Gold-Catalysed Oxidative Cycloisomerisation of 1,6-Diyne Acetates to 1-Naphthyl Ketones
Andrew Thomas Holm A , Sanatan Nayak A and Philip Wai Hong Chan A B CA School of Chemistry, Monash University, Clayton, Vic. 3800, Australia.
B Department of Chemistry, University of Warwick, Coventry CV4 7AL, UK.
C Corresponding author. Email: phil.chan@monash.edu
Australian Journal of Chemistry 72(11) 881-889 https://doi.org/10.1071/CH19330
Submitted: 16 July 2019 Accepted: 27 August 2019 Published: 14 October 2019
Abstract
A synthetic method to prepare 1-naphthyl ketones from gold(i)-catalysed oxidative cycloisomerisation of 1,6-diyne acetates is described. The proposed mechanism involves cyclopropenation–cycloreversion of the 1,6-diyne motif initiated by a 1,2-acyloxy migration. This is followed by nucleophilic attack of the ensuing gold carbenoid species by a molecule of water and autoxidation to give the aromatic product.
Introduction
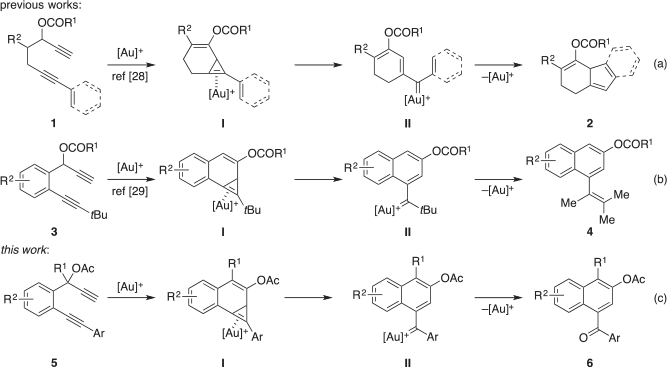
An emerging synthetic tool in organic synthesis to rapidly achieve molecular complexity and diversity is homogeneous gold catalysis.[1–54] In recent years, the field has witnessed a myriad of elegant methods to assemble an array of compounds of potential synthetic value, such as carbocycles and heterocycles, from readily accessible propargyl substrates in a single step.[18,30] An example of this is the gold(i)-catalysed preparation of 2,4a-dihydro-1H-fluorenes from 1,6-diyne esters in which computational studies revealed the likely involvement of the cyclopropene adduct I and gold carbenoid species II (Scheme 1a).[28] Closely following this work, the participation of these type of intermediates was proposed in the cycloisomerisation of 1,6-diyne esters to vinyl-substituted β-napthols (Scheme 1b).[29] Building on these initial studies, we were drawn to the potential reactivity of aryl-substituted 1,6-diyne esters containing a benzene tether (Scheme 1c). We anticipated that such substrates would form the corresponding cyclopropene intermediate I in the presence of a gold(i) complex.[47] Cycloreversion might then give the gold carbenoid species II. In the absence of a pronucleophilic motif or external reagent, this newly formed organogold species might then be susceptible to hydrolysis and autoxidation to give the 1-naphthyl ketone derivative 6. Herein, we describe the details of this chemistry, which provides an expedient route to the aromatic carbonyl compound under reaction conditions that did not require an external oxidant.
Results and Discussion
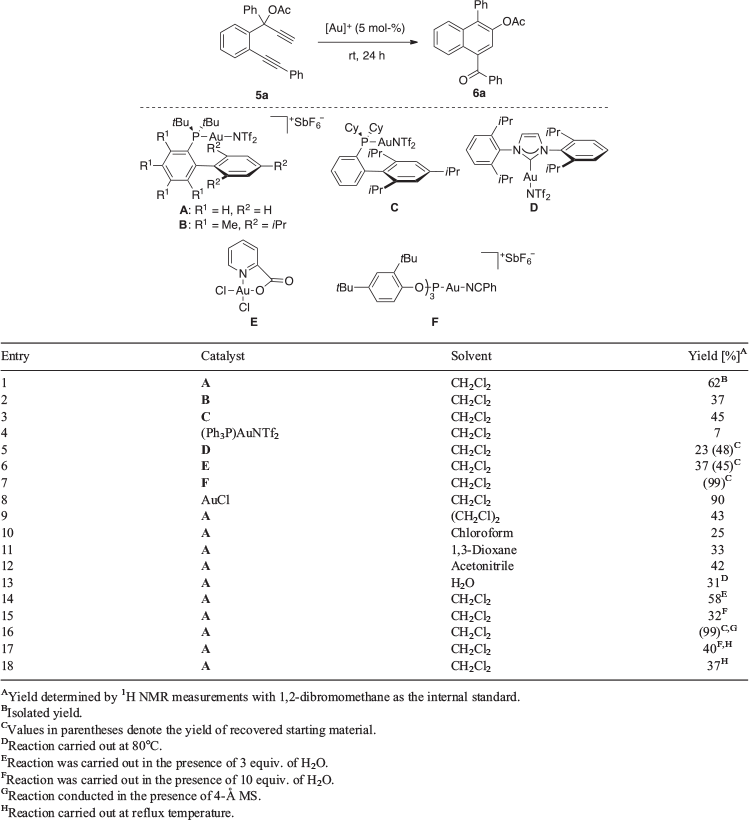
We began our investigations by examining the gold-catalysed cycloisomerisations of 1,6-diyne ester 5a to establish the optimum reaction conditions (Table 1). This initially revealed subjecting the substrate to 5 mol-% of gold(i) phosphine complex A in dichloromethane at room temperature for 24 h gave the best result (entry 1). Under these conditions, the desired 1-naphthyl ketone 6a was afforded in 62 % yield. Lower product yields of 7–45 % were obtained on repeating the reaction with the gold(i) phosphine complexes B, C, and PPh3PAuNTf2 (Tf = triflate) in place of A as the catalyst (entries 2–4). Control experiments with the NHC-AuI (NHC = N-heterocyclic carbene) complex D or gold(iii) complex E were found to result in incomplete consumption of the starting material, which was obtained along with 6a in respective yields of 48 and 23 %, and 45 and 37 % (entries 5 and 6). In contrast, the use of the gold(i) phosphite complex F as the catalyst was found to lead to no reaction and the near-quantitative recovery of the substrate (entry 7). Although the analogous reaction mediated by AuCl was initially found to give a product yield of 90 %, unfortunately this could not be consistently replicated (entry 8). A survey of other solvents such as 1,2-dichloroethane, chloroform, 1,3-dioxane or acetonitrile in place of dichloromethane was shown to give product yields of 25–43 % (entry 9–12). Likewise, comparable product yields of 31–58 % were afforded in control reactions in dichloromethane with 3 or 10 equiv. of water or only the latter as the solvent (entries 13–15). In a final set of control experiments, the introduction of 4-Å molecular sieves (MS) was observed to result in no reaction and near-quantitative recovery of the substrate (entry 16). On the other hand, conducting the reaction at reflux temperature in dichloromethane or the chlorinated solvent containing 10 equiv. of H2O was found to provide product yields of 40 and 37 %, respectively (entries 17 and 18).
|
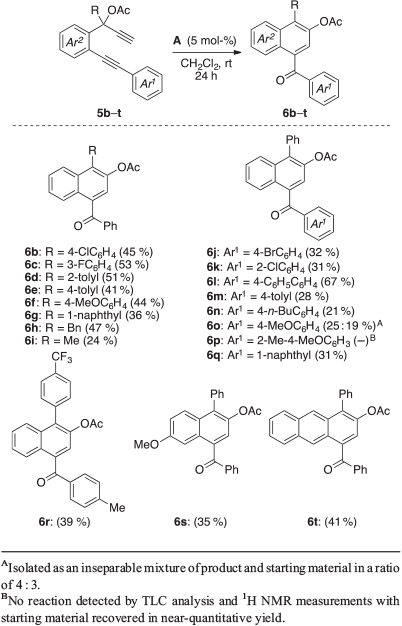
The generality of the transformation was next investigated with a variety of 1,6-diyne esters (Table 2). With gold(i) phosphine complex A as the catalyst, the reaction conditions were found to be broad, and a range of 1-naphthyl ketones with a variety of substitution patterns were furnished in 21–67 % yield from the corresponding substrates 5b–t. Reactions of substrates where the ester carbon centre contained a phenyl motif with an electron-withdrawing (5b and 5c) or donating (5d–f) group at either the o-, m- or p-position of the ring were shown to be well tolerated and gave 6b–f in 41–53 % yield. Likewise, 1,6-diyne acetates with a 1-naphthyl (5g), methyl (5h) or benzyl (5i) substituent at the R position were found to proceed well and give 6g–i in 24–47 % yield. Reactions with the introduction of other phenyl-substituted motifs (5j–n) or a 1-naphthyl group (5q) at the Ar1 position of the starting acetate were also found to proceed well, affording the corresponding products 6j–n and 6q in 21–67 % yield. The reactions of substrates containing a p-anisyl (5o) or 2-Me-4-MeO-substituted phenyl (5p) group at the Ar1 position were observed to be the only exceptions. In the case of the former, an inseparable mixture of 6o and the starting material in a ratio of 4 : 3 and 44 % overall yield was observed. For the latter, no reaction was found and the starting material was recovered in near-quantitative yield. In contrast, reaction of a substrate containing both a p-CF3 and p-Me substituent on the phenyl rings at the respective R and Ar1 positions (5r) was found to give corresponding ketone 6r in 39 % yield. Similarly, reactions of substrates in which the Ar2 of the substrate is a p-anisyl (5s) or 2-naphthyl (5t) moiety were found to proceed well. Under the gold(i)-catalysed standard reaction conditions, these experiments gave the anticipated aryl ketone adducts 6s and 6t in respective yields of 35 and 41 %.
