Do habitat fragmentation and fire influence variation of plant species composition, structure and diversity within three regional ecosystems on the Sunshine Coast, Queensland, Australia?
Rohan Etherington A and Alison Shapcott A BA Genecology Research Centre, Faculty Science Health Education Engineering, University Sunshine Coast, Maroochydore DC 4558, Qld Australia.
B Corresponding author. Email: ashapcot@usc.edu.au
Australian Journal of Botany 62(1) 36-47 https://doi.org/10.1071/BT13232
Submitted: 27 September 2013 Accepted: 5 March 2014 Published: 23 April 2014
Abstract
Habitat fragmentation is considered to be one of the greatest threats to biodiversity. Species richness is predicted to decrease with decreasing patch size and increasing isolation, and this has been shown in some ecosystems. However, few studies have specifically investigated the effects of fragmentation on specific vegetation types, or compared different vegetation types within the same region. In this study, we assessed the influence of habitat fragmentation and time since fire on the floristic composition, structure and diversity of three ecosystems with varying fire proneness within the Sunshine Coast region. This study found that the tall-open forest ecosystem (RE 12.9-10.14) had higher overall species richness within fixed sample areas used for this study than did either open forest (RE 12.5.3) or gallery rainforest (RE 12.3.1), because it was composed of species typical of each of these ecosystem types. Open forest species richness was found mostly in the lower stratum, whereas gallery rainforest diversity was found in the upper stratum. Species richness decreased with increasing isolation in the open forest ecosystem where seeds are mostly abiotically dispersed. However, this study did not find strong evidence for reduced species richness within smaller patches in any ecosystem type studied; instead, finding species richness decreased with increasing patch size in the open forest ecosystem. Overall, across ecosystems, time since fire affected vegetation structure, but in fire-prone ecosystems, time since fire was not a determinant of species richness within the sites studied.
Additional keywords: diversity, functional groups, fire, isolation, open forest, patch size, rainforest.
Introduction
Habitat fragmentation is considered to be one of the greatest threats to biodiversity on a global scale, converting connected forests into a mosaic of patches, of varying size and isolation (Fahrig 2003; Ewers and Didham 2006; Alados et al. 2009; Archibald et al. 2011; Laurance et al. 2011; Parsons and Gosper 2011; Rodriguez-Loinaz et al. 2012). The species–area relationship (SAR) hypothesises that smaller patches have a less heterogeneous environment, supporting fewer species (Gleason 1922, 1925; Williams 1943; MacArthur and Wilson 1967; Connor and McCoy 1979). Recent studies by Godefroid and Koedam (2003), Cagnolo et al. (2006), Aparicio et al. (2008), Brown and Boutin (2009), Gonzalez et al. (2010) and Laurance et al. (2011) have supported this relationship. Smaller, and therefore more isolated, patches are predicted to support smaller, genetically similar species populations that are vulnerable to localised extinction from disease, population decline and altered microclimatic conditions (Connor and McCoy 1979; Baldwin and Bradfield 2007; Shapcott et al. 2009).
Isolation occurs both spatially (distance between patches of the same type) and temporally (how long the patches have been separated) (Collard et al. 2011). Isolation can impede the dispersal of seed and pollen both abiotically and biotically, therefore reducing emigration and immigration between communities (White et al. 2004; Cramer et al. 2007; Alados et al. 2009). Isolated patches, irrespective of patch size are therefore still vulnerable to population decline and, therefore, localised to extinctions (Baldwin and Bradfield 2007; Shapcott et al. 2009). This is important with respect to keystone species, because a significant alteration of structure and therefore microclimate can lead to altered disturbance regimes (e.g. fire) at the patch level, leaving other species vulnerable to further population declines and/or extinctions (Alados et al. 2009; Parsons and Gosper 2011; Knox and Clarke 2012).
Fire is responsible for shaping the structure, composition and diversity of many Australian plant communities (Bradstock and Myerscough 1981; Russell-Smith et al. 2004; Scott et al. 2012). Increased fire frequency can decrease species richness by removing or reducing the fecundity of obligate seeder species (Knox and Morrison 2005; Fisher et al. 2009). High-frequency fire regimes have also been shown to increase fine fuel loads such as grasses, in turn further increasing the frequency of fire (Hoffmann et al. 2012; Scott et al. 2012). Conversely, an absence of fire can cause understorey strata to develop to a stage where fine fuels are scarce and the probability of fire is decreased (Close et al. 2011; Scott et al. 2012; Williams et al. 2012). Habitat fragmentation can alter the frequency and intensity of fire regimes (Driscoll et al. 2010). Alterations in patch size have been shown to alter fire regimes with respect to how a fire behaves and the probability of fire reoccurring in small patches (Ross et al. 2002; Parsons and Gosper 2011).
Many studies investigating the effects of fragmentation have employed broad vegetation classification systems that include varying geologies and vegetation types (Cagnolo et al. 2006; Alados et al. 2009). This may weaken the results because different ecosystems may respond differently to fragmentation, fire and other anthropogenic disturbances. By comparing vegetation structure and composition within defined communities, variation can be attributed to exogenous pressures rather than variation in environmental qualities (Adams et al. 2011; Collard et al. 2011). Sampling effects may also affect comparability of results and most field assessments of diversity are based on subsampling within fixed plots. Management and conservation decisions for vegetation communities in Queensland are largely based on regional ecosystem (RE) mapping (Wilson et al. 2002; Carter et al. 2009). This mapping was developed by the Queensland Herbarium and is at a scale of ~1 : 100000 (Wilson et al. 2002). Compared with other Australian states, and other countries globally, this mapping is specific, differentiating among different bioregions, geologies, community structures and compositions (Sattler and Williams 1999; Wilson et al. 2002; Fensham 2008). Therefore, this detailed mapping provides the opportunity to study fragmentation within specific ecosystems.
The Sunshine Coast is located in the subtropics of the eastern coast of Australia (Fig. 1). As a result of variation in climate, geology and topography, the Sunshine Coast has high biodiversity, supporting a wide range of different ecosystem types, such as coastal heath (Brownlie et al. 2009), rainforests (Moran et al. 2009; Shapcott et al. 2009) and a variety of dry and wet eucalypt communities (Callaghan et al. 2011). As a result of urban development over the past 40 years, the landscape of this area is heavily fragmented (Brownlie et al. 2009; Carter et al. 2009; Moran et al. 2009). Fragmentation of these important ecosystems has been identified as having a major impact on both fauna and flora of the area (Moran et al. 2009; Shapcott et al. 2009; Callaghan et al. 2011). Therefore, investigation into the impact of habitat fragmentation and its effects on biodiversity is important, particularly because urban development is expected to increase rapidly into the future (Carter et al. 2009).
