Applying resource-selection functions to assess host preference in the endemic endoparasite Pilostyles hamiltoniorum (Apodanthaceae) and its principal host Daviesia (Fabaceae)
Ryan J. Craig A * , Bethany Pittway A , Tingting Wu A , Shane R. Turner
A * , Bethany Pittway A , Tingting Wu A , Shane R. Turner  B and Jacqueline Batley A
B and Jacqueline Batley A
A
B
Abstract
Pilostyles are a genus of endoparasitic plants specific to the Fabaceae family. In Australia, three species are restricted to the South-west floristic region.
This study aimed to assess the use of resource-selection functions (RSFs) on populations of Pilostyles hamiltoniorum to understand host preference to the known host species of Daviesia.
Forty sites were chosen along the known distribution of P. hamiltoniorum, and infected and uninfected hosts were recorded at each site. The Manly resource-usage function was applied to the data to assess host use in populations of P. hamiltoniorum.
Only 9 of the 40 sites had populations large enough to assess host use. Out of these surveys, Pilostyles presented high preference for four hosts species, namely, Daviesia angulata, D. physodes, D. preisii and D. rhombifolia, with D. decurrens, observed to be the least favoured host.
Resource-selection indices showed to be a potential tool in understanding host preference within the genus Pilostyles, with evidence here indicating that host use is not equal within the environment.
The patterns of host use show that there are some unknown factors between each host affecting infection, along with the identification of strongly preferred hosts that could lead to future research in ex vitro cultivation.
Keywords: Apodanthaceae, Daviesia, endoparasite, host preference, parasite, parasitic plant, Pilostyles, resource, selection functions.
Introduction
Throughout all kingdoms of life, parasitism has evolved as a way of using the resources of another organism, and many parasites play important roles within the ecosystem they occupy (Westwood et al. 2010; Poulin and Randhawa 2015; Nickrent 2020). According to Nickrent (2020), approximately 1% of angiosperm plants are parasites and can be found in many ecosystems around the world in a variety of forms (Westwood et al. 2010; Poulin and Randhawa 2015; Nickrent 2020). Identifying the ecological interactions with their hosts is important in understanding how they function within their ecosystem (Watson 2009a, 2009b; Watson et al. 2022). Parasitic plants live on a spectrum of hosts (Milner et al. 2020), from host generalists, such as Viscum album, which has been shown to use over 400 plant species (Krasylenko et al. 2020), to strict specialists, such as genera within the Rafflesiaceae family (e.g. Sapria, Rhizanthes and Rafflesia spp.), where they are associated only with plants in the genus Tetrastigma (Vitaceae) (Pelser et al. 2016). Host preference is affected by a number of qualities of the potential host, such as greater access to water and nutrients, and specific beneficial mutualisms held by the host species (Watson 2009a). Understanding the patterns of host preference is more important in understanding the ecology of parasitic plant species, than is simply listing what hosts they target. This can be important in conserving threatened parasitic plant species because it can help target specific host species for ex situ experiments and inform restoration efforts as to where this species may better be placed (Pelser et al. 2016; Watson et al. 2017; Milner et al. 2020; Thorogood et al. 2022).
It cannot be assumed that a parasitic plant uses all their known hosts uniformly. Although a parasite may vary in specificity, there is often a level of preference given to a particular host/s (Giorgi et al. 2004; Milner et al. 2020). One way in which host preference could be quantified is to assess resource usage (Kelly et al. 1988). Resource usage is tested using various functions, termed resource-selection functions (RSF), that consider the level of used and unused resources by an organism. These functions are commonly prescribed to animal studies where resources are defined by food or habitat. Habitat selection is one of the more common uses of RSFs. These RSFs can be important in assessing habitat selection of organisms in the face of climate change and urbanisation (Dias et al. 2020; Gili et al. 2020; Jones et al. 2020). RSFs can also inform ecologists about behavioural responses to resource competition among habitat types (Ordiz et al. 2020), and the identification of suitable environments for rare species translocation (Barras et al. 2020). However, the use of RSFs is underutilised in plants, although they could be useful in the study of host preference within parasitic species. Host preference in parasitic plants has mostly been assessed using mistletoe species, which are easy to assess in the environment because of their distinctive vegetative state being above ground, making them easily visible to study (Lemaitre et al. 2012; Watson et al. 2017; Hódar et al. 2018; Sultan et al. 2018; Milner et al. 2020; Atencio et al. 2021). Currently, only one study has been published utilising RSFs for parasitic plants. Milner et al. (2020) assessed several mistletoe species across three different spatial scales, namely, habitat, state and bioregion. Their study confirmed that the qualitative descriptions of host preference and specialisation of the three mistletoe species (Amyema lucasii, Amyena quongdong and Lysiana exocarpi) is quantitatively supported using RSFs and specialisation indices. Amyena lucasii was confirmed as a strict specialist, only occurring on Flindersia maculosa, having the highest degree of specialisation and host species turn-over. Whereas A. quongdong was shown to be a genus specialist on Acacia and L. exocarpi was demonstrated to be the most generalist species over all spatial scales. This variation among mistletoe species over a given spatial scale has highlighted the use of such functions in understanding host preference and specialisation within parasitic plants (Milner et al. 2020). Nevertheless, for other parasitic plants, the use of RSFs may be limited to species where evidence of infection is easy to assess and thus readily quantifiable.
Many cryptic species, such as endoparasites, have gone unnoticed based on the difficulty in finding such species. These endoparasites are found in a variety of plant families across the globe, such as the mistletoes, Tristrex aphylla, Viscum minimum and Arceuthobium spp., which come from the Loranthaceae and Viscaceae families (Mauseth and Rezaei 2013; Lucero et al. 2014). Worldwide, there are now four strict endoparasitic plant families recognised, namely (1) the Apodanthaceae, containing Apodanthes and Pilostyles (Bellot and Renner 2014), (2) the Rafflesiaceae, which contains the well-known endoparasites Rafflesia, Sapria and Rhizanthes (Arunachalam et al. 2004; Pelser et al. 2016; Norhazlini et al. 2021; Wicaksono et al. 2022), (3) the Cytinaceae, which contains two genera, Cytinus and Bdallophytum (García-Franco and Rico-Gray 1997; Thorogood and Hiscock 2007; Govaerts and Nickrent 2010; Rios-Carrasco and Vázquez-Santana 2021), and (4) the Mitrastemonaceae, which contains the genus Mitrostemon (Teixeira-Costa and Suetsugu 2023). To add to these four families, another recent confirmed endoparasitic species is Lathrophytum peckoltii, from the Balanophoraceae (Pellissari et al. 2022).
