Last Updated: 22 Aug 2024
Cornforth Review Series
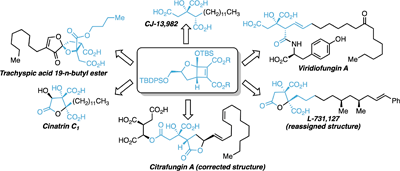
This review outlines the synthesis of alkyl citrate natural products using cyclobutene diester precursors. This highly stereoselective approach gives the citrate core with the correct oxidation state and allows for the synthesis of a large selection of these interesting natural products. Furthermore, stepwise oxidation provides access to the higher oxidised alkyl citrates from a common intermediate. (Image credit: Nikolai Rossouw.)
CH20371The Future of Retrosynthesis and Synthetic Planning: Algorithmic, Humanistic or the Interplay?

Will the computer ever compete with human retrosynthetic design and the art of organic synthesis?

Polarity inversion through 1,4-addition of N-heterocyclic carbenes (NHCs) to conjugate acceptors was first reported in 2006 and has subsequently evolved as an active subfield of NHC-organocatalysis. In this review, this emerging area of NHC-organocatalysis is discussed with comprehensive coverage.
CH14568Synthetic Amino Acids for Applications in Peptide Ligation–Desulfurization Chemistry

Native chemical ligation followed by desulfurization is a powerful strategy for the chemical synthesis of protein targets. This review focusses on the current synthetic approaches to access amino acid building blocks bearing suitably positioned β-, γ- or δ-thiol ligation auxiliaries that have greatly expanded the scope of the ligation–desulfurization manifold.