Sunlight and red to far-red ratio impact germination of tropical montane cloud forest species
Gemma L. Hoyle A * , Amelia V. Stevens
A * , Amelia V. Stevens  A , Lydia K. Guja
A , Lydia K. Guja  A B , Karen D. Sommerville
A B , Karen D. Sommerville  C , Stuart Worboys
C , Stuart Worboys  D and Darren M. Crayn D
D and Darren M. Crayn D
A National Seed Bank, Australian National Botanic Gardens, Parks Australia, Clunies Ross Street, Acton, ACT, Australia.
B Centre for Australian National Biodiversity Research, (a joint venture between Parks Australia and CSIRO), CSIRO, Clunies Ross Street, Acton, ACT, Australia.
C The Australian PlantBank, Botanic Gardens of Sydney, Narellan Road, Mount Annan, NSW, Australia.
D Australian Tropical Herbarium, James Cook University Nguma-Bada Campus, McGregor Road, Smithfield, Qld, Australia.
Australian Journal of Botany - https://doi.org/10.1071/BT22126
Submitted: 3 November 2022 Accepted: 8 March 2023 Published online: 20 April 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Australia’s tropical montane cloud forests (TMCF) exhibit exceptional species richness and endemism. Determinants of regeneration via seed of these species are next to unknown, limiting our ability to quantify and project their vulnerability to climate change. The ratio of red to far-red light (R:FR) has been shown to influence seed germination of many tropical species.
Aims: We investigated germination of six previously unstudied TMCF species in relation to the presence or absence of light (light/dark) and light quality (R:FR). We hypothesised that increased R:FR would lead to increased germination and that small-seeded species would be more likely to have a light requirement and be less sensitive to R:FR compared to larger-seeded species.
Methods: Sunlight and polyester filters were used to create a gradient of R:FR ranging from 0.1 to 1.14. Seeds were also sown in constant darkness.
Key results: Across species we saw varying germination responses. Three of the four smallest-seeded species exhibited an absolute light requirement for germination and did not discriminate between different R:FR. Germination of the small-seeded TMCF endemic Dracophyllum increased exponentially with increasing R:FR. Germination of the largest-seeded species was inhibited by both low and high R:FR, and germination was higher in constant darkness than diurnal light/dark. All six species were able to germinate at remarkably low R:FR values.
Conclusions: Light affects seed germination of Australia’s TMCF plant species in a variety of ways.
Implications: The findings of this study provide insights into plant recruitment in situ, and the acclimation potential of these species under reduced R:FR predicted for the future.
Keywords: climate change, cloud forest, light, R:FR, red to far-red ratio, seed ecology, seed germination, seed mass.
Introduction
In tropical montane systems worldwide, mid to upper elevations often harbour evergreen forests that experience persistent, frequent, or seasonal cloud at vegetation level (Bruijnzeel et al. 2011; Ray 2013). These ‘tropical montane cloud forests’ (TMCF) typically exhibit exceptional species richness and endemism (Martin and Bellingham 2016). Globally, tropical montane ecosystems are recognised as highly threatened by climate change (McJannet et al. 2008; Helmer et al. 2019; Karger et al. 2021). In TMCF, we can expect rises in temperature, reductions in cloudiness and increased drought periods leading to species extinction, altitudinal shifts in species’ ranges and TMCF community reshuffling (Costion et al. 2015; Elsen et al. 2022). Yet these extraordinary ecosystems remain under-documented, and their persistence under climate change insufficiently quantified, due to their complex terrain and isolation (Martin and Bellingham 2016). Plant regeneration from seed is critical for species persistence in the landscape under future climate scenarios (Walck et al. 2011). This is particularly the case in montane environments where an upward shift in distribution of plant species to cooler, wetter refuges may be the only option for survival (Pauli et al. 2012; Auld et al. 2022). The ability of seed-bearing TMCF plants to shift their distribution in response to climate change will depend on the ability of their seed to germinate under new environmental conditions in their current location and/or at higher altitudes. However, our understanding of the regeneration and distribution of tropical montane plant species (Baskin and Baskin 2014; Baskin et al. 2021), and particularly TMCF species (Martin and Bellingham 2016), is limited.
Australia’s TMCF occurs within the Wet Tropics Bioregion of northeast Queensland. We estimate that these complex, diverse TMCF systems contain ~930 seed-bearing plant species including ~70–80 endemics, all with a distributional range encompassing tropical montane rain forest at elevations of ≥900 m above sea level. Costion et al. (2015) modelled changes in the distribution and extent of suitable habitat for 19 endemic species and predicted a decline in area of suitable habitat of 81% by 2040, and 95% by 2080 (mean for all species). Roeble (2018) expanded on this work, modelling 37 species based on improved occurrence data from additional field surveys, and a mean habitat loss of 63% was predicted for all species by 2085. These projections are alarming. However, they must assume that the current niche occupied by each species is essential for its survival because very little is known about environmental factors determining the distribution of TMCF species, or their capacity to regenerate. Better understanding of each species’ current germination niche would improve our ability to quantify and project the vulnerability of TMCF flora to climate change, to inform modelling, conservation, management and future restoration (Christmann and Menor 2021).
The main factors controlling germination are temperature, moisture and light (Baskin and Baskin 2014). In TMCF, where rainfall is high throughout the year and there is little seasonal variation in temperature, light may be the main driver of germination. Light-sensitive species utilise light to indicate conditions suitable for germination (Pearson et al. 2003), with light controlling the induction, maintenance and release of seed dormancy via hormone metabolism and signalling (Yan and Chen 2020; Yang et al. 2020). In tropical montane regions, an absolute light requirement for germination or a preference for high light quantity has been reported for species in wet forest (Everham et al. 1996) and rain forest (Zang et al. 2008). ‘Smaller’ seeds (with seed mass <2 mg) of herbaceous and woody species from tropical habitats are more likely to require light for germination than ‘larger’ seeded species (Milberg et al. 2000; Pearson et al. 2002; Jankowska-Blaszczuk and Daws 2007; Aud and Ferraz 2012; Tiansawat and Dalling 2013). Restoration efforts in Costa Rican tropical montane forest reported low germination of small-seeded tree species due to the canopy reducing light (Sady et al. 2010).
Light/dark studies provide some information on the germination requirements of species, but vegetation does not consist of a binary patchwork of open sun and full shade (Lieberman et al. 1989). The spectral composition of light depends on the degree of filtering due to soil, canopy and cloud cover, geographical factors such as latitude and altitude, and time of day (Anderson 1964). At midday, unfiltered sunlight contains red light (600–700 nm; R) and far-red light (700–800 nm; FR) in a ratio of ~1.2. As light passes through a leaf canopy, more red light is absorbed relative to FR light and the R:FR decreases. As light passes through a secondary or under canopy, the R:FR is even lower, ~0.3 (Lee 1987). The R:FR can be as low as 0.1 beneath leaf litter layers on the ground (Vazquez-Yanes et al. 1990). Thus, R:FR varies continuously from deep shade to open sky conditions, providing a sensitive indicator of canopy vegetation and leaf litter conditions of the ground surface. In a typical phytochrome-mediated response, the increase in proportion of FR light below the canopy inhibits seed germination, while germination is promoted by light with a high R:FR in more open areas and below gaps in the canopy (Cone and Kendrick 1986). For example, Válio and Scarpa (2001) found that low R:FR inhibited germination of seven Brazilian tropical pioneer species. However, other studies report species-specific R:FR for maximum germination. For example, in tropical montane grassland of South America, some Vellozia species presented maximum germination at the greatest R:FR tested (0.77) while others reached maximum germination at the lowest R:FR tested (0.02 and 0.08) (Vieira et al. 2018). In Mexico, tropical rain forest species required high R:FR for germination while high altitude lava field species germinated at both low and high R:FR (Vàzquez-Yanes and Orozco-Segovia 1990). Seed size has also been linked to R:FR sensitivity. For 22/29 tropical taxa (mostly trees), there was a significant positive correlation between seed mass and R:FR50 (the R:FR at 50% of maximum germination; Tiansawat and Dalling 2013). In other words, smaller seeds (<2 mg) were able to germinate at low R:FR as well as high R:FR. Similarly, eight small-seeded species from tropical rain forest in Singapore germinated under a spectral composition similar to canopy shade (Metcalfe 1996), and small-seeded tropical species from Central America germinated under a wider range of R:FR values than larger-seeded species (Pearson et al. 2003). In contrast, small-seeded, light-dependent temperate taxa (herbaceous species) are likely to be more sensitive to R:FR, not less sensitive (Jankowska-Blaszczuk and Daws 2007; Tiansawat and Dalling 2013).
