Structural Systematics of Lanthanide(iii) Picrate Solvates: Molecular, Mononuclear Ln(pic)3(trimethylphosphate)3 Arrays
Eric J. Chan A , Jack M. Harrowfield A B D , Brian W. Skelton A , Alexandre N. Sobolev A and Allan H. White A CA School of Molecular Sciences, M310, University of Western Australia, 35 Stirling Hwy, Perth, WA 6009, Australia.
B Current address: Institut de Science et d’Ingénierie Supramoléculaires, Université de Strasbourg, 8, allée Gaspard Monge, Strasbourg, 67083, France.
C Deceased.
D Corresponding author. Email: harrowfield@unistra.fr
Australian Journal of Chemistry 73(6) 462-467 https://doi.org/10.1071/CH19176
Submitted: 18 April 2019 Accepted: 6 June 2019 Published: 3 July 2019
Abstract
Adducts of the form Ln(pic)3(tmp)3 (Ln = lanthanide(iii); pic = picrate = 2,4,6-trinitrophenoxide; tmp = trimethylphosphate, (MeO)3PO) have been prepared for extremal Ln = La, Lu and some intermediate members, also Y, and characterised by single crystal X-ray structure determinations as unsolvated, mononuclear, molecular species. The lanthanide atom has nine-coordinate, tri-capped trigonal prismatic stereochemistry in all cases, the picrate components behaving as O,O′-bidentate ligands chelating through the phenoxy- and an adjacent O-nitro oxygen atom, thus: [Ln(tmp-O)3(pic-O,O′)3]. Two isomeric forms are found, one mer in which the three unidentate tmp-O ligands coordinate in cis-sites spanning the upper and lower triangles and a capping site of the coordination sphere, and fac, in which all three unidentate ligands occupy the mutually cis-sites of one triangular face. The mer isomer has been described as an isomorphous series in a monoclinic P21/c, Z 4, form, for Ln = La, Ce, Pr, Nd, Sm, Gd, Lu, and Y, presumptively accessible for the full gamut of Ln. The fac-isomer also crystallises in a monoclinic P21/c form, Z 8, two independent molecules of similar stereochemistry here comprising the asymmetric unit and described for Ln = Eu, Lu(isomorphous); it has also been described in a triclinic P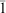 , Z 2 form for Ln = La.
, Z 2 form for Ln = La.
References
[1] Z. Asfari, E. J. Chan, J. M. Harrowfield, B. W. Skelton, A. N. Sobolev, P. Thuéry, A. H. White, Aust. J. Chem. 2019, in press.[2] V. Gutmann, Coordination Chemistry in Non-Aqueous Solvents 1968 (Springer: Vienna).
[3] Y. Marcus, Ion Solvation 1985 (Wiley: Chichester).
[4] W. W. Schulz, L. L. Burger, J. D. Navratil, Science and Technology of Tributylphosphate, Vol. III 1990 (CRC Press: Boca-Raton, FL).
[5] National Research Council (USA), Nuclear Wastes: Technologies for Separation and Transmutation 1996 (National Academy Press: Washington, D.C.).
[6] W. W. Butcher, F. H. Westheimer, J. Am. Chem. Soc. 1955, 77, 2420.
| Crossref | GoogleScholarGoogle Scholar |
[7] A. M. Sargeson, P. Hendry, Prog. Inorg. Chem. 1990, 38, 201.
[8] (a) J. Sumaoka, Y. Yamamoto, Y. Kitamura, M. Komiyama, Curr. Org. Chem. 2007, 11, 463.
| Crossref | GoogleScholarGoogle Scholar |
(b) M. Komiyama, Met. Ions Biol. Syst. 2003, 40, 463.
(c) T. Takarada, M. Yashiro, M. Komiyama, Chem. – Eur. J. 2000, 6, 3906.
| Crossref | GoogleScholarGoogle Scholar |
[9] V. Dokukin, S. K. Silvermann, Chem. Sci. 2012, 3, 1707.
| Crossref | GoogleScholarGoogle Scholar | 23243490PubMed |
[10] L. Daumann, Angew. Chem. Int. Ed. 2019, in press.
| Crossref | GoogleScholarGoogle Scholar |
[11] (a) V. G. Lebedev, K. K. Palkina, S. I. Maksimova, E. N. Lebedeva, O. V. Galaktionova, Zh. Neorg. Khim. 1982, 27, 2980.
(b) C. Huang, T. Yi, Y. Lu, G. Xu, H. Ling, G. Ma, Chin. Chem. Lett. 1992, 3, 947.
[12] E. Kuhlmann, S. Himmler, H. Giebelhaus, P. Wasserschied, Green Chem. 2007, 9, 233.
| Crossref | GoogleScholarGoogle Scholar |
[13] E. J. Chan, manuscript in preparation.
[14] J. M. Harrowfield, in Metal Ions in Biological Systems (Eds A. Sigel, H. Sigel) 2003, Vol. 40, Ch. 4, pp. 105–159 (Marcel Dekker: New York, NY).
[15] C. Den Auwer, C. Lecouteux, M. C. Charbonnel, C. Madic, R. Guillaumont, Polyhedron 1997, 16, 2233.and references therein.
| Crossref | GoogleScholarGoogle Scholar |
[16] J. M. Harrowfield, M. Mocerino, B. J. Peachey, B. W. Skelton, A. H. White, J. Chem. Soc., Dalton Trans. 1996, 1687.
| Crossref | GoogleScholarGoogle Scholar |
[17] (a) J. M. Harrowfield, W. Lu, B. W. Skelton, A. H. White, Aust. J. Chem. 1994, 47, 321.
| Crossref | GoogleScholarGoogle Scholar |
(b) J. M. Harrowfield, W. Lu, B. W. Skelton, A. H. White, Aust. J. Chem. 1994, 47, 339.
| Crossref | GoogleScholarGoogle Scholar |
(c) J. M. Harrowfield, W. Lu, B. W. Skelton, A. H. White, Aust. J. Chem. 1994, 47, 349.
| Crossref | GoogleScholarGoogle Scholar |
(d) J. M. Harrowfield, B. W. Skelton, A. H. White, Aust. J. Chem. 1994, 47, 359.
| Crossref | GoogleScholarGoogle Scholar |
[18] G. M. Sheldrick, Acta Crystallogr. Sect. C 2015, 71, 3.
| Crossref | GoogleScholarGoogle Scholar |
[19] C. F. Mackenzie, P. R. Spackman, D. Jayatilaka, M. A. Spackman, IUCrJ 2017, 4, 575.
| Crossref | GoogleScholarGoogle Scholar | 28932404PubMed |
[20] S. K. Wolff, D. J. Grimwood, J. J. McKinnon, M. J. Turner, D. Jayatilaka, M. A. Spackman, CrystalExplorer 2012 (University of Western Australia: Perth).
[21] H. Lumpe, A. Pol, H. J. M. Op den Camp, L. J. Daumann, Dalton Trans. 2018, 47, 10463.
| Crossref | GoogleScholarGoogle Scholar | 30020281PubMed |


