Using vital statistics and core-habitat maps to manage critically endangered orchids in the Western Australian wheatbelt
Mark C. BrundrettSchool of Plant Biology, Faculty of Natural and Agricultural Sciences, University of Western Australia, Crawley, WA 6009, Australia; and Department of Parks and Wildlife, Swan Region, Locked Bag 104, Bentley Delivery Centre, WA 6983, Australia. Email: mark.brundrett@dpaw.wa.gov.au, mark.brundrett@uwa.edu.au
Australian Journal of Botany 64(1) 51-64 https://doi.org/10.1071/BT15087
Submitted: 8 April 2015 Accepted: 26 November 2015 Published: 12 February 2016
Journal Compilation © CSIRO Publishing 2016 Open Access CC BY-NC-ND
Abstract
Vital-statistics data concerning population viability were gathered for four of the rarest orchids in Western Australia using surveys to define population sizes and habitat areas and annual measurements of plant demographics. These orchids were Caladenia melanema, C. graniticola, C. williamsiae and Drakaea isolata from the wheatbelt of Western Australia. This agricultural area has a Mediterranean climate with unreliable rainfall, and is >80% cleared of native vegetation. Surveys with 10–30 volunteers increased population-size estimates by up to 10 times and provided spatial data to define core habitat areas. These areas included most of the individuals of a species, but were only 2–10 ha in size. Within these areas, orchids were often highly aggregated in patches a few metres wide, potentially resulting in a high degree of intraspecific competition. Vital statistics were obtained using 4-m wide and 30–50-m-long transects to measure rates of emergence, flowering, grazing and seed-set for each orchid. Plants emerging at the same position in different years were considered to be the same individual, but most emerged in new positions. Many plants emerged just once in 4 years, and 2–3 years of dormancy was common. Emergence frequencies were used to provide estimates of population sizes that were two or three times larger than suggested by data from a single year. Seed production was typically very low. Grazing by kangaroos and rabbits was most severe for C. melanema, but was greatly reduced by fencing. Severe drought prevented flowering of C. graniticola in the driest year, whereas other species were more resilient. These orchids are likely to persist as long as there are some years where rainfall is sufficient for flowering and seed set followed by a year with adequate rain for seed germination. Populations of all these orchids were stable or increasing, but they are still at high risk of extinction because of the impacts of increasing soil salinity or fire on their habitats. These species are unlikely to spread elsewhere in the highly cleared and fragmented wheatbelt. Intervention by hand-pollination, grazing protection and translocation to new locations is required to mitigate these risks. Results were summarised in vital statistics report cards with thresholds set to inform conservation management for these species. Core habitat maps and vital-statistics report cards should also be valuable new tools for terrestrial-orchid conservation in other biomes.
Additional keywords: demographics, orchid conservation, pollination, rare flora, seed set.
Introduction
The South-west Floristic Region of Western Australia is a globally recognised centre of plant species richness and endemism, coupled with a high degree of habitat loss (Myers et al. 2000; Hopper and Gioia 2004; www.biodiversityhotspots.org, accessed 2009). This high plant diversity is linked to a long period since major tectonic or glacial disturbance, highly infertile soils and periodic minor disturbances such as drought (Hopper 2009). Unfortunately, the exceptionally high biodiversity in south-western Western Australia (WA) faces many threats that are primarily of human origin. This is one of the most stressed regions in Australia, with a high degree of land clearing (>80%) and fragmentation, as well as declining vegetation health linked to secondary soil salinity, drought, weeds and other factors (Commonwealth of Australia 2002).
As the largest plant family globally (>25 000 species), the orchids are considered to have the highest rate of speciation, the highest rate of extinctions and the most rare species of any plant family (Molvray et al. 2000; Chase et al. 2015). Most WA orchids occur in the south-west, which has a Mediterranean-type climate with cool, wet winters, followed by 5–8 months of summer drought when orchids aestivate as dormant tubers. Despite the long, dry summer, south-western WA is one of the world’s diversity hotspots for terrestrial orchids with ~400 species, most of which are endemic; however, their diversity is highest in coastal areas with more rainfall (Brundrett 2014). In 2013, there were 40 taxa of WA orchids designated as Rare Flora and 55 as Priority Species requiring further surveys (florabase.dpaw.wa.gov.au, accessed December 2003). The Rare Flora meet IUCN criteria for population size and habitat area, suggesting that they are threatened with extinction (IUCN 2012; Table 1). The majority of both rare and common orchids in WA have highly specific associations with mycorrhizal fungi and insect pollinators and this may explain why some of them are restricted to very small habitat areas, despite having wind-dispersed seeds (Brundrett 2007). Orchids in the present study are listed as Critically Endangered (the most threatened category of Declared Rare Flora in WA) because they are likely to become extinct in the wild without intervention. The main threats to these orchids result from the scarcity and fragmentation of suitable new habitats and the impacts of factors such as weeds, herbivores, infrequent pollination, salinity, drought and fire (Brown et al. 1998).

|
This publication presents some of the results of the Wheatbelt Orchid Rescue (WOR) Project, which was a Lotterywest-funded collaboration between the Western Australian Native Orchid Study and Conservation Group (WANOSCG), the Friends of Kings Park and the Department of Environment and Conservation (DEC). The work presented here concerns four of the rarest orchids in WA, including the granite spider orchid (Caladenia graniticola), the ballerina orchid (C. melanema), William’s spider orchid (C. williamsiae) and the lonely hammer orchid (Drakaea isolata). The present research aimed to help conserve these Critically Endangered orchids by obtaining knowledge required for conservation management and directly contributing to actions listed in existing recovery plans (Table 1). More specifically, the present research aimed to (1) provide better estimates of population sizes by harnessing volunteer assistance, (2) measure orchid mortality, seed set and recruitment, (3) measure orchid habitat areas and (4) identify the most important threats to species. The second phase of the present project, which concerned recovery actions such as the propagation and translocation of orchids, will be described in a subsequent paper.
