Frameworks for identifying priority plants and ecosystems most impacted by major fires
Tony D. Auld A B C * , David A. Keith
A B C * , David A. Keith  A B , Rachael V. Gallagher
A B , Rachael V. Gallagher  D E , Mark Tozer A B , Mark K. J. Ooi
D E , Mark Tozer A B , Mark K. J. Ooi  B , Tom Le Breton B , Stuart Allen D , Colin Yates
B , Tom Le Breton B , Stuart Allen D , Colin Yates  F , Stephen van Leeuwen
F , Stephen van Leeuwen  G , Richard J. Williams H and Berin D. E. Mackenzie
G , Richard J. Williams H and Berin D. E. Mackenzie  A B
A B
A NSW Department of Planning and Environment, Parramatta, NSW 2124, Australia.
B Centre for Ecosystem Science, University of NSW, Kensington, NSW 2052, Australia.
C School of Earth, Atmospheric and Life Sciences, University of Wollongong, Wollongong, NSW 2522, Australia.
D Department of Biological Sciences, Macquarie University, North Ryde, NSW 2109, Australia.
E Hawkesbury Institute for the Environment, Western Sydney University, Richmond, NSW 2753, Australia.
F WA Department of Biodiversity, Conservation and Attractions, Kensington, WA 6151, Australia.
G Faculty of Science & Engineering, School of Molecular & Life Sciences, Curtin University, Kent Street, Bentley, WA 6102, Australia.
H Department of Environment and Genetics, La Trobe University, Bundoora, Vic. 3086, Australia.
Handling Editor: James Camac
Australian Journal of Botany 70(7) 455-493 https://doi.org/10.1071/BT22009
Submitted: 25 January 2022 Accepted: 14 October 2022 Published: 9 December 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Globally, many species and ecosystems are experiencing landscape-scale wildfires (‘megafires’) and these events are predicted to increase in frequency and severity as the climate warms. Consequently, the capability to rapidly assess the likely impacts of such large fires and identify potential risks they pose to the persistence of species and ecosystems is vital for effective conservation management. In this review, we propose novel frameworks to identify which plant species and ecosystems are most in need of management actions as a result of megafires. We do this by assessing the impacts of a fire event on plants and ecosystems in the context of the whole fire regime (current fire event combined with recent fire history) and its interactions with other threatening processes, rather than simply considering the amount of habitat burnt. The frameworks are based on a combination of key species’ traits related to mechanisms of decline, components of the fire regime that are most likely to have adverse impacts on species or ecosystem recovery, and biotic and environmental factors that may amplify fire impacts or pose barriers to post-fire recovery. We applied these frameworks to guide management priorities and responses following the extensive 2019/2020 fires in southern Australia, and we illustrate their application here via a series of worked examples that highlight the various mechanisms of post-fire decline the frameworks address. The frameworks should be applicable to a broader range of fire-prone biomes worldwide. Our approach will (1) promote the development of foundational national datasets for assessing megafire impacts on biodiversity, (2) identify targeted priority actions for conservation, (3) inform planning for future fires (both prescribed burning and wildfire suppression), and (4) build awareness and understanding of the potential breadth of factors that threaten plants and ecosystems under changing fire regimes.
Keywords: disease, drought, fire frequency, fire history, fire planning, fire regime, fire response, fire severity, fire spatial extent, herbivory, life history traits, recovery actions, threats, weeds.
Fires as a driving force in Australian vegetation
Australian vegetation has been shaped by fire over evolutionary timescales (Kemp 1981; Lamont et al. 2019) because fire is a major factor affecting the life histories and ongoing persistence of plants, animals and ecosystems. Increasing fire activity in the landscape since the mid- to late-Tertiary (30–60 million years ago) is supported by evidence of increasing deposits of charcoal in the paleorecord, notwithstanding periodic fluctuations (Lynch et al. 2007). Similarly, the rising importance of fire on Australian vegetation through time is evidenced by the co-incident proliferation of traits associated with recovery from fire, such as epicormic resprouting, serotiny, fire-stimulated seed germination and the expansion of fire-prone biomes across the continent (Clarke et al. 2015). Fire continues to maintain contemporary vegetation structure across Australia, with vast areas (∼70%) of the continent’s vegetation being dependant on fire as the major disturbance promoting successional processes (Russell-Smith et al. 2007). Further, the non-linear patterns of fire behaviour define and maintain some vegetation boundaries, most notably between rainforest and sclerophyll communities (Bowman 2000).
Fire regimes vary across the landscape because fire-regime components (frequency, intensity/severity, season and type; Box 1) exhibit biogeographic patterns related to climatic factors at regional, continental and subglobal scales (Bradstock et al. 2005; Murphy et al. 2013; Young et al. 2017). However, fire regimes also vary markedly across the landscape owing to environmental factors such as topography (Miller and Murphy 2017), local weather and vegetation feedbacks (Zylstra 2018), along with anthropogenic factors (Bird et al. 2016). Ecosystems occurring in different climatic zones thus comprise species that evolved under different fire regimes and vary in their sensitivity to changes in fire-regime components, as well as the magnitude and direction of those changes. Just because a species or ecosystem is burnt in a fire does not mean there has been an adverse impact on that entity. Understanding the likely impacts of fires on plant species and ecosystems requires knowledge of how fire regimes affect species and their life histories in the context of recent and historical fire regimes, and the varying sensitivities of ecosystems across the landscape to different components of the fire regime. Persistence of species depends on interactions between population processes and fire regimes (Keith 1996, 2012). Declines may occur when one or more components of the fire regime moves outside a species’ tolerable range. For example, population declines are likely to occur in obligate-seeding or non-resprouting plants (sensu Pausas et al. 2004) if fires recur before these species can replenish their seed banks.
| Box 1. The fire regime and related components that cause declines in biodiversity |
| What is the fire regime? |
There are four main elements of the fire regime (Gill 1975; Department of Agriculture, Water and the Environment 2022) and several additional fire components that can affect biodiversity. The impacts they can have on plants and ecosystems can result from individual elements or the interactions among them. Biological impacts are described below at the level of species’ populations, but each has implications for ecosystems depending on the structural, functional and compositional roles of the species in their ecosystems (Akçakaya et al. 2020). Biological impacts are as follows: |
| Core elements |
Definition: The number of fires per unit time at a point in the landscape. Impacts: Fire frequency can affect two main components of plant life histories:
|
Definition: Energy output or heat release from fire per unit time at a point in the landscape. Impacts: see Fire severity below. |
Definition: The time of year of a specified fire event. Impacts: There are at least eight ways in which fire season can affect plant life cycles (see Miller et al. 2019 and Keith et al. 2020c for details). Fire season may limit population persistence through reductions in adult survival and growth, post-fire flowering and seed production, the magnitude of seed banks, juvenile growth and maturation, tolerance of seeds to heat, post-fire seed survival and establishment, and dispersal distances. |
Definition: Whether a specified fire event at a point in the landscape burns at or above ground level (consuming live/dead biomass), or below ground level (consuming semi-decomposed organic matter, such as peat, coal). Impacts: Relative to above-ground fires, below-ground fires can cause elevated mortality of seeds and regenerative organs, leading to greatly reduced post-fire recovery (Keith et al. 2022a). |
| Additional fire components that can affect biodiversity (after Nolan et al. 2021; Department of Agriculture, Water and the Environment 2022) |
Definition: The amount of organic matter consumed in a fire event at a point in the landscape (see Keeley 2009 for review of terms fire severity, fire intensity and burn severity). Impacts: Effects of both fire intensity and severity depend on the exposure of critical plant tissues to lethal temperatures (both above and below ground). In turn, this depends on aspects of fire behaviour as well as the location of the critical plant tissues. Both fire intensity and fire severity can be useful indicators of exposure to lethal temperatures under particular circumstances, but neither precisely represents temperature exposure. Whereas, fire intensity is challenging to measure in real time (Alexander 1982), a number of real and proxy metrics are available to estimate fire severity, either on-ground or from remote sensors, with before/after fire comparisons or from post-fire observations only (Keeley 2009). Each of these methods involves simplifying assumptions that fail to hold in some circumstances. Positive and negative effects of high exposure to lethal temperatures (i.e. fire severity) are known.
|
Definition: Area within the spatial boundary of a fire event. Impacts:
|
Definition: The spatial configuration of patches with different fire characteristics (varied levels of severity, including unburnt) within a specified area. Impacts:
|
The survival of populations of plants and animal species is strongly influenced by the fire regime (Box 1; Gill et al. 1981; Whelan 1995; Keith 1996; Bradstock et al. 2002, 2012; Bowman et al. 2019), population vital rates (survival, stage transitions, recruitment) in combination with environmental factors such as climatic fluctuations (e.g. drought), anthropogenic impacts (clearing and fragmentation that affect fire behaviour and spread), and herbivory and competition with other species (native or exotic). The impacts of a fire event therefore depend on the interactive effects of environmental factors, fire regime components and species’ life histories. Further, as changing climates reduce plant survival and reproductive rates, the range of fire regimes that facilitate species persistence are predicted to become more restricted (the concept of interval squeeze, Enright et al. 2015), potentially leaving many species and ecosystems vulnerable to decline. More extreme fire weather is predicted under a changing climate, likely increasing the frequency and severity of fires (and potentially reducing fire patchiness and fire refugia), along with the extent and severity of droughts and storms (Bradstock 2010; Cary et al. 2012; Miller and Murphy 2017; Abram et al. 2021), all of which affect ecological responses to fires (Miller and Murphy 2017). The widespread fires that occurred in south-eastern Australia in the summer of 2019/2020 are consistent with predicted outcomes of global warming (Nolan et al. 2020a), as are recent large wildfires in California (Keeley and Syphard 2021) and Mediterranean Europe (Ruffault et al. 2020).
Fire management for biodiversity conservation relies on an ability to accurately identify and implement fire regimes that facilitate species persistence and maintain diverse plant communities and ecosystems. In essence, this means identifying and avoiding those fire regimes that are likely to be detrimental to the persistence of species and ecosystems. Species and associated vegetation evolved under lightning-ignited fires driven by climatic, topographic and edaphic drivers until the arrival of Indigenous Australians (at least 50–60 000 years ago) who used fire and initiated additional human ignitions. In the past 230 years, fires initiated by European settlers include prescribed ignitions for management purposes (e.g. pastoral management and more recently, hazard reduction), accidental ignitions and arson. Ignitions have therefore not only increased in frequency with human intervention, but also in their spatial and temporal distribution across landscapes, seasons and years. Such ignitions have led to fire regimes diverging in different directions from natural patterns through different eras of human activity, as well as because of past and current climate changes. Fire regimes in contemporary Australia are a product of fires that originate from both natural (primarily lightning) and human ignitions (Indigenous burning and planned fires for pastoralism, fuel reduction or conservation, along with arson or accidental fire escapes; Bowman et al. 2020). Understanding of how alterations to fire regimes affect plants and other biota has only recently begun to develop (Parson and Gosper 2011; Keith 2012; Kelly et al. 2020).
Fires of human origin add to the natural complexity of fire regimes in the landscape; however, conversely, European land management practices may also supress the outcomes of natural ignitions through active fire suppression and landscape fragmentation (Parsons and Gosper 2011). Many current applications of fire by humans aim to reduce fuel loads, either over broad-scale landscapes, or in and around areas that contain dwellings and other assets. Fire is also used to promote regeneration in logged forests. Unplanned fires (wildfires resulting from lightning or unintentional human ignitions) combine with this prescribed fire matrix to create complex patterns of fire regimes in different parts of the landscape (Bradstock et al. 2005). The consequences are expressed in the interval, timing and spatial dimensions of fire regimes (Bond and van Wilgen 1996). For example, some patches are frequently burnt or have short intervals between some successive fires, whereas others are rarely burnt or remain entirely unburnt, either because fragmentation has isolated them from fire pathways, or because feedbacks have allowed them to develop low-flammability properties. Event characteristics, including fire severity, seasonality and extent, all vary spatially and temporally across different landscape and pyroclimate types, (e.g. see Russell-Smith et al. (2007) for variation in fire seasonality across Australia). In summary, landscapes have both complex spatial patterns of fire regimes, as well as complex requirements for the persistence of the full diversity of plant species, vegetation communities and ecosystems under recurring fires.