The study specifically aimed to compare and contrast the species composition, structure and diversity among three different ecosystems and assess the influence of habitat fragmentation on fire-prone and non-fire-prone communities within the Sunshine Coast. We predicted that study sites located within larger, more connected patches would have higher floristic diversity because they would be less disturbed, therefore maintaining a more stable vegetation structure and composition than smaller, isolated patches. Furthermore, the influence of isolation on floristic composition was investigated with respect to dispersal mechanisms. We aimed to reduce statistical noise in the study by consistency in sample size, replication and vegetation type.
Materials and methods
Ecosystem selection
The study expanded on the vegetation component of a wider biodiversity assessment undertaken on the Sunshine Coast; thus, some components of vegetation and site selection were determined as part of this wider study and expanded on for this study. Three REs were selected for comparison on the basis of perceived differences in structural complexity and frequency of fire and were identified as of significance for fauna within the wider study (Specht 1970; Knox and Clarke 2012; Queensland Herbarium 2012). RE 12.5.3 is an open forest of Eucalyptus racemosa (Specht 1970; Eyre 2006; Queensland Herbarium 2012). This ecosystem is a typical fire-prone community, with fires every 5–10 years maintaining an open canopy with a grassy to shrubby understorey (Parsons and Gosper 2011; Queensland Herbarium 2012). Once common on the sandy-loam soils of the Sunshine Coast, the RE is now listed as Endangered in Queensland (Vegetation Management (VM) Act 1999). RE 12.9-10.14 is a tall-open forest dominated by E. pilularis on sedimentary rocks (Specht 1970; Queensland Herbarium 2012). Fires have been estimated to typically occur every 10–20 years, and without fire, a rainforest understorey can develop (Queensland Herbarium 2012). The third ecosystem, RE 12.3.1, is characterised by an absence of fire and is gallery rainforest occurring on alluvial substrates (Queensland Herbarium 2012). This ecosystem is listed as Endangered in Queensland (VM Act) and, as a lowland subtropical rainforest, it is listed as Critically Endangered federally (Environmental Protection and Biodiversity Conservation (EPBC) Act 1999).
Site selection
The most current version of the Regional Ecosystem Mapping (v6.1) was used to identify the distribution of the three study REs (State of Queensland – Department of Natural Resources and Mines 2012a) in ArcMap 10 (ESRI 2011). Ten sites per vegetation type (RE) were selected to represent their distribution within the Sunshine Coast local government area (SCLGA). These sites were also selected to represent their range of varying patch sizes and degrees of isolation (Fig. 1). Sites were selected with a minimum patch width of 100 m, to allow surveying to occur more than 50 m from the edge of the patch to reduce impact of edge effects (Gosper et al. 2010). Gallery rainforest was associated with watercourses; therefore, sites were selected to avoid connectivity between study areas by allowing only one site per watercourse or drainage system. Logistical constraints, including access, and permitting also determined site selection within these parameters.
Isolation and patch area
To test the species–area relationship (SAR), the area of RE patch (ARE) and total area of remnant/high-value regrowth within a 1 km radius of the sample site (ATOTAL) was measured in ArcMap 10 (ESRI 2011). Patches were manually delineated, with ‘spurs’ of less than 50 m width being removed. Patch area was calculated by drawing a rectangle over the patch, estimating the proportion of the rectangle taken up by the patch and then multiplying that proportion by the area of the rectangle. Isolation from patches of same RE type (ISO) was measured by calculating the average distance to the nearest five patches of same RE type (Broadhurst and Young 2006). In addition, to quantify the fragmentation of the surrounding landscape, a score was calculated using the following three components: patch size, connectivity and context, consistent with the methodology developed for Queensland Biocondition assessments (Eyre et al. 2011). This methodology relates to vegetation deemed to be ‘high-value’ by the VM Act. Therefore, in addition to the Regional Ecosystem Mapping v6.1, the High-value Regrowth Vegetation v2.1 layer was also used to calculate patch size (ATOTAL), connectivity and context (State of Queensland – Department of Natural Resources and Mines 2012b). Patch size was calculated as the total area of high-value vegetation within which the study site was located (ATOTAL; Eyre et al. 2011). The connectivity component scores the level of linkage a patch has to surrounding patches (Eyre et al. 2011). Context is the amount of high-value vegetation within a 500 m radius of the study site (Eyre et al. 2011). The sum of these three components formed the landscape score (LS), which is a score out of 20 (Eyre et al. 2011).
Vegetation sampling
A vegetation-survey methodology consistent with Neldner et al. (2005) was used at each site to enable the data to be added to the Queensland Herbarium CORVEG database. Vegetation sampling was performed within the interior of the patch more than 50 m from the edge (Gosper et al. 2011). In extremely thin, linear gallery rainforest patches, this was often unachievable because of the presence of waterways and six sites were surveyed less than 50 m inside the patch. The location of plots was undertaken to be as consistent as possible within each vegetation type and sampled along rather than across creek lines. At each site, species richness and composition were determined within a 50 m by 100 m plot (or an equivalent area in thin, linear patches), which was systematically surveyed (Neldner et al. 2005). This methodology recorded species presence only and, thus, did not enable other diversity indices to be calculated. Herbarium vouchers were made and lodged with the University of the Sunshine Coast Herbarium. Specimens were named according to the Census of Queensland Flora (Bostock and Holland 2010). Identification was confirmed by local botanists or the Queensland Herbarium.
Community structure was sampled in a 10 × 50 m belt transect, located within the 50 × 100 m plot consistent with Neldner et al. (2005) and Eyre et al. (2011). Community structure was classified by identifying the strata; emergent (E), canopy (T1), subcanopy (T2), shrub layer (S1) and/or ground layer (G) present (Neldner et al. 2005). For each stratum (excluding the ground layer), the following variables were recorded: minimum, median and maximum stratum height was estimated in metres with a clinometer and measuring tape, following standard methods (Neldner et al. 2005). The number of stems within each stratum was counted, where practical, in the belt transect (Austin 1978; Neldner et al. 2005). However, if the density of stems was too high to count accurately, counting was performed within a subsampled 50 × 5 m or 50 × 2 m belt transect. Total percentage crown cover was calculated through the 50 m transect by using the line intercept method (Greig-Smith 1964; Neldner et al. 2005).
