Lack of fire rather than pollinator absence may drive population decline in the critically endangered Banksia conferta (Proteaceae)
Stephen A. J. Bell A * , Nigel Hunter B and Andrew Steed B
A * , Nigel Hunter B and Andrew Steed B
A Conservation Science Research Group, School of Environmental and Life Sciences, University of Newcastle, Callaghan, NSW 2308, Australia.
B Department of Planning, Industry and Environment, PO Box 488G, Newcastle, NSW 2300, Australia.
Australian Journal of Botany 70(5) 372-383 https://doi.org/10.1071/BT21143
Submitted: 25 November 2021 Accepted: 25 July 2022 Published: 16 August 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context and aim: Stands of the critically endangered Banksia conferta in north-eastern New South Wales show low recruitment and were examined to elucidate whether pollinator absence or fire history best explained this.
Methods: Motion-detection cameras were deployed at three sites to identify potential pollinators, and age structure within stands and past follicle production were assessed through tree measurements and follicle counts.
Key results: In total, 691 fauna-triggered image sequences were recorded between June and December 2019. Six mammal and 10 bird species were detected, but only 10 of these were observed probing inflorescences. White-cheeked Honeyeater and Sugar Glider combined comprised 66% of all fauna interactions, and of all 400 Banksia probes, 45% were from White-cheeked Honeyeaters and 18% were by Sugar Gliders. Different size structures of B. conferta were evident at each site, consistent with older and younger populations post-fire. Significantly more Banksia individuals (5×) were present at the younger site, likely governing pollinator composition through interspecific competition within pollinator guilds, and past follicle production was also greater here.
Conclusions: Pollinator absence is not the cause of low recruitment in this population, and current stand structure and follicle production reflect past fire history. The three sites differed in their dominant fauna foragers, suggesting that B. conferta is a pollinator-generalist. Birds appear to operate as the key pollinating species during the day but are replaced by small mammals at night. Follicle production is higher in younger post-fire plants.
Implications: The absence of fire from older populations for over 55 years is of concern, and population decline seems likely without fire intervention.
Keywords: Banksia, Honeyeaters, mammals, pollinator, population decline, recruitment, threatened, wildfire.
Introduction
Understanding the ecology of threatened species aids effective long-term management (Scheele et al. 2018), and appreciation of the role of pollinator networks and fire are key management objectives in many species (Brown et al. 2017; Carbone et al. 2019). Identifying the balance between managing habitat for pollinators and all other co-habiting species and ecological processes, while also ensuring optimal fire-related recruitment conditions for a target taxon can be problematic, yet without this knowledge some species may undergo insidious decline (Driscoll et al. 2010; McLauchlan et al. 2020). For example, the removal of fire disturbance from habitats in which plant species require this for vital life stages can expedite population decline and result in local extinction (Gill and Bradstock 1995; Keith 1996), and high-frequency fire events may exhaust seed reserves or progressively weaken plants and similarly cause species loss (Keith 1996; Gallagher et al. 2021). Fire impacts on habitat for pollinators can also be dramatic, resulting in temporal shifts in fauna populations while recovery occurs, followed by a return to the structural habitat elements required for pollinators (Potts et al. 2003; Brown et al. 2017; García et al. 2018).
Pollination in the woody shrubs and small trees comprising Banksia (Proteaceae) appears uncomplicated because of their large and showy inflorescences, highly sought after by nectivorous fauna at peak flowering for their copious nectar. Despite this, several studies have shown low rates of seed production in this genus (Collins and Rebelo 1987), irrespective of high visitation rates by pollinators (Vaughton 1988; Carthew 1993a). Pollination in Banksia can be enacted either through vertebrate (mammal, bird) or invertebrate (insects) interactions, including flying (Carthew 1993b; O’Rourke et al. 2020) and non-flying mammals (Carpenter 1978; Goldingay et al. 1991; Hackett and Goldingay 2001; Wooller and Wooller 2001, 2002, 2003; Thavornkanlapachai et al. 2019), and birds (Ramsey 1988; Carthew 1993b; Krauss et al. 2009, 2018; Llorens et al. 2012). Several Banksia species are suspected of being pollinated by multiple faunal groups (Hopper 1980; Evans and Bunce 2000; Wooller and Wooller 2002), with such species commonly being referred to as pollinator-generalists (Saffer 2004). Field observations suggest that birds are the most common visitors to Banksia flowers, but in some cases, flower morphology may favour insects (Sedgley et al. 1993) over vertebrates, and those emitting strong-scented nectar at anthesis tend to attract mammals (Carpenter 1978; Whelan and Burbidge 1980).
Fire is an important ecological process in the life history of many Banksia species, where it is often necessary to promote seed release from an infructescence and permit new recruitment (George 1981; Lamont et al. 2007). A long absence of fire may consequently threaten the persistence of such fire-dependent species in an area (Gill and Mcmahon 1986; Gill and Bradstock 1995; Lamont et al. 2007), and to manage for this, an understanding of species-specific seed production and plant longevity is paramount (Gosper et al. 2013). Seeds in Banksia are either released spontaneously over a short time span (‘non-serotinous’) or are retained unopened in the crown until fire (‘serotinous’). In some cases, differing site conditions in populations of the same species display different levels of serotiny (Whelan et al. 1998; Lamont et al. 2020), and this can affect habitat-management strategies for different populations of these species.
Banksia conferta is a serotinous critically endangered shrub or small tree that in New South Wales (NSW) occurs only in Coorabakh National Park (NSW Scientific Committee 2007). Widely disjunct populations are also present in the Lamington Plateau, Mount Barney and Glasshouse Mountains regions of south-eastern Queensland (George 1981, 1999; Harris et al. 2007), where the species is listed as vulnerable under Queensland legislation. Although reportedly non-lignotuberous and likely killed by fire events (George 1999; Griffith 2005), NSW populations are known to resprout from a plate-like lignotuber (Bell 2017), which can develop into a bulbous mass beneath the ground surface. Flowering occurs over winter, with inflorescences opening florets bottom-to-top, and, just prior to anthesis, a strong musky ‘wet towel’ odour is released (becoming sweeter at anthesis) during prolific flowering events (pers. obs.). Key pollinators in NSW populations are unknown, although Eastern Pygmy Possums are thought to play an important role in Queensland (Harris et al. 2007), and, elsewhere, other Banksia species are favoured dietary foods of this small arboreal possum (Turner 1984, 1985; Bladon et al. 2002; Tulloch and Dickman 2006; Harris et al. 2014). Banksia conferta flowers possess attributes indicative of vertebrate pollination (Sedgley et al. 1993; Ladd et al. 1996), and together with the presence of a strong odour at anthesis suggest mammalian pollination to be important.
Previous research hypothesised that for NSW populations of B. conferta, an absence of arboreal mammals and their pollinating services in the extensively logged former wood production forests of Coorabakh National Park may explain observed low follicle production (Bell 2017). An absence of records for the Eastern Pygmy Possum and other arboreal possums such as the Feather-tail Glider, Squirrel Glider and Sugar Glider (all known nectar feeders of other Banksia spp.) in fauna databases was postulated to correlate with an observed low abundance of the hollow-bearing trees they require (Smith 1973; Harris 2008, 2015). Although numerous records for Sugar Gliders (but few for the other three species) existed for surrounding areas, none was present within Coorabakh National Park. To test this hypothesis (i.e. that low follicle production was due to a dearth of mammalian pollinators), the present study conducted camera-trapping during the 2019 flowering season to document potential pollinators of B. conferta. Additionally, data on population size class (as a surrogate for plant age) and follicle presence (a measure of pollination) were examined against fire history to further investigate poor recruitment in this species. Specifically, this research aimed to determine whether an absence of arboreal mammals within flowering Banksia stands limits pollination and new recruitment, or, alternatively, whether stand structure as determined by fire history provides a better explanation for low seed production.
