Improving weed management by targeting the seed ecology of blackberry (Rubus anglocandicans) in a biodiversity hotspot
Caroline Delaisse A B C , Paul B. Yeoh
A B C , Paul B. Yeoh  A , Raphael K. Didham
A , Raphael K. Didham  A B , Wolfgang Lewandrowski
A B , Wolfgang Lewandrowski  B D , John K. Scott
B D , John K. Scott  A B and Bruce L. Webber
A B and Bruce L. Webber  A B E *
A B E *
A CSIRO Health & Biosecurity, Private Bag 5, Wembley PO, Wembley, WA 6913, Australia.
B School of Biological Sciences, University of Western Australia, Crawley, WA 6009, Australia.
C CSIRO Health & Biosecurity, PO Box 1700, Canberra, ACT 2601, Australia.
D Kings Park Science, Department of Biodiversity, Conservation and Attractions, Kattidj Close, Kings Park, WA 6009, Australia.
E Western Australian Biodiversity Science Institute, 133 St Georges Terrace, Perth, WA 6000, Australia.
Australian Journal of Botany 71(1) 28-42 https://doi.org/10.1071/BT22041
Submitted: 26 April 2022 Accepted: 20 January 2023 Published: 20 February 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial 4.0 International License (CC BY-NC)
Abstract
Context: Germination is a vulnerable life stage for plants, therefore understanding the dynamics of seed ecology is essential to guiding management recommendations for highly invasive weeds.
Aim: We addressed the knowledge gap for how seeds contribute to the invasion process for European blackberry (Rubus anglocandicans), a threatening weed across the riparian ecosystems of south-western Australia.
Methods: We performed mechanical, chemical and thermal treatments on seeds to test for changes in germination success and conducted seed-burial trials to monitor seed viability over time in the soil seedbank.
Key results: In germination trials, freshly picked and frugivore egested seeds failed to germinate with the endocarp intact. With the endocarp removed, germination remained lower at 4–6 months compared with 10–28 months after collection, indicating a significant after-ripening period. Seeds in intact endocarps survived water immersion for more than 2 months, indicating an ability to survive winter flooding. Acid immersion did not improve germination. The germination success of seeds with endocarp removed increased linearly above 11°C, was greatest at 30°C and thereafter declined rapidly (no survival at 40°C). In a 5-year seed-burial trial, germination varied from 7.6 to 48.4% and was significantly lower closer to a river, and in areas where ‘blackberry decline’ syndrome was present.
Conclusions: While germination of seed without its pyrene coat occurred over a range of controlled conditions, the natural processes needed to break the pyrene remain unknown.
Implications: High germination success and the long-term survival of seeds in soil clarifies that the management of blackberry remains a difficult challenge in Australia.
Keywords: after-ripening, bet hedging, dormancy, ecophysiology, frugivory, invasive species, riparian restoration, seedbank, seed germination, seed persistence.
Introduction
Understanding plant recruitment is an essential prerequisite to developing strategies for the management of vegetation for both conservation and agricultural purposes. An example of the critical role that recruitment biology represents in the management of plant populations is the reduction of weed seeds in agricultural lands by cultivation, selective grazing, or pre-emergent herbicides; all techniques are improved by a knowledge of germination behaviour and conditions (Gallagher 2000; Bewley et al. 2013; Baskin and Baskin 2014). More broadly, the management of seeds in ecosystems can include manipulating fire frequencies to enable seeds to germinate with the aid of smoke water (Dixon et al. 2009), leading to considerable seedbank depletion following a fire (Keith 1996). Given that establishment is one of the most vulnerable phases of a plant’s ontogenetic progression, conditions for seed production and germination need to be explicitly considered as part of the entire cycle of vegetation management, from eradication, to containment, control and restoration of ecosystems (Wilson et al. 2017).
Knowledge of seed persistence (Long et al. 2015) and germination requirements (Bewley et al. 2013) are critical to extirpating plant populations from, or reducing abundance in, targeted areas. For example, the failure of many weed-control programs is often caused by the seedbank outliving the resources available for control (Panetta 2004). Because most seeds are hidden in the soil ‘out of sight’, with management programs focusing primarily on controlling established plants, their biology and ecology are often unknown. This knowledge gap has certainly been the case for many decades of weed management in natural environments globally.
Within Australia, species of European blackberry (Rubus fruticosus L. species aggregate; Evans et al. 2007) are declared as Weeds of National Significance, and have negative impacts both economically and environmentally (Thorp and Lynch 2000; Bradshaw et al. 2021). The most prominent of the weedy European blackberries within Australia is Rubus anglocandicans A.Newton (Evans et al. 2007), which is thought to have originated from populations in England (Evans and Weber 2003; Clark et al. 2013). Across southern Australia, the fast-growing R. anglocandicans dominates along water bodies and riparian ecosystems, for example, in the biodiversity hotspot of south-western Western Australia (SWWA; Hancock et al. 1996; Fig. 1). A monoculture several metres high of blackberry can line river banks, preventing easy access for amenity use for recreation or fishing and severely impeding access for emergency situations such as bushfire (Amor et al. 1998). Even though the structure of the infestation can provide shelter for some native mammals and increase or maintain mammal diversity in native and novel ecosystems (Packer et al. 2016), the negative impacts on native vegetation are significant (Yeoh et al. 2016). Blackberry infestations result in a reduction of plant species richness by ~50%, can outcompete native plants after disturbance from fires and flooding, and can further provide favourable habitat for exotic fauna such as feral pigs and rabbits (Amor et al. 1998).
|
|
Control strategies for R. anglocandicans in Australia include foliar herbicides, physical removal of plants, and classical biological control. One biological control agent that has been released is a strain of leaf-rust fungus, Phragmidium violaceum, which has been used to control multiple European blackberry species across Australia (Morin and Evans 2012). Whereas the leaf-rust-focused biocontrol strategy has worked for controlling R. anglocandicans in some areas of south-eastern Australia, it is not as effective in south-western Australia, probably because of the lack of rainfall necessary to complete its lifecycle (Morin and Evans 2012). These control methods are primarily aimed at adult blackberry plants, whereas seeds and the seedbank are commonly not considered. Buddenhagen and Tye (2015) gave examples where spread and persistence of species in soil seedbanks have been underestimated (including some Rubus species), impeding their control or extirpation. Knowledge of the seed ecology, including persistence and germination triggers, is important for determining how an area should be monitored and managed to prevent the risk of recolonisation and infestation.
A further need for change in control strategies has arisen with the emergence of a pathogen in the Warren Region of SWWA. Identified as a disease complex involving a recently described pathogenic strain of Phytophthora, the pathogen has caused a rapid decline in R. anglocandicans abundance (Aghighi et al. 2012, 2014). Observations of this decline include the rotting of roots in adult plants, prevention of new growth, and, eventually, death of the adult plant. The widespread death of adult plants has resulted in opening of understorey areas that were previously invaded, giving new opportunities for restoration of native vegetation, especially along riparian zones (Yeoh et al. 2016). However, what is not known is how the R. anglocandicans seedbank responds to the widespread dieback of vegetative material.
Despite a high weed prioritisation status, the lack of information on R. anglocandicans establishment ecology continues to hamper the design of effective management strategies. Blackberry can reproduce from vegetative regrowth (shoot tip and root suckering) as well as from seeds (Amor 1974). However, whereas current management strategies and the opportunity presented by dieback affect live plants (i.e. vegetative establishment), the potential risk for reinvasion remains if there is a viable seedbank. Most research specifically covering the seed biology of various Rubus species is based on cultivated or native species in Europe or America, and rarely on the species as a weed or in an Australian context. Moreover, many findings for one Rubus species cannot always be applied to another Rubus species, because there is considerable variation in seed properties across the genus. Such variation results in contrasting responses to certain environmental conditions, making generalising among species difficult (Amor 1974; Wada and Reed 2011b; Choi et al. 2016). Thus, an understanding of blackberry seed ecology is the primary limiting step to effective management of blackberry invasions, including progressing the restoration of native vegetation in affected areas.
