H), 7.52 (ddd, J 8.2, 7.1, 1.1, 1H), 7.37–7.09 (m, 10H), 4.52 (s, 2H), 2.55 (s, 3H). δC (75 MHz, DMSO-d6) 148.9, 144.3, 138.2, 134.5, 131.9, 131.7, 131.0, 130.2, 129.9, 128.6, 128.1, 127.8, 126.4, 126.0, 121.9, 119.5, 116.6, 115.0, 103.1, 34.2, 19.8. νmax (KBr)/cm−1 3422, 3079, 3024, 2223, 1620, 1584, 1466, 1452, 1444, 1372, 1387, 1298, 1195, 1086, 1007, 823, 761, 743, 730. HRMS (+ESI) m/z 440.0981, [M + H]+; calcd for [C26H18ClN3S + H] 440.0988.
2-Benzyl-1-(butylamino)-3-methylbenzo[4,5]imidazo[1,2-a]pyridine-4-carbonitrile (8e)
A mixture of compound 7a (100 mg, 0.30 mmol) and n-butylamine (33.0 mg, 0.45 mmol) in DMF (10 mL) was heated to 80°C for 4 h. The reaction mixture was cooled to room temperature. Water (30 mL) was added to precipitate a yellow solid which was collected by filtration. The crude product was recrystallised from acetone to afford compound 8e as a yellow solid (95 mg, 85 %). Mp 179–181°C. δH (300 MHz, acetone-d6) 8.29 (ddd, J 8.4, 1.2, 0.7, 1H), 7.82 (ddd, J 8.2, 1.2, 0.7, 1H), 7.53 (ddd, J 8.3, 7.2, 1.2, 1H), 7.40–7.27 (m, 3H), 7.27–7.18 (m, 3H), 5.36 (t, J 6.8, 1H), 4.36 (d, J 1.0, 2H), 3.23–3.11 (m, 2H), 2.58 (s, 3H), 1.74–1.61 (m, 2H), 1.25 (dq, J 14.7, 7.4, 2H), 0.81 (t, J 7.3, 3H). δC (75 MHz, acetone-d6) 151.3, 149.1, 146.3, 140.2, 130.2, 129.6, 128.7, 127.3, 126.4, 121.5, 120.0, 117.0, 116.7, 112.1, 95.4, 48.2, 33.1, 32.9, 20.8, 19.8, 14.0. νmax (KBr)/cm−1 3241, 3091, 3050, 2952, 2866, 2216, 1707, 1628, 1595, 1369, 1311, 1133, 765. HRMS (+ESI) m/z 369.2068, [M + H]+; calcd for [C24H24N4 + H] 369.2079.
2-Benzyl-1-(cyclohexylamino)-3-methylbenzo[4,5]imidazo[1,2-a]pyridine-4-carbonitrile (8f)
A mixture of compound 7a (100 mg, 0.30 mmol) and cyclohexylamine (52.0 mg, 0.45 mmol) in DMF (10 mL) was heated to 80°C for 4 h. The reaction mixture was cooled to room temperature and water (30 mL) was added to precipitate a yellow solid which was collected by filtration. The crude product was recrystallised from acetone to afford compound 8f as a yellow solid (84.5 mg, 71 %). Mp 256–257°C. δH (300 MHz, DMSO-d6) 8.26 (d, J 8.4, 1H), 7.89–7.81 (m, 1H), 7.54 (ddd, J 8.2, 7.2, 1.0, 1H), 7.38 (ddd, J 8.4, 7.2, 1.2, 1H), 7.34–7.24 (m, 2H), 7.24–7.19 (m, 1H), 7.16–7.10 (m, 2H), 6.04 (d, J 10.4, 1H), 4.29 (s, 2H), 3.27–3.06 (m, 1H), 2.51 (m, 1H), 2.41 (s, 3H), 1.85 (d, J 12.2, 2H), 1.63 (d, J 11.4, 2H), 1.46 (t, J 11.5, 3H), 1.16–0.82 (m, 2H). νmax (KBr)/cm−1 3373, 3021, 2847, 2366, 2217, 1627, 1595, 1476, 1446, 1306, 737, 720, 698. HRMS (+ESI) m/z 403.1910, [M + H]+; calcd for [C27H26N4 + H] 403.1923.
2-Benzyl-1-(benzylamino)-3-methylbenzo[4,5]imidazo[1,2-a]pyridine-4-carbonitrile (8g)
A mixture of compound 7a (100 mg, 0.30 mmol) and benzylamine (48.4 mg, 0.45 mmol) in DMF (10 mL) was heated to 80°C for 4 h. The reaction mixture was cooled to room temperature. Water (30 mL) was added to precipitate a yellow solid which was collected by filtration. The crude product was recrystallised from acetone to afford compound 8g as a yellow solid (89.9 mg, 74 %). Mp 234–236°C. δH (300 MHz, DMSO-d6) 8.31 (d, J 8.4, 1H), 7.87 (d, J 8.1, 1H), 7.55 (ddd, J 8.2, 7.1, 1.1, 1H), 7.39–7.14 (m, 7H), 7.14–7.06 (m, 2H), 7.06–6.97 (m, 2H), 6.64 (d, J 6.9, 1H), 4.28 (d, J 6.2, 2H), 3.92 (s, 2H), 2.35 (s, 3H). νmax (KBr)/cm−1 3373, 3021, 2847, 2366, 2217, 1627, 1595, 1476, 1446, 1306, 737, 720, 689. HRMS (+ESI) m/z 403.1910, [M + H]+; calcd for [C27H22N4 + H] 403.1923.
