An icosanuclear silver(I) cluster supported by bis(thiosemicarbazonato) ligands†
Brett M. Paterson A , Jonathan M. White
A , Jonathan M. White  A * and Paul S. Donnelly
A * and Paul S. Donnelly  A *
A *
A School of Chemistry and Bio21 Molecular Science Biotechnology Institute, University of Melbourne, Melbourne, 3010, Vic., Australia.
Handling Editor: George Koutsantonis
Australian Journal of Chemistry 75(9) 631-635 https://doi.org/10.1071/CH21335
Submitted: 15 December 2021 Accepted: 17 January 2022 Published: 10 March 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
The synthesis and structural characterisation of an icosanuclear silver(I) cluster complex is reported here. The complex includes twenty silver(I) ions supported by eighteen bis(thiosemicarbazonato) ligands. The cluster of silver(I) ions involves several close Ag⋯Ag contacts suggesting some degree of argentophilic interactions and the bis(thiosemicarbazonato) ligands are present in three different conformations highlighting the ability of thiosemicarbazone ligands to coordinate to metal ions in different modes.
Keywords: argentophillic interactions, cluster of 20 silver ions, cluster, silver, sulfur ligands, thiosemicarbazone.
Introduction
Thiosemicarbazones and bis(thiosemicarbazones) are versatile ligands containing both nitrogen and sulfur donor atoms that can coordinate to metal ions as either anionic or charge neutral mono- or multidentate ligands.[1] As part of an exploratory study on the synthesis of zinc(II) complexes with glyoxal-bis(4-phenyl-3-thiosemicarbazone) (H2gtsp, Fig. 1) we isolated a trinuclear [3 × Zn(II) + 3 × ligand] prism-like complex, and recrystallisation of this complex in the presence of either CO2, CS2 or CH3CN led to the formation of tetranuclear [4 × Zn(II) + 4 × ligand] coordination nanotubes.[2] In a continuation of this work, we have investigated the coordination chemistry of H2gtsp with silver(I).
|
The closed d10 valence shell of silver(I) tolerates a variety of different coordination geometries. Simple silver(I) complexes can be either 2-coordinate and approximately linear or 3- or 4-coordinate,[3] but the use of chelating ligands often leads to polymeric species or multinuclear clusters.[1b] The resonance delocalisation within thiosemicarbazones contributes to them acting as ‘masked thiolate’ donors that are less reducing than conventional thiols. This masked thiolate nature contributes to thiosemicarbazones forming a vast array of coordination complexes with thiophilic silver(I). The first structural characterisation by X-ray crystallography of a silver(I) salicylaldehyde thiosemicarbazone complex of silver(I) was reported in 2004 and revealed a hexameric complex with the Ag6S6N6 core consisting of two six-membered Ag3S3 rings in a chair configuration, stacked one above the other and linked by bridging ligands.[4] Each silver(I) cation is 3-coordinate, coordinated to two thiolate sulfur atoms and one hydrazinic nitrogen atom, while each sulfur bridges two silver(I) cations. Since this initial report, a range of different silver clusters[1b,5] and helicates[5b,6] supported by thiosemicarbazones have been reported as well as some discrete complexes featuring stabilising phosphine[5a,7] or 1,10-phenanthroline co-ligands.[8]
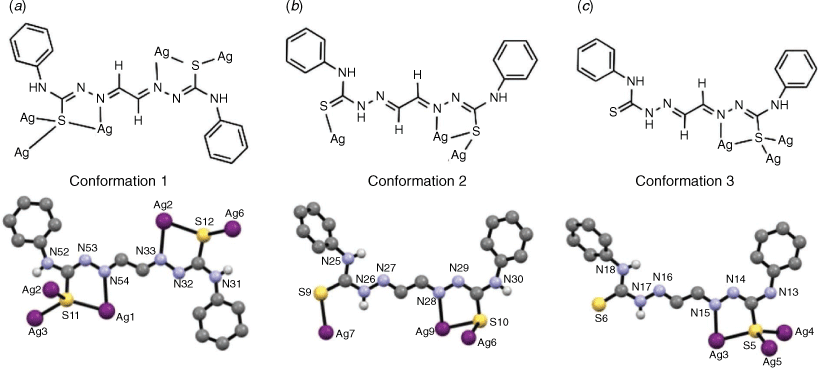
There are relatively few reports of silver(I) complexes with bis(thiosemicarbazone) ligands derived from 1,2-diones, such as H2gtsp (Fig. 1), although glyoxal-bis(thiosemicarbazone) (H2gts, Fig. 1) has previously been investigated as a spectrophotometric reagent with silver(I), but the product was not structurally characterised.[9] Reaction of pyridyl-bis(3-hexamethyleneiminyl thiosemicarbazone) (H2Plhexim, Fig. 1) with various silver(I) salts and different metal-ligand ratios allowed isolation of mono-, di- and tetranuclear complexes.[10] In this work, we report the synthesis and structural characterisation of a complex comprising 20 silver(I) ions supported by 18 bis(thiosemicarbazone) ligands.