Materials and methods
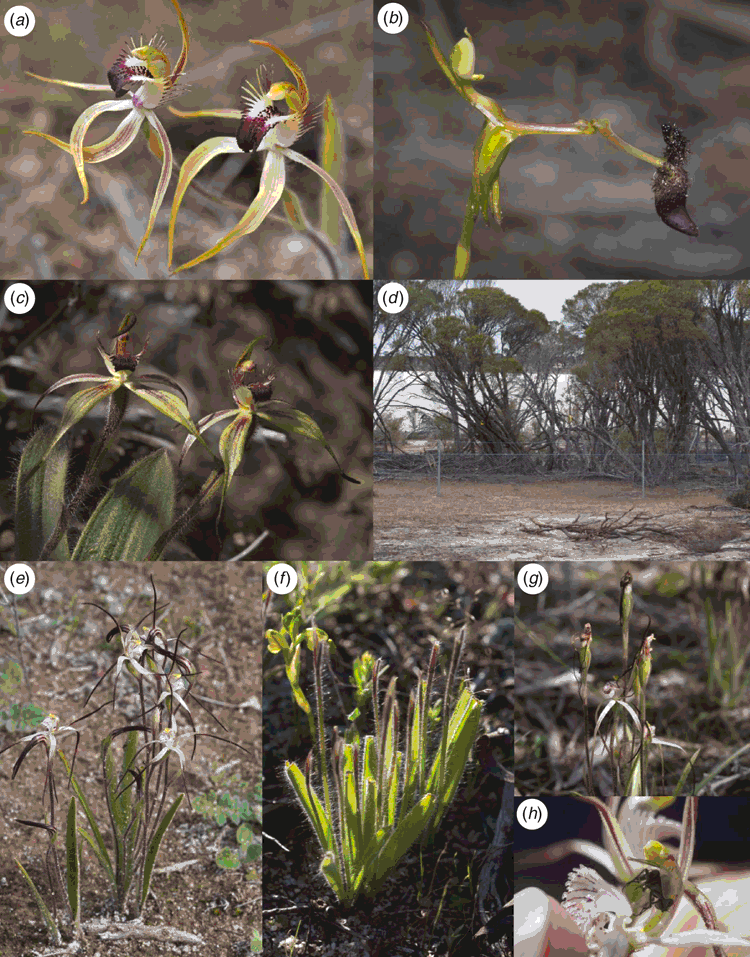
Characteristics of species and their habitat types, rainfall and the approximate locations of the orchid populations studied are summarised in Table 1 and Fig. 1. The orchids studied were all long-lived perennial geophytes that are readily identifiable and have been monitored by periodical surveys for several decades (Fig. 2). Soils of these sites were sandy loams with very low fertility levels (Brundrett 2011).

|
Extensive habitat-area surveys
All surveys were run by the author and attended by DEC conservation staff whenever possible. These survey trips were attended by up to 30 volunteers (WANOSCG members) who had up to five decades of experience in recognising and locating uncommon orchids. There was a substantial commitment from volunteers because trips lasted 3–6 days and involved travel distances well over 1000 km (Brundrett 2012). At each location, volunteers and staff were divided into several groups, each of which recorded numbers of leaves, plants, flowers and seed associated with a GPS coordinate. The impacts of threats such as grazing or weeds were also recorded. The number of plants counted was compared with data published in Interim Recovery Plans for each species (Table 1) to assess population-size trends and investigate the impact of survey effort and rainfall on numbers of orchids detected at the same locations. The overall condition of vegetation was assessed using Landmonitor vegetation-change imagery based on satellite images from 1988 and 2013 (landgate.wa.gov.au, accessed 2011). Recorded GPS coordinates were used to map areas of ‘critical’ and ‘core’ habitats for each species, as defined below.
Critical habitat is identified in the Australian Environment Protection and Biodiversity Conservation Act 1999 as being habitat essential for the survival of a listed threatened species or community. Habitat means the biophysical medium or media (1) occupied (continuously, periodically or occasionally) by an organism or group of organisms, or (2) once occupied (continuously, periodically or occasionally) by an organism or group of organisms, and into which organisms of that kind have the potential to be reintroduced.
Core habitat, as defined here, is the most essential area(s) for survival of the taxa with highest densities of and/or the majority of currently known individuals. This area is also the most susceptible to threats such as, for example, disturbance, fire, weeds and animal grazing. Multiple separate areas, if defined, are ranked in order of importance.
Intensive demographics studies
Permanent transects were used to measure flowering, seed set and survival rates in a fixed area for 4 years in a row for three orchid species (C. melanema, C. graniticola, C. williamsiae). For each orchid, a 30–50-m long × 4-m wide transect was established in the largest (or only known) population. The length of transects was dictated by the size of habitats and was oriented to include as many individuals as possible in the densest area of occupation for each species. Transects established in 2007 were marked with steel posts at both ends and monitored several times each year until 2010. Plants on transects were counted during spring (August or September) to assess plant emergence, flowering and preliminary seed set, and again in late spring to determine final seed set (October or November). Grazing was assessed in each visit because it often increased during the year. The relationship between total annual or winter rainfall and the number of emerging and flowering plants was also investigated using data from nearby weather stations published by the Australian Bureau of Meteorology (www.bom.gov.au, accessed 2011).
For each orchid plant, the distance along the transect axis and perpendicular distance from it was recorded. These coordinates were used to identify plants that were assumed to be the same individual if they emerged at the same location on different years (within 2 cm). The accuracy in measurements was increased by inserting permanent steel pegs as fixed reference points every 5 m along transects. The identification of individual plants was used to determine how often each plant emerged or flowered over the 4-year period of observation.
For Drakaea isolata, photo monitoring of tagged plants was used instead of establishing a transect because of the widely scattered nature of clumps of individuals and the existence of a series of older photos showing the relative position of the prostrate leaves within groups of plants next to a fixed point (a steel peg with label). Photographs of the same location taken on different years were compared by tracing outlines of leaves from these photos. The resulting circles were rotated and transformed in a vector-drawing program (Adobe Illustrator, Adobe, San Jose, CA, USA), so that the relative positions of the fixed post and leaves were as close together as possible. These transformations were necessary because each photo was taken from a different direction and angle downward.
Results
Population surveys
The population survey for C. graniticola occurred in 2008, on a year when substantial numbers of orchids emerged as a result of above-average rainfall in winter (Fig. 3). A team of 30 experienced volunteers searched in suitable habitats for 3 days within Dragon Rocks Nature Reserve, where three populations of this orchid were known to occur. This survey resulted in the discovery of over 300 plants, being an order of magnitude increase in the size of populations from earlier surveys (Fig. 4). The majority of plants were flowering; however, it was also possible to reliably identify non-flowering individuals by distinguishing their leaves from those of other orchids. These orchids were primarily associated with sheoak woodlands (Allocasuarina huegliana and A. campestris) over granite rock, although some were in other habitat types nearby. All of the areas occupied by C. graniticola were very small patches, totalling 18 ha in a very large nature reserve (332 km2). For this species, the number of plants emerging and flowering is strongly influenced by rainfall (Fig. 5a). The most important core habitat area contained 217 plants and was only 150 × 150 m in size. These plants are densely aggregated into two smaller areas less than 50 m wide. In total, there were ~300 plants in all three local populations, so the granite spider orchid is a highly threatened species (small populations occur in 2 other reserves).