Materials and methods
Study area
Coorabakh National Park is positioned between the Lansdowne and Comboyne State Forests (part of the Lansdowne Plateau) and lies on the mid-northern coast of New South Wales, approximately 25 km north of the regional town of Taree (Fig. 1). Formerly part of Landsdowne State Forest and including the Big Nellie Flora Reserve, this 1800 ha national park conserves a range of rainforest, wet and dry sclerophyll forests, and minor areas of heath vegetation. Most of the reserve supports tall wet sclerophyll forests of Eucalyptus pilularis, E. agglomerata, E. microcorys, E. saligna, Lophostemon confertus and Syncarpia glomulifera, with subtropical rainforest dominated by Ficus spp., Dendrocnide excelsa, Ceratopetalum apetalum and Archontophoenix cunninghamiana in sheltered gullies (NSW National Parks and Wildlife Service 2007). Habitats supporting B. conferta are drier and often constrained to shallow soils on rock substrate (Redpath et al. 2008). A long history of logging has resulted in high volumes of even-aged trees throughout the park, with very few old-growth trees remaining.
|
|
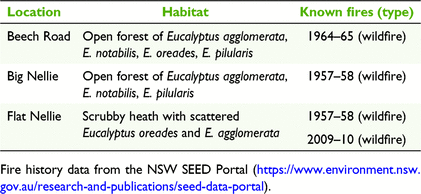
The following three populations of B. conferta (at 450–480 m elevation) were investigated for potential pollinators: Flat Nellie, Beech Road, and Big Nellie. One of these (Flat Nellie) has shown abundant seed production in recent years, whereas the other two (Big Nellie and Beech Road) have displayed negligible seed production (Bell 2017). Four fire events have affected Coorabakh and populations of B. conferta over several decades, during 1957–59, 1963–65, 1990–92 and 2009–10. Study populations were last burnt 11 (Flat Nellie), 56 (Beech Road) and 63 (Big Nellie) years previously (Table 1). On its initial discovery in 2017, the Flat Nellie population was flowering (8 years post-fire), and in 2018 infructescences were recorded on plants from 1 m in height.
|
Camera placement
Fourteen motion-detection cameras (Reconyx Hyperfire HC600) were installed within these three stands of B. conferta on 30 May 2019, including seven at Flat Nellie, five at Beech Road and two at Big Nellie (Fig. 1). These focused on between 1 and 4 inflorescences each (nine at Beech Road, four at Big Nellie, 17 at Flat Nellie; 30 in total) and when triggered were programmed to fire three successive images in quick succession (approximately one every second), so that each trigger captured a three-image sequence. All cameras were positioned near Banksia inflorescences that were in late bud or had not yet reached full anthesis, to maximise chances of capturing fauna interactions. Most inflorescences within the populations had already attained or passed full anthesis, so targeting the last few remaining inflorescences would likely focus fauna activity here. One consequence of this may be that fauna behaviour observed was not representative of that occurring across the full flowering season; however, focusing survey effort on the last remaining inflorescences would ensure sufficient data capture for analysis.
Banksia size class, inflorescence density and follicle presence
Within each site, single randomly placed belt transects (100 × 5 m) were sampled in 2020 (after the completion of camera trapping) to collect data on Banksia size classes, past flowering effort and successful production of follicles. To assess size class and past flowering, diameter-at-breast-height (DBH; later aggregated into six size classes) and height (to the nearest 0.5 m) of all individuals within transects were measured, and the numbers of current and old inflorescences on each individual were tallied. If present, seedlings and individuals less than breast height (1.6 m) were included in the lowest size class. Follicle presence was measured separately on 10 randomly selected Banksia individuals within each transect (with a required minimum of five inflorescences per individual), by counting the number of old inflorescences (irrespective of age) with and without follicles on each plant, assessed by feeling for protuberances beneath the retained filaments. In this context, follicle presence was used as an indicator of successful seed production, but the number of follicles per infructescence was not recorded and no attempt was made to quantify the seedbank.
Data analysis
Camera-capture data were viewed on-screen to detect fauna interactions. All interactions were tabulated against date and time, and whether clear interest was shown in the target inflorescences (recorded as a ‘probe’). Separate interactions within a short time period were differentiated if images captured the subject animal leaving or approaching the target; uncertain movements potentially comprising a single visit were combined and recorded as a single interaction. Univariate statistics were used to determine the most frequent faunal visitors for each site and to assess Banksia density, whereas a linear regression assessed Banksia structure (testing whether height could predict girth) and inflorescence abundance (testing whether height or girth could predict flowering). An independent measures ANOVA was used to test for significance in relative follicle production among the three populations, with Tukey’s HSD post hoc test being used to examine pair-wise comparisons.
Results
Camera monitoring
Cameras were triggered 691 times by fauna (3% of all images); all other images were presumably in response to wind events. Total fauna-triggered image spans varied for each camera, from 23 to 146 days (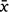 = 67, std = 51), and four cameras returned no fauna images. Although Camera 1 at Flat Nellie commenced recording on the 30 May 2019, it was a month later before the first fauna-triggered image was captured, and all collection finished in that camera by the 22 July. Three cameras (Camera 2 at Flat Nellie, and Cameras 10 and 11 at Beech Road) captured fauna interactions over a total period of 133 and 146 days. The months of June and July were the most active for interactions, corresponding to peak flowering in B. conferta, with the last fauna interactions on 24 October 2019.
= 67, std = 51), and four cameras returned no fauna images. Although Camera 1 at Flat Nellie commenced recording on the 30 May 2019, it was a month later before the first fauna-triggered image was captured, and all collection finished in that camera by the 22 July. Three cameras (Camera 2 at Flat Nellie, and Cameras 10 and 11 at Beech Road) captured fauna interactions over a total period of 133 and 146 days. The months of June and July were the most active for interactions, corresponding to peak flowering in B. conferta, with the last fauna interactions on 24 October 2019.