The blackberry seed and seed coat are contained within a pyrene (a collective term for the seed and the surrounding woody endocarp), which, in turn, is contained in a druplet that is aggregated with many other druplets into a single fruit (a berry; Fig. 2a). For the purposes of this paper, a pyrene with the endocarp removed is called a bare seed). Vertebrate vectors consume the fleshy blackberry fruit (Nybom 1987), enabling long distance seed dispersal. In Australia, the main consumers of fruits, and hence dispersers of R. anglocandicans pyrenes, are native emus (Dromaius novaehollandiae) and non-native foxes (Vulpes vulpes). In SWWA, emus focus on patrolling blackberry bushes, selectively harvesting berries as they ripen. Brunner et al. (1976) estimated that this targeted frugivory can result in between 1000 and 4000 viable pyrenes per emu scat during the fruiting season.
|
|
Rubus pyrenes commonly display innate dormancy traits (Baskin and Baskin 1998) and are typically characterised by low germination rates, even after treatment (Wada and Reed 2011b). Seed dormancy in Rubus is characterised by a physiologically dormant embryo (Lasheen and Blackhurst 1956). The endocarp additionally acts as a physical barrier to the seed, restricting the amount of oxygen and water taken up, and reducing the embryo growth potential during germination (Nybom 1980). Moreover, the thickness of the endocarp varies among Rubus species, with thicker, harder endocarps contributing towards lower seed germination rates (Wada and Reed 2011a). Artificial breaking of seed dormancy has been achieved in various Rubus seeds by scarifying or removing the endocarp (Lasheen and Blackhurst 1956; Mian et al. 1995; Wada et al. 2011; Choi et al. 2016). Under natural conditions, the gradual breakdown of the endocarp coat is likely to be influenced by after-ripening in the soil seedbank, through changes in seasonal temperature and moisture fluctuations (Rose 1919). Whereas seasonal variation in temperature and moisture may regulate dormancy and subsequent germination, there is currently a limited understanding of the environmental requirements for germination and the temporal persistence of ungerminated seeds in the seedbank for all Rubus species. Therefore, understanding the regulation of seed dormancy and the survival of seeds is likely to be critical for the management of invasive Rubus species.
Taken together, understanding the seed ecology of R. anglocandicans is particularly important for successful management, because establishment of a single seed may be enough for the re-invasion of whole landscapes through clonal growth and clonal seed production (the species is apomictic). Therefore, the overall aim of this study was to develop an understanding of the seed biology and ecology of R. anglocandicans to underpin more effective weed-control strategies. Because R. anglocandicans is a major weed across Australia and elsewhere, these findings are likely to be valuable for weed management in other regions. To achieve this outcome, we
-
assessed the physiological or morphological factors influencing seed germination in R. anglocandicans; we focused on the role of after-ripening and the endocarp in controlling germination; scanning electron microscopy was used to assess endocarp morphology and modification due to interactions with frugivores;
-
determined what impacts microclimatic conditions have on germination and seedling development, focusing on controlled condition studies manipulating moisture and temperature;
-
measured 5-year viability of seed buried in soil in field conditions and assessed the presence of seed dormancy; and
-
developed prioritised recommendations as to how to improve the effectiveness and efficiency of control programs by a better understanding of seed persistence and germination.
Materials and methods
Study location
The field study area and source of seeds was the banks of the Warren River, between Manjimup, Pemberton and Walpole (330 km S of Perth) in the south-west of Western Australia (SWWA). The vegetation structure, topology, and blackberry infestation are described in Aghighi et al. (2014) and White et al. (2021). The area has a Mediterranean-type climate with wet cool winters and hot dry summers that are becoming less wet and hotter as a result of climate change (White et al. 2021).
Source of pyrenes
Rubus anglocandicans pyrenes were sourced from ripe fruit picked from bushes or from fresh emu scats found within 100 m of adult plants across several years. Pyrenes were primarily collected from a site ‘Sonia’, located beside the Warren River near Pemberton in SWWA (34.502°S; 116.085°E) in March 2013 and 2014. Owing to low numbers of accessible ripe fruit on plants at this site in 2014 and 2015 (they had already been eaten by emus), in 2015 bush-picked fruit were sourced from an additional site, Smith’s Brook (34.364°S, 116.201°E), close (~10 km) to the Sonia site.
Ripened fruit were left for less than a week at room temperature for the flesh to decay so as to mimic field conditions, and then washed to remove fruit flesh remnants from pyrenes. Following the method described by Wada and Reed (2010), pyrenes were washed in a solution of 20 mL of pectinase and 750 mL of water. Pyrenes were left in the solution on a magnetic stirrer (Cole Parmer Model 51450) for between 24 and 48 h to break down the remnant fruit flesh from pyrenes, before being rinsed clean in tap water. Pyrenes that sank in water (a sign of a filled, healthy seed) were retained and air dried in laboratory conditions for ~1 week. The collected pyrenes were then stored in containers or bags at room temperature (22–25°C) and with some exposure to artificial light.
Pyrenes collected in fresh emu scats in 2013 and 2014 were washed and separated from other organic matter by using sieves and water; then, healthy seeds were separated, dried and stored in the same manner as those from ripened fruit.
Pyrene morphology
To assess endocarp morphology and modification from interactions with frugivores, pyrenes from all years and sources were imaged with a scanning electron microscope (SEM; JCM-6000PLUS NeoScope Benchtop SEM, JEOL). Prior to photographing, pyrenes were sectioned or left intact, dried overnight in silicon, and gold coated with a sputter-coater (EMITECH K550X, Quorum Technologies Ltd, Kent, UK).
Germination media
For the following experiments, intact pyrenes and treated seeds were incubated on an agar media consisting of 1% H2O-agar (Woods and Woods Agar Powder 1000, New South Wales, Australia) and 0.0002% plant preservative mixture (PPM; a broad-spectrum biocide and fungicide for plant-tissue culture; PhytoTechnology Laboratories, Lenexa, KS, USA) in 90-mm Petri dishes. Each Petri dish contained seven seeds, and six dishes (i.e. replicates) per treatment were used (unless specified differently). Petri dishes were arranged on a tray and placed within a resealable plastic sandwich bag to help maintain a more consistent environment around the dishes.
Incubation conditions were maintained using refrigerated incubators (Refrigerated Incubator LMRIl-5, Lindner + May, Australia) that were set at different temperatures with a light cycle of 14-h days and 10-h nights. For the influence of light on germination, see the ‘Germination in relation to light’ section in the Supplementary material. Data loggers (HOBO Pendant or HOBO u23 pro V2, Onset, Australia) were placed within the incubators to monitor temperature, light, and humidity over time in the incubators. For all experiments, seed germination was defined as the moment the emerged radicle was at least 1 mm long (Bewley et al. 2013).
Influence of the endocarp on germination
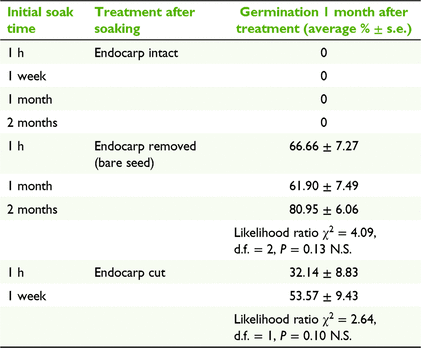
To determine the effects of an intact endocarp on seed germination, experiments were run with the following three endocarp manipulation treatments, after first soaking the pyrene for multiple time intervals: (1) endocarp intact (after soak times of 1 h, 1 week, 1 month and 2 months); (2) endocarp removed manually (after soak times of 1 h, 1 month and 2 months) with a scalpel and tweezers while being viewed under a dissecting microscope to ensure that the seed and seed coat remained intact; and (3) the radicle end of the endocarp cut with a scalpel (after soak times of 1 h and 1 week), which has previously been shown to trigger germination in a raspberry species (Rubus idaeus L.; Nesme 1985). In parallel, we ran a trial investigating the effect of long term immersion in water on germination and found that the endocarp did not break down naturally with next to no germination (see the ‘Germination after long term immersion’ section in the Supplementary material). All treatments were placed in an incubator, running at a constant 25°C. We first analysed variation in germination percentages between soak times within each endocarp treatment (separately), and then analysed variation in germination percentages between endocarp treatments where soak-time effects were consistent. Analyses were performed using a binomial generalised linear model (GLM) in R (ver. 4.1.0, R Foundation for Statistical Computing, Vienna, Austria, see https://www.r-project.org/), with the number of germinated seeds (of seven) as the response variable in each of the six replicates per treatment combination. Model residuals were tested for overdispersion by using package ‘DHARMa’ (ver. 0.4.4, F. Hartig, see https://cran.r-project.org/package=DHARMa/), but none was detected.