Host preference and host use are difficult to determine for many of these species because they can be hard to find when not flowering, owing to many of these parasitic plants residing mostly within the host root tissue existing as endoparasites, making them virtually impossible to easily find (Thorogood and Hiscock 2007; Pelser et al. 2019; Rios-Carrasco and Vázquez-Santana 2021; Teixeira-Costa and Suetsugu 2023). However, for some species such as Pilostyles (Apodanthaceae), the use of RSFs could be possible. Pilostyles leaves clear signs of infection throughout the year, either because of the presence of flowers and fruits of the parasite along the host plant stems or the distinct scarring left behind on host plants (Dell et al. 1982; Kuijt et al. 1985; González and Pabón-Mora 2017). Pilostyles species, although specific to the Fabaceae, show some generalism to a number of species within their respective host genera. For example, P. hamiltoniorum is specific to Daviesia spp., P. collina on Gastrolobium and P. coccoidea on Jacksonia, although compared with some parasitic plants that parasitise a variety of different host genera (e.g. Nuytsia floribunda), their affinity to one genus of host leads to them being considered highly specialised (Bellot and Renner 2014). Therefore, they are an interesting group to target so as to understand host usage in a cryptic endoparasite. The Australian species Pilostyles hamiltoniorum (syn. P. hamiltonii) has been recorded to parasitise 10 species within the genus Daviesia and is widespread from Eneabba to Margaret River in south-western Western Australia. The restricted distribution of P. hamiltoniorum follows a narrow band along the Darling Scarp, yet the host species are found over much larger ranges (Thiele et al. 2008). This species infects the largest variety of hosts within its specific host genus compared with the two other Australian Pilostyles species, P. coccoidea and P. collina, which infect two species of Jacksonia and five species of Gastrolobium respectively. However, the pattern of host use throughout this distribution has not been well documented. Therefore, RSFs could be useful in assessing host use between P. hamiltoniorum and their known host Daviesia to improve the understanding of P. hamiltoniorum host use.
Although the species does not fall under conservation concern, the cryptic nature and lack of information regarding its resilience to impacts brought on by climate change (i.e. drought and fire) and broad-scale land-clearing, means that an understanding of host use will help not only fill in some gaps in the understanding of the unusual distribution of P. hamiltoniorum, but also identify hosts that could be important in the study of germination, restoration and ex situ cultivation. Furthermore, the potential to map host preference in P. hamiltoniorum by using RSFs could lead to similar surveys being conducted on other Pilostyles species. Here, the Manley Resource Selection Ratio (Manly et al. 2002) is applied to the known Daviesia hosts species to assess P. hamiltoniorum host preference. This is an approach that has not been applied to the genus, and up until now, the extent of host preference has not been fully mapped out for this species. Pilostyles hamiltoniorum could be uniformly using hosts throughout its distribution or using a select few species of Daviesia over others. Because host preference has not been accurately assessed for P. hamiltoniorum it cannot be assumed that P. hamiltoniorum uses the host Daviesia uniformly. Here, the aim is to assess the host preference of P. hamiltoniorum on the Daviesia species known to be susceptible to P. hamiltoniorum infection, specifically the level of preference towards differing Daviesia species within the environment.
Methods
Study area
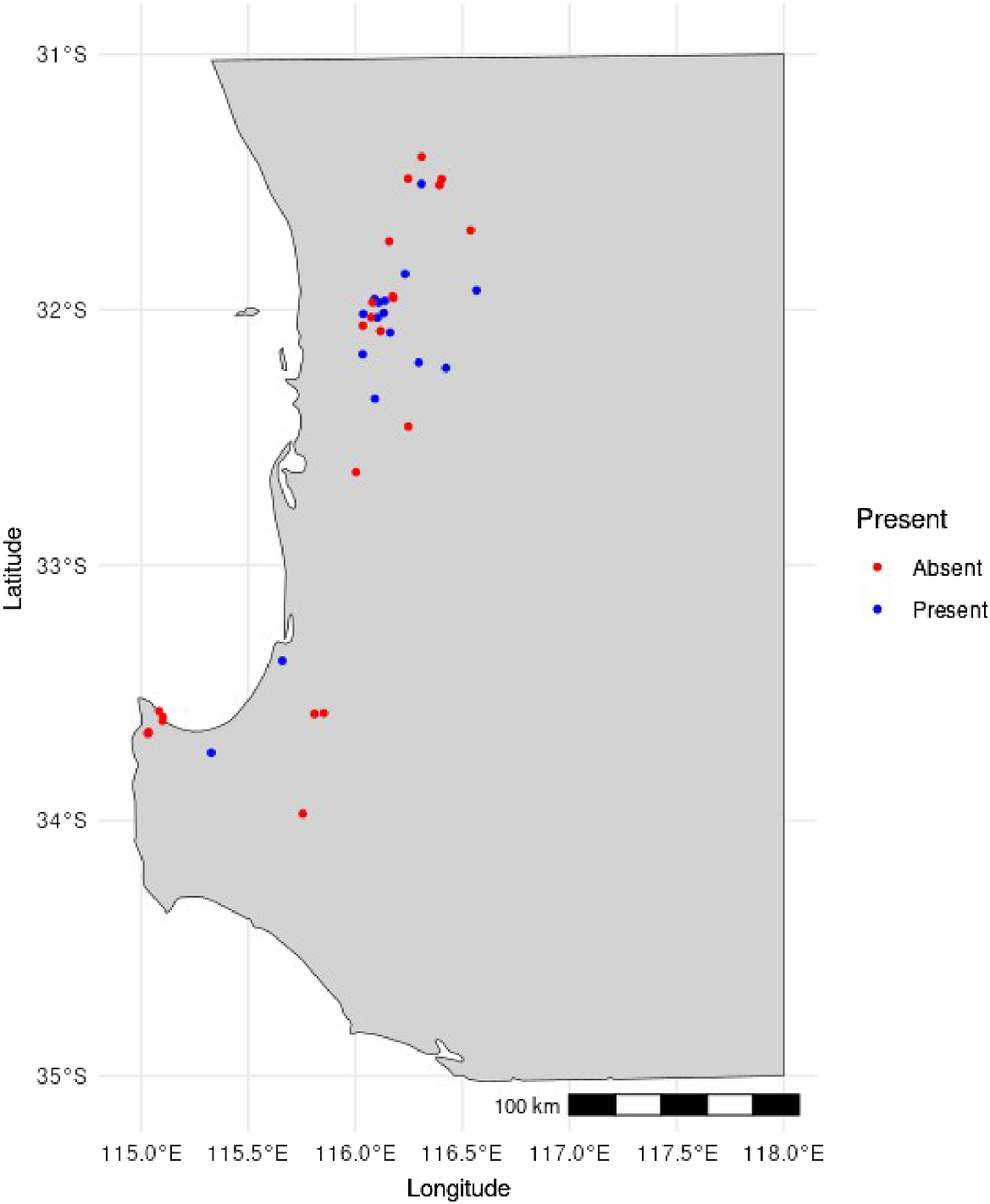
Surveys were conducted from Bindoon to Dunsborough within the South-west of Western Australia, mostly within the Jarrah Forest IBRA (Interim Biogeographic Regionalisation of Australia) region, but locations also fell within the Wheatbelt region east of Perth. Survey locations were restricted to walking tracks to allow easy access to habitat and to minimise the impact on native vegetation and the spread of dieback disease (Phytophthora spp.). Forty sites were selected on the basis of herbarium records for Pilostyles hamiltoniorum (Fig. 1).