The ground layer was sampled within a 1 m2 quadrat at five points along the 50 m transect, with the mean of these values used to represent that site. Minimum, median and maximum heights (cm) of the ground layer were recorded. Percentage cover of grasses, forbs and non-grass species, woody plant seedlings and shrubs, litter, bare rock and cryptogams were also recorded consistent with the methods of Neldner et al. (2005) and Eyre et al. (2011).
All species recorded were later grouped for analyses by taxonomy (genus and family), life form (large trees >20 m, small–medium trees <20 m, shrubs <6 m, ferns, forbs and herbs, and grasses, vines and epiphytes), species origin (native or exotic to Australia; Bostock and Holland 2010) and dispersal mechanism (abiotic or biotic; Cagnolo et al. 2006; Campetella et al. 2011; Pekin et al. 2012). To identify the contribution each group of species made to their respective functional grouping, each sample site was standardised by dividing the number of species in that grouping by the total species richness of the site (Figueroa et al. 2011; Pekin et al. 2012). Thirteen species were not able to be identified because of time constraints and were not included in functional grouping analysis; however, they were used in estimates of species richness.
Fire history
All available fire-history data for each site were obtained from Queensland Parks and Wildlife Service (QPWS), Sunshine Coast Council (SCC) and local Rural Fire Brigades (RFB), but was often limited. The time-since-fire (TSF) data were the most consistently available data; however, these data were not uniformly accurate across sites; therefore, each site was classified as exposed to fire (1) 0–4 years ago, (2) 5–10 years ago, (3) >10 years ago or (4) not on record. We attempted to select sample sites that covered the range of fire histories of the vegetation types.
Statistical analysis
Multivariate analyses were used to compare sites within and among ecosystem types. Multivariate distance matrices, using Euclidian distance, were calculated for life form, species origin and dispersal mechanism in PRIMER v6.1.5 (Clarke and Gorley 2006). The values of species richness of each vegetation type were standardised for each sample before the Euclidian distance matrix was generated. Community structure variables were standardised by the maximum, before generating a Euclidian distance matrix of multivariate structure. A Bray–Curtis dissimilarity matrix of species presence and absence at each site was constructed for each of the three ecosystems and across all sites in PRIMER v6.1.5 (Clarke and Gorley 2006). Non-metric multidimensional scaling (NMDS) analysis was performed on each matrix, using a repetition value of 9999 to test for clustering of sites within and among ecosystem types according to these different attributes on a two- and three-dimensional plane, using PRIMER v6.1.5, (Clarke and Gorley 2006). It was detected from the preliminary assessment of community structure and composition in addition to MDS outputs of species composition that sites grouped according to community structure. To test this relationship, paired distance matrices of multivariate species composition and community structure were compared with a Mantel test, using the RELATE analysis with 9999 random permutations in PRIMER v6.1.5 (Clarke and Gorley 2006).
The three ecosystem types were tested for significant differences in their fragmentation, structure and species diversity, and composition variables were measured using ANOVA or Kruskal –Wallis tests, followed by Duncan’s post hoc test in SPSS Statistics v19 (IBM 2010). Within ecosystem types, Spearman’s rank correlations were used to test whether RE patch size (ARE) and or isolation were significantly correlated with taxonomic- and functional-grouping species richness and community structure variables, to assess the influence of patch size and isolation on structural complexity and diversity in SPSS Statistics v19 (IBM 2010). Accurate fire-history data were available for the open forest system and the effect of time-since-fire (TSF) and community composition and community structure variables was tested using Spearman’s rank correlation in SPSS Statistics v19 (IBM 2010). To avoid a Type 1 error, a Bonferroni correction was applied to results tables (Townend 2002). Spearman’s rank coefficients that were rejected after the Bonferroni correction, but had a strong bivariate relationship (i.e. rs ≥ 0.5 or rs ≤ –0.5), were accepted.
Results
Comparisons among vegetation types
In total, 473 species of flora from 108 families were recorded across the 30 sites, with 38 of these being exotic species (Table 1). In contrast to expectations, the tall-open forest ecosystem recorded higher species richness (275) than did open forest (230) or gallery rainforest (198) sites (Table 1). The open forest and gallery rainforest sites had distinctly different species composition. In open forest and gallery rainforest communities, 100 and 98 species were exclusive to these ecosystems, respectively (Table 1). In contrast, the tall-open forest ecosystem had only two species (Denhamia celastroides and Xanthorrhoea macronema) that were unique to this ecosystem.
Of the 460 species positively identified, 64 were large trees, 97 species were small–medium trees, 106 were shrubs, 107 species were ferns, forbs or herbs, 31 were species of grass and 67 species were vines or epiphytes (Table 1). Large trees (25.79%; F = 37.674, P < 0.05) and small–medium trees (24.16%; F = 8.019, P < 0.05) were significantly more dominant life forms in the gallery rainforest system (Table 2). In contrast, forbs, ferns and herbs (31.33%; F = 37.349, P < 0.05) and grasses (9.85%; F = 20.785, P < 0.05) were the dominant life forms in the fire-prone open forest system, in comparison to the tall-open forest and gallery rainforest (Table 2). However, their abundance and diversity were not significantly (P > 0.05) correlated with differences in canopy cover. The open forest sites had a vegetation structure different from that in the other two vegetation communities (Fig. 2). The open forest sites had significantly lower canopy height than did tall-open forest and gallery rainforest (18.701 m; F = 5.069, P < 0.05, Table 2). The tall-open forest and gallery rainforest sites were not significantly (P < 0.05) different for most structure variables measured and clustered together (Fig. 2). However, the gallery rainforest had a significantly lower ground strata vegetation cover and a higher litter cover than did the other two vegetation types where vegetation cover in the ground layer was greater than 50% (Table 2).
However, species composition was distinctly different for each vegetation type (Fig. 3). The sclerophyll communities, open forest and tall-open forest were more similar in species composition. Two gallery rainforest sites (41 and 43) exhibited species composition and structural patterns that suggested they were at a later successional stage because their canopies were composed of late-successional species, such as Ficus macrophylla, Elaeocarpus grandis and Castenospermum australe. In contrast, canopies of the other sites were dominated by secondary rainforest species such as Neolitsea dealbata or sclerophyll species Lophostemon confertus, Syncarpia glomifera or Eucalyptus grandis. The majority of species sampled utilised abiotic dispersal mechanisms (272 species), with 188 species bearing fleshy fruits (Table 1). The proportion of biotically dispersed species per site in gallery rainforest was significantly higher than that in the open forest and tall-open forest communities (F = 72.925, P < 0.05; Table 2). There was a weak yet significant relationship between species composition and community structure across the study sites (rs = 0.295, P < 0.05; Table 3).