|

|

|
Surveys for the ballerina orchid (Caladenia melanema), with the assistance of several volunteers, occurred over several years in Melaleuca lateriflora shrublands on the margins of salt lakes. These surveys found four new subpopulations of this orchid and two new populations that were ~10 km from the main population. These, presumably, arose recently by seed dispersal, because the number of plants within all subpopulations has increased substantially since monitoring began (Fig. 5d). Even though a greater number of plants and several new populations were found, 90% of known plants of this species were confined to small patches of fringing shrubland within chain of salt lakes with a total area 3 × 1 km in size (Fig. 6a). Satellite imagery provides evidence that the canopy of shrubs under which C. melanema grows has substantially declined in cover over the past 20 years (Fig. S1, available as supplementary material for this paper). Because most C. melanema plants occur under the canopy of M. lateriflora, the long-term sustainability of these shrubs is likely to be critical for orchid populations. Recruitment of M. lateriflora seedlings has not been observed at the site and needs to be encouraged by grazing protection or planting seedlings raised in a nursery.

|
A full survey of suitable habitats in the nature reserve where Caladenia williamsiae occurs was undertaken by three people in 2010 to count individuals and to accurately map habitat areas for this extremely rare orchid. Despite the low rainfall in 2010 (Fig. 3), considerably more plants were counted than expected on the basis of earlier surveys of the same area (450), but these were concentrated in a very narrow strip of core habitat ~2 ha in size (Fig. 6b) and plants were grouped together in small patches within this area. All populations of this orchid occur within a critical habitat area of ~1 km2 in a single nature reserve. This orchid was discovered in 1999 and surveys of the same area between 2000 and 2006 detected from 6 to 143 plants (Department of Environment and Conservation 2007b). The apparent increase in population size following the 2010 survey is probably due to increased survey effort, which was required to overcome detectability problems, as C. williamsiae is a small orchid that often grows under shrubs.
Survey data for Drakaea isolata in 2007 with 29 volunteers revealed almost 300 individuals, of which 50 were flowering. The number of plants found was determined, to a large extent, by the number of people searching for them, because earlier surveys found as few as 77 plants in the same area, whereas a major survey with volunteer assistance found 250 plants in 1989 (Phillimore et al. 2000). Thus, the population size and habitat area for this orchid have not changed much since 1989. Leaves of this species were aggregated into 56 groups, which may represent the number of individuals of this species, because up to 50 leaves were found in each group and probably arise from clonal division. Almost all known plants of D. isolata occur in a small square area of ~10 ha. This area is at a very high risk from rising saline groundwater, because it is next to a salt lake where severely salt-affected areas are expanding (Fig. S1).
Detailed demographic studies
Caladenia graniticola
Severe winter drought had a substantial impact on flowering of of C. graniticola, especially in 2010 when rainfall was less than half of average (Fig. 3) and all plants aborted before flowering. Emergence and flowering of C. graniticola were correlated with rainfall (Fig. 5a).
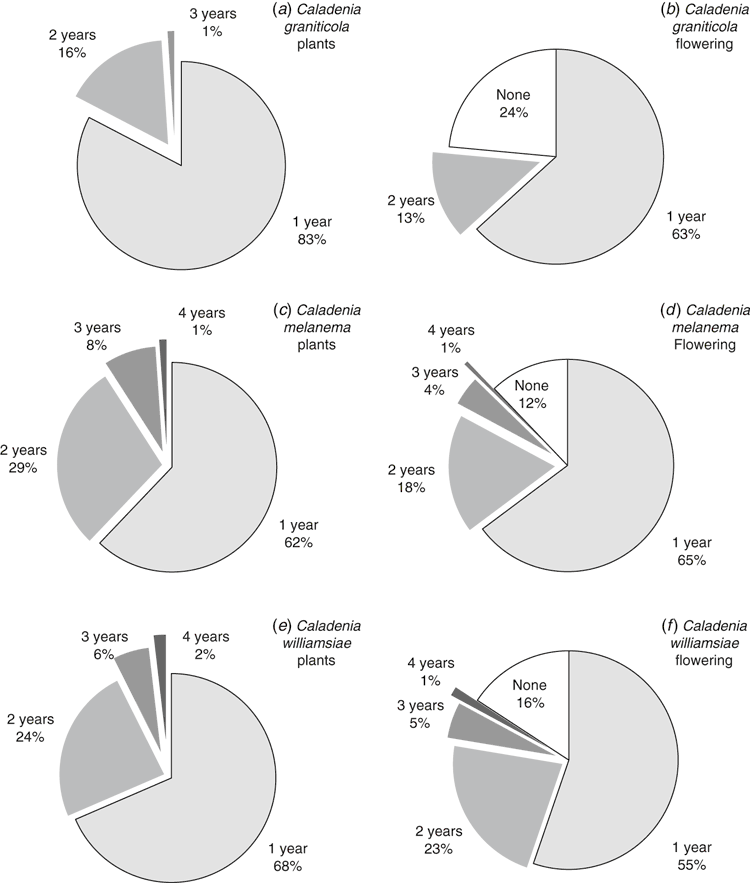
The emergence of plants at different positions on the transect (Fig. 7a) allowed individual plant emergence to be observed over 3 years (data from Year 4 were not available because of severe drought). For this orchid, only ~1/2 of emergent plants produced a single flower and very few set seed or produced two flowers, even on years with a relatively wet winter (Figs 8a, 9a). As shown by Fig. 10a, b, on average, 37% of plants emerged each year and 17% flowered. It seems that ~1/2 of the orchids that exist as dormant tubers emerged in 2008, which was a wet year, whereas only 1/3 or 1/4 of these plants emerged in years with winter drought. There were limited impacts of grazing at this site. This orchid has a relatively low frequency of annual emergence from dormant tubers and the proportion of plants that emerge, but fail to flower, is also greater than for other species and none flowered more than twice over 4 years (Fig. 10a, b). The average length of dormancy was close to 2 years, but was measured only for 3 years because of drought in Year 4 (Fig. 11a).