The ratio of R to FR light is particularly interesting in TMCF since far-red light is affected by water vapour in the atmosphere such that the R:FR increases as atmospheric water content increases (Górski 1976). In a temperate mountain cloud forest, Reinhardt et al. (2010) recorded 25–30% higher R:FR below the canopy on low cloud and cloud immersed days relative to sunny days. Cloud immersion may somewhat counteract the effect that canopy cover has on the R:FR. The Australian TMCF canopy is dense and closed. In the future, predicted reduction in atmospheric moisture and cloud immersion in TMCF (Helmer et al. 2019), may lead to a reduction in R:FR below the canopy. Despite this, we know of no previous studies examining the effect of light quality on germination of TMCF seed.
Here, we investigated the germination niche of six previously unstudied TMCF species in relation to absolute light requirement (light/dark) and light quality (R:FR). Previous studies on species from a range of habitats have investigated germination responses to R:FR using coloured bulbs or light filters (Steinbauer and Grigsby 1957; Toledo et al. 1990; Drake 1993; Válio and Scarpa 2001; Daws 2002; Steadman 2004; Jankowska-Blaszczuk and Daws 2007; Lund et al. 2007; Vieira et al. 2018). However, these studies may have limited application to the interpretation of responses of plants in the field since they used artificial light sources. We used sunlight to create a gradient of seven different R:FR reminiscent of that which seeds would experience in situ. We asked, do seeds of TMCF species require light for germination and do they differentiate between different R:FR? We hypothesised that increased R:FR would lead to increased germination and that small-seeded species would be more likely to have a light requirement and be less sensitive to R:FR compared to larger-seeded species. The ecological relevance of findings for the current germination niche and acclimation potential of each species under future light conditions are discussed.
Materials and methods
Seed collecting and processing
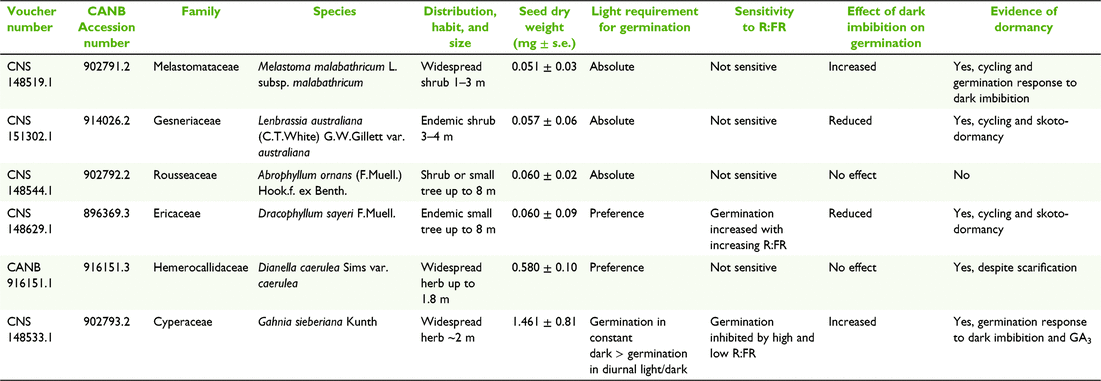
Mature fruits/seeds of Dracophyllum sayeri F.Muell. (Ericaceae), Melastoma malabathricum L. subsp. malabathricum (Melastomataceae), Abrophyllum ornans (F.Muell.) Hook.f. ex Benth. (Rousseaceae), Lenbrassia australiana (C.T.White) G.W.Gillett var. australiana (Gesneriaceae), Dianella caerulea Sims var. caerulea (Hemerocallidaceae) and Gahnia sieberiana Kunth (Cyperaceae) were collected between April 2019 and December 2020 on Mt Lewis (16°30′S, 145°16E) and Mt Bellenden Ker (17°15′S, 145°52′E) in the Wet Tropics Bioregion of northeast Queensland, Australia (Table 1; Fig. 1). The Wet Tropics Bioregion is extremely wet for much of the year: the weather station on Mt Bellenden Ker (1593 m) records mean annual rainfall of 7150 mm (Xu et al. 2014a). Most of the rain falls during the ‘wet’ season (typically November–April), however, vegetation strips the clouds of moisture during the ‘dry’ season (May–September) which evens out seasonality (McJannet et al. 2008). Mean maximum/minimum air temperatures over the period 1983–2012 were 22/16°C for summer (December, January, February) and 15/11°C for winter (June, July, August; Xu et al. 2014b, 2014c). The climate and vegetation of the Wet Tropics Bioregion are described in greater detail elsewhere (Jarvis and Mulligan 2010; Eller et al. 2020).
|
The study species will be referred to as Dracophyllum, Melastoma, Abrophyllum, Lenbrassia, Dianella and Gahnia from here on. These taxa were sampled due to the presence of multiple plants producing sufficient mature seed at the point of natural dispersal at the time of collection, and represent a range of growth forms including herbs, shrubs and trees (Zich et al. 2020; Table 1). Dracophyllum and Lenbrassia are endemic to TMCF in the Wet Tropics Bioregion and the other species are more widespread to the south/southeast.
For all but one species (Dianella), vouchers were lodged at the Australian Tropical Herbarium, Cairns (CNS) and duplicates provided to the Australian National Herbarium, Canberra (CANB). The Dianella collection voucher was only lodged at the Australian National Herbarium, Canberra (CANB; Table 1).
Fleshy fruited species (Abrophyllum, Melastoma, Lenbrassia and Dianella) were collected into polythene zip lock bags and stored for up to seven days in chilled, insulated boxes before being posted to the Australian PlantBank (Australian Botanic Gardens, Mount Annan, NSW) or the National Seed Bank (Australian National Botanic Gardens, Canberra, ACT). Dry fruited species (Dracophyllum and Gahnia) were stored and transported in paper bags. Abrophyllum, Melastoma, Dracophyllum and Gahnia were processed at the Australian PlantBank before being sent to the National Seed Bank. Lenbrassia and Dianella were processed at the National Seed Bank. Where necessary, dehiscent fruits were held at ambient laboratory conditions for up to two weeks to facilitate the release of seeds. Upon receipt at the National Seed Bank, seed collections were provided with CANB accession numbers linked to the herbarium vouchers (Table 1). Seeds of all species were confirmed to be orthodox and stored in a drying facility at approximately 15 ± 2°C and 15–20% relative humidity (RH) until the experiment began in February 2021.
Seed weight
Dry seed weight was determined for each species by drying seeds to 15% equilibrium RH, weighing three replicates of 50 seeds per species and calculating an average seed weight.