Study species
Pilostyles hamiltoniorum is a highly specific endoparasitic plant that currently is known to infect 10 species of Daviesia, namely D. angulata, D. decurrens, D. hakeaoides, D. preisii, D. polyphylla, D. incrassata, D. rhombifolia, D. quadrilatera, D. physodes and D. nudiflora (DBCA 2021). These hosts are a key component of the understorey layer of woodland (mainly jarrah, Eucalyptus marginata) communities, and often comprise the dominant shrub layer (Dell and Burbidge 1981). They are disturbance-responding species, and so are often found near fire breaks, tracks and burnt patches, making them easy to locate. Pilostyles hamiltoniorum can be found flowering (Fig. 2d) from February to April, and fruit (Fig. 2c) between July and November. Throughout the year, distinct highly visible scarring left by P. hamiltoniorum flowers can be seen (on its hosts), from the previous year’s flowering, allowing for easy identification in and out of the flowering period. If not for this scarring, it would be impossible to casually observe because of its endophytic nature (Fig. 2a).
Survey
Each site was visited and traversed along established walking trails, with all species of Daviesia spotted from the trail being quickly assessed for signs of P. hamiltoniorum infection. If there were no signs of infection, the trek along the path continued until an infected host plant was identified. Intensive surveys commenced of all individual Daviesia within a location, once a potential host was discovered to be infected. Survey activity involved quantifying all infected and uninfected Daviesia within the general area and stopped once 25 individuals of Daviesia were sampled within the locality of the first identified individual. Counting 25 infected hosts was chosen on the basis of previous studies by Milner et al. (2020) on mistletoe (Milner et al. 2020). Host Daviesia individuals were assessed for presence of scarring, fruits, or flowers, regardless of whether the infection was active. In some areas it was noted that burns did occur (Jarrahdale) and for these areas scarring was present on burnt stems; however, there is little research on whether Pilostyles survives fire (within host tissues), and these individuals were still recorded as infected. Because of the high specificity of host use, only known hosts (D. angulata, D. decurrens, D. hakeaoides, D. preisii, D. polyphylla, D. incrassata, D. rhombifolia, D. quadrilatera, D. physodes and D. nudiflora) were captured in the non-infected group, with up to 100 potential host Daviesia individuals being scored as uninfected. The uninfected population was scored as the closest individuals in a radius around the infected Daviesia population. This allowed for scoring all the known host Daviesia species at the site.
Host preference: resource-selection functions
Pilostyles hamiltoniorum host preference was estimated using the Manly resource-selection ratio, which compares the proportion of used resources (i.e. infected Daviesia) with unused resources (i.e. non-infected Daviesia). The data fit the assumptions for Study design 1, Sampling protocol A, by using the the following resource-usage ratio equation (from Manly et al. 2002, eqn 4.14):
Here, σi denotes the used-resource proportion in Category i, (e.g. the number of individuals of an infected Daviesia species/the total number of infected Daviesia species within a site), and πi is the proportional availability of resources in Category i (e.g. the number of a non-infected Daviesia species/the total number of non-infected Daviesia within a site). Preference to a host is suggested when ωi > 1. Subsequent confidence intervals based on the Bonferroni correction were applied, as was done by Milner et al. (2020), with a lower confidence interval of >1 indicating that the preference to a particular host is significant. Whereas, if the upper confidence interval is <1, then this indicates that the host was significantly avoided. Of all the sites, only those that had more than five infected individuals reliably show significance.