Significance of the Australian 2019/2020 wildfires
The fires that occurred in south-eastern Australia in the 2019/2020 fire season were among the most extensive of southern Australia’s European era (Davey and Sarre 2020; Nolan et al. 2020a). The fires burnt ∼7 Mha across south-eastern Australia between September 2019 and March 2020 and burnt a greater proportion of Australia’s temperate broadleaf and mixed forest biome than any other global forest biome in the past 20 years (Boer et al. 2020; Collins et al. 2021). Much of the area was estimated to have burnt at high severity (Collins et al. 2021) and the fires affected a substantial proportion of the known ranges of large numbers of vertebrate fauna (Ward et al. 2020; Legge et al. 2022), vascular plant species (Auld et al. 2020; Gallagher et al. 2021), terrestrial (Lindenmayer and Taylor 2020; Keith et al. 2022b) and aquatic (Silva et al. 2020) ecosystems. This created huge challenges for land managers dealing with the conservation of biodiversity and demonstrated the need for frameworks to guide what species and ecosystems are most likely to have been adversely affected as a result of the 2019/2020 Australian wildfires.
The scale and severity (sensu Box 1) of the 2019/2020 fires was clearly unprecedented and influenced by the trend for increased area of forest burning driven by climate change (Canadell et al. 2021). Diagnosing the potential impacts of these fires is not straightforward because fire is a recurring event, even if only rarely, in virtually all of the ecosystems affected (Keith and Tozer 2017; Miller and Murphy 2017). To forecast potential declines, a variety of factors need to be evaluated, including the complex patterns of antecedent fire history, regional variability in pre- and post-fire weather, diverse land uses within the fire footprint and an array of threats posed by alien predators, herbivores, competitors and pathogens that pose considerable risks of adverse impacts to plant species and ecosystem recovery. Timely assessments across the full range of biota and their national or global distributions are essential to inform effective management responses.
Frameworks for rapidly predicting impacts of fire events on plant species and ecosystems
Framework aims
Here we present novel predictive frameworks developed to identify, first, plant species and, second, ecosystems that are expected to have been most affected following a major wildfire event, so that those most in need of management actions can be identified. These frameworks go beyond simple reporting of how much habitat was burnt in a fire. The Frameworks assume that risks of decline from fire-related impacts are related to the proportion of the species/ecosystem range affected by a given mechanism of decline, in a comparable way to assessing risk ranking on the basis of geographic distribution in IUCN Red List Criteria (Keith et al. 2018). They build on the understanding that fire regime impacts (Box 1) are important in the response of biodiversity to individual fire events (Bradstock et al. 2012; Keith 2012; Miller and Murphy 2017) as are both biotic and abiotic factors, including threats from, in particular, weeds, pests and pathogens and human impacts. We integrated these components into a scheme that permits comparisons of relative exposure to impacts across either species or ecosystems. The frameworks are decision support tools to guide identification of species and ecosystems that are likely to need active management during post-fire recovery, including what factors may need to be addressed in such recovery. We provide worked examples of applications of these frameworks from the emergency response to the 2019/2020 Australian bushfire season, noting that they are equally applicable to other major wildfire events within Australia and globally. The frameworks
-
provide transparent, logical pathways for decision-making that supports well reasoned strategic policy, management and resourcing responses in an emotionally charged post-fire social environment when fires occur;
-
identify the key issues that need to be addressed to ameliorate impacts of megafires on plants and ecosystems. This includes consideration of the fire regime and its components (Box 1), species’ life histories, ecosystem processes, environmental conditions and other biotic and abiotic threats;
-
use available data to prioritise species and ecosystems for post-fire conservation management, including field-impact assessments and any necessary recovery actions (both immediate and medium- to long-term); and
-
allow for ongoing re-evaluation of which species and ecosystems are most likely to be at risk from future fires and landscape-scale threats.
Summary of framework elements
Here we describe related individual frameworks that were designed for species and ecosystems respectively. The frameworks predicted the likelihood of poor post-fire recovery via three components (mechanisms, sensitivity and exposure):
-
Mechanisms of decline and their interactions, e.g. combinations of life-history traits and threats that make species prone to population declines or local extinctions if they are affected within the spatial extent of a fire;
-
Sensitivity of species and ecosystems to the identified mechanisms of decline (e.g. sensitivity to high fire frequency or to fire-promoted pathogens (such as Phytophthora or myrtle rust)); and
-
Exposure in the landscape where these mechanisms are most likely to be expressed (e.g. the overlap/intersection of species’ or ecosystem distributions with the spatial extent of the fire event being investigated (excluding unburnt patches and refugia that do not burn) AND the particular threat of concern).
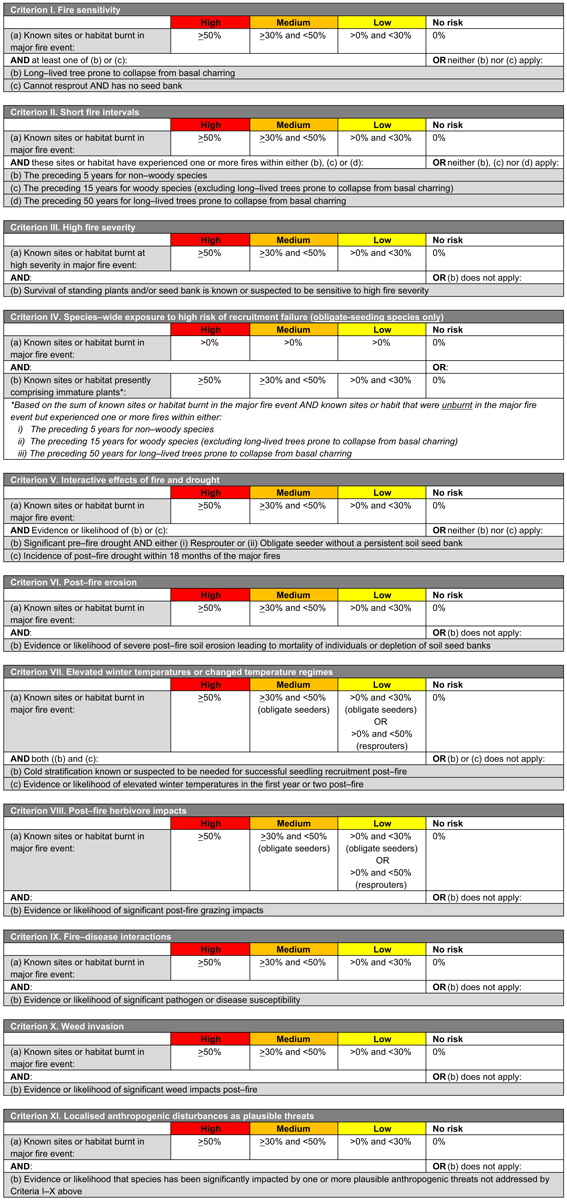
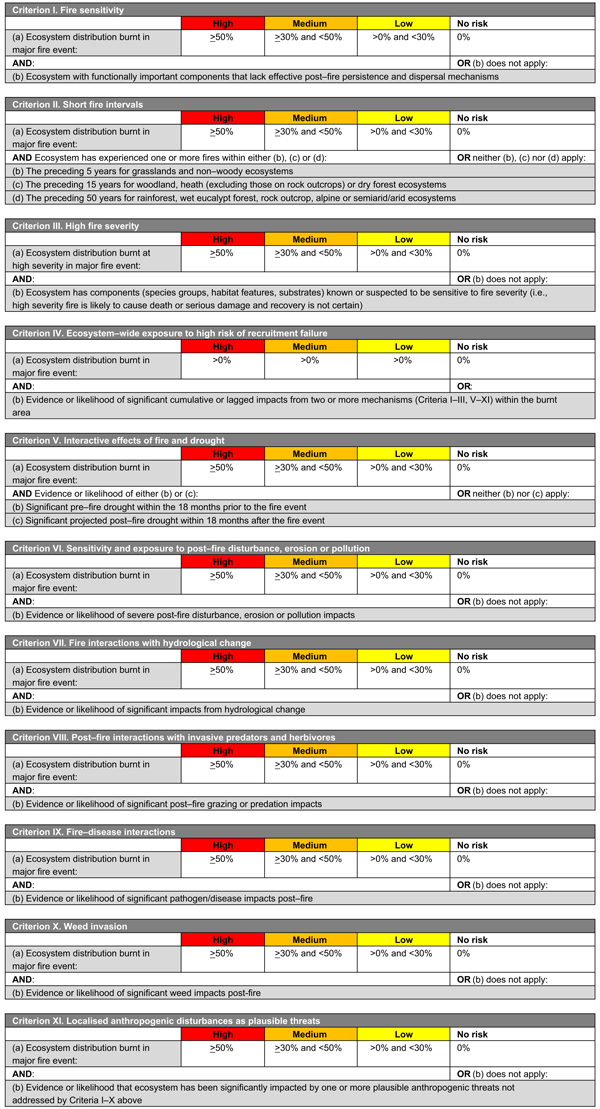
Each framework includes 11 criteria or mechanisms of decline (across four main themes, see below) related to the fire regime, environmental conditions, life history of species and concurrent threats (Fig. 1, Tables 1, 2). These mechanisms are as follows:
|
|
|
|
-
Components of the fire regime (see Box 1 for explanation terms and potential impacts on biodiversity) that are most likely to have adverse impacts on species or ecosystem recovery through disruption of life-history processes (I, fire sensitivity; II, short fire intervals; III, high fire severity; IV, recruitment failure)
-
Fire-environment abiotic interactions, including prevailing environmental conditions (for species: V, drought; VI, erosion; VII, elevated temperatures; or for ecosystems: V, drought; VI, erosion, disturbance or pollution; and VII, altered hydrology)
-
Fire-biotic interactions, including sensitivity of species or ecosystems to a fire and biotic threats to post-fire recovery (VIII, herbivore impacts; IX, disease; X, weed invasions)
-
Fire–human interactions, including XI, localised anthropogenic disturbances such as disturbances from vehicles or foot traffic, rubbish dumping, clearing of habitat and logging (among others).
Framework application
The two frameworks (Fig. 1, Tables 1, 2) were developed and implemented nationally in Australia, for species and ecosystems respectively, following the 2019/2020 fire season. The criteria enable each species or ecosystem assessed to be assigned to an ordinal category (‘high’, medium’, ‘low’, ‘no impact’ or ‘data deficient’) on the basis of spatial thresholds drawn from the IUCN risk-assessment protocols (Bland et al. 2017). Categories are indicative of the risk of recovery failure and decline, as follows:
-
HIGH: high degree of exposure to the risk of decline. An urgent assessment of initial fire impacts and threats to recovery is required and post-fire monitoring of recovery where impacts are significant.
-
MEDIUM: medium degree of exposure to the risk of decline. Assessment of initial fire impacts and threats to recovery is required and post-fire monitoring is recommended.
-
LOW: low degree of exposure to the risk of decline. Post-fire monitoring may be conducted opportunistically during site visits.
-
NO IMPACT: negligible exposure to the risk of decline or not burnt in the fires.
-
DATA DEFICIENT: insufficient data to enable an assessment.
Species or ecosystems should be assessed against as many criteria as possible, depending on available data. Outcomes of each criterion are evaluated concurrently, with the highest category of concern across all criteria being used to allocate a species or ecosystem to an overall category, recognising that factors driving decline may operate independently or interact in complex ways, and hence the criteria are best not combined in an additive or multiplicative manner (Burgman et al. 1999; Keith 2009). This emulates the approach used in established frameworks such as the IUCN Red List Criteria for Threatened Species (IUCN 2022) and IUCN Red List Criteria for Ecosystems (Bland et al. 2017). Separate reporting on each criterion also allows an informed comparison of the factors driving the highest likelihood of decline for groups of interest, such as groups of species or higher taxa (e.g. families), life-form groups, fire-response groups, or regional floras.
Species or ecosystems for which the highest ranking is ‘NO IMPACT’ on the basis of assessments of at least three criteria are assigned an overall rank of NO IMPACT, but otherwise must be assigned to the ‘DATA DEFICIENT’ category until at least three criteria are assessed. This is an important distinction and highlights species or ecosystems with important knowledge gaps that require addressing for their effective conservation management.