After-ripening
Presence of the endocarp is not the only factor that might inhibit or limit germination. Even with the endocarp removed, seeds may have reduced germination during an after-ripening period. After-ripening refers to the process of gradual release of primary physiological dormancy of the seeds, which starts during seed maturation and continues over a post-maturation period of variable length. After-ripening in the context of our study would be expressed as germination failure for seeds collected from the mother plant during fruit maturation in summer, or seeds collected immediately after dispersal (usually by a frugivore), but then increasing germination percentages after the first summer and winter have passed. To test this, we collected seeds from two sources: (1) pre-dispersal seeds from freshly picked ripe fruit, and (2) post-dispersal seeds from fresh emu scat. We compared germination percentages at five time points following collection and storage at ambient laboratory conditions, namely, 4, 6 and 10 months after collection in March 2015, 16 months after collection in March 2014, and 28 months after collection in March 2013. Unfortunately, no seed collection from fresh fruits was available from the March 2014 collections as we could not source them in the field at this time. In all cases, bare seeds (i.e. seeds with their endocarp removed) were placed in an incubator and germination success was recorded after 1 month. Analysis was performed using a binomial GLM, as described above, with fixed predictor effects for the interaction between seed source and time since collection. No overdispersion of model residuals was detected.
Pyrene treatments – scarification with acids
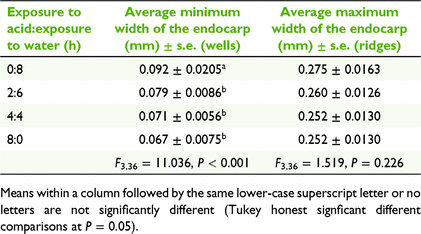
A common method to ensure germination in blackberry cultivation is to scarify pyrenes through acid treatments, such as sulfuric acid (Moore et al. 1974; Wada and Reed 2011a, 2011b). We measured the effect of acid on endocarp thickness in pyrenes collected from fresh emu scats in 2013. Approximately 300 pyrenes were processed in a single batch spread evenly across four conical flasks (i.e. ~85 pyrenes per flask) in 20 mL of 35% H2SO4, shaken on an orbital shaker for 0, 2, 4 and 8 h respectively, then drained, and then shaken in water for 8, 6, 4 and 0.5 h respectively (i.e. to standardise total shaking time). When transferring the pyrenes from acid to water bath, the acid was drained off, pyrenes were rinsed in water five times, and then the pyrenes were soaked in sodium hydrogen carbonate (NaHCO3) to neutralise remaining acid. Pyrenes were washed twice more in water and left to air-dry on filter paper overnight. A sample of 10 pyrenes per treatment was drawn out and each pyrene was cut cross-sectionally, so that the thickness of the endocarp could be measured at 50× magnification at three maximum and three minimum cross-sections per pyrene. The minimum sections correspond to what we refer to as ‘wells’ in the endocarp surface (Fig. 2e, f). Comparable acid treatment of pyrenes was also undertaken with 98% H2SO4, but seeds became brittle to the touch and were unable to be measured without the pyrene disintegrating.
The effect of acid treatment on endocarp thickness was analysed using a linear model (LM) in R, with minimum or maximum thickness as the response variable (in separate models) and acid treatment as a fixed categorical predictor. Assumptions of normality and homogeneity of variances were tested in package ‘performance’ in R (ver. 0.10.0, see https://cran.r-project.org/package=performance; Lüdecke et al. 2021), and post hoc multiple comparisons of means tests were performed using Tukey honest significant differences in the ‘TukeyHSD’ function in R.
For each acid treatment, two sets of 10 seeds with endocarp removed were placed on 1% agar plates with PPM in incubators set at 30°C:15°C, 10 h:14 h, day:night. Seeds were observed weekly for 1 month and germination was recorded. Another two sets of 10 seeds were drawn from each acid treatment in the same manner, but in this case the pyrene was kept intact, and pyrenes were planted into soil media (University of California potting mix) in a glasshouse.
Variation in germination rate with increasing incubation temperature
Pyrenes were sourced from fresh emu scats collected in 2013, and the endocarp was removed manually (as described previously), immediately prior to each experiment. In 2015, bare seeds (six replicate Petri dishes per treatment with seven seeds per dish) were incubated in controlled-temperature cabinets at eight constant temperatures: 5, 10, 15, 20, 25, 30, 35 and 40°C. Data loggers confirmed that the observed mean temperatures within individual incubators varied slightly to calibrated set points: 3.7, 11.1, 14.2, 20.6, 25.5, 31.6, 35.6, and 40.5°C respectively. Because of the limited availability of incubators and logistics feasibility, critical parameters (humidity and light intensity) were carefully monitored and cross-checked between incubators for uniformity, and plate trays were rotated daily in position and within shelves of the incubators, rather than rotating the experiment between incubators.
Because of short-term hardware failures, trials in the 10 and 15°C incubators were repeated in 2016, and these results were cross-referenced with an additional repeated trial in the 25°C incubator. As no differences in germination outcomes were evident between years, all trials were combined into a single analysis. A further trial was set up in a glasshouse, in which seeds were placed on 1% agar plates with PPM and exposed to natural light and temperature conditions from May to August 2015. The glasshouse was fitted with an evaporative air-conditioning system and roof vents that operated when the temperature exceeded 25°C. Data loggers showed the glasshouse to average 16.6°C (minimum 7.5°C, maximum 25.4°C) during the experiment.
For consistency with past studies, we used an approach similar to Andrewartha and Birch (1954) to calculate a rate of germination of seeds across a range of temperatures. For germination, we recorded number of seeds germinated each day through time, and once cumulative maximum germination had been reached, we divided it by the number of days to estimate rate of germination per day. The advantage of this is that degree-days (DD) could then be calculated through the equation:
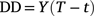
where Y is the average number of days to reach germination, T is the constant temperature, and t is the basal temperature for development (i.e. lower temperature for development; Asante et al. 1991).
Seeds were observed daily during the first 2 weeks of the experiment. Germination experiments running at the temperatures of 25, 30 and 40°C were terminated after 1 month, (the seeds had germinated or were dead), whereas at the other temperatures, experiments were run up to 200 days or until all healthy seeds had germinated.
Inspection of the data indicated clear non-linearity in the effect of temperature on germination rate. Therefore, we tested for non-linear ‘break points’ in temperature responses using segmented regression models with the package ‘segmented’ (ver. 1.3-4, see https://CRAN.R-project.org/package=segmented; Muggeo 2008, 2017) in R. We specified a Gaussian error structure (with identity link) and confirmed that model residuals did not depart significantly from normality and homogeneity of variances. We used the ‘selgmented’ function to automatically select the optimal number of breakpoints in the data, on the basis of model comparisons using the Bayesian information criterion (BIC; Muggeo 2008), and Davies test for the significance of changes in slope across breakpoints (Muggeo 2017). Note that all temperature experiments were repeated on pyrenes with the endocarp intact, but no seeds germinated at any of the temperatures tested and the data were not analysed.
Seed-burial trial
To test the ability of buried seeds to maintain viability through time, pyrenes were buried for 5 years at the following four field sites along the Warren River in SWWA: Sonia (34.507°S, 116.085°E), Log Bridge Crossing (34.515°S, 116.100°E), Wheatley Coast Road (34.365°S, 116.267°E) and Shields (34.391°S, 116.220°E). The Sonia and Log Bridge Crossing sites had extensive evidence of ‘blackberry decline’ syndrome (Aghighi et al. 2012, 2014), which is most likely related to blackberry mortality owing to Phytophthora infestation. The Wheatley Coast Road and Shields sites had healthy blackberry plants present. Each site was further divided into two locations: ‘low’-elevation locations of blackberry infestation (typically 1–2 m above the river’s normal water level during summer); and ‘high’-level locations at the highest point of blackberry infestation at the site (typically 3–5 m above normal summer water levels). At low-elevation locations, inundation during winter floods was for at least a few weeks in most winters, whereas at high-elevation locations flooding occurred for only brief periods of time (a few days to 1 week), if at all, in years with higher than normal rainfall.