|
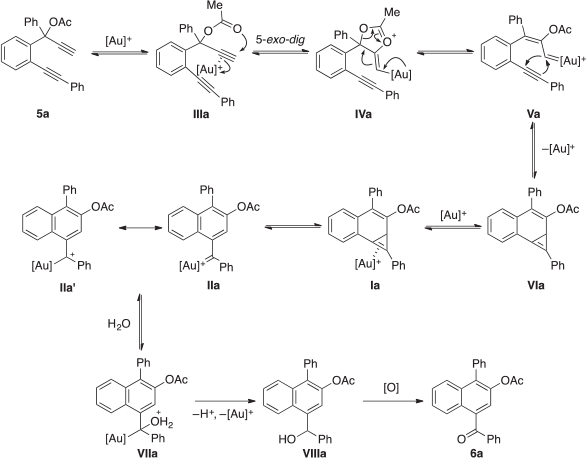
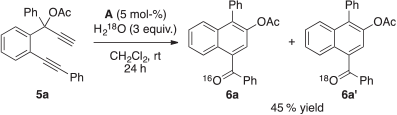
A tentative mechanism for the present gold(i)-catalysed 1-naphthyl ketone forming reaction is outlined in Scheme 2. With 5a as a representative example, this may initially involve activation of the propargyl moiety of the substrate by the AuI catalyst to give the gold(i)-coordinated complex IIIa. This results in the [2,3]-sigmatropic rearrangement of the acetyl functional group to produce the gold carbenoid species Va via the 1,3-dioxin-1-ium intermediate IVa. Trapping of this newly formed organogold species by the remaining alkyne motif may then provide the putative cyclopropene adduct VIa.[47,55] Further coordination of the Group 11 metal complex to the C=C bond of the tricyclic intermediate may next provide the gold(i)-activated species Ia. Ensuing electrophilic ring-opening of the gold(i)-activated three-membered ring in Ia would deliver the dibenzyl-stabilised gold carbenoid species IIa and its gold-stabilised allylic carbocation isomer IIa′. This is the active species that is prone to intermolecular nucleophilic attack by a molecule of water to form the hydrated organogold intermediate VIIa.[59] A sequential deprotonation and protodeauration process may then be anticipated to give the benzylic alcohol species VIIIa, which undergoes autoxidation to yield the 1-naphthyl ketone 6a. The proposed role of water in providing the oxygen source for the carbonyl functional group formation through addition to the gold carbenoid species IIa would be in good agreement with our earlier findings described in Table 1, entry 16 showing no reaction when 4-Å MS were added to the reaction conditions. It is also supported by the following control experiment (Scheme 3). Treatment of 5a with 3 equiv. of H218O in distilled dichloromethane predried using 4-Å MS yielded the desired 1-naphthyl ketones 6a and 6a′ as an inseparable mixture of isotopic isomers in an overall yield of 45 %. The incorporation of 18O-content in 6a′ was detected by mass spectrometric measurements.
|
|
Conclusion
In summary, we have demonstrated that propargyl esters containing a benzene-tethered alkyne undergo a 1,2-acyloxy migration–cyclopropenation–ring-opening sequence to access a dibenzyl-stabilised gold-carbenoid intermediate. In the absence of a pronucleophilic group or external reagent, addition of a molecule of water is followed by a deprotonation–protodeauration–autoxidation cascade to give access to a range of 1-naphthyl ketones. This successful utilisation of stabilised gold-carbenoid intermediates of this type should potentially lead to further useful transformations to compounds of synthetic value.
Experimental
General Considerations
Unless specified, all reagents, solvents, and starting materials were purchased from commercial sources and used as received. The propargyl alcohol precursor to substrate 5 was prepared following literature procedures.[28] Analytical thin-layer chromatography (TLC) was performed using precoated silica gel plates and visualisation was achieved with UV light (254 nm). Flash chromatography was performed using silica gel and a gradient solvent system (toluene or EtOAc/n-hexane as eluent). 1H and 13C spectra were measured on 400 and 600 MHz spectrometers. Chemical shifts (ppm) were recorded with respect to TMS in CD2Cl2 and CDCl3. Multiplicities are given as: s (singlet), brs (broad singlet), d (doublet), dd (doublet of doublets), ddd (doublet of doublets of doublets), t (triplet), dt (doublet of triplets), q (quartet) or m (multiplet). The number of protons (n) for a given resonance is indicated by nH. Coupling constants are reported as a J value in hertz. Infrared spectra were recorded on an IR spectrometer. Low- and high-resolution mass spectra (electrospray ionisation, ESI) were obtained using a liquid chromatography/high resolution mass spectrometry-time-of-flight (LC/HRMS TOF) spectrometer fitted with an analytical electrospray source using NaI for accurate mass calibration. Mass spectral data are reported in units of mass to charge ratio (m/z).
General Experimental Procedure for the Preparation of 1,6-Diyne Acetate Substrate 5[28]
To a solution of the propargyl alcohol (1equiv.) and 4-dimethylaminopyridine (DMAP, 0.6 equiv.) in distilled CH2Cl2 (1 mL per 0.1 mmol) were sequentially added N,N-diisopropylethylamine (DIPEA, 4 equiv.) and acetic anhydride (3 equiv.). The reaction mixture was stirred at room temperature for 18 h. On completion, the reaction mixture was quenched by adding saturated aqueous NH4Cl (10 mL) and extracted with CH2Cl2 (2 × 20 mL). The combined organic layers were washed with saturated aqueous NH4Cl (2 × 20 mL), H2O (15 mL), and brine (15 mL), dried over Na2SO4, and concentrated under reduced pressure. Purification of the crude mixture by flash column chromatography on silica gel (eluent: n-hexanes/EtOAc, gradient 14 : 1 to 9 : 1) gave the 1,6-diyne acetate substrate.
1-Phenyl-1-(2-(phenylethynyl)phenyl)prop-2-yn-1-yl Acetate (5a)
Orange solid (438 mg, 98 % yield); mp 155.5–156.3°C. δH (600 MHz, CD2Cl2) 7.99 (s, 1H), 7.56–7.51 (m, 3H), 7.42 (td, J 7.7, 1.5, 1H), 7.39–7.25 (m, 9H), 3.05 (s, 1H), 2.12 (s, 3H). δC (151 MHz, CD2Cl2) 168.44, 142.49, 142.48, 141.67, 141.66, 134.94, 134.93, 131.48, 131.46, 128.80, 128.78, 128.77, 128.55, 128.41, 128.30, 128.29, 127.08, 123.67, 121.12, 95.78, 88.46, 82.32, 79.14, 78.59, 21.65. νmax (NaCl, neat)/cm−1 3252, 2113, 1745, 1491, 1442, 1364, 1226. m/z (HRMS ESI) 351.1277 [M + H]+; calc. for C25H18O2: 351.1385.
1-(4-Chlorophenyl)-1-(2-(phenylethynyl)phenyl)prop-2-yn-1-yl Acetate (5b)
Yellow solid (386 mg, 96 % yield); mp 114.0–116.0°C. δH (600 MHz, CD2Cl2) 7.99 (dd, J 8.0, 0.9, 1H), 7.53 (dd, J 7.6, 1.5, 1H), 7.50–7.47 (m, 2H), 7.43 (td, J 7.7, 1.5, 1H), 7.38–7.34 (m, 6H), 7.30–7.26 (m, 2H), 3.06 (s, 1H), 2.12 (s, 3H). δC (151 MHz, CDCl3) 168.26, 141.66, 139.87, 134.86, 134.02, 131.20, 128.54, 128.53, 128.45, 128.42, 128.24, 127.88, 123.41, 120.94, 95.85, 88.21, 81.62, 78.71, 78.52, 21.63. νmax (NaCl, neat)/cm−1 3267, 2221, 2113, 1740, 1490, 1442, 1228. m/z (HRMS ESI) 385.0963 [M + H]+; calc. for C25H17ClO2: 385.0995.