Experimental light gradient
In a temperature-controlled glasshouse in Canberra we created a gradient of seven R:FR ratios using sunlight (Górski and Górska 1979; Tiansawat and Dalling 2013) and coated polyester filters (Lee Filters, Media Vision, Sydney, Australia): 0.11 (filter #242), 0.2 (filter #121), 0.38 (filter #088), 0.54 (filter #244) 0.74 (filter #245), 0.91 (filter #246) and 1.14 (no filter). Using a hand-held spectrometer to measure light wavelengths (SpectraPen, Photon Systems Instruments, www.psi.cz) the R:FR ratio was calculated using the mean red wavelengths (657–664 nm) and the mean far-red wavelengths (726–734 nm). Total irradiance was also calculated. We also took light readings in various TMCF locations in the Wet Tropics Bioregion to help validate our experimental light gradient (Supplementary material, Table S1). The R:FR was highest at the edge of the canopy in direct sunlight (1.0), and lowest below the canopy and leaf litter (0.3). The R:FR was higher below the canopy on an overcast day (0.7) when compared to below the canopy on a sunny day (0.4). This was in line with the known effect of atmospheric moisture on the R:FR of sunlight (Górski 1976; Reinhardt et al. 2010). Total irradiance decreased as R:FR decreased, both in situ and in experimental conditions (Supplementary material, Table S1 and Fig. S1). The R:FR and total irradiance inside the glasshouse, across the entire gradient, were comparable to those measured directly outside the glasshouse, if a little lower (Fig. S1). Light readings were also taken hourly between 06:00 and 18:00 hours during a pilot study in September 2020 and were consistent over the day, except for some expected decrease at dusk (Fig. S2). To determine whether R:FR changed over the duration of the experiment, filtered and unfiltered light readings were taken in the glasshouse, hourly, between 09:00 and 15:00 hours in Week 1, Week 6 and Week 13 of the experiment. The R:FR of unfiltered and filtered light changed very little over the duration of the experiment (e.g. mean R:FR of unfiltered light at 12:00 hours in Weeks 1, 6 and 13 was 1.17, 1.29 and 1.23 respectively; Fig. S3). Supplementary material is available on request.
Effect of light on germination
Three replicates of 25 seeds per species per treatment (three replicates of 10 seeds of Gahnia) were sown into 5 or 9 cm diameter plastic Petri dishes containing 0.8–1.0% plain water-agar (Sigma lot # SLBH0519V) that had been autoclaved at 121°C for 30 min and brought to room temperature prior to use. There are numerous challenges associated with collecting seed in TMCF, therefore, the quantity of seeds used was determined by availability and minimised to balance seed use with ex situ conservation in seed banks. Gahnia required a scarification pre-treatment to assist with radicle emergence, prior to being sown onto agar containing 200 mg/L gibberellic Acid (GA3, Sigma-Aldrich, Sydney, Australia) to overcome what is likely to be physiological dormancy (G. Liyanage, pers. comm.). GA3 is a plant hormone known to promote germination of physiologically dormant seeds. The seed coat of Gahnia was chipped with a scalpel away from the embryonic axis. Scarified Gahnia seeds were also sown onto plain agar due to uncertainty about the interactive effect of GA3 and light (Bachelard 1967). Dianella seeds were also scarified to alleviate a potential physiological dormancy (Vening et al. 2017). Petri dishes of seeds were sealed using Parafilm (Thermoline Scientific, Sydney, NSW, Australia), to avoid agar desiccation, before being placed directly into an open-topped aluminium foil tray (325 mm × 270 mm × 95 mm) that was covered with a coated polyester filter and sealed with Sellotape. Dark treatment replicates were double wrapped with aluminium foil and control replicates had no filter Sellotaped over the tray. Three trays were created per light treatment and contained one replicate of each species. Treatments were arranged at random on a 265 cm × 80 cm steel bench in the glasshouse. i-Button data loggers (Thermochron DS1921G, Temperature Technology, Adelaide) were placed in trays along the length of the glasshouse bench to monitor temperature throughout the experiment. Temperatures in the trays in the glasshouse ranged from 22 to 35°C in the day (at 07:00–18:30 hours, mean 30.87 ± 2.6°C) to 14–19°C at night (at 18:31–06:59 hours, mean 16 ± 0.6°C). Final germination was scored at 10 weeks to prevent the transient exposure of seeds to light which might impact on germination. For all species, germination was defined as radicle emergence by >1 mm. At 10 weeks, all ungerminated seeds in the constant dark treatment were exposed to unfiltered sunlight for a further 4 weeks and final germination then scored. Average daylength was 12.12 ± 0.1 h during the first 10 weeks, and 10.34 ± 0.03 h during the final 4 weeks. Following termination of the experiment, all remaining intact seeds were dissected with a scalpel under a microscope. Seeds with a firm, intact endosperm and embryo were deemed viable and seeds empty of an embryo were deducted from the total when calculating mean final percent germination.
Data analysis
All statistical analyses were performed using R statistical software (ver. 4.0.2; R Core Team 2021). Final germination percentages ± light and ± dark imbibition were compared using a Pearson’s chi-squared test. To examine the effect that R:FR had on final germination we applied the Brain–Cousens model (Brain and Cousens 1989) using the ‘seedreg’ package in R. The Brain–Cousens model is a type of regression analysis that extends the log-logistic model to take into account inverse u-shaped response effects. The model is more commonly applied to seed temperature gradient studies, but we believe it can also be applied to light gradient analysis due to the similarities between the effect of temperature and light on germination.
Results
Seed mass
Species are listed from smallest to largest by seed mass in Table 1. Melastoma, Lenbrassia, Abrophyllum and Dracophyllum seed were all <0.07 mg. Dianella seeds were an order of magnitude larger at 0.58 mg and Gahnia seeds were the largest at 1.46 mg (Table 1; Fig. 1).
Light requirement for germination
Light was critical for germination of three of the four smallest-seeded species. Melastoma, Lenbrassia and Abrophyllum exhibited an absolute light requirement for germination i.e. zero germination in constant darkness compared to high germination in unfiltered light/dark (55, 89 and 94% respectively; P < 0.0001; Fig. 2a, b, c). Of the remaining three species, some germination occurred in dark. Germination of small-seeded Dracophyllum was significantly greater in unfiltered light/dark (91%) when compared to germination in constant darkness (19%; P < 0.0001; Fig. 2d). Germination of medium-seeded Dianella was greater in light/dark (43%) compared to constant darkness (17%), although not significantly (P = 0.058; Fig. 2e). Conversely, germination of the largest-seeded species, Gahnia, was significantly greater in the dark (60%) compared to light/dark (24.4%; P < 0.05; Fig. 2f). The reduced germination of Gahnia in the light was overcome by application of GA3 (Fig. 2f inset).
Effect of R:FR on germination
All six species exhibited at least 20% germination under all the red to far-red ratios investigated, however, we found variation in the sensitivity of species to light quality. Despite an absolute light requirement for germination, Melastoma, Lenbrassia and Abrophyllum exhibited little to no sensitivity to light quality (Fig. 3a, b, c). Similarly, R:FR had no effect on germination of Dianella (Fig. 3e). Germination of Dracophyllum increased exponentially with increasing R:FR (Fig. 3d), indicating a preference for high R:FR. Interestingly, germination of Gahnia was inhibited at both high and low R:FR (Fig. 3f), and this was overcome by GA3 (Fig. 3f inset).
|
|
Effect of dark imbibition on germination
The effect of dark imbibition on germination in the light was investigated. Following 10 weeks in the constant dark treatment, ungerminated seeds were exposed to diurnal natural light for a further 4 weeks. Germination of Melastoma was significantly greater in diurnal sunlight following dark imbibition when compared to control seeds (P < 0.05; Fig. 4a). Results suggest that 10 weeks in darkness alleviated dormancy of these seeds. In contrast, germination of Lenbrassia and Dracophyllum seeds was significantly lower following dark imbibition when compared to germination in constant light/dark (P < 0.0001; Fig. 4b, d). Dark imbibition had no effect on germination of Abrophyllum or Dianella (Fig. 4c, e). No further germination of the dark treatment Gahnia seeds occurred following exposure to light (Fig. 4f).