Blackberry pyrenes were all collected from the same site (Sonia) and were stratified by seed source, i.e. extracted from fresh fruit in 2013, fresh emu scats in 2013, and fresh emu scats in 2014. After collection, pyrenes were stored in paper bags in the laboratory at ambient conditions until burial.
Mesh bags (10 × 10 cm; 1.8-mm diagonal aperture size) were made from Alspec black fibreglass screening (Hanover Wire Cloth, the Mesh Company). Mesh bags were heat-sealed using a bag sealer and contained either 200 pyrenes per bag for each of the two emu samples or 50 pyrenes per bag for the pyrenes extracted from fresh fruit (as fewer were available), as well as a small internal identifying label (laser printed on waterproof paper).
Within each low and high elevation at a site, there were up to five replicates spaced so that each replicate was at least 2 m from other replicates. For each replicate, a pair of survey pegs were placed 7 m apart (to allow a tape measure to be placed and samples relocated in subsequent years) and were buried between these pegs (1-m spacing) for 5 years. The experiment was set up for five other time periods but these samples were not used for this study. Because we had fewer pyrenes sourced from fruit, it was not possible to place pyrenes from all three sources in all replicates. Instead, pyrenes from all sources were buried in Replicates 1, 2 and 3 but only pyrenes from emu scats collected in 2013 and 2014 were buried in Replicates 4 and 5.
Mesh bags were buried 10 cm below the soil surface by using a spade to lift a wedge of topsoil and replace it back on top of the seed bags. The bags were buried on 19–20 March and 15–16 May 2014, when the river was at its normal low level following summer and before the winter rains.
Mesh bags were retrieved on 17–18 October 2019 by randomly choosing one of the five sets from each replicate. Thus, the number of bags excavated in 2019 was 10–12 per site and 93 overall. Pyrenes retrieved from the bags were stored in the laboratory at ambient temperature and assessed within 3 months of collection.
Following burial, each mesh bag was cut open and the number of pyrenes and opened pyrenes (as occurs with germination) were counted. A subsample of 40 pyrenes per bag were selected randomly for dissection to score obvious mortality (seed decay, necrosis) and extract putatively live seed. Each live seed (endocarp removed) was placed in a 90-mm Petri dish on agar and PPM solution (as described previously) that was placed in a resealable sandwich plastic bag (to maintain high humidity). These bagged plates were placed in an incubator at 20°C and a 16 h:14 h day:night cycle.
Germination was scored initially at 4–5 days and this continued each 2–3 days through to 15 days. Seeds that had not germinated after 15 days were examined under the microscope and noted as dead (e.g. if imbibition did not take place, the seed coat was black, the seed was shrunken and the filling was grey or black), failed to germinate (imbibition took place, seed coat split around the middle of the seed as occurs prior to germination, but no radicle produced) or potentially alive (otherwise healthy, no imbibition).
Analysis was performed using a binomial generalised linear mixed model (GLMM) to test the interaction among fixed predictor effects of blackberry decline (decline v. clean), elevation (low v. high) and seed source (2013 fruit, 2013 emu and 2014 emu) on the number of seeds alive (of 40) per mesh bag. Random effects in the model were specified for site identity and for elevation within site, to account for the spatial non-independence of bags. Initial model tests in the package ‘lme4’ (ver. 1.1-27.1, see https://CRAN.R-project.org/package=lme4; Bates et al. 2015) showed significant overdispersion of model residuals when tested in ‘DHARMa’. Therefore, we used a beta-binomial GLMM in package ‘glmmTMB’ (ver. 1.1.2.3, see https://cran.r-project.org/package=glmmTMB; Brooks et al. 2017) to deal with overdispersion, and added a model term to accommodate significant zero-inflation of seed-survival counts. We conducted a model simplification procedure to identity the minimal adequate model on the basis of comparison of Akaike information criterion (AIC) values in models with subsets of the predictor effects. We used a ΔAIC of 2.0 as a threshold for model improvement (Burnham and Anderson 2002).
Results
Pyrene morphology
The fruit of R. anglocandicans comprises multiple drupelets (42–119 per fruit; Strik et al. 1996), each containing a single pyrene (Fig. 2a, b). Each pyrene contained a single seed. The endocarps of pyrenes that had been egested by emus and pyrenes that were collected from fruit (Fig. 2a, b) did not appear to differ in thickness on the basis of visual assessment under a microscope. The endocarp of R. anglocandicans pyrenes was clearly distinctive (Fig. 2c, d) and contained ‘wells’ (Fig. 2e, f). The surface of the endocarp appeared more degraded having been egested by emus, than did picked-fruit pyrenes (Fig. 2e, f).
Influence of the pyrene on germination
None of the pyrenes with the endocarp intact germinated, regardless of the length of time they were soaked in water (Table 1). Pyrenes that had the radicle end of the endocarp cut with a scalpel had intermediate levels of germination (mean ± 1 s.e., 42.9 ± 6.6%), but this did not differ significantly among soak times (Table 1). Pyrenes with the endocarp completely removed had significantly higher levels of germination (69.8 ± 4.1%) than did pyrenes with a cut endocarp (GLM, likelihood ratio χ2 = 11.76, d.f. = 1, P < 0.001), but again there was no significant effect of soak time on germination for bare seeds without an endocarp (Table 1).
|
After-ripening
There was a strong effect of time since collection, indicating the presence of after-ripening or the presence of some level of dormancy in the initial stage of seed development since dispersal (Fig. 3). No difference in germination was found among the source of seeds, emu scat or fruit-derived, despite the time since collection nor on the interaction of time and source (ANOVA glm: time χ2 = 56.4, d.f. = 4, P < 0.001; source χ2 = 0.07, d.f. = 1, P < 0.80; time × source χ2 = 3.9, d.f. = 3, P = 0.28).
Pyrene treatments – scarification with acids
There was no significant reduction in the average maximum thickness of the pyrene at any duration of 35% H2SO4 acid treatment (F3,36 = 1.519, P = 0.226; Table 2). However, the minimum thickness of pyrenes did vary significantly among acid treatments (F3,36 = 11.036, P < 0.001), with pyrenes soaked for 1, 2 or 8 h in acid being significantly thinner than those in the no acid treatment (Table 2).
|
Despite a reduction in pyrene thickness, the acid treatments did not improve germination, because the seeds with an intact endocarp still did not germinate following acid treatment and planting in soil (no statistical analysis possible on zero-germination outcomes). We confirmed that this was not due to seeds being killed by the acid treatment, because pyrenes with the endocarp removed germinated at the same proportion as did other samples (that were also acid-treated) and germinated at the same proportion as did others, indicating that seeds were not killed by the acid treatment.
Variation in germination rate with incubation temperature
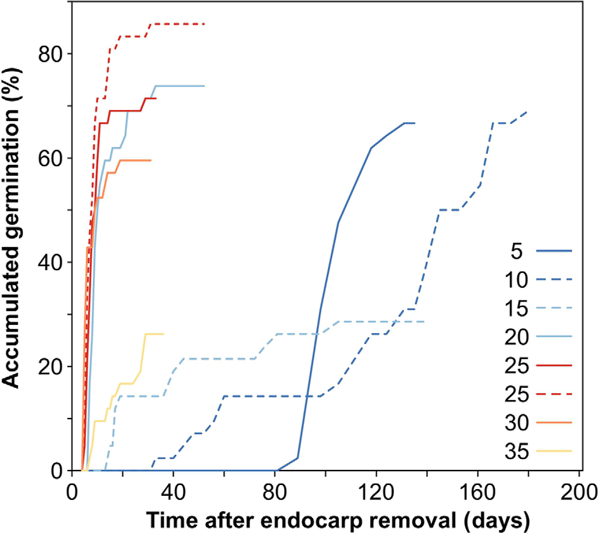
Germination of one or more bare seeds (with the endocarp removed) occurred at all temperatures from 3.9 to 35.6°C, but not at 40.5°C (Fig. 4). However, time to germination varied dramatically with temperature (Fig. 4). Below 10°C, a large portion of seeds germinated only after 3 months, whereas most germination occurred within 1 month at temperatures between 20 and 30°C (Fig. 4).
|
In the segmented model analysis, the automatic selection of breakpoints identified two breaks in the curve (mean ± s.e. at 11.35 ± 1.361°C, and 30.88 ± 0.525°C; Fig. 5). Below 11.35°C, there was a flat slope that was not significantly different from zero (estimated slope −0.00005 ± 0.0019, t = −0.03). Between 11.35 and 30.88°C, there was a highly significant positive effect of temperature on germination percentage (estimated slope 0.00888 ± 0.0009, t = 9.86, P < 0.01). Above 30.88°C, there was a sharp decline in germination percentage (estimated slope −0.02002 ± 0.0017, t = −11.81, P < 0.001), with all seeds at 40°C blackening and dying (without germinating) within 3 weeks (Fig. 5).