|

|

|
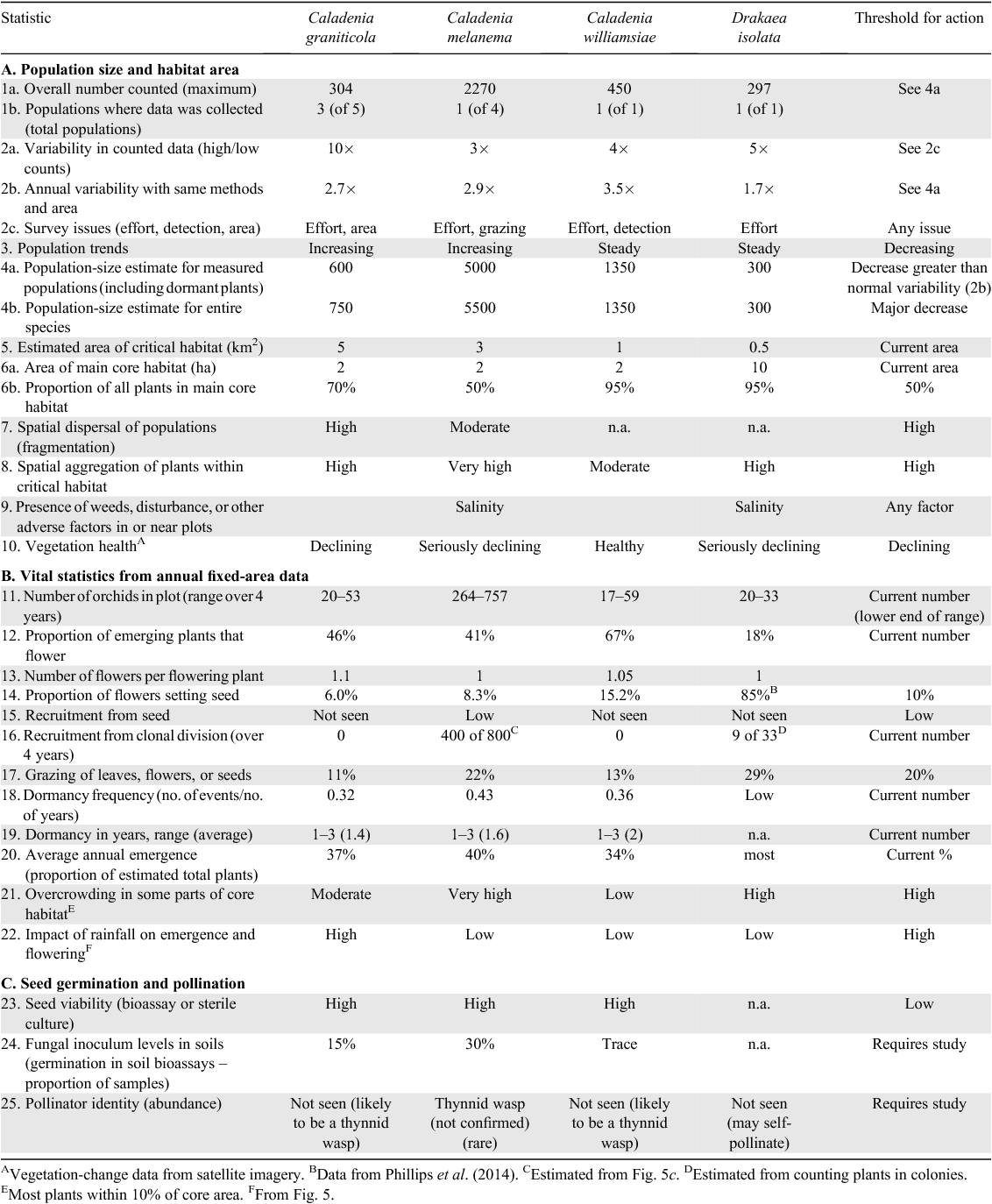
Data on orchid emergence rates were used in combination with survey data, to provide a revised estimate for orchid population size. Because ~1/2 of the detected plants on the transect emerged in 2008 when 300 plants were counted during surveys, it would suggest that there are ~600 plants in total in all three populations in the single nature reserve where most plants of this species occur (Table 2). This confirms that C. graniticola is at high risk of extinction (populations occur in 2 other reserves, but contain less than 150 additional plants).
|
Caladenia melanema
There was no strong correlation between rainfall and plant numbers for C. melanema, but annual trends tended to be overwhelmed by the steady population-growth trend over time (Fig. 5b, d). As shown in Fig. 8b, plants were very densely aggregated at several points along the 50-m transect where clumps of plants are almost touching, with 50–62% of all plants on the transect growing in a single patch ~1 m wide. This made identifying individual plants within the most crowded area impossible, so only plants in the first 15 m of the transect were identified by location (Fig. 7b). Dense clumps of orchids result from clonal division by daughter-tuber production next to the parent tuber, and these locations were also hotspots for reproduction from seed, resulting in new clumps of orchids within close proximity to each other. Each clump had 1–30 leaves and 1–14 flowers, with an average of 4.6 leaves and 2.5 flowers per clump in 2009. All flowers were single (Fig. 9b). There was good pollination of C. melanema flowers, but many were grazed before seeds matured (Fig. 8b). A thynnid wasp (Rhytidothynnus sp.) was observed to be a potential pollinator, whereas flies were abundant on years with warm winter weather and became stuck in flowers. Flies block the stigma because they are not strong enough to escape, and so prevent pollination by other insects (Fig. 2h).
Data on relative plant positions (Fig. 7b) showed that, on average, 40% emerged each year and 16% flowered, whereas seed set was only 2% of all plants (dormant plus emergent). Over 60% of plants emerged and flowered only once in 4 years (Fig. 10c, d), and 3 years of consecutive dormancy or two episodes of dormancy within 4 years were most common (Fig. 11b). These data suggest that there were ~1350 plants on the transect (including dormant plants), so there was an estimated total of 4000–5500 plants within the 3-km2 critical habitat area of this species (Table 2).
In response to severe grazing of plants observed throughout the critical habitat area in 2007, a 10 × 10 m enclosure of 1-m-high rabbit-proof fencing was erected on the transect that summer when plants were dormant. Subsequently, 75–100% of uneaten seedpods on the transect were located in the enclosure. The type of grazing differed within the enclosure, where flowers were sometimes eaten, but there was almost no grazing of leaves. Four new enclosures were erected in 2009 to protect other subpopulations and these also greatly reduced grazing, but did not eliminate it. It is not clear what caused grazing within fenced enclosures.
Caladenia williamsiae
Monitoring plants on a 50 × 4 m transect for 4 years showed that grazing and infrequent seed production were the most significant threats to C. williamsiae (Figs 7c, 8c). Grazing impacts, especially of flowers, varied from 6% of plants in 2008 and 2010, to 26% in 2009 (Fig. 8c). The numbers of plants emerging or flowering varied considerably from year to year, but remained low each year and were not strongly linked to rainfall (Fig. 5d). Relatively few plants set seed, except in 2007, where 28% had capsules (Fig. 8c), so pollination (presumably by a thynnid wasp) is unreliable. Overall, rates of seed set are probably adequate, whereas the volume of seed in capsules is low compared with that in most other Caladenia species. As shown in Fig. 9c, from 60% to 90% of plants flowered, and most of these produced a single flower. The fact that most plants remain dormant each year limits their reproductive potential and may be due to the exposed habitat of this orchid, which grows on upland ridges within very well drained lateritic gravel. It was also observed that grazed plants are often growing in the open, whereas those protected under the canopy of shrubs were less likely to be grazed. There is clear evidence of high kangaroo population levels at the site (tracks, scats and sleeping areas).