β-specificity
β-specificity is an evaluation of host use among different sites. This allows for a pairwise comparison between sites that shows the spatial turnover of hosts between each site. This can then indicate whether a parasite, such as Pilostyles hamiltoniorum, is using a specific host within a particular area, or whether there is no specificity among sites. β-specificity is calculated using the Chao–Sorensen similarity index; this index uses abundance data and corrects for unseen shared species (Chao et al. 2005, 2006). This was all performed in EstimateS (ver. 9.1.0, https://www.robertkcolwell.org/pages/1407; Colwell 2013). β-specificity was calculated from total number of infected Daviesia from each site, using EstimateS. β-specificity was assessed over the different sites surveyed along the range of P. hamiltoniorum. This index states that a parasite with high β-specificity (specialist) will present a score close to one, whereas a parasite with less β-specificity (generalist) will have a score close to zero (Milner et al. 2020).
Results
Host preference
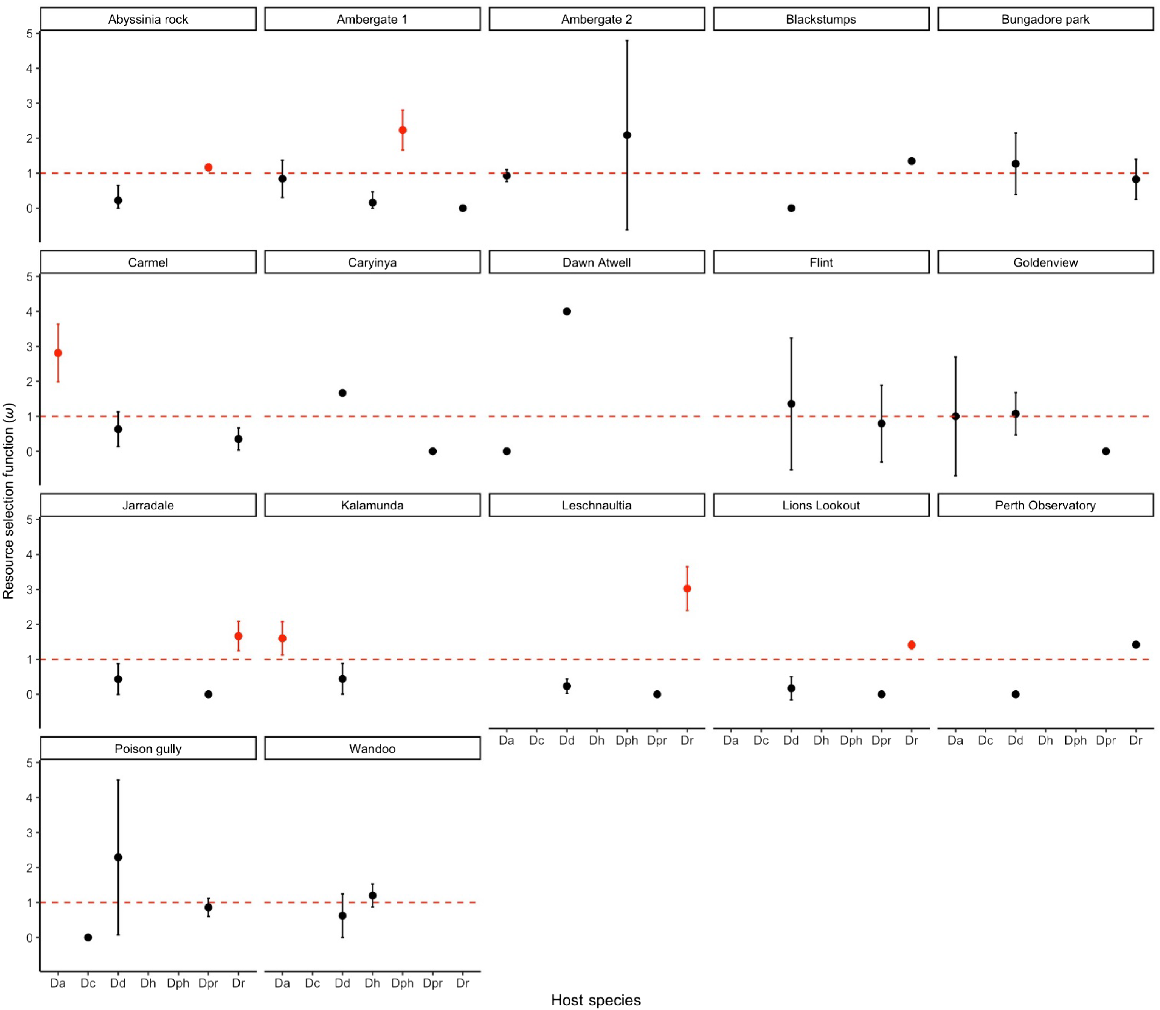
Of the 40 sites surveyed across south-western Western Australia, 17 were observed to have Pilostyles hamiltoniorum present, with the remaining 23 sites showing no presence of P. hamiltoniorum. Five host species were significantly preferred by P. hamiltoniorum at the following seven sites: Abysinnia Rock, Ambergate, Carmel, Jarrahdale, Kalamunda, Lions Lookout and Leschnaultia (Fig. 3). Daviesia angulata was the preferred host at two sites, namely, Carmel (ω = 2.81) and Kalamunda (ω = 1.60). D. physodes was the preferred at one site, Ambergate (ω = 2.23). D. preisii was the preferred at one site, Abysinnia Rock (ω = 1.17). D. rhombifolia was the preferred at the following three sites: Jarrahdale (ω = 1.67), Lions Lookout (ω = 1.41) and Leschenaultia (ω = 3.02). Overall, many of the sites contained infected hosts of D. angulata, D. rhombifolia and D. decurrens (Fig. 3). With D. hakeoides (Ambergate and Wandoo), D. physodes (Ambergate) and D. preisii (Abyssinia Rock) being infected at only a few sites. D. decurrens is repeatedly a low-preference host species over several sites (Fig. 3), including at Abyssinia Rock (ω = 0.22), Carmel (ω = 0.63), Jarrahdale (ω = 0.44), Kalamunda (ω = 0.44), Lions Lookout (ω = 0.17), Leschenaultia (ω = 0.25) and Wandoo (ω = 0.82). Sites Blackstumps, Caryinya, Goldenview and Perth Observatory (Fig. 3) all show P. hamiltoniorum having no preference to D. decurrens; however, the low number of infected Daviesia suggests this may be only an indicator of low preference at these sites. Poison Gully presents D. decurrens to have the highest preference score of ω = 2.29, but shows no significance on the basis of the lower confidence interval being less that one.