From the variation in germination percentages through time, we calculated that it took ~245 ± 62 degree-days (DD) to germinate, and a further 88 ± 2 DD for cotyledons to emerge. In total, 333 ± 64 DD were necessary to fully germinate with cotyledons.
Seed-burial trial
Ninety three of the 96 buried mesh bags were retrieved (Table 3). The bags were not damaged, although occasionally traversed by a root. The bags retained most of the pyrenes, with 87.7% of 18 600 pyrenes still present in the 93 retrieved bags after 5 years in the soil (Table 3).

|
Very few of the buried pyrenes had germinated during the 5 years (indicated by the presence of two sides of the pyrene opened to resemble a butterfly). The total number of seeds germinated prior to retrieval was 283, with most (269) from one location, Log Bridge Crossing (low elevation), where five mesh bags were inadvertently exposed to light by erosion of the river bank during a high water-flow event. The percentage germination in these five bags were 19, 20, 33, 34, and 54%. Otherwise, the high number of unopened pyrenes retained in other mesh bags confirmed that there were few germinations over the 5 years of burial.
Following pyrene dissection and germination trials, seed mortality was found to be >90% at the two sites, Sonia and Log Bridge Crossing, that exhibited ‘blackberry decline’, compared with much higher survival at the two ‘clean’ sites, Shield and Wheatley Coast Road (Table 3). The best-fit GLMM model (Supplementary Table S1) found significant additive effects of decline status, elevation and seed source on seed survival (Fig. 6), but no significant interaction effects among predictors (Table S1). Sites with blackberry decline had significantly lower seed survival than did clean sites (χ2 = 45.6537, d.f. = 1, P < 0.0001; Fig. 6). Within sites, seed survival was significantly lower at lower elevation than at higher elevation above the river (χ2 = 7.3892, d.f. = 1, P < 0.0066; Fig. 6). Seed survival also varied significantly depending on seed source (χ2 = 13.0105, d.f. = 2, P < 0.0015; Fig. 6), with seeds sourced from fresh fruit in 2013 having higher survival than seeds sourced from emu scats in either 2013 or 2014 (model coefficients did not differ significantly between 2013-emu and 2014-emu, z = −1.647, P = 0.0996; Fig. 6). There was negligible influence of site-level or location-level random effects on model estimates (random variance components were close to zero), but there was significant zero-inflation detected (log-odds estimate = −1.954 ± 0.424s.e., z = −4.609, P < 0.0001).
Discussion
Our study demonstrated seed dormancy in R. anglocandicans to be a multilayered interaction of physiological dormancy, and the endocarp surrounding the seed restricting the germination process. Through prolonged after-ripening of up to 10 months, physiological dormancy was alleviated, and germination proceeded only after the removal of the endocarp from after-ripened seeds. Additionally, field studies demonstrated that seeds have the capacity to persist without germinating in the seedbank, for in excess of 5 years with the endocarp intact, and that germination was still possible after the removal of the endocarp following this period of burial. Additionally, there does not appear to be a chemical control of germination by the endocarp because germination was still able to proceed from pyrenes with the endocarp chipped to expose the seed. Seeds sourced from fresh fruit in 2013 have a higher survival than seeds sourced from emu scats in either 2013 or 2014, but the degree of survival is still likely to enable wide dispersal. Taken together, these results suggest that for successful establishment in the soil seedbank, the endocarp must split or become somewhat degraded to allow for germination before the seed loses viability. The mechanism by which the endocarp splits to open is unknown. We hypothesise that daily and seasonal temperature and moisture conditions in Mediterranean climate of south-western Australia, with its large swings from hot dry summers to cool wet winters, might provide suitable microclimatic conditions in the soil seedbank to promote dormancy alleviation. Alternatively, seeds have the capacity to persist in the seedbank, with the physiological dormancy and the endocarp surrounding the seed providing important mechanisms restricting germination behaviour. This interaction might promote a bet-hedging strategy for recruitment during unfavourable environmental periods, which combined with increased persistence and the accumulation of seeds through dispersal from maternal plants in the seedbank could lead to reinfestations under conditions more favourable for germination and establishment. Taken together, these results could have significant implications for the effective management of R. anglocandicans infestations. More specifically, control efforts should place a greater emphasis on long-term monitoring of previously infested areas to mitigate the risk of re-infestation from the soil seedbank.
Initial factors influencing germination
Critical to future management considerations is the knowledge we have quantified from seed germination through targeted experimentation in controlled conditions and field scenarios. Under controlled conditions, investigations into the characteristics of the endocarp surrounding the seed provide five insights into the dynamics that are critical for germination.
First, the pyrene is permeable to moisture, a prerequisite for germination, with moisture taken up rapidly within 2–3 h, leading to at least 20% of fresh mass increase before reaching a plateau of ~38% over the subsequent 8 days (Supplementary Fig. S1). Similarly, in R. cuneifolius, an invasive species in subtropical South Africa, imbibition to 15% in fresh-mass increase is completed within 40 min (van Staden and Campbell 1984), demonstrating the rapid nature of moisture uptake into the pyrene.
Second, cutting the endocarp was found to promote germination, consistent with van Staden and Campbell (1984) who found that germination in R. cuneifolius could be achieved as long as the endocarp was scarified through cutting, or acid treatment. In contrast, acid treatment of the endocarp did not improve germination in R. anglocandicans, which was consistent with previous studies in a number of blackberry species (Wada and Reed 2011b; Choi et al. 2016). Choi et al. (2016) found that for R. parvifolius, R. phoenicolasius, and R. takesimensis scarification (with sulphuric acid) needed to be combined with cold stratification (8 weeks at 4°C) to improve germination, owing to physiological dormancy reported in these species (Bewley et al. 2013), whereas other species such as R. buergeri and R. corchorifolius did not respond to any imposed treatments. In contrast, van Staden and Campbell (1984) found that the most effective treatment of R. cuneifolius was immersion in concentrated sulfuric acid for 1.5 h. In R. anglocandicans, the physical suppression of germination still held despite acid treatments and germination occurred only once the endocarp was removed or damaged. Such variability in germination requirements reinforces the findings of previous studies that there are few generalisations to make for germination requirements among Rubus species.
Third, germination in numerous plant species is known to be enhanced through bird or mammal gut passage (Traveset 1998). However, within Rubus, vertebrate egestion can have positive (Fedriani and Delibes 2009; Molefe et al. 2020; García-Rodriguez et al. 2021) or negative (Brunner et al. 1976) effects on seed germination. As blackberry fruit are commonly consumed by emus in SWWA, there was an expectation that through natural pyrene scarification in the emu gut, the endocarp would become degraded enough to increase germination. Germination percentages of bare seeds (i.e. seeds with the endocarp manually removed) sourced from intact pyrenes from emu scats germinated at similar proportions to that of seeds of pyrenes from bush-sourced fruit (Fig. 3), but a smaller proportion survived the 5-year burial period (Fig. 6), indicating that emu gut-passage has a negative effect on the viability of R. anglocandicans seeds. Some insight on possible impacts of emu gut passage on seed germination traits was provided by a SEM analysis of the endocarp. When consumed by emus, pyrenes pass through digestive areas of high acidity between a pH of 2.5 and 2.8 (Davies 1978), and are exposed to small ingested stones and grit that assist with the grinding of gut contents (Herd 1985). However, the rate at which food passes through the gut of the emu is quick (4.1–5.5 h) relative to other birds (Herd and Dawson 1984; Herd 1985), particularly as the gut is relatively short. Gut throughput times for blackberry seeds may be even shorter because of the high water content of blackberry fruit; therefore, the duration may not be enough to scarify the pyrene surface to cause endocarp weakening.
