Supramolecular Polymers
Tom F.A. de Greef A and E. W. Meijer A BA Institute for Complex Molecular Systems and Laboratory of Macromolecular and Organic Chemistry, Eindhoven University of Technology, P.O. Box 513, 5600 MB Eindhoven, the Netherlands.
B Corresponding author. Email: E.W.Meijer@tue.nl
Australian Journal of Chemistry 63(4) 596-598 https://doi.org/10.1071/CH10097
Published: 8 April 2010
Chemistry has witnessed a remarkable transition in scales since the original discovery of the synthesis of urea in 1828 by Wöhler and that of Bakelite – a poly(phenol-formaldehyde) – in 1907 by Baekeland. Synthesis in the 19th and 20th century has mainly been focussed on the manipulation of individual chemical bonds using functional group transformations and a large variety of synthetic methods to prepare an almost unlimited number of complex (macro)molecules have been disclosed. At the end of the 20th century, chemistry witnessed the rise of supramolecular chemistry due to the seminal work on molecular recognition by Pedersen, Cram, and Lehn. Chemists learned to manipulate ensembles of molecules by the use of strong and directional non-covalent interactions, ultimately also leading to supramolecular polymers. These dynamic and living polymers represent a topic of increasing interest due to their enormous potential in a variety of applications.
Therefore, this special issue of the Australian Journal of Chemistry is focussed on several aspects of supramolecular polymers. However, it escaped many scientists their attention that molecular polymerization via associative interactions between molecules was already proposed as an idea by Louis Henry in 1878.[1,2] It was at the same time that van der Waals proposed his famous equation of state that took intermolecular interactions in liquids into account and only 50 years after Berzelius coined the term polymers.[3,4] In the decades that followed the proposal of Henry’s idea, many chemists struggled to measure degrees of polymerization of associative molecules in liquids.[2,5] A beautiful book written by Turner[5] illustrates the ingenuity of chemists in the early 1900s in their efforts to probe molecular association in solution by concentration dependent viscosity measurements. With the advent of infrared spectroscopy, Buswell, Rodebush, and Roy reported evidence for the hydrogen-bonded oligomerization of amides in the mid-1930s.[6] Somewhat later, Kempter, Mecke, and Kreuzer measured the hydrogen-bonded oligomerization of phenol in benzene using infrared spectroscopy and analyzed their results using a simple association model.[7,8] Although the molecular association between these monomers cannot be regarded as a true polymerization process due to the low association constant between the monomers and the short lifetime of the non-covalent bonds, these studies reflect the first investigations on ‘polymerization by molecular association’, what is now called ‘supramolecular polymerization’.
During the subsequent decades and certainly after the revolutionary proposal by Staudinger that polymers are high molecular weight macromolecules, polymer chemistry focussed on the synthesis of these macromolecules using different mechanisms; step-, chain-, and ring-opening polymerization. The success of this field is extraordinary and today we cannot imagine a world without polymers, either for their use as mechanical strong materials or for their properties in electronics and medicine. It was the late Reimund Stadler and his group, who introduced the concept to make materials out of low molecular weight structures, which were brought together by multiple hydrogen bonds.[9] In 1990, this was followed by Lehn, who reported the first main-chain supramolecular polymer based on the self-assembly of ditopic monomers in which complementary three-fold hydrogen-bonding groups were separated via a flexible tether.[10] Due to the low association constant between these monomers, a high degree of polymerization was only obtained in the liquid crystalline state. For our group, the crucial step in the development of supramolecular polymers was the introduction of a strong, self-complementary, quadruple hydrogen bonding unit, the 2-ureido-4-pyrimidinone or UPy-unit in 1997 (Fig. 1).[11] A monomer with two of these self-complementary UPy-units connected via a flexible tether, self-assembles into a one-dimensional supramolecular polymer with high degrees of polymerization. The relatively long lifetime of the UPy-UPy bond (0.1–1 s) was essential for making polymers that displayed excellent material properties. Since then the field of supramolecular polymerization has exploded. Today UPy-based polymers are marketed by e.g. SupraPolix and others, and they are produced at a 10 ton scale. Recently, also the self-healing properties of supramolecular polymers based on hydrogen bonding are receiving increasing attention.[12] Besides hydrogen-bonding, chemists have used other non-covalent interactions such ionic and dipole-dipole interactions in creating one-dimensional supramolecular polymers from ditopic monomers.[13] Furthermore, supramolecular polymers based on shape-persistent monomers that self-assemble into ordered nanostructures and filaments are attracting considerable interest. The formation of these ordered structures, however, often occurs via a different mechanism than the formation of supramolecular polymers from ditopic substituted monomers (vide infra).