2-Ethyl-3-methyl-1-(p-tolylamino)benzo[4,5]imidazo[1,2-a]pyridine-4-carbonitrile (8h)
A mixture of compound 7b (100 mg, 0.36 mmol) and p-toluidine (57.3 mg, 0.54 mmol) in DMF (10 mL) was heated to 80°C for 48 h. The reaction mixture was cooled to room temperature. Water (30 mL) was added to precipitate a yellow solid which was collected by filtration. The crude product was recrystallised from acetone to afford compound 8h as a yellow solid (110.5 mg, 91 %). δH (300 MHz, CDCl3) 7.94 (dd, J 8.2, 1.0, 1H), 7.79 (dd, J 8.4, 1.0, 1H), 7.43 (ddd, J 8.2, 7.1, 1.0, 1H), 7.10 (ddd, J 8.4, 7.1, 1.1, 1H), 7.00 (d, J 8.3, 2H), 6.47 (d, J 8.3, 2H), 6.06 (bs, 1H), 2.93 (s, 3H), 2.71 (q, J 7.5, 2H), 2.24 (s, 3H), 1.24 (t, J 7.5, 3H). δC (75 MHz, CDCl3) 150.1, 149.2, 146.5, 144.6, 142.2, 140.0, 138.8, 131.6, 128.6, 128.5, 126.0, 121.6, 120.9, 120.8, 119.5, 116.0, 20.6, 19.1, 18.9, 14.3. νmax (KBr)/cm−1 3296, 2849, 2533, 2225, 1635, 1585, 1511, 1478, 1457, 1370, 1307, 1273, 1215, 1220, 1165, 1010, 814, 757. HRMS (+ESI) m/z 341.1760, [M + H]+; calcd for [C22H20N4 + H] 341.1766.
2-Benzyl-3-methyl-1-(p-tolylamino)benzo[4,5]imidazo[1,2-a]pyridine-4-carbonitrile (8i)
A mixture of compound 7a (100 mg, 0.30 mmol) and p-toluidine (323.4 mg, 3.02 mmol) was heated to 120°C for 1 h. The crude material was purified by flash chromatography on silica gel using 2 : 3 ethyl acetate/hexane to afford compound 8i as a yellow solid (103.3 mg, 85 %). δH (300 MHz, CDCl3) 8.17 (br s, 1H), 8.02 (dd, J 8.4, 1.0, 1H), 7.82 (dd, J 8.2, 1.0, 1H), 7.45 (ddd, J 8.2, 7.2, 1.0, 1H), 7.17–7.26 (m, 5H), 7.13 (ddd, J 8.4, 7.2, 1.0, 1H), 6.99 (d, J 8.12, 2H), 6.67 (d, J 8.44, 2H), 4.28 (s, 2H), 2.57 (s, 3H). δC (75 MHz, CDCl3) 150.1, 149.2, 146.8, 145.3, 144.6, 143.4, 142.2, 141.2, 140.0, 138.8, 131.6, 128.6, 128.5, 126.0, 121.6, 120.9, 120.8, 119.5, 117.3, 116.0, 112.4, 105.6, 33.7, 20.6, 18.9. νmax (KBr)/cm−1 3396, 2839, 2543, 2215, 1645, 1595, 1510, 1480, 1467, 1422, 1370, 1307, 1283, 1265, 1240, 1165, 1080, 814, 757. HRMS (+ESI) m/z 403.1914, [M + H]+; calcd for [C27H22N4 + H] 403.1923.
2-Benzyl-1-((3-fluorophenyl)amino)-3-methylbenzo[4,5]imidazo[1,2-a]pyridine-4-carbonitrile (8j)
A mixture of compound 7a (100 mg, 0.30 mmol) and m-fluoroaniline (335.2 mg, 3.02 mmol) was heated to 120°C for 1 h. The crude material was purified by flash chromatography on silica gel using 2 : 3 ethyl acetate/hexane to afford compound 8j as a yellow solid (90.8 mg, 74 %). Mp 234–236°C. δH (300 MHz, acetone-d6) 8.57 (s, 1H), 8.04 (dt, J 8.5, 1.0, 1H), 7.82 (dt, J 8.2, 1.0, 1H), 7.46 (ddd, J 8.3, 7.1, 1.1, 1H), 7.19 (m, 7H), 6.69–6.55 (m, 3H), 4.30 (s, 2H), 2.57 (s, 3H). δC (75 MHz, acetone-d6) 166.5, 163.2, 151.4, 148.1, 146.6, 146.5, 145.9, 139.9, 139.6, 132.2, 132.1, 129.8, 129.4, 128.8, 127.1, 126.5, 122.2, 120.3, 120.1, 116.8, 116.0, 111.4, 111.3, 107.9, 107.6, 102.7, 102.3, 100.6, 55.0, 33.3, 19.8. νmax (KBr)/cm−1 3405, 3365, 2220, 1610, 1560, 1476, 1453, 1420, 1389, 1374, 1230, 1200, 1163, 1124, 1045, 845, 740. HRMS (+ESI) m/z 429.1486, [M + Na]+; calcd for [C26H19FN4 + Na] 429.1491.