Comparison of the same plants showed that most appeared only once in 4 years and flowering was even less frequent (Fig. 10e, f). Only 32% of plants emerged more than once at the same position, only 29% of plants flowered more than once and 16% did not flower at all in 4 years. The majority of plants were dormant for 2 or 3 years in total, with one or two dormancy episodes in 4 years (Fig. 11c). The number of individuals on the transect was estimated to be 108, of which 34% emerged and 22% flowered, on average, each year (Table 2). The transect emergence data in combination with survey data provided a revised estimate for an orchid population size of ~1400 plants for all populations of the William’s spider orchid (Table 2).
Drakaea isolata
Photographs taken by DEC staff of a tagged group of leaves in 2003 and 2004 were compared with those taken by the author in 2007 at the same coordinates to compare leaf and flower numbers (Fig. S2, available as Supplementary material for this paper). Comparisons of photos required image transformation to overcome varying camera positions. Many leaves occupy similar positions in all photos, whereas others present in 2007 appear to have recruited since 2004. There are also two leaves present in the first photos that may have died since 2003, or were dormant. In 2004, there were eight inflorescences at this location, but all were grazed. Comparison of five groups of tagged plants showed that most of the plants observed in 2003 were also found in 2007; however, Groups 4 and 5 may no longer exist (Brundrett 2011). It seems that the larger groups of D. isolata are stable over time. Unlike the Caladenia species, there was little evidence that substantial numbers of D. isolata plants were dormant, because most leaves appear in all three photographs of each group. Leaves of this orchid are relatively xeromorphic (Brundrett 2014). Each leaf is connected to a separate tuber, whereas all those in a group are likely to be the same clonal individual.
Discussion
The Wheatbelt Orchid Rescue Project established that surveys that included substantial numbers of highly experienced volunteers were very effective at both counting numbers of individuals and mapping their core habitat areas within large nature reserves. Both the time spent at locations and areas covered increased considerably in surveys with volunteers. Comparisons with earlier surveys established that surveys with one or two people substantially underestimated numbers of plants in many cases. Unfortunately, the large number of rare species in WA has resulted in very limited resources to survey many of these species (Coates and Atkins 2001; West Australian Auditor General 2009). For example, there are 125 plants designated as Rare Flora in the wheatbelt region of WA (florabase.dpaw.wa.gov.au, accessed April 2015), but only two Flora Conservation Officers to manage them. The comparative merits and risks of including volunteers in surveys need to be considered (if locations are not already fairly well known), and it also needs to be recognised that effective monitoring is not possible without volunteer support if areas to be surveyed are very large. In WA, locations of rare orchids are generally fairly well known by enthusiasts, who often have key roles in discovering and monitoring these locations.
A key outcome of surveys was the identification and mapping of core habitat and critical habitat areas for each species, which is required to manage orchid populations within nature reserves, especially from disturbance and fire. These areas were defined, listed in order of importance and provided to the land manager as reports and GIS datasets (Brundrett 2011). Core habitat areas are the highest priority for fire protection, grazing control or weed management. These areas were very small for rare wheatbelt orchids, varying from 2 to 10 ha. Core habitats are smaller than the area of occupancy of a rare species, as defined by the IUCN (2012), and arise because these rare orchids had aggregated distributions where most individuals occur in a few small patches within their area of occupancy. For example, ~50% of all known individuals of C. graniticola occur within a 1-ha area that contains the largest of its five populations, whereas the area of occupancy is much larger (5 km2). Unfortunately, many of the core habitat areas are highly vulnerable to fire or salinity (Table 2).
The distributions of orchids were aggregated at three or more nested spatial scales, including (1) critical habitat areas of a few square kilometres enclosing each population and other habitat with similar vegetation and landforms, (2) core habitat areas of 2–10 ha where most individuals occur and (3) dense patches of orchids a few metres wide within the core habitat where many individuals grow. The aggregated distributions of the orchids studied here suggest that intense competition for resources, such as nutrients provided by mycorrhizal fungi or pollinator visitations, is likely to occur in the densest patches. The resources required by mycorrhizal fungi would primarily consist of coarse soil organic matter and litter where the inoculum of orchid fungi is most concentrated (Brundrett et al. 2003). Individual insect pollinators quickly learn to avoid areas where sexually deceptive flowers occur (Alcock 2000; Menz et al. 2013), so pollination is more frequent if plants are widely dispersed. Density-dependent impacts on orchid fitness may be less severe in non-productive years when more plants remain dormant. In the case of C. melanema, overcrowding is most severe in one densely occupied area, with over 500 plants within 1 m2. In the case of clonal orchids such as the C. melanema, it is recommended that some tubers be moved to less crowded areas to determine whether this is beneficial to future flowering and division. It should be possible to do this when plants are dormant in the summer without harming them, as dormant tubers can be handled without harm when they are re-potted in ex situ collections. For C. melanema, individuals in the same clump are likely to be genetically identical because of clonal division.
Estimating mortality and recruitment for these orchids would require longer survey times. However, there is already sufficient evidence that these populations were steady (2 species) or growing (2 species), on the basis of long-term monitoring of specific subpopulations (see Table 2). Clonal recruitment-rate estimates were also provided for C. melanema and D. isolata. There has been a steady increase in numbers of individuals and populations for C. melanema, from a few individuals discovered in 1985 to over 5000 in 2010. This trend and the absence of records before 1985 suggest that this species is of recent hybrid origin. It is part of a large species complex that includes many similar species requiring further taxonomic investigation (Brundrett 2014).
Annual assessments that identify orchids by their locations within a fixed area each year for 4 years were used to accurately measure vital statistics such as emergence, flowering, pollination and grazing rates. These studies revealed annual variations in numbers of plants that emerge from dormant tubers, produce flowers, set seeds or are grazed, so it was much easier to determine the impacts of these factors with 4 years of data. However, longer surveys are required to measure rates of recruitment and estimate the lifespan of individuals (see below). Vital-statistics data gathered from fixed areas were used in combination with overall population-size data from surveys, to provide an overall estimate of orchid population size, based on an estimated emergence rate for the year of the survey. This approach overcame problems with previous population data for these species that were not based on a consistent area or survey effort and occurred irregularly.
Because only a fraction of dormant orchids emerged each year, long-term monitoring of the same areas was required to allow population-size estimates to be obtained and to investigate the impact of climatic factors such as rainfall on orchid emergence and flowering. For the three Caladenia species, emergence frequencies were lower than expected, with many plants emerging only once in the 4-year period. These data revealed that orchids in dry habitats are time travellers that tend to skip multiple years, even in years with relatively wet winters. This makes accurate counting of these rare terrestrial orchids much more difficult. Other studies of terrestrial orchids have found that they can remain dormant for 1–2 years (Shefferson et al. 2005), or 1–4 years (Kéry et al. 2005); however, occasionally they have been observed to re-emerge after as long as 7–18 years (Light and MacConaill 2005). In these studies, it was observed that orchids that remain dormant for more than 4 years were likely to be dead.