Fauna visitation
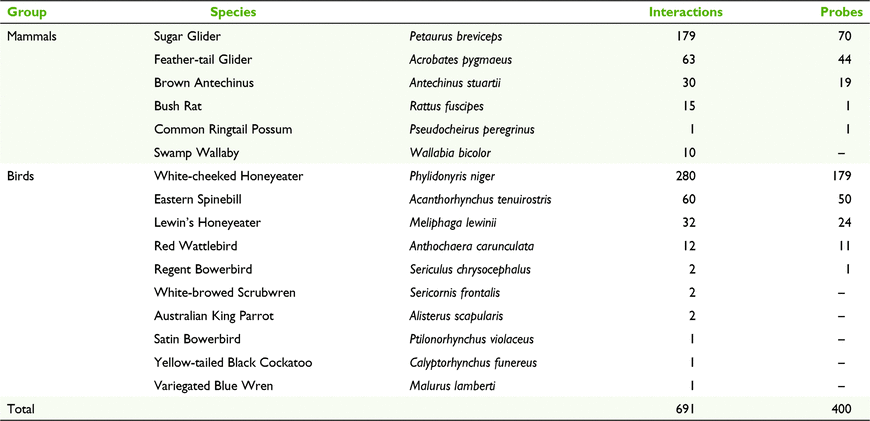
Six mammal and 10 bird species were captured interacting with B. conferta plants, with 10 species seen to probe inflorescences (Table 2). White-cheeked Honeyeaters and Sugar Gliders combined comprised 66% of all fauna interactions (n = 691), and of all 400 Banksia probes, 45% were from White-cheeked Honeyeaters and 18% from Sugar Gliders. Six fauna species (one mammal, five birds) interacted with inflorescences but did not probe flowers. Highest activity was seen at Flat Nellie (188 bird and 75 mammal probes), followed by Beech Road (77 bird, 77 mammal) and then Big Nellie (0 birds, 18 mammals).
|
The three survey sites differed in the dominant fauna foragers (Fig. 2). White-cheeked Honeyeaters clearly dominated captures and probes at Flat Nellie (although predominantly at Camera 5, presumably because of a superior flowering effort here), whereas this species was virtually non-existent at both Beech Road (two probes) and Big Nellie (absent). At Beech Road, two species (Sugar Glider and Eastern Spinebill) comprised the bulk of all probes, and this site supported the greatest diversity of fauna foragers (nine species). Only two species were detected at Big Nellie, with the Sugar Glider comprising almost all interactions, but no birds were recorded.
Banksia size class
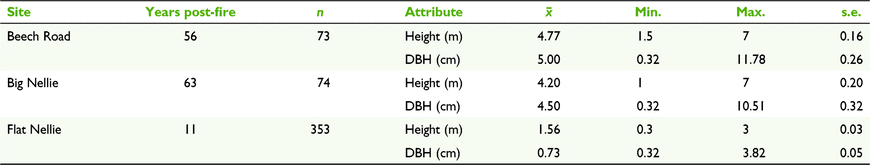
Flat Nellie (n = 353; 7060 plants/ha) supported more than double the density of Banksia than did Beech Road (n = 73; 1460 plants/ha) and Big Nellie (n = 74; 1480 plants/ha) combined. Four Banksia individuals at Flat Nellie attained a maximum height of 3 m (mean 1.56 m ± s.e. 0.03), whereas at both Beech Road (mean 4.77 m ± s.e. 0.16) and Big Nellie (mean 4.20 m ± s.e. 0.20), the largest individuals reached 7 m (seven and six individuals respectively). There were differences in Banksia height and girth at all sites (Table 3), and linear regression identified significant relationships predicting girth from height within all three; strong relationships were evident at Beech Road (R2 = 0.68, F(1,71) = 147.94, P < 0.001; y = −1.4536 + 1.3537x) and Big Nellie (R2 = 0.79, F(1,72) = 273.11, P < 0.001; y = −1.4766 + 1.4215x), but a moderate relationship only occurred at Flat Nellie (R2 = 0.33, F(1,351) = 171.52, P < 0.001; y = −0.6843 + 0.9076x). The latter result may be attributed to sampling issues relating to the smaller size of plants at this site, and/or differing habitat conditions (open heath/low woodland vs open forest). Collectively, linear regression on inflorescence presence (current and old) with Banksia size found a strong predictive relationship with girth (R2 = 0.60, F(1,498) = 735.04, P < 0.001; y = −3.8236 + 6.7845x), but only a moderate predictive relationship with height (R2 = 0.47, F(1,498) = 447.57, P < 0.001; y = −12.1222 + 8.7893x).
|
Structurally, the three Banksia populations displayed differing proportions of large and small individuals as determined through DBH data, reflecting fire history (Fig. 3). Beech Road supported a diverse profile of many individuals in the 2.1–8 cm DBH classes, but with limited numbers in smaller and larger classes. Flat Nellie supported no individuals >4 cm DBH, and presumably any larger standing individuals that may have remained after the wildfire in 1957–58 were consumed during the more recent fire in 2009–10. Big Nellie supported 89% of individuals within the 0–8 cm classes, suggesting that either a more recent recruitment event may have occurred well after the 1957–58 fire event, or, more likely, that resprouts from subsurface roots of larger individuals influenced size distribution in the data. Resprouting is previously unknown for B. conferta (discussed later) and was noted only at Big Nellie but may have also occurred at the other two populations, limiting interpretation of the structural profiles for each population.
Follicle production and fire history
The collective number of infructescences with follicles compared with those without across 10 Banksia individuals in each population at Beech Road (63 with, 154 without) and Big Nellie Road (78 with, 203 without) contrasted with those at Flat Nellie (110 with, 58 without). Relative follicle production differed among the three populations (F = 10.05, P < 0.001; Fig. 4), and Tukey’s HSD test identified significant differences between Flat Nellie with Beech Road (Q = 5.29, P = 0.003), and Flat Nellie and Big Nellie (Q = 5.67, P = 0.001). There was no significant difference in follicle presence between Beech Road and Big Nellie. The Flat Nellie Banksia population (last burnt 11 years ago) produced more fertile infructescences than did either of the other two populations (burnt 55 and 62 years previously), and although available data are scant, there is a trend suggestive of reduced reproductive output with time.
|
|
Discussion
Potential pollinators
The hypothesis that an absence or low density of arboreal mammals within flowering Banksia stands at Coorabakh was limiting pollination opportunities was not supported in this study. Two of four postulated pollinating mammals (Feather-tail Glider and Sugar Glider) were detected at all three sites investigated. Sugar Gliders were dominant at Big Nellie, and this species (and Eastern Spinebill) was equally dominant at Beech Road. Additionally, Bush Rats and Common Ringtail Possums (both Flat Nellie), and Brown Antechinus (Beech Road) were (rarely) captured visiting inflorescences. Five bird species (White-cheeked Honeyeater, Eastern Spinebill, Lewin’s Honeyeater, Red Wattlebird and Regent Bowerbird) were observed probing inflorescences, with White-cheeked Honeyeaters predominating in the only stand consistently producing infructescences (Flat Nellie). Very few instances of invertebrates foraging on flowers were captured by cameras, with ants and moths noted on <10 occasions; however, cameras were not set to target this fauna group. Given the range of faunal visitors, B. conferta is likely to be a pollinator-generalist (see Saffer 2004), with birds (and particularly White-cheeked Honeyeater) operating as the key species during the day and small mammals (particularly Sugar Glider) at night. Morphological features of pollen presenters and styles in B. conferta (George 1981) are also indicative of a species likely to be pollinated by vertebrates; rigid styles are a trait adapted to vertebrate pollination, particularly in the role this rigidity plays in forcing pollen entry into the pollination chamber via the stigmatic groove (Sedgley et al. 1993; Ladd et al. 1996).
White-cheeked Honeyeaters have been implicated in the pollination of other Banksia species elsewhere (Collins and Spice 1986; Dalgleish 1999); however, in some areas pollen transfer via this species appears limited. For example, Hackett and Goldingay (2001) found only low pollen loads of Banksia ericifolia and B. integrifolia on White-cheeked Honeyeaters in comparison to Silvereyes (Zosterops lateralis), suggesting that on the NSW northern coast this honeyeater was not an important pollinator for those species. A similar trend was found by Paton and Turner (1985) for Banksia ericifolia near Sydney. In Western Australia, Hopper (1980) observed that New Holland Honeyeaters (Phylidonyris novaehollandiae) carried considerably more pollen from Banksia occidentalis than did White-cheeked Honeyeaters, but concluded that this was perhaps related to territorial and aggressive interactions between these two species.