2-Benzyl-1-((2-fluorophenyl)amino)-3-methylbenzo[4,5]imidazo[1,2-a]pyridine-4-carbonitrile (8k)
A mixture of compound 7a (100 mg, 0.30 mmol) and o-fluoroaniline (335.2 mg, 3.02 mmol) was heated to 120°C for 1 h. The crude material was purified by column chromatography on silica gel using 2 : 3 ethyl acetate/hexane to afford compound 8k as a yellow solid (80.8 mg, 65 %). Mp 226–228°C. δH (300 MHz, acetone-d6) 8.16 (br s, 1H), 7.97 (dt, J 8.4, 1.0, 1H), 7.84 (ddd, J 8.3, 1.2, 0.7, 1H), 7.48 (ddd, J 8.3, 7.2, 1.1, 1H), 7.31–7.11 (m, 7H), 6.92–6.78 (m, 2H), 6.58–6.45 (m, 1H), 4.30(s, 2H), 2.60 (s, 3H). δC (75 MHz, acetone-d6) 154.3, 151.4, 151.1, 148.1, 145.9, 139.9, 139.7, 132.4, 132.3, 130.0, 129.4, 128.9, 127.2, 126.6, 126.0, 125.9, 122.2, 122.0, 121.9, 120.8, 120.3, 116.8, 116.5, 116.5, 116.2, 116.0, 33.3, 19.9. νmax (KBr)/cm−1 3531, 3385, 2233, 1618, 1599, 1591, 1477, 1448, 1433, 1292, 1374, 1304, 1231, 764, 699. HRMS (+ESI) m/z 429.1486, [M + Na]+; calcd for [C26H19FN4 + Na] 429.1491.
2-Benzyl-3-methyl-1-((4-(trifluoromethyl)phenyl)amino)benzo[4,5]imidazo[1,2-a]pyridine-4-carbonitrile (8l)
A mixture of compound 7a (100 mg, 0.30 mmol) and p-(trifluoromethyl)aniline (0.49 g, 3.02 mmol) was heated to 120°C for 3 h. The crude material was purified by flash chromatography on silica gel using 2 : 3 ethyl acetate/hexane to afford compound 8l as a yellow solid (74.4 mg, 54 %). Mp 226–228°C. δH (300 MHz, acetone-d6) 8.77 (br s, 1H), 8.01 (ddd, J 8.5, 0.7, 1H), 7.85 (ddd, J 8.3, 1.2, 0.7, 1H), 7.55–7.42 (m, 3H), 7.29–7.11 (m, 6H), 6.96 (dt, J 8.2, 0.8, 2H), 4.29 (d, J 0.7, 2H), 2.59 (s, 3H). δC (75 MHz, acetone-d6) 151.4, 148.1, 148.0, 146.0, 139.6, 139.4, 129.8, 129.5, 128.9, 127.9, 127.9, 127.2, 126.6, 122.3, 122.1, 120.9, 120.4, 116.6, 115.9, 115.2, 101.1, 33.4, 19.8. νmax (KBr)/cm−1 3384, 3133, 3064, 3024, 2931, 2222, 1622, 1527, 1495, 1408, 1371, 1354, 1321, 1260, 1200, 1184, 1109, 1064, 970, 848, 757, 738. HRMS (+ESI) m/z 479.1465, [M + Na]+; calcd for [C27H19F3N4 + Na] 479.1460.
2-Benzyl-1-((3,5-dimethoxyphenyl)amino)-3-methylbenzo[4,5]imidazo[1,2-a]pyridine-4-carbonitrile (8m)
A mixture of compound 7a (100 mg, 0.30 mmol) and 3,5-dimethoxyaniline (51.7 mg, 3.02 mmol) was heated to 120°C for 3 h. The crude product was purified by flash chromatography on silica gel using 2 : 3 ethyl acetate/hexane to afford compound 8m as a yellow solid (85.3 mg, 63 %). Mp 244–246°C. δH (300 MHz, acetone-d6) 8.22 (br s, 1H), 8.10 (ddd, J 8.5, 1.1, 0.7, 1H), 7.83 (ddd, J 8.3, 1.2, 0.7, 1H), 7.48 (ddd, J 8.3, 7.2, 1.1, 1H), 7.30–7.14 (m, 6H), 6.03 (t, J 2.1, 1H), 5.96 (d, J 2.2, 2H), 4.29 (d, J 0.8, 2H), 3.63 (s, 6H), 2.57 (s, 3H). δC (75 MHz, acetone-d6) 163.2, 151.5, 148.1, 146.4, 145.9, 140.5, 139.9, 130.1, 129.4, 128.9, 127.2, 126.5, 122.1, 120.2, 120.1, 117.0, 116.1, 94.3, 93.3, 55.5, 33.4, 19.8. νmax (KBr)/cm−1 2898, 2603, 2220, 1605, 1545, 1524, 1493, 1446, 1423, 1354, 1305, 1207, 1158, 1051, 823, 740, 678. HRMS (+ESI) m/z 471.1791, [M + Na]+; calcd for [C28H24N4O2 + Na] 471.1797.
General Procedure A: Buchwald–Hartwig Amination
An oven-dried resealable Schlenk tube was evacuated and backfilled with argon. The Schlenk tube was charged with Pd2dba3 (1 mol-%) and XPhos (5 mol-%). If the aryl halide (1.0 mmol), amine (1.1–1.5 mmol), or base (1.5–4.0 mmol) were solids, they were also added at this time. The Schlenk tube was evacuated and backfilled with argon or nitrogen (this sequence was repeated three times) and then capped with a rubber septum. If the aryl halide, amine or base were liquids, they were added to the Schlenk tube at this time along with the solvent. The reaction mixture was allowed to stir at room temperature for 1 h and quenched with water and extracted with ethyl acetate. The organic layer was dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure. The crude material was purified by column chromatography on silica gel (eluting with ethyl acetate/hexane mixtures).