Results and discussion
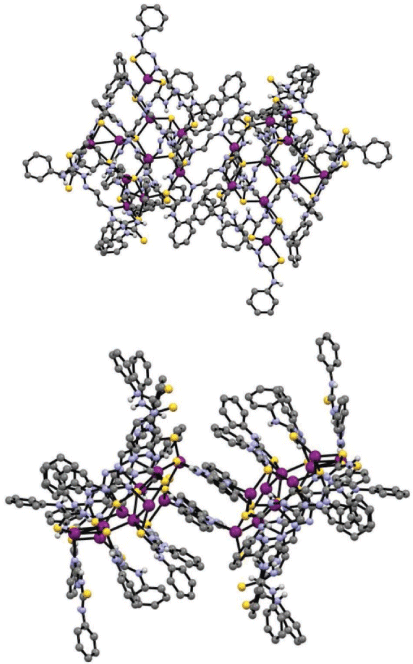
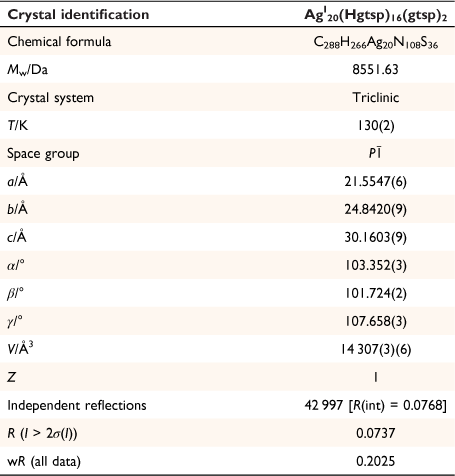
Addition of one equivalent of silver tetrafluoroborate (AgBF4) to a solution of H2gtsp in deoxygenated DMF in the presence of triethylamine resulted in the formation of a bright yellow/orange solution. Diffusion of diethyl ether to this mixture allowed isolation of yellow/orange crystals suitable for analysis by X-ray crystallography. The crystals were light stable but rapidly lost crystallinity when removed from the solvent, presumably due to desolvation. Plunging the crystals directly into Parobar low temperature oil followed by flash-cooling to 130 K allowed for suitable data for the crystals to be obtained. The X-ray analysis revealed the complex to be AgI20(Hgtsp)16(gtsp)2 (x.DMF, y.diethylether) (Fig. 2, Table 1). The large volume of solvent accessible voids (7310.1 Å3) that account for just over 50% of the total volume of AgI20(Hgtsp)16(gtsp)2·(x.DMF, y.diethylether), consisted of 2058 electrons that were modelled by the procedure SQUEEZE as implemented within the PLATON suite of programs.[11] Presumably, this solvent accessible void is occupied by a mixture of DMF and diethyl ether molecules, but the composition could not be determined.
|
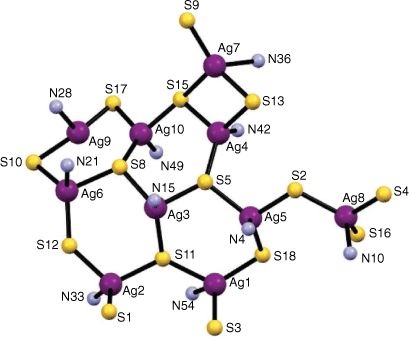
There are a total of 18 ligands and 20 silver atoms in the complex with two centrosymmetric charge neutral AgI10(Hgtsp)8(gtsp) clusters that are situated on a crystallographic centre of inversion. The two AgI10(Hgtsp)8(gtsp) clusters are linked to each other by two gtsp2− ligands that are related by the inversion centre and coordinate as a bidentate N,S donor to both Ag1 and Ag2 (Fig. 2).