Dealing with interactions across criteria
While the frameworks require assessment of each criterion individually, interactions among criteria may also arise. In such cases, it is useful to recognise that individual criterion outcomes may be affected by other factors. For example, a pre-fire drought may exacerbate the impact of high fire severity. This may occur where pre-fire drought reduces the capacity for species to resprout post-fire, although prior depletion of carbohydrate reserves and the impact of this effect vary with fire severity. In many such cases, the measurement of impacts (post-fire recovery) will necessarily include outcomes of the interaction among factors because each factor cannot be clearly separated when assessing (or sampling) post-fire impacts. In other interactions among criteria, there may be temporal differences in the expected timing of impact; for example, the interaction between high fire frequency and post-fire herbivory is a two-step process, with initial impacts of high fire frequency and subsequent impacts of herbivory on recovery. When examining the strength of these effects through one-off sampling of post-fire recovery, often all that can be measured is their combined interaction. Hence, in most cases, major interaction effects are included by recognising the combined effects on species or ecosystems under each driving criterion in the frameworks. When the level of impact of individual factors within interactive effects can be identified, one option is for the rank to be increased by one level when there is a synergistic (or additive) interaction of threats, and decreased by one level where there is a compensatory interaction (in the same fashion as application of the IUCN Red List criteria at regional or national scales; IUCN 2012).
Data issues
Data requirements
Assessing priorities for post-fire conservation action requires data on the spatial distribution and fire-response traits of species and ecosystems, as well as the distribution, extent and severity of threats and other relevant environmental factors. Assessing impacts after a major fire event specifically requires data on fire spatial extent and fire-severity mapping for the fire event being investigated, as well as reliable fire-history mapping. Data availability will vary among assessment areas, taxa and ecosystems, and it is important to aim for standardised national fire-related datasets (Bowman et al. 2020). Here, we provide several examples to illustrate the types of data that could be used in the assessments and the need to address standardisation, licensing, transparency and efficient workflows, drawing on the assessments of potential impacts for plants (Auld et al. 2020; Gallagher 2020; Gallagher et al. 2021, 2022) and ecosystems (Keith et al. 2020a, 2022b) conducted in response to the Australian 2019/2020 fire season.
Assembling species and ecosystem lists
An appropriate target list of species or ecosystems forms the foundation of any assessment of impacts or management needs after a fire event. Ideally, all species co-occurring within the spatial extent of the fire event should be targeted for assessment. This requires species-occurrence information (see below) and validation of current species nomenclature against a taxonomic authority, including identifying taxonomic synonyms. For instance, the Australian Plant Census provides up-to-date information on the status of taxonomic names of Australian plants, and similar resources exist in many countries (e.g. USA: https://www.itis.gov/) and globally (e.g. The Plant list, http://www.theplantlist.org/ and Plants of the World Online http://www.plantsoftheworldonline.org/; Catalogue of Life: https://www.catalogueoflife.org/). Checking of taxonomy can be automated through workflows in R using packages such as taxise (Chamberlain and Szöcs 2013), or via online portals such as the Taxonomic Resolution Naming Service (TNRS; https://tnrs.biendata.org/).
For ecosystems, the analogous data required are a typology that identify all the ecosystem types within a study area at a suitable level of thematic resolution. The units of the typology should represent the critical processes and dependencies that sustain ecosystem components and functions. IUCN’s Global Ecosystem Typology (GET, Keith et al. 2020d) provides a suitable framework for this purpose. The GET facilitates the integration of national or jurisdictional-level typologies based, for example, on the classification of vegetation communities under a hierarchy of types, with similar functional responses to fire regimes in combination with the posited threats.
Spatial data on species and ecosystem occurrence
National and international databases can be used to access georeferenced information on occurrences of species. For instance, international resources such as the Global Biodiversity Information Facility (GBIF; https://www.gbif.org/) or national resources such as the Australasian Virtual Herbarium (AVH; https://avh.chah.org.au/) provide open access to vouchered records of species occurrence. Herbarium specimens have several advantages over unvouchered records of species-occurrence information, including verification of taxonomic identity by experts, the ability to trace information back to a vouchered collection and to update analyses following taxonomic revisions (Heberling and Isaac 2017). However, bias in spatial patterns of sampling in herbarium specimens towards roads and access tracks may limit the accuracy of species ranges inferred from them (Haque et al. 2017). To address this issue, secondary sources of information on species range may be used in conjunction with herbarium occurrence data, such as records with lower standards of verification (e.g. research-grade observations in iNaturalist, https://www.inaturalist.org/), data from systematic vegetation surveys (e.g. BioNet systematic flora surveys, https://www.environment.nsw.gov.au/atlaspublicapp/UI_Modules/YETI_/FloraSearch.aspx), expert-derived range maps, or the spatial projections from species distribution models. Ideally, multiple lines of evidence about species occurrence should be used in parallel when applying the frameworks and the highest risk (most precautionary) category across the different sources used to rank species.
Steps should be taken to minimise spatial inaccuracies of species-occurrence records. For example, the full, original, as-held data for any unique threatened species records should always be obtained directly from the original custodian to ensure accurate locality descriptions and georeferences (because sensitive information is often denatured prior to incorporation into third-party repositories). Checking georeferences for consistency against the location description allows detection of erroneously georeferenced points that may otherwise bias predictive modelling of species distributions or population-level assessments of fire impact/escape. This highlights the need for resourcing to maintain and update databases of reliable species occurrence records so that georeferences are an accurate representation of the location information.
For ecosystems, maps of proxy units such as vegetation types can be obtained from government repositories or scientific literature, or generated via a wide range of remote-sensing and modelling approaches (Bredenkamp et al. 1998). Similar verification and error-correction procedures should be applied as those recommended above for species.
Fire-response traits for species and ecosystems
The framework criteria use a suite of traits to identify species or ecosystems that are susceptible to different fire-related causes of decline. Traits are the measurable characteristics of organisms that shape their ecological performance (Westoby et al. 2002; Gallagher et al. 2021) and in the context of post-fire assessment frameworks, specifically include factors such as habit, woodiness, fire-response strategy (capacity to resprout versus only recruit via post-fire seedings, sensu Pausas et al. 2004; Clarke et al. 2015; Prior and Bowman 2020), seed-bank type (canopy- vs soil-stored), seed-dormancy types (physical, physiological and morphophysiological, among others) as well as various germination requirements and drought-avoidance mechanisms. It is now possible to source data on plant species traits directly from large, aggregated databases such as TRY (Kattge et al. 2020), LEDA (Kleyer et al. 2008), BIEN (Maitner et al. 2018) and AusTraits (Falster et al. 2021), as well as from more bespoke datasets for particular plant groups (e.g. bryophytes; Bernhardt-Römermann et al. 2018; or plant parts; e.g. roots; Iversen et al. 2017). Fire-response traits may also be sourced from environmental management agencies that use ecological information in planning for prescribed burning.
For some species, multiple observations of the same trait may lead to conflicts in assigning species to categories (e.g. because of natural variation, variation owing to particular factors such as fire severity, observer error etc.), and strategies are required for choosing the appropriate trait value. For instance, consensus across datasets may be used to assign a species as woody or non-woody. Similarly, continuous or ordinal trait values may need to be partitioned or aggregated to form appropriate categories. This may include grouping information on growth forms (e.g. tree, shrub, grass, herb) and standing plant longevity (e.g. annual, biennial, perennial) to inform woody and non-woody categories. As per species-occurrence records, prior to use, taxonomic names associated with trait observations need to be assessed for consistency and taxonomic synonyms to ensure alignment with the species list of interest.
The requirement for large, comprehensive datasets on plant traits and plant responses to components of the fire regime (including variation in responses), in addition to comprehensive and spatially accurate databases of species-occurrence records, highlights the need for forward planning and investment. These databases need to be established and maintained at the national scale in advance of future megafire events to enable a rapid and effective responses.
Framework criteria and their assessment
The 11 criteria representing key mechanisms of post-fire decline identified in each framework (Tables 1, 2) and the potential methods for assessing each criterion are presented below.
Criteria relating to morphological traits
I. Fire sensitivity
Some plant species, including some functionally important species within ecosystems, have no means of in situ persistence through fire events, because they lack regenerative organs, and on-site propagule storage (e.g. R-P- of Pausas et al. 2004) or long-distance dispersal traits to facilitate recolonisation. Some species or ecosystems may currently persist in disequilibrium states and may be incapable of re-establishment under present-day conditions. Examples include certain rainforest species (Boxes 2, 3) and peatlands, forests or heaths dominated by paleo-endemic species (e.g. Kirkpatrick et al. 2010; Bliss et al. 2021). A single fire event may eliminate these entities or substantially diminish their role in the community, an effect that persists until they slowly disperse and re-establish from unburnt patches.
| Box 2. Species criteria: fire sensitivity (Criterion I) and fire-disease interactions (Criterion IX) |
| Case study: Eidothea hardeniana (nightcap oak, Proteaceae). |
| Background: Eidothea hardeniana is a rainforest species the life-history of which is poorly known. It is restricted to the Nightcap Range in north-eastern New South Wales, Australia. The total population is very small. |
| Conservation status: Critically Endangered (IUCN Red List, Forster et al. (2020); EPBC Act). |
| Relevant life-history traits |
|
Biotic/abiotic/fire regime threats: some impact of weeds (Cinnamomum camphorum Camphor Laurel, NSW Saving our Species 2021). May be adversely affected by the introduced pathogen Phytophthora cinnamomi. |
Estimate of known sites/habitat burnt in the 2019/2020 fires: over 90%. |
Assessment against framework criteria for species: predicted to be at HIGH risk via Criteria I and IX (Table 1). |
Management response: post-fire survey suggests that approximately a quarter of individuals were burnt in the 2019/2020 fires with approximately two-thirds of these plants being killed and most of the rest reduced to resprouting from the base of the trunk (NSW Saving our Species 2021). A very long fire-free period (at least 50–100 years) is considered necessary to allow any recovery; so active protection of the known sites from fire is essential. |
| Box 3. Species criteria: fire sensitivity (Criterion I); short fire intervals (Criterion II); fire and drought (Criterion V) and fire-disease interactions (Criterion IX) |
| Case study: Wollemia nobilis, the Wollemi Pine (Araucariaceae), a long-lived tree prone to scarring and collapse from prolonged basal charring. |
| Background: Wollemia nobilis is a recently discovered rainforest species. It is a monotypic genus, with Wollemia pollen being abundant 65–34 million years ago, then steadily declining in response to cooling and drying during the northward movement of Australia (NSW Department of Environment and Conservation 2006). It is now known from one population (four small stands) in warm temperate rainforest in Wollemi National Park New South Wales (NSW; Benson and Allen 2007). Endemic to NSW, Australia. Restricted to gorges in Wollemi National Park, west of Sydney, Australia. |
| Conservation status: Critically Endangered (IUCN Red List, Mackenzie and Auld, in press; EPBC Act). |
| Relevant life-history traits |
|
Biotic/abiotic/fire regime threats: affected by of exotic pathogens (Phytophthora spp.; Bullock et al. 2000; Puno et al. 2015). Major threats of increased fire frequency in combination with climatic drying (Mackenzie et al. 2021). |
Estimate of known sites/habitat burnt in the 2019–2020 fires: 100%. |
Assessment against framework criteria for species: predicted to be at HIGH risk via Criteria I, II, V and IX (Table 1). |
Management response: post-fire survey confirmed that all sites were burnt in the 2019/2020 fires, albeit at low severity, whereas some plants experienced basal charring of trunks and trunk loss (Mackenzie et al. 2021). Most of the juvenile bank of plants was killed, although a few juvenile plants escaped being burnt. A fire-free period of at least 50–100 years is recommended for recovery (Mackenzie et al. 2021). |
Assessing fire sensitivity
There is no globally comprehensive database on fire sensitivity of plant species, although geographically scoped fire-response databases and classification schemes (e.g. Gill and Bradstock 1992) can inform fire sensitivity, as may data held in global or regional databases such as TRY (Kattge et al. 2020) and AusTraits (Falster et al. 2021). Pausas et al. (2004) compiled data on a small number of species that lacked regenerative organs and seed banks to support post-fire recovery by sprouting or seeding respectively (R-P-), and this was also flagged by Prior and Bowman (2020). Information on the susceptibility of species to single fire events can also be based on available scientific literature (e.g. Athrotaxis, Bliss et al. 2021) and expert opinion.