2-Benzyl-1-((3,5-bis(trifluoromethyl)phenyl)amino)-3-methylbenzo[4,5]imidazo[1,2-a]pyridine-4-carbonitrile (8n)
Following the general procedure A, compound 7a (100 mg, 0.30 mmol) was reacted with 3,5-bis(trifluoromethyl)aniline (103.8 mg, 0.45 mmol) in the presence of potassium tert-butoxide (107.7 mg, 0.96 mmol). The crude material was purified by column chromatography on silica gel using 2 : 3 ethyl acetate/hexane to afford compound 8n as a yellow solid (57.0 mg, 36 %). Mp 236–238°C. δH (600 MHz, acetone-d6) 8.04 (dt, J 8.5, 1.0, 1H), 7.86 (dt, J 8.2, 1.0, 1H), 7.48 (ddd, J 8.3, 7.2, 1.1, 1H), 7.42–7.34 (m, 3H), 7.22–7.15 (m, 5H), 7.15–7.09 (m, 1H), 4.31 (s, 2H), 2.62 (s, 3H). δC (150 MHz, acetone-d6) 151.36, 148.16, 146.86, 145.93, 139.56, 138.80, 133.42, 129.78, 129.39, 128.84, 127.15, 126.66, 125.17, 123.37, 122.44, 121.22, 120.39, 116.34, 115.95, 115.30, 113.73, 33.40, 19. νmax (KBr)/cm−1 3326, 3195, 3022, 2917, 2855, 2227, 1614, 1562, 1466, 1453, 1327, 774, 749. HRMS (+ESI) m/z 547.1337, [M + Na]+; calcd for [C28H18F6N4 + Na] 547.1333.
2-Benzyl-3-methyl-1-((2,4,6-trifluorophenyl)amino)benzo[4,5]imidazo[1,2-a]pyridine-4-carbonitrile (8o)
Following the general procedure A, compound 7a (100 mg, 0.30 mmol) was reacted with 2,4,6-trifluoroaniline (66.6 mg, 0.45 mmol) in the presence of potassium tert-butoxide (107.7 mg, 0.96 mmol). The crude material was purified by column chromatography on silica gel using 2 : 3 ethyl acetate/hexane to afford compound 8o as a yellow solid (45.4 mg, 34 %). Mp 230–232°C. δH (600 MHz, acetone-d6) 8.34 (d, J 8.4, 1H), 7.82 (dt, J 8.2, 1.0, 1H), 7.51 (ddd, J 8.3, 7.2, 1.1, 1H), 7.25 (ddd, J 8.4, 7.2, 1.2, 1H), 7.18–7.10 (m, 3H), 7.03–6.98 (m, 2H), 6.74 (t, J 8.9, 2H), 4.17 (s, 2H), 2.56 (s, 3H). δC (150 MHz, acetone-d6) 158.01, 156.41, 155.37, 153.81, 153.75, 153.71, 153.65, 150.75, 147.89, 139.21, 130.56, 129.20, 128.15, 126.95, 126.67, 122.05, 118.90, 116.98, 116.39, 101.32, 33.10, 19.53. νmax (KBr)/cm−1 3431, 3358, 2224, 1610, 1593, 1492, 1475, 1448, 1423, 1346, 1189, 892, 742, 689. HRMS (+ESI) m/z 465.1296, [M + Na]+; calcd for [C26H17F3N4 + Na] 465.1303.
2-Benzyl-1-((2-benzyl-1-chloro-4-cyanobenzo[4,5]imidazo[1,2-a]pyridin-3-yl)methyl)-3-methylbenzo[4,5]imidazo[1,2-a]pyridine-4-carbonitrile (13)
Following the general procedure, compound 7a (100 mg, 0.30 mmol) was reacted with 2,4,6-trifluoroaniline (66.6 mg, 0.45 mmol) in the presence of potassium tert-butoxide (107.7 mg, 0.96 mmol). The crude material was purified by column chromatography on silica gel using 2 : 3 ethyl acetate/hexane to afford compound 13 as a yellow solid (60.1 mg, 30 %). Mp 240–242°C. δH (400 MHz, CDCl3) 8.63 (dt, J 8.6, 0.9, 1H), 8.04 (dt, J 8.3, 1.0, 2H), 7.64 (ddd, J 8.3, 7.2, 1.1, 1H), 7.49 (ddd, J 8.6, 7.2, 1.2, 1H), 7.43 (ddd, J 8.2, 6.6, 1.5, 1H), 7.21–7.06 (m, 5H), 7.04–6.89 (m, 5H), 6.85–6.78 (m, 2H), 4.99–4.79 (m, 2H), 4.45 (d, J 16.9, 1H), 4.12–3.84 (m, 3H), 2.59 (s, 3H). δC (100 MHz, CDCl3) 148.28, 146.47, 146.16, 145.35, 144.83, 142.86, 137.67, 136.35, 135.02, 134.74, 130.35, 129.27, 129.22, 128.77, 127.76, 127.33, 127.28, 127.14, 126.80, 126.04, 123.36, 123.02, 121.99, 121.17, 120.99, 119.47, 115.88, 114.94, 113.23, 112.07, 102.83, 100.85, 35.22, 34.81, 34.01, 19.68. νmax (KBr)/cm−1 3444, 3308, 3177, 2217, 1647, 1644, 1615, 1590, 1502, 1455, 1400, 1325, 1270, 1178, 738, 707. HRMS (+ESI) m/z 627.2056 [M + H]+; calcd for [C40H27ClN6 + H] 627.2064.
