Seed biology can inform conservation actions: a case study on Geijera parviflora
Ganesha S. Liyanage A * , Amy-Marie Gilpin B C , Catherine A. Offord A and Amelia J. Martyn Yenson A B
A * , Amy-Marie Gilpin B C , Catherine A. Offord A and Amelia J. Martyn Yenson A B
A
B
C
Abstract
Knowledge of seed biology is imperative for effective curation and utilisation of seeds.
We studied the seed biology and reproduction of Geijera parviflora, a species suitable for ecological restoration and ornamental horticulture that has largely been overlooked because of issues with unreliable germination and viability.
Germination in response to dormancy-breaking treatments and soil seed burial, and variation in germination at an inter-population level were assessed for G. parviflora. Seed storability in a conventional seedbank was tested. Floral phenology was assessed.
Germination of untreated and seed coat removed seeds were 0 ± 0.0% and 67 ± 5.5% respectively. Seed germination varied between 21.9 ± 1.8 and 66.6 ± 5.5% at an inter-population level. Seeds buried in soil for 6 months showed 11.7 ± 0.8% germination without any treatment. All seeds buried for 12 months were non-viable. Drying seeds from 11.4 ± 0.19% to 5.79 ± 0.17% moisture content resulted in 40% less germination. Visual observation of floral attributes confirmed the presence of orange-coloured pollen grains for 3.5 ± 0.48 days from floral opening. The stigmatic surface became shiny 3.5 ± 0.21 days after floral opening.
Seeds of G. parviflora demonstrate physiological dormancy; treatments that remove seed coat resistance against embryo growth enable germination. Local environmental conditions may explain the inter-population variation in germination. Seeds are short-lived in soil and ex situ seedbank conditions. In soil, seeds release dormancy at the end of first winter, so seeds are available to germinate the following spring/summer with high rainfall. Floral phenology assessment indicated protandrous sequential hermaphroditism, which may reduce the probability of self-pollination in G. parviflora.
This knowledge supports use of G. parviflora in ecological restoration and horticulture.
Keywords: ecology, plant conservation, reproduction, restoration, seed banking, seed dormancy, seed germination, seed storage.
Introduction
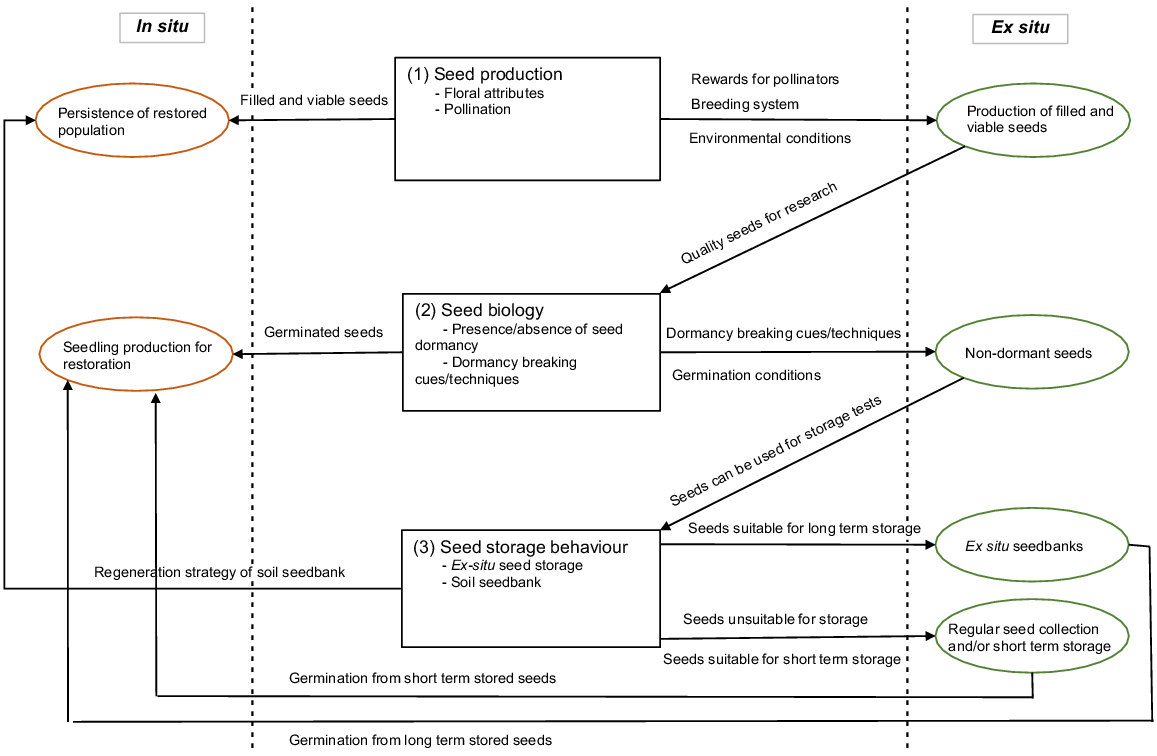
Seed biology and reproduction studies are important for implementing effective conservation actions in both in situ and ex situ (Fig. 1). Reproduction studies (i.e. pollination and floral biology) provide evidence on the availability of quality (i.e. filled, viable and germinable) seeds and thereby persistence of the population. When quality seeds are available, ex situ conservation of seeds, in seedbanks, provides one of the most efficient and cost-effective ways of accessing genetic diversity for re-establishment of wild populations (Laliberté 1997; Offord et al. 2004; Cochrane et al. 2007; Li and Pritchard 2009). Use of stored seeds in re-establishment practices depends on successful germination. Periodic germination testing of seeds stored in seedbanks is imperative to assess the quality of stored seeds and produce seedlings for re-establishment. Understanding seed biology (i.e. seed dormancy, dormancy breaking cues, germination conditions) and reproduction biology, therefore, is essential for effective curation and utilisation of storedseed collections (Fig. 1).
Diagram showing avenues of investigation and their relationship to conservation outcomes. Boxes in the middle represent the focused experimental areas of Geijera parviflora in this study and circles represent results used in in situ (brown) and ex situ (green) conservation practices.
Geijera parviflora, known as wilga, sheepbush and dogwood, is a species that has been identified as suitable for ecological restoration, as well as ornamental horticulture but, until now, has largely been overlooked primarily because of a lack of understanding surrounding the seed biology. Geijera parviflora has characteristics beneficial for horticulture and restoration of degraded drylands (e.g. mining sites) because it occurs over a wide range of soil types, has a low moisture requirement for adult plant growth (i.e. it is drought tolerant with adaptations to withstand low water potentials) and the capacity to withstand heatwave conditions and frost (Warnock et al. 2007; Curran et al. 2008; Tabassum et al. 2021). In Australia, G. parviflora is widespread in dryland areas of western New South Wales (NSW), north-eastern South Australia and Queensland, but rare in Victoria (Duretto 1999). In NSW, G. parviflora is a common species and constitutes a well-developed shrub layer of the Hunter Valley Weeping Myall Woodland in the Sydney Basin Bioregion that is listed as a Critically Endangered Ecological Community under the NSW Biodiversity Conservation Act 2016 and as Hunter Valley Weeping Myall (Acacia pendula) Woodland under the Commonwealth Environment Protection and Biodiversity Conservation Act 1999. This species occurs in many small and isolated remnants of dry rainforests, which may disappear as a result of an unsuitable climate in the future, with increasing climate change, fire damage, mining, grazing and weed infestation (Tozer et al. 2010; Commonwealth of Australia 2015). Geijera parviflora flowering mainly occurs from June to November, producing large quantities of coccus type fruit with a spherical black seed. Seed dispersal has been identified as unaided (Navie et al. 1997; Atlas of Living Australia Website (ALA) 2023). It belongs to the plant family Rutaceae, which is well-known to have seed germination difficulties associated with dormancy and low production of filled seeds (Auld 2001; Floyd 2008; Martyn et al. 2009). Its suitability for ex situ seedbank storage is predicted on the basis of species from the same family (Society for Ecological Restoration, International Network for Seed Based Restoration, Royal Botanic Gardens Kew 2023), but not yet confirmed using standard seed storage behaviour tests because of a lack of reliable dormancy-breaking techniques.