Discussion
This study aimed to investigate germination of six previously unstudied tropical montane cloud forest (TMCF) species in relation to the presence of light (light/dark) and light quality (R:FR). The climate niche of these species has been estimated (Costion et al. 2015), but the determinants of their distribution and regeneration are next to unknown. This limits our ability to quantify and project their vulnerability to climate change. Light is a main driver of seed germination in tropical rain forests and predicted changes in atmospheric moisture and cloud immersion in TMCF (Helmer et al. 2019) may lead to reductions in R:FR beneath the canopy (Górski 1976; Reinhardt et al. 2010). Across six species we saw varying responses to light/dark and R:FR suggesting divergent germination strategies. Three of the four smallest-seeded species (Melastoma, Lenbrassia and Abrophyllum), exhibited an absolute light requirement for germination and did not discriminate between different R:FR. This is in accordance with previous studies that concluded that small-seeded tropical species are more likely to require light for germination and germinate under a wider range of R:FR values, when compared to larger-seeded species (Milberg et al. 2000; Pearson et al. 2002, 2003; Jankowska-Blaszczuk and Daws 2007; Aud and Ferraz 2012; Tiansawat and Dalling 2013). A light response and seed mass may have coevolved to ensure that small-seeded species germinate when close to the soil surface to avoid seedlings being unable to reach the surface and initiate photosynthesis before energetic compounds stored in the seeds are exhausted (Milberg et al. 2000; Batlla and Benech-Arnold 2014). A light requirement for germination may also contribute to the persistence of seeds in the soil seed bank (Grime et al. 1981; Benvenuti 1995; Milberg et al. 2000). In situ, Melastoma, Lenbrassia and Abrophyllum may be adapted to germinate in response to disturbance events that expose buried seeds to light, rather than changes in R:FR brought about by canopy gaps. However, germination of small-seeded Dracophyllum increased exponentially with increasing R:FR, a response more typical of small-seeded temperate taxa (Jankowska-Blaszczuk and Daws 2007; Tiansawat and Dalling 2013). Results suggest that Dracophyllum is a gap-colonising species. This is consistent with unpublished data (due to A. Stevens, B. Lautgier, G. Hoyle, L. Guja, S. Worboys, D. Crayn, and K. Sommerville). These workers observed increased germination in response to increases in daily soil temperature, temperature amplitude and light, indicating that D. sayeri seeds likely take advantage of canopy gaps. Seeds held in dry storage for several months may lose some of their light requirement for germination (Baskin and Baskin 2014), which may explain why some Dracophyllum and Dianella germination was seen in the dark. As hypothesised, germination of the largest-seeded species, Gahnia, was inhibited by low R:FR (Válio and Scarpa 2001; Pearson et al. 2003; Tiansawat and Dalling 2013). Interestingly, germination of Gahnia was also inhibited by high R:FR. To our knowledge, an inverse u-shaped response to increasing R:FR, as seen for Gahnia, is unusual. We suggest that this species has the potential for fine scale differentiation of transient safe sites for germination. Evidence of changes in seed germinability in the light following 10 weeks of dark imbibition (Melastoma, Lenbrassia and Dracophyllum) suggests that some species may cycle in and out of dormancy when adequate germination conditions are not met. Varying germination responses to light/dark and R:FR may contribute to species coexistence by reducing interspecific competition. All six species were able to germinate at remarkably low R:FR values, suggesting acclimation potential to reductions in light quality under climate change.
Dracophyllum and Lenbrassia are endemic to Australian TMCF. Dracophyllum is a rare species that grows on exposed, rocky ridges in stunted rain forest (Zich et al. 2020). Consistent with adult plant occurrence, Dracophyllum showed an adaptation to germinate more in high R:FR and less under diffuse light in dense forest. Results suggest that Dracophyllum may have efficient phytochrome activation mechanisms that may act as an indicator of the degree of canopy opening, as described in (Vàzquez-Yanes and Orozco-Segovia 1990). Germination in open, vegetatively sparse areas may lead to increased risk of lethal seedling desiccation. However, Dracophyllum plants have tough, hard leaves that possess lots of sclerenchyma – an unusual plant trait in rain forest ecosystems and one that may support growth in sun-exposed sites. In the future, regeneration via seed of Dracophyllum may be inhibited by reduced R:FR linked to reduced cloud cover. On the other hand, germination of Dracophyllum is not restricted to microsites of high R:FR, suggesting acclimation potential to change.
Lenbrassia is an endemic understory plant found in well-developed rain forest (Zich et al. 2020). Consistent with this, results suggest Lenbrassia seeds germinate regardless of R:FR. Future changes in light quality are unlikely to impact on regeneration of this species, however, light quality may be an important factor in the establishment of these seedlings. Like Lenbrassia, Streptocarpus rexii (also Gesneriaceae), required light for germination and seedlings under red light did not develop normally (Nishii et al. 2012). Investigation into the effect of R:FR on Dracophyllum and Lenbrassia seedling establishment would help us to understand how climate change will affect regeneration of these endemic species more fully.
We saw reduced germination of Dracophyllum and Lenbrassia in diurnal light/dark following 10 weeks of dark imbibition. This change in germinability may be explained by dormancy imposed by prolonged imbibition in the dark, otherwise known as skoto-dormancy (Thanos and Georghiou 1988; Koutsovoulou and Thanos 2010). Post dispersal, seeds continually adjust their dormancy status in response to a range of environmental signals (Finch-Savage and Footitt 2017), and an alleviation and re-induction of dormancy constitutes an important ecological process known as dormancy cycling (Footitt et al. 2013). If Dracophyllum and Lenbrassia seeds do not receive light for germination (along with other requirements such as adequate moisture and temperatures), they may postpone germination by becoming even more dormant. Dormancy cycling is thought to bet-hedge against events that trigger germination but are insufficient for plant maturation (Walck et al. 2011) and may explain changes in Dracophyllum and Lenbrassia seed germinability in the dark. Current bet-hedging mechanisms may be compromised in the future by increased seed germinability (Ooi et al. 2009). Continuous FR light reversed the skoto-dormancy of Lactuca sativa seeds (Thanos and Georghiou 1988) and could help to confirm dark-induced dormancy of Dracophyllum and Lenbrassia. Alternatively, reduced germination following dark imbibition may be a result of seed deterioration over time and/or inadequate time to reach final germination.
While occurring in TMCF, Melastoma, Abrophyllum, Dianella and Gahnia also have wider southern and/or eastern distributions (Zich et al. 2020) and may exhibit germination strategies that are not specific to TMCF. Abrophyllum grows as an understory tree in well-developed rain forest but more frequently as a regrowth species in disturbed areas (Zich et al. 2020). Melastoma plants also grow in disturbed areas including roadsides (Zich et al. 2020). Adult plant occurrence in developed rain forest and disturbed areas corresponds with our seed germination data, which demonstrate a light requirement and ability to germinate at low R:FR values. M. malabathricum seeds collected from tropical rain forest in Singapore germinated only in unfiltered sunlight and not beneath ‘a green polyester filter that filtered out red and blue wavelengths’ (Metcalfe 1996). These results contrast with our data and suggest variation between populations of the same species. One theory is that low survival of small-seeded species due to fungal pathogens (Pearson et al. 2002) may have imposed selection for a less discriminating germination physiology (Pearson et al. 2003). Alternatively, the soil surface in partially shaded microsites of low R:FR retains more moisture than soil below canopy gaps, creating lower risk of lethal desiccation for seedlings, particularly of small-seeded species (Jankowska-Blaszczuk and Daws 2007). Regardless of the ecological significance, a lack of sensitivity to R:FR may benefit species distribution and regeneration in the future by spreading germination across a wide range of microsites.
Seed dormancy is a means of spreading germination over time. We saw almost 20% more germination of Melastoma seeds in diurnal light/dark following 10 weeks of dark imbibition when compared to control seeds. This was despite only 4 weeks of sunlight and no prior germination. Results suggest that a fraction of the Melastoma seeds possessed dormancy that was alleviated by dark imbibition. In situ, some Melastoma seeds may postpone germination regardless of light conditions, until a time when other environmental factors have proven consistent for seedling growth and survival. Dianella seeds were scarified to alleviate a potential physiological dormancy as per Vening et al. (2017). However, germination remained <50% suggesting some remaining dormancy. Future studies might explore increasing evidence that warm stratification treatments promote germination of Dianella species (Wolkis et al. 2018; Hodges et al. 2019).