General Procedure B: Suzuki Coupling Reaction
To a stirred solution of intermediate 7a (100 mg, 0.30 mmol) in dioxane(4 mL) in a re-sealable Schlenk tube, was added boronic acid (0.60 mmol) followed by Na2CO3 (95.8 mg, 0.90 mmol) dissolved in water (1 mL) at room temperature and the reaction mixture was purged with argon for 10 min. Pd(PPh3)4 (34.8 mg, 0.03 mmol) was then added and the reaction mixture purged with argon for the second time for 5 min. The reaction mixture was sealed and heated at 90°C for 16 h. The reaction mixture was cooled to room temperature, water added (20 mL), and the mixture extracted with EtOAc (2 × 25 mL). The organic layers were combined, washed with brine solution (15 mL), dried over anhydrous Na2SO4, and concentrated under reduced pressure. The residue obtained was purified by silica gel column chromatography using methanol/dichloromethane (0–10 %) to afford impure products that were recrystallised from EtOAc/acetonitrile to obtain pure compounds as yellow solids.
2-Benzyl-1-(4-methoxyphenyl)-3-methylbenzo[4,5]imidazo[1,2-a]pyridine-4-carbonitrile (14a)
Following the general procedure B, compound 7a (100 mg, 0.30 mmol) was reacted with 4-methoxyphenylboronic acid (91.6 mg, 0.60 mmol) in the presence of Na2CO3 (95.8 mg, 0.90 mmol) and Pd(PPh3)4 (34.8 mg, 0.03 mmol). The residue obtained was purified by silica gel column chromatography using methanol/dichloromethane (0–10 %) to afford impure product, which was then recrystallised from EtOAc/acetonitrile to afford pure compound 14a as a yellow solid (35.1 mg, 29 %). Mp 296–298°C. δH (400 MHz, CD2Cl2) 7.93–7.87 (m, 1H), 7.42 (ddd, J 8.3, 7.2, 1.1, 1H), 7.33–7.28 (m, 2H), 7.28–7.16 (m, 3H), 7.13–7.06 (m, 2H), 7.01–6.94 (m, 3H), 6.14–6.09 (m, 1H), 3.93 (s, 2H), 3.91 (s, 3H), 2.52 (s, 3H). δC (100 MHz, CD2Cl2) 161.7, 150.1, 147.1, 145.4, 143.7, 139.4, 130.5, 129.1, 127.9, 126.8, 126.1, 125.0, 121.6, 121.3, 120.1, 115.7, 115.6, 115.1, 101.5, 55.9, 34.8, 19.9. νmax (KBr)/cm−1 2929, 2221, 1594, 1490, 1442, 1292, 1243, 1028, 834, 735. HRMS (+ESI) m/z 404.1756 [M + H]+; calcd for [C27H21N3O + H] 404.1757.
2-Benzyl-3-methyl-1-(4-(methylamino)phenyl)benzo[4,5]imidazo[1,2-a]pyridine-4-carbonitrile (14b)
Following the general procedure B, compound 7a (100 mg, 0.30 mmol) was reacted with 4-aminophenylboronic acid pinacol ester (140.5 mg, 0.60 mmol) in the presence of Na2CO3 (95.8 mg, 0.90 mmol) and Pd(PPh3)4 (34.8 mg, 0.03 mmol). The residue obtained was purified by silica gel column chromatography using methanol/dichloromethane (0–10 %) to afford impure product, which was then recrystallised from EtOAc/acetonitrile to afford pure compound 14b as a yellow solid (45.2 mg, 37 %). Mp 269–271°C. δH (400 MHz, CD2Cl2) 7.89 (dt, J 8.2, 1.0, 1H), 7.42 (ddd, J 8.2, 7.1, 1.1, 1H), 7.30–7.22 (m, 2H), 7.22–7.11 (m, 3H), 7.03–6.95 (m, 3H), 6.78–6.73 (m, 2H), 6.31 (dt, J 8.5, 1.0, 1H), 4.23 (q, J 5.2, 1H), 3.98 (s, 2H), 2.91 (d, J 5.1, 3H), 2.50 (s, 3H). δC (100 MHz, CD2Cl2) 151.4, 150.2, 147.3, 145.4, 144.7, 139.7, 130.7, 130.0, 129.0, 128.0, 126.7, 125.9, 121.4, 121.3, 120.6, 120.0, 115.8, 115.5, 113.1, 101.1, 34.9, 30.6, 19.9. νmax (KBr)/cm−1 3435, 3026, 2922, 2222, 1611, 1491, 1441, 1396, 1262, 1180, 824, 739. HRMS (+ESI) m/z 403.1916 [M + H]+; calcd for [C27H22N4 + H] 403.1917.