Irregular or delayed germination owing to dormancy can impede planning for ex situ curation, seed science research and production of seedlings for restoration purposes. Within natural environments, seed dormancy helps species maintain long-term soil seedbanks, which avoids the risk of seed germination in environments unfavourable for subsequent seedling establishment (Venable and Brown 1988; Baskin and Baskin 2014). Dormancy studies on a number of other species from Rutaceae family have confirmed the presence of physiological dormancy in genus Acronychia, Boronia, Diplolaena, Rhadinothamnus (Mackenzie et al. 2016; Liyanage et al. 2020; Just et al. 2023); however, dormancy is still not classified for G. parviflora. Seed dormancy is broadly classified into the following five classes on the basis of time to germination and various seed characteristics: (1) physical (PY), in which an impermeable seed coat prevents the imbibition of water and gases needed for germination; (2) physiological (PD), in which one of a variety of mechanisms inhibits embryo growth and emergence of the radicle; (3) morphological (MD), in which the embryo is underdeveloped and must grow before the radicle can emerge; (4) morphophysiological (MPD), in which the seed has both physiological dormancy and an underdeveloped embryo; and (5) combinational dormancy (PY + PD), in which the seed has both physical and physiological dormancy (Baskin and Baskin 2004). Understanding the type of dormancy a seed possesses is helpful for determining the treatments needed to relieve it.
In recent years, much research has been conducted on seed traits, such as dormancy, germination, viability, and after-ripening to achieve restoration goals (Larson et al. 2015; Baskin and Baskin 2020; Commander et al. 2020). Commander et al. (2020) studied in situ seed dormancy, germination and emergence of eight Australian native species to understand the limitations to plant recruitment in effective restoration of Australian semi-arid zone ecosystems. They identified that knowledge of methods to break seed dormancy was one of the key limitations in restoration initiatives. Under laboratory or nursery conditions depending on the dormancy type, different dormancy-breaking techniques can be used to germinate seeds and produce seedlings (Hirst et al. 2021). For example, PY can be relieved by creating an opening in the seed coat with a scalpel or by rubbing the seed on sandpaper, which enable the imbibition of water and/or gas to initiate germination. Techniques to relieve PD depend on the mechanism inhibiting embryo growth but can include a period of dry after-ripening, treatment with gibberellic acid, precision nicking, mimicking seasonal conditions (e.g. cold-moist stratification) that occur in soil seedbank of their habitat (Kucera et al. 2005; Mackenzie et al. 2016; Liyanage et al. 2020). Availability of reliable methods to break seed dormancy is essential to perform seed storage behaviour experiments. Use of this ex situ conservation method for a species depends on the seed ability to withstand storage conditions. Seeds are dried to low moisture contents so as to withstand the sub-zero temperatures (usually −20°C) used in conventional ex situ seedbanks (Martyn Yenson et al. 2021). Seed storage behaviour tests provide evidence on species’ suitability to store in these conventional ex situ seedbanks, while maintaining quality seeds for longer periods.
Predicting the time and extent of seedling emergence, for species that produce dormant seeds, is more reliable when the changes in dormancy over time in the field are known (Batlla and Benech-Arnold 2010). Soil seedbank experiments, along with laboratory-based dormancy studies, help understand these changes, thereby identifying environmental cues that break dormancy in natural environment (Auld 1986; Baskin and Baskin 2014; Ooi 2015; Liyanage and Ooi 2017). Daily and seasonal temperature fluctuations within soil can effect physiological changes to seeds and cause physical deterioration of seed structures, thereby changing the dormancy-breaking response in stored seeds (Liyanage and Ooi 2017). Studies on soil seedbank behaviour therefore provide key knowledge on factors that contribute to determining recruitment and robustly predicting population persistence.
Large-scale restoration projects require large quantities of viable seeds collected from wild populations (Merritt and Dixon 2011) and this can be a problem in species that usually produce seeds with a low viability or poor seed fill. Factors such as inbreeding in small populations, pollen limitation and seed predation can cause low seed fill or decrease viability (Hardner and Potts 1995; Del Castillo and Trujillo 2008). External factors such as prolonged drought during pollination or seed development and predation can also cause low seed fill (Martyn et al. 2009). Interactions between plant and pollinators are mediated by floral attributes (i.e. colour, presence of nectar, amount of nectar) (Kearns and Inouye 1993). Floral attributes provide insight into pollination success and help identify the features that potentially can improve the chances of pollination to make quality seeds (Schemske and Bradshaw 1999; E-Vojtkó et al 2022). Using species in restoration without this pivotal knowledge neglects the role of pollination in quality seed production.
In this study, we investigated dormancy-breaking techniques, and changes to the germination of stored seeds of G. parviflora in both the soil seedbank and conventional ex situ seedbank conditions. This knowledge can aid development of reliable methods to germinate seeds and assess the viability of stored seeds. In addition, we assessed the floral biology, floral ontogeny and stigma receptivity of G. parviflora. We also report our findings from a hand-pollination experiment that sought to identify the breeding system and potential causes of low seed fill within G. parviflora. Understanding seed biology, including both dormancy and reproduction, provides crucial knowledge to implement conservation strategies for species and their associated vegetation communities (Fig. 1). Our findings support understanding of G. parviflora for both its ecology in the wild and its use in restoration and cultivation.
Materials and methods
Seed collection
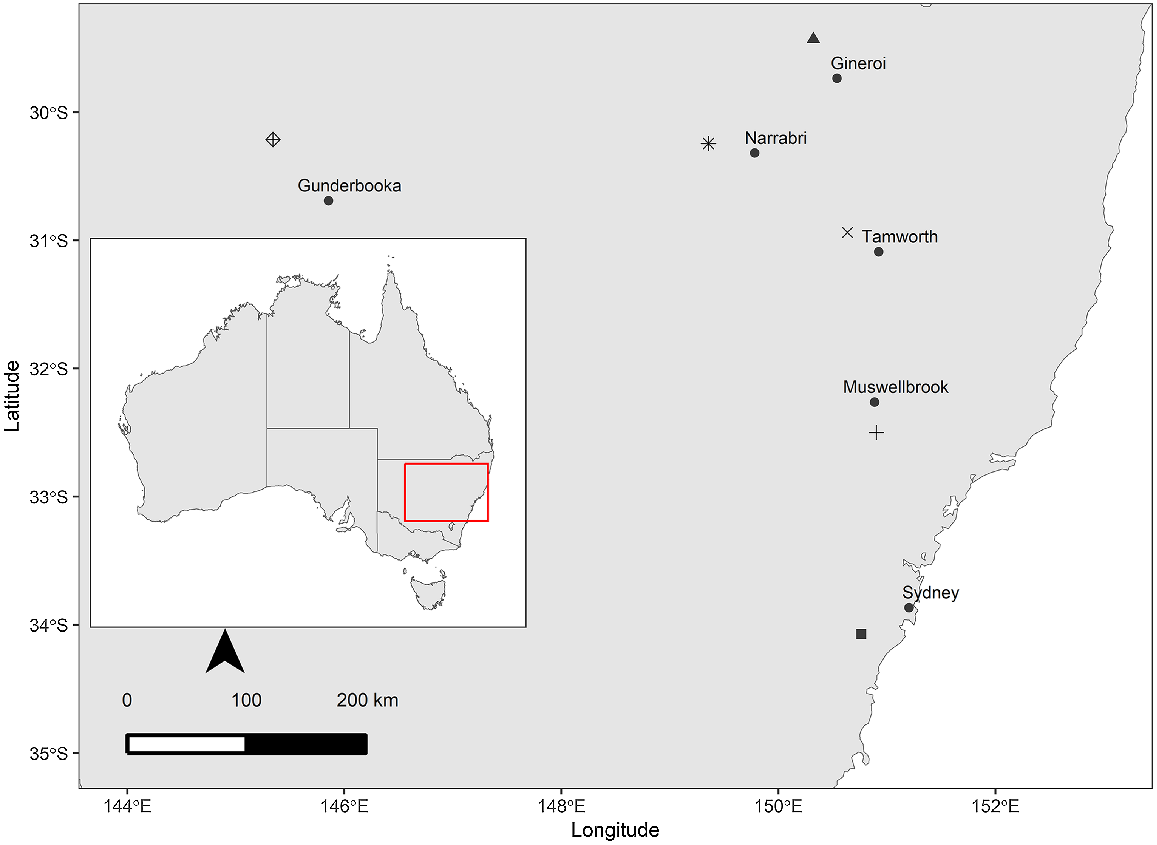
Six seed collections of G. parviflora were made from six different populations distributed across New South Wales, Australia, in 2019, 2020 and 2021 (Fig. 2). Five collections were made from naturally occurring plant populations (Brigalow Nature Reserve, Jerrys Plains, Gundabooka State Conservation Area, Somerton, Gineroi) and one collection was made from garden-grown plants (sourced from a wild population in Dubbo State Forest, NSW, and accessioned as A2001-1086/5-10) at the Australian Botanic Gardens, Mount Annan, Australia (ABG) (Table 1). For each population, mature fruits were collected from 5–10 individual plants and bulked within population before commencing the experiments. Seeds were extracted from the fruits at the Australian PlantBank. Healthy looking seeds, those that had intact and fully formed seed coats, were selected and stored in paper bags, under room temperature conditions, until the commencement of experiments. Tests on seed fill (i.e. seeds with well-developed firm endosperm and embryo), viability (i.e. seeds with the ability to produce a radicle, which is considered germination), dormancy and germination for each collection were started within 2–3 weeks of seed collection.
Map showing six populations of Geijera parviflora distributed across New South Wales, Australia, used in this study to collect seeds from and closest town to each population. Different shapes represent locations of (1) Brigalow Nature Reserve (*), (2) Australian Botanic Gardens (⊠), (3) Jerrys Plains (+), (4) Gundabooka State Conservation Area (⊠), (5) Somerton (×), (6) Gineroi (▲) and (7) nearest town to the seed collection site (●).
| Population (accession) | Time of collection | Total number of seeds used for experiments | Seed fill (%) A | Seed viability (%) A | |
|---|---|---|---|---|---|
| Brigalow Nature Reserve (P2020-0399) | September 2020 | 106 | 97.8 ± 2.2a | 93.3 ± 0.0a | |
| Australian Botanic Gardens (ABG) (P2020-0846) | February 2020 | 138 | 100 ± 0.0a | 96.8 ± 1.8a | |
| Jerrys Plains (P2020-0847) | March 2020 | 720 | 81.7 ± 4.4a | 80.0 ± 5.0a | |
| Gundabooka State Conservation Area (P2020-0044) | January 2020 | 134 | 88.3 ± 7.3a | 88.3 ± 7.3a | |
| Somerton (P2020-0845) | April 2020 | 790 | 79.2 ± 2.1a | 77.1 ± 2.1a | |
| Gineroi (P2019-0206) | January 2019 | 112 | 45.8 ± 2.1b | 39.6 ± 2.1b |
A large quantity of seeds was collected from the Somerton population and this collection was used to study the effect of different dormancy-breaking treatments on germination success and ex situ seed storage behaviour tests. A soil seedbank experiment was performed to understand soil seedbank behaviour of G. parviflora at Jerrys Plains Cemetery, NSW, Australia, by using a seed collection made from a plant population found at the same location. A hand pollination experiment was also performed in field on the plant population found at the Jerrys Plains Cemetery.
Identification of dormancy-breaking techniques for G. parviflora
To identify an effective dormancy-breaking treatment, the following seven laboratory-based treatments were applied: (1) intact seeds incubated on agar, incorporating 250 mg/L gibberellic acid, (2) leaching of seeds for 24 h under running water, (3) leaching of seeds for 48 h under running water, (4) seed scarification with a scalpel, (5) seed coat removed using a vice, (6) seed coat removed using a vice and incubation on agar incorporating 250 mg/L gibberellic acid, and (7) seed coat removed using a vice and incubation with crushed seed coats. Treatments 1, 3, 4, 5 and 6 were incubated in Petri dishes containing 0.8% water agar. These treatments are the most commonly used dormancy-breaking techniques under both nursery and laboratory conditions (Hirst et al. 2021). When removing the seedcoat by using a vice, the following steps were applied to avoid embryo and endosperm damage. The seed was held by a pair of forceps such that the clear suture along the longitudinal plane around the seed coat was held against the panel of the vice, and the lever of the vice was slowly turned until the seed coat split open and the endosperm containing the embryo could be extracted. For the leaching treatment, seeds were stored in a clamp-style tea infuser in a beaker placed under a tap, with steady water stream overflowing continuously for 24 or 48 h. Three replicates of 20 seeds on three Petri dishes (60 seeds per treatment) containing 0.8% water agar medium were used for each treatment. All Petri dishes were sealed with cling wrap to reduce water loss and placed in an incubator set at 25°C/10°C diurnal alternating temperature and 12/12 h light/dark conditions. Germination was recorded every second day for 6 weeks. The germination was scored at 1–2 mm radicle protrusion. After 6 weeks of incubation, ungerminated seeds were pressed gently and firm seeds were dissected under the microscope to assess viability. The number of germinated seeds, ungerminated mushy (i.e. considered viable at the beginning of the experiment and may have deteriorated over time) and ungerminated firm (i.e. seeds with green/white embryo and endosperm) seeds were scored as initially viable seeds. Intact seeds were surface sterilised using 2% bleach (White King® containing sodium hypochlorite 42 g/L) for 10 min in a shaker, followed by three rinses in sterilised water before setting up all germination experiments described below.
Assessment of inter-population seed characteristics of G. parviflora
Seeds from each of the six populations were collected at dispersal and used for experiments within 2 weeks of collection. Twenty-five fresh seeds were used in the seed fill test, with each seed cut in half using secateurs and observed under the microscope to score seed fill.
Three replicates of 16–20 intact seeds from each seed collection (48–60 seeds per population) were placed on three Petri dishes containing 0.8% water agar and incubated as explained in the section ‘Identification of dormancy-breaking techniques for G. parviflora’. The number of germinated seeds during incubation were recorded for 6 weeks and viability was assessed as explained above.
Treatment 1 was identified as the most effective dormancy-breaking treatment from the ‘Identification of dormancy-breaking techniques for G. parviflora’ section, enabling the inter-population comparison of germination. Two sets of three replicates of 16–20 seeds from each population (48–60 seeds per population) were used to compare the germination among populations. Seed coats of one set were removed using a vice and a germination test was performed as explained in Treatment 1 of the section ‘Identification of dormancy-breaking techniques for G. parviflora’. Intact seeds in remaining set of three replicates (control replicates) were incubated along with the treated seed replicates in same incubation conditions as explained above.
Soil seedbank behaviour of G. parviflora
To explore the cues to seed dormancy break under natural conditions, as well as seed persistence within the soil seedbank, a seed burial experiment was conducted (Hirst et al. 2021) at Jerrys Plains, NSW (32°29′59.4″S, 150°54′12.6″E). Five hundred and forty seeds from the Jerrys Plains seed collection were used in the soil seed burial experiment and seeds were divided into three sets of 180 seeds. Sixty seeds were stored in each of nine nylon mesh bags (20 × 10 cm) with 2 mm mesh size. Each bag was filled with sand and seeds were mixed into the soil before placing three replicate bags in each of three locations within the existing G. parviflora population at Jerrys Plains Cemetery. Three bags were buried along with a temperature data logger (Thermochron® ibutton) within the top 2 cm soil layer at each location to record temperatures during storage. One mesh bag from each location was retrieved at 6-, 12- and 18-month time intervals and subsequent germination, dormancy and viability were assessed in the laboratory by using bulked seeds from three locations to overcome any microclimatic effects. Dormancy was assessed using intact seeds and seeds treated with Treatment 1 as the best dormancy-breaking treatment from section ‘Identification of dormancy-breaking techniques for G. parviflora’.
Ex situ seed storage and germination of G. parviflora
Laboratory seed storage tests were performed to understand the seed storage behaviour and suitability of G. parviflora seeds for storage in conventional ex situ seedbank conditions. An oven-drying method was used to determine seed moisture content (SMC) of both fresh seeds and seeds equilibrated to 15% relative humidity (eRH) and 15°C.
Two hundred fresh seeds, from the Somerton population (P2020-0845), were dried by placing them in a drying room maintained at 15% relative humidity (RH) and 15°C (100 extra seeds were added on top of 200 seeds to the sample to ensure enough filled seeds were available for the germination test after storage treatments). The relative humidity of those seeds was measured weekly using a Rotronic® eRH meter (Model HP23-AW-A; Pryde Measurement, Ingleburn NSW) with a Rotronic® water activity probe (Model HC2-AW; Pryde Measurement, Ingleburn NSW) until the seeds reached ~15% RH. To determine the seed moisture content of fresh seeds, 10 small aluminium trays were weighed individually with and without 10 seeds and then dried in an oven at 103°C for 17.5 ± 0.5 h. Seeds were reweighed individually after cooling down in a sealed box containing silica gel to avoid moisture absorption from the atmosphere during cooling. These seed masses were used to calculate the SMC by using the formula:
where, Mf = mass of fresh seed, and Md = mass of oven-dried seeds (ISTA 2007).
Seed moisture content was also determined for dried seeds using 10 seeds, as for fresh seeds.
The germination of dried seeds was assessed by using 60 dried seeds. Treatment 1 from section ‘Identification of dormancy breaking techniques for G. parviflora’ was used as the most effective dormancy-breaking treatment and germination test was performed as described. The remaining seeds were divided into two sets of 60 seeds and packed in separate vacuum-sealed aluminium foil bags and stored in a fridge (4°C) or freezer (−20°C at the Australian PlantBank) for a month. Stored seeds were retrieved after a month and germination was assessed as for dried seeds. The sealed foil bags retrieved from each storage condition were thawed at room temperature for 24 h before starting the germination test, so as to avoid seeds experiencing temperature shock. Treatment 1 from section ‘Identification of dormancy-breaking techniques for G. parviflora’ was used to test germination. The short-term storage potential of G. parviflora seeds was also assessed by using a separate set of 120 fresh seeds, vacuum sealed in two foil bags and stored in the fridge and freezer (as above) without drying to 15% RH. These seeds were also retrieved from storage after 1 month and germination was tested as above.
Quantifying floral attributes, nectar availability and the breeding system of G. parviflora
To observe the floral parts of G. parviflora, 10 freshly opened florets from five inflorescences were collected, and floral parts were observed by dissecting under a stereomicroscope (M60; Leica Microsystems, Macquarie Park).
To observe the floral phenology of G. parviflora, 15 unopened floret buds from six inflorescences on each of six plants at the Australian Botanic Garden, Mount Annan, were followed over a period of 1 month. The presence of an androecium and/or a gynoecium was recorded, along with categorisation of reproductive structures as immature, mature or senesced by visual observation with an ARC Magnifier Triplet (×20 magnification, sight 21 mm) every weekday. Observation of orange-coloured stamens with pollen grains was considered as the indication of androecium maturity and green-coloured stigmas with shiny surfaces indicated gynoecium maturity (stigmatic receptivity).
Nectar quantity was measured by bagging six inflorescences, with a total of 24 buds, across six individual plants, by using organza bags (15 × 15 cm) to prevent pollinator visitation to flowers once they opened. Once the flowers were open, 12 of the 24 flowers were measured in the morning (about 10 am) and the remaining 12 flowers were measured in the afternoon (about 2 pm). Each day, flowers that displayed a shiny stigma (sexually receptive) were used to measure the amount of nectar harvested into 1 μL capillary tubes. Nectar was measured while flowers were attached to the plant. The flowers that had nectar harvested were removed from the plants after measurement, to avoid repeated measurements.
Bagging experiment to determine the breeding system of G. parviflora
A field hand pollination experiment was conducted to determine the breeding system of Geijera parviflora in October 2020. Nine plants that had buds and at least one open flower, were selected from two clusters of plants (five trees at Cluster 1 and four trees at Cluster 2) within the Jerrys Plains Cemetery, NSW, Australia. The two clusters of plants were separated by approximately 1 km and included the most widely separated flowering study trees within Jerrys Plains Cemetery area. For this reason, these two clusters were selected as pollen donor and recipient clusters.
On each tree, a group of unopened buds on a single inflorescence or branchlet was selected and randomly assigned to one of the following five pollination treatments: (1) open pollination, (2) manipulative self-pollination, (3) autonomous self-pollination, (4) manipulative outcrossing and (5) bag control treatment (Supplementary material S1). Any open flowers on each of the selected branchlets were removed using a pair of forceps prior to the start of the experiment and branchlets assigned to each of the treatments were denoted by a small amount of five different-colour nail polish and thread before the treatments.
In Cluster 1, five study trees were selected, with five replicates of five pollination treatments assigned to each tree. In Cluster 2, four trees had five replicates of Treatments 1 and 4 only, owing to low numbers of flower buds ready to open within the experimental time-period. For 3 days, all five experimental trees at Cluster 1 and for 2 days all four experimental trees at Cluster 2 were subjected to all of the assigned pollination treatments.
The bags used during the hand-pollination and, subsequently, to capture produced seeds after hand-pollination treatments, were fashioned from tea bags by the method of Reiter et al. (2021). The bags were emptied of tea to create an open-ended bag, which was fitted over the flower and fastened with twine to prevent insect visitation to the flowers, or over the developing fruit to capture seeds. Any unopened buds at the conclusion of the experiment were removed from all treatments. The remaining experimental recipient flowers were bagged until returning to the study site to collect in January 2021.
Statistical analysis
Two separate one-factor generalised linear models (GLM) were performed to compare the effect of dormancy-breaking treatments and populations on germination. Treatment and population were considered as the explanatory variables in each model and a binomial error structure with logit-link function was used. When the germination proportions were significantly different among treatments or populations, Tukey’s pair-wise comparison test was performed using glht command of the multcomp package (Hothorn et al. 2008). One-factor GLMs were performed for soil-stored and seedbank-stored seeds separately, so as to compare initial dormancy, viability, and germination between fresh and stored seeds. Storage time and storage condition were used as the explanatory variable for soil- and seedbankstored seeds respectively. Models did not converge for experiments with no germination within at least one of the treatments. To enable model convergence, one ‘germinant’ was added to one replicate from the treatments with zero germination (Collette and Ooi 2021). We assessed the model fit for all models by checking overdispersion by using the residual degrees of freedom against residual deviance. All data analyses were performed using R 3.5.1 statistical platform (R Development Core Team 2018).
Results
Identification of dormancy-breaking techniques for G. parviflora
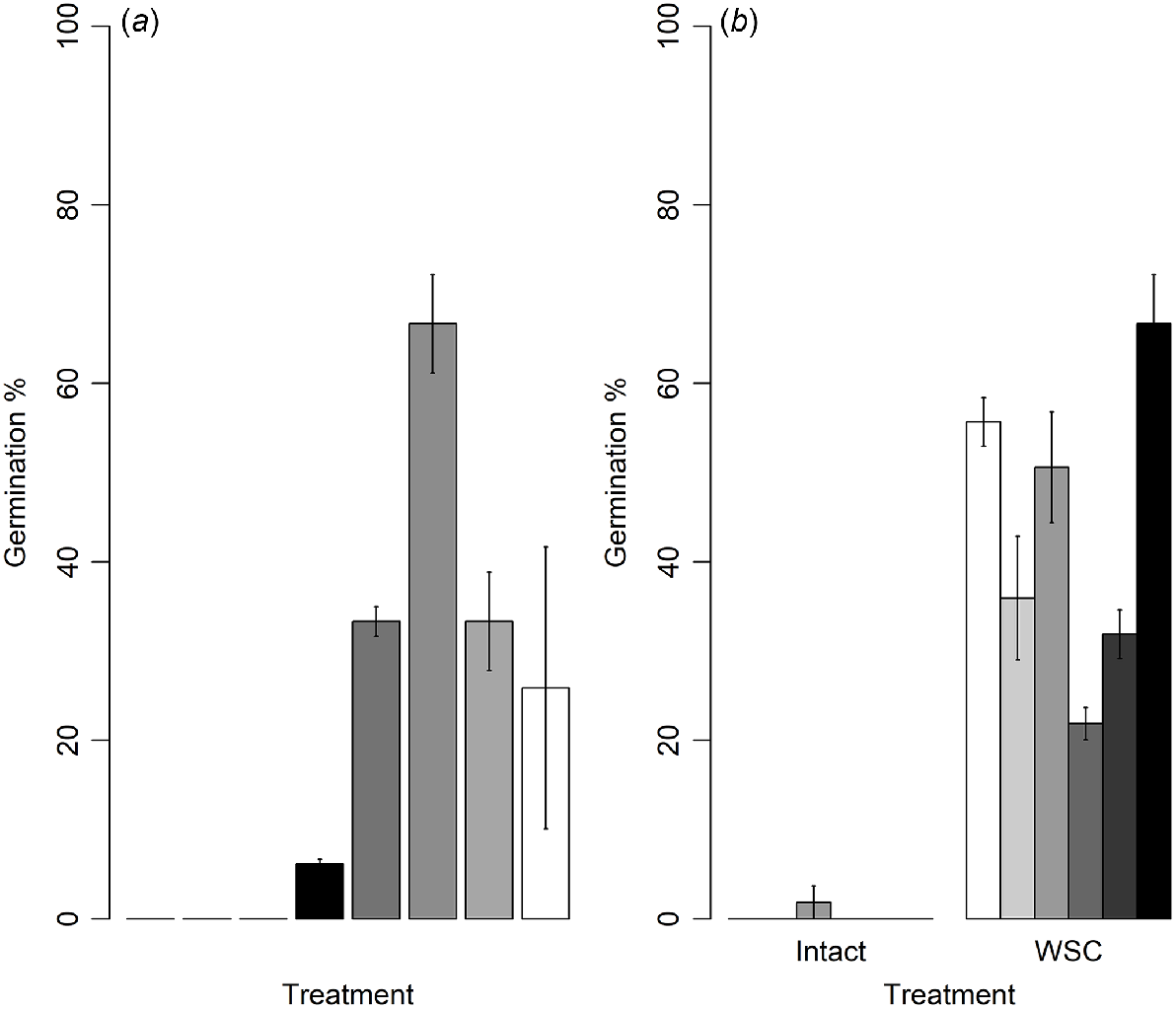
The final seed germination percentages showed significantly different responses to the dormancy-breaking treatments (GLM, d.f. = 7, χ2 = 7.6, P < 0.01, Fig. 3a). Intact seeds did not germinate, and significantly higher germination (67 ± 5.5%) was observed when seed coats were removed by using a vice (Treatment 5) (Tukey’s test, d.f. = 7, Z = 2.96, P = 0.03). The presence of gibberellic acid in the agar (Treatment 1) did not promote germination of intact seeds (Tukey’s test, d.f. = 7, Z = 0.05, P = 1.00). However, when the seed coat was removed, the presence of gibberellic acid (Treatment 6) reduced germination by nearly 50% compared with the seeds incubated without gibberellic acid (Treatment 5) (Tukey’s test, d.f. = 7, Z = 1.88, P = 0.51). Low levels of seed germination (<35%, Fig. 3a) were observed for the following treatments: seed-coat removal by using a vice and incubation in 250 mg/L gibberellic acid-incorporated agar (Treatment 6), seed-coat removal by using a vice and incubation with crushed seed coats (Treatment 7), seed scarification near the micropylar end by using a scalpel (Treatment 4) and, leaching of seeds for 24 (Treatment 2) and 48 h under running water (Treatment 3).
Mean germination percentages (±s.e.) of Geijera parviflora seeds (a) after different dormancy-breaking treatments and (b) from six different populations. In graph (a), differently shaded bars from left to right represent germination of (1) intact seeds incubated in 0.8% water agar, (2) intact seeds incubated on agar incorporating 250 mg/L gibberellic acid, (3) seeds leached for 24 h under running water and incubated in 0.8% agar, (4) seeds leached for 48 h under running water and incubated in 0.8% agar ( ), (5) seeds scarified with a scalpel (
), (5) seeds scarified with a scalpel ( ), (6) seeds with seed coat removed by using a vice (
), (6) seeds with seed coat removed by using a vice ( ), (7) seeds with seed coat removed by using a vice and incubation on agar incorporating 250 mg/L gibberellic acid (
), (7) seeds with seed coat removed by using a vice and incubation on agar incorporating 250 mg/L gibberellic acid ( ), (8) seeds with seed coat removed by using a vice and incubated with crushed seedcoats (
), (8) seeds with seed coat removed by using a vice and incubated with crushed seedcoats ( ). In graph (b) differently shaded bars in each graph represent seeds collected from Australian Botanic Gardens (
). In graph (b) differently shaded bars in each graph represent seeds collected from Australian Botanic Gardens ( ), Brigalow Nature Reserve (
), Brigalow Nature Reserve ( ), Gineroi (
), Gineroi ( ), Gundabooka (
), Gundabooka ( ), Jerrys Plains (
), Jerrys Plains ( ) or Somerton (
) or Somerton ( ) populations. (1) Intact represent the germination of untreated seeds and (2) WSC represent the germination of seeds with seed coat removed using a vice.
) populations. (1) Intact represent the germination of untreated seeds and (2) WSC represent the germination of seeds with seed coat removed using a vice.
Assessment of inter-population seed characteristics
Comparison of seed characteristics among six G. parviflora plant populations showed a significant difference in seed fill (GLM, d.f. = 5, χ2 = 0.67, P < 0.01), initial viability (GLM, d.f. = 5, χ2 = 0.73, P < 0.01) and germination (GLM, d.f. = 5, χ2 = 3.33, P < 0.01) (Fig. 3b, Table 1). Both seed fill and viability were highest in seeds collected from ABG (100 ± 0.0 and 96.8 ± 1.8 respectively) and lowest in seeds collected from Gineroi (45.8 ± 2.1 and 39.6 ± 2.1 respectively).
Seed germination was much more variable among populations than were seed fill or viability (Fig. 3b). The seeds from Brigalow, Jerrys Plains and Somerton showed >50% germination and the seed germination of remaining three populations was <36% when the most effective dormancy-breaking treatment was applied. The lowest germination (21.9 ± 1.8%) was observed for seeds collected from the Gundabooka population, which was significantly lower than the germination of all other populations; Brigalow (d.f. = 5, Z = 5.19, P < 0.01), Jerrys Plains (d.f. = 5, Z = 4.49, P < 0.01), Somerton (d.f. = 5, Z = 6.22, P < 0.01) except ABG (d.f. = 5, Z = 2.70, P = 0.07) and Gineroi (d.f. = 5, Z = 1.83, P = 0.44). None of the populations had germination of their untreated seeds except for <2% germination of seeds from Jerrys Plains.