|
Fragmentation
The three ecosystems did not differ significantly in size of RE patches and remnant areas; however, gallery rainforest sites were significantly more isolated than were the sites of the other two ecosystems studied (Table 1). In contrast to expectations, open forest total species richness and native species richness were significantly (P < 0.05) negatively correlated with the total area of the patch (ATOTAL; rs = –0.872; rs = –0.839), but were not correlated with RE patch area (ARE; Table 4). Species richness was not significantly (P > 0.05) correlated with RE patch or total patch area in the tall-open forest or gallery rainforest (Table 4). The percentage of rainforest shrubs was negatively correlated with increasing isolation (Table 4). In the open forest ecosystem, native species richness was negatively correlated with isolation (rs = –0.685; Table 4).
The structure of any of the vegetation communities was not generally correlated with patch size or isolation. However, the dominance of different strata in the open forest and gallery rainforest ecosystems was related to habitat fragmentation variables. The percentage of forbs was positively correlated with increasing isolation (rs = 0.632), landscape scores (rs = 0.647) and total remnant patch area in the open forest system (rs = 0.726; Table 4). However, the percentage of shrubs (rs = –0.809) and small–medium tree species (rs = –0.697) decreased as gallery rainforest patches became more isolated (Table 4). This study found that small–medium tree species contributed more to species richness in tall-open forest sites with higher landscape scores (rs = 0.714). The percentage of abiotically dispersed species in open forest sites increased with greater isolation (rs = 0.648). In contrast, the percentage of biotically dispersed species increased with increasing isolation (rs = 0.600) in gallery rainforest (Table 4).
Time since fire
Open forest sites had been burnt either within the past 4 years (Class 1) or between 5 and 10 years ago (Class 2). Tall-open forest sites were mostly burnt more than 10 years ago (Class 3) or had no fire records (Class 4). No gallery rainforest sites had any recorded fire events (Class 4). Tall-open forest sites sampled less than 14 years following fire (Sites 2, 3, 5, 6, 7 and 9) were more similar to open forest sites (Fig. 2). Within the fire-prone open forest ecosystem, there was no significant correlation between time since fire and species richness, whereas the percentage of biotic dispersed species increased with time since fire (rs = 0.798; Table 5). The canopy and understorey of the open forest system developed in the absence of fire, with canopy cover (rs = 0.724) and shrub height (rs = 0.805) being positively correlated and bare ground negatively correlated with time since fire (rs = –0.889; Table 5).

|
Discussion
Rainforest ecosystems are known for their high diversity of species (Janzen 1970; Connell 1971; Hubbell 1979; Denslow 1987). Rainforests generally have a diverse upper stratum, with a closed canopy maintaining a sparse understorey (Janzen 1970; Connell 1971; Hubbell 1979, 1980; Mangan et al. 2010). The species richness of the studied gallery rainforest community was highest in the tree layers. Conversely, open forest sites had low tree diversity and higher diversity in the grass and herbaceous layers. However, the gallery rainforest community did not have the highest species richness of the ecosystems in this study, but was still comparable with the open forest in terms of total species richness. Le Brocque (1998) also found that diversity of the tree layer was a poor indicator of total species richness across various different ecosystems; particularly in open forests and closed forests (i.e. rainforests). The tall-open forest in this study had overall highest diversity and did not possess the distinctive partitioning of species to different layers that we observed in the open forest and gallery rainforest systems. Instead, the tall-open forest was composed of species found in both the open forest and gallery rainforest ecosystems.
In contrast to the tall-open forest community, the open forest is more fire prone because of higher cover of herbaceous and grass species, as shown by this study (Queensland Herbarium 2012). Increased time since fire was significantly correlated with changing vegetation structure, particularly with increased shrub-layer height, in the open forest community. Other studies have found the understorey (shrub and subcanopy) of sclerophyll systems to become structurally complex in the prolonged intervals between fire events (Russell-Smith et al. 2004; Close et al. 2011; Parsons and Gosper 2011; Gosper et al. 2012; Scott et al. 2012). Lewis et al. (2012) reported that understorey fire-prone species were replaced by rainforest species when fire intervals were increased in a long-term experimental plot located within the same tall-open forest community as was studied here. There were obvious differences in composition between recently burnt and long-unburnt sites within this study. Recently burnt tall-open forest sites had species composition similar to that of the fire-prone open forest system. Conversely, long-unburnt tall-open forest sites possessed many fire-intolerant species that they shared with the rainforest ecosystem. This is likely to have implications for wet sclerophyll forests in the metropolitan south-eastern Queensland area into the future, because prescribed burns are a key management tool utilised to maintain community structure and composition (Kuenzi et al. 2008; Knox and Clarke 2012; Scott et al. 2012).
The species–area relationship predicts that larger patches will support higher species diversity of plants and animals (Gleason 1922, 1925; Williams 1943; MacArthur and Wilson 1967; Connor and McCoy 1979). Several recent studies have provided empirical evidence to support its theoretical predictions in relation to assessing the influence of altered patch size, caused by habitat fragmentation, on vascular plant diversity (Cagnolo et al. 2006; Aparicio et al. 2008; Brown and Boutin 2009; Gonzalez et al. 2010; Laurance et al. 2011). Laurance et al. (2011) reported that species richness of many different taxa decreased in smaller patches in the Amazonian rainforest. It was expected that patch size would affect species richness at rainforest sites more than at sclerophyll sites because rainforest sites are less resilient to disturbance which is often amplified in fragmented landscapes (Laurance et al. 2011). However, although species richness was weakly correlated with total patch area in the open forest, we found no significant relationship between species richness and RE patch or total patch area in the gallery rainforest or tall-open forest ecosystems. Conversely, the results of this study were more consistent with Ross et al. (2002) and Rodriguez-Loinaz et al. (2012) who found larger habitat patches to support fewer species than did smaller fragments, with species richness being significantly negatively correlated with an increasing total patch size in the open forest ecosystem of this study.