2-Benzyl-3-methyl-1-phenylbenzo[4,5]imidazo[1,2-a]pyridine-4-carbonitrile (14c)
Following the general procedure B, compound 7a (100 mg, 0.30 mmol) was reacted with phenyl boronic acid (73.5 mg, 0.60 mmol) in the presence of Na2CO3 (95.8 mg, 0.90 mmol) and Pd(PPh3)4 (34.8 mg, 0.03 mmol). The residue obtained was purified by silica gel column chromatography using methanol/dichloromethane (0–10 %) to afford impure product, which was then recrystallised from EtOAc/acetonitrile to afford pure compound 14c as a yellow solid (85.1 mg, 76 %). Mp 263–265°C. δH (400 MHz, CD2Cl2) 7.91 (dt, J 8.3, 1.0, 1H), 7.71–7.64 (m, 1H), 7.61 (ddt, J 8.5, 6.7, 1.4, 2H), 7.42 (ddd, J 8.8, 7.6, 1.3, 3H), 7.29–7.15 (m, 3H), 7.02–6.96 (m, 2H), 6.94 (ddd, J 8.5, 7.1, 1.2, 1H), 5.94 (dt, J 8.6, 0.9, 1H), 3.92 (s, 2H), 2.53 (s, 3H). δC (100 MHz, CD2Cl2) 150.1, 147.1, 145.4, 143.6, 139.3, 133.0, 131.0, 130.4, 130.3, 129.2, 129.1, 127.9, 126.8, 126.1, 121.6, 120.9, 120.2, 115.6, 114.9, 101.7, 54.4, 54.1, 54.0, 53.8, 53.8, 53.6, 53.3, 34.8, 19.9. νmax (KBr)/cm−1 2917, 2225, 1742, 1628, 1595, 1483, 1446, 1306, 1206, 775, 735, 713. HRMS (+ESI) m/z 374.1649 [M + H]+; calcd for [C26H19N3 + H] 374.1651.
2-Benzyl-1-(4-fluorophenyl)-3-methylbenzo[4,5]imidazo[1,2-a]pyridine-4-carbonitrile (14d)
Following the general procedure B, compound 7a (100 mg, 0.30 mmol) was reacted with 4-fluorophenyl boronic acid (84.6 mg, 0.60 mmol) in the presence of Na2CO3 (95.8 mg, 0.90 mmol) and Pd(PPh3)4 (34.8 mg, 0.03 mmol). The residue obtained was purified by silica gel column chromatography using methanol/dichloromethane (0–10 %) to afford impure product, which was then recrystallised from EtOAc/acetonitrile to afford pure compound 14d as a yellow solid (78.0 mg, 67 %). Mp 276–278°C. δH (400 MHz, CD2Cl2) 7.92 (dt, J 8.2, 1.0, 1H), 7.44 (ddd, J 8.2, 7.1, 1.1, 1H), 7.42–7.36 (m, 2H), 7.34–7.16 (m, 5H), 7.02–6.92 (m, 3H), 6.04 (dt, J 8.6, 0.9, 1H), 3.91 (s, 2H), 2.54 (s, 3H). δC (100 MHz, CD2Cl2) 165.6, 163.1, 150.0, 147.0, 145.5, 142.5, 139.1, 131.5, 131.4, 130.3, 129.1, 129.0, 129.0, 127.9, 126.9, 126.2, 121.8, 121.3, 120.4, 117.7, 117.4, 115.5, 114.7, 102.0, 34.7, 19.9. νmax (KBr)/cm−1 3032, 2931, 2223, 1601, 1485, 1447, 1367, 1294, 1219, 1018, 836, 750, 695. HRMS (+ESI) m/z 392.1556 [M + H]+; calcd for [C26H18FN3 + H] 392.1557.
2-Benzyl-1-(4-cyanophenyl)-3-methylbenzo[4,5]imidazo[1,2-a]pyridine-4-carbonitrile (14e)
Following the general procedure B, compound 7a (100 mg, 0.30 mmol) was reacted with 4-cyanophenyl boronic acid (88.6 mg, 0.60 mmol) in the presence of Na2CO3 (95.8 mg, 0.90 mmol) and Pd(PPh3)4 (34.8 mg, 0.03 mmol). The residue obtained was purified by silica gel column chromatography using methanol/dichloromethane (0–10 %) to afford impure product, which was then recrystallised from EtOAc/acetonitrile to afford pure compound 14e as a yellow solid (30.6 mg, 25 %). Mp 289–292°C. δH (400 MHz, CD2Cl2) 7.96–7.86 (m, 3H), 7.62–7.52 (m, 2H), 7.44 (ddd, J 8.3, 7.2, 1.1, 1H), 7.32–7.18 (m, 3H), 7.00 (ddd, J 8.5, 7.2, 1.2, 1H), 6.96–6.87 (m, 2H), 5.96 (dt, J 8.6, 0.9, 1H), 3.86 (s, 2H), 2.53 (s, 3H). δC (100 MHz, CD2Cl2) 149.9, 146.7, 145.4, 141.2, 138.7, 137.1, 134.1, 130.4, 130.0, 129.2, 127.8, 127.1, 126.4, 122.1, 121.1, 120.5, 118.2, 115.3, 115.2, 114.3, 102.4, 34.7, 19.9. νmax (KBr)/cm−1 3031, 2224, 1592, 1488, 1447, 1300, 1266, 1182, 1022, 844, 756, 696. HRMS (+ESI) m/z 399.1603 [M + H]+; calcd for [C27H18N4 + H] 399.1604.