|
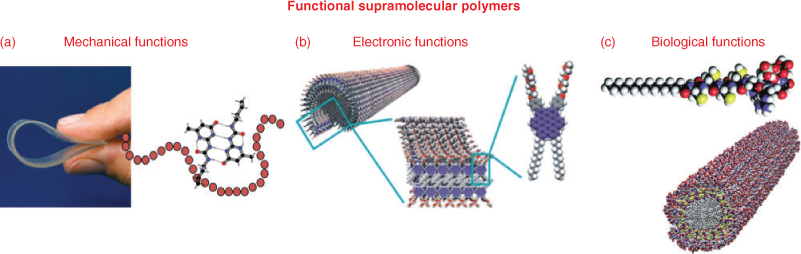
The choice in mechanism of formation, the huge variety of self-assembling units and the stabilities of the structures formed, offer an enormous range of frequencies in the dynamic properties of these supramolecular polymers. This control yields unique processing capabilities of responsive materials, but also creates a modular approach to construct functional materials. Three major classes of functional supramolecular polymers can be discriminated (Fig. 1), where in recent years progress has been enormous: (1) functions based on excellent and unique mechanical properties with ease in processing due to the dynamic character of the bonding,[11,12,14] (2) electronic functions based on π-conjugated repeating units leading to supramolecular electronics,[15] and (3) biomedical functions in regenerative medicine of biologically active supramolecular polymers.[16]
In spite of this success, in many cases the preparation of one-dimensional supramolecular polymers and nanostructures from their respective monomers is guided by serendipity as a complete understanding of the physical mechanisms of the self-assembly process is still lacking. In recent work, we have elaborated on the various growth mechanisms by which one-dimensional supramolecular polymers can grow.[17] For supramolecular polymers based on ditopic monomers and having intriguing mechanical properties, growth of the polymers occurs via an isodesmic mechanism and thermodynamically stable products are easily obtained. In contrast, the growth of shape-persistent monomers into ordered supramolecular polymers occurs in most cases via a nucleated or cooperative growth mechanism.[17c] Hence, kinetic factors and meta-stable traps can easily yield thermodynamically unstable materials. Although temperature dependent optical measurements are useful as a first diagnostic to establish whether a supramolecular polymerization occurs via a cooperative or nucleated or isodesmic growth mechanism, such equilibrium measurements fail to give any information on the nucleus size, or whether nucleation occurs via a homogeneous or heterogeneous pathway.[17d]
Based on the arguments above, an increasing number of kinetic studies on cooperative supramolecular polymerizations will be conducted in the years to come. Much can be learned from the field of protein assembly, notably the cooperative aggregation of shape-persistent amyloidal proteins, were kinetic studies[18] have yielded a remarkable mechanistic insight into the self-assembly mechanism. It is now generally accepted that in many amyloidal aggregations, besides homogeneous nucleation,[18a,b] secondary nucleation either by fragmentation[18c,e,f] of filaments or surface-assisted[18c,d] nucleation plays a dominant role in the self-assembly process. Given the importance of secondary nucleation in the crystallization of small molecules[19a] and polymers,[19b] understanding which chemical features in a monomer lead to secondary nucleation in the self-assembly pathway from monomer to supramolecular polymer will be a huge step in understanding the relation between structure and function. Furthermore, as secondary nucleation has autocatalytic features; it can play an important role in the design of self-organizing supramolecular polymers (vide infra).
In many areas of science, but supramolecular chemistry in particular, nature serves both as an inspiration and a challenge. It is tempting to compare one-dimensional supramolecular polymers with their natural counterparts: the cytoskeletal polymers such as actin and tubulin. However, in contrast to supramolecular polymers which are formed by self-assembly, the formation of cytoskeletal polymers occurs by self-organization via reaction-diffusion processes. As a result cytoskeletal structures display unique properties such as for example spatio-temporal synchronization between growing and shrinking individual cytoskeletal polymers. The design and characterization of artificial self-organizing supramolecular polymers will be a significant achievement in the years to come. In order to design these self-organizing supramolecular polymers, three goals will have to be met: (1) engineering of dissipative aspects to supramolecular polymerizations for example using light or chemical energy, (2) engineering of positive (autocatalytic) and negative (physico)chemical feedback cycles in the chemical self-assembly, and (3) understanding diffusion-driven instabilities in non-covalent systems. Although this seems a daunting task, a recent example in which inorganic self-organizing reactions are performed in a micro-heterogeneous medium has shown that even relatively simple chemistry can yield dynamic behaviour resembling that displayed by multicellular organisms.[20]
Although with some delay, the recognition of non-covalent forces as a tool to direct the ordering and function of molecules has now been widely acknowledged and respected. Currently supramolecular polymers are explored at the interface between chemistry, biology, physics, medicine, soft-condensed matter, and nanoscience. This multi-disciplinary approach will result in a better understanding of these systems through both experimental and theoretical studies, and undoubtedly will result in more innovative materials and new applications sought. During these advances, supramolecular materials will find their way into everyday life to enable technologies not previously possible.
[1]
L. Henry,
Annales de la Société scientifique de Bruxelles 1878, 3, 267.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
| Crossref | GoogleScholarGoogle Scholar |
CAS |
|
CAS |
|
CAS |
| Crossref | GoogleScholarGoogle Scholar |
CAS |
| Crossref | GoogleScholarGoogle Scholar |
CAS |
| Crossref | GoogleScholarGoogle Scholar |
CAS |
| Crossref | GoogleScholarGoogle Scholar |
CAS |
| Crossref | GoogleScholarGoogle Scholar |
CAS |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
CAS |
| Crossref | GoogleScholarGoogle Scholar |
CAS |
| Crossref | GoogleScholarGoogle Scholar |
CAS |
| Crossref | GoogleScholarGoogle Scholar |
CAS |
| Crossref | GoogleScholarGoogle Scholar |
CAS |
| Crossref | GoogleScholarGoogle Scholar |
CAS |
| Crossref | GoogleScholarGoogle Scholar |
CAS |
| Crossref | GoogleScholarGoogle Scholar |
CAS |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
CAS |