1-(3-Fluorophenyl)-1-(2-(phenylethynyl)phenyl)prop-2-yn-1-yl Acetate (5c)
Beige solid (275 mg, 81 % yield); mp 146.7–148.2°C. δH (600 MHz, CD2Cl2) 8.03–8.00 (m, 1H), 7.54 (dd, J 7.6, 1.1, 1H), 7.45 (td, J 7.7, 1.5, 1H), 7.41–7.33 (m, 7H), 7.31–7.27 (m, 2H), 7.03–6.96 (m, 1H), 3.08 (s, 1H), 2.14 (s, 3H). δC (151 MHz, CDCl3) 168.30, 163.59, 161.97, 144.32, 141.88, 135.01, 131.46, 129.91, 128.84, 128.77, 128.50, 128.34, 123.54, 122.94, 121.12, 115.25, 115.10, 114.52, 114.36, 95.96, 88.25, 81.79, 78.95, 78.53, 21.60. νmax (NaCl, neat)/cm−1 3250, 1742, 1592, 1491, 1441, 1368, 1223. m/z (HRMS ESI) 369.1285 [M + H]+ calc. for C25H17FO2: 369.1291.
1-(2-(Phenylethynyl)phenyl)-1-(o-tolyl)prop-2-yn-1-yl Acetate (5d)
Yellow oil (85 mg, 84 % yield). δH (600 MHz, CD2Cl2) 7.90–7.86 (m, 1H), 7.71 (dd, J 8.0, 1.4, 1H), 7.60–7.54 (m, 1H), 7.41–7.35 (m, 2H), 7.22 (td, J 7.4, 1.3, 1H), 7.19–7.15 (m, 1H), 7.15–7.10 (m, 1H), 3.09 (s, 1H), 2.23 (s, 3H), 2.09 (s, 3H). δC (151 MHz, CD2Cl2) 168.22, 141.75, 138.45, 136.55, 134.99, 132.73, 131.51, 129.52, 129.22, 128.71, 128.66, 128.59, 128.05, 125.47, 123.75, 121.76, 96.01, 88.61, 81.39, 79.77, 78.94, 21.66, 21.60. νmax (NaCl, neat)/cm−1 3284, 2931, 2114, 1750, 1598, 1570, 1492, 1442, 1365, 1223. m/z (HRMS ESI) 365.1520 [M + H]+; calc. for C26H20O2: 365.1542.
1-(2-(Phenylethynyl)phenyl)-1-(p-tolyl)prop-2-yn-1-yl Acetate (5e)
White oil (132 mg, 97 % yield). δH (400 MHz, CDCl3) 7.94 (dd, J 7.9, 1.3, 1H), 7.52 (dt, J 7.6, 1.0, 1H), 7.47–7.42 (m, 2H), 7.41–7.28 (m, 7H), 7.14–7.09 (m, 2H), 2.99 (d, J 0.9, 1H), 2.32 (s, 3H), 2.13 (d, J 0.8, 3H). δC (101 MHz, CDCl3) 168.44, 142.35, 138.36, 137.83, 134.77, 131.27, 128.81, 128.44, 128.33, 128.13, 128.11, 127.96, 126.81, 123.71, 120.99, 95.55, 88.56, 82.24, 79.08, 78.22, 77.48, 77.36, 77.16, 76.84, 21.67, 21.25, 1.17. νmax (NaCl, neat)/cm−1 3283, 2923, 2113, 1751, 1492, 1365, 1224. m/z (HRMS ESI) 365.1532 [M + H]+; calc. for C26H20O2: 365.1542.
1-(4-Methoxyphenyl)-1-(2-(phenylethynyl)phenyl)prop-2-yn-1-yl Acetate (5f)
Yellow oil (70.5 mg, 97 % yield). δH (600 MHz, CDCl3) 7.93 (d, J 8.0, 1H), 7.53 (dd, J 7.6, 1.1, 1H), 7.49–7.46 (m, 2H), 7.40–7.35 (m, 3H), 7.34–7.29 (m, 4H), 6.85–6.81 (m, 2H), 3.76 (s, 3H), 3.00 (s, 1H), 2.13 (s, 3H). δC (151 MHz, CDCl3) 168.41, 159.26, 142.38, 134.71, 133.39, 131.23, 128.41, 128.32, 128.27, 128.10, 128.07, 127.76, 123.61, 120.96, 113.37, 95.60, 88.47, 82.16, 78.87, 78.12, 77.38, 77.17, 76.96, 55.31, 55.27, 29.81, 21.66. νmax (NaCl, neat)/cm−1 3286, 2927, 1752, 1608, 1510, 1494, 1443, 1368, 1229, 1176. m/z (HRMS ESI) 381.1481 [M + H]+; calc. for C26H20O3: 381.1491.
1-(Naphthalen-2-yl)-1-(2-(phenylethynyl)phenyl)prop-2-yn-1-yl Acetate (5g)
Yellow oil (87 mg, 87 % yield). δH (400 MHz, CDCl3) 8.11 (s, 1H), 8.01 (dt, J 8.0, 1.6, 1H), 7.80–7.68 (m, 3H), 7.57 (dt, J 8.7, 2.0, 1H), 7.50 (dt, J 7.6, 1.6, 1H), 7.46–7.36 (m, 3H), 7.33–7.18 (m, 7H), 3.04 (d, J 1.6, 1H), 2.17 (d, J 1.2, 3H). δC (101 MHz, CDCl3) 168.32, 141.95, 138.12, 134.72, 132.89, 132.72, 131.18, 128.53, 128.32, 128.27, 128.23, 128.11, 127.97, 127.89, 127.58, 126.42, 126.21, 126.03, 124.51, 123.41, 120.96, 95.67, 88.34, 81.92, 79.11, 78.62, 77.42, 77.10, 76.78, 21.60, 21.11, 14.26, 1.10. νmax (NaCl, neat)/cm−1 3286, 2169, 1751, 1600, 1494, 1226. m/z (HRMS ESI) 401.1523 [M + H]+; calc. for C29H20O2: 401.1542.
1-Phenyl-2-(2-(phenylethynyl)phenyl)but-3-yn-2-yl Acetate (5h)
Yellow oil (211 mg, 86 % yield). δH (600 MHz, CDCl3) 7.64–7.58 (m, 3H), 7.43–7.36 (m, 3H), 7.28 (dd, J 7.5, 1.4, 1H), 7.24–7.22 (m, 1H), 7.21–7.15 (m, 4H), 7.11–7.07 (m, 2H), 3.79 (d, J 13.2, 1H), 3.67 (d, J 13.3, 1H), 2.85 (s, 1H), 2.08 (s, 3H). δC (101 MHz, CDCl3) 168.57, 140.70, 134.83, 134.55, 131.26, 131.15, 128.58, 128.53, 128.50, 128.12, 127.82, 127.55, 126.91, 123.49, 119.01, 95.13, 88.54, 81.39, 79.43, 77.93, 77.34, 77.03, 76.71, 46.61, 21.27. νmax (NaCl, neat)/cm−1 2184, 2162, 2140, 1750, 1559, 1540, 1522, 1507, 1496, 1457, 1437, 1419, 1225. m/z (HRMS ESI) 365.1532 [M + H]+; calc. for C26H20O2: 365.1541.
2-(2-(Phenylethynyl)phenyl)but-3-yn-2-yl Acetate (5i)
Yellow oil (121 mg, 71 % yield). δH (600 MHz, CD2Cl2) 7.90 (dd, J 7.9, 0.9, 1H), 7.58–7.53 (m, 3H), 7.42–7.35 (m, 4H), 7.31 (td, J 7.5, 1.2, 1H), 2.89 (s, 1H), 2.10 (s, 3H), 2.02 (s, 3H). δC (151 MHz, CD2Cl2) 168.95, 142.97, 134.74, 131.58, 128.92, 128.87, 128.66, 128.12, 127.44, 123.81, 119.45, 95.36, 88.52, 83.45, 76.14, 75.82, 29.93, 21.37. νmax (NaCl, neat)/cm−1 3283, 2933, 2116, 1744, 1598, 1570, 1493, 1441, 1224. m/z (HRMS ESI) 289.1211 [M + H]+; calc. for C20H16O2: 289.1229.
1-(2-((4-Bromophenyl)ethynyl)phenyl)-1-phenylprop-2-yn-1-yl Acetate (5j)
Yellow solid (480 mg, 78 % yield); mp 133.2–135.2°C. δH (600 MHz, CD2Cl2) 7.98 (dd, J 9.2, 0.4, 1H), 7.53–7.49 (m, 3H), 7.49–7.47 (m, 2H), 7.44 (td, J 7.7, 1.5, 1H), 7.35 (td, J 7.5, 1.3, 1H), 7.33–7.26 (m, 3H), 7.24–7.20 (m, 2H), 3.05 (s, 1H), 2.11 (s, 3H). δC (151 MHz, CD2Cl2) 168.40, 142.58, 141.63, 134.88, 132.93, 132.04, 128.64, 128.59, 128.38, 128.32, 127.08, 122.87, 122.70, 120.84, 94.67, 89.62, 82.21, 79.08, 78.69, 21.66. νmax (NaCl, neat)/cm−1 3269, 211, 1742, 1491, 1441, 1235, 1192. m/z (HRMS ESI) 429.0479 [M + H]+; calc. for C25H17BrO2: 429.0490.