There is a trade-off in terms of duration of demographic studies relative to the accuracy of estimates of population size, because increases or decreases in populations over longer time-frames would become more important than annual fluctuations in emergent orchids. In the present study, it was not possible to confirm that plants flowering at the same location (within 2 cm) were the same individual, because it is possible that several orchids emerged on different years, which is a potential source of underestimation for population size. However, there were also potential sources of overestimation errors in identifying individual orchids by their position, such as position-measuring errors and the fact that growth from tubers is not always vertical (they usually grow upward through the remains of the previous year’s plant base). Excavation of these rare species to confirm that assumptions are correct was not permitted. Grazing can also have an impact on numbers of orchids counted, because it may not be possible to separate dormant orchids from those that are eaten (in most cases, leaf bases could still be counted). Another assumption was that newly observed plants were not seedlings; however, this can be ruled out as most were of flowering size. None of these issues is likely to substantially affect numbers of plants counted.
Several different approaches have been used to estimate the size and dynamics of orchid populations where dormancy is common. Kéry et al. (2005) recommend multistate capture–recapture models after comparing methods to estimate survival rate. Pfeifer et al. (2006) used matrix models to estimate long-term survival probabilities on the basis of rates of transition between life states. Tremblay et al. (2009a) used a Bayesian capture–recapture multistate analysis for nine Australian Caladenia species to estimate dormancy and survival probabilities. Only C. graniticola is included in both Tremblay et al. (2009a) and the present study, allowing comparisons to be made. For this species, most of the parameters estimated by modelling approaches are similar to those measured in the present study, except one, which is different (dormancy for C. graniticola was several years longer in the present study). It is worth noting that the population of C. graniticola where data for the Tremblay et al. (2009b) were collected seemed to be relatively viable in the past (their quasi extinction rate for 50% of populations was 24 years); however, no plants have been seen in the plot where these data were obtained for a decade (they are still growing nearby). This highlights a problem with estimates of long-term population viability produced by models, because they must assume that habitat conditions are relatively constant or change gradually and predictably. However, this is often not the case for many of the WA rare orchids because impacts such as fire, rising saline groundwater, the severity and frequency of drought, grazing pressure, or weed invasion have the capacity to rapidly change habitat conditions and often become more severe over time.
Numbers of flowering, vegetative, seedling and dormant plants need to be accurately measured to determine orchid lifespans and dormancy; however, some of these cannot be directly measured (germination and dormancy are subterranean). In the case of the rare WA orchids studied here, it was easy to measure numbers of emergent, flowering and seeding plants, but seedlings were rarely observed. It will not be possible to use these data to model the probability of long-term persistence of these populations without more data on seedling establishment. The low rates of seed production noted here would be expected to cause low recruitment. However, very good seed germination was observed for two of these species when seed and organic matter were combined in a controlled environment off site, showing that both seed and mycorrhizal fungi within natural habitats were viable (Brundrett 2011).
Seed germination in these habitats seems to either be very rare or, else, is more dependent on soil moisture than are emergence and flowering. It may be necessary to have two consecutive years with sufficient rainfall, the first to support flowering and seed set, coinciding with conditions suitable for pollinator activity, and the second to support fungal activity and seed germination. Thus, we should expect that the productivity of some orchids may be lower in the future because of expected climatic trends, because rainfall is expected to decline and become less consistent in the WA wheatbelt (http://www.environment.gov.au/climate-change/climate-science/impacts/wa, accessed 2015). Fortunately, these orchids are time travelers that often remain dormant for multiple years, so should persist as long as there are some years when rainfall is sufficient. If seed germination is less than plant mortality, it is reasonable to assume that populations will eventually become extinct. This contrasts with observations summarised in Table 2 that show that the populations studied here seem to be stable or increasing over several decades of observations. In the case of C. melanema, this primarily results from the multiplication of tubers and D. isolata also spreads clonally. The other species probably had occasional years when reproduction was sufficient to maintain or expand populations of these long-lived individuals. The present 4-year study was sufficient to estimate population sizes; however, longer-term studies are required to measure recruitment form seeds or mortality, because these were infrequent.
The capacity of rare orchids in WA to spread to new habitats is expected to be very low owing to infrequent pollination, grazing impacts on seeds and the highly cleared and extremely fragmented landscapes where they grow (Brundrett 2014). Consequently, they require intervention in the form of supplemental pollination, watering to promote seed germination, protection from grazing and translocation of plants or seeds to new locations to reduce the risk of extinction. This risk is especially high for C. melanema and D. isolata because of rising saline groundwater, whereas the habitats where C. graniticola and C. williamsiae grow may be highly vulnerable to fire. Thus, evidence that populations were stable in the past does not guarantee future survival if habitat conditions deteriorate or change suddenly. In the case of C. graniticola tree-canopy decline, possible linked to drought or an extended time since the last fire, has resulted in increased light levels in the understorey and a substantial increase in the numbers of plants observed recently (Brundrett 2011). In the longer term, declining canopy vigour could also be detrimental to orchids because of increased competition from other native plants and weeds in the understorey. Research on impacts of climate change and the duration of intervals between fires on rare orchids in WA is required.
For C. williamsiae, low seed set and small population size, coupled with a small area of occupation (2 ha), were major threats to the long-term survival of this unique orchid that has no close relatives. The long-term viability of all the rare orchids studied here is threated by altered rainfall patterns in the wheatbelt of WA linked to climate change, because this area is already on the margin of the orchid diversity hotspot in the South-west Floristic Region of WA (Brundrett 2014). Changes in rainfall patterns may also affect orchids indirectly, if canopy decline opens up the habitat and results in increased competition with other species. The available evidence suggests that C. graniticola is more susceptible to drought than are the other species examined and this species occurs in a lower-rainfall area. These impacts should be evaluated by long-term monitoring of transects established in the present study.
Additional research is required to develop an understanding of habitat specificity and why the majority of apparently suitable habitat is unoccupied for most orchids. The role of mycorrhizal fungi in determining habitat preferences should also be investigated further. This requires seed-baiting experiments, where seed germination is used to detect suitable fungi in soil, and compatibility testing of mycorrhizal fungi to determine whether they also associate with co-occurring orchids that are more common (Brundrett et al. 2003; Bonnardeaux et al. 2007). For the orchids studied here, seed-baiting trials confirmed that: (1) seed of these orchids was highly viable; (2) compatible fungi were more widespread than their orchids in most suitable habitats; and (3) sites likely to be suitable for translocation of these orchids could be identified (Brundrett 2011).