Species: long-lived trees that are prone to collapse from prolonged basal charring during fires are candidates for Criterion I (Table 1, Boxes 2, 3). For example, Gallagher (2020) used trait and spatial information to identify 463 rainforest-tree taxa greater than 30 m in maximum height (taking height as a proxy for longevity) using the AusTraits database. Long-lived tree species sensitive to fire may also occur in low-productivity environments and require substantial time (>30–50 years) to regenerate and set seed post-fire.
Ecosystems: Keith et al. (2020a) identified ecosystems that rarely experience fire and were sensitive to a single fire event, because species with key structural or functional roles lack regenerative organs and seed banks that enable autogenic recovery. The main ecosystem types that include such species are rainforests and samphire shrublands and herbfields, sphagnum bogs, as well as peatland ecosystems, because peat is combustible should substrate fires occur, and may take many decades or centuries to re-accumulate to similar depths. Most rainforest trees have thin bark (Lawes et al. 2013) and, even though a surprising number has basal regenerative buds, top-kill resulting from fires with scorch heights as low as 2 m may result in structural transformation of large stands that may take many decades to re-establish their mesic micro-climate, arboreal substrates for tree-dependent flora and fauna and structural complexity.
Exposure of fire-sensitive species and ecosystems to any fire event can be estimated by intersection of their distributions with the fire extent (e.g. for the Australian 2019/2020 fires – the National Indicative Aggregated Fire Extent Dataset; Department of Agriculture, Water and the Environment 2020).
Ecosystems: Keith et al. (2020a) identified ecosystems that rarely experience fire and were sensitive to a single fire event, because species with key structural or functional roles lack regenerative organs and seed banks that enable autogenic recovery. The main ecosystem types that include such species are rainforests and samphire shrublands and herbfields, sphagnum bogs, as well as peatland ecosystems, because peat is combustible should substrate fires occur, and may take many decades or centuries to re-accumulate to similar depths. Most rainforest trees have thin bark (Lawes et al. 2013) and, even though a surprising number has basal regenerative buds, top-kill resulting from fires with scorch heights as low as 2 m may result in structural transformation of large stands that may take many decades to re-establish their mesic micro-climate, arboreal substrates for tree-dependent flora and fauna and structural complexity.
Exposure of fire-sensitive species and ecosystems to any fire event can be estimated by intersection of their distributions with the fire extent (e.g. for the Australian 2019/2020 fires – the National Indicative Aggregated Fire Extent Dataset; Department of Agriculture, Water and the Environment 2020).
Application of the assessment frameworks
Spatial scale of application
The framework for species has been applied to plants after the Australian 2019/2020 fires with both a national focus (Gallagher 2020; Gallagher et al. 2021, 2022) and a regional one (the state of NSW, Auld et al. 2020). These assessments applied the framework to assess fire impacts across a species’ national distribution so that priority outcomes were not biased by local/regional impacts. Similarly, Keith et al. (2020a, 2022b) applied the ecosystem framework at the national scale to ecosystems recognised in national and state jurisdictions (the latter particularly for ecosystems largely endemic to a particular state).
The frameworks can be applied to any area of management interest, but it is also important to consider the total distribution of species or ecosystems to provide relevant context. This may help inform management priorities at the scale of reserve networks or bioregions, with species or ecosystems exposed to high impacts throughout their distribution being assigned a higher priority than those exposed to high impacts in the assessment area, but not throughout their range. The benefits of applying the criteria at global or national scales for megafires include a more comprehensive understanding of relative risks to different functional groupings of plants or types of ecosystems, and the factors driving reduced post-fire recovery, along with targeting of subsequent management actions to where they are most needed. Improvements in national datasets on plant species responses to fire and ecological traits will enhance the capacity of such comparisons. Gallagher et al. (2021) and Godfree et al. (2021) both estimated the percentage of Australian native plant species affected by the 2019/2020 fires, which helped convey the magnitude of potential impacts to a wide audience. However, the additional application of the risk framework for plant species allowed Gallagher et al. (2021) to identify key mechanisms driving potential decline, which is the first critical step towards development and implementation of strategies for risk reduction and impact mitigation.
Application to threatened entities
The frameworks can be applied to threatened species and ecosystems to identify those most in need of management action. For example, Gallagher (2020) and Gallagher et al. (2021) found that some 67 nationally threatened plants in Australia were likely to decline as a result of the 2019/2020 fires (i.e. medium or high risk) owing to too frequent fire. The framework has also been used to identify priority species for possible statutory listing, and a number of conservation status assessments are currently underway (see https://www.dcceew.gov.au/environment/biodiversity/threatened/seap). This fills an important gap, given that the current statutory listings markedly underestimate the number of plant species (and ecosystems) that are likely to be threatened (Alfonzetti et al. 2020). Auld et al. (2020) found that over 100 nationally threatened plants that occurred in NSW were likely to decline as a result of the 2019/2020 fires, with an additional 60 NSW endemic species listed as threatened either under the IUCN Red List of Threatened Species or the NSW Biodiversity Conservation Act also being likely to decline. These latter 60 species potentially need to be added to the national Environment Protection and Biodiversity Conservation Act threatened species list. Finally, some 230 NSW endemics that are not currently listed as threatened under any legislation were identified by Auld et al. (2020) as potentially declining because of the megafires. These species are candidates for possible threatened species listings, should the predicted impacts and threats to natural recovery result in population declines in response to the 2019/2020 fires.
Application to other priority conservation assets or functional groupings
As well as individual species and ecosystems, the framework criteria could be applied to a range of other priority conservation assets affected by megafires, including keystone species, refugia and key biodiversity areas (sensu IUCN 2016). For example, Gallagher et al. (2021) examined the spread of impacts for the 2019/2020 Australian megafires across different plant family groupings, highlighting high levels of impacts in three major families (Proteaceae, Fabaceae and Myrtaceae).
Recommended recovery actions
Despite uncertainties in assessments, in many cases the impacts of a number of key threats are sufficiently understood to recommend specific conservation actions for affected species and ecosystems (Table 4). Some actions are needed in the short term (0–2 years after fire), whereas others may require longer-term implementation (>5 years post-fire, Fig. 2). Key recovery actions should be identified for species or ecosystems that are most likely to decline or fail to recover after fire. Prioritisation and implementation of actions should recognise that fire plays a key role in the life history of many plant and animal species, and that the abundance of certain groups such as obligate seeders may fluctuate in response to fire as a consequence of their natural population dynamics (i.e. sustained population declines should be distinguished from population fluctuations, sensu IUCN 2022). Other species may recover vegetatively after a fire, but their detectability may be highest during post-fire flowering (e.g. pyrogenic species of terrestrial orchids), with many plants persisting below ground in the intervening period. In these cases, population trends can be difficult to detect and require monitoring designs that are cognisant of life-history dynamics.
Immediate post-fire actions
For species and ecosystems ranked with the potential for medium to high declines (Table 4), the primary recommendation is to undertake post-fire field surveys. These are essential to assess realised impacts and the degree of recovery, verify causes of decline inferred in the assessment, identify any emerging, previously unidentified threats to recovery, and to devise actions to mitigate the threats. This is particularly critical for species that have sites/populations affected by high fire frequency (Criterion II) and species sensitive to fire (Criterion I). For long-lived rainforest trees prone to top-kill and/or collapse from basal charring (Box 5), there is an urgent requirement to conduct field inspections within the first year post-fire so as to assess the scale of tree loss or damage and the rehabilitation actions required.
Development of risk-reduction strategies is essential if an understanding of impacts is to be translated into positive conservation outcomes for both species and ecosystems. Field surveys should inform the relative conservation priorities for recovery actions and the nature and timing of the actions needed for recovery (Fig. 2, Table 4, Box 5) as well as identifying any new or emerging threats. This is essential to modify and update proposed management actions as circumstances change, and to adjust priorities where required, so as to ensure the best possible investment of the limited resources available for post-fire recovery.
Medium- to long-term actions
Depending on the particular threats identified, actions required to assist recovery include the following:
-
Development of a fire management plan to reduce the likelihood of future fires burning over recovering sites (Criteria I–III). For example, avoiding additional fires (including hazard reduction burns) in all recently burnt habitat is necessary to allow time for recovery of species and ecosystems.
-
Protecting unburnt parts of a species’ range, including fire refugia (i.e. no burning or clearing or logging in that habitat) to provide insurance and avoid having the entire species’ range at risk from a future fire recurrence at the one time (i.e. avoid risks from Criterion IV for species) and to protect any refugia.
-
Avoid works that may affect surface or subsurface drainage or increase erosion risks (Criteria V and VI (species) or V, VI and VII (ecosystems)). In emergency situations, consider options for supplementary watering if post-fire drought conditions are evident (Criterion V).
-
Exclusion or control of invasive herbivores or predators (Criterion VIII).
-
Maintain suitable disease hygiene and mitigation (Criterion IX).
-
Post-fire weed control (Criterion X).
-
Minimise any localised site disturbances (Criterion XI).
-
Monitoring species’ recovery to determine the time required
-
-
– to replenish seed banks (especially in obligate seeders);
-
– for juveniles resprouting plants to become fire resistant; and
-
– for top-killed trees to recover their stature, structure and reproductive capacity.
-
Species or ecosystems that show limited post-fire recovery may require further interventions. These may include collections of seed or vegetative material for various conservation purposes, including restoration and translocation, reintroductions and establishing seed orchards (e.g. Bassett et al. 2015; Commander et al. 2018; Martyn Yenson et al. 2021, Box 15). For some species, emergency germplasm collection may be needed (Martyn Yenson et al. 2021), for example because of post-fire impacts of myrtle rust.
| Box 15. Seed collection and translocation as management responses |
| It is critically important to allow natural systems to recover after fire without intervention. Post-fire recovery can take months or years (and even longer for some species). The focus in the first 12 months after fires should be on eliminating threats to natural recovery rather than on translocation (which itself needs to be well planned and thought out and requires significant lead time, Commander et al. 2018). |
| When to use translocation |
If it can be demonstrated that species fail to recover effectively at a site or within an ecosystem after a fire, then consideration of translocation (seed addition or supplementary planting) may be necessary. Note that some fires may kill standing plants, yet not promote seedling recruitment post-fire. A soil seed bank may still be present and this needs to be considered in any assessment of whether translocation is required. Species with certain dormancy types (PD or MPD) may have post-fire germination delayed for over 12 months, depending on timing of fires and favourable conditions for recruitment. Decisions to proceed with translocation should be based on rigorous post-fire site assessments of recovery and should follow appropriate national guidelines on translocation (Commander et al. 2018). |
| Seed collection after fire – risks and benefits |
The resilience of many species to fire is dependent on the maintenance of persistent soil or canopy seed banks. Seed banks allow post-fire seedling recruitment and the size of the seed bank (along with fire-related factors such as heat and smoke) and post-fire rainfall, govern the magnitude of post-fire seedling recruitment. Canopy seed banks may be exhausted by a single fire (if all plants are burnt). Soil seed banks are likely to provide some buffer against successive fires because of residual seeds surviving in the soil after a fire (not all seeds will germinate), but soil seed banks too can be locally exhausted in a single fire (Auld and Denham 2006). For population persistence, seed banks need to be sufficiently replenished after a fire before the next fire occurs, otherwise decline will occur. The length of time required to replenish seed banks varies among species and is dependent on life-history attributes. As examples, some taxa have mass-flowering and fruiting soon after fire (e.g. Actinotus spp. (Kubiak 2009), Acacia suaveolens (Auld 1987)), whereas others may flower early but take 5–10 years to be large enough to produce sufficient seed to replenish their seed banks (e.g. Grevillea caleyi, Darwinia biflora (Auld and Scott 1997)). |
Seed collection (e.g. for ex situ conservation or other restoration activities) prior to adequate post-fire replenishment of in situ seed banks may limit species’ persistence capacity, especially because more frequent fires are predicted under a changing climate, along with a reduction in favourable windows for recruitment (interval squeeze of Enright et al. 2015). Consequently, seed collection should be limited for any species until its seed bank has been sufficiently replenished to enable population recovery in the event of a subsequent fire. Cases of urgent ex situ conservation may be an exception, and, in such cases, seed collection should be conducted in a way to minimise impacts on in situ seed-bank accumulation. |
Conclusions
Species and ecosystems that have a long evolutionary history of persisting under recurring fires have developed various mechanisms and strategies for recovering their populations or structure, function and composition. Their continued ability to do so is dependent on their tolerance to changing fire regimes, in combination with how other threatening processes compromise resistance, resilience or regenerative responses to the fire event. Here we have highlighted a novel mechanistic approach to rapidly predict the impacts of megafires on plant and ecosystem biodiversity. Key advances on unstructured methods of assessment include the incorporation of (1) fire-regime data (see Box 1; e.g. fire frequency, severity, season, type and fire patchiness), rather than fire extent alone, (2) species life histories, biology and ecosystem properties to define potential mechanisms of decline, and (3) interactions between fire events with biotic and abiotic threats to survival and recovery. The frameworks explicitly recognise that knowledge of the antecedent fire regime in combination with that of coincident biotic and abiotic threats in the landscape are required to predict and understand the impacts of megafire events on species and ecosystems. The frameworks help integrate and contextualise the interpretation of single fire events into fire-regime impacts.