Fourth, for intact pyrenes that were soaked for up to 2 months and incubated at room temperature with fluctuating temperature conditions, splitting of the endocarp was observed only after 7 weeks. The total number of seeds that germinated after 52 weeks was still very low. A possible cause of the low germination rates could be the lack of other factors that would naturally drive the endocarp breakdown in the field. One such factor is the activity of soil fungi, which degrade non-lignified pericarp cells as part of overcoming mechanical dormancy as shown for Lepidium didymium (Sperber et al. 2017).
Because of how robust the endocarp is and how intact pyrenes did not germinate even after 2 months of soaking in water, it is unlikely that intact pyrenes will germinate directly after a flooding event. In south-eastern Australia, flooding events in lowland rivers create bare patches because of deposited sediments in the riparian regions. These bare patches were rapidly colonised by R. anglocandicans through clonal vegetative growth; yet, no new seedlings were observed (Greet et al. 2015). Although this finding was from a single study that does not accord with observations on seedling colonisation in SWWA, in combination with our findings, it does suggest that flooding events are not likely to be a primary driver in breaking down the endocarp and ensuring germination after a flooding disturbance. We conclude that a combination of other contributing factors must be involved.
Finally, in this study, stratification of R. anglocandicans pyrenes was not assessed experimentally. Stratification refers to storage of seeds in conditions similar to the habitat of origin (e.g. by incubating seeds under alternating temperatures reminiscent of different seasons, or under constant cold or warm wet conditions), prior to germination. Whereas previous studies have demonstrated improved germination after stratifying seeds at 5°C in subtropical Rubus species (e.g. van Staden and Campbell 1984), additional studies into whether cold stratification reduces time for after-ripening might be useful in understanding further germination cues for R. anglocandicans. Further studies could also investigate dry–wet cycles that mimic the field environmental clues that have an influence on dormancy loss (Hoyle et al. 2008).
Germination and growth in relation to temperature
Our results have also given an insight into the thermal thresholds limiting germination in R. anglocandicans, with an optimum germination temperature between 20 and 30°C. These findings indicated the temperature requirement for germination to be higher than that for a number of native plant species throughout SWWA (Bell 1994; Bell et al. 1995). In contrast, seeds incubated at cooler temperatures (5–15°C) took a longer time to germinate than did seeds at 25°C. However, most seeds at 5°C germinated within 40 days of each other and reached 64.3% germination. Although seeds at 10°C began germinating earlier, they took a longer time period (150 days) to reach 69% germination.
The broad range of temperatures (5–35°C) at which R. anglocandicans can germinate may be a factor as to why this species has been able to colonise areas in SWWA that other introduced European blackberries have not (Evans and Weber 2003; Evans et al. 2007). Such attributes are advantageous when competing with native species that have a lower temperature requirement and may further become beneficial in an increasingly warmer climate in SWWA (Hope et al. 2015).
Seed persistence
To our knowledge, this is the first study to assess experimentally the persistence of R. anglocandicans seeds in the field. After 5 years of burial, pyrenes remained viable and germinated rapidly once extracted from the field and the endocarp was removed. Seed buried for 5 years germinated as quickly as samples studied at the initiation of the project. However, there was considerable among-site variation in buried-seed viability.
Soil erosion of one end of a site resulted in germination in an inadvertently exposed bag. This serendipitous observation points to the need for some form of soil disturbance or stratification while moisture is present (for imbibition).
The among site comparison indicated a strong association between seed viability and the presence of ‘blackberry decline’ syndrome. Pyrene survival in decline areas was very low; however, the cause of this excess mortality needs further explanation. For example, there is also an association with waterlogging at the decline sites; see Aghighi et al. (2014) for a listing of associations that possibly cause ‘blackberry decline’. It should be emphasised that none of the buried pyrenes showed internal evidence of presence of hyphae. A more detailed mycological study could elucidate a potential pathogen association.
Implications for management of Rubus anglocandicans and its seedbank
The broad range of thermal and light conditions in which R. anglocandicans can germinate and establish provides further justification for how this species has managed to become so invasive in Australia. The upper temperature threshold for development is higher than in many native species in SWWA (Bell et al. 1995). Therefore, when planning weed control, considering the effects of warmer climates that we are projected to face in the future will be important.
There is potential for a long-lived seedbank (at least 5 years). There is evidence that there is a viable seedbank even in areas that have experienced ‘blackberry decline’. This implies that long-term monitoring of sites will be necessary wherever the weed was once present. Even so, it appears that ‘blackberry decline’ has also dramatically reduced the abundance of the seedbank seeds, which makes restoration of the riparian ecosystem feasible by following-up with herbicide treatments.
Another set of bags containing R. anglocandicans seeds remains in situ in the Warren study area and feasibly could be assessed after 10 years (i.e. March to May 2024). Very few studies into the management of Rubus species have included the seedbank. Renteria et al. (2012) gave an example of a 5-year study into the management options (eradication or control) for an invasive Rubus species on the Galapagos Islands, concluding that it is very difficult to avoid off-target damage in either option. This would be the same situation in our study area.
Conclusions
This study has exposed important aspects of seed ecology and biology of R. anglocandicans that are crucial to incorporate into effective management strategies for this weed in the future. Without prior research, weed control strategies can be ineffective or unsuccessful, unnecessarily wasting already limited funds and allowing threats to native biodiversity to continue. Particularly, as the climate changes, understanding seed biological attributes as a critical life stage through which the majority of weeds must pass can inform better plans for future control methods. Although a number of the higher-priority seed-ecology characteristics have been addressed for R. anglocandicans in this study, there are still aspects to be covered so as to develop a better understanding of this problematic weed to better manage this species in both restoration and non-restoration areas.
Supplementary material
Supplementary material is available online.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
This work was funded as part of ‘Clean Energy Future – Biodiversity Fund’ of the Australian Department of Sustainability, Environment, Water, Population and Communities. B. L. Webber was supported in part by a CSIRO Julius Career Award.
Acknowledgements
We thank Kathryn Batchelor, Lee Fontanini, Helen White and Shane Turner for help in the field and laboratory, Andy Russell (Warren Catchment Council) for sourcing seeds from fresh emu scats, Pieter Poot (University of Western Australia) for co-supervisory input on the BSc(Hons) thesis of C. Delaisse, and Mariana Campos for comments on an earlier version of the manuscript.
References
Aghighi S, Hardy GESJ, Scott JK, Burgess TI (2012) Phytophthora bilorbang sp. nov., a new species associated with the decline of Rubus anglocandicans (European blackberry) in Western Australia. European Journal of Plant Pathology 133, 841–855.| Phytophthora bilorbang sp. nov., a new species associated with the decline of Rubus anglocandicans (European blackberry) in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Aghighi S, Fontanini L, Yeoh PB, Hardy GESJ, Burgess TI, Scott JK (2014) A conceptual model to describe the decline of European blackberry (Rubus anglocandicans), a weed of national significance in Australia. Plant Disease 98, 580–589.
| A conceptual model to describe the decline of European blackberry (Rubus anglocandicans), a weed of national significance in Australia.Crossref | GoogleScholarGoogle Scholar |
Amor RL (1974) Ecology and control of blackberry (Rubus fruticosus L. agg.). II. Reproduction. Weed Research 14, 231–238.
| Ecology and control of blackberry (Rubus fruticosus L. agg.). II. Reproduction.Crossref | GoogleScholarGoogle Scholar |
Amor RL, Richardson RG, Pritchard GH, Bruzzese E (1998) Rubus fruticosus L. agg. In ‘The biology of Australian weeds’. Vol. 2. (Eds FD Panetta, RH Groves, RCH Shepherd) pp. 225–246. (RG and FJ Richardson: Melbourne, Vic., Australia)
Andrewartha HG, Birch LC (1954) ‘The distribution and abundance of animals.’ (University of Chicago Press: Chicago, IL, USA)
Asante SK, Danthanarayana W, Heatwole H (1991) Bionomics and population-growth statistics of apterous virginoparae of woolly apple aphid (Eriosoma lanigerum) at constant temperatures. Entomologia Experimentalis et Applicata 60, 261–270.