Preference scores (ω) for Pilostyles hamiloniorum from all 17 sites surveyed. The dashed line shows the ω = 1, where, if the lower confidence interval of preference score is >1, then significance in the score is shown, with significant points marked red. Da, Daviesia angulata; Dc, Daviesia cordata; Dd, Daviesia decurrens; Dh, Daviesia hakeioides; Dph, Daviesia physodes; Dpr, Daviesia preisii; Dr, Daviesia rhombifolia.
Host Daviesia species turn-over between sites (β-specificity)
Because of the specificity of P. hamiltoniorum, most of the site comparisons did not change, which is largely due to the widespread distribution of the known host Daviesia. Most of the Perth Hills sites shown in Table 1 (Caryinya, Goldenview, Carmel, Jarrahdale, Kalamunda, Lions Lookout, Perth Observatory, Leschnaultia, Blackstumps and Dawn Atwell) showed high similarity (β = 1), owing to the same hosts being shared among these sites. Abyssinia Rock (Table 1) was one of the few sites in the Perth Hills region that presented low similarity scores, owing to most of the Daviesia found being D. preisii. Owing to D. preisii being infected at only Abyssinia rock, Poison Gully and Flint (Table 1), these site all showed high similarity among each other (β = 1). Ambergate and Wandoo (Table 1) also showed lower β-specificity than did other sites, owing to the presence of less frequently recorded hosts. Ambergate was the only place to have presented D. physodes, with most of the β-specificity scores present being low (β = <0.5). D. hakeoides, also found in Wandoo, was another rarely recorded species and, so, comparison scores (Table 1) among all sites were also low (β = <0.4).
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 3 | 0 | 0.98 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 5 | 0.076 | 0 | 0 | 0.667 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 6 | 0.07 | 0.379 | 0.739 | 0.276 | 0.529 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 7 | 0.077 | 0 | 0 | 0 | 0.667 | 0.333 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 8 | 0.077 | 0.298 | 0.4 | 0 | 0.6 | 1 | 0.857 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 9 | 0.07 | 0 | 0 | 0.889 | 1 | 0.529 | 0.333 | 0.316 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 10 | 0.072 | 0.399 | 0.819 | 0 | 0.316 | 0.913 | 0.375 | 1 | 0.214 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 11 | 0.059 | 0 | 0 | 0.98 | 1 | 0.626 | 0.077 | 0.077 | 1 | 0.072 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 12 | 0 | 0 | 0 | 1 | 0.667 | 0.276 | 0 | 0 | 0.889 | 0 | 0.98 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 13 | 1 | 0 | 0 | 0 | 0.324 | 0.218 | 0.387 | 0.364 | 0.218 | 0.235 | 0.072 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 14 | 0.069 | 0 | 0 | 0.909 | 1 | 0.529 | 0.286 | 0.273 | 1 | 0.194 | 1 | 0.909 | 0.197 | 0 | 0 | 0 | 0 | |
| 15 | 0.071 | 0.074 | 0 | 0 | 0.3 | 0.207 | 0.353 | 0.333 | 0.207 | 0.222 | 0.071 | 0 | 0.226 | 0.188 | 0 | 0 | 0 | |
| 16 | 1 | 0 | 0 | 0 | 0.556 | 0.333 | 0.667 | 0.652 | 0.333 | 0.366 | 0.094 | 0 | 1 | 0.294 | 0.349 | 0 | 0 | |
| 17 | 0.077 | 0 | 0 | 0 | 0.667 | 0.333 | 1 | 0.857 | 0.333 | 0.375 | 0.077 | 0 | 0.387 | 0.286 | 0.353 | 0.667 | 0 |
*1, Abyssinia Rock; 2, Ambergate; 3, Ambergate 2; 4, Blackstumps; 5, Bungendore Park; 6, Carmel; 7, Caryinya; 8, Goldenview; 9, Jarrahdale; 10, Kalamunda; 11, Lions Lookout; 12, Perth Observatory; 13, Poison Gully; 14, Leschnaultia; 15, Wandoo; 16, Flint; and 17, Dawn Atwell Reserve.
Discussion
Even though Pilostyles is specific to parasitising species from the Fabaceae, its affinity to parasitise multiple hosts species within its respected host genera, means that there is feasibility in the use of resource-usage functions (RSFs). This study is the first to use RSFs to assess host preference in Pilostyles and serves as a template for future studies on other parasitic plant species. The results from this study suggest that host use is uneven among different host species of Daviesia. Daviesia decurrens was identified as an unfavourable host at all sites, whereas D. angulata and D. rhombifolia were observed to be the preferred hosts over many of the surveyed areas. Similar results were seen in the surveys conducted by Dell and Burbidge (1981), where it was observed within the study plots that D. angulata represented more of the infected hosts within the area (12%) than did D. decurrens, which showed limited infection within the site (7%) (Dell and Burbidge 1981). Further study is needed to capture the full extent of host preference in this species, particularly for capturing D. physodes and D. preisii, because at some sites, these presented as preferred hosts, but they were not infected or present at every site.