In contrast to the other species, germination of Gahnia seeds was inhibited by both high and low R:FR, and germination was greater in constant darkness than diurnal light/dark. Relatively few species are known to germinate to higher percentages in darkness than in light (Baskin and Baskin 2014). Gahnia is a tall, tufted, broad-leaved sedge that typically occurs in disturbed areas of well-developed rain forest, on a variety of sites (Zich et al. 2020). Although the plant can be found in sunny sites, results indicate that germination is more likely to occur in the dark or moderate shade. Gahnia seed that is dispersed short distances and remains beneath adult plants would have very little light. Germination in the shade of adult plants may be a seedling survival advantage that enables Gahnia seedlings to establish in protected sites with reduced risk of desiccation. Light-inhibited germination of Gahnia was overcome by GA3, providing further evidence that Gahnia has a germination strategy that involves physiological dormancy. Dormancy benefits seed persistence in the soil since dormant seeds typically take longer to germinate than non-dormant seeds (Long et al. 2015). Since light inhibited germination of Gahnia, and germination shows sensitivity to R:FR, this species may use the spectral composition of light to sense optimal depth for germination in the soil profile (Woolley and Stoller 1978; Benvenuti 1995; Batlla and Benech-Arnold 2014), i.e. not too deep, not too shallow. Persistence in the soil seed bank of larger-seeded TMCF species such as Gahnia warrant investigation because it may help explain the greater discrimination of R:FR that stimulates germination in larger-seeded tropical taxa (Tiansawat and Dalling 2013). For Gahnia, there may be no match between adult plant occurrence and the germination niche in terms of their tolerance to abiotic factors. Quantifying environmental niches in different phases of the life cycle of TMCF plants may provide important insights into community assembly processes (Del Vecchio et al. 2020).
In using sunlight to create a R:FR gradient we were able to avoid complications associated with artificial light sources including reduced/limited spectral content of light-emitting diode (LED) bulbs and cool white fluorescent tubes (Toole 1963; Lund et al. 2007; Vieira et al. 2018), and additional heat created by tungsten and incandescent bulbs (Steinbauer and Grigsby 1957; Toledo et al. 1990; Válio and Scarpa 2001; Vieira et al. 2018). Our experimental light gradient was comparable to that which we collected in situ where seed collections were made, and our data suggests that light quality is unlikely to change significantly during a typical 10–14 week study. There is an annual cycle in R:FR of sunlight and, in central Europe, the ratio is at its maximum in spring and minimum in late summer and autumn (Górski 1980). It is possible that these seasonality effects are less pronounced closer to the equator. However, for the study of species that exhibit a distinct spring or autumn germination, we would suggest running experiments that use sunlight to coincide with the season when germination is expected to occur in situ. Simultaneously recording cloud cover would aid interpretation of light readings. The R:FR of sunlight drops at twilight (Holmes and Smith 1977), and this is important for plant growth (Lund et al. 2007) and possibly also seed germination. Comparative studies of the effect of R:FR on seed germination using both sunlight and artificial light sources are warranted.
With this study we have begun to address the deficit of seed ecology knowledge for Australian TMCF plant species. All six species showed acclimation potential to changes in light quality under climate change. One potential explanation is that TMCF flora pre-date previous changes in climate in the geological timescale. In Hawaiian TMCF, species composition has been relatively stable over the past seven millennia and shows resilience to climate-driven disturbances (Crausbay et al. 2014). However, germination response to light is expected to be correlated with other environmental factors (Pons 2000; Pearson et al. 2002; Aud and Ferraz 2012). Next steps are to investigate interactive effects of light, temperature and soil moisture on germination, in current scenarios and in the context of climate change. Species such as Gahnia and Dianella, that also occur at sea level and as far south as Tasmania, provide the opportunity to investigate whether light requirements and sensitivity to R:FR vary along latitudinal and elevational gradients. Such studies are needed to support conclusions regarding acclimation potential (e.g. Hoyle et al. 2014; Cochrane et al. 2015). This study raises questions regarding seed persistence in the soil. Tropical rain forest species are renowned for having short-lived soil seed banks (Vazquez-Yanes and Orozco-Segovia 1993; Sautu et al. 2006), but this is on the assumption that most rain forest species produce desiccation sensitive seeds (Tweddle et al. 2003). Unpublished data (G. Hoyle, K. Sommerville, G. Liyanage, S. Worboys, L. Guja, A. Stevens, and D. Crayn) suggests that many of the shrub and herb species in Australian TMCF, as well as at least half of the tree species, will be desiccation tolerant. Soil seed banks have been reported in tropical montane forest in Mexico (Williams-Linera 1993; Alvarez-Aquino et al. 2005) and China (Zang et al. 2008), but whether they were transient or persistent was not addressed. Soil seed bank persistence in Australian TMCF species warrants further investigation. Following on from germination, investigation into the impact of light quality on TMCF seedling establishment and growth is warranted. It is likely that light quality impacts different developmental stages of some TMCF species. For example, tropical ‘pioneer’ species are understood to persist as small seedlings for a long time before taking advantage of canopy gaps and growing rapidly (Swaine and Whitmore 1988), which may be in response to changes in R:FR. In addition, cloud cover has been shown to impact photosynthesis and plant growth in understory species (Johnson and Smith 2006), which may also be linked to R:FR. The use of sunlight and coated polyester filters to create a gradient of R:FR is an effective, ecologically valid and economical approach to investigating these questions. Increased knowledge of the determinants of species regeneration via seed would facilitate more accurate predictive modelling of species distribution under climate change, inform target species for conservation and assist those involved in TMCF management and restoration of these highly diverse environments.
Supplementary material
Supplementary material is available online.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
This study is part of the Tropical Mountaintop Plant Science project (2019–2023) funded by the Ian Potter Foundation. The Ian Potter Foundation had no role in the design, conduct or publication of this review or associated research.
Acknowledgements
We acknowledge the Traditional Custodians of the land on which the seed for study was collected, the Yirrganydji, Ma:Mu, Muluridji, Western Yalanji and Eastern Kuku Yalanji Peoples, and the land on which this research was conducted, the Ngunnawal and Ngambri People. Thank you to staff and volunteers at the National Seed Bank for their help in collecting and processing seed. Thanks to Rhys Tooth for statistical support, Dr Richard Poire-Lassus at the Australian Plant Phenomics Facility for loan of the SpectraPen, John Fitz Gerald for capturing seed images, Julie Percival and Mitchell Korda for collecting light data in situ and Nursery staff at the Australian National Botanic Gardens for glasshouse space and assistance.