| Bionomics and population-growth statistics of apterous virginoparae of woolly apple aphid (Eriosoma lanigerum) at constant temperatures.Crossref | GoogleScholarGoogle Scholar |
Baskin CC, Baskin JM (1998) ‘Seeds ecology, biogeography, and evolution of dormancy and germination.’ (Academic Press: San Diego, CA, USA)
Baskin CC, Baskin JM (2014) ‘Seeds ecology, biogeography, and evolution of dormancy and germination.’ 2nd edn. (Academic Press: San Diego, CA, USA)
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67, 1–48.
| Fitting linear mixed-effects models using lme4.Crossref | GoogleScholarGoogle Scholar |
Bell DT (1994) Interaction of fire, temperature and light in the germination response of 16 species from the Eucalyptus marginata forest of south-western Western Australia. Australian Journal of Botany 42, 501–509.
| Interaction of fire, temperature and light in the germination response of 16 species from the Eucalyptus marginata forest of south-western Western Australia.Crossref | GoogleScholarGoogle Scholar |
Bell DT, Rokich DP, McChesney CJ, Plummer JA (1995) Effects of temperature, light and gibberellic acid on the germination of seeds of 43 species native to Western Australia. Journal of Vegetation Science 6, 797–806.
| Effects of temperature, light and gibberellic acid on the germination of seeds of 43 species native to Western Australia.Crossref | GoogleScholarGoogle Scholar |
Bewley JD, Bradford KJ, Hilhorst HWM, Nonogaki H (2013) ‘Seeds physiology of development, germination and dormancy.’ 3rd edn. (Springer: New York, NY, USA)
Bradshaw CJA, Hoskins AJ, Haubrock PJ, et al. (2021) Detailed assessment of the reported economic costs of invasive species in Australia. NeoBiota 67, 511–550.
| Detailed assessment of the reported economic costs of invasive species in Australia.Crossref | GoogleScholarGoogle Scholar |
Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Mächler M, Bolker BM (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. The R Journal 9, 378–400.
| glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling.Crossref | GoogleScholarGoogle Scholar |
Brunner H, Harris RV, Amor RL (1976) A note on the dispersal of seeds of blackberry (Rubus procerus P.J. Muell.) by foxes and emus. Weed Research 16, 171–173.
| A note on the dispersal of seeds of blackberry (Rubus procerus P.J. Muell.) by foxes and emus.Crossref | GoogleScholarGoogle Scholar |
Buddenhagen CE, Tye A (2015) Lessons from successful plant eradications in Galapagos: commitment is crucial. Biological Invasions 17, 2893–2912.
| Lessons from successful plant eradications in Galapagos: commitment is crucial.Crossref | GoogleScholarGoogle Scholar |
Burnham KP, Anderson DR (2002) ‘Model selection and inference: a practical information-theoretical approach.’ 2nd edn. (Springer-Verlag)
Choi GE, Ghimire B, Lee H, et al. (2016) Scarification and stratification protocols for breaking dormancy of Rubus (Rosaceae) species in Korea. Seed Science and Technology 44, 239–252.
| Scarification and stratification protocols for breaking dormancy of Rubus (Rosaceae) species in Korea.Crossref | GoogleScholarGoogle Scholar |
Clark LV, Evans KJ, Jasieniuk M (2013) Origins and distribution of invasive Rubus fruticosus L. agg. (Rosaceae) clones in the Western United States. Biological Invasions 15, 1331–1342.
| Origins and distribution of invasive Rubus fruticosus L. agg. (Rosaceae) clones in the Western United States.Crossref | GoogleScholarGoogle Scholar |
Davies SJJF (1978) Food of emus. Australian Journal of Ecology 3, 411–422.
| Food of emus.Crossref | GoogleScholarGoogle Scholar |
Dixon KW, Merritt DJ, Flematti GR, Ghisalberti EL (2009) Karrikinolide – a phytoreactive compound derived from smoke with applications in horticulture, ecological restoration and agriculture. ISHS Acta Horticulturae 813, 155–170. https://doi.org/10.17660/ActaHortic.2009.813.20
Evans KJ, Weber HE (2003) Rubus anglocandicans (Rosaceae) is the most widespread taxon of European blackberry in Australia. Australian Systematic Botany 16, 527–537.
| Rubus anglocandicans (Rosaceae) is the most widespread taxon of European blackberry in Australia.Crossref | GoogleScholarGoogle Scholar |
Evans KJ, Symon DE, Whalen MA, Hosking JR, Barker RM, Oliver JA (2007) Systematics of the Rubus fruticosus aggregate (Rosaceae) and other exotic Rubus taxa in Australia. Australian Systematic Botany 20, 187–251.
| Systematics of the Rubus fruticosus aggregate (Rosaceae) and other exotic Rubus taxa in Australia.Crossref | GoogleScholarGoogle Scholar |
Fedriani JM, Delibes M (2009) Functional diversity in fruit-frugivore interactions: a field experiment with Mediterranean mammals. Ecography 32, 983–992.
| Functional diversity in fruit-frugivore interactions: a field experiment with Mediterranean mammals.Crossref | GoogleScholarGoogle Scholar |
Gallagher RS (2000) ‘Seeds: the ecology of regeneration in plant communities.’ 3rd edn. (CAB International: Wallingford, UK)
García-Rodriguez A, Albrecht J, Szczutkowska S, Valido A, Farwig N, Selva N (2021) The role of the brown bear Ursus arctos as a legitimate megafaunal seed disperser. Scientific Reports 11, 1282
| The role of the brown bear Ursus arctos as a legitimate megafaunal seed disperser.Crossref | GoogleScholarGoogle Scholar |
Greet J, Webb JA, Cousens RD (2015) Floods reduce the prevalence of exotic plant species within the riparian zone: evidence from natural floods. Applied Vegetation Science 18, 503–512.
| Floods reduce the prevalence of exotic plant species within the riparian zone: evidence from natural floods.Crossref | GoogleScholarGoogle Scholar |
Hancock CN, Ladd PG, Froend RH (1996) Biodiversity and management of riparian vegetation in Western Australia. Forest Ecology and Management 85, 239–250.
| Biodiversity and management of riparian vegetation in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Herd RM (1985) Anatomy and histology of the gut of the emu Dromaius novaehollandiae. Emu – Austral Ornithology 85, 43–46.
| Anatomy and histology of the gut of the emu Dromaius novaehollandiae.Crossref | GoogleScholarGoogle Scholar |
Herd RM, Dawson TJ (1984) Fiber digestion in the emu, Dromaius novaehollandiae, a large bird with a simple gut and high-rates of passage. Physiological Zoology 57, 70–84.
| Fiber digestion in the emu, Dromaius novaehollandiae, a large bird with a simple gut and high-rates of passage.Crossref | GoogleScholarGoogle Scholar |
Hope P, Abbs D, Bhend J, Chiew F, Church J, Ekström M, Kirono D, Lenton A, Lucas C, McInnes K, Moise A, Monselesan D, Mpelasoka F, Timbal B, Webb L, Whetton P (2015) ‘Southern and south-western flatlands cluster report. Climate change in Australia. Projections for Australia’s NRM regions.’ (Eds M Ekström, P Whetton, C Gerbing, M Grose, L Webb, J Risbey) (CSIRO and Bureau of Meteorology: Canberra, ACT, Australia) Available at https://www.climatechangeinaustralia.gov.au/media/ccia/2.2/cms_page_media/168/SSWFLATLANDS_CLUSTER_REPORT.pdf
Hoyle GL, Daws MI, Steadman KJ, Adkins SW (2008) Mimicking a semi-arid tropical environment achieves dormancy alleviation for seeds of Australian native Goodeniaceae and Asteraceae. Annals of Botany 101, 701–708.
| Mimicking a semi-arid tropical environment achieves dormancy alleviation for seeds of Australian native Goodeniaceae and Asteraceae.Crossref | GoogleScholarGoogle Scholar |
Jacobs B, Donoghue MJ, Bouman F, Huysmans S, Smets E (2008) Evolution and phylogenetic importance of endocarp and seed characters in Viburnum (Adoxaceae). International Journal of Plant Sciences 169, 409–431.
| Evolution and phylogenetic importance of endocarp and seed characters in Viburnum (Adoxaceae).Crossref | GoogleScholarGoogle Scholar |
Keith D (1996) Fire-driven extinction of plant populations: a synthesis of theory and review of evidence from Australian vegetation. Proceedings of the Linnean Society of New South Wales 116, 37–78.