At Coorabakh, Eastern Spinebill foraged predominantly at the Beech Road site where White-cheeked Honeyeaters were rare. At this location, this species dominated diurnal visitation to B. conferta, but nocturnal visitation by Sugar Gliders was equally important. Eastern Spinebill is low in the hierarchy of foraging guilds (Ford 1979; Ford and Paton 1982), and other studies have inferred that spinebills (Eastern and Western) avoid feeding in Banksia stands dominated by larger honeyeaters (Hopper 1980; Newland and Wooller 1985; Paton and Turner 1985; Armstrong 1991). The presence of a resident population of White-cheeked Honeyeaters at Flat Nellie is, therefore, likely to competitively exclude extensive foraging of B. conferta by Eastern Spinebill at that location. The dominance of White-cheeked Honeyeaters at Flat Nellie, and its absence or low abundance at both Big Nellie and Beech Road, may reflect resource availability. At Flat Nellie, Banksia densities of 7060 plants per hectare are substantially greater than the 1460–1480 plants per hectare at Beech Road and Big Nellie respectively. This represents a considerable contrast in nectar resource availability, and it is possible that insufficient nectar at Beech Road and Big Nellie may preclude a resident population of White-cheeked Honeyeaters persisting in these areas. Such a situation was reported by Ford and Pursey (1982), who found Eastern Spinebills to be the most abundant honeyeater at their site in north-eastern NSW and suggested that perhaps the available nectar supply was inadequate to maintain larger species there.
The Eastern Pygmy Possum is thought to be an important pollinator of B. conferta in Queensland (Harris et al. 2007), and Banksia pollen forms a major dietary component in this species (van Tets and Whelan 1997). The lack of captures of Eastern Pygmy Possums at any site in the Coorabakh stands of B. conferta (even in the recently burnt Flat Nellie site) suggests this species to be of low importance here, pollination being effected primarily by other species. However, low rates of capture of Eastern Pygmy Possums in surveys and seasonal variations in abundance mean that population densities are often under-estimated (Harris and Goldingay 2005; Goldingay and Keohan 2017), and there remains the possibility that this species may still frequent the area. Other arboreal mammals captured in the present study are also known feeders and potential pollinators of Banksia species (see Goldingay et al. 1991; Carthew 1993b; Hackett and Goldingay 2001; O’Rourke et al. 2020), although none has been reported feeding on B. conferta. Brown Antechinus and Bush Rat have occasionally been recorded frequenting Banksia flowers (Goldingay et al. 1987; van Tets and Whelan 1997; Goldingay 2000); however, this does not seem to be widespread (see Watts and Braithwaite 1978; Hall 1980; Fox and Archer 1984; Carron et al. 1990; Gray et al. 2016). Although primarily a ground-dwelling mammal, Bush Rats have been observed foraging for food and nectar at heights of up to 4 m (Carpenter 1978; O’Rourke et al. 2020); so, the single record of this species probing B. conferta flowers at Coorabakh is not unusual.
Stand structure and follicle production
The three stands of B. conferta at Coorabakh display different size classes consistent with older (Beech Road, Big Nellie) and younger (Flat Nellie) populations, although during data collection it was discovered that B. conferta is capable of resprouting from lateral subsurface roots when older individuals are dying or are under severe stress (discussed later). This may have affected categorisation of a small component of the younger cohort of individuals, particularly when assessing older dead individuals at Big Nellie; however, we have no way to retrospectively ascertain the extent of this. Despite this, the strong correlation between plant height and DBH evident in the older stands was not replicated at the younger stand. Significantly more individuals were present at Flat Nellie than at Beech Road or Big Nellie, with this site supporting nearly five times the number of plants of the other two. As noted earlier, such high densities of Banksia may well determine the pollinator composition present at a site, in this case allowing White-cheeked Honeyeaters to dominate the area.
Follicle production was significantly greater at Flat Nellie than at Beech Road and Big Nellie and could be aligned with fire history. Incidental observations of follicles on older plants at Beech Road and Big Nellie showed many to have been in a decayed state, implying that any seed contained therein may be inviable. This has implications on future recruitment, particularly in long-unburnt stands that are poorly serviced by pollinators and where new seed production is consequently limited. These plants may ultimately reach a point where low attention by pollinators restricts input into the canopy seed bank, and the species is lost from that area on the death of standing individuals.
Inter-fire recruitment and asexual reproduction
Very few true seedlings (<20 cm high) have been observed within stands of B. conferta at Coorabakh, and those that are present rarely seem to persist for long. This trait has been reported in other Banksia species and is representative of serotinous species that release seed predominantly after fire. Enright et al. (1996) found that no seedlings of Banksia hookeriana establishing between fire events survived for more than 1 year, likely being due to competition with other plants. For B. conferta, no seedlings were recorded within study transects, but scattered individuals elsewhere in the populations suggest that sporadic establishment does occur. Assuming that some of these seedlings do persist, distinguishing them from resprouts emanating via subsurface roots is difficult without careful excavation. During data collection, it was noted at Big Nellie that resprouts from roots were associated predominantly with older trees. These suckering resprouts (∼1 m high) may be a response to water stress or non-fire-related tree damage, given that fire has been absent from this site for 62 years; however, this is unclear.
The suckering habit from lateral roots is rare in the Banksia genus, with B. elegans, B. paludosa and certain forms of B. marginata and B. integrifolia being examples of the few known (George 1981). He et al. (2011) suggested that such clonality via suckering is an advanced growth trait in Banksia and allows individuals to persist through drought and fire for up to 1000 years. For the functionally sterile Banksia elegans, Lamont (1988) found the suckering habit to be significant in persistence and spread after fire, although subsequent post-fire flowering relied on epicormic recovery of larger plants and medium-sized individuals resprouting from lignotubers, with limited flowering in suckers. In the context of the present study, it is plausible that, like in Banksia elegans, recovery from fire in B. conferta is aided by both a lignotuber and suckering resprouts from subsurface lateral roots; however, it is yet to be determined how important seedling recruitment post-fire is in this species. Both flowering and fruiting were more abundant at the most recently burnt Flat Nellie site than at Beech Road or Big Nellie, suggesting that seed-bank development is more likely to occur here than at the two other sites, which are currently persisting primarily through asexual reproduction.
Lack of fire and implications
A substantially variable at the sites examined during this study is fire history and how it influences stand structure, floristic composition, and habitat. Flat Nellie, the only site where abundant follicles have been produced in recent years, has burnt twice in recorded history, namely, 63 and 11 years ago. In contrast, Beech Road and Big Nellie last burnt 56 and 63 years ago respectively, and the current individuals at those two sites are now of considerable age. The more recent fire at Flat Nellie explains the prevalence of Banksia individuals of shorter stature (presumably all new germinants, but possibly also older resprouted individuals from lignotubers and/or lateral root suckers), and it is likely that better flowering and fruiting has occurred here because plants (or ramets) are younger. Elapsed time since fire and its influence on flowering and fruiting can differ in resprouting versus fire-sensitive Banksia species (e.g. most seed production in the resprouting Banksia oblongifolia occurred <10 years versus mostly >16 years in the fire-sensitive Banksia ericifolia; Zammit and Westoby 1987; and see Gill and Mcmahon 1986; Enright et al. 1996; McCaw 2008). For B. conferta, time to first flowering at Flat Nellie was observed to be 8 years, possibly shorter, and such a timeframe fits the model of other resprouting species.