References
Alvarez-Aquino C, Williams-Linera G, Newton AC (2005) Disturbance effects on the seed bank of Mexican cloud forest fragments. Biotropica 37, 337–342.| Disturbance effects on the seed bank of Mexican cloud forest fragments.Crossref | GoogleScholarGoogle Scholar |
Aud FF, Ferraz IDK (2012) Seed size influence on germination responses to light and temperature of seven pioneer tree species from the Central Amazon. Anais da Academia Brasileira de Ciências 84, 759–766.
| Seed size influence on germination responses to light and temperature of seven pioneer tree species from the Central Amazon.Crossref | GoogleScholarGoogle Scholar |
Anderson MC (1964) Light relations of terrestrial plant communities and their measurement. Biological Reviews 39, 425–481.
| Light relations of terrestrial plant communities and their measurement.Crossref | GoogleScholarGoogle Scholar |
Auld J, Everingham SE, Hemmings FA, Moles AT (2022) Alpine plants are on the move: quantifying distribution shifts of Australian alpine plants through time. Diversity and Distributions 28, 943–955.
| Alpine plants are on the move: quantifying distribution shifts of Australian alpine plants through time.Crossref | GoogleScholarGoogle Scholar |
Bachelard EP (1967) Effects of gibberellic acid, kinetin, and light on the germination of dormant seeds of some eucalypt species. Australian Journal of Botany 15, 393–401.
| Effects of gibberellic acid, kinetin, and light on the germination of dormant seeds of some eucalypt species.Crossref | GoogleScholarGoogle Scholar |
Baskin CC, Baskin JM (2014) ‘Seeds: ecology, biogeography and evolution of dormancy and germination.’ 2nd edn. (Academic Press: San Diego, CA, USA)
Baskin CC, Baskin JM, Yoshinaga A, Wolkis D (2021) Physiological dormancy in seeds of tropical montane woody species in Hawaii. Plant Species Biology 36, 60–71.
| Physiological dormancy in seeds of tropical montane woody species in Hawaii.Crossref | GoogleScholarGoogle Scholar |
Batlla D, Benech-Arnold RL (2014) Weed seed germination and the light environment: implications for weed management. Weed Biology and Management 14, 77–87.
| Weed seed germination and the light environment: implications for weed management.Crossref | GoogleScholarGoogle Scholar |
Benvenuti S (1995) Soil light penetration and dormancy of Jimsonweed (Datura stramonium) seeds. Weed Science 43, 389–393.
| Soil light penetration and dormancy of Jimsonweed (Datura stramonium) seeds.Crossref | GoogleScholarGoogle Scholar |
Brain P, Cousens R (1989) An equation to describe dose responses where there is stimulation of growth at low doses. Weed Research 29, 93–96.
| An equation to describe dose responses where there is stimulation of growth at low doses.Crossref | GoogleScholarGoogle Scholar |
Bruijnzeel LA, Scatena FN, Hamilton LS (2011) ‘Tropical montane cloud forests: science for conservation and management.’ (Cambridge University Press: Cambridge, UK)
Christmann T, Menor IO (2021) A synthesis and future research directions for tropical mountain ecosystem restoration. Scientific Reports 11, 23948
| A synthesis and future research directions for tropical mountain ecosystem restoration.Crossref | GoogleScholarGoogle Scholar |
Cochrane A, Yates CJ, Hoyle GL, Nicotra AB (2015) Will among-population variation in seed traits improve the chance of species persistence under climate change? Global Ecology and Biogeography 24, 12–24.
| Will among-population variation in seed traits improve the chance of species persistence under climate change?Crossref | GoogleScholarGoogle Scholar |
Cone JW, Kendrick RE (1986) Photocontrol of seed germination. In ‘Photomorphogenesis in plants’. (Eds RE Kendrick, GHM Kronenberg) pp. 443–465. (Springer)
Costion CM, Simpson L, Pert PL, Carlsen MM, John Kress W, Crayn D (2015) Will tropical mountaintop plant species survive climate change? Identifying key knowledge gaps using species distribution modelling in Australia. Biological Conservation 191, 322–330.
| Will tropical mountaintop plant species survive climate change? Identifying key knowledge gaps using species distribution modelling in Australia.Crossref | GoogleScholarGoogle Scholar |
Crausbay S, Genderjahn S, Hotchkiss S, Sachse D, Kahmen A, Arndt SK (2014) Vegetation dynamics at the upper reaches of a tropical montane forest are driven by disturbance over the past 7300 years. Arctic, Antarctic, and Alpine Research 46, 787–799.
| Vegetation dynamics at the upper reaches of a tropical montane forest are driven by disturbance over the past 7300 years.Crossref | GoogleScholarGoogle Scholar |
Daws M (2002) Mechanisms of plant species coexistence in a semi-deciduous tropical forest in Panamá. PhD Thesis, University of Aberdeen, U160354. ProQuest Dissertations Publishing, UK.
Del Vecchio S, Fantinato E, Roscini M, Acosta ATR, Bacchetta G, Buffa G (2020) The germination niche of coastal dune species as related to their occurrence along a sea–inland gradient. Journal of Vegetation Science 31, 1112–1121.
| The germination niche of coastal dune species as related to their occurrence along a sea–inland gradient.Crossref | GoogleScholarGoogle Scholar |
Drake DR (1993) Germination requirements of Metrosideros polymorpha, the dominant tree of Hawaiian lava flows and rain forests. Biotropica 25, 461–467.
| Germination requirements of Metrosideros polymorpha, the dominant tree of Hawaiian lava flows and rain forests.Crossref | GoogleScholarGoogle Scholar |
Eller CB, Meireles LD, Sitch S, Burgess SSO, Oliveira RS (2020) How climate shapes the functioning of tropical montane cloud forests. Current Forestry Reports 6, 97–114.
| How climate shapes the functioning of tropical montane cloud forests.Crossref | GoogleScholarGoogle Scholar |
Elsen PR, Saxon EC, Simmons BA, Ward M, Williams BA, Grantham HS, Kark S, Levin N, Perez-Hammerle K-V, Reside AE, Watson JEM (2022) Accelerated shifts in terrestrial life zones under rapid climate change. Global Change Biology 28, 918–935.
| Accelerated shifts in terrestrial life zones under rapid climate change.Crossref | GoogleScholarGoogle Scholar |
Everham EM, Myster RW, VanDeGenachte E (1996) Effects of light, moisture, temperature, and litter on the regeneration of five tree species in the tropical montane wet forest of Puerto Rico. American Journal of Botany 83, 1063–1068.
| Effects of light, moisture, temperature, and litter on the regeneration of five tree species in the tropical montane wet forest of Puerto Rico.Crossref | GoogleScholarGoogle Scholar |
Finch-Savage WE, Footitt S (2017) Seed dormancy cycling and the regulation of dormancy mechanisms to time germination in variable field environments. Journal of Experimental Botany 68, 843–856.
| Seed dormancy cycling and the regulation of dormancy mechanisms to time germination in variable field environments.Crossref | GoogleScholarGoogle Scholar |
Footitt S, Huang Z, Clay HA, Mead A, Finch-Savage WE (2013) Temperature, light and nitrate sensing coordinate Arabidopsis seed dormancy cycling, resulting in winter and summer annual phenotypes. The Plant Journal 74, 1003–1015.
| Temperature, light and nitrate sensing coordinate Arabidopsis seed dormancy cycling, resulting in winter and summer annual phenotypes.Crossref | GoogleScholarGoogle Scholar |
Grime JP, Mason G, Curtis AV, Rodman J, Band SR (1981) A comparative study of germination characteristics in a local flora. The Journal of Ecology 69, 1017–1059.
| A comparative study of germination characteristics in a local flora.Crossref | GoogleScholarGoogle Scholar |
Górski T (1976) Red and far red radiation at sunset. Naturwissenschaften 63, 530–531.
Górski T (1980) Annual cycle of the red and far red radiation. International Journal of Biometeorology 24, 361–365.
| Annual cycle of the red and far red radiation.Crossref | GoogleScholarGoogle Scholar |
Górski T, Górska K (1979) Inhibitory effects of full daylight on the germination of Lactuca sativa L. Planta 144, 121–124.
| Inhibitory effects of full daylight on the germination of Lactuca sativa L.Crossref | GoogleScholarGoogle Scholar |
Helmer EH, Gerson EA, Baggett LS, Bird BJ, Ruzycki TS, Voggesser SM (2019) Neotropical cloud forests and páramo to contract and dry from declines in cloud immersion and frost. PLoS ONE 14, e0213155
| Neotropical cloud forests and páramo to contract and dry from declines in cloud immersion and frost.Crossref | GoogleScholarGoogle Scholar |
Hodges JA, Price JN, Nimmo DG, Guja LK (2019) Evidence for direct effects of fire-cues on germination of some perennial forbs common in grassy ecosystems. Austral Ecology 44, 1271–1284.