The two assessment frameworks were applied to guide conservation responses to the Australian 2019/2020 mega-fires across southern Australia (Gallagher et al. 2021; Keith et al. 2020a, 2022b). They enabled rapid assessment of potential impacts of large fires on plant species and ecosystems, establishing immediate priorities for post-fire surveys, which are now guiding management and monitoring strategies, as well as new statutory listings to protect threatened species and ecosystems.
The post-fire assessment frameworks support rapid decision-making, in part because of their flexibility to utilise a combination of readily available remotely sensed data, detailed species-trait data, available literature and expert opinion. They allow for addition of further criteria should novel threats emerge that affect species and ecosystem recovery. A structured approach that leverages diverse sources of existing knowledge provides a way of informing prioritisation for resourcing of urgent and on-going field inspections and recovery actions.
Although the frameworks were developed as an immediate response to the unprecedented Australian 2019/2020 wildfires, the frameworks can be applied to any fire and any landscape, provided the combination of fire variables, life-history traits, threats to recovery and environmental variables are known or can be estimated or inferred. We highlight that post-fire surveys of prioritised species and ecosystems are essential to ensure that management priorities are robust and well supported, and to identify the most effective management responses. Finally, the frameworks highlight the need for development and maintenance of national and global plant databases to inform plant and ecosystem responses to fire and other threats.
Data availability
Data used in this study are available on request from the senior author (tony.auld@environment.nsw.gov.au).
Conflicts of interest
Mark Ooi is one of the current EiCs and Richard Williams is the previous EiC of the Australian Journal of Botany, but they were blinded from the peer-review process for this paper. The authors declare that they have no other conflicts of interest.
Declaration of funding
This research did not receive any specific funding.
Acknowledgements
Anonymous reviewers provided helpful comments on this manuscript. Rebecca Gibson and Jane De Gabriel kindly reviewed the manuscript. Nicola Auld sorted the referencing. Genevieve Wright and Geoff Robertson kindly offered insights drawn from their research on the population dynamics of Eucalyptus delegatensis.
References
Abram NJ, Henley BJ, Sen Gupta A, Lippmann TJR, Clarke H, Dowdy AJ, Sharples JJ, Nolan RH, Zhang T, Wooster MJ, Wurtzel JB, Meissner KJ, Pitman AJ, Ukkola AM, Murphy BP, Tapper NJ, Boer MM (2021) Connections of climate change and variability to large and extreme forest fires in southeast Australia. Communications Earth & Environment 2, 8| Connections of climate change and variability to large and extreme forest fires in southeast Australia.Crossref | GoogleScholarGoogle Scholar |
Airey Lauvaux C, Skinner CN, Taylor AH (2016) High severity fire and mixed conifer forest-chaparral dynamics in the southern Cascade Range, USA. Forest Ecology and Management 363, 74–85.
| High severity fire and mixed conifer forest-chaparral dynamics in the southern Cascade Range, USA.Crossref | GoogleScholarGoogle Scholar |
Akçakaya HR, Rodrigues ASL, Keith DA, Milner-Gulland EJ, Sanderson EW, Hedges S, Mallon DP, Grace MK, Long B, Meijaard E, Stephenson PJ (2020) Assessing ecological function in the context of species recovery. Conservation Biology 34, 561–571.
| Assessing ecological function in the context of species recovery.Crossref | GoogleScholarGoogle Scholar |
Alexander ME (1982) Calculating and interpreting forest fire intensities. Canadian Journal of Botany 60, 349–357.
| Calculating and interpreting forest fire intensities.Crossref | GoogleScholarGoogle Scholar |
Alfonzetti M, Rivers MC, Auld TD, Le Breton T, Cooney T, Stuart S, Zimmer H, Makinson R, Wilkins K, Delgado E, Dimitrova N, Gallagher RV (2020) Shortfalls in extinction risk assessments for plants. Australian Journal of Botany 68, 466–471.
| Shortfalls in extinction risk assessments for plants.Crossref | GoogleScholarGoogle Scholar |
Allen CD, Breshears DD, McDowell NG (2015) On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 6, art129
| On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene.Crossref | GoogleScholarGoogle Scholar |
Andela N, Morton DC, Giglio L, Paugam R, Chen Y, Hantson S, van der Werf GR, Randerson JT (2019) The global fire atlas of individual fire size, duration, speed and direction. Earth System Science Data 11, 529–552.
| The global fire atlas of individual fire size, duration, speed and direction.Crossref | GoogleScholarGoogle Scholar |
Andersen AN (1988) Immediate and longer-term effect of fire on seed predation by ants in sclerophyllous vegetation in south-easten Australia. Australian Journal of Ecology 13, 285–293.
| Immediate and longer-term effect of fire on seed predation by ants in sclerophyllous vegetation in south-easten Australia.Crossref | GoogleScholarGoogle Scholar |
Auld TD (1987) Population dynamics of the shrub Acacia suaveolens (Sm.) Willd.: survivorship throughout the life cycle, a synthesis. Australian Journal of Ecology 12, 139–151.
| Population dynamics of the shrub Acacia suaveolens (Sm.) Willd.: survivorship throughout the life cycle, a synthesis.Crossref | GoogleScholarGoogle Scholar |
Auld TD (1990) The survival of juvenile plants of the resprouting shrub Angophora hispida (Myrtaceae) after a simulated low-intensity fire. Australian Journal of Botany 38, 255–260.
| The survival of juvenile plants of the resprouting shrub Angophora hispida (Myrtaceae) after a simulated low-intensity fire.Crossref | GoogleScholarGoogle Scholar |
Auld TD, Denham AJ (2005) A technique to estimate the pre-fire depth of burial of Grevillea seeds by using seedlings after fire. Australian Journal of Botany 53, 401–405.
| A technique to estimate the pre-fire depth of burial of Grevillea seeds by using seedlings after fire.Crossref | GoogleScholarGoogle Scholar |
Auld TD, Denham AJ (2006) How much seed remains in the soil after a fire? Plant Ecology 187, 15–24.
| How much seed remains in the soil after a fire?Crossref | GoogleScholarGoogle Scholar |
Auld TD, O’Connell MA (1991) Predicting patterns of post-fire seed germination in 35 eastern Australian Fabaceae. Australian Journal of Ecology 16, 53–70.
| Predicting patterns of post-fire seed germination in 35 eastern Australian Fabaceae.Crossref | GoogleScholarGoogle Scholar |
Auld T, Scott J (1997) Conservation of endangered plants in urban fire-prone habitats. In ‘Fire effects on rare and endangered species and habitats conference’. Coeur D’Alene, Idaho, USA. (Ed. JM Greenlee) pp. 163–171. (International Association of Wildland Fire: Fairfield, WA, USA)
Auld TD, Scott J (2004) Estimating population abundance in plant species with dormant life-stages: fire and the endangered plant Grevillea caleyi R. Br. Ecological Management and Restoration 5, 125–129.
Auld TD, Mackenzie BDE, Le Breton T, Keith DA, Ooi MKJ, Allen S, Gallagher RV (2020) A preliminary assessment of the impact of the 2019/2020 fires on NSW plants of national significance. NSW Department of Planning, Industry and Environment, Australia.
Australian Government Bureau of Meteorology (2022) Climate Data Online. Available at http://www.bom.gov.au/climate/data [verified 25 July 2022]
Ayre DJ, Ottewell KM, Krauss SL, Whelan RJ (2009) Genetic structure of seedling cohorts following repeated wildfires in the fire-sensitive shrub Persoonia mollis ssp. nectens. Journal of Ecology 97, 752–760.
| Genetic structure of seedling cohorts following repeated wildfires in the fire-sensitive shrub Persoonia mollis ssp. nectens.Crossref | GoogleScholarGoogle Scholar |
Baird IRC, Benson D (2021) Population ecology of two endemic, fire-sensitive, Blue Mountains Banksia taxa (Proteaceae) in response to fire. Proceedings of the Linnean Society of New South Wales 143, 87–108.
Barker W, Keith D (2020) Hakea pachyphylla. In ‘The IUCN Red List of Threatened Species 2020: e.T117511628A129453427’. Available at https://dx.doi.org/10.2305/IUCN.UK.2020-2.RLTS.T117511628A129453427.en [Verified 18 January 2022]
Barrett S, Yates CJ (2015) Risks to a mountain summit ecosystem with endemic biota in southwestern Australia. Austral Ecology 40, 423–432.
| Risks to a mountain summit ecosystem with endemic biota in southwestern Australia.Crossref | GoogleScholarGoogle Scholar |
Baskin CC, Baskin JM (2014) ‘Seeds: ecology, biogeography, and evolution of dormancy and germination.’ 2nd edn. (Elsevier: San Diego, CA, USA)
Bassett OD, Prior LD, Slijkerman CM, Jamieson D, Bowman DMJS (2015) Aerial sowing stopped the loss of alpine ash (Eucalyptus delegatensis) forests burnt by three short-interval fires in the Alpine National Park, Victoria, Australia. Forest Ecology and Management 342, 39–48.
| Aerial sowing stopped the loss of alpine ash (Eucalyptus delegatensis) forests burnt by three short-interval fires in the Alpine National Park, Victoria, Australia.Crossref | GoogleScholarGoogle Scholar |
Benson DH (1985) Aspects of the ecology of a rare tree species, Eucalyptus benthamii, at Bents Basin, Wallacia. Cunninghamia 1, 371–383.
Benson J, Allen C (2007) Vegetation associated with Wollemia nobilis (Araucariaceae). Cunninghamia 10, 255–262.
Benson D, McDougall L (1994) Ecology of Sydney plants species Part 2: Dicotyledon families Asteraceae to Buddlejaceae. Cunninghamia 3, 789–995.
Benson D, McDougall L (2000) Ecology of Sydney plants species Part 7b: Dicotyledon families Proteaceae to Rubiaceae. Cunninghamia 6, 1016–1202.