Host preference within parasitic plants has been noted in several groups, with many of the examples supporting the host-quality hypothesis. This hypothesis takes the host quality into account, where hosts with better access to water and nutrients are viewed as superior hosts compared with those with limited access to these resources. In some cases, preference is based on the relatedness of host species, e.g. Pilostyles affinity to the Fabaceae family, or Rafflesiaceae specificity to Tetrastigma (Arunachalam et al. 2004; Bellot and Renner 2014; Pelser et al. 2016; Norhazlini et al. 2021; Wicaksono et al. 2022). Examples of how host quality can affect preference can also be seen within age classes of a host. For example, Conopholis Americanum is a species of root parasite within the Orobanchaceae and shows preference to older hosts compared with younger hosts that may not have access to the depth of resources as much as the older hosts would (Holmes 2022). Host quality can also be seen in some mistletoes, and Santalum, where they have been noted to benefit from hosts growing near water sources, compared with hosts that do not (Watson 2009a). The preference to Fabaceae that Pilostyles hold may be due to this hypothesis as well, because this family is well known for nitrogen fixation (Sprent 1999). This is already seen in the parasitic tree Santalum acuminatum, preferring Acacia saligna over other hosts because of the increased source of nitrogen provided by this host (Nge et al. 2019). Many Daviesia species present mutualism with nitrogen-fixating bacteria, which is also observed in many other Fabaceae as well (Werner et al. 2014; Crisp et al. 2017). Some Daviesia species have also been observed to form cluster roots, which are modified roots for phosphorus acquisition, thus increasing access to this important element (Nge et al. 2020). Daviesia species are also well protected from herbivory, with the presence of their phyllodes often having sharp tips to them (Crisp et al. 2017). Both benefits from these nutrient-acquisition strategies and herbivory defence may in turn benefit P. hamiltoniorum and lead to its preference to Daviesia. From observations while undertaking the surveys, many of the highly preferred hosts such as D. angulata and D. rhombifolia were much healthier than was D. decurrens. Although this was not assessed in depth, this does present as an interesting aspect for future research.
Pilostyles hamiltoniorum appears to prefer some of the more commonly found host species that are found over the survey area. With the β-specificity (Table 1) showing that between sites there is an even spread of host use over all the survey areas, with minor host changes with the addition of D. physodes in the south. This may be because many of these hosts show wide overlaps in distribution across the South-west of Western Australia (Crisp et al. 2017). Although they may prefer the more common hosts, this highlights the restricted distribution of P. hamiltoniorum. When comparing other species of Pilostyles, it is evident that most share a restricted distribution similar to their hosts. Pilostyles boyacensis is restricted to a smaller area than is the widespread host (Dalea cuatrecasasii), and is found in only 10 populations at high elevation in dry forests (Gonzalez and Pabon-Mora 2014; Pabón-Mora 2021). Likewise, P. maya is restricted compared with its host, Bauhinia erythrocalyx, which is a widespread liana, yet P. maya is found only in the state of Campeche (Ortega-gonzález et al. 2020). Pilostyles berteroi is also considered scarce in its habitat with minimal information available, but its host Adesmia spp. shows a much wider distribution (Novoa 2005). Calliandra houstoniana is also widespread in central America compared with P. mexicana that infects it (Macqueen and Hernández 1997; Ortega-gonzález et al. 2020). These are just several examples of species where sufficient information is available and it may be that many others show a similar habitat and range restriction when survey work is completed on populations of other Pilostyles and their host species.
This study has shown that the use of RSFs can be an informative tool in Pilostyles surveys. However, it should be noted that hosts may be missed owing to early infection or owing to recent fire. The methods outlined in this study require populations to be mature and unaffected by disturbance events. However, with the use of RSFs, this study was able to show that P. hamiltoniorum does not infect its hosts uniformly across the known distribution. Further surveys may tease apart more questions around its host use and may help us piece together the restricted distribution P. hamiltoniorum has within its range in comparison to its host species. Additional work on identification of suitable germination conditions and dormancy breakers as well as work on the molecular interactions between Pilostyles and their hosts may further piece together their preference to Daviesia over other Fabaceae.
Declaration of funding
Funding for this research was provided by the Ecological Society of Australia’s Holsworth wildlife endowment grant (PG: 51009900) to Ryan Craig.
Acknowledgements
Ryan Craig acknowledges and thanks volunteers, Bethany Pittway, Tingting Wu, Ashlee Adams, Khoa Mai, Meg Drummond-Wilson and Calum Hamilton, for taking the time to search for populations of Pilostyles hamiltoniorum and record data. I also acknowledge William J. W. Thomas for providing helpful feedback and edits to the manuscript.
References
Arunachalam A, Adhikari D, Sarmah R, Majumder M, Khan ML (2004) Population and conservation of Sapria himalayana Griffith. in Namdapha national park, Arunachal Pradesh, India. Biodiversity & Conservation 13, 2391-2397.
| Crossref | Google Scholar |
Atencio NO, Vidal-Russell R, Chacoff N, Amico GC (2021) Host range dynamics at different scales: host use by a hemiparasite across its geographic distribution. Plant Biology 23(4), 612-620.
| Crossref | Google Scholar | PubMed |
Barras AG, Marti S, Ettlin S, Vignali S, Resano-Mayor J, Braunisch V, Arlettaz R (2020) The importance of seasonal environmental factors in the foraging habitat selection of Alpine Ring Ouzels Turdus torquatus alpestris. Ibis 162(2), 505-519.
| Crossref | Google Scholar |
Bellot S, Renner SS (2014) The systematics of the worldwide endoparasite family Apodanthaceae (Cucurbitales), with a key, a map, and color photos of most species. PhytoKeys 36(36), 41-57.
| Crossref | Google Scholar |
Chao A, Chazdon RL, Colwell RK, Shen T-J (2005) A new statistical approach for assessing similarity of species composition with incidence and abundance data. Ecology Letters 8(2), 148-159.
| Crossref | Google Scholar |
Chao A, Chazdon RL, Colwell RK, Shen T-J (2006) Abundance-based similarity indices and their estimation when there are unseen species in samples. Biometrics 62(2), 361-371.
| Crossref | Google Scholar | PubMed |
Crisp MD, Cayzer L, Chandler GT, Cook LG (2017) A monograph of Daviesia (Mirbelieae, Faboideae, Fabaceae). Phytotaxa 300(1), 001-308.
| Crossref | Google Scholar |
Dell B, Burbidge AH (1981) Notes on the biology of Pilostyles (Rafflesiaceae) in Western Australia. Western Australian Herbarium Research Notes 5, 71-79.
| Google Scholar |
Dell B, Kuo J, Burbidge AH (1982) Anatomy of Pilostyles hamiltonii C.A.Gardner (Rafflesiaceae) in stems of Daviesia. Australian Journal of Botany 30(1), 1-9.