Fire plays a major role in the ecology of seed release in many Banksia species (Lamont et al. 2007; Huss et al. 2019). Queensland populations of B. conferta are reportedly serotinous (George 1981), releasing seeds from the canopy only after a fire event. The very few seedlings and numerous closed follicles observed at Coorabakh in recent years suggest that these populations also display this trait. Serotiny can be variable within a species and reflect environmental conditions (Cowling and Lamont 1985; Whelan et al. 1998; Lamont et al. 2020), but, nevertheless, it compromises persistence in some species when fire is excluded from a population for extended periods of time (Lamont et al. 2007). For example, Gill and Mcmahon (1986) estimated the viable canopy seed store of Banksia ornata to increase with stand age up to 38 years post-fire, but declined by 50 years owing to dieback and senescence. There is currently no data on the collective number of infructescences and viable seed store within the canopy of B. conferta populations, but it is plausible that many hundreds or thousands of seeds may be present. Data from this study found a total of 665 old and current inflorescences on 30 plants across all three populations; however, only 251 of these had follicles developed (no counts of viable seeds per infructescence were undertaken). Despite this, the absence of fire from both Beech Road and Big Nellie populations for over 55 years remains a concern, and with observations reported here of low follicle production (despite the presence of pollinators) and decaying older follicles, it seems likely that little new recruitment will occur without intervention. Fortunately, the ability of this species to resprout from lignotubers and subsurface roots should ensure persistence through drought and other stressors while awaiting fire.
Deriving appropriate fire prescriptions is difficult for many ecosystems and target species (Gosper et al. 2013), and these can vary across different landscapes. The results from this study suggest that a fire (prescribed or wildfire) in one or both of Beech Road and Big Nellie will be beneficial to rejuvenate habitat and promote germination of those viable seeds that remain in the canopy before further follicle decay occurs. Importantly, this should be followed by a fire-free interval of perhaps 10 years to allow maturation and restoration of the canopy seed store. Smith et al. (2021) highlighted how short intervals between fires threaten the ability of this species to mature and produce seed in Queensland. However, at the Coorabakh populations, lack of fire, at least initially, appears to be constraining replenishment of the population, but fires in quick succession, which restricts seed development, will remain a threat to this species.
Ongoing studies into the ecology and viability of pollination and seed development in B. conferta are also needed to further inform management of this species. Pollination in Banksia is typically low across the genus (Collins and Rebelo 1987; Goldingay and Carthew 1998), and in part this is due to the inactivity and inconsistency of pollinators (Copland and Whelan 1989). Controlled pollination studies (e.g. Collins and Spice 1986; Ramsey 1988) aimed at identifying limitations to pollination would be informative in this regard. Seed production is highly variable over most species, and poor follicle development can follow copious flowering events across individuals and populations, with few clear reasons (Vaughton 1988; Carthew 1993a). Successful fruit set as low as 0.92–1.77% has been reported for Banksia spinulosa (Carthew 1993a), but up to 9.5% for B. neoanglica (Vaughton 1988), and the average fruit set across 15 species was found to be just 2.5% by Collins and Rebelo (1987). Gaining an understanding of seed production and expected rates of fruit set in B. conferta will assist future management of this species after fire events.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
Data collected and analysed for this project were completed under contract to the first author, funded via the NSW Government Saving our Species initiative and supported in-kind by remaining authors.
Acknowledgements
Thanks go to Luke Foster and Michael Murray for assistance in camera placement/retrieval and fauna identifications, and the anonymous reviewers and the editors for constructive comments on an earlier draft. Funding for parts of this work was made available through the NSW Governments Saving Our Species program.
References
Armstrong DP (1991) Aggressiveness of breeding territorial honeyeaters corresponds to seasonal changes in nectar availability. Behavioral Ecology and Sociobiology 29, 103–111.| Aggressiveness of breeding territorial honeyeaters corresponds to seasonal changes in nectar availability.Crossref | GoogleScholarGoogle Scholar |
Bell S (2017) New insights into the ecology of the critically endangered ‘Banksia conferta’ (Proteaceae) from the mid-north coast of NSW. Australasian Plant Conservation 26, 15–18. https://search.informit.org/doi/10.3316/informit.178254394062662
Bladon RV, Dickman CR, Hume ID (2002) Effects of habitat fragmentation on the demography, movements and social organisation of the eastern pygmy-possum (Cercartetus nanus) in northern New South Wales. Wildlife Research 29, 105–116.
| Effects of habitat fragmentation on the demography, movements and social organisation of the eastern pygmy-possum (Cercartetus nanus) in northern New South Wales.Crossref | GoogleScholarGoogle Scholar |
Brown J, York A, Christie F, McCarthy M (2017) Effects of fire on pollinators and pollination. Journal of Applied Ecology 54, 313–322.
| Effects of fire on pollinators and pollination.Crossref | GoogleScholarGoogle Scholar |
Carbone LM, Tavella J, Pausas JG, Aguilar R (2019) A global synthesis of fire effects on pollinators. Global Ecology and Biogeography 28, 1487–1498.
| A global synthesis of fire effects on pollinators.Crossref | GoogleScholarGoogle Scholar |
Carpenter FL (1978) Hooks for mammal pollination? Oecologia 35, 123–132.
| Hooks for mammal pollination?Crossref | GoogleScholarGoogle Scholar |
Carron PL, Happold DCD, Bubela TM (1990) Diet of two sympatric Australian subalpine rodents, Mastacomys fuscus and Rattus fuscipes. Australian Wildlife Research 17, 479–489.
| Diet of two sympatric Australian subalpine rodents, Mastacomys fuscus and Rattus fuscipes.Crossref | GoogleScholarGoogle Scholar |
Carthew SM (1993a) Patterns of flowering and fruit production in a natural-population of Banksia spinulosa. Australian Journal of Botany 41, 465–480.
| Patterns of flowering and fruit production in a natural-population of Banksia spinulosa.Crossref | GoogleScholarGoogle Scholar |
Carthew SM (1993b) An assessment of pollinator visitation to Banksia spinulosa. Australian Journal of Ecology 18, 257–268.
| An assessment of pollinator visitation to Banksia spinulosa.Crossref | GoogleScholarGoogle Scholar |
Collins BG, Rebelo T (1987) Pollination biology of the Proteaceae in Australia and southern Africa. Australian Journal of Ecology 12, 387–421.
| Pollination biology of the Proteaceae in Australia and southern Africa.Crossref | GoogleScholarGoogle Scholar |
Collins BG, Spice J (1986) Honeyeaters and the pollination biology of Banksia prionotes (Proteaceae). Australian Journal of Botany 34, 175–185.
| Honeyeaters and the pollination biology of Banksia prionotes (Proteaceae).Crossref | GoogleScholarGoogle Scholar |
Copland BJ, Whelan RJ (1989) Seasonal variation in flowering intensity and pollination limitation of fruit set in four co-occurring Banksia species. Journal of Ecology 77, 509–523.