The silver atoms are coordinated to three sulfur atoms and an azomethinic nitrogen atom except for Ag9, which is coordinated to two sulfur atoms and an azomethinic nitrogen atom in a distorted T-shaped trigonal coordination geometry (Fig. 3). The coordination environment of the other nine Ag(I) ions is tending toward a distorted trigonal–pyramidal geometry. The shortest Ag⋯Ag distances, Ag9–Ag10 (2.8577(18) Å) and Ag4–Ag7 (3.0551(12) Å), are less than twice the van der Waals radius for silver (3.44 Å) consistent with some degree of argentophilic Ag–Ag interactions.[4,5] The Ag–Ag distance in metallic silver is 2.889 Å.[7a]
|
The Ag–S bond distances range from 2.395(4) Å (Ag9–S17) to 2.769(3) Å (Ag7–S15). There are four sulfur atoms acting as μ3-bridges (S5, S8, S11, and S15) and six sulfur atoms acting as μ2-donors between silver atoms (S2, S10, S12, S13, S17, and S18). The Ag–N bond distances range from 2.176(9) Å (Ag10–N49) to 2.444(10) Å (Ag9–N28) and are fairly typical for Ag(I) thiosemicarbazone complexes.[4]
The ligand adopts three different conformations within the AgI20(Hgtsp)16(gtsp)2 complex highlighting the flexibility and versatility of bis(thiosemicarbazone) ligands. In each conformation the ligand adopts a s-trans conformation about the C–C backbone of the ligand. Two of the ligands are doubly deprotonated (gtsp2−) whilst sixteen ligands are singly deprotonated (Hgtsp−) to give an overall charge neutral cluster. One ligand in the asymmetric unit has ‘conformation 1’ (Fig. 4a), which involves a Z configuration about the two C–Nhydrazinic bonds (C145–N53, C88–N32) and is doubly deprotonated with the loss of two protons from the hydrazinic nitrogen atoms (N53, N32). The ligand coordinates to both Ag1 and Ag2 through the azomethinic nitrogen (N54 and N33) and thiolate sulfur atoms resulting in two 5-membered chelate rings. The thiolate-like sulfur atoms also have the ability to act as a bridge between silver atoms. The deprotonation and change in resonance structure of the ligand is supported by an increase in the C–S bond distances which average 1.74 Å when compared to the structure of the charge neutral free ligand, H2gtsp·0.5Et2O, where the C=S bond distances average 1.68 Å (Supplementary material) consistent with a shift toward thiolate-like rather than thione-like in nature. The deprotonation also results in a reduction in the length of the C–Nhydrazinic (e.g. C145–N53) bonds to an average value of 1.31 Å compared to an average 1.36 Å in H2gtsp consistent with the thiolate resonance form predominating.
There are five ligands in the asymmetric unit which adopt ‘conformation 2’ (Fig. 4b) with one thiosemicarbazone moiety in a Z configuration about the C–Nhydrazinic bond (e.g. C75–N29). The hydrazinic nitrogen of this moiety has lost a proton giving the ligand a 1− charge. Both the azomethinic nitrogen (e.g. N28) and thiolate sulfur atoms coordinate to a silver atom resulting in a five-membered chelate ring. Some of the thiolate sulfur atoms of this conformation also act as μ2 and μ3 bridges between silver atoms. The second moiety has the sulfur atom s-trans to the azomethinic nitrogen similar to the structure of H2gtsp·0.5Et2O (Supplementary material). In this moiety, the hydrazinic nitrogen (e.g. N26) is not deprotonated. Whilst the sulfur atom in this moiety coordinates a silver atom, the azomethinic nitrogen atom (e.g. N27) does not participate in silver coordination. This charge neutral moiety has an average C–S bond length of 1.68 Å, which is more thione than thiolate-like, and an average C–Nhydrazinic bond length of 1.33 Å.
There are three ligands in the asymmetric unit with ‘conformation 3’ (Fig. 4c). This conformation has one thiosemicarbazone moiety deprotonated and adopting the Z configuration giving the ligand a 1− charge. The second thiosemicarbazone moiety is not deprotonated with the sulfur atom s-trans to the azomethinic nitrogen atom (N16) and not participating in silver coordination. The average C–S and C–Nhydrazinic bond lengths in the ligand are 1.79 Å and 1.31 Å for the 1− moiety and 1.65 Å and 1.34 Å for the neutral moiety, respectively.
The isolation of this cluster was repeatable from several separate synthetic preparations using the same procedure, but also when a different silver salt, Ag(CF3SO3) was used as the starting material. The 1H NMR spectrum of crystals of AgI20(Hgtsp)16(gtsp)2 dissolved in d6-DMSO was broad and unresolved, which is consistent with the ligands being present in both coordinated and non-coordinated forms as well as charge neutral and deprotonated forms. An attempt at obtaining ESI-MS spectra failed to provide any characterisation data consistent with the AgI20 cluster, which may indicate that the complex is unstable in the solution and gas phases.