2-Benzyl-3-methyl-1-(pyridin-3-yl)benzo[4,5]imidazo[1,2-a]pyridine-4-carbonitrile (14f)
Following the general procedure B, compound 7a (100 mg, 0.30 mmol) was reacted with 3-pyridinyl boronic acid (74.1 mg, 0.60 mmol) in the presence of Na2CO3 (95.8 mg, 0.90 mmol) and Pd(PPh3)4 (34.8 mg, 0.03 mmol). The residue obtained was purified by silica gel column chromatography using methanol/dichloromethane (0–10 %) to afford impure product, which was then recrystallised from EtOAc/acetonitrile to afford pure compound 14f as a yellow solid (32.2 mg, 29 %). Mp 253–256°C. δH (400 MHz, CD2Cl2) 8.90 (dd, J 4.9, 1.7, 1H), 8.65 (dd, J 2.2, 0.9, 1H), 7.93 (dt, J 8.3, 1.0, 1H), 7.74 (ddd, J 7.8, 2.3, 1.7, 1H), 7.55 (ddd, J 7.9, 4.9, 0.9, 1H), 7.44 (ddd, J 8.2, 7.1, 1.1, 1H), 7.29–7.20 (m, 3H), 7.02–6.91 (m, 3H), 5.99 (dt, J 8.6, 0.9, 1H), 3.90 (s, 2H), 2.56 (s, 3H). δC (100 MHz, CD2Cl2) 152.2, 149.8, 149.7, 146.9, 145.5, 140.3, 138.8, 137.1, 130.3, 129.2, 129.2, 128.2, 127.9, 127.1, 126.3, 124.7, 122.0, 121.9, 120.6, 115.3, 114.4, 102.5, 34.8, 19.9. νmax (KBr)/cm−1 3024, 2915, 2222, 1590, 1507, 1443, 1299, 1183, 1021, 815, 733. HRMS (+ESI) m/z 375.1604 [M + H]+; calcd for [C25H18N4 + H] 375.1604.
2-Benzyl-3-methyl-1-(thiophen-3-yl)benzo[4,5]imidazo[1,2-a]pyridine-4-carbonitrile (14g)
Following the general procedure B, compound 7a (100 mg, 0.30 mmol) was reacted with 3-thienylboronic acid (77.2 mg, 0.60 mmol) in the presence of Na2CO3 (95.8 mg, 0.90 mmol) and Pd(PPh3)4 (34.8 mg, 0.03 mmol). The residue obtained was purified by silica gel column chromatography using methanol/dichloromethane (0–10 %) to afford impure product, which was then recrystallised from EtOAc/acetonitrile to afford pure compound 14g as a yellow solid (39.3 mg, 34 %). Mp 268–270°C. δH (400 MHz, CD2Cl2) 7.91 (dt, J 8.3, 1.0, 1H), 7.67 (dd, J 5.0, 2.9, 1H), 7.52–7.40 (m, 2H), 7.29–7.20 (m, 3H), 7.14 (dd, J 5.0, 1.3, 1H), 7.10–6.90 (m, 3H), 6.11 (dt, J 8.5, 1.0, 1H), 4.00–3.87 (m, 2H), 2.52 (s, 3H). δC (100 MHz, CD2Cl2) 149.7, 147.0, 145.4, 139.4, 139.1, 132.7, 130.3, 129.1, 128.9, 128.1, 127.9, 127.2, 126.9, 126.2, 122.1, 121.8, 120.2, 115.5, 114.6, 101.9, 34.9, 19.8. νmax (KBr)/cm−1 2221, 1589, 1542, 1489, 1442, 1347, 1303, 1078, 830, 741, 692. HRMS (+ESI) m/z 380.1215 [M + H]+; calcd for [C24H17N3S + H] 380.1215.
2-Benzyl-1-(furan-3-yl)-3-methylbenzo[4,5]imidazo[1,2-a]pyridine-4-carbonitrile (14h)
Following the general procedure B, compound 7a (100 mg, 0.30 mmol) was reacted with 3-furanylboronic acid (67.5 mg, 0.60 mmol) in the presence of Na2CO3 (95.8 mg, 0.90 mmol) and Pd(PPh3)4 (34.8 mg, 0.03 mmol). The residue obtained was purified by silica gel column chromatography using methanol/dichloromethane (0–10 %) to afford impure product, which was then recrystallised from EtOAc/acetonitrile to afford pure compound 14h as a yellow solid (36.1 mg, 33 %). Mp 252–254°C. δH (400 MHz, CD2Cl2) 7.92 (dt, J 8.2, 1.0, 1H), 7.78 (t, J 1.7, 1H), 7.61 (dd, J 1.6, 0.9, 1H), 7.48 (ddd, J 8.2, 7.1, 1.1, 1H), 7.32–7.18 (m, 3H), 7.13 (ddd, J 8.5, 7.1, 1.2, 1H), 7.03–6.95 (m, 2H), 6.81 (dt, J 8.6, 0.9, 1H), 6.57 (dd, J 1.9, 0.9, 1H), 4.11–3.90 (m, 2H), 2.52 (s, 3H). δC (100 MHz, CD2Cl2) 149.5, 147.0, 145.4, 142.4, 139.4, 135.9, 130.4, 129.2, 127.9, 126.9, 126.2, 122.9, 121.8, 120.3, 117.6, 115.5, 115.1, 111.7, 102.1, 35.0, 19.9. νmax (KBr)/cm−1 3108, 2222, 1588, 1489, 1447, 1364, 1302, 1189, 1157, 1084, 939, 873, 739. HRMS (+ESI) m/z 364.1443 [M + H]+; calcd for [C24H17N3O + H] 364.1444.
Supplementary Material
1H and 13C NMR spectra of all synthesised compounds are available on the Journal’s website.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgements
The authors thank the University of New South Wales and the Kids Cancer Alliance for financial support. The authors thank the Mark Wainwright Analytical centre for the analytical support.
References
[1] E. S. A. M. Badawey, T. Kappe, Eur. J. Med. Chem. 1999, 34, 663.[2] J. S. Bae, D. W. Lee, D. H. Lee, D. S. Jeong, WO2007011163A1 2007.