| Seasonal variation in flowering intensity and pollination limitation of fruit set in four co-occurring Banksia species.Crossref | GoogleScholarGoogle Scholar |
Cowling RM, Lamont BB (1985) Variation in serotiny of three Banksia species along a climatic gradient. Australian Journal of Ecology 10, 345–350.
| Variation in serotiny of three Banksia species along a climatic gradient.Crossref | GoogleScholarGoogle Scholar |
Dalgleish E (1999) Effectiveness of invertebrate and vertebrate pollinators and the influence of pollen limitation and inflorescence position on follicle production of Banksia aemula (family Proteaceae). Australian Journal of Botany 47, 553–562.
| Effectiveness of invertebrate and vertebrate pollinators and the influence of pollen limitation and inflorescence position on follicle production of Banksia aemula (family Proteaceae).Crossref | GoogleScholarGoogle Scholar |
Driscoll DA, Lindenmayer DB, Bennett AF, Bode M, Bradstock RA, Cary GJ, Clarke MF, Dexter N, Fensham R, Friend G, Gill M, James S, Kay G, Keith DA, MacGregor C, Russell-Smith J, Salt D, Watson JEM, Williams RJ, York A (2010) Fire management for biodiversity conservation: key research questions and our capacity to answer them. Biological Conservation 143, 1928–1939.
| Fire management for biodiversity conservation: key research questions and our capacity to answer them.Crossref | GoogleScholarGoogle Scholar |
Enright NJ, Lamont BB, Marsula R (1996) Canopy seed bank dynamics and optimum fire regime for the highly serotinous shrub, Banksia hookeriana. Journal of Ecology 84, 9–17.
| Canopy seed bank dynamics and optimum fire regime for the highly serotinous shrub, Banksia hookeriana.Crossref | GoogleScholarGoogle Scholar |
Evans KM, Bunce A (2000) A comparison of the foraging behaviour of the eastern pygmy-possum (Cercartetus nanus) and nectarivorous birds in a Banksia integrifolia woodland. Australian Mammalogy 22, 81–86.
| A comparison of the foraging behaviour of the eastern pygmy-possum (Cercartetus nanus) and nectarivorous birds in a Banksia integrifolia woodland.Crossref | GoogleScholarGoogle Scholar |
Ford HA (1979) Interspecific competition in Australian honeyeaters: depletion of common resources. Australian Journal of Ecology 4, 145–164.
| Interspecific competition in Australian honeyeaters: depletion of common resources.Crossref | GoogleScholarGoogle Scholar |
Ford HA, Paton DC (1982) Partitioning of nectar sources in an Australian honeyeater community. Australian Journal of Ecology 7, 149–159.
| Partitioning of nectar sources in an Australian honeyeater community.Crossref | GoogleScholarGoogle Scholar |
Ford HA, Pursey JF (1982) Status and feeding of the Eastern Spinebill Acanthorhynchus tenuirostris at New England National Park, north-eastern NSW. Emu - Austral Ornithology 82, 203–211.
| Status and feeding of the Eastern Spinebill Acanthorhynchus tenuirostris at New England National Park, north-eastern NSW.Crossref | GoogleScholarGoogle Scholar |
Fox BJ, Archer E (1984) The diets of Sminthopsis murina and Antechinus stuartii (Marsupialia: Dasyuridae) in sympatry. Australian Wildlife Research 11, 235–248.
| The diets of Sminthopsis murina and Antechinus stuartii (Marsupialia: Dasyuridae) in sympatry.Crossref | GoogleScholarGoogle Scholar |
Gallagher RV, Allen S, Mackenzie BDE, et al. (2021) High fire frequency and the impact of the 2019–2020 megafires on Australian plant diversity. Diversity and Distributions 27, 1166–1179.
| High fire frequency and the impact of the 2019–2020 megafires on Australian plant diversity.Crossref | GoogleScholarGoogle Scholar |
García Y, Castellanos MC, Pausas JG (2018) Differential pollinator response underlies plant reproductive resilience after fires. Annals of Botany 122, 961–971.
| Differential pollinator response underlies plant reproductive resilience after fires.Crossref | GoogleScholarGoogle Scholar |
George AS (1981) The genus Banksia L.f. (Proteaceae). Nuytsia 3, 239–473. https://biostor.org/reference/217708
George AS (1999) Banksia (Proteaceae). In ‘Flora of Australia. Vol. 17B’. (Ed. A Wilson) pp. 175–251 (ABRS/CSIRO Australia: Melbourne, Vic., Australia) Available at https://www.dcceew.gov.au/sites/default/files/env/pages/6d8c5c3b-8545-437e-b9b3-944ac95ee07a/files/flora-australia-17b-proteaceae-3-hakea-dryandra.pdf
Gill AM, Bradstock R (1995) Extinction of biota by fires. In ‘Conserving biodiversity: threats and solutions’. (Eds R Bradstock, T Auld, D Keith, R Kingsford, D Lunney, D Sivertsen) pp. 309–322. (Surrey Beatty and Sons: Sydney, NSW, Australia) Available at http://hdl.handle.net/102.100.100/233144?index=1
Gill AM, Mcmahon A (1986) A postfire chronosequence of cone, follicle and seed production in Banksia ornata. Australian Journal of Botany 34, 425–433.
| A postfire chronosequence of cone, follicle and seed production in Banksia ornata.Crossref | GoogleScholarGoogle Scholar |
Goldingay RL (2000) Small dasyurid marsupials: are they effective pollinators? Australian Journal of Zoology 48, 597–606.
| Small dasyurid marsupials: are they effective pollinators?Crossref | GoogleScholarGoogle Scholar |
Goldingay RL, Carthew SM (1998) Breeding and mating systems of Australian Proteaceae. Australian Journal of Botany 46, 421–437.
| Breeding and mating systems of Australian Proteaceae.Crossref | GoogleScholarGoogle Scholar |
Goldingay RL, Keohan J (2017) Population density of the eastern pygmy-possum in a heath–woodland habitat. Australian Journal of Zoology 65, 391–397.
| Population density of the eastern pygmy-possum in a heath–woodland habitat.Crossref | GoogleScholarGoogle Scholar |
Goldingay RL, Carthew SM, Whelan RJ (1987) Transfer of Banksia spinulosa pollen by mammals: implications for pollination. Australian Journal of Zoology 35, 319–325.
| Transfer of Banksia spinulosa pollen by mammals: implications for pollination.Crossref | GoogleScholarGoogle Scholar |
Goldingay RL, Carthew SM, Whelan RJ (1991) The importance of non-flying mammals in pollination. Oikos 61, 79–87.
| The importance of non-flying mammals in pollination.Crossref | GoogleScholarGoogle Scholar |
Gosper CR, Prober SM, Yates CJ (2013) Estimating fire interval bounds using vital attributes: implications of uncertainty and among-population variability. Ecological Applications 23, 924–935.
| Estimating fire interval bounds using vital attributes: implications of uncertainty and among-population variability.Crossref | GoogleScholarGoogle Scholar |
Gray EL, Burwell CJ, Baker AM (2016) Benefits of being a generalist carnivore when threatened by climate change: the comparative dietary ecology of two sympatric semelparous marsupials, including a new endangered species (Antechinus arktos). Australian Journal of Zoology 64, 249–261.
| Benefits of being a generalist carnivore when threatened by climate change: the comparative dietary ecology of two sympatric semelparous marsupials, including a new endangered species (Antechinus arktos).Crossref | GoogleScholarGoogle Scholar |
Griffith SJ (2005) Banksia conferta subsp. conferta in Coorabakh National Park: Preliminary observations and guidelines for fire management. Unpublished Report to Manning Area of the Parks and Wildlife Division, Department of Environment and Conservation.