Lasheen AM, Blackhurst HT (1956) Biochemical changes associated with dormancy and after-ripening of blackberry seed. Proceedings of the American Society for Horticultural Science 67, 331–340.
Long RL, Gorecki MJ, Renton M, et al. (2015) The ecophysiology of seed persistence: a mechanistic view of the journey to germination or demise. Biological Reviews 90, 31–59.
| The ecophysiology of seed persistence: a mechanistic view of the journey to germination or demise.Crossref | GoogleScholarGoogle Scholar |
Lüdecke D, Ben-Shachar MS, Patil I, Waggoner P, Makowsk D (2021) performance: an R package for assessment, comparison and testing of statistical models. Journal of Open Source Software 6, 3139
| performance: an R package for assessment, comparison and testing of statistical models.Crossref | GoogleScholarGoogle Scholar |
Mian MAR, Skirvin RM, Norton MA, Otterbacher AG (1995) Drying interferes with germination of blackberry (Rubus sp.) seeds in vitro. HortScience 30, 124–126.
| Drying interferes with germination of blackberry (Rubus sp.) seeds in vitro.Crossref | GoogleScholarGoogle Scholar |
Molefe KL, Tedder MJ, Thabethe V, Rushworth I, Downs CT (2020) Role of native avian frugivores in germination facilitation and potential dispersal of invasive American bramble (Rubus cuneifolius) in South Africa. Biological Invasions 22, 1109–1120.
| Role of native avian frugivores in germination facilitation and potential dispersal of invasive American bramble (Rubus cuneifolius) in South Africa.Crossref | GoogleScholarGoogle Scholar |
Moore JN, Brown GR, Lundergan C (1974) Effect of duration of acid scarification on endocarp thickness and seedling emergence of blackberries. HortScience 9, 204–205.
| Effect of duration of acid scarification on endocarp thickness and seedling emergence of blackberries.Crossref | GoogleScholarGoogle Scholar |
Morin L, Evans KJ (2012) Rubus fruticosus L. aggregate – European blackberry. In ‘Biological control of weeds in Australia’. (Eds M Julien, R McFadyen, J Cullen) pp. 499–509. (CSIRO Publishing: Melbourne, Vic., Australia)
Muggeo VMR (2008) segmented: an R package to fit regression models with broken-line relationships. R News 8, 20–25.
Muggeo VMR (2017) Interval estimation for the breakpoint in segmented regression: a smoothed score-based approach. Australian & New Zealand Journal of Statistics 59, 311–322.
| Interval estimation for the breakpoint in segmented regression: a smoothed score-based approach.Crossref | GoogleScholarGoogle Scholar |
Nesme X (1985) Respective effects of endocarp, testa and endosperm, and embryo on the germination of raspberry (Rubus idaeus L.) seeds. Canadian Journal of Plant Science 65, 125–130.
| Respective effects of endocarp, testa and endosperm, and embryo on the germination of raspberry (Rubus idaeus L.) seeds.Crossref | GoogleScholarGoogle Scholar |
Nybom H (1980) Germination in Swedish blackberries (Rubus L. subgenus Rubus). Botaniska Notiser 133, 619–631.
Nybom H (1987) A demographic study of the apomictic blackberry, Rubus nessensis (Rosaceae). Nordic Journal of Botany 7, 365–372.
| A demographic study of the apomictic blackberry, Rubus nessensis (Rosaceae).Crossref | GoogleScholarGoogle Scholar |
Packer JG, Delean S, Kueffer C, Prider J, Abley K, Facelli JM, Carthew SM (2016) Native faunal communities depend on habitat from non-native plants in novel but not in natural ecosystems. Biodiversity and Conservation 25, 503–523.
| Native faunal communities depend on habitat from non-native plants in novel but not in natural ecosystems.Crossref | GoogleScholarGoogle Scholar |
Panetta FD (2004) Seed banks: the bane of the weed eradicator. In ‘Proceedings of the 14th Australian Weeds Conference’, 6–9 September 2004, Wagga Wagga, NSW, Australia. (Eds BM Sindel, SB Johnson) pp. 523–526. (Weed Society of New South Wales)
Renteria JL, Gardener MR, Panetta FD, Crawley MJ (2012) Management of the Invasive Hill Raspberry (Rubus niveus) on Santiago Island, Galapagos: eradication or indefinite control? Invasive Plant Science and Management 5, 37–46.
| Management of the Invasive Hill Raspberry (Rubus niveus) on Santiago Island, Galapagos: eradication or indefinite control?Crossref | GoogleScholarGoogle Scholar |
Rose RC (1919) After-ripening and germination of seeds of Tilia, Sambuchus, and Rubus. Botanical Gazette 67, 281–308.
| After-ripening and germination of seeds of Tilia, Sambuchus, and Rubus.Crossref | GoogleScholarGoogle Scholar |
Sperber K, Steinbrecher T, Graeber K, et al. (2017) Fruit fracture biomechanics and the release of Lepidium didymum pericarp-imposed mechanical dormancy by fungi. Nature Communications 8, 1868
| Fruit fracture biomechanics and the release of Lepidium didymum pericarp-imposed mechanical dormancy by fungi.Crossref | GoogleScholarGoogle Scholar |
Strik BC, Mann J, Finn CE (1996) Percent druplet set varies between blackberry genotypes. Journal of the American Society for Horticultural Science 121, 371–373.
| Percent druplet set varies between blackberry genotypes.Crossref | GoogleScholarGoogle Scholar |
Thorp JR, Lynch R (2000) ‘The determination of Weeds of National Significance.’ (National Weeds Strategy Executive Committee: Launceston, Tas., Australia)
Traveset A (1998) Effect of seed passage through vertebrate frugivores’ guts on germination: a review. Perspectives in Plant Ecology, Evolution and Systematics 1, 151–190.
| Effect of seed passage through vertebrate frugivores’ guts on germination: a review.Crossref | GoogleScholarGoogle Scholar |
van Staden J, Campbell PL (1984) A complex dormancy mechanism in seeds of the weed Rubus cuneifolius. South African Journal of Plant and Soil 1, 48–50.
| A complex dormancy mechanism in seeds of the weed Rubus cuneifolius.Crossref | GoogleScholarGoogle Scholar |
Wada S, Reed BM (2010) Seed coat morphology differentiates blackberry cultivars. Journal of the American Pomological Society 64, 152–161.
Wada S, Reed BM (2011a) Optimized scarification protocols improve germination of diverse Rubus germplasm. Scientia Horticulturae 130, 660–664.
| Optimized scarification protocols improve germination of diverse Rubus germplasm.Crossref | GoogleScholarGoogle Scholar |
Wada S, Reed BM (2011b) Standardizing germination protocols for diverse raspberry and blackberry species. Scientia Horticulturae 132, 42–49.
| Standardizing germination protocols for diverse raspberry and blackberry species.Crossref | GoogleScholarGoogle Scholar |
Wada S, Kennedy JA, Reed BM (2011) Seed-coat anatomy and proanthocyanidins contribute to the dormancy of Rubus seed. Scientia Horticulturae 130, 762–768.
| Seed-coat anatomy and proanthocyanidins contribute to the dormancy of Rubus seed.Crossref | GoogleScholarGoogle Scholar |
White HA, Scott JK, Didham RK (2021) Evidence of range shifts in riparian plant assemblages in response to multidecadal streamflow declines. Frontiers in Ecology and Evolution 9, 605951
| Evidence of range shifts in riparian plant assemblages in response to multidecadal streamflow declines.Crossref | GoogleScholarGoogle Scholar |
Wilson JR, Panetta FD, Lindgren C (2017) ‘Detecting and responding to alien plant incursions.’ (Cambridge University Press: Cambridge, UK)
Yeoh PB, Fontanini L, Carley JA, Russell AT, Dawson KE, Scott JK, Delaisse CYM, Webber BL (2016) Landscape transformations following blackberry decline in the south-west of Western Australia: successful restoration or back to blackberry? In ‘Proceedings of the 20th Australasian Weeds Conference’, 11–15 September 2016, Perth, WA, Australia. (Eds R Randall, S Lloyd, C Borger) pp. 146–151. (WA Weeds Society)