Conclusions and recommendations
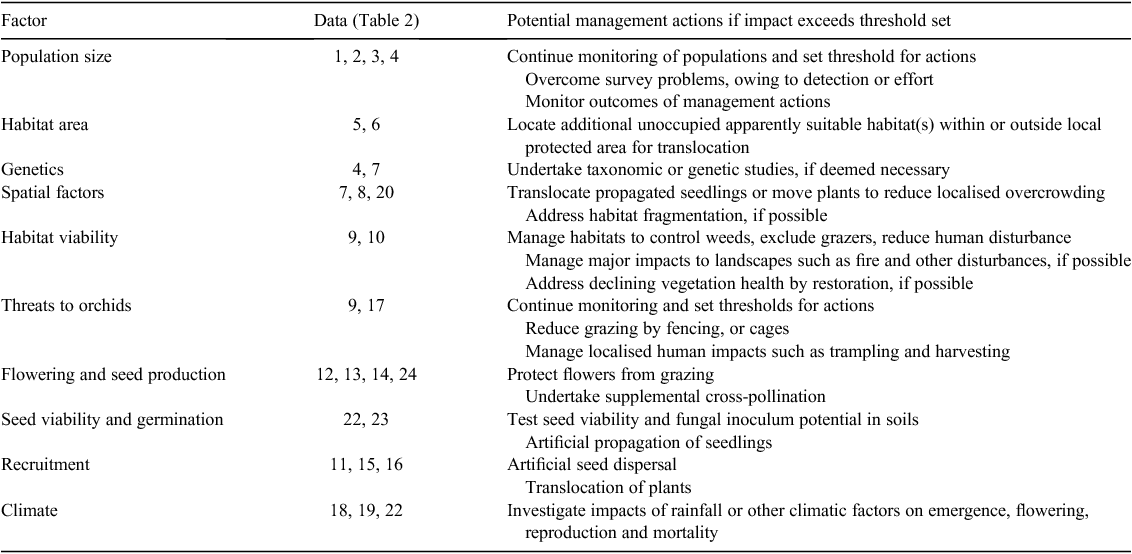
Table 2 provides an example of a report card for gathering and interpreting vital statistics using orchids from the present study. This report card identified the main threats to these rare-orchid populations as grazing, low seed set and inconsistent recruitment. These statistics were also used to set thresholds for management actions for each species (Table 2). In most cases, thresholds were set at levels close to those measured during the present study, because these species are already designated as Critically Endangered owing to low populations sizes and small areas of occupation following IUCN Criteria (IUCN 2012). Management actions that are available to reverse a gradual population decline include supplemental pollination, grazing control, weed management and habitat restoration. However, vital-statistics measurements may not detect impacts of catastrophic events such as fire or rising saline groundwater in time to prevent the loss of populations or subpopulations. Many of the vital statistics are rainfall dependant, so can also be used to monitor impacts of climate on orchid populations (i.e. Factors 11–22 in Table 2).
Making conservation plans for rare orchids is complicated by the fact that some of the key data from Table 2 are often missing. Further research is required to determine whether we can use surrogate data from other closely related species growing in similar habitats. Table 3 demonstrates how vital-statistics data can be combined to identify key threats to the viability of orchid species and provides examples of management actions that could address these threats. For example, the identification of grazing as a threat during the present study resulted in fences being erected to protect core habitat areas for C. melanema and substantially increasing seed set in subsequent years. Further information about conservation actions for these species are provided in reports (Brundrett 2011) and summarised in the Australian Species Profile and Threats Database (www.environment.gov.au/sprat, accessed 2015). Propagation and translocation trials for these orchids will be described in a subsequent publication.
|
I determined that vital statistics data were relatively easy to obtain from fixed area transects within the time constraints caused by travel to remote locations (2–4 h per site) and should be feasible despite the limited resources available for monitoring rare flora in WA. Population-size estimates required 3 or 4 years of data to compensate for annual variations in emergence and 1–3-year dormancy periods. Long-term measurements of fixed plots are the best approach to obtain population-size and -viability data for orchids. Pfeifer et al. (2006) found that 4 years of data are usually adequate for modelling population size for terrestrial orchids, whereas longer periods of observation are better for studying population dynamics. Unfortunately, there are no annually repeated measurements of fixed plots for most of the rare orchids in WA and the existing population-size census data for these species is of limited use for assessing plant population size because of infrequent surveys, variations in sampling intensity and detectability problems (West Australian Auditor General 2009; Brundrett 2011). It is recommended that monitoring protocols that allow population size and viability to be better estimated be adopted in the future. Volunteers from community groups are increasingly becoming major contributors to rare flora surveys in WA (Brundrett 2011; Adopt and Orchid Project, available at www.dpaw.wa.gov.au, accessed 2015) and would have the capacity and skills (with some additional training) to undertake annual fixed-area surveys for rare orchids. Planning for new monitoring programs of this type is currently underway.
Vital-statistics data are essential decision-making tools for orchid conservation in WA, because they allow changes to population size and viability to be detected before populations are lost and can be used to allocate conservation resources to the most threatened orchids. These data can be gathered from a relatively small fixed area, so are an efficient means of determining the most important threats to orchid populations without having an impact on them. It is recommended that these data are summarised in a report-card format, with thresholds set for management actions for key criteria such as population size and reproduction. Mapping of core habitat areas was also found to be a vital tool for conservation planning for rare species and management of the areas where they occur. Vital-statistics report cards with thresholds set for conservation actions should also be valuable tools for the management of rare terrestrial orchids in other biomes.
Acknowledgements
The Wheatbelt Orchid Rescue Project was funded primarily by Lotterywest. Andrew Brown of DEC Species and Communities Branch and Conservation Officers Beth Laudon, Erica Shedley, Marie Edgley and Kris Brooks made major contributions to project planning and survey work. I especially acknowledge Ann and Barry Rick of Newdegate who provided invaluable assistance, accommodation and advice and Judy Williams of Brookton for field trip assistance and knowledge of habitats. I also thank the Western Australian Native Orchid Study and Conservation Group volunteers who assisted in surveys and helped acquire data along the transects, especially Mayne Merritt, Margaret Petridis, Pam Goodman and Ray Grant as well as Jocelyn Ward and Lucy Skipsey from wheatbelt community groups. Graham Brown identified wasp species.
References
Alcock J (2000) Interactions between the sexually deceptive orchid Spiculaea ciliata and its wasp pollinator Thynnoturneria sp. (Hymenoptera: Thynninae). Journal of Natural History 34, 629–636.| Interactions between the sexually deceptive orchid Spiculaea ciliata and its wasp pollinator Thynnoturneria sp. (Hymenoptera: Thynninae).Crossref | GoogleScholarGoogle Scholar |
Bonnardeaux Y, Brundrett M, Batty A, Dixon K, Koch J, Sivasithamparam K (2007) Diversity of mycorrhizal fungi of Western Australian terrestrial orchids: Compatibility webs, brief encounters, lasting relationships and alien invasions. Mycological Research 111, 51–61.