| Evidence for direct effects of fire-cues on germination of some perennial forbs common in grassy ecosystems.Crossref | GoogleScholarGoogle Scholar |
Holmes MG, Smith H (1977) The function of phytochrome in the natural environment–I. Characterization of daylight for studies in photomorphogenesis and photoperiodism. Photochemistry and Photobiology 25, 533–538.
| The function of phytochrome in the natural environment–I. Characterization of daylight for studies in photomorphogenesis and photoperiodism.Crossref | GoogleScholarGoogle Scholar |
Hoyle GL, Cordiner H, Good RB, Nicotra AB (2014) Effects of reduced winter duration on seed dormancy and germination in six populations of the alpine herb Aciphyllya glacialis (Apiaceae). Conservation Physiology 2, cou015
| Effects of reduced winter duration on seed dormancy and germination in six populations of the alpine herb Aciphyllya glacialis (Apiaceae).Crossref | GoogleScholarGoogle Scholar |
Jankowska-Blaszczuk M, Daws MI (2007) Impact of red:far red ratios on germination of temperate forest herbs in relation to shade tolerance, seed mass and persistence in the soil. Functional Ecology 21, 1055–1062.
| Impact of red:far red ratios on germination of temperate forest herbs in relation to shade tolerance, seed mass and persistence in the soil.Crossref | GoogleScholarGoogle Scholar |
Jarvis A, Mulligan M (2010) Chapter 3: The climate of cloud forests. In ‘Tropical montane cloud forests: science for conservation and management’. (Eds LA Bruijnzeel, FN Scatena, LS Hamilton) pp. 39–56. (Cambridge University Press: Cambridge, UK)
Johnson DM, Smith WK (2006) Low clouds and cloud immersion enhance photosynthesis in understory species of a southern Appalachian spruce–fir forest (USA). American Journal of Botany 93, 1625–1632.
| Low clouds and cloud immersion enhance photosynthesis in understory species of a southern Appalachian spruce–fir forest (USA).Crossref | GoogleScholarGoogle Scholar |
Karger DN, Kessler M, Lehnert M, Jetz W (2021) Limited protection and ongoing loss of tropical cloud forest biodiversity and ecosystems worldwide. Nature Ecology & Evolution 5, 854–862.
| Limited protection and ongoing loss of tropical cloud forest biodiversity and ecosystems worldwide.Crossref | GoogleScholarGoogle Scholar |
Koutsovoulou K, Thanos CA (2010) Light requirement and skotodormancy in Campanulaceae an ecophysiological approach. In ‘Proceedings of the seed ecology III conference’, Salt Lake City, Utah, USA. (Eds R Pendleton, S Meyer, B Schultz) pp. 98–99.
Lee DW (1987) The spectral distribution of radiation in two neotropical rainforests. Biotropica 19, 161–166.
| The spectral distribution of radiation in two neotropical rainforests.Crossref | GoogleScholarGoogle Scholar |
Lieberman M, Lieberman D, Peralta R (1989) Forests are not just Swiss cheese: canopy stereogeometry of non-gaps in tropical forests. Ecology 70, 550–552.
| Forests are not just Swiss cheese: canopy stereogeometry of non-gaps in tropical forests.Crossref | GoogleScholarGoogle Scholar |
Long RL, Gorecki MJ, Renton M, Scott JK, Colville L, Goggin DE, Commander LE, Westcott DA, Cherry H, Finch-Savage WE (2015) The ecophysiology of seed persistence: a mechanistic view of the journey to germination or demise. Biological Reviews 90, 31–59.
| The ecophysiology of seed persistence: a mechanistic view of the journey to germination or demise.Crossref | GoogleScholarGoogle Scholar |
Lund JB, Blom TJ, Aaslyng JM (2007) End-of-day lighting with different red/far-red ratios using light-emitting diodes affects plant growth of Chrysanthemum × morifolium Ramat. ‘Coral Charm’. HortScience 42, 1609–1611.
| End-of-day lighting with different red/far-red ratios using light-emitting diodes affects plant growth of Chrysanthemum × morifolium Ramat. ‘Coral Charm’.Crossref | GoogleScholarGoogle Scholar |
Martin PH, Bellingham PJ (2016) Towards integrated ecological research in tropical montane cloud forests. Journal of Tropical Ecology 32, 345–354.
| Towards integrated ecological research in tropical montane cloud forests.Crossref | GoogleScholarGoogle Scholar |
McJannet D, Wallace J, Fitch P, Disher M, Reddell P (2008) Hydrological processes in the tropical rainforests of Australia. In ‘Living in a dynamic tropical forest landscape’. (Eds NE Stork, SM Turton) pp. 197–209. (Blackwell Publishing: Melbourne, Vic.)
Metcalfe DJ (1996) Germination of small-seeded tropical rain forest plants exposed to different spectral compositions. Canadian Journal of Botany 74, 516–520.
| Germination of small-seeded tropical rain forest plants exposed to different spectral compositions.Crossref | GoogleScholarGoogle Scholar |
Milberg P, Andersson L, Thompson K (2000) Large-seeded spices are less dependent on light for germination than small-seeded ones. Seed Science Research 10, 99–104.
| Large-seeded spices are less dependent on light for germination than small-seeded ones.Crossref | GoogleScholarGoogle Scholar |
Nishii K, Nagata T, Wang C-N, Möller M (2012) Light as environmental regulator for germination and macrocotyledon development in Streptocarpus rexii (Gesneriaceae). South African Journal of Botany 81, 50–60.
| Light as environmental regulator for germination and macrocotyledon development in Streptocarpus rexii (Gesneriaceae).Crossref | GoogleScholarGoogle Scholar |
Ooi MKJ, Auld TD, Denham AJ (2009) Climate change and bet-hedging: interactions between increased soil temperatures and seed bank persistence. Global Change Biology 15, 2375–2386.
| Climate change and bet-hedging: interactions between increased soil temperatures and seed bank persistence.Crossref | GoogleScholarGoogle Scholar |
Pauli H, Gottfried M, Dullinger S, Abdaladze O, Akhalkatsi M, Alonso JLB, et al. (2012) Recent plant diversity changes on Europe’s mountain summits. Science 336, 353–355.
| Recent plant diversity changes on Europe’s mountain summits.Crossref | GoogleScholarGoogle Scholar |
Pearson TRH, Burslem DFRP, Mullins CE, Dalling JW (2002) Germination ecology of neotropical pioneers: interacting effects of environmental conditions and seed size. Ecology 83, 2798–2807.
| Germination ecology of neotropical pioneers: interacting effects of environmental conditions and seed size.Crossref | GoogleScholarGoogle Scholar |
Pearson TRH, Burslem DFRP, Mullins CE, Dalling JW (2003) Functional significance of photoblastic germination in neotropical pioneer trees: a seed’s eye view. Functional Ecology 17, 394–402.
| Functional significance of photoblastic germination in neotropical pioneer trees: a seed’s eye view.Crossref | GoogleScholarGoogle Scholar |
Pons TL (2000) Seed responses to light. In ‘Seeds: the ecology of regeneration in plant communities’. (Ed. M. Fenner) pp. 237–260. (CABI)
R Core Team (2021) ‘R: a language and environment for statistical computing.’ (R Foundation for Statistical Computing: Vienna, Austria) Available at https://www.R-project.org/ [Accessed 1 November 2021]
Ray DK (2013) Chapter 5.05: Tropical mountain cloud forests. In ‘Climate vulnerability: understanding and addressing threats to essential resources’. (Ed. RA Pielke) (Academic Press: Oxford, UK)
Reinhardt K, Smith WK, Carter GA (2010) Clouds and cloud immersion alter photosynthetic light quality in a temperate mountain cloud forest. Botany 88, 462–470.
| Clouds and cloud immersion alter photosynthetic light quality in a temperate mountain cloud forest.Crossref | GoogleScholarGoogle Scholar |
Roeble E (2018) Modelling the vulnerability of endemic montane flora to climate change in the Australian Wet Tropics. MSc Thesis, Imperial College London, UK.