1-(2-((2-Chlorophenyl)ethynyl)phenyl)-1-phenylprop-2-yn-1-yl Acetate (5k)
Yellow oil (87 mg, 86 % yield). δH (400 MHz, CDCl3) 7.94 (ddd, J 8.0, 1.3, 0.5, 1H), 7.59 (ddd, J 7.5, 1.5, 0.5, 1H), 7.56–7.52 (m, 2H), 7.44–7.34 (m, 3H), 7.33–7.27 (m, 4H), 7.25–7.17 (m, 2H), 3.01 (s, 1H), 2.14 (s, 3H). δC (101 MHz, CD2Cl2) 168.55, 142.50, 141.58, 135.74, 135.34, 133.34, 129.84, 129.67, 128.80, 128.56, 128.41, 128.37, 128.34, 127.10, 126.96, 123.51, 120.74, 93.43, 92.48, 82.30, 79.11, 78.75, 21.67. νmax (NaCl, neat)/cm−1 3288, 2921, 1752, 1487, 1227. m/z (HRMS ESI) 325.0767 [M – OAc]−; calc. for C25H17ClO2: 325.0784.
1-(2-([1,1’-Biphenyl]-4-ylethynyl)phenyl)-1-phenylprop-2-yn-1-yl Acetate (5l)
White oil (98 mg, 87 % yield). δH (600 MHz, CD2Cl2) 7.99 (d, J 8.0, 1H), 7.65–7.58 (m, 4H), 7.58–7.52 (m, 3H), 7.49–7.41 (m, 5H), 7.40–7.26 (m, 5H), 3.07 (s, 1H), 2.14 (s, 3H). δC (151 MHz, CD2Cl2) 168.47, 142.49, 141.68, 141.39, 140.56, 134.93, 131.92, 129.29, 128.56, 128.42, 128.36, 128.32, 128.12, 127.40, 127.31, 127.11, 122.60, 121.16, 95.73, 89.21, 82.34, 79.16, 78.61, 21.70. νmax (NaCl, neat)/cm−1 3284, 2925, 1725, 1599, 1489, 1448, 1226. m/z (HRMS ESI) 367.1471 [M – OAc]−; calc. for C31H22O2: 367.1487.
1-Phenyl-1-(2-(p-tolylethynyl)phenyl)prop-2-yn-1-yl Acetate (5m)
Yellow solid (211 mg, 83 % yield); mp 142.0–143.2°C. δH (600 MHz, CD2Cl2) 7.97 (dd, J 8.0, 1.3, 1H), 7.55–7.52 (m, 2H), 7.50 (dd, J 7.6, 1.5, 1H), 7.41 (td, J 7.7, 1.5, 1H), 7.35–7.24 (m, 6H), 7.18–7.14 (m, 2H), 3.05 (s, 1H), 2.36 (s, 3H), 2.12 (s, 3H). δC (151 MHz, CD2Cl2) 168.46, 142.35, 141.68, 139.16, 134.84, 131.34, 129.54, 128.50, 128.29, 128.27, 128.24, 128.20, 127.08, 121.32, 120.59, 96.04, 87.84, 82.37, 79.15, 78.51, 21.66, 21.62. νmax (NaCl, neat)/cm−1 3269, 2920, 2855, 2213, 2116, 1743, 1509, 1440, 1363, 1238. m/z (HRMS ESI) 365.1519 [M + H]+; calc. For C26H20O2: 365.1542.
1-(2-((4-Butylphenyl)ethynyl)phenyl)-1-phenylprop-2-yn-1-yl Acetate (5n)
Yellow oil (498 mg, 73 % yield). δH (600 MHz, CD2Cl2) 7.99 (d, J 8.0, 1H), 7.59–7.55 (m, 2H), 7.55–7.51 (m, 1H), 7.42 (td, J 7.8, 1.5, 1H), 7.37–7.27 (m, 6H), 7.18 (d, J 8.2, 2H), 3.06 (s, 1H), 2.67–2.61 (m, 2H), 2.14 (s, 3H), 1.65–1.58 (m, 2H), 1.38 (h, J 7.4, 2H), 0.96 (t, J 7.4, 3H). δC (151 MHz, CD2Cl2) 168.46, 144.16, 142.35, 141.69, 134.88, 131.37, 128.92, 128.72, 128.51, 128.30, 128.29, 128.25, 128.20, 127.10, 126.29, 121.36, 120.80, 96.12, 87.87, 82.39, 79.18, 78.54, 35.95, 33.85, 22.77, 21.68, 14.12. νmax (NaCl, neat)/cm−1 3283, 2955, 2927, 2215, 2117, 1725, 1509 1449, 1225. m/z (HRMS ESI) 407.1992 [M + H]+; calc. for C29H26O2: 407.2011.
1-(2-((4-Methoxyphenyl)ethynyl)phenyl)-1-phenylprop-2-yn-1-yl Acetate (5o)
Yellow solid (134 mg, 98 % yield); mp 121.0–122.0°C. δH (600 MHz, CD2Cl2) 7.96 (dd, J 8.0, 0.9, 1H), 7.55–7.52 (m, 2H), 7.49 (dd, J 7.6, 1.1, 1H), 7.39 (td, J 7.7, 1.5, 1H), 7.35–7.25 (m, 6H), 6.90–6.84 (m, 2H), 3.82 (s, 3H), 3.04 (s, 1H), 2.12 (s, 3H). δC (151 MHz, CD2Cl2) 168.18, 159.95, 141.91, 141.43, 134.42, 132.65, 128.22, 128.02, 127.99, 127.95, 127.74, 126.81, 121.21, 115.46, 114.15, 95.68, 86.94, 82.11, 78.90, 78.21, 55.44, 21.39. νmax (NaCl, neat)/cm−1 3271, 2935, 2842, 2218, 2113, 1741, 1508, 1442, 1363, 1247, 1224, 1181. m/z (HRMS ESI) 380.1441 [M]+; calc. for C26H20O3: 380.1413.
1-(2-((4-Methoxy-2-methylphenyl)ethynyl)phenyl)-1-phenylprop-2-yn-1-yl Acetate (5p)
Yellow solid (155 mg, 81 % yield); mp 131.7–132.7°C. δH (600 MHz, CD2Cl2) 7.96 (dd, J 7.9, 1.0, 1H), 7.54–7.50 (m, 3H), 7.40 (td, J 7.7, 1.5, 1H), 7.35 (dd, J 7.5, 1.4, 1H), 7.33–7.26 (m, 3H), 7.25 (d, J 8.4, 1H), 6.74 (d, J 2.6, 1H), 6.71 (dd, J 8.4, 2.6, 1H), 3.79 (s, 3H), 3.05 (s, 1H), 2.29 (s, 3H), 2.11 (s, 3H). δC (151 MHz, CD2Cl2) 168.54, 160.14, 142.28, 141.72, 141.64, 134.93, 133.19, 128.48, 128.34, 128.30, 127.93, 127.17, 121.82, 115.66, 115.37, 111.67, 95.04, 90.89, 82.55, 79.32, 78.59, 55.62, 21.65, 20.97. νmax (NaCl, neat)/cm−1 3283, 2936, 2837, 2207, 1751, 1604, 1501, 1449, 1237. m/z (HRMS ESI) 395.1660 [M + H]+; calc. for C27H22O3: 395.1647.
1-(2-(Naphthalen-1-ylethynyl)phenyl)-1-phenylprop-2-yn-1-yl Acetate (5q)
White oil (40.2 mg, 77 % yield). δH (600 MHz, CDCl3) 8.14–8.10 (m, 1H), 8.02 (ddd, J 7.9, 1.3, 0.5, 1H), 7.83 (ddt, J 12.4, 8.6, 0.9, 2H), 7.70 (ddd, J 7.6, 1.5, 0.5, 1H), 7.61–7.56 (m, 3H), 7.53–7.47 (m, 2H), 7.47–7.42 (m, 2H), 7.39 (td, J 7.5, 1.3, 1H), 7.36–7.28 (m, 3H), 3.05 (s, 1H), 2.10 (s, 3H). δC (151 MHz, CDCl3) 168.65, 141.62, 141.57, 141.30, 135.10, 133.27, 130.13, 128.82, 128.60, 128.38, 128.27, 128.23, 128.20, 128.18, 127.78, 127.67, 127.03, 126.71, 126.60, 126.55, 125.37, 29.86. νmax (NaCl, neat)/cm−1 3234, 2918, 1749, 1558, 1506, 1228. m/z (HRMS ESI) 341.1316 [M – OAc]−; calc. for C29H20O2: 341.13303.