Conclusions
A complex with a cluster of 20 silver(I) ions supported by eighteen bis(thiosemicarbazonato) ligands is reported. The formation of this high nuclearity cluster involves the ligand adopting three different conformations highlighting the versatility of bis(thiosemicarbazone) ligands that are capable of coordinating to metal ions in a variety of different modes. The reproducible self-assembly of this cluster of twenty silver(I) ions is presumably supported by several close Ag⋯Ag contacts and the resulting argentophilic interactions as well as the flexible coordination geometries of closed shell silver(I).
|
Experimental
Glyoxal bis(4-phenylthiosemicarbazone), H2gtsp
This compound was synthesised utilising a similar published procedure for the synthesis of glyoxal bis(thiosemicarbazone).[13] To a solution of 4-phenyl-3-thiosemicarbazide (2.02 g, 12.1 mmol) in a mixture of boiling water (7.5 mL), ethanol (10 mL) and acetic acid (0.76 mL), a mixture of aqueous glyoxal (40 wt%, 0.35 g, 6.05 mmol) in water (14 mL) was added. A precipitate formed instantly, which was stirred for 5 min. The solid was collected by hot filtration and washed with water, ethanol and diethyl ether, then dried under vacuum at room temperature (1.72 g, 80%). (Found: C, 54.09; H, 4.64; N, 23.70; calcd for C16H16N6S2: C, 53.91; H, 4.52; N, 23.58). 1H NMR (d6-DMSO, 500 MHz): δ = 7.19, 2H, m, Ar–H4; 7.35, 4H, m, Ar–H3; 7.56, 4H, m, Ar–H2; 7.90, 2H, s, H–C=N; 10.18, 2H, s, NH–Ar; 12.14, 2H, s, NH–N. 13C{1H) NMR (125 MHz): δ = 125.3, ArC4; 125.5, ArC2; 128.1, ArC3; 138.9, ArC1; 140.7, C=N; 176.0, C=S. MS: (+ve ion) m/z 100% [M + H+] 356.09 (experimental), 356.09 (calculated). Crystals suitable for single crystal X-ray crystallography were grown from vapour diffusion diethyl ether into a mixture of H2gtsp dissolved in DMF (Supplementary material).
AgI20(Hgtsp)16(gtsp)2.(x.DMF, y.diethylether)
To a degassed solution of H2gtsp (0.10 g, 0.28 mmol) and triethylamine (40 μL, 0.28 mmol) in DMF (2 mL) was added AgBF4 (0.05 g, 0.28 mmol). Vapour diffusion of diethyl ether into the yellow/orange solution resulted in yellow crystals of AgI20(Hgtsp)16(gtsp)2·solvent (0.08 g) after 12 h that were suitable for analysis by X-ray crystallography. The crystals were collected by filtration and dried before they were dissolved in d6DMSO for analysis by 1H NMR spectroscopy. 1H NMR (d6-DMSO, 500 MHz): δ = 2.73, CH3, DMF; 2.89, CH3, DMF; 6.68, m, Ar–H; 6.85, m, Ar–H; 7.19, Ar–H; 7.35, m, Ar–H; 7.55, m, Ar–H; 7.65, m, Ar–H; 7.84, s, H–C=N; 7.89, s, H–C=N; 7.95, DMF; 9.66, s, NH; 10.16, s, NH. 13C{1H} NMR (125.7 MHz): δ = 30.5, CH3, DMF; 35.6, CH3, DMF, 120.6, Ar–C; 125.4, Ar–C; 125.5, Ar–C; 128.1, Ar–C; 138.8, C=N; 140.8, C=N; 162.3, DMF, CH; 176.0, C=S.
X-ray crystallography
Crystals were mounted in a low temperature oil then flash cooled to 130 K using an Oxford low temperature device. Intensity data were collected at 130 K with an Oxford XCalibur X-ray diffractometer with Sapphire CCD detector using Cu-Kα radiation (graphite crystal monochromator λ = 1.54184 Å) or a Bruker SMART Apex CCD detector using Mo-Kα radiation (graphite crystal monochromator λ = 0.71073 Å). Data were reduced and corrected for absorption. The structure was solved by direct methods and difference Fourier synthesis using the SHELX suite of programs[14] as implemented within the WINGX software.[15] The solvent was extensively disordered to the extent that modelling the disorder was not practical given the number of restraints that would be required and given that the identity of the solvent was questionable. The large (7310.1 Å3) amount of solvent accounts for just over 50% of the total volume AgI20(Hgtsp)16(gtsp)2 consisted of 2058 electrons and is presumably occupied by a combination of DMF and diethylether molecules.[11]
Data availability
The data that support this study are available from the Cambridge Crystallographic Data Centre CCDC 2128093 and CCDC 2128318.
Conflicts of interest
Jonathan M. White is an Associate Editor for the Australian Journal of Chemistry but was blinded from the peer review of this manuscript.
Declaration of funding
We thank the Australian Research Council for financial support of this research.
Supplementary material
The details of the crystal structure of H2gtsp·Et2O are available in Supplementary material online.
References
[1] (a) BM Paterson, PS Donnelly, Copper complexes of bis(thiosemicarbazones): from chemotherapeutics to diagnostic and therapeutic radiopharmaceuticals. Chem Soc Rev 2011, 40, 3005.| Copper complexes of bis(thiosemicarbazones): from chemotherapeutics to diagnostic and therapeutic radiopharmaceuticals.Crossref | GoogleScholarGoogle Scholar | 21409228PubMed |
(b) TS Lobana, S Khanna, R Sharma, G Hundal, R Sultana, M Chaudhary, et al. Versatility of Thiosemicarbazones in the Construction of Monomers, Dimers and Hydrogen-Bonded Networks of Silver(I) Complexes. Cryst Growth Des 2008, 8, 1203.
| Versatility of Thiosemicarbazones in the Construction of Monomers, Dimers and Hydrogen-Bonded Networks of Silver(I) Complexes.Crossref | GoogleScholarGoogle Scholar |
[2] BM Paterson, KF White, JM White, BF Abrahams, PS Donnelly, Guest-induced Assembly of Bis(thiosemicarbazonato) Zinc(II) Coordination Nanotubes. Angew Chem, Int Ed 2017, 56, 8370.
| Guest-induced Assembly of Bis(thiosemicarbazonato) Zinc(II) Coordination Nanotubes.Crossref | GoogleScholarGoogle Scholar |
[3] Lancashire RK. Silver. In: Wilkinson G, Gillard RD, McCleverty JA, editors. In: Comprehensive Coordination Chemistry. Pergamon; 1987. Vol 5. pp. 775–859.
[4] LJ Ashfield, AR Cowley, JR Dilworth, PS Donnelly, Functionalized Thiosemicarbazone Clusters of Copper(I) and Silver(I). Inorg Chem 2004, 43, 4121.
| Functionalized Thiosemicarbazone Clusters of Copper(I) and Silver(I).Crossref | GoogleScholarGoogle Scholar | 15236522PubMed |
[5] (a) K Onodera, NC Kasuga, T Takashima, A Hara, A Amano, H Murakami, et al. Synthesis, reaction and structure of a highly light-stable silver(I) cluster with an Ag4S4N4 core having a tridentate 4N-morpholinyl-2-acetylpyridine thiosemicarbazone ligand: Use of water-soluble silver(I) carboxylates as a silver(I) source. Dalton Trans 2007, 3646.
| Synthesis, reaction and structure of a highly light-stable silver(I) cluster with an Ag4S4N4 core having a tridentate 4N-morpholinyl-2-acetylpyridine thiosemicarbazone ligand: Use of water-soluble silver(I) carboxylates as a silver(I) source.Crossref | GoogleScholarGoogle Scholar | 17700827PubMed |
(b) MR Bermejo, AM Gonzalez-Noya, M Martinez-Calvo, R Pedrido, MJ Romero, MV Lopez, Checking the route to cluster helicates. Eur J Inorg Chem 2008, 3852.
| Checking the route to cluster helicates.Crossref | GoogleScholarGoogle Scholar |
[6] MR Bermejo, AM González-Noya, RM Pedrido, MJ Romero, M Vázquez, Route to Cluster Helicates. Angew Chem Int Ed 2005, 44, 4182.
| Route to Cluster Helicates.Crossref | GoogleScholarGoogle Scholar | 15929158PubMed |
[7] (a) A Castineiras, R Pedrido, Factors Involved in the Nuclearity of Silver Thiosemicarbazone Clusters: Cocrystallization of Two Different Sized Tetranuclear Silver(I) Clusters Derived from a Phosphinothiosemicarbazone Ligand. Inorg Chem 2008, 47, 5534.