Sady GC, Holl KD, Cole RJ, Zahawi RA (2010) Germination and survival of tree seeds in a tropical montane forest restoration study (Costa Rica). Ecological Restoration 28, 121–124.
| Germination and survival of tree seeds in a tropical montane forest restoration study (Costa Rica).Crossref | GoogleScholarGoogle Scholar |
Sautu A, Baskin JM, Baskin CC, Condit R (2006) Studies on the seed biology of 100 native species of trees in a seasonal moist tropical forest, Panama, Central America. Forest Ecology and Management 234, 245–263.
| Studies on the seed biology of 100 native species of trees in a seasonal moist tropical forest, Panama, Central America.Crossref | GoogleScholarGoogle Scholar |
Steadman KJ (2004) Dormancy release during hydrated storage in Lolium rigidum seeds is dependent on temperature, light quality, and hydration status. Journal of Experimental Botany 55, 929–937.
| Dormancy release during hydrated storage in Lolium rigidum seeds is dependent on temperature, light quality, and hydration status.Crossref | GoogleScholarGoogle Scholar |
Steinbauer GP, Grigsby B (1957) Interaction of temperature, light, and moistening agent in the germination of weed seeds. Weeds 5, 175–182.
| Interaction of temperature, light, and moistening agent in the germination of weed seeds.Crossref | GoogleScholarGoogle Scholar |
Swaine MD, Whitmore TC (1988) On the definition of ecological species groups in tropical rain forests. Vegetatio 75, 81–86.
| On the definition of ecological species groups in tropical rain forests.Crossref | GoogleScholarGoogle Scholar |
Thanos CA, Georghiou K (1988) On the mechanism of skotodormancy induction in Grand Rapids lettuce (Lactuca sativa L.) seeds. Journal of Plant Physiology 133, 580–584.
| On the mechanism of skotodormancy induction in Grand Rapids lettuce (Lactuca sativa L.) seeds.Crossref | GoogleScholarGoogle Scholar |
Tiansawat P, Dalling JW (2013) Differential seed germination responses to the ratio of red to far-red light in temperate and tropical species. Plant Ecology 214, 751–764.
| Differential seed germination responses to the ratio of red to far-red light in temperate and tropical species.Crossref | GoogleScholarGoogle Scholar |
Toledo JR, Rincón E, Vázquez-Yanes C (1990) A light quality gradient for the study of red:far red ratios on seed germination. Seed Science and Technology 18, 277–282.
Toole VK (1963) Light control of seed germination. Proceedings of the Association of Official Seed Analysts. JSTOR 53, 124–143.
Tweddle JC, Dickie JB, Baskin CC, Baskin JM (2003) Ecological aspects of seed desiccation sensitivity. Journal of Ecology 91, 294–304.
| Ecological aspects of seed desiccation sensitivity.Crossref | GoogleScholarGoogle Scholar |
Válio IFM, Scarpa FM (2001) Germination of seeds of tropical pioneer species under controlled and natural conditions. Brazilian Journal of Botany 24, 79–84.
| Germination of seeds of tropical pioneer species under controlled and natural conditions.Crossref | GoogleScholarGoogle Scholar |
Vazquez-Yanes C, Orozco-Segovia A (1993) Patterns of seed longevity and germination in the tropical forest. Annual Review of Ecology and Systematics 24, 69–87.
Vàzquez-Yanes C, Orozco-Segovia A (1990) Ecological significance of light controlled seed germination in two contrasting tropical habitats. Oecologia 83, 171–175.
| Ecological significance of light controlled seed germination in two contrasting tropical habitats.Crossref | GoogleScholarGoogle Scholar |
Vazquez-Yanes C, Orozco-Segovia A, Rincon E, Sanchez-Coronado ME, Huante P, Toledo JR, Barradas VL (1990) Light beneath the litter in a tropical forest: effect on seed germination. Ecology 71, 1952–1958.
| Light beneath the litter in a tropical forest: effect on seed germination.Crossref | GoogleScholarGoogle Scholar |
Vening GS, Guja LK, Spooner PG, Price JN (2017) Seed dormancy and germination of three grassy woodland forbs required for diverse restoration. Australian Journal of Botany 65, 625–637.
| Seed dormancy and germination of three grassy woodland forbs required for diverse restoration.Crossref | GoogleScholarGoogle Scholar |
Vieira BC, Rodrigues BMA, Garcia QS (2018) Light exposure time and light quality on seed germination of Vellozia species (Velloziaceae) from Brazilian campo rupestre. Flora 238, 94–101.
| Light exposure time and light quality on seed germination of Vellozia species (Velloziaceae) from Brazilian campo rupestre.Crossref | GoogleScholarGoogle Scholar |
Walck JL, Hidayati SN, Dixon KW, Thompson K, Poschlod P (2011) Climate change and plant regeneration from seed. Global Change Biology 17, 2145–2161.
| Climate change and plant regeneration from seed.Crossref | GoogleScholarGoogle Scholar |
Williams-Linera G (1993) Soil seed banks in four lower montane forests of Mexico. Journal of Tropical Ecology 9, 321–337.
| Soil seed banks in four lower montane forests of Mexico.Crossref | GoogleScholarGoogle Scholar |
Wolkis D, Baskin CC, Baskin JM (2018) Dormancy-breaking and germination requirements of seeds of the Hawaiian endemic Dianella sandwicensis (Xanthorrhoeaceae). Australian Journal of Botany 66, 213–217.
| Dormancy-breaking and germination requirements of seeds of the Hawaiian endemic Dianella sandwicensis (Xanthorrhoeaceae).Crossref | GoogleScholarGoogle Scholar |
Woolley JT, Stoller EW (1978) Light penetration and light-induced seed germination in soil. Plant Physiology 61, 597–600.
| Light penetration and light-induced seed germination in soil.Crossref | GoogleScholarGoogle Scholar |
Xu T, Kesteven J, Hutchison M (2014a) Daily total precipitation: ANUClimate 1.0, 0.01 degree, Australian Coverage, 1970–2014. (Australian National University: Canberra, ACT) Available at https://portal.tern.org.au/daily-total-precipitation-1970-2014/20312 [Accessed 4 November 2020]
Xu T, Kesteven J, Hutchison M (2014b) Daily maximum temperature: ANUClimate 1.1, 0.01 degree, Australian Coverage, 1970–2014. (Australian National University: Canberra, ACT) Available at https://portal.tern.org.au/daily-maximum-temperature-1970-2014/20314 [Accessed 4 November 2020]
Xu T, Kesteven J, Hutchison M (2014c) Daily minimum temperature: ANUClimate 1.1, 0.01 degree, Australian Coverage, 1970–2014. (Australian National University: Canberra, ACT) Available at https://portal.tern.org.au/daily-minimum-temperature-1970-2014/20313 [Accessed 4 November 2020]
Yan A, Chen Z (2020) The control of seed dormancy and germination by temperature, light and nitrate. The Botanical Review 86, 39–75.
| The control of seed dormancy and germination by temperature, light and nitrate.Crossref | GoogleScholarGoogle Scholar |
Yang L, Liu S, Lin R (2020) The role of light in regulating seed dormancy and germination. Journal of Integrative Plant Biology 62, 1310–1326.
| The role of light in regulating seed dormancy and germination.Crossref | GoogleScholarGoogle Scholar |
Zang R-G, Ding Y, Zhang W-Y (2008) Seed dynamics in relation to gaps in a tropical montane rainforest of Hainan Island, South China: (II) Seed Bank. Journal of Integrative Plant Biology 50, 513–521.
| Seed dynamics in relation to gaps in a tropical montane rainforest of Hainan Island, South China: (II) Seed Bank.Crossref | GoogleScholarGoogle Scholar |
Zich FA, Hyland BPM, Whiffin T, Kerrigan RA (2020) Australian tropical rainforest plants. Edition 8. Available at https://apps.lucidcentral.org/rainforest/text/intro/index.html [Accessed 12 September 2022]