Soil seedbank behaviour of G. parviflora
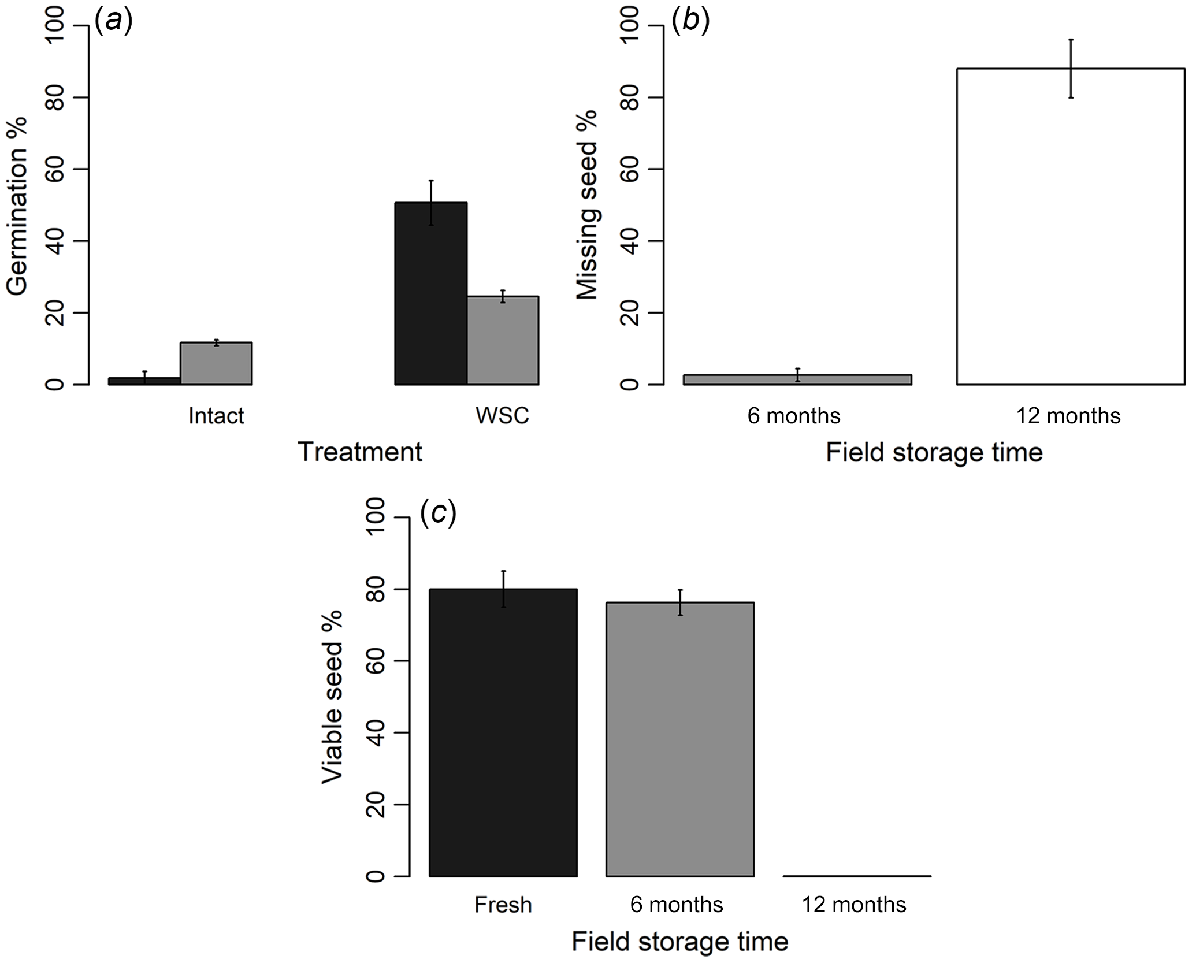
After soil burial, germination of intact seeds was significantly increased, from almost 2% in fresh seeds to 12% in seeds buried for 6 months in soil (GLM, d.f. = 1, χ2 = 1.96, P = 0.01, Fig. 4), indicating that the proportion of the seeds that were dormant was decreased. However, a significant decrease in germination response to the most effective dormancy-breaking treatment (seed-coat removal) was observed in seeds buried for 6 months (24.57 ± 1.70%) in comparison to fresh seeds (50.61 ± 6.20%) (Tukey’s test, d.f. = 2, Z = 4.68, P < 0.01). Within the first 6 months of soil burial, 2% of seeds were observed to produce radicles within bags, indicating initiation of seed germination. Viability did not decrease after 6 months of soil burial, but drastically declined after 12 months of soil burial (GLM, d.f. = 2, χ2 = 0.27, P < 0.01, Fig. 4) and retrieved seeds did not show any germination. After 12 months of burial, 88 ± 4.7% seeds were missing from bags at retrieval. No radicle emerged seeds were observed within bags at 12-month retrieval. Because of the lack of viable seeds at 12 months retrieval, we concluded the experiment and did not assess the seeds retrieved at 18 months.
Characteristics of Geijera parviflora seeds buried in soil seedbank for different time periods. (a) Mean germination percentages (±s.e.) of intact (untreated) seeds and WSC (seed coat removed) seeds by using a vice. (b) Mean of missing seed percentage (±s.e.). (c) Mean of viable-seed percentage (±s.e.). Differently shaded bars in each graph represent seeds stored for 0 ( ), 6 (
), 6 ( ) or 12 (
) or 12 ( ) months.
) months.
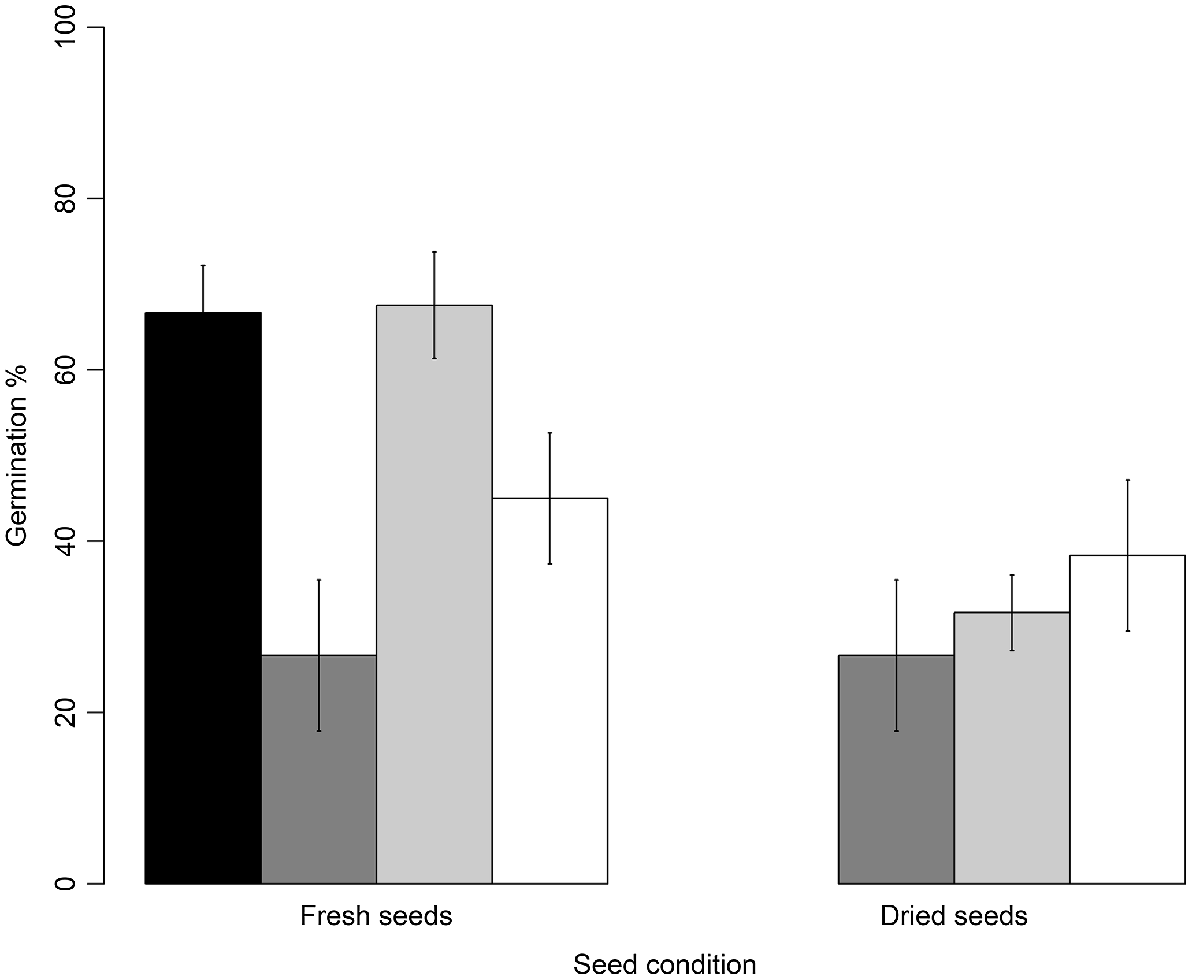
Ex situ seed storage and germination of G. parviflora
The initial seed moisture content of fresh seeds was 11.4 ± 0.19%, whereas dried seeds had a moisture content of 5.79 ± 0.17%. Seed drying significantly reduced germination from 67 ± 5.51% (fresh seed) to 27 ± 8.82% (dried seed) (d.f. = 3, Z = 2.825, P = 0.02, Fig. 5). No further reductions in germination were found after storing dried seeds in the fridge (d.f. = 3, Z = 0.46, P = 0.97) or freezer (d.f. = 3, Z = 0.97, P = 0.76). However, when fresh seeds were stored in both the freezer and fridge (without drying), germination of fridge-stored seeds was similar to germination of fresh seeds (Tukey’s test, d.f. = 3, Z = 0.08, P = 0.99, Fig. 5). The germination of freezer-stored seeds was significantly lower than the germination of fresh seeds (Tukey’s test, d.f. = 3, Z = 2.99, P < 0.01).