Bernhardt-Römermann M, Poschlod P, Hentschel J (2018) BryForTrait – A life-history trait database of forest bryophytes. Journal of Vegetation Science 29, 798–800.
| BryForTrait – A life-history trait database of forest bryophytes.Crossref | GoogleScholarGoogle Scholar |
Bird RB, Taylor N, Codding BF, Bird DW (2013) Niche construction and dreaming logic: aboriginal patch mosaic burning and varanid lizards (Varanus gouldii) in Australia. Proceedings of the Royal Society B 280, 20132297
| Niche construction and dreaming logic: aboriginal patch mosaic burning and varanid lizards (Varanus gouldii) in Australia.Crossref | GoogleScholarGoogle Scholar |
Bird RB, Bird DW, Codding BF (2016) People, El Niño southern oscillation and fire in Australia: fire regimes and climate controls in hummock grasslands. Philosophical Transactions of the Royal Society B: Biological Sciences 371, 20150343
| People, El Niño southern oscillation and fire in Australia: fire regimes and climate controls in hummock grasslands.Crossref | GoogleScholarGoogle Scholar |
Bland LM, Keith DA, Miller RM, Murray NJ, Rodríguez JP (Eds) (2017) ‘Guidelines for the application of IUCN Red List of Ecosystems Categories and Criteria, Version 1.1’. (IUCN: Gland, Switzerland)
Bliss A, Prior LD, Bowman DMJS (2021) Lack of reliable post-fire recovery mechanisms makes the iconic Tasmanian conifer Athrotaxis cupressoides susceptible to population decline. Australian Journal of Botany 69, 162–173.
| Lack of reliable post-fire recovery mechanisms makes the iconic Tasmanian conifer Athrotaxis cupressoides susceptible to population decline.Crossref | GoogleScholarGoogle Scholar |
Boer MM, Resco de Dios V, Bradstock RA (2020) Unprecedented burn area of Australian mega forest fires. Nature Climate Change 10, 171–172.
| Unprecedented burn area of Australian mega forest fires.Crossref | GoogleScholarGoogle Scholar |
Bond WJ, van Wilgen BW (1996) ‘Fire and plants.’ (Springer Science & Business Media)
Bond WJ, Honig M, Maze KE (1999) Seed size and seedling emergence: an allometric relationship and some ecological implications. Oecologia 120, 132–136.
| Seed size and seedling emergence: an allometric relationship and some ecological implications.Crossref | GoogleScholarGoogle Scholar |
Bowman DMJS (2000) ‘Australian rainforests: islands of green in a land of fire.’ (Cambridge University Press: Cambridge, UK)
Bowman DMJS, Bliss A, Bowman CJW, Prior LD (2019) Fire caused demographic attrition of the Tasmanian palaeoendemic conifer Athrotaxis cupressoides. Austral Ecology 44, 1322–1339.
| Fire caused demographic attrition of the Tasmanian palaeoendemic conifer Athrotaxis cupressoides.Crossref | GoogleScholarGoogle Scholar |
Bowman DMJS, Kolden CA, Abatzoglou JT, Johnston FH, van der Werf GR, Flannigan M (2020) Vegetation fires in the Anthropocene. Nature Reviews Earth & Environment 1, 500–515.
| Vegetation fires in the Anthropocene.Crossref | GoogleScholarGoogle Scholar |
Bradstock RA (2008) Effects of large fires on biodiversity in south-eastern Australia: disaster or template for diversity? International Journal of Wildland Fire 17, 809–822.
| Effects of large fires on biodiversity in south-eastern Australia: disaster or template for diversity?Crossref | GoogleScholarGoogle Scholar |
Bradstock RA (2010) A biogeographic model of fire regimes in Australia: current and future implications. Global Ecology and Biogeography 19, 145–158.
| A biogeographic model of fire regimes in Australia: current and future implications.Crossref | GoogleScholarGoogle Scholar |
Bradstock RA, Myerscough PJ (1988) The survival and population response to frequent fires of two woody resprouters Banksia serrata and Isopogon anemonifolius. Australian Journal of Botany 36, 415–431.
| The survival and population response to frequent fires of two woody resprouters Banksia serrata and Isopogon anemonifolius.Crossref | GoogleScholarGoogle Scholar |
Bradstock RA, Gill AM, Hastings SM, Moore PHR (1994) Survival of serotinous seedbanks during bushfires: comparative studies of Hakea species from southeastern Australia. Australian Journal of Ecology 19, 276–282.
| Survival of serotinous seedbanks during bushfires: comparative studies of Hakea species from southeastern Australia.Crossref | GoogleScholarGoogle Scholar |
Bradstock RA, Williams JE, Gill MA (2002) ‘Flammable Australia: the fire regimes and biodiversity of a continent.’ (Cambridge University Press: Cambridge, UK)
Bradstock RA, Bedward M, Gill AM, Cohn JS (2005) Which mosaic? A landscape ecological approach for evaluating interactions between fire regimes, habitat and animals. Wildlife Research 32, 409–423.
| Which mosaic? A landscape ecological approach for evaluating interactions between fire regimes, habitat and animals.Crossref | GoogleScholarGoogle Scholar |
Bradstock RA, Gill AM, Williams RJ (2012) ‘Flammable Australia: fire regimes, biodiversity and ecosystems in a changing world.’ (CSIRO Publishing: Melbourne, Vic., Australia)
Bredenkamp G, Chytrý M, Fischer HS, Neuhäuslová Z, van der Maarel E (1998) Vegetation mapping: theory, methods and case studies: introduction. Applied Vegetation Science 1, 162–164.
| Vegetation mapping: theory, methods and case studies: introduction.Crossref | GoogleScholarGoogle Scholar |
Bullock S, Summerell BA, Gunn LV (2000) Pathogens of the Wollemi Pine, Wollemia nobilis. Australasian Plant Pathology 29, 211–214.
| Pathogens of the Wollemi Pine, Wollemia nobilis.Crossref | GoogleScholarGoogle Scholar |
Burgman MA, Keith DA, Rohlf FJ, Todd CR (1999) Probabilistic classification rules for setting conservation priorities. Biological Conservation 89, 227–231.
| Probabilistic classification rules for setting conservation priorities.Crossref | GoogleScholarGoogle Scholar |
Cahill DM, Rookes JE, Wilson BA, Gibson L, McDougall KL (2008) Phytophthora cinnamomi and Australia’s biodiversity: impacts, predictions and progress towards control. Australian Journal of Botany 56, 279–310.
| Phytophthora cinnamomi and Australia’s biodiversity: impacts, predictions and progress towards control.Crossref | GoogleScholarGoogle Scholar |
Canadell JG, Meyer CP, Cook GD, Dowdy A, Briggs PR, Knauer J, Pepler A, Haverd V (2021) Multi-decadal increase of forest burned area in Australia is linked to climate change. Nature Communications 12, 6921
| Multi-decadal increase of forest burned area in Australia is linked to climate change.Crossref | GoogleScholarGoogle Scholar |
Carter O, Walsh N (2010) ‘National recovery plan for the Dwarf Kerrawang (Rulingia prostrata).’ (Department of Sustainability and Environment, Melbourne) Available at https://www.awe.gov.au/sites/default/files/documents/rulingia-prostrata.pdf [verified 25 January 2022]
Cary GJ, Bradstock RA, Gill AM, Williams RJ (2012) Global change and fire regimes in Australia. In ‘Flammable Australia: fire regimes, biodiversity and ecosystems in a changing world’. (Eds RA Bradstock, AM Gill, RJ Williams) pp. 149–169. (CSIRO Publishing: Melbourne, Vic., Australia)
Cavieres LA, Sierra-Almeida A (2018) Assessing the importance of cold-stratification for seed germination in alpine plant species of the High-Andes of central Chile. Perspectives in Plant Ecology, Evolution and Systematics 30, 125–131.
| Assessing the importance of cold-stratification for seed germination in alpine plant species of the High-Andes of central Chile.Crossref | GoogleScholarGoogle Scholar |
Chamberlain SA, Szöcs E (2013) Taxize: taxonomic search and retrieval in R. F1000Research 2, 191
| Taxize: taxonomic search and retrieval in R.Crossref | GoogleScholarGoogle Scholar |
Choat B, Brodribb TJ, Brodersen CR, Duursma RA, López R, Medlyn BE (2018) Triggers of tree mortality under drought. Nature 558, 531–539.
| Triggers of tree mortality under drought.Crossref | GoogleScholarGoogle Scholar |
Clarke PJ, Knox KJ, Campbell ML, Copeland LM (2009) Post-fire recovery of woody plants in the New England Tableland Bioregion. Cunninghamia 11, 221–239.
Clarke PJ, Knox KJE, Butler D (2010) Fire intensity, serotiny and seed release in 19 woody species: evidence for risk spreading among wind-dispersed and resprouting syndromes. Australian Journal of Botany 58, 629–636.
| Fire intensity, serotiny and seed release in 19 woody species: evidence for risk spreading among wind-dispersed and resprouting syndromes.Crossref | GoogleScholarGoogle Scholar |
Clarke PJ, Lawes MJ, Murphy BP, Russell-Smith J, Nano CEM, Bradstock R, Enright NJ, Fontaine JB, Gosper CR, Radford I, Midgley JJ, Gunton RM (2015) A synthesis of postfire recovery traits of woody plants in Australian ecosystems. Science of The Total Environment 534, 31–42.
| A synthesis of postfire recovery traits of woody plants in Australian ecosystems.Crossref | GoogleScholarGoogle Scholar |
Collette JC, Ooi MKJ (2021) Distribution of seed dormancy classes across a fire-prone continent: effects of rainfall seasonality and temperature. Annals of Botany 127, 613–620.
| Distribution of seed dormancy classes across a fire-prone continent: effects of rainfall seasonality and temperature.Crossref | GoogleScholarGoogle Scholar |
Collins L, Bradstock RA, Clarke H, Clarke MF, Nolan RH, Penman TD (2021) The 2019/2020 mega-fires exposed Australian ecosystems to an unprecedented extent of high-severity fire. Environmental Research Letters 16, 044029
| The 2019/2020 mega-fires exposed Australian ecosystems to an unprecedented extent of high-severity fire.Crossref | GoogleScholarGoogle Scholar |
Commander LE, Coates D, Broadhurst L, Offord CA, Makinson RO, Matthes M (2018) ‘Guidelines for the translocation of threatened plants in Australia.’ (Australian Network for Plant Conservation)
Davey SM, Sarre A (2020) Editorial: the 2019/20 Black Summer bushfires. Australian Forestry 83, 47–51.
| Editorial: the 2019/20 Black Summer bushfires.Crossref | GoogleScholarGoogle Scholar |
De Kauwe MG, Medlyn BE, Ukkola AM, Mu M, Sabot MEB, Pitman AJ, Meir P, Cernusak LA, Rifai SW, Choat B, Tissue DT, Blackman CJ, Li X, Roderick M, Briggs PR (2020) Identifying areas at risk of drought-induced tree mortality across South-Eastern Australia. Global Change Biology 26, 5716–5733.
| Identifying areas at risk of drought-induced tree mortality across South-Eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Denham AJ, Auld TD (2012) Population ecology of waratahs, Telopea speciosissima (Proteaceae): implications for management of fire-prone habitats. Proceedings of the Linnean Society of New South Wales 134, B101–B111.
Denham AJ, Vincent BE, Clarke PJ, Auld TD (2016) Responses of tree species to a severe fire indicate major structural change to Eucalyptus–Callitris forests. Plant Ecology 217, 617–629.
| Responses of tree species to a severe fire indicate major structural change to Eucalyptus–Callitris forests.Crossref | GoogleScholarGoogle Scholar |
Department of Agriculture, Water and the Environment (2020) National indicative aggregated fire extent datasets. Available at https://www.environment.gov.au/fed/catalog/search/resource/details.page?uuid=%7B9ACDCB09-0364-4FE8-9459-2A56C792C743%7D [verified 21 January 2022]
Department of Agriculture, Water and the Environment (2022) Fire regimes that cause declines in biodiversity as a key threatening process. Department of Agriculture, Water and the Environment, Canberra. April. CC BY 4.0. Available at https://www.awe.gov.au/environment/biodiversity/threatened/key-threatening-processes [verified 12 August 2022]
Department of the Environment (2014) Approved Conservation Advice Leionema ralstonii. Available at http://www.environment.gov.au/biodiversity/threatened/species/pubs/64926-conservation-advice.pdf [verified 21 January 2022]
Department of the Environment, Water, Heritage and the Arts (2008a) Approved Conservation Advice for Budawangia gnidioides (Budawangs cliff-heath). Available at http://www.environment.gov.au/biodiversity/threatened/species/pubs/55850-conservation-advice.pdf [verified 21 January 2022]
Department of the Environment, Water, Heritage and the Arts (2008b) Approved Conservation Advice for Bossiaea oligosperma. Available at http://www.environment.gov.au/biodiversity/threatened/species/pubs/10059-conservation-advice.pdf [verified 21 January 2022]
Eldridge DJ, Travers SK, Val J, Zaja A, Veblen KE (2019) Horse activity is associated with degraded subalpine grassland structure and reduced habitat for a threatened rodent. Rangeland Ecology and Management 72, 467–473.
| Horse activity is associated with degraded subalpine grassland structure and reduced habitat for a threatened rodent.Crossref | GoogleScholarGoogle Scholar |
Emery NJ, Offord CA (2018) Managing Persoonia (Proteaceae) species in the landscape through a better understanding of their seed biology and ecology. Cunninghamia 18, 89–107.