Brown A, Thomson-Dans C, Marchant N (1998) ‘Western Australia’s threatened flora.’ (Department of Conservation and Land Management, Perth, Western Australia)
Brundrett MC (2007) Scientific approaches to terrestrial orchid conservation with particular reference to Western Australia. Australian Journal of Botany 55, 293–307.
| Scientific approaches to terrestrial orchid conservation with particular reference to Western Australia.Crossref | GoogleScholarGoogle Scholar |
Brundrett M (2011) ‘Wheatbelt Orchid Rescue Project: case studies of collaborative orchid conservation in Western Australia.’ Available at http://repository.uwa.edu.au [28 January 2016]
Brundrett M (2012) The role of community groups in rare orchid monitoring in the West Australian wheatbelt. Australasian Plant Conservation 20, 3–5.
Brundrett M (2014) ‘Identification and ecology of southwest Australian orchids.’ (West Australian Naturalists’ Club Inc.: Perth)
Brundrett MC, Scade A, Batty AL, Dixon KW, Sivasithamparam K (2003) Development of in situ and ex situ seed baiting techniques to detect mycorrhizal fungi from terrestrial orchid habitats. Mycological Research 107, 1210–1220.
| Development of in situ and ex situ seed baiting techniques to detect mycorrhizal fungi from terrestrial orchid habitats.Crossref | GoogleScholarGoogle Scholar | 14635769PubMed |
Coates DJ, Atkins KA (2001) Priority setting and the conservation of Western Australia’s diverse and highly endemic flora. Biological Conservation 97, 251–263.
| Priority setting and the conservation of Western Australia’s diverse and highly endemic flora.Crossref | GoogleScholarGoogle Scholar |
Chase MW, Cameron KM, Freudenstein JV, Pridgeon AM, Salazar G, Berg C, Schuiteman A (2015) An updated classification of Orchidaceae. Botanical Journal of the Linnean Society 177, 151–174.
Commonwealth of Australia (2002) ‘Australian terrestrial biodiversity assessment 2002.’ (National Land and Water Resources Audit: Canberra)
Department of Environment and Conservation (2007a) ‘Ballerina orchid (Caladenia melanema) interim recovery plan 2007–2012. Interim recovery plan no. 276.’ (Department of Environment and Conservation, Kensington)
Department of Environment and Conservation (2007b) ‘Williams’ Spider Orchid (Caladenia williamsiae) Interim Recovery Plan 2007–2012. Interim Recovery Plan No. 277.’ (Department of Environment and Conservation, Kensington)
Hopper SD (2009) OCBIL theory: towards an integrated understanding of the evolution, ecology and conservation of biodiversity on old, climatically buffered, infertile landscapes. Plant and Soil 322, 49–86.
Hopper SD, Gioia P (2004) The southwest Australian floristic region: evolution and conservation of a global hot spot of biodiversity. Annual Review of Ecology, Evolution, and Systematics 35, 623–650.
| The southwest Australian floristic region: evolution and conservation of a global hot spot of biodiversity.Crossref | GoogleScholarGoogle Scholar |
IUCN (2012) ‘IUCN red list categories and criteria: version 3.1.’ 2nd edn. (IUCN: Gland, Switzerland)
Kershaw K, Loudon B, Beecham B, Durell G, Brown A (2003) ‘Interim recovery plan no. 123. Pingaring spider orchid (Caladenia hoffmanii subsp. graniticola) 2003–2008.’ (Department of Conservation and Land Management, Perth)
Kéry M, Gregg KB, Schaub M (2005) Demographic estimation methods for plants with unobservable life-states. Oikos 108, 307–320.
| Demographic estimation methods for plants with unobservable life-states.Crossref | GoogleScholarGoogle Scholar |
Light MHS, MacConaill M (2005) Long-term studies: a case for orchid species survival. Selbyana 26, 174–188.
Menz MH, Phillips RD, Dixon KW, Peakall R, Didham RK (2013) Mate-searching behaviour of common and rare wasps and the implications for pollen movement of the sexually deceptive orchids they pollinate. PLoS One 8, e59111
| Mate-searching behaviour of common and rare wasps and the implications for pollen movement of the sexually deceptive orchids they pollinate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXksFGjs74%3D&md5=7d0fa78673db140fe6623dd594e2fa77CAS | 23536860PubMed |
Molvray M, Kores PJ, Chase MW (2000) Polyphyly of mycoheterotrophous orchids and functional influences on floral and molecular characters. In ‘Monocots: systematics and evolution’. (Eds KL Wilson, DA Mossison) pp. 441–448. (CSIRO: Melbourne)
Myers N, Mittermeier RA, Mittermeier CG, Da Fonseca GA, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403, 853–858.
Pfeifer M, Wiegand K, Heinrich W, Jetchke G (2006) Long-term demographic fluctuations in an orchid species driven by weather implications for conservation planning. Journal of Applied Ecology 43, 313–324.
| Long-term demographic fluctuations in an orchid species driven by weather implications for conservation planning.Crossref | GoogleScholarGoogle Scholar |
Phillimore R, Stack G, Brown A (2000) ‘Drakaea isolata ms interim recovery plan no. 81, 2000–2003.’ (Department of Conservation and Land Management, Kensington)
Phillips RD, Peakall R, Hutchinson MF, Linde CC, Xu T, Dixon KW, Hopper SD (2014) Specialized ecological interactions and plant species rarity: the role of pollinators and mycorrhizal fungi across multiple spatial scales. Biological Conservation 169, 285–295.
| Specialized ecological interactions and plant species rarity: the role of pollinators and mycorrhizal fungi across multiple spatial scales.Crossref | GoogleScholarGoogle Scholar |
Shefferson RP, Kull T, Tali K (2005) Adult whole-plant dormancy induced by stress in long-lived orchids. Ecology 86, 3099–3104.
| Adult whole-plant dormancy induced by stress in long-lived orchids.Crossref | GoogleScholarGoogle Scholar |
Tremblay RL, Perez ME, Larcombe M, Brown A, Quarmby J, Bickerton D, French G, Bould A (2009a) Dormancy in Caladenia: a Bayesian approach to evaluating latency. Australian Journal of Botany 57, 340–350.
| Dormancy in Caladenia: a Bayesian approach to evaluating latency.Crossref | GoogleScholarGoogle Scholar |
Tremblay RL, Perez ME, Larcombe M, Brown A, Quarmby J, Bickerton D, French G, Bould A (2009b) Population dynamics of Caladenia: Bayesian estimates of transition and extinction probabilities. Australian Journal of Botany 57, 351–360.
| Population dynamics of Caladenia: Bayesian estimates of transition and extinction probabilities.Crossref | GoogleScholarGoogle Scholar |
West Australian Auditor General (2009) ‘Rich and rare: conservation of threatened species.’ Available at audit.wa.gov.au/reports-and-publications/reports/ [28 January 2016]