Quantifying floral attributes, nectar availability and the breeding system of G. parviflora
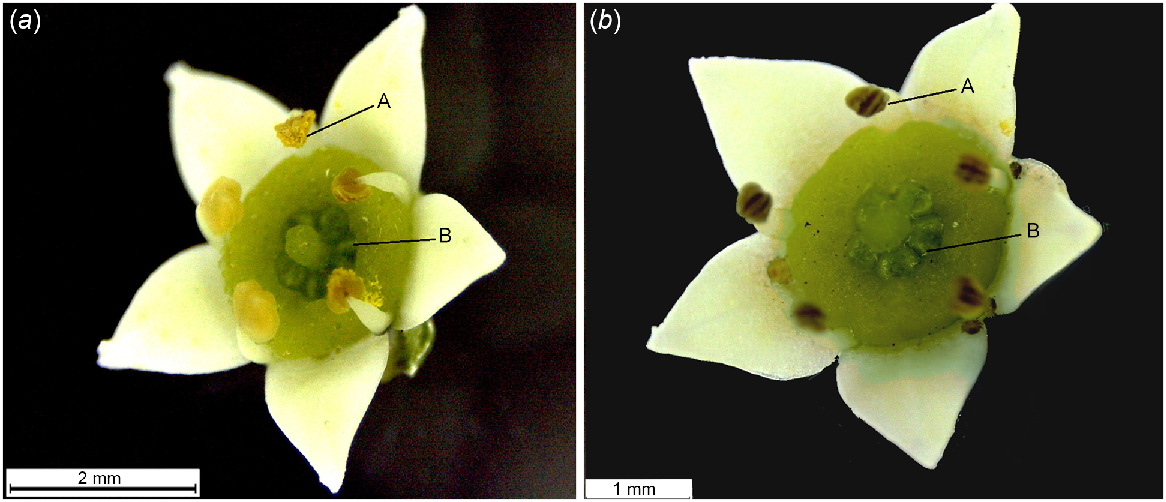
Flowers of G. parviflora had an androecium and a gynoecium on the same flower (Fig. 6a). Five stamens were clearly visible on each flower. Observations of phenology on 15 florets from six inflorescences indicated that petals typically remained open for more than 2 weeks until fruit initiation or senescence, but reproductive parts became dry and brown coloured after 6.25 ± 0.52 days unless they developed fruit. Mature, dehisced anthers with orange-coloured pollen grains were visible within 24 h of flower opening and remained visible for 3.5 ± 0.48 days (Fig. 6a). The stigmatic surface became shiny after 3.5 ± 0.21 days in floral opening (Fig. 6b).
Fully opened Geijera parviflora floret. (a) Mature androecium with immature gynoecium: mature pollen (A), pale green-coloured stigmatic surface (B). (b) Mature gynoecium with senesced androecium: dried anthers (A), shiny stigmatic surface (B).
All 24 florets observed produced nectar; however, nectar production was consistently less than 1 μL and was unable to be quantified accurately. Nectar was present in both sets of floret observed in the morning and afternoon.
Pollination experiment to determine the breeding system of G. parviflora
Three months after the application of pollination treatments, plants were checked for mature seed. This was the anticipated time frame for seed maturity, on the basis of personal observation of cultivated plants at ABG. Initial inspection confirmed the presence of mature fruits on all plants. However, examination of treatment branchlets showed that an overwhelming majority of the inflorescences failed to set seed among all treatments and that the majority of the bags (89%) placed during the experiment had been damaged, presumably by wind, storm or vandals. The majority of inflorescences within intact bags that were retained on the tree (11%) did not produce any seeds in any of the pollination treatments except production of one fruit from the open pollination treatment, which was not viable.
Discussion
The main objectives of this study were to fill the knowledge gap regarding the seed biology and reproduction of Geijera parviflora to aid critical conservation projects that include this species. Seed-dormancy experiments confirmed the presence of physiological dormancy in G. parviflora that was overcome by removing the full seed coat. We observed variation in seed characteristics among seed provenances, especially, in germination response to the most effective dormancy-breaking treatment. Both laboratory and soil seedbank storage experiments aligned and confirmed the short lifespan of G. parviflora seeds in storage, which highlights the importance of frequent replenishment of seed collections for conservation practices. Protandrous sequential hermaphroditism, which reduces self-compatibility, was evident in G. parviflora and provides information on the pollination strategies of G. parviflora. Results of this study highlighted the importance of understanding seed storage biology; knowing how to break seed dormancy and germinate seeds is integral to securing quality seeds in ex situ seedbanks and producing seedlings for restoration when necessary (Hong and Ellis 1996; Cochrane et al. 2002; Pritchard et al. 2004).
All fresh seeds of G. parviflora were dormant at dispersal and did not germinate without a dormancy-breaking treatment. Complete seed coat removal enabled the highest germination in comparison to all other laboratory treatments used in this study, suggesting that the seed coat of G. parviflora acts as a barrier to germination. However, treatment with scarification alone did not alleviate dormancy in comparison to the full seed coat removal of G. parviflora. An imbibition test performed to compare the seed weight of intact and scarified seeds confirmed the permeability of seed coats of G. parviflora (data not shown), which ruled out the presence of PY. A precision-nicking scarification technique that clears around micropylar end, or full seed coat removal, can eliminate resistance of the seed coat against radicle protrusion in species with physiological or morphophysiological dormancy (Briggs et al. 2016; Liyanage et al. 2020; Martyn Yenson et al. 2021; Liyanage et al. 2022); the highest germination in this study was observed when the complete seed coat was removed. Species in the Rutaceae family are known to have well-developed embryos (Baskin and Baskin 2014) and we confirmed this for G. parviflora by cut-tests, ruling out morphological or morphophysiological dormancy. We can therefore confirm the presence of physiological dormancy in G. parviflora.
Germination was comparatively lower when extracted seeds were incubated with crushed seed coats than without crushed seed coats and in all the replicates with partial seed coat scarification. These low germination results link to the microbial growth when full or partial seed coats were present during incubation. Surface sterilisation using mild concentrations of bleach solution, and introducing sterilised seeds onto incubating surfaces under aseptic conditions, have been suggested to overcome outbreaks of contamination (Bauer et al. 2004). However, the sterilisation of seeds using 2% bleach in this study did not completely control the microbial contamination of G. parviflora. This could be a result of endophytic microbial activity, as observed in Persoonia species (Emery and Offord 2019). Contamination may also have derived from the incubating surface, because the plates and agar were not sterilised. However, complete seed-coat removal effectively reduced seed coat-borne contamination while achieving high germination.