Enright NJ, Fontaine JB, Bowman DMJS, Bradstock RA, Williams RJ (2015) Interval squeeze: altered fire regimes and demographic responses interact to threaten woody species persistence as climate changes. Frontiers in Ecology and the Environment 13, 265–272.
| Interval squeeze: altered fire regimes and demographic responses interact to threaten woody species persistence as climate changes.Crossref | GoogleScholarGoogle Scholar |
Etchells H, O’Donnell AJ, McCaw WL, Grierson PF (2020) Fire severity impacts on tree mortality and post-fire recruitment in tall eucalypt forests of southwest Australia. Forest Ecology and Management 459, 117850
| Fire severity impacts on tree mortality and post-fire recruitment in tall eucalypt forests of southwest Australia.Crossref | GoogleScholarGoogle Scholar |
Falster D, Gallagher R, Wenk EH, et al. (2021) AusTraits, a curated plant trait database for the Australian flora. Scientific Data 8, 254
| AusTraits, a curated plant trait database for the Australian flora.Crossref | GoogleScholarGoogle Scholar |
Fensham RJ, Fairfax RJ, Archer SR (2005) Rainfall, land use and woody vegetation cover change in semi-arid Australian savanna. Journal of Ecology 93, 596–606.
| Rainfall, land use and woody vegetation cover change in semi-arid Australian savanna.Crossref | GoogleScholarGoogle Scholar |
Fernández-Pascual E, Carta A, Mondoni A, Cavieres L, Rosbakh S, Venn S, Satyanti A, Guja L, Briceño VF, Vandelook F, Mattana E, Saatkamp A, Bu H, Sommerville K, Poschlod P, Liu K, Nicotra A, Jiménez-Alfaro B (2021) The seed germination spectrum of alpine plants: a global meta-analysis. New Phytologist 229, 3573–3586.
| The seed germination spectrum of alpine plants: a global meta-analysis.Crossref | GoogleScholarGoogle Scholar |
Forster P, Ford A, Griffith S, Benwell A (2020) Eidothea hardeniana. In ‘The IUCN Red List of Threatened Species 2020: e.T112631200A113309140’. Available at https://dx.doi.org/10.2305/IUCN.UK.2020-2.RLTS.T112631200A113309140.en [verified 19 January 2022]
Gallagher RV (2020) ‘National prioritisation of Australian plants affected by the 2019–2020 bushfire season.’ (Commonwealth Department of Agriculture, Water and Environment: Canberra, ACT, Australia) Available at https://www.dcceew.gov.au/sites/default/files/env/pages/289205b6-83c5-480c-9a7d-3fdf3cde2f68/files/final-national-prioritisation-australian-plants-affected-2019-2020-bushfire-season.pdf [verified 24 January 2022]
Gallagher RV, Allen S, Mackenzie BDE, Yates CJ, Gosper CR, Keith DA, Merow C, White MD, Wenk E, Maitner BS, He K, Adams VM, Auld TD (2021) High fire frequency and the impact of the 2019–2020 megafires on Australian plant diversity. Diversity and Distributions 27, 1166–1179.
| High fire frequency and the impact of the 2019–2020 megafires on Australian plant diversity.Crossref | GoogleScholarGoogle Scholar |
Gallagher RV, Allen SP, Mackenzie BDE, Keith DA, Nolan RH, Rumpff L, Gosper CR, Pegg G, van Leeuwen S, Ooi MKJ, Yates CJ, Merow C, Williams RJ, Nikolopoulos EI, Beaumont LJ, Auld TD (2022) An integrated approach to assessing abiotic and biotic threats to post-fire plant species recovery: lessons from the 2019–2020 Australian fire season. Global Ecology and Biogeography 31, 2056–2069.
| An integrated approach to assessing abiotic and biotic threats to post-fire plant species recovery: lessons from the 2019–2020 Australian fire season.Crossref | GoogleScholarGoogle Scholar |
George A (1996) Notes on Banksia Lf (Proteaceae). Nuytsia 11, 21–24.
Gibson R, Danaher T, Hehir W, Collins L (2020) A remote sensing approach to mapping fire severity in south-eastern Australia using sentinel 2 and random forest. Remote Sensing of Environment 240, 111702
| A remote sensing approach to mapping fire severity in south-eastern Australia using sentinel 2 and random forest.Crossref | GoogleScholarGoogle Scholar |
Giljohann KM, McCarthy MA, Keith DA, Kelly LT, Tozer MG, Regan TJ (2017) Interactions between rainfall, fire and herbivory drive resprouter vital rates in a semi-arid ecosystem. Journal of Ecology 105, 1562–1570.
| Interactions between rainfall, fire and herbivory drive resprouter vital rates in a semi-arid ecosystem.Crossref | GoogleScholarGoogle Scholar |
Gill AM (1975) Fire and the Australian flora: a review. Australian Forestry Journal 38, 4–25.
| Fire and the Australian flora: a review.Crossref | GoogleScholarGoogle Scholar |
Gill AM, Bradstock RA (1992) A national register for the fire responses of plant species. Cunninghamia 2, 653–660.
Gill AM, Groves RH, Noble IR (1981) ‘Fire and the Australian biota.’ (Australian Academy of Science: Canberra, ACT, Australia)
Godfree RC, Knerr N, Encinas-Viso F, Albrecht D, Bush D, Cargill DC, Clements M, Gueidan C, Guja LK, Harwood T, Joseph L, Lepschi B, Nargar K, Schmidt-Lebuhn A, Broadhurst LM (2021) Implications of the 2019–2020 megafires for the biogeography and conservation of Australian vegetation. Nature Communications 12, 1023
| Implications of the 2019–2020 megafires for the biogeography and conservation of Australian vegetation.Crossref | GoogleScholarGoogle Scholar |
Gosper CR, Yates CJ, Cook GD, Harvey JM, Liedloff AC, McCaw WL, Thiele KR, Prober SM (2018) A conceptual model of vegetation dynamics for the unique obligate-seeder eucalypt woodlands of south-western Australia. Austral Ecology 43, 681–695.
| A conceptual model of vegetation dynamics for the unique obligate-seeder eucalypt woodlands of south-western Australia.Crossref | GoogleScholarGoogle Scholar |
Haque MM, Nipperess DA, Gallagher RV, Beaumont LJ (2017) How well documented is Australia’s flora? Understanding spatial bias in vouchered plant specimens. Austral Ecology 42, 690–699.
| How well documented is Australia’s flora? Understanding spatial bias in vouchered plant specimens.Crossref | GoogleScholarGoogle Scholar |
Heberling JM, Isaac BL (2017) Herbarium specimens as exaptations: new uses for old collections. American Journal of Botany 104, 963–965.
| Herbarium specimens as exaptations: new uses for old collections.Crossref | GoogleScholarGoogle Scholar |
IUCN (2012) ‘Guidelines for Application of IUCN Red List Criteria at Regional and National Levels: Version 4.0.’ (IUCN: Gland, Switzerland) Available at https://portals.iucn.org/library/sites/library/files/documents/RL-2012-002.pdf [verified 24 January 2022]
IUCN (2016) ‘A Global Standard for the Identification of Key Biodiversity Areas, Version 1.0.’ 1st edn. (International Union for Conservation of Nature: Gland, Switzerland) Available at https://portals.iucn.org/library/sites/library/files/documents/2016-048.pdf [verified 25 January 2022]
IUCN (2022) ‘Guidelines for Using the IUCN Red List Categories and Criteria, Version 15.0’. (Standards and Petitions Subcommittee of the IUCN Species Survival Commission: Gland, Switzerland) Available at https://www.iucnredlist.org/resources/redlistguidelines [verified 18 July 2022]
Iversen C, Powell A, McCormack M, Blackwood C, Freschet G, Kattge J, Roumet C, Stover D, Soudzilovskaia N, Valverde-Barrantes O (2017) Fine-Root Ecology Database (FRED): a global collection of root trait data with coincident site, vegetation, edaphic, and climatic data, version 1. ORNLTESSFA (Oak Ridge National Lab’s Terrestrial Ecosystem Science Scientific Focus Area (ORNL TES SFA))
Jones DA, Wang W, Fawcett R (2009) High-Quality spatial climate data-sets for Australia. Australian Meteorological and Oceanographic Journal 58, 233–248.
Jones W, Hill KD, Allen JM (1995) Wollemia nobilis, a new living Australian genus and species in the Araucariaceae. Telopea 6, 173–176.
| Wollemia nobilis, a new living Australian genus and species in the Araucariaceae.Crossref | GoogleScholarGoogle Scholar |
Judd TS, Ashton DH (1991) Fruit clustering in the Myrtaceae: seed survival in capsules subjected to experimental heating. Australian Journal of Botany 39, 241–245.
| Fruit clustering in the Myrtaceae: seed survival in capsules subjected to experimental heating.Crossref | GoogleScholarGoogle Scholar |
Kattge J, Bönisch G, Díaz S, Lavorel S, Prentice IC, Leadley P, Tautenhahn S, Werner GD, Aakala T, Abedi M, et al. (2020) TRY plant trait database – enhanced coverage and open access. Global Change Biology 26, 119–188.
| TRY plant trait database – enhanced coverage and open access.Crossref | GoogleScholarGoogle Scholar |
Keeley JE (2009) Fire intensity, fire severity and burn severity: a brief review and suggested usage. International Journal of Wildland Fire 18, 116–126.
| Fire intensity, fire severity and burn severity: a brief review and suggested usage.Crossref | GoogleScholarGoogle Scholar |
Keeley JE, Brennan TJ (2012) Fire-driven alien invasion in a fire-adapted ecosystem. Oecologia 169, 1043–1052.
| Fire-driven alien invasion in a fire-adapted ecosystem.Crossref | GoogleScholarGoogle Scholar |
Keeley JE, Pausas JG (2019) Distinguishing disturbance from perturbations in fire-prone ecosystems. International Journal of Wildland Fire 28, 282–287.
| Distinguishing disturbance from perturbations in fire-prone ecosystems.Crossref | GoogleScholarGoogle Scholar |
Keeley JE, Syphard AD (2021) Large California wildfires: 2020 fires in historical context. Fire Ecology 17, 22
| Large California wildfires: 2020 fires in historical context.Crossref | GoogleScholarGoogle Scholar |
Keith D (1996) Fire-driven extinction of plant populations: a synthesis of theory and review of evidence from Australian vegetation. Proceedings of the Linnean Society of New South Wales 116, 37–78.