| Factors Involved in the Nuclearity of Silver Thiosemicarbazone Clusters: Cocrystallization of Two Different Sized Tetranuclear Silver(I) Clusters Derived from a Phosphinothiosemicarbazone Ligand.Crossref | GoogleScholarGoogle Scholar | 18533649PubMed |
(b) TS Lobana, S Khanna, G Hundal, B-J Liaw, CW Liu, The influence of substituents at the C2 carbon of thiosemicarbazones on bonding and nuclearity of silver(I) complexes. Polyhedron 2008, 27, 2251.
| The influence of substituents at the C2 carbon of thiosemicarbazones on bonding and nuclearity of silver(I) complexes.Crossref | GoogleScholarGoogle Scholar |
(c) A Castineiras, R Pedrido, Novel Fluorescent Cationic Silver Thiosemicarbazone Clusters Containing Different Eight-Membered Ag4S4 Metallacycles. Inorg Chem 2009, 48, 4847.
| Novel Fluorescent Cationic Silver Thiosemicarbazone Clusters Containing Different Eight-Membered Ag4S4 Metallacycles.Crossref | GoogleScholarGoogle Scholar |
(d) TS Lobana, S Khanna, G Hundal, P Kaur, B Thakur, S Attri, et al. Coinage metal derivatives of salicylaldehyde thiosemicarbazones: Synthesis, structures, bond isomerism and H-bonded networks. Polyhedron 2009, 28, 1583.
| Coinage metal derivatives of salicylaldehyde thiosemicarbazones: Synthesis, structures, bond isomerism and H-bonded networks.Crossref | GoogleScholarGoogle Scholar |
[8] DES Silva, AB Becceneri, MC Solcia, JVB Santiago, MB Moreira, JA Gomes Neto, et al. Cytotoxic and apoptotic effects of ternary silver(I) complexes bearing 2-formylpyridine thiosemicarbazones and 1,10-phenanthroline. Dalton Trans 2020, 49, 5264.
| Cytotoxic and apoptotic effects of ternary silver(I) complexes bearing 2-formylpyridine thiosemicarbazones and 1,10-phenanthroline.Crossref | GoogleScholarGoogle Scholar | 32242564PubMed |
[9] BW Budesinsky, J Svec, Photometric determination of silver and mercury with glyoxal dithiosemicarbazone. Anal Chim Acta 1971, 55, 115.
| Photometric determination of silver and mercury with glyoxal dithiosemicarbazone.Crossref | GoogleScholarGoogle Scholar |
[10] A Castineiras, N Fernandez-Hermida, I Garcia-Santos, JL Perez-Lustres, I Rodriguez-Gonzalez, Luminescent complexes of silver(I) with pyridylbis(3-hexamethyleneiminyl thiosemicarbazone): effect of the counterion on the nuclearity. Dalton Trans 2012, 41, 3787.
| Luminescent complexes of silver(I) with pyridylbis(3-hexamethyleneiminyl thiosemicarbazone): effect of the counterion on the nuclearity.Crossref | GoogleScholarGoogle Scholar | 22354121PubMed |
[11] AL Spek, PLATON SQUEEZE: a tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr C 2015, 71, 9.
| PLATON SQUEEZE: a tool for the calculation of the disordered solvent contribution to the calculated structure factors.Crossref | GoogleScholarGoogle Scholar |
[12] M Jansen, Homoatomic d10–d10 Interactions: Their Effects on Structure and Chemical and Physical Properties. Angew Chem Int Ed 1987, 26, 1098.
| Homoatomic d10–d10 Interactions: Their Effects on Structure and Chemical and Physical Properties.Crossref | GoogleScholarGoogle Scholar |
[13] FA French, BL Freelander, Carcinostatic action of polycarbonyl compounds and their derivatives. IV. Glyoxal bis(thiosemicarbazone) and derivatives. Cancer Res 1958, 18, 1290.
| 13608436PubMed |
[14] GM Sheldrick, Crystal structure refinement with SHELXL. Acta Crystallogr C 2015, 71, 3.
| Crystal structure refinement with SHELXL.Crossref | GoogleScholarGoogle Scholar |
[15] LJ Farrugia, WinGX and ORTEP for Windows: an update. J Appl Crystallogr 2012, 45, 849.
| WinGX and ORTEP for Windows: an update.Crossref | GoogleScholarGoogle Scholar |
† Dedicated to Prof. Glen B. Deacon, on the occasion of his 85th birthday and in recognition of his many outstanding contributions to inorganic chemistry.