Inter-population variation of seed traits might reflect both variation in genetic drift and localised adaptations (Hudson et al. 2015). Understanding potential environmental effects on those seed trait variations will enable identification of populations at risk under changing climate and critical for defining ex situ plant conservation protocols (Mira et al. 2018). The presence of high dormancy levels at dispersal was confirmed across all populations tested in this study. However, interestingly, the inter-population level seed germination response to the most effective dormancy-breaking treatment varied considerably among populations. After the dormancy-breaking treatment, all seeds were incubated at the same temperature. The variation in germination observed may be related to the incubation temperature, with the ideal germination temperature associated with temperature range occuring in the respective habitat. Initial seed fill and viability were high for all populations, except for the seeds collected from Gineroi population, which had <50% filled and viable seeds in the seed lot. Prolonged drought during pollination or seed development has been suggested as a cause for low seed fill in species from the Rutaceae family (Martyn et al. 2009) and the effect of 2017–2019 extreme drought that much of New South Wales, Australia, experienced (Bureau of Meteorology (BOM) 2020) may be evident in the Gineroi collection. This was the only collection made within this period (January 2019). Seeds collected from the plants grown at ABG had the highest-quality seeds compared with seeds from wild populations. This may be a result of favourable growing conditions provided during plant maintenance at the garden. Overall, seed fill and viability did not show variation among all other populations, which may indicate a strong link to the genetic control of those seed traits in G. parviflora. We also observed higher contamination in seeds from some populations than in others during incubation. Contamination may have hindered germination of some seeds, especially because those seeds were extracted from seed coat and were more prone to fungal attack. Further investigation of temperatures on seed germination is recommended.
Interestingly, the soil seedbank experiment showed an increase in germination of intact seeds after 6 months of soil burial (retrieved in early September). This may indicate the start of dormancy release within soil at the end of winter, making non-dormant seeds available for germination the following spring. During winter, seeds experienced constantly low temperatures and typically low rainfall. Low and comparatively constant temperatures observed in Jerrys Plains (soil seedbank experimental site) during autumn and winter, in comparison to other seasons (Supplementary material S2, Tables 1 and 2), may have the effect of stratifying the soil-buried seeds and alleviating their dormancy so as to enable germination in warmer spring/summer conditions, a common strategy in many physiologically dormant species dispersed around autumn (Baskin and Baskin 2014). Rainfall data from the weather station closest to the field site (Doyles Creek [Wood Park], Station Number 61130, Bureau of Meteorology (BOM) 2022) showed higher rainfall in spring/summer in comparison to autumn/winter. So, germination during those seasons is ideal, because high rainfall in spring and summer can favour the seedling establishment followed by germination. However, seed burial for 12 months resulted in complete depletion of seeds within bags (>90% of missing seeds) where seedcoats of germinated seeds and/or ungerminated seeds were completely degraded. This indicates a presence of a short-term soil seedbank (<12 months) in G. parviflora. We observed nearly all-year-around seed production in G. parviflora (G. Liyanage, pers. obs.), which may frequently replenish its short-term soil seedbank.
The soil seedbank results aligned with results of the ex situ seed storage behaviour experiments, which indicated that longevity of G. parviflora seeds is low. There was a significant decrease in germination when seeds of G. parviflora were dried down to 50% of their initial moisture content. This confirmed desiccation sensitivity of G. parviflora seeds, which renders them unsuitable for long-term storage in conventional seedbank conditions. Interestingly, when fresh seeds were retrieved after storage in the fridge, they germinated as well as fresh seeds, indicating that fresh seeds can be stored under fridge conditions for a short period of time. The time frame for survival of fresh seeds in the fridge needs further investigation, comparing the germination of seeds retrieved after different storage-time periods in a fridge, providing practical information to support restoration efforts.
Knowledge of a plant floral biology and breeding system is important in guiding the collection of seeds for ex situ conservation and in situ restoration (van der Merwe et al. 2021). Little is known about the breeding system of Geijrea; although G. salicifolia was found to be outcrossing by Adam and Williams (2001), caution should be applied because congeners may display different breeding systems. In one study, differences in breeding system exhibited by two Hakea species found lower rates of diversity in the species with a higher level of selfing, which would necessitate collecting from up to twice as many sites to capture diversity at a regional scale than is needed for the largely outcrossing species (Lu-Irving et al. 2023). Geijera parviflora flowers possess protandrous sequential hermaphroditism, with stamens maturing before the carpels or stigma are receptive. Although our hand pollination experiment did not confirm the breeding system of G. parviflora, the presence of protandry in G. parviflora may indicate a level of outcrossing in this species. Pollen maturity and stigma receptivity timing of G. parviflora were predicted on the basis of visual observations, and slight overlap between those two events therefore is still possible. In preliminary testing, we used esterase and peroxidase enzymatic activity tests on different florets, representing different ages of opening, to assess the suitability of those tests for stigma receptivity. Both tests showed positive results (darkening on stigmatic surface by esterase activity test and production of air bubbles by peroxidase test) in florets of 0–11 days of age. A slight change in intensity of the reactions was evident, but it did not show a pattern (data not shown) indicating potential for false positive results.
The experimental design that sought to determine the breeding system of G. parviflora followed conventional protocols and treatments widely employed in other studies (see Kearns and Inouye 1993). The observed seed set of trees outside of treatment branchlets indicates that successful pollination is occurring at each of the study clusters. In this study, we were unable to determine the breeding system of G. parviflora because of a lack of seed set across all treatments. This is likely to be an outcome of the high floral output of the species and a comparatively low seed set. Studies that aim to further investigate the reproductive biology of G. parviflora should aim to account for this pattern, using very high replication at a flower level to accommodate the predicted low seed set. The use of tea bag material to construct exclusion bags is ideal for fragile flowers such as orchids, because the lightweight material minimises stem and flower damage (Reiter et al. 2021). However, the inherent fragility of the exclusion bags, coupled with their visibility to members of the public, led to issues with their longevity under the experimental field conditions. To overcome these issues, the authors highly recommend regular inspection and subsequent replacement of damaged bags to ensure continuity of pollinator exclusion and seed collection or investigation of studier options suitable for the task. Whereas we sought to find distant flowering donor and recipient flowering populations, the travel of field researchers was limited by COVID-19 travel restrictions at the time of the flowering; future studies may seek to use more distant sites. The distance between clusters may not have been sufficient to guarantee an outcross pollen source; however, lack of seed set under all experimental treatments prevented the determination of the breeding system.
Conclusions
Our results addressed several issues leading to poor seed germination and shed light on the previously unknown seed storage behaviour of G. parviflora, in both in situ and ex situ conditions. We identified physiological dormancy in G. parviflora. Complete seed coat removal is recommended as the ideal laboratory dormancy-breaking technique to achieve high germination while minimising microbial contamination. Variation in seed germination in response to the effective dormancy-breaking technique was evident among populations and may be related to environmental conditions in their local habitat. The soil seed burial experiment showed evidence that stratification of seeds in low and constant winter temperatures may alleviate dormancy allowing them to germinate in spring/summer with high rainfall. Seeds of G. parviflora are not long-lived in either natural soil seedbanks or conventional ex situ seedbanks. This indicates that other germplasm conservation techniques should be considered to save genetic diversity of this species, if long-term storage is necessary for conservation. In the meantime, we recommend short-term storage in fridge conditions, or regular seed collections to ensure that fresh seeds are used for germination. Along with complete depletion of viable seeds in the soil seedbank after 12 months, we did not observe any established seedlings within 1–2 m radius around three buried sites during our field work even when mass seed set was clearly noticeable (G. Liyanage, pers. obs.). Studies on the conditions for seedling establishment of G. parviflora are required to better understand the lack of seedling establishment in their natural habitat. Although the study also aimed to understand the reproductive biology of G. parviflora, the hand-pollination experiment did not result in any seed set. We believe that the use of high replication at the flower level across the flowering season, coupled with frequent inspection and replacement of damaged exclusion bags to ensure their integrity, may help maximise the success of the experiment should it be repeated. The knowledge obtained through these experiments enables the use of G. parviflora in ecological restoration projects into the future.


 ), 4°C fridge (
), 4°C fridge (