Ross et al. (2002) also worked in coastal, fire-prone open forests on the central coast of New South Wales and found lower species richness with increased time since fire in fragmented and unfragmented patches, with the relationship being more pronounced on patches of 5–25 ha. Although no direct relationship was established between increased time since fire and a decline in species richness by this study, a negative relationship between species richness and landscape context and connectivity was detected. This may involve interplay between habitat fragmentation and altered disturbance regimes. Parsons and Gosper (2011) studied the relationship between patch area and fire regime in fire-prone ecosystems of Western Australia, concluding that large patches (100–500 ha) maintain fire regimes by allowing higher proportions of the patch to be burnt. Therefore, larger patches of fire-prone vegetation may be less species rich because small patches may have a more heterogeneous environment as a result of components of the patch being at varying stages of succession following a fire.
Patch area and connectivity to the surrounding landscape have been shown to influence the composition of species with different dispersal mechanisms (Aparicio et al. 2008; Alados et al. 2009; Brown and Boutin 2009; Alofs and Fowler 2010; Jesus et al. 2012). In this study, the open and tall-open forest ecosystems had mostly abiotically dispersed fruits, whereas gallery rainforest contained species with mostly fleshy biotically dispersed fruits. Jesus et al. (2012) found wind-dispersed seeds to decrease in quantity in larger, more connected patches. Consistent with this, our study found species richness to be negatively correlated with increasing isolation in the open forest ecosystem. Isolation was expected to negatively affect dispersal in gallery rainforest sites as a result of biotic dispersal being impeded (White et al. 2004; Cramer et al. 2007; Aparicio et al. 2008; Moran et al. 2009). Cramer et al. (2007) found that large-seeded species are dispersed shorter distances than are small-seeded species in fragmented patches, hypothesising that their result was due to an absence of large mammalian frugivores in these patches. Moran et al. (2009) found frugivore species richness and abundance to decline with increasing fragmentation in their study of rainforest patches in the Sunshine Coast hinterland. We found that the rainforest patches were more isolated than were the other two vegetation types studied and were composed largely of frugivore-dispersed species. However, we did not find that species richness of gallery rainforest sites declined with increasing isolation, or with decreasing patch size. This may have been due to the riparian nature of this system allowing species to employ hydrochory as a key dispersal mechanism (Jansson et al. 2005).
Conclusions
This study found that tall-open forest sites had a higher overall species richness within fixed sample areas used for the study than did either open forest or gallery rainforest because it was composed of species typical of both of these ecosystem types. Open forest species richness was found mostly in the lower strata, whereas gallery rainforest diversity was found in the tree layers. Species richness negatively correlated with increasing isolation in the open forest ecosystem where seeds are mostly being dispersed abiotically. However, this study did not find strong evidence for reduced species richness with decreasing RE or total patch size in any ecosystem type studied when using a consistent survey size and found that species richness decreased with increasing patch size in the open forest ecosystem. Time since fire affected vegetation structure in fire-prone ecosystems but was not a determinant of species richness within the sites studied.
Acknowledgements
We especially thank Greg Smyrell, Kris Kupsch and Peter Dufourq for their botanical expertise and field assistance, as well as, Laura Simmons, Brad Jeffers, Rosie McVeigh, Marion Howard, Steven Williams, Maree Clancy, Lidia Davidovics and Bruce and Jarrod Etherington for field assistance. We also thank Neil Tindale and Katie O’Connor for encouragement and editorial assistance. Part of this study was undertaken within a larger regional assessment of biodiversity in the Sunshine Coast and we especially thank Scott Burnett and his team. This project was funded by Sunshine Coast Council and the University of the Sunshine Coast.
References
Adams MP, Smith PL, Beattie AJ (2011) Impact of spatial disjunction within biophysical classes on plant species composition: implications for conservation planning. Austral Ecology 36, 453–460.| Impact of spatial disjunction within biophysical classes on plant species composition: implications for conservation planning.Crossref | GoogleScholarGoogle Scholar |
Alados CL, Navarro T, Komac B, Pascual V, Martinez F, Cabezudo B, Pueyo Y (2009) Do vegetation patch spatial patterns disrupt the spatial organization of plant species? Ecological Complexity 6, 197–207.
| Do vegetation patch spatial patterns disrupt the spatial organization of plant species?Crossref | GoogleScholarGoogle Scholar |
Alofs KM, Fowler NL (2010) Habitat fragmentation caused by woody plant encroachment inhibits the spread of an invasive grass. Journal of Applied Ecology 47, 338–347.
| Habitat fragmentation caused by woody plant encroachment inhibits the spread of an invasive grass.Crossref | GoogleScholarGoogle Scholar |
Aparicio A, Albaladejo RG, Olalla-Tárraga MÁ, Carrillo LF, Rodríguez MÁ (2008) Dispersal potentials determine responses of woody plant species richness to environmental factors in fragmented Mediterranean landscapes. Forest Ecology and Management 255, 2894–2906.
| Dispersal potentials determine responses of woody plant species richness to environmental factors in fragmented Mediterranean landscapes.Crossref | GoogleScholarGoogle Scholar |
Archibald RD, Craig MD, Bialkowski K, Howe C, Burgess TI, Hardy GESJ (2011) Managing small remnants of native forest to increase biodiversity within plantation landscapes in the south west of Western Australia. Forest Ecology and Management 261, 1254–1264.
| Managing small remnants of native forest to increase biodiversity within plantation landscapes in the south west of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Austin MP (1978) Vegetation In ‘Land use of the South Coast of New South Wales’ . Biophysical background studies. (Ed. RH Gunn) pp. 44–67. (CSIRO: Melbourne)
Baldwin LK, Bradfield GE (2007) Bryophyte responses to fragmentation in temperate coastal rainforests: a functional group approach. Biological Conservation 136, 408–422.
| Bryophyte responses to fragmentation in temperate coastal rainforests: a functional group approach.Crossref | GoogleScholarGoogle Scholar |
Bostock PD, Holland AE (Eds) (2010) ‘Census of the Queensland flora 2010.’ (Queensland Herbarium, Department of Environment and Resource Management: Brisbane)
Bradstock RA, Myerscough PJ (1981) Fire effects on seed release and the emergence and establishment of seedlings in Banksia evicifolia L.f. Australian Journal of Botany 29, 521–531.
| Fire effects on seed release and the emergence and establishment of seedlings in Banksia evicifolia L.f.Crossref | GoogleScholarGoogle Scholar |
Broadhurst LM, Young AG (2006) Reproductive constraints for the long-term persistence of fragmented Acacia dealbata (Mimosaceae) populations in southeast Australia. Biological Conservation 133, 512–526.
| Reproductive constraints for the long-term persistence of fragmented Acacia dealbata (Mimosaceae) populations in southeast Australia.Crossref | GoogleScholarGoogle Scholar |
Brown CD, Boutin C (2009) Linking past land use, recent disturbance, and dispersal mechanism to forest composition. Biological Conservation 142, 1647–1656.
| Linking past land use, recent disturbance, and dispersal mechanism to forest composition.Crossref | GoogleScholarGoogle Scholar |
Brownlie H, Playford J, Wallace H, Shapcott A (2009) Population ecology and genetics of the vulnerable Acacia attenuata (Mimosaceae) and their significance for its conservation, recovery and translocation. Australian Journal of Botany 57, 675–687.
| Population ecology and genetics of the vulnerable Acacia attenuata (Mimosaceae) and their significance for its conservation, recovery and translocation.Crossref | GoogleScholarGoogle Scholar |
Cagnolo L, Cabido M, Valladares G (2006) Plant species richness in the Chaco Serrano woodland from central Argentina: ecological traits and habitat fragmentation effects. Biological Conservation 132, 510–519.
| Plant species richness in the Chaco Serrano woodland from central Argentina: ecological traits and habitat fragmentation effects.Crossref | GoogleScholarGoogle Scholar |
Callaghan J, McAlpine C, Mitchell D, Thompson J, Bowen M, Rhodes J, de Jong C, Domalewski R, Scott A (2011) Ranking and mapping koala habitat quality for conservation planning on the basis of indirect evidence of tree-species use: a case study of Noosa Shire, south-eastern Queensland. Wildlife Research 38, 89–102.
| Ranking and mapping koala habitat quality for conservation planning on the basis of indirect evidence of tree-species use: a case study of Noosa Shire, south-eastern Queensland.Crossref | GoogleScholarGoogle Scholar |
Campetella G, Botta-Dukát Z, Wellstein C, Canullo R, Gatto S, Chelli S, Mucina L, Bartha S (2011) Patterns of plant trait–environment relationships along a forest succession chronosequence. Agriculture, Ecosystems & Environment 145, 38–48.
| Patterns of plant trait–environment relationships along a forest succession chronosequence.Crossref | GoogleScholarGoogle Scholar |
Carter J, Moscato V, Tindale N (2009) GIS as a rapid decision-support tool for raptor conservation planning in urbanising landscapes. The Australian Geographer 40, 471–494.
| GIS as a rapid decision-support tool for raptor conservation planning in urbanising landscapes.Crossref | GoogleScholarGoogle Scholar |
Clarke KR, Gorley RN (2006) ‘PRIMER v6: user manual/tutorial.’ (Primer: Plymouth, UK)
Close DC, Davidson NJ, Swanborough PW (2011) Fire history and understorey vegetation: Water and nutrient relations of Eucalyptus gomphocephala and E. delegatensis overstorey trees. Forest Ecology and Management 262, 208–214.
| Fire history and understorey vegetation: Water and nutrient relations of Eucalyptus gomphocephala and E. delegatensis overstorey trees.Crossref | GoogleScholarGoogle Scholar |
Collard SJ, Le Brocque AF, Zammit C (2011) Effects of local-scale management on herbaceous plant communities in Brigalow (Acacia harpophylla) agroecosystems of southern Queensland, Australia. Agriculture, Ecosystems & Environment 142, 176–183.
| Effects of local-scale management on herbaceous plant communities in Brigalow (Acacia harpophylla) agroecosystems of southern Queensland, Australia.Crossref | GoogleScholarGoogle Scholar |
Connell JH (1971) On the role of natural enemies in preventing competitive exclusion in some marine animals and in rain forest trees. In ‘Dynamics of populations’. (Eds PJ Den Boer, G Gradwell) pp. 298–312. (Pudoc: Wageningen, The Netherlands)
Connor EF, McCoy ED (1979) The statistics and biology of the species-area relationship. American Naturalist 113, 791–833.
| The statistics and biology of the species-area relationship.Crossref | GoogleScholarGoogle Scholar |
Cramer JM, Mesquita RCG, Bruce Williamson G (2007) Forest fragmentation differentially affects seed dispersal of large and small-seeded tropical trees. Biological Conservation 137, 415–423.
| Forest fragmentation differentially affects seed dispersal of large and small-seeded tropical trees.Crossref | GoogleScholarGoogle Scholar |
Denslow JS (1987) Tropical rainforest gaps and tree species diversity. Annual Review of Ecology and Systematics 18, 431–451.
| Tropical rainforest gaps and tree species diversity.Crossref | GoogleScholarGoogle Scholar |
Driscoll DA, Lindenmayer DB, Bennett AF, Bode M, Bradstock RA, Cary GJ, Clarke MF, Dexter N, Fensham R, Friend G, Gill M, James S, Kay G, Keith DA, MacGregor C, Russell-Smith J, Salt D, Watson JEM, Williams RJ, York A (2010) Fire management for biodiversity conservation: key research questions and our capacity to answer them. Biological Conservation 143, 1928–1939.
| Fire management for biodiversity conservation: key research questions and our capacity to answer them.Crossref | GoogleScholarGoogle Scholar |
ESRI (2011) ‘ArcGIS desktop: release 10.’ (Environmental Systems Research Institute: Redlands, CA)
Ewers RM, Didham RK (2006) Confounding factors in the detection of species responses to habitat fragmentation. Biological Reviews of the Cambridge Philosophical Society 81, 117–142.
| Confounding factors in the detection of species responses to habitat fragmentation.Crossref | GoogleScholarGoogle Scholar |
Eyre TJ (2006) Regional habitat selection of large gliding possums at forest stand and landscape scales in southern Queensland, Australia. Forest Ecology and Management 235, 270–282.
| Regional habitat selection of large gliding possums at forest stand and landscape scales in southern Queensland, Australia.Crossref | GoogleScholarGoogle Scholar |
Eyre TJ, Kelly AL, Neldner VJ, Wilson BA, Ferguson DJ (2011) ‘BioCondition: a condition assessment framework for terrestrial biodiversity in Queensland. Assessment methodology manual. Version 2.0.’ (Department of Environment and Resource Management (DERM), Biodiversity and Ecosystem Sciences: Brisbane)
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annual Review of Ecology Evolution and Systematics 34, 487–515.
| Effects of habitat fragmentation on biodiversity.Crossref | GoogleScholarGoogle Scholar |
Fensham RJ (2008) A protocol for assessing applications to selectively clear vegetation in Australia. Land Use Policy 25, 249–258.