1-(2-(p-Tolylethynyl)phenyl)-1-(4-(trifluoromethyl)phenyl)prop-2-yn-1-yl Acetate (5r)
Orange oil (43 mg, 39 % yield). δH (600 MHz, CDCl3) 7.99 (dd, J 8.0, 1.0, 1H), 7.63 (d, J 8.2, 2H), 7.51 (d, J 8.3, 2H), 7.47 (ddd, J 7.6, 1.5, 0.5, 1H), 7.38 (td, J 7.7, 1.5, 1H), 7.30 (td, J 7.5, 1.3, 1H), 7.16–7.13 (m, 2H), 7.10 (d, J 7.9, 2H), 3.00 (s, 1H), 2.34 (s, 3H), 2.13 (s, 3H). δC (151 MHz, CDCl3) 168.20, 145.12, 141.18, 138.82, 134.83, 131.06, 129.32, 128.61, 128.14, 128.03, 127.34, 125.08, 125.06, 121.13, 120.20, 96.21, 87.42, 81.42, 79.05, 29.86, 22.34, 21.67, 21.60. νmax (NaCl, neat)/cm−1 3297, 2921, 2210, 2182, 1755, 1326, 1226. m/z (HRMS ESI) 373.1187 [M – OAc]−; calc. for C27H19F3O2: 373.1204.
1-(4-Methoxy-2-(phenylethynyl)phenyl)-1-phenylprop-2-yn-1-yl Acetate (5s)
Brown solid (83 mg, 74 % yield); mp 116.6–117.1°C. δH (600 MHz, CDCl3) 7.79 (dd, J 8.8, 1.4, 1H), 7.49 (dt, J 8.2, 1.6, 2H), 7.30–7.24 (m, 7H), 7.23–7.19 (m, 1H), 7.00 (dd, J 2.6, 1.1, 1H), 6.86 (dd, J 8.8, 2.7, 1H), 3.76 (s, 3H), 2.95 (s, 1H), 2.07 (s, 3H). δC (151 MHz, CDCl3) 168.47, 159.05, 141.65, 134.51, 131.23, 129.76, 128.39, 128.04, 127.95, 126.71, 123.40, 122.11, 119.31, 114.01, 95.22, 88.23, 82.29, 78.81, 78.37, 55.49, 29.80, 21.65. νmax (NaCl, neat)/cm−1 3283, 2925, 1752, 1601, 1569, 1225. m/z (HRMS ESI) 381.1448 [M + H]+; calc. for C26H20O3: 381.1491.
1-Phenyl-1-(3-(phenylethynyl)naphthalen-2-yl)prop-2-yn-1-yl Acetate (5t)
White oil (10 mg, 42 % yield). δH (600 MHz, CDCl3) 8.40 (s, 1H), 8.06 (s, 1H), 7.90 (dd, J 6.3, 2.9, 1H), 7.78 (dd, J 6.3, 2.7, 1H), 7.60–7.56 (m, 2H), 7.56–7.50 (m, 2H), 7.39–7.27 (m, 8H), 3.08 (d, J 0.7, 1H), 2.17 (d, J 0.8, 3H). δC (151 MHz, CDCl3) 168.55, 141.15, 138.12, 135.16, 132.66, 132.17, 131.29, 128.72, 128.46, 128.32, 128.17, 128.13, 127.87, 127.38, 127.36, 127.28, 127.03, 123.68, 118.55, 94.58, 88.84, 82.18, 79.24, 78.84, 77.37, 77.16, 76.95, 21.82. νmax (NaCl, neat)/cm−1 3286, 2225, 1752, 1598, 1495, 1449, 1226. m/z (HRMS ESI) 401.1528 [M + H]+; calc. for C29H20O2: 401.1542.
General Experimental Procedure for the Gold(i)-Catalysed Oxidative Cycloisomerisation of 5
To a reaction vessel containing the 1,6-diyne acetate substrate 5 (0.1 mmol) and gold(i) phosphine complex A (5 mol-%) under atmospheric conditions was added CH2Cl2 (2 mL) and the resulting reaction mixture was stirred for 24 h. The crude reaction mixture was purified by flash column chromatography on silica gel (eluent: n-hexane/EtOAc 14 : 1) to give the 1-naphthyl ketone product 6.
4-Benzoyl-1-phenylnaphthalen-2-yl Acetate (6a)
Yellow solid (33 mg, 62 % yield); mp 126.1–127.8°C. δH (400 MHz, CDCl3) 8.19–8.13 (m, 1H), 8.00–7.95 (m, 2H), 7.70–7.60 (m, 2H), 7.55–7.42 (m, 8H), 7.41–7.35 (m, 3H), 1.97 (s, 3H). δC (151 MHz, CD2Cl2) 196.83, 169.86, 144.57, 138.33, 137.51, 135.01, 134.46, 134.28, 133.88, 130.75, 130.48, 129.80, 128.97, 128.72, 128.42, 127.42, 127.06, 126.14, 123.88, 20.69. νmax (NaCl, neat)/cm−1 2923, 1761, 1494, 1197, 1176. m/z (HRMS ESI) 367.1333 [M + H]+; calc. for C25H18O3: 367.1334.
4-Benzoyl-1-(4-chlorophenyl)naphthalen-2-yl Acetate (6b)
Yellow oil (24 mg, 45 % yield). δH (600 MHz, CD2Cl2) 8.13–8.09 (m, 1H), 7.95–7.90 (m, 2H), 7.67–7.62 (m, 2H), 7.54–7.47 (m, 6H), 7.38 (s, 1H), 7.35–7.32 (m, 2H), 2.00 (s, 3H). δC (151 MHz, CD2Cl2) 196.74, 169.75, 144.62, 138.22, 137.92, 134.44, 134.08, 133.95, 133.58, 133.10, 132.02, 130.74, 129.76, 129.01, 127.63, 127.17, 126.73, 126.23, 123.69, 20.74. νmax (NaCl, neat)/cm−1 2924, 1763, 1661, 1491, 1197, 1176. m/z (HRMS ESI) 401.0930 [M + H]+; calc. for C25H17ClO3: 401.0945.
4-Benzoyl-1-(3-fluorophenyl)naphthalen-2-yl Acetate (6c)
White oil (28.5 mg, 53 % yield). δH (400 MHz, CD2Cl2) 8.12–8.06 (m, 1H), 7.95–7.89 (m, 2H), 7.68–7.60 (m, 2H), 7.55–7.44 (m, 5H), 7.38 (s, 1H), 7.25–7.08 (m, 4H), 1.99 (s, 3H). δC (101 MHz, CD2Cl2) 196.73, 169.73, 164.30, 161.85, 144.58, 138.22, 138.02, 137.29, 137.21, 133.99, 133.97, 132.96, 132.94, 130.75, 130.49, 130.41, 129.74, 129.01, 127.66, 127.19, 126.74, 126.49, 126.46, 126.22, 123.69, 117.66, 117.44, 115.47, 115.26, 54.24, 20.70. νmax (NaCl, neat)/cm−1 1764, 1582, 1489, 1449, 1437, 1369, 1338, 1269, 1242, 1201, 1172, 1123, 1020. m/z (HRMS ESI) 385.1233 [M + H]+; calc. for C25H17FO3: 385.1240.
4-Benzoyl-1-(o-tolyl)naphthalen-2-yl Acetate (6d)
White oil (17 mg, 51 % yield). δH (400 MHz, CDCl3) 8.18 (dt, J 8.4, 1.1, 1H), 8.00–7.95 (m, 2H), 7.63 (ddt, J 7.9, 6.9, 1.3, 1H), 7.54–7.36 (m, 8H), 7.34–7.28 (m, 1H), 7.20 (dd, J 7.2, 1.2, 1H), 2.02 (s, 3H), 1.92 (s, 3H). δC (151 MHz, CDCl3) 196.87, 169.58, 144.74, 138.37, 137.70, 137.37, 134.48, 134.10, 134.04, 133.85, 130.78, 130.44, 129.71, 128.96, 128.68, 127.54, 127.09, 126.78, 126.26, 126.03, 124.06, 30.09, 20.61, 19.86. νmax (NaCl, neat)/cm−1 2920, 2851, 1765, 1663, 1449, 1201. m/z (HRMS ESI) 381.1467 [M + H]+; calc. for C26H20O3: 381.1491.
4-Benzoyl-1-(p-tolyl)naphthalen-2-yl Acetate (6e)
Yellow oil (18 mg, 41 % yield). δH (400 MHz, CDCl3) 8.15 (dd, J 8.3, 1.6, 1H), 7.96 (dt, J 7.1, 1.4, 2H), 7.72–7.58 (m, 2H), 7.53–7.40 (m, 4H), 7.37 (s, 1H), 7.32 (d, J 7.8, 2H), 7.28–7.22 (m, 2H), 2.46 (s, 3H), 1.99 (s, 3H). δC (101 MHz, CDCl3) 196.63, 169.55, 144.09, 137.95, 137.70, 136.86, 134.23, 134.10, 133.42, 131.56, 130.53, 129.96, 129.57, 129.02, 128.57, 126.92, 126.79, 126.71, 125.81, 123.58, 77.33, 77.01, 76.69, 21.36, 20.57, 1.02. νmax (NaCl, neat)/cm−1 2957, 2923, 1763, 1661, 1595, 1579, 1508, 1449, 1231, 1200. m/z (HRMS ESI) 381.1487 [M + H]+; calc. for C26H20O3: 381.1491.