Hackett DJ, Goldingay RL (2001) Pollination of Banksia spp. by non-flying mammals in north-eastern New South Wales. Australian Journal of Botany 49, 637–644.
| Pollination of Banksia spp. by non-flying mammals in north-eastern New South Wales.Crossref | GoogleScholarGoogle Scholar |
Hall S (1980) The diets of two coexisting species of Antechinus (Marsupialia: Dasyuridae). Australian Wildlife Research 7, 365–378.
| The diets of two coexisting species of Antechinus (Marsupialia: Dasyuridae).Crossref | GoogleScholarGoogle Scholar |
Harris JM (2008) Cercartetus nanus (Diprotodontia: Burramyidae). Mammalian Species 815, 1–10.
| Cercartetus nanus (Diprotodontia: Burramyidae).Crossref | GoogleScholarGoogle Scholar |
Harris JM (2015) Acrobates pygmaeus (Diprotodontia: Acrobatidae). Mammalian Species 47, 32–44.
| Acrobates pygmaeus (Diprotodontia: Acrobatidae).Crossref | GoogleScholarGoogle Scholar |
Harris JM, Goldingay RL (2005) Detection of the eastern pygmy-possum Cercartetus nanus (Marsupialia: Burramyidae) at Barren Grounds Nature Reserve, New South Wales. Australian Mammalogy 27, 85–88.
| Detection of the eastern pygmy-possum Cercartetus nanus (Marsupialia: Burramyidae) at Barren Grounds Nature Reserve, New South Wales.Crossref | GoogleScholarGoogle Scholar |
Harris JM, Gynther I, Eyre TJ, Goldingay RL, Mathieson MT (2007) Distribution, habitat and conservation status of the eastern pygmy-possum Cercartetus nanus in Queensland. Australian Zoologist 34, 209–221.
| Distribution, habitat and conservation status of the eastern pygmy-possum Cercartetus nanus in Queensland.Crossref | GoogleScholarGoogle Scholar |
Harris JM, Goldingay RL, Brooks LO (2014) Population ecology of the eastern pygmy-possum (Cercartetus nanus) in a montane woodland in southern New South Wales. Australian Mammalogy 36, 212–218.
| Population ecology of the eastern pygmy-possum (Cercartetus nanus) in a montane woodland in southern New South Wales.Crossref | GoogleScholarGoogle Scholar |
He T, Lamont BB, Downes KS (2011) Banksia born to burn. New Phytologist 191, 184–196.
| Banksia born to burn.Crossref | GoogleScholarGoogle Scholar |
Hopper SD (1980) Bird and mammal pollen vectors in Banksia communities at Cheyne Beach, Western Australia. Australian Journal of Botany 28, 61–75.
| Bird and mammal pollen vectors in Banksia communities at Cheyne Beach, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Huss JC, Fratzl P, Dunlop JWC, Merritt DJ, Miller BP, Eder M (2019) Protecting offspring against fire: lessons from Banksia seed pods. Frontiers in Plant Science 10, 283
| Protecting offspring against fire: lessons from Banksia seed pods.Crossref | GoogleScholarGoogle Scholar |
Keith D (1996) Fire-driven extinction of plant populations: a synthesis of theory and review of evidence from Australian vegetation. Proceedings of the Linnean Society of NSW 116, 37–78. https://biostor.org/reference/68152
Krauss SL, He T, Barrett LG, Lamont BB, Enright NJ, Miller BP, Hanley ME (2009) Contrasting impacts of pollen and seed dispersal on spatial genetic structure in the bird-pollinated Banksia hookeriana. Heredity 102, 274–285.
| Contrasting impacts of pollen and seed dispersal on spatial genetic structure in the bird-pollinated Banksia hookeriana.Crossref | GoogleScholarGoogle Scholar |
Krauss SL, Roberts DG, Phillips RD, Edwards C (2018) Effectiveness of camera traps for quantifying daytime and nighttime visitation by vertebrate pollinators. Ecology and Evolution 8, 9304–9314.
| Effectiveness of camera traps for quantifying daytime and nighttime visitation by vertebrate pollinators.Crossref | GoogleScholarGoogle Scholar |
Ladd PG, Alkema AJ, Thomson GJ (1996) Pollen presenter morphology and anatomy in Banksia and Dryandra. Australian Journal of Botany 44, 447–471.
| Pollen presenter morphology and anatomy in Banksia and Dryandra.Crossref | GoogleScholarGoogle Scholar |
Lamont BB (1988) Sexual versus vegetative reproduction in Banksia elegans. Botanical Gazette 149, 370–375.
| Sexual versus vegetative reproduction in Banksia elegans.Crossref | GoogleScholarGoogle Scholar |
Lamont BB, Enright NJ, Witkowski ETF, Groeneveld J (2007) Conservation biology of banksias: insights from natural history to simulation modelling. Australian Journal of Botany 55, 280–292.
| Conservation biology of banksias: insights from natural history to simulation modelling.Crossref | GoogleScholarGoogle Scholar |
Lamont BB, Pausas JG, He T, Witkowski ETF, Hanley ME (2020) Fire as a selective agent for both serotiny and nonserotiny over space and time. Critical Reviews in Plant Sciences 39, 140–172.
| Fire as a selective agent for both serotiny and nonserotiny over space and time.Crossref | GoogleScholarGoogle Scholar |
Llorens TM, Byrne M, Yates CJ, Nistelberger HM, Coates DJ (2012) Evaluating the influence of different aspects of habitat fragmentation on mating patterns and pollen dispersal in the bird-pollinated Banksia sphaerocarpa var. caesia. Molecular Ecology 21, 314–328.
| Evaluating the influence of different aspects of habitat fragmentation on mating patterns and pollen dispersal in the bird-pollinated Banksia sphaerocarpa var. caesia.Crossref | GoogleScholarGoogle Scholar |
McCaw L (2008) Variation in age to first flowering and fruiting of Banksia baxteri and Banksia coccinea at the Stirling Range, south-western Australia. Journal of the Royal Society of Western Australia 91, 269–273. https://www.rswa.org.au/publications/Journal/91(4)/VOL%2091%20PT%204%20269-273.pdf
McLauchlan KK, Higuera PE, Miesel J, et al. (2020) Fire as a fundamental ecological process: research advances and frontiers. Journal of Ecology 108, 2047–2069.
| Fire as a fundamental ecological process: research advances and frontiers.Crossref | GoogleScholarGoogle Scholar |
Newland CE, Wooller RD (1985) Seasonal changes in a honeyeater assemblage in Banksia woodland near Perth, Western Australia. New Zealand Journal of Zoology 12, 631–636.
| Seasonal changes in a honeyeater assemblage in Banksia woodland near Perth, Western Australia.Crossref | GoogleScholarGoogle Scholar |
NSW National Parks and Wildlife Service (2007) Coorabakh National Park plan of management. NSW National Parks and Wildlife Service, Department of Environment and Climate Change NSW, Australia.