| A protocol for assessing applications to selectively clear vegetation in Australia.Crossref | GoogleScholarGoogle Scholar |
Figueroa JA, Teillier S, Castro SA (2011) Diversity patterns and composition of native and exotic floras in central Chile. Acta Oecologica 37, 103–109.
| Diversity patterns and composition of native and exotic floras in central Chile.Crossref | GoogleScholarGoogle Scholar |
Fisher JL, Loneragan WA, Dixon K, Delaney J, Veneklaas EJ (2009) Altered vegetation structure and composition linked to fire frequency and plant invasion in a biodiverse woodland. Biological Conservation 142, 2270–2281.
| Altered vegetation structure and composition linked to fire frequency and plant invasion in a biodiverse woodland.Crossref | GoogleScholarGoogle Scholar |
Gleason HA (1922) On the relation between species and area. Ecology 3, 158–162.
| On the relation between species and area.Crossref | GoogleScholarGoogle Scholar |
Gleason HA (1925) Species and area. Ecology 6, 66–74.
| Species and area.Crossref | GoogleScholarGoogle Scholar |
Godefroid S, Koedam N (2003) How important are large vs. small forest remnants for the conservation of the woodland flora in an urban context? Global Ecology and Biogeography 12, 287–298.
| How important are large vs. small forest remnants for the conservation of the woodland flora in an urban context?Crossref | GoogleScholarGoogle Scholar |
Gonzalez M, Ladet S, Deconchat M, Cabanettes A, Alard D, Balent G (2010) Relative contribution of edge and interior zones to patch size effect on species richness: an example for woody plants. Forest Ecology and Management 259, 266–274.
| Relative contribution of edge and interior zones to patch size effect on species richness: an example for woody plants.Crossref | GoogleScholarGoogle Scholar |
Gosper CR, Yates CJ, Prober SM, Williams MR (2010) Fire does not facilitate invasion by alien annual grasses in an infertile Australian agricultural landscape. Biological Invasions 13, 533–544.
Gosper CR, Yates CJ, Prober SM, Williams MR (2011) Fire does not facilitate invasion by alien annual grasses in an infertile Australian agricultural landscape. Biological Invasions 13, 533–544.
| Fire does not facilitate invasion by alien annual grasses in an infertile Australian agricultural landscape.Crossref | GoogleScholarGoogle Scholar |
Gosper CR, Yates CJ, Prober SM, Parsons BC (2012) Contrasting changes in vegetation structure and diversity with time since fire in two Australian Mediterranean-climate plant communities. Austral Ecology 37, 164–174.
| Contrasting changes in vegetation structure and diversity with time since fire in two Australian Mediterranean-climate plant communities.Crossref | GoogleScholarGoogle Scholar |
Greig-Smith P (1964) ‘Quantitative plant ecology.’ (Butterworths: London)
Hoffmann WA, Jaconis SY, McKinley KL, Geiger EL, Gotsch SG, Franco AC (2012) Fuels or microclimate? Understanding the drivers of fire feedbacks at savanna–forest boundaries. Austral Ecology 37, 634–643.
| Fuels or microclimate? Understanding the drivers of fire feedbacks at savanna–forest boundaries.Crossref | GoogleScholarGoogle Scholar |
Hubbell SP (1979) Tree dispersion, abundance, and diversity in a tropical dry forest. Science 203, 1299–1309.
| Tree dispersion, abundance, and diversity in a tropical dry forest.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cvjtVGktg%3D%3D&md5=37b9bffb9b076d9d17a1f0a3509beef2CAS | 17780463PubMed |
Hubbell SP (1980) Seed predation and the coexistence of tree species in tropical forests. Oikos 35, 214–229.
| Seed predation and the coexistence of tree species in tropical forests.Crossref | GoogleScholarGoogle Scholar |
IBM (2010) ‘IBM SPSS statistics for Windows, version 19.0.’ (IBM Corporation: Armonk, NY)
Jansson R, Zinko U, Merritt DM, Nilsson C (2005) Hydrochory increases riparian plant species richness: a comparison between a free-flowing and a regulated river. Journal of Ecology 93, 1094–1103.
| Hydrochory increases riparian plant species richness: a comparison between a free-flowing and a regulated river.Crossref | GoogleScholarGoogle Scholar |
Janzen DH (1970) Herbivores and the number of tree species in forests. American Naturalist 104, 501–528.
| Herbivores and the number of tree species in forests.Crossref | GoogleScholarGoogle Scholar |
Jesus FM, Pivello VR, Meirelles ST, Franco GADC, Metxger JP (2012) The importance of landscape structure for seed dispersal in rain forest fragments. Journal of Vegetation Science 23, 1126–1136.
| The importance of landscape structure for seed dispersal in rain forest fragments.Crossref | GoogleScholarGoogle Scholar |
Knox KJE, Clarke PJ (2012) Fire severity, feedback effects and resilience to alternative community states in forest assemblages. Forest Ecology and Management 265, 47–54.
| Fire severity, feedback effects and resilience to alternative community states in forest assemblages.Crossref | GoogleScholarGoogle Scholar |
Knox KJE, Morrison DA (2005) Effects of inter-fire intervals on the reproductive output of resprouters and obligate seeders in the Proteaceae. Austral Ecology 30, 407–413.
| Effects of inter-fire intervals on the reproductive output of resprouters and obligate seeders in the Proteaceae.Crossref | GoogleScholarGoogle Scholar |
Kuenzi AM, Fulé PZ, Sieg CH (2008) Effects of fire severity and pre-fire stand treatment on plant community recovery after a large wildfire. Forest Ecology and Management 255, 855–865.
| Effects of fire severity and pre-fire stand treatment on plant community recovery after a large wildfire.Crossref | GoogleScholarGoogle Scholar |
Laurance WF, Camargo JLC, Luizão RCC, Laurance SG, Pimm SL, Bruna EM, Stouffer PC, Bruce Williamson G, Benítez-Malvido J, Vasconcelos HL (2011) The fate of Amazonian forest fragments: a 32-year investigation. Biological Conservation 144, 56–67.
| The fate of Amazonian forest fragments: a 32-year investigation.Crossref | GoogleScholarGoogle Scholar |
Le Brocque AF (1998) Richness of species and growth-forms within sclerophyll and mesophyll vegetation in eastern Australia. Australian Journal of Ecology 23, 168–176.
| Richness of species and growth-forms within sclerophyll and mesophyll vegetation in eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Lewis T, Reif M, Prendergast E, Tran C (2012) The effect of long-term repeated burning and fire exclusion on above- and below-ground blackbutt (Eucalyptus pilularis) forest vegetation assemblages. Austral Ecology 37, 767–778.