4-Benzoyl-1-(4-methoxyphenyl)naphthalen-2-yl Acetate (6f)
White oil (32.3 mg, 44 % yield). δH (600 MHz, CDCl3) 8.16 (d, J 8.4, 1H), 7.96 (d, J 8.2, 2H), 7.71 (d, J 8.4, 1H), 7.64–7.60 (m, 1H), 7.53–7.42 (m, 4H), 7.37 (s, 1H), 7.32–7.28 (m, 2H), 7.08–7.03 (m, 2H), 3.91 (s, 3H), 2.01 (s, 3H). δC (151 MHz, CDCl3) 196.78, 169.71, 159.47, 144.37, 138.07, 136.95, 134.38, 134.11, 133.58, 131.44, 130.67, 129.73, 128.72, 127.09, 126.89, 126.87, 126.78, 125.97, 123.71, 55.46, 20.74. νmax (NaCl, neat)/cm−1 2927, 1759, 1578, 1507, 1448, 1366, 1244, 1196, 1172. m/z (HRMS ESI) 397.1412 [M + H]+; calc. for C26H20O4: 397.1440.
4-Benzoyl-(1,1’-binaphthalen)-2-yl Acetate (6g)
White oil (20 mg, 36 % yield). δH (600 MHz, CD2Cl2) 8.14 (d, J 8.1, 1H), 8.02 (d, J 8.4, 1H), 7.99–7.91 (m, 4H), 7.87 (s, 1H), 7.71–7.68 (m, 1H), 7.66 (tt, J 7.8, 1.3, 1H), 7.61–7.56 (m, 2H), 7.55–7.48 (m, 4H), 7.47–7.42 (m, 2H), 1.91 (s, 3H). δC (151 MHz, CD2Cl2) 196.84, 169.87, 144.78, 138.34, 137.62, 134.39, 134.30, 133.90, 133.60, 133.32, 132.55, 130.77, 129.83, 129.67, 128.99, 128.49, 128.36, 128.31, 128.16, 127.49, 127.13, 127.11, 126.89, 126.82, 126.19, 123.93, 54.02, 20.72. νmax (NaCl, neat)/cm−1 2925, 1763, 1662, 1200, 1175. m/z (HRMS ESI) 417.1451 [M + H]+; calc. for C29H20O3: 417.1491.
4-Benzoyl-1-benzylnaphthalen-2-yl Acetate (6h)
Yellow oil (5.8 mg, 47 % yield). δH (600 MHz, CDCl3) 8.17–8.12 (m, 1H), 8.06–8.02 (m, 1H), 7.96–7.91 (m, 2H), 7.62 (ddt, J 7.7, 7.1, 1.3, 1H), 7.52–7.44 (m, 4H), 7.38 (s, 1H), 7.28–7.23 (m, 2H), 7.21–7.16 (m, 3H), 4.45 (s, 2H), 2.27 (s, 3H). δC (125 MHz, CDCl3) 194.83, 170.61, 142.99, 139.17, 136.56, 133.85, 132.69, 130.42, 129.99, 128.51, 126.78, 126.37, 125.43, 33.51, 20.02. νmax (NaCl, neat)/cm−1 3062, 3028, 2922, 2853, 2360, 2341, 2250, 1761, 1659, 1594, 1515, 1495, 1449, 1368, 1339, 1281, 1255, 1199, 1176, 1082, 1011, 907. m/z (HRMS ESI) 381.1483 [M + H]+; calc. for C26H20O3: 380.1412.
4-Benzoyl-1-methylnaphthalen-2-yl Acetate (6i)
White oil (15 mg, 24 % yield). δH (400 MHz, CDCl3) 8.16 (ddd, J 8.5, 1.4, 0.7, 1H), 8.09 (ddd, J 8.6, 1.3, 0.7, 1H), 7.91–7.87 (m, 2H), 7.63–7.57 (m, 2H), 7.53–7.44 (m, 3H), 7.30 (s, 1H), 2.58 (s, 3H), 2.37 (s, 3H). δC (151 MHz, CD2Cl2) 169.68, 145.17, 143.66, 138.55, 135.72, 134.22, 133.67, 130.68, 129.57, 128.97, 128.87, 127.33, 126.88, 126.61, 124.94, 124.03, 100.40, 30.09, 21.04, 12.27. νmax (NaCl, neat)/cm−1 2922, 2853, 1759, 1594, 1199. m/z (HRMS ESI) 305.1170 [M + H]+; calc. for C20H16O3: 305.1178.
4-(4-Bromobenzoyl)-1-phenylnaphthalen-2-yl Acetate (6j)
Yellow oil (20 mg, 32 % yield). δH (400 MHz, CDCl3) 8.14 (ddd, J 8.4, 1.5, 0.7, 1H), 7.86–7.79 (m, 2H), 7.69–7.63 (m, 3H), 7.55–7.42 (m, 6H), 7.39–7.34 (m, 3H), 1.98 (s, 3H). δC (151 MHz, CD2Cl2) 195.78, 169.86, 144.53, 137.19, 136.81, 134.90, 134.80, 134.31, 132.30, 132.26, 130.44, 129.69, 129.01, 128.74, 128.47, 127.51, 127.21, 127.11, 126.02, 124.08, 70.87, 20.68. νmax (NaCl, neat)/cm−1 2865, 1763, 1583, 1201. m/z (HRMS ESI) 445.0431 [M + H]+; calc. for C25H17BrO3: 445.0440.
4-(2-Chlorobenzoyl)-1-phenylnaphthalen-2-yl Acetate (6k)
Brown solid (24 mg, 31 % yield); mp 137.0–138.0°C. δH (400 MHz, CDCl3) 8.87 (d, J 8.6, 1H), 7.70–7.61 (m, 2H), 7.58 (d, J 7.2, 1H), 7.55–7.44 (m, 6H), 7.43–7.32 (m, 4H), 1.93 (s, 3H). δC (151 MHz, CD2Cl2) 196.09, 169.70, 144.59, 139.65, 136.89, 135.76, 134.83, 134.54, 132.45, 132.40, 130.84, 130.34, 129.78, 128.69, 128.53, 128.07, 127.59, 127.33, 127.18, 127.15, 126.33, 20.63. νmax (NaCl, neat)/cm−1 2926, 1751, 1674, 1582, 1197. m/z (HRMS ESI) 401.0938 [M + H]+; calc. for C25H17ClO3: 401.0945.
4-([1,1’-Biphenyl]-4-carbonyl)-1-phenylnaphthalen-2-yl Acetate (6l)
Yellow solid (20 mg, 67 % yield); mp 126.4–127.2°C. δH (600 MHz, CDCl3) 8.18 (d, J 8.4, 1H), 8.08–8.03 (m, 2H), 7.73 (d, J 8.2, 2H), 7.69–7.64 (m, 3H), 7.54–7.37 (m, 11H), 1.99 (s, 3H). δC (151 MHz, CDCl3) 196.34, 169.72, 146.37, 144.22, 139.96, 137.32, 136.67, 134.80, 134.23, 134.10, 131.30, 130.24, 129.67, 129.14, 128.48, 128.15, 127.48, 127.40, 127.20, 126.94, 126.86, 126.00, 123.56, 20.67. νmax (NaCl, neat)/cm−1 2923, 1761, 1655, 1600, 1260, 1198, 1176. m/z (HRMS ESI) 443.1641 [M + H]+; calc. for C31H22O3: 443.1647.
4-(4-Methylbenzoyl)-1-phenylnaphthalen-2-yl Acetate (6m)
Yellow oil (14.9 mg, 28 % yield). δH (400 MHz, CDCl3) 8.16–8.10 (m, 1H), 7.92–7.85 (m, 2H), 7.68–7.63 (m, 1H), 7.54–7.41 (m, 5H), 7.39–7.35 (m, 3H), 7.32–7.28 (m, 2H), 2.45 (s, 3H), 1.98 (s, 3H). δC (101 MHz, CDCl3) 196.42, 169.64, 144.63, 144.19, 137.56, 135.43, 134.81, 134.01, 133.94, 130.82, 130.22, 129.62, 129.42, 128.42, 128.08, 127.08, 126.77, 125.97, 123.28, 77.45, 77.33, 77.13, 76.81, 21.88, 20.62, 1.14. νmax (NaCl, neat)/cm−1 2926, 1763, 1604, 1495, 1201. m/z (HRMS ESI) 381.1461 [M + H]+; calc. for C26H20O3: 381.1491.
4-(4-Butylbenzoyl)-1-phenylnaphthalen-2-yl Acetate (6n)
Orange oil (12.7 mg, 21 % yield). δH (400 MHz, CDCl3) 8.05 (d, J 8.1, 1H), 7.82 (d, J 8.3, 2H), 7.61–7.54 (m, 1H), 7.46–7.34 (m, 5H), 7.32–7.28 (m, 3H), 7.26–7.20 (m, 2H), 2.66–2.59 (m, 2H), 1.90 (s, 3H), 1.63–1.53 (m, 2H), 1.31 (dq, J 14.7, 7.3, 3H), 0.87 (t, J 7.3, 3H). δC (151 MHz, CD2Cl2) 168.47, 145.15, 144.45, 144.15, 142.33, 141.67, 134.88, 134.47, 131.46, 131.35, 128.92, 128.86, 128.72, 128.51, 128.49, 128.39, 128.32, 128.28, 128.18, 127.08, 126.91, 126.27, 35.93, 33.81, 22.75, 21.67, 14.09. νmax (NaCl, neat)/cm−1 2925, 2859, 1765, 1605, 1456, 1201. m/z (HRMS ESI) 423.1929 [M + H]+; calc. for C29H26O3: 423.19604.