| Crossref | Google Scholar |
Dias TC, Stabach JA, Huang Q, Labruna MB, Leimgruber P, Ferraz KMPMB, Lopes B, Luz HR, Costa FB, Benatti HR, Correa LR, Nievas AM, Monticelli PF, Piovezan U, Szabó MPJ, Aguiar DM, Brites-Neto J, Port-Carvalho M, Rocha VJ (2020) Habitat selection in natural and human-modified landscapes by capybaras (Hydrochoerus hydrochaeris), an important host for Amblyomma sculptum ticks. PLoS ONE 15(8), e0229277.
| Crossref | Google Scholar |
García-Franco JG, Rico-Gray V (1997) Reproductive biology of the holoparasitic endophyte Bdallophyton bambusarum (Rafflesiaceae). Botanical Journal of the Linnean Society 123(3), 237-247.
| Crossref | Google Scholar |
Gili F, Newson SE, Gillings S, Chamberlain DE, Border JA (2020) Bats in urbanising landscapes: habitat selection and recommendations for a sustainable future. Biological Conservation 241, 108343.
| Crossref | Google Scholar |
Giorgi MS, Arlettaz R, Guillaume F, Nusslé S, Ossola C, Vogel P, Christe P (2004) Causal mechanisms underlying host specificity in bat ectoparasites. Oecologia 138, 648-654.
| Crossref | Google Scholar | PubMed |
Gonzalez F, Pabon-Mora N (2014) Pilostyles boyacensis, a new species of Apodanthaceae (Cucurbitales) from Colombia. Phytotaxa 178(2), 138-145.
| Crossref | Google Scholar |
González F, Pabón-Mora N (2017) Floral development and morphoanatomy in the holoparasitic Pilostyles Boyacensis (Apodanthaceae, Cucurbitales) reveal chimeric half-staminate and half-carpellate flowers. International Journal of Plant Sciences 178(7), 522-536.
| Crossref | Google Scholar |
Govaerts R, Nickrent DL (2010) 658. Cytinus ruber: Cytinaceae. Curtis’s Botanical Magazine 26(4), 314-321.
| Crossref | Google Scholar |
Hódar JA, Lázaro-González A, Zamora R (2018) Beneath the mistletoe: parasitized trees host a more diverse herbaceous vegetation and are more visited by rabbits. Annals of Forest Science 75(3), 77.
| Crossref | Google Scholar |
Holmes MA (2022) Host quality, mediated by land-use history and landscape position, shapes distributions of parasitic plants in postagricultural forests. International Journal of Plant Sciences 183(5), 348-356.
| Crossref | Google Scholar |
Jones GM, Kramer HA, Whitmore SA, Berigan WJ, Tempel DJ, Wood CM, Hobart BK, Erker T, Atuo FA, Pietrunti NF, Kelsey R, Gutiérrez RJ, Peery MZ (2020) Habitat selection by spotted owls after a megafire reflects their adaptation to historical frequent-fire regimes. Landscape Ecology 35(5), 1199-1213.
| Crossref | Google Scholar |
Kelly CK, Venable DL, Zimmerer K (1988) Host specialization in Cuscuta costaricensis: an assessment of host use relative to host availability. Oikos 53(3), 315-320.
| Crossref | Google Scholar |
Krasylenko Y, Sosnovsky Y, Atamas N, Popov G, Leonenko V, Janošíková K, Sytschak N, Rydlo K, Sytnyk D (2020) The European mistletoe (Viscum album l.): distribution, host range, biotic interactions, and management worldwide with special emphasis on Ukraine. Botany 98(9), 499-516.
| Crossref | Google Scholar |
Kuijt J, Bray D, Olson AR (1985) Anatomy and ultrastructure of the endophytic system of Pilostyles thurberi (Rafflesiaceae). Canadian Journal of Botany 63(7), 1231-1240.
| Crossref | Google Scholar |
Lemaitre AB, Troncoso AJ, Niemeyer HM (2012) Host preference of a temperate mistletoe: disproportional infection on three co-occurring host species influenced by differential success. Austral Ecology 37(3), 339-345.
| Crossref | Google Scholar |
Lucero F, Botto-Mahan C, Medel R, Fontúrbel FE (2014) New insights on the mistletoe Tristerix aphyllus (Loranthaceae): interaction with diurnal and nocturnal frugivorous species. Gayana. Botánica 71(2), 270-272.
| Crossref | Google Scholar |
Macqueen DJ, Hernández HM (1997) A revision of Calliandra series Racemosae (Leguminosae: Mimosoideae). Kew Bulletin 52(1), 1-50.
| Crossref | Google Scholar |
Manly BFJ, Mcdonald LL, Thomas DL, Mcdonald TL, Erickson WP (2002) ‘Resource selection by animals: statistical design and analysis for field studies (second).’ (Springer: Dordrecht, Netherlands). 10.1007/0-306-48151-0
Mauseth JD, Rezaei K (2013) Morphogenesis in the parasitic plant Viscum minimum (Viscaceae) is highly altered, having apical meristems but lacking roots, stems, and leaves. International Journal of Plant Sciences 174(5), 791-801.
| Crossref | Google Scholar |
Milner KV, Leigh A, Gladstone W, Watson DM (2020) Subdividing the spectrum: quantifying host specialization in mistletoes. Botany 98(9), 533-543.
| Crossref | Google Scholar |
Nge FJ, Ranathunge K, Kotula L, Cawthray GR, Lambers H (2019) Strong host specificity of a root hemi-parasite (Santalum acuminatum) limits its local distribution: beggars can be choosers. Plant and Soil 437(1), 159-177.
| Crossref | Google Scholar |
Nge FJ, Cambridge ML, Ellsworth DS, Zhong H, Lambers H (2020) Cluster roots are common in Daviesia and Allies (Mirbelioids; Fabaceae). Journal of the Royal Society of Western Australia 103, 111-118.