| The effect of long-term repeated burning and fire exclusion on above- and below-ground blackbutt (Eucalyptus pilularis) forest vegetation assemblages.Crossref | GoogleScholarGoogle Scholar |
MacArthur RH, Wilson EO (1967) ‘The theory of island biogeography.’ (Princeton University Press: Princeton, NJ)
Mangan SA, Schnitzer SA, Herre EA, Mack KM, Valencia MC, Sanchez EI, Bever JD (2010) Negative plant-soil feedback predicts tree-species relative abundance in a tropical forest. Nature 466, 752–755.
| Negative plant-soil feedback predicts tree-species relative abundance in a tropical forest.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtVSmu7rN&md5=45604f7b5a5b5f78eceb53f563a10f05CAS | 20581819PubMed |
Moran C, Catterall C, Kanowski J (2009) Reduced dispersal of native plant species as a consequence of the reduced abundance of frugivore species in fragmented rainforest. Biological Conservation 142, 541–552.
| Reduced dispersal of native plant species as a consequence of the reduced abundance of frugivore species in fragmented rainforest.Crossref | GoogleScholarGoogle Scholar |
Neldner VJ, Wilson BA, Thompson EJ, Dilleward HA (2005) ‘Methodology for survey and mapping of regional ecosystems and vegetation communities in Queensland. Version 3.1.’ (Queensland Herbarium, Environmental Protection Agency: Brisbane)
Parsons BC, Gosper CR (2011) Contemporary fire regimes in a fragmented and an unfragmented landscape: implications for vegetation structure and persistence of the fire-sensitive malleefowl. International Journal of Wildland Fire 20, 184–194.
| Contemporary fire regimes in a fragmented and an unfragmented landscape: implications for vegetation structure and persistence of the fire-sensitive malleefowl.Crossref | GoogleScholarGoogle Scholar |
Pekin BK, Wittkuhn RS, Boer MM, Macfarlane C, Grierson PF (2012) Response of plant species and life form diversity to variable fire histories and biomass in the jarrah forest of south-west Australia. Austral Ecology 37, 330–338.
| Response of plant species and life form diversity to variable fire histories and biomass in the jarrah forest of south-west Australia.Crossref | GoogleScholarGoogle Scholar |
Queensland Herbarium (2012) ‘Regional ecosystem description database (REDD) version 6.1.’ (Department of Science, Information Technology, Innovation and the Arts, Queensland Government: Brisbane)
Rodríguez-Loinaz G, Amezaga I, Onaindia M (2012) Does forest fragmentation affect the same way all growth-forms? Journal of Environmental Management 94, 125–131.
| Does forest fragmentation affect the same way all growth-forms?Crossref | GoogleScholarGoogle Scholar | 21924813PubMed |
Ross KA, Fox BJ, Fox MD (2002) Changes to plant species richness in forest fragments: fragment age, disturbance and fire history may be as important as area. Journal of Biogeography 29, 749–765.
| Changes to plant species richness in forest fragments: fragment age, disturbance and fire history may be as important as area.Crossref | GoogleScholarGoogle Scholar |
Russell-Smith J, Stanton PJ, Whitehead PJ, Edwards A (2004) Rain forest invasion of eucalypt-dominated woodland savanna, Iron Range, north-eastern Australia: I. Successional processes. Journal of Biogeography 31, 1293–1303.
| Rain forest invasion of eucalypt-dominated woodland savanna, Iron Range, north-eastern Australia: I. Successional processes.Crossref | GoogleScholarGoogle Scholar |
Sattler PS, Williams R (1999). ‘The conservation status of Queensland’s bioregional ecosystems.’ (Environmental Protection Agency, Queensland Government: Brisbane)
Scott K, Setterfield SA, Douglas MM, Parr CL, Schatz JON, Andersen AN (2012) Does long-term fire exclusion in an Australian tropical savanna result in a biome shift? A test using the reintroduction of fire. Austral Ecology 37, 693–711.
| Does long-term fire exclusion in an Australian tropical savanna result in a biome shift? A test using the reintroduction of fire.Crossref | GoogleScholarGoogle Scholar |
Shapcott A, Bau B, Powell M (2009) Fragmentation and genetic diversity in Romnalda (Daspogonaceae), a rare rain forest herbaceous genus from New Guinea and Australia. Biotropica 41, 128–136.
| Fragmentation and genetic diversity in Romnalda (Daspogonaceae), a rare rain forest herbaceous genus from New Guinea and Australia.Crossref | GoogleScholarGoogle Scholar |
Specht RL (1970) Vegetation In ‘The Australian environment’. (Ed. GW Leeper) pp. 44–67. (CSIRO and Melbourne University Press: Melbourne)
State of Queensland – Department of Natural Reseources and Mines (2012a) ‘Vegetation Management Act. Regional ecosystems version 6.1 – SEQ.’ (Queensland Government Information Service: Brisbane)
State of Queensland – Department of Natural Reseources and Mines (2012b) ‘Vegetation Management Act. High-value regrowth vegetation version 2.1.’ (Queensland Government Information Service: Brisbane)
Townend J (2002) ‘Practical statistics for environmental and biological sciences.’ (John Wiley and Sons: Oxford, UK)
White E, Tucker N, Meyers N, Wilson J (2004) Seed dispersal to revegetated isolated rainforest patches in north Queensland. Forest Ecology and Management 192, 409–426.
| Seed dispersal to revegetated isolated rainforest patches in north Queensland.Crossref | GoogleScholarGoogle Scholar |
Williams CB (1943) Area and number of species. Nature 152, 264–267.
| Area and number of species.Crossref | GoogleScholarGoogle Scholar |
Williams PR, Parsons M, Jensen R, Tran C (2012) Mechanisms of rainforest persistence and recruitment in frequently burnt wet tropical eucalypt forests. Austral Ecology 37, 268–275.
| Mechanisms of rainforest persistence and recruitment in frequently burnt wet tropical eucalypt forests.Crossref | GoogleScholarGoogle Scholar |
Wilson BA, Neldner VJ, Accad A (2002) The extent and status of remnant vegetation in Queensland and its implications for statewide vegetation management and legislation. Journal of Rangeland Ecology 24, 6–35.
| The extent and status of remnant vegetation in Queensland and its implications for statewide vegetation management and legislation.Crossref | GoogleScholarGoogle Scholar |





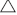 ) and gallery rainforest (■).
) and gallery rainforest (■).