4-(4-Methoxybenzoyl)-1-phenylnaphthalen-2-yl Acetate (6o)
Orange oil (42 mg, 25 % yield, obtained as inseparable mixture of 5o and 6o in a ratio 4 : 1). δH (400 MHz, CD2Cl2) 8.07–8.02 (m, 1H), 7.95–7.91 (m, 2H), 7.73–7.65 (m, 2H), 7.57–7.27 (m, 7H), 7.02–6.97 (m, 2H), 3.89 (s, 3H), 1.98 (s, 3H). δC (101 MHz, CD2Cl2) 169.89, 164.50, 156.57, 144.62, 142.18, 134.69, 133.12, 132.92, 131.29, 131.04, 130.51, 128.26, 128.22, 128.01, 126.98, 126.84, 126.17, 123.00, 87.21, 82.38, 79.16, 78.50, 56.01, 55.69, 21.67. νmax (NaCl, neat)/cm−1 3282, 2959, 2934, 2214, 2114, 1751, 1511, 1247, 1226. m/z (HRMS ESI) 397.1417 [M + H]+; calc. for C26H20O4: 397.1440.
4-(1-Naphthoyl)-1-phenylnaphthalen-2-yl Acetate (6q)
Yellow oil (12.9 mg, 31 % yield). δH (600 MHz, CDCl3) 8.69 (d, J 8.4, 1H), 8.54 (d, J 8.5, 1H), 8.06 (d, J 8.2, 1H), 7.96 (d, J 7.6, 1H), 7.74 (dd, J 7.1, 1.1, 1H), 7.68 (d, J 8.5, 2H), 7.60 (ddd, J 8.1, 6.8, 1.3, 1H), 7.55 (ddd, J 8.4, 6.8, 1.4, 1H), 7.53–7.45 (m, 5H), 7.40 (s, 1H), 7.38–7.33 (m, 2H), 1.91 (s, 3H). δC (151 MHz, CDCl3) 198.48, 169.59, 144.25, 138.36, 136.49, 135.37, 134.76, 134.19, 134.08, 133.14, 131.38, 130.18, 130.00, 128.66, 128.46, 128.29, 128.19, 127.39, 127.25, 126.90, 126.84, 126.17, 126.04, 125.74, 124.57, 14.27. νmax (NaCl, neat)/cm−1 2920, 1745, 1653, 1578, 1173. m/z (HRMS ESI) 417.1489 [M + H]+; calc. for C29H20O3: 417.1491.
4-(4-Methylbenzoyl)-1-(4-(trifluoromethyl)phenyl)naphthalen-2-yl Acetate (6r)
Orange solid (20 mg, 35 % yield); mp 75.0–76.0°C. δH (600 MHz, CDCl3) 8.12 (d, J 8.6, 1H), 7.87 (d, J 8.2, 2H), 7.79 (d, J 8.0, 2H), 7.56–7.44 (m, 6H), 7.37 (s, 1H), 7.32–7.29 (m, 2H), 2.45 (s, 3H), 2.00 (s, 3H). δC (151 MHz, CDCl3) 196.25, 169.50, 144.88, 144.22, 138.85, 138.41, 135.26, 133.57, 132.25, 130.84, 130.82, 129.59, 129.52, 127.53, 127.03, 126.27, 126.20, 125.51, 125.48, 125.45, 125.43, 123.38, 123.02, 21.94, 20.67. νmax (NaCl, neat)/cm−1 2918, 1766, 1574, 1323, 1176. m/z (HRMS ESI) 365.1159 [M + H]+; calc. for C23H15F3O: 365.1153.
4-Benzoyl-6-methoxy-1-phenylnaphthalen-2-yl Acetate (6s)
Orange oil (30 mg, 35 % yield). δH (600 MHz, CDCl3) 7.96 (d, J 7.2, 2H), 7.67 (d, J 2.5, 1H), 7.65–7.61 (m, 1H), 7.57–7.44 (m, 7H), 7.39 (s, 1H), 7.37–7.33 (m, 2H), 7.11 (dd, J 9.3, 2.6, 1H), 3.83 (s, 3H), 1.96 (s, 3H). δC (151 MHz, CDCl3) 197.08, 169.83, 158.65, 142.43, 138.43, 134.93, 134.91, 134.89, 133.39, 131.28, 130.65, 130.15, 129.44, 128.70, 128.43, 128.31, 128.13, 125.15, 120.03, 104.28, 55.46, 20.63. νmax (NaCl, neat)/cm−1 2931, 1765, 1513, 1471, 1203. m/z (HRMS ESI) 397.1418 [M + H]+; calc. for C26H20O4: 397.1440.
4-Benzoyl-1-phenylanthracen-2-yl Acetate (6t)
White oil (3 mg, 41 % yield). δH (600 MHz, CDCl3) 8.84 (s, 1H), 8.19 (s, 1H), 8.04 (s, 2H), 7.96–7.92 (m, 1H), 7.84–7.80 (m, 1H), 7.67–7.62 (m, 1H), 7.60–7.56 (m, 2H), 7.55–7.51 (m, 3H), 7.48–7.42 (m, 4H), 7.40 (s, 1H), 2.00 (s, 3H). δC (151 MHz, CDCl3) 196.81, 169.77, 143.27, 138.21, 137.08, 134.95, 133.80, 133.64, 132.13, 132.04, 131.93, 130.75, 130.31, 128.78, 128.71, 128.61, 128.28, 127.56, 126.52, 126.31, 125.95, 125.48, 124.85, 29.90, 20.70, 14.28. νmax (NaCl, neat)/cm−1 1758, 1655, 1459, 1258. m/z (HRMS ESI) 417.1468 [M + H]+; calc. for C29H20O3: 417.1491.
General Experimental Procedure for the Gold(i)-Catalysed Oxidative Cycloisomerisation of 5a
To a reaction vessel containing the 1,6-diyne acetate substrate 5a (41.2 mg, 0.1 mmol), H218O (6.4 μL, 0.3 mmol), and gold(i) phosphine complex A (4.5 mg, 5 mol-%) under atmospheric conditions was added CH2Cl2 (2 mL) and the resulting reaction mixture was stirred for 24 h. The crude reaction mixture was purified by flash column chromatography on silica gel (eluent: n-hexane/EtOAc 14 : 1) to give an inseparable mixture of the 1-naphthyl ketone products 6a and 6a′ (19.6 mg, 45 % yield).
Supplementary Material
1H and 13C NMR spectra for all starting materials and products are available on the Journal’s website.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgements
This work was supported by a Discovery Project Grant (DP160101682) from the Australian Research Council.
References
[1] For selected reviews on gold catalysis, see refs [2–10].[2] K. Holzschneider, S. F. Kirsch, Isr. J. Chem. 2018, 58, 596.
| Crossref | GoogleScholarGoogle Scholar |
[3] Y. Wei, M. Shi, ACS Catal. 2016, 6, 2515.
| Crossref | GoogleScholarGoogle Scholar |
[4] D. Pflästerer, A. S. K. Hashmi, Chem. Soc. Rev. 2016, 45, 1331.
| Crossref | GoogleScholarGoogle Scholar | 26673389PubMed |
[5] R. Dorel, A. M. Echavarren, Chem. Rev. 2015, 115, 9028.
| Crossref | GoogleScholarGoogle Scholar | 25844920PubMed |
[6] Gold Catalysis: A Homogeneous Approach (Eds F. D. Toste, V. Michelet) 2014 (Imperial College Press: London).
[7] A. S. K. Hashmi, Acc. Chem. Res. 2014, 47, 864.
| Crossref | GoogleScholarGoogle Scholar |
[8] Modern Gold-Catalyzed Synthesis (Eds A. S. K. Hashmi, F. D. Toste) 2012 (Wiley-VCH: Weinheim).
[9] F. Miege, C. Meyer, J. Cossy, Beilstein J. Org. Chem. 2011, 7, 717.
| Crossref | GoogleScholarGoogle Scholar | 21804867PubMed |
[10] A. Fürstner, Chem. Soc. Rev. 2009, 38, 3208.
| Crossref | GoogleScholarGoogle Scholar | 19847352PubMed |
[11] For selected reviews on gold-catalysed cyclisation of propargyl esters, see refs [12–17].
[12] A. M. Asiri, A. S. K. Hashmi, Chem. Soc. Rev. 2016, 45, 4471.
| Crossref | GoogleScholarGoogle Scholar | 27385433PubMed |
[13] D. P. Day, P. W. H. Chan, Adv. Synth. Catal. 2016, 358, 1368.
| Crossref | GoogleScholarGoogle Scholar |
[14] L. Fensterbank, M. Malacria, Acc. Chem. Res. 2014, 47, 953.
| Crossref | GoogleScholarGoogle Scholar | 24564512PubMed |
[15] B. J. Ayers, P. W. H. Chan, Synlett 2015, 1305.