[3] H. L. Koo, H. L. Dupont, Curr. Opin. Gastroenterol. 2010, 26, 17.
| Crossref | GoogleScholarGoogle Scholar | 19881343PubMed |
[4] S. K. Kotovskaya, Z. M. Baskakova, V. N. Charushin, O. N. Chupakhin, E. F. Belanov, N. I. Bormotov, S. M. Balakhnin, O. A. Serova, JP 659160 1962.
[5] M. Hammad, A. Mequid, M. El Ananni, N. Shafik, Egypt. J. Chem. 1987, 29, 5401.
[6] M.Takemura, H. Takashi, K. Kawakami, H. Takeshita, Y. Kimura, J. Watanabe, Y. Sugimoto, A. Kitamura, R. Nakajima, K. Kanai, T. Fujisawa, EP 1479681 2004.
[7] A. J. Ndakala, R. K. Gessner, P. W. Gitari, N. October, K. L. White, A. Hudson, F. Fakorede, D. M. Shackleford, M. Kaiser, C. Yeates, S. A. Charman, K. Chibale, J. Med. Chem. 2011, 54, 4581.
| Crossref | GoogleScholarGoogle Scholar | 21644541PubMed |
[8] S. M. Rida, F. S. G. Soliman, E. S. A. M. Badawey, T. Kappe, J. Heterocycl. Chem. 1988, 25, 1725.
| Crossref | GoogleScholarGoogle Scholar |
[9] E. Badawey, T. Kappe, Eur. J. Med. Chem. 1995, 30, 327.
| Crossref | GoogleScholarGoogle Scholar |
[10] S. A. M. El-Hawash, E. A. M. Badawey, T. Kappe, Pharmazie 1999, 54, 341.
[11] S. V. Ryabukhin, A. S. Plaskon, D. M. Volochnyuk, A. A. Tolmachev, Synthesis 2007, 3155.
[12] (a) H. Wang, Y. Wang, C. Peng, J. Zhang, Q. Zhu, J. Am. Chem. Soc. 2010, 132, 13217.
| Crossref | GoogleScholarGoogle Scholar | 20822153PubMed |
(b) K. S. Masters, T. R. M. Rauws, A. K. Yadav, W. A. Herrebout, B. Van der Veken, B. U. W. Maes, Chem.– Eur. J. 2011, 17, 6315.
| Crossref | GoogleScholarGoogle Scholar |
[13] M. Alajarin, A. Vidal, F. Tovar, Tetrahedron Lett. 2000, 41, 7029.
| Crossref | GoogleScholarGoogle Scholar |
[14] H. M. Refaat, Med. Chem. Res. 2012, 21, 1253.
| Crossref | GoogleScholarGoogle Scholar |
[15] S. Caddick, Tetrahedron 1995, 51, 10403.
| Crossref | GoogleScholarGoogle Scholar |
[16] P. Lidstrom, J. Tierney, B. Wathey, J. Westman, Tetrahedron 2001, 57, 10229.
| Crossref | GoogleScholarGoogle Scholar |
[17] K. Tanaka, F. Toda, Chem. Rev. 2000, 100, 1025.
| Crossref | GoogleScholarGoogle Scholar | 11749257PubMed |
[18] M. A. P. Martins, C. P. Frizzo, D. N. Moreira, L. Buriol, P. Machado, Chem. Rev. 2009, 109, 4140.
| Crossref | GoogleScholarGoogle Scholar |
[19] B. Schlummer, U. Scholz, Adv. Synth. Catal. 2004, 346, 1599.
| Crossref | GoogleScholarGoogle Scholar |
[20] D. S. Surry, S. L. Buchwald, Chem. Sci. 2010, 1, 13.
| Crossref | GoogleScholarGoogle Scholar | 22384310PubMed |
[21] K. W. Anderson, R. E. Tundel, T. Ikawa, R. A. Altman, S. L. Buchwald, Angew. Chem. Int. Ed. 2006, 45, 6523.
| Crossref | GoogleScholarGoogle Scholar |
[22] N. Marion, E. C. Ecarnot, O. Navarro, D. Amoroso, A. Bell, S. P. Nolan, J. Org. Chem. 2006, 71, 3816.
| Crossref | GoogleScholarGoogle Scholar | 16674054PubMed |
[23] A. Shafir, S. L. Buchwald, J. Am. Chem. Soc. 2006, 128, 8742.
| Crossref | GoogleScholarGoogle Scholar | 16819863PubMed |
[24] R. A. Altman, B. P. Fors, S. L. Buchwald, Nat. Protoc. 2007, 2, 2881.
| Crossref | GoogleScholarGoogle Scholar | 18007623PubMed |
[25] X. H. Huang, S. L. Buchwald, Org. Lett. 2001, 3, 3417.
| Crossref | GoogleScholarGoogle Scholar |
[26] K. W. Anderson, R. E. Tundel, T. Ikawa, R. A. Altman, S. L. Buchwald, Angew. Chem. Int. Ed. 2006, 45, 6523.
| Crossref | GoogleScholarGoogle Scholar |
[27] J. P. Wolfe, S. L. Buchwald, Angew. Chem. Int. Ed. 1999, 38, 2413.
| Crossref | GoogleScholarGoogle Scholar |
[28] C. W. Cheung, S. L. Buchwald, J. Org. Chem. 2012, 77, 7526.
| Crossref | GoogleScholarGoogle Scholar | 22838632PubMed |
[29] Crystallographic data for the structures in this paper have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication no. CCDC 2006266 (14a), CCDC 2006267 (14d) and CCDC 2017491 (13). X-Ray crystal structures were obtained by Donald Craig, Crystallography Laboratory, UNSW Analytical Centre, Sydney, Australia.