NSW Scientific Committee (2007) Banksia conferta A.S.George subsp. conferta: critically endangered species listing. Final Determination. Available at https://www.environment.nsw.gov.au/Topics/Animals-and-plants/Threatened-species/NSW-Threatened-Species-Scientific-Committee/Determinations/Final-determinations/2004-2007/Banksia-conferta-A-S-George-subsp-conferta-critically-endangered-species-listing
O’Rourke RL, Anson JR, Saul AM, Banks PB (2020) Limits to alien black rats (Rattus rattus) acting as equivalent pollinators to extinct native small mammals: the influence of stem width on mammal activity at native Banksia ericifolia inflorescences. Biological Invasions 22, 329–338.
| Limits to alien black rats (Rattus rattus) acting as equivalent pollinators to extinct native small mammals: the influence of stem width on mammal activity at native Banksia ericifolia inflorescences.Crossref | GoogleScholarGoogle Scholar |
Paton DC, Turner V (1985) Pollination of Banksia ericifolia Smith: birds, mammals and insects as pollen vectors. Australian Journal of Botany 33, 271–286.
| Pollination of Banksia ericifolia Smith: birds, mammals and insects as pollen vectors.Crossref | GoogleScholarGoogle Scholar |
Potts SG, Vulliamy B, Dafni A, Ne’eman G, O’Toole C, Roberts S, Willmer P (2003) Response of plant-pollinator communities to fire: changes in diversity, abundance and floral reward structure. Oikos 101, 103–112.
| Response of plant-pollinator communities to fire: changes in diversity, abundance and floral reward structure.Crossref | GoogleScholarGoogle Scholar |
Ramsey MW (1988) Differences in pollinator effectiveness of birds and insects visiting Banksia menziesii (Proteaceae). Oecologia 76, 119–124.
| Differences in pollinator effectiveness of birds and insects visiting Banksia menziesii (Proteaceae).Crossref | GoogleScholarGoogle Scholar |
Redpath P, Steed A, Kendall P, Snelson B (2008) Banksia conferta A. S. George subspecies conferta: a critically endangered species, its habitat, condition, extent and management. Scientific Services Division and Conservation Protection and Regulation Division, DECC, North Coast Region.
Saffer VM (2004) Are diel patterns of nectar production and anthesis associated with other floral traits in plants visited by potential bird and mammal pollinators? Australian Journal of Botany 52, 87–92.
| Are diel patterns of nectar production and anthesis associated with other floral traits in plants visited by potential bird and mammal pollinators?Crossref | GoogleScholarGoogle Scholar |
Scheele BC, Legge S, Armstrong DP, Copley P, Robinson N, Southwell D, Westgate MJ, Lindenmayer DB (2018) How to improve threatened species management: an Australian perspective. Journal of Environmental Management 223, 668–675.
| How to improve threatened species management: an Australian perspective.Crossref | GoogleScholarGoogle Scholar |
Sedgley M, Sierp M, Wallwork MA, Fuss AM, Thiele K (1993) Pollen presenter and pollen morphology of Banksia L.f. (Proteaceae). Australian Journal of Botany 41, 439–464.
| Pollen presenter and pollen morphology of Banksia L.f. (Proteaceae).Crossref | GoogleScholarGoogle Scholar |
Smith MJ (1973) Petaurus breviceps. Mammalian Species 30, 1–5.
| Petaurus breviceps.Crossref | GoogleScholarGoogle Scholar |
Smith I, Velasquez E, Pickering C (2021) Quantifying potential effect of 2019 fires on national parks and vegetation in South-East Queensland. Ecological Management & Restoration 22, 160–170.
| Quantifying potential effect of 2019 fires on national parks and vegetation in South-East Queensland.Crossref | GoogleScholarGoogle Scholar |
Thavornkanlapachai R, Byrne M, Yates CJ, Ladd PG (2019) Degree of fragmentation and population size do not adversely affect reproductive success of a rare shrub species, Banksia nivea (Proteaceae), in a naturally fragmented community. Botanical Journal of the Linnean Society 191, 261–273.
| Degree of fragmentation and population size do not adversely affect reproductive success of a rare shrub species, Banksia nivea (Proteaceae), in a naturally fragmented community.Crossref | GoogleScholarGoogle Scholar |
Tulloch AI, Dickman CR (2006) Floristic and structural components of habitat use by the eastern pygmy-possum (Cercartetus nanus) in burnt and unburnt habitats. Wildlife Research 33, 627–637.
| Floristic and structural components of habitat use by the eastern pygmy-possum (Cercartetus nanus) in burnt and unburnt habitats.Crossref | GoogleScholarGoogle Scholar |
Turner V (1984) Banksia pollen as a source of protein in the diet of two Australian marsupials Cercartetus nanus and Tarsipes rostratus. Oikos 43, 53–61.
| Banksia pollen as a source of protein in the diet of two Australian marsupials Cercartetus nanus and Tarsipes rostratus.Crossref | GoogleScholarGoogle Scholar |
Turner V (1985) The ecology of the eastern pygmy possum Cercartetus nanus and its association with Banksia. PhD Thesis, Monash University, Melbourne, Vic., Australia.
van Tets IG, Whelan RJ (1997) Banksia pollen in the diet of Australian mammals. Ecography 20, 499–505.
| Banksia pollen in the diet of Australian mammals.Crossref | GoogleScholarGoogle Scholar |
Vaughton G (1988) Pollination and seed set of Banksia spinulosa: evidence for autogamy. Australian Journal of Botany 36, 633–642.
| Pollination and seed set of Banksia spinulosa: evidence for autogamy.Crossref | GoogleScholarGoogle Scholar |
Watts CHS, Braithwaite RW (1978) The diet of Rattus lutreolus and five other rodents in southern Victoria. Australian Wildlife Research 5, 47–57.
| The diet of Rattus lutreolus and five other rodents in southern Victoria.Crossref | GoogleScholarGoogle Scholar |
Whelan RJ, Burbidge AH (1980) Flowering phenology, seed set and bird pollination of five Western Australian Banksia species. Australian Journal of Ecology 5, 1–7.
| Flowering phenology, seed set and bird pollination of five Western Australian Banksia species.Crossref | GoogleScholarGoogle Scholar |
Whelan RJ, de Jong NH, von der Burg S (1998) Variation in bradyspory and seedling recruitment without fire among populations of Banksia serrata (Proteaceae). Australian Journal of Ecology 23, 121–128.
| Variation in bradyspory and seedling recruitment without fire among populations of Banksia serrata (Proteaceae).Crossref | GoogleScholarGoogle Scholar |
Wooller SJ, Wooller RD (2001) Seed set in two sympatric banksias, Banksia attenuata and B. baxteri. Australian Journal of Botany 49, 597–602.
| Seed set in two sympatric banksias, Banksia attenuata and B. baxteri.Crossref | GoogleScholarGoogle Scholar |
Wooller SJ, Wooller RD (2002) Mixed mating in Banksia media. Australian Journal of Botany 50, 627–631.
| Mixed mating in Banksia media.Crossref | GoogleScholarGoogle Scholar |
Wooller RD, Wooller SJ (2003) The role of non-flying animals in the pollination of Banksia nutans. Australian Journal of Botany 51, 503–507.
| The role of non-flying animals in the pollination of Banksia nutans.Crossref | GoogleScholarGoogle Scholar |
Zammit C, Westoby M (1987) Population structure and reproductive status of two Banksia shrubs at various times after fire. Vegetatio 70, 11–20. http://www.jstor.org/stable/20038124