[16] A. S. K. Hashmi, Angew. Chem. Int. Ed. 2010, 49, 5232.
| Crossref | GoogleScholarGoogle Scholar |
[17] E. Jimenez-Nunez, A. M. Echavarren, Chem. Rev. 2008, 108, 3326.
| Crossref | GoogleScholarGoogle Scholar | 18636778PubMed |
[18] For selected recent examples of gold-catalysed carbocyclic synthesis, see refs [19–29].
[19] M. Mathiew, J. K. Tan, P. W. H. Chan, Angew. Chem. Int. Ed. 2018, 57, 14235.
| Crossref | GoogleScholarGoogle Scholar |
[20] P. T. Bohan, F. D. Toste, J. Am. Chem. Soc. 2017, 139, 11016.
| Crossref | GoogleScholarGoogle Scholar | 28771334PubMed |
[21] S. K. Thummanapelli, S. Hosseyni, Y. Su, N. G. Akhmedov, X. Shi, Chem. Commun. 2016, 7687.
| Crossref | GoogleScholarGoogle Scholar |
[22] W. Rao, J. W. Boyle, P. W. H. Chan, Chem. – Eur. J. 2016, 22, 6532.
| Crossref | GoogleScholarGoogle Scholar | 26945940PubMed |
[23] X. Chen, D. P. Day, W. T. Teo, P. W. H. Chan, Org. Lett. 2016, 18, 5936.
| Crossref | GoogleScholarGoogle Scholar | 27791382PubMed |
[24] E. Rettenmeier, M. M. Hansmann, A. Ahrens, K. Rubenacker, T. Saboo, J. Massholder, C. Meier, M. Rudolph, F. Rominger, A. S. Hashmi, Chem. – Eur. J. 2015, 21, 14401.
| Crossref | GoogleScholarGoogle Scholar | 26291466PubMed |
[25] W. Rao, D. Susanti, B. J. Ayers, P. W. H. Chan, J. Am. Chem. Soc. 2015, 137, 6350.
| Crossref | GoogleScholarGoogle Scholar | 25905645PubMed |
[26] J. Yan, G. L. Tay, C. Neo, B. R. Lee, P. W. H. Chan, Org. Lett. 2015, 17, 4176.
| Crossref | GoogleScholarGoogle Scholar | 26291118PubMed |
[27] W. Zi, H. Wu, F. D. Toste, J. Am. Chem. Soc. 2015, 137, 3225.
| Crossref | GoogleScholarGoogle Scholar | 25710515PubMed |
[28] W. Rao, M. J. Koh, D. Li, H. Hirao, P. W. H. Chan, J. Am. Chem. Soc. 2013, 135, 7926.
| Crossref | GoogleScholarGoogle Scholar | 23627597PubMed |
[29] T. Lauterbach, S. Gatzweiler, P. Nösel, M. Rudolph, F. Rominger, A. S. K. Hashmi, Adv. Synth. Catal. 2013, 355, 2481.
| Crossref | GoogleScholarGoogle Scholar |
[30] For selected examples of gold-catalysed heterocyclic synthesis, see refs [21], [26] and [31–46].
[31] X. Cheng, Z. Wang, C. D. Quintanilla, L. Zhang, J. Am. Chem. Soc. 2019, 141, 3787.
| Crossref | GoogleScholarGoogle Scholar | 30789268PubMed |
[32] M. Bao, X. Wang, L. Qiu, W. Hu, P. W. H. Chan, X. Xu, Org. Lett. 2019, 21, 1813.
| Crossref | GoogleScholarGoogle Scholar | 30840467PubMed |
[33] D. Allegue, J. González, S. Fernández, J. Santamaría, A. Ballesteros, Adv. Synth. Catal. 2019, 361, 758.
| Crossref | GoogleScholarGoogle Scholar |
[34] M. E. Muratore, A. I. Konovalov, H. Armengol-Relats, A. M. Echavarren, Chem. – Eur. J. 2018, 24, 15613.
| Crossref | GoogleScholarGoogle Scholar | 30066978PubMed |
[35] J. Zhao, W. Xu, X. Xie, N. Sun, X. Li, Y. Liu, Org. Lett. 2018, 20, 5461.
| Crossref | GoogleScholarGoogle Scholar | 30102048PubMed |
[36] J. Jin, Y. Zhao, E. M. L. Sze, P. Kothandaraman, P. W. H. Chan, Adv. Synth. Catal. 2018, 360, 4744.
| Crossref | GoogleScholarGoogle Scholar |
[37] Y.-C. Hsu, S.-A. Hsieh, P.-H. Li, R.-S. Liu, Chem. Commun. 2018, 2114.
| Crossref | GoogleScholarGoogle Scholar |
[38] X. Chen, J. T. Merrett, P. W. H. Chan, Org. Lett. 2018, 20, 1542.
| Crossref | GoogleScholarGoogle Scholar | 29481090PubMed |
[39] B. Zhang, T. Wang, Z. Zhang, J. Org. Chem. 2017, 82, 11644.
| Crossref | GoogleScholarGoogle Scholar | 28967246PubMed |
[40] W. Rao, Sally, S. N. Berry, P. W. H. Chan, Chem. – Eur. J. 2014, 20, 13174.
| Crossref | GoogleScholarGoogle Scholar | 25113644PubMed |
[41] W. Rao, P. W. H. Chan, Chem. – Eur. J. 2014, 20, 713.
| Crossref | GoogleScholarGoogle Scholar | 24323953PubMed |
[42] W. T. Teo, W. Rao, M. J. Koh, P. W. H. Chan, J. Org. Chem. 2013, 78, 7508.
| Crossref | GoogleScholarGoogle Scholar | 23883133PubMed |
[43] C. Gronnier, G. Boissonnat, F. Gagosz, Org. Lett. 2013, 15, 4234.
| Crossref | GoogleScholarGoogle Scholar | 23909764PubMed |
[44] W. Rao, M. J. Koh, P. Kothandaraman, P. W. H. Chan, J. Am. Chem. Soc. 2012, 134, 10811.
| Crossref | GoogleScholarGoogle Scholar | 22663059PubMed |
[45] A. S. K. Hashmi, M. Rudolph, H.-U. Siehl, M. Tanaka, J. W. Bats, W. Frey, Chem. – Eur. J. 2008, 14, 3703.
| Crossref | GoogleScholarGoogle Scholar |
[46] A. S. K. Hashmi, M. Wölfle, F. Ata, M. Hamzic, R. Salathé, W. Frey, Adv. Synth. Catal. 2006, 348, 2501.
| Crossref | GoogleScholarGoogle Scholar |
[47] For a review on the gold-catalysed chemistry of cyclopropenes, see ref. [9]. For selected examples, see refs [26], [28], [29], [41] and [48–54].
[48] N. A. Rajabi, M. J. Atashgah, R. BabaAhmadi, C. Hyland, A. Ariafard, J. Org. Chem. 2013, 78, 9553.
| Crossref | GoogleScholarGoogle Scholar | 23977881PubMed |
[49] P. C. Young, M. S. Hadfield, L. Arrowsmith, K. M. Macleod, R. J. Mudd, J. A. Jordan-Hore, A.-L. Lee, Org. Lett. 2012, 14, 898.
| Crossref | GoogleScholarGoogle Scholar | 22272604PubMed |
[50] M. S. Hadfield, A. L. Lee, Chem. Commun. 2011, 1333.
| Crossref | GoogleScholarGoogle Scholar |
[51] E. Seraya, E. Slack, A. Ariafard, B. F. Yates, C. J. T. Hyland, Org. Lett. 2010, 12, 4768.
| Crossref | GoogleScholarGoogle Scholar | 20873827PubMed |
[52] M. S. Hadfield, J. T. Bauer, P. E. Glen, A. L. Lee, Org. Biomol. Chem. 2010, 8, 4090.
| Crossref | GoogleScholarGoogle Scholar | 20623054PubMed |
[53] M. S. Hadfield, A. L. Lee, Org. Lett. 2010, 12, 484.
| Crossref | GoogleScholarGoogle Scholar | 20050603PubMed |
[54] J. T. Bauer, M. S. Hadfield, A. L. Lee, Chem. Commun. 2008, 6405.
| Crossref | GoogleScholarGoogle Scholar |
[55] For recent reviews on the chemistry of cyclopropenes, see [56–58].
[56] I. Marek, S. Simaan, A. Masarwa, Angew. Chem. Int. Ed. 2007, 46, 7364.
| Crossref | GoogleScholarGoogle Scholar |
[57] M. Rubin, M. Rubina, V. Gevorgyan, Chem. Rev. 2007, 107, 3117.
| Crossref | GoogleScholarGoogle Scholar | 17622181PubMed |
[58] M. Rubin, M. Rubina, V. Gevorgyan, Synthesis 2006, 1221.
[59] For precedence of water serving as the oxygen atom source, see refs [39], [45] and [46].