| Google Scholar |
Nickrent DL (2020) Parasitic angiosperms: how often and how many? Taxon 69(1), 5-27.
| Crossref | Google Scholar |
Norhazlini MZ, Latiff A, Farah KK, Nasihah M, Siti-Munirah MY, Zulhazman H (2021) A review of species diversity of Rafflesia in Malaysia. Malayan Nature Journal 73(4), 531-552.
| Google Scholar |
Novoa P (2005) Hallazgo de Pilostyles berteroi Guill. (Rafflesiaceae) en el Jardín Botánico Nacional de Viña del Mar, Región de Valparaíso (V). Chloris Chilensis: Revista Chilena de Flora y Vegetacion 8(1), 1.
| Google Scholar |
Ordiz A, Uzal A, Milleret C, Sanz-Pérez A, Zimmermann B, Wikenros C, Wabakken P, Kindberg J, Swenson JE, Sand H (2020) Wolf habitat selection when sympatric or allopatric with brown bears in Scandinavia. Scientific Reports 10(1), 9941.
| Crossref | Google Scholar |
Ortega-González PF, Rios-Carrasco S, González-Martínez CA, Bonilla-Cruz N, Vázquez-Santana S (2020) Pilostyles maya, a novel species from Mexico and the first cleistogamous species in Apodanthaceae (Cucurbitales). Phytotaxa 440(4), 255-267.
| Crossref | Google Scholar |
Pabón-Mora N (2021) My favourite flowering image: two in one – a holoparasite growing inside a shrubby legume. Journal of Experimental Botany 72(8), 2818-2821.
| Crossref | Google Scholar | PubMed |
Pellissari LCO, Teixeira-Costa L, Ceccantini G, Tamaio N, Cardoso LJT, Braga JMA, Barros CF (2022) Parasitic plant, from inside out: endophytic development in Lathrophytum peckoltii (Balanophoraceae) in host liana roots from tribe Paullineae (Sapindaceae). Annals of Botany 129(3), 331-342.
| Crossref | Google Scholar | PubMed |
Pelser PB, Nickrent DL, Barcelona JF (2016) Untangling a vine and its parasite: host specificity of Philippine Rafflesia (Rafflesiaceae). Taxon 65(4), 739-758.
| Crossref | Google Scholar |
Pelser PB, Nickrent DL, van Ee BW, Barcelona JF (2019) A phylogenetic and biogeographic study of Rafflesia (Rafflesiaceae) in the Philippines: limited dispersal and high island endemism. Molecular Phylogenetics and Evolution 139, 106555.
| Crossref | Google Scholar |
Poulin R, Randhawa HS (2015) Evolution of parasitism along convergent lines: from ecology to genomics. Parasitology 142(S1), S6-S15.
| Crossref | Google Scholar |
Rios-Carrasco S, Vázquez-Santana S (2021) Comparative morphology and ontogenetic patterns of Bdallophytum species (Cytinaceae, Malvales): insight into the biology of an endoparasitic genus. Botany 99(4), 221-238.
| Crossref | Google Scholar |
Sprent JI (1999) Nitrogen fixation and growth of non-crop legume species in diverse environments. Perspectives in Plant Ecology, Evolution and Systematics 2(2), 149-162.
| Crossref | Google Scholar |
Sultan A, Tate JA, de Lange PJ, Glenny D, Ladley JJ, Heenan P, Robertson AW (2018) Host range, host specificity, regional host preferences and genetic variability of Korthalsella Tiegh. (Viscaceae) mistletoes in New Zealand. New Zealand Journal of Botany 56(2), 127-162.
| Crossref | Google Scholar |
Teixeira-Costa L, Suetsugu K (2023) Neglected plant parasites: mitrastemonaceae. Plants, People, Planet 5(1), 5-13.
| Crossref | Google Scholar |
Thiele KR, Wylie SJ, Maccarone L, Hollick P, McComb JA (2008) Pilostyles coccoidea (Apodanthaceae), a new species from Western Australia described from morphological and molecular evidence. Nuytsia – The Journal of the Western Australian Herbarium 18, 273-284.
| Crossref | Google Scholar |
Thorogood CJ, Hiscock SJ (2007) Host Specificity in the Parasitic Plant Cytinus hypocistis. Research Letters in Ecology 2007, 84234.
| Crossref | Google Scholar |
Thorogood C, Witono JR, Mursidawati S, Fleischmann A (2022) Parasitic plant cultivation: examples, lessons learned and future directions. Sibbaldia: the International Journal of Botanic Garden Horticulture [21] 109-136.
| Crossref | Google Scholar |
Watson DM (2009a) Determinants of parasitic plant distribution: the role of host quality. Botany 87(1), 16-21.
| Crossref | Google Scholar |
Watson DM (2009b) Parasitic plants as facilitators: more Dryad than Dracula? Journal of Ecology 97(6), 1151-1159.
| Crossref | Google Scholar |
Watson DM, Milner KV, Leigh A (2017) Novel application of species richness estimators to predict the host range of parasites. International Journal for Parasitology 47(1), 31-39.
| Crossref | Google Scholar | PubMed |
Watson DM, McLellan RC, Fontúrbel FE (2022) Functional roles of parasitic plants in a warming world. Annual Review of Ecology, Evolution, and Systematics 53, 25-45.
| Crossref | Google Scholar |
Werner GDA, Cornwell WK, Sprent JI, Kattge J, Kiers ET (2014) A single evolutionary innovation drives the deep evolution of symbiotic N2-fixation in angiosperms. Nature Communications 5, 4087.
| Crossref | Google Scholar |
Westwood JH, Yoder JI, Timko MP, dePamphilis CW (2010) The evolution of parasitism in plants. Trends in Plant Science 15(4), 227-235.
| Crossref | Google Scholar | PubMed |
Wicaksono A, Cristy GP, Raihandhany R, Mursidawati S, Teixeira da Silva JA, Susatya A (2022) Rhizanthes, the forgotten relative of Rafflesia in the Rafflesiaceae. The Botanical Review 88(1), 130-143.
| Crossref | Google Scholar |