Keith D (2009) Red listing: deciding which species are at risk. Significance 6, 100–104.
| Red listing: deciding which species are at risk.Crossref | GoogleScholarGoogle Scholar |
Keith DA (2012) Functional traits: their roles in understanding and predicting biotic responses to fire regimes from individuals to landscapes. In ‘Flammable Australia: fire regimes, biodiversity and ecosystems in a changing world’. (Eds RA Bradstock, AM Gill, RJ Williams) pp. 97–125. (CSIRO Publishing: Melbourne, Vic., Australia)
Keith DA, Bradstock RA (1994) Fire and competition in Australian heath: a conceptual model and field investigations. Journal of Vegetations Science 5, 347–354.
| Fire and competition in Australian heath: a conceptual model and field investigations.Crossref | GoogleScholarGoogle Scholar |
Keith DA, Tozer MG (2017) Girt: a continental synthesis of Australian Vegetation. In ‘Australian vegetation’. (Ed. DA Keith) pp. 3–39. (Cambridge University Press: Cambridge, UK)
Keith DA, Tozer MG, Regan TJ, Regan HM (2007) The persistence niche: what makes it and what breaks it for two fire-prone plant species. Australian Journal of Botany 55, 273–279.
| The persistence niche: what makes it and what breaks it for two fire-prone plant species.Crossref | GoogleScholarGoogle Scholar |
Keith DA, Lindenmayer D, Lowe A, Russell-Smith J, Barrett S, Enright NJ, Fox BJ, Guerin G, Paton DC, Tozer MG (2014) Heathlands. In ‘Biodiversity and environmental change: monitoring, challenges and direction’. (Eds D Lindenmayer, E Burns, N Thurgate, A Lowe) pp. 215–285. (CSIRO Publishing: Melbourne, Vic., Australia)
Keith DA, Akçakaya HR, Murray NJ (2018) Scaling range sizes to threats for robust predictions of risks to biodiversity. Conservation Biology 32, 322–332.
| Scaling range sizes to threats for robust predictions of risks to biodiversity.Crossref | GoogleScholarGoogle Scholar |
Keith D, Auld T, Barrett S, English V, Gallagher R, Gray R, van Leeuwin S, McIlwee A, Mitchell D, Tozer M, Williams D, Yates C, Neldner J, Buchan A, White MD, Rogers D, West A, Simpson C (2020a) Ecological Communities: priorities for listing assessments. Unpublished report to expert panel for bushfire recovery.
Keith DA, Benson DH, Krogh M, Watts L, Simpson CC, Mason TL (2020b) Newnes Plateau Shrub Swamp: monitoring responses to the 2019-2020 bushfires and interactions with other threatening processes. Centre for Ecosystem Science, University of NSW, Sydney, NSW, Australia.
Keith DA, Dunker B, Driscoll DA (2020c) Dispersal: the eighth fire seasonality effect on plants. Trends in Ecology & Evolution 35, 305–307.
| Dispersal: the eighth fire seasonality effect on plants.Crossref | GoogleScholarGoogle Scholar |
Keith DA, Ferrer-Paris JR, Nicholson E, Kingsford RT (2020d) ‘IUCN Global Ecosystem Typology 2.0. Descriptive profiles for biomes and ecosystem functional groups.’ (IUCN: Gland, Switzerland)
Keith DA, Benson DH, Baird IRC, Watts L, Simpson CC, Krogh M, Gorissen S, Ferrer-Paris JR, Mason TJ (2022a) Interactions between anthropogenic stressors and recurring perturbations mediate ecosystem resilience or collapse. Conservation Biology,
Keith DA, Allen SP, Gallagher RV, Mackenzie BDE, Auld TD, Barrett S, Buchan A, English V, Gosper C, Kelly D, McIllwee A, Melrose RT, Miller BP, Neldner VJ, Simpson CC, Tolsma AD, Rogers D, van Leeuwen S, White MD, Yates CJ, Tozer MG (2022b) Fire-related threats and transformational change in Australian ecosystems. Global Ecology and Biogrography 31, 2070–2084.
| Fire-related threats and transformational change in Australian ecosystems.Crossref | GoogleScholarGoogle Scholar |
Kelly LT, Giljohann KM, Duane A, Aquilué N, Archibald S, Batllori E, Bennett AF, Buckland ST, Canelles Q, Clarke MF, et al. (2020) Fire and biodiversity in the Anthropocene. Science 370, eabb0355
| Fire and biodiversity in the Anthropocene.Crossref | GoogleScholarGoogle Scholar |
Kemp EM (1981) Pre-Quaternary fire in Australia. In ‘Fire and the Australian biota’. (Eds AM Gill, RH Groves, IR Noble) pp. 3–21. (Australian Academy of Science: Canberra, ACT, Australia)
Kirkpatrick JB, Bridle KL, Dickinson KJM (2010) Decades-scale vegetation change in burned and unburned alpine coniferous heath. Australian Journal of Botany 58, 453–462.
| Decades-scale vegetation change in burned and unburned alpine coniferous heath.Crossref | GoogleScholarGoogle Scholar |
Kleyer M, Bekker RM, Knevel IC, Bakker JP, Thompson K, Sonnenschein M, Poschlod P, Van Groenendael JM, Klimeš L, Klimešová J, et al. (2008) The LEDA Traitbase: a database of life-history traits of the Northwest European flora. Journal of Ecology 96, 1266–1274.
| The LEDA Traitbase: a database of life-history traits of the Northwest European flora.Crossref | GoogleScholarGoogle Scholar |
Knight TM, Holt RD (2005) Fire generates spatial gradients in herbivory: an example from a Florida sandhill ecosystem. Ecology 86, 587–593.
| Fire generates spatial gradients in herbivory: an example from a Florida sandhill ecosystem.Crossref | GoogleScholarGoogle Scholar |
Kubiak P (2009) Fire responses of bushland plants after the January 1994 wildfires in northern Sydney. Cunninghamia 11, 131–165.
Lamont BB, He T, Yan Z (2019) Evolutionary history of fire-stimulated resprouting, flowering, seed release and germination. Biological Reviews 94, 903–928.
| Evolutionary history of fire-stimulated resprouting, flowering, seed release and germination.Crossref | GoogleScholarGoogle Scholar |
Lawes MJ, Midgley JJ, Clarke PJ (2013) Costs and benefits of relative bark thickness in relation to fire damage: a savanna/forest contrast. Journal of Ecology 101, 517–524.
| Costs and benefits of relative bark thickness in relation to fire damage: a savanna/forest contrast.Crossref | GoogleScholarGoogle Scholar |
Legge S, Woinarski JCZ, Scheele BC, Garnett ST, Lintermans M, Nimmo DG, Whiterod NS, Southwell DM, Ehmke G, Buchan A, et al. (2022) Rapid assessment of the biodiversity impacts of the 2019–2020 Australian megafires to guide urgent management intervention and recovery and lessons for other regions. Diversity and Distributions 28, 571–591.
| Rapid assessment of the biodiversity impacts of the 2019–2020 Australian megafires to guide urgent management intervention and recovery and lessons for other regions.Crossref | GoogleScholarGoogle Scholar |
Leigh JH, Wimbush DJ, Wood DH, Holgate MD, Slee AV, Stanger MG, Forrester RI (1987) Effects of rabbit grazing and fire on a sub-alpine environment. I. Herbaceous and shrubby vegetation. Australian Journal of Botany 35, 433–464.
| Effects of rabbit grazing and fire on a sub-alpine environment. I. Herbaceous and shrubby vegetation.Crossref | GoogleScholarGoogle Scholar |
Lindenmayer DB, Taylor C (2020) New spatial analyses of Australian wildfires highlight the need for new fire, resource, and conservation policies. Proceedings of the National Academy of Sciences of the United States of America 117, 12481–12485.
| New spatial analyses of Australian wildfires highlight the need for new fire, resource, and conservation policies.Crossref | GoogleScholarGoogle Scholar |
Lindenmayer DB, Hobbs RJ, Likens GE, Krebs CJ, Banks SC (2011) Newly discovered landscape traps produce regime shifts in wet forests. Proceedings of the National Academy of Sciences of the United States of America 108, 15887–15891.
| Newly discovered landscape traps produce regime shifts in wet forests.Crossref | GoogleScholarGoogle Scholar |
Lindenmayer DB, Blanchard W, McBurney L, Blair D, Banks SC, Driscoll D, Smith AL, Gill AM (2013) Fire severity and landscape context effects on arboreal marsupials. Biological Conservation 167, 137–148.
| Fire severity and landscape context effects on arboreal marsupials.Crossref | GoogleScholarGoogle Scholar |
Lunt ID, Zimmer HC, Cheal DC (2011) The tortoise and the hare? Post-fire regeneration in mixed EucalyptusCallitris forest. Australian Journal of Botany 59, 575–581.
| The tortoise and the hare? Post-fire regeneration in mixed EucalyptusCallitris forest.Crossref | GoogleScholarGoogle Scholar |
Lynch AH, Beringer J, Kershaw P, Marshall A, Mooney S, Tapper N, Turney C, Van Der Kaars S (2007) Using the paleorecord to evaluate climate and fire interactions in Australia. Annual Review of Earth and Planetary Sciences 35, 215–239.
| Using the paleorecord to evaluate climate and fire interactions in Australia.Crossref | GoogleScholarGoogle Scholar |
Mackenzie BDE, Auld TD (in press) Wollemia nobilis. In ‘The IUCN Red List of Threatened Species 2022’. Available at https://www.iucnredlist.org
Mackenzie BDE, Keith DA (2009) Adaptive management in practice: conservation of a threatened plant population. Ecological Management & Restoration 10, S129–S135.
| Adaptive management in practice: conservation of a threatened plant population.Crossref | GoogleScholarGoogle Scholar |
Mackenzie BDE, Clarke SW, Zimmer HC, Liew ECY, Phelan MT, Offord CA, Menke LK, Crust DW, Bragg J, McPherson H, Rossetto M, Coote DM, Yap J-YS, Auld TD (2021) Ecology and conservation of a living fossil: Australia’s Wollemi Pine (Wollemia nobilis). In ‘Imperiled: the encyclopedia of conservation. Vol. 1’. (Eds D DiPaolo, J Villella) pp. 884–894. (Elsevier) https://doi.org/10.1016/b978-0-12-821139-7.00188-4
Maitner BS, Boyle B, Casler N, et al. (2018) The BIEN R package: a tool to access the Botanical Information and Ecology Network (BIEN) database. Methods in Ecology and Evolution 9, 373–379.
| The BIEN R package: a tool to access the Botanical Information and Ecology Network (BIEN) database.Crossref | GoogleScholarGoogle Scholar |
Martyn Yenson AJ, Offord CA, Meagher PF, Auld T, Bush D, Coates DJ, Commander LE, Guja LK, Norton SL, Makinson RO, Stanley R, Walsh N, Wrigley D, Broadhurst L (2021) ‘Plant germplasm conservation in Australia: strategies and guidelines for developing, managing and utilising ex situ collections.’ 3rd edn. (Australian Network for Plant Conservation: Canberra, ACT, Australia)
Mason TJ, Krogh M, Popovic GC, Glamore W, Keith DA (2021) Persistent effects of underground longwall coal mining on freshwater wetland hydrology. Science of The Total Environment 772, 144 772
| Persistent effects of underground longwall coal mining on freshwater wetland hydrology.Crossref | GoogleScholarGoogle Scholar |
McKee TB, Doesken NJ, Kleist J (1993) The relationship of drought frequency and duration to time scales. In ‘Proceedings of the 8th conference on applied climatology’. 17–22 January 1993, Anaheim, California, USA. pp. 179–183. (American Meteorological Society)
Meddens AJH, Kolden CA, Lutz JA, Smith AMS, Cansler CA, Abatzoglou JT, Meigs GW, Downing WM, Krawchuk MA (2018) Fire refugia: what are they, and why do they matter for global change? BioScience 68, 944–954.
| Fire refugia: what are they, and why do they matter for global change?Crossref | GoogleScholarGoogle Scholar |
Merritt DJ, Turner SR, Clarke S, Dixon KW (2007) Seed dormancy and germination stimulation syndromes for Australian temperate species. Australian Journal of Botany 55, 336–344.
| Seed dormancy and germination stimulation syndromes for Australian temperate species.Crossref | GoogleScholarGoogle Scholar |
Milberg P, Lamont BB (1995) Fire enhances weed invasion of roadside vegetation in southwestern Australia. Biological Conservation 73, 45–49.
| Fire enhances weed invasion of roadside vegetation in southwestern Australia.Crossref | GoogleScholarGoogle Scholar |
Miller BP, Murphy BP (2017) Fire and Australian Vegetation. In ‘Australian vegetation’. 3rd edn. (Ed. DA Keith) pp. 113–134. (Cambridge University Press: Cambridge, UK)
Miller G, Friedel M, Adam P, Chewings V (2010) Ecological impacts of buffel grass (Cenchrus ciliaris L.) invasion in central Australia – does field evidence support a fire-invasion feedback? The Rangeland Journal 32, 353–365.
| Ecological impacts of buffel grass (Cenchrus ciliaris L.) invasion in central Australia – does field evidence support a fire-invasion feedback?Crossref | GoogleScholarGoogle Scholar |
Miller RG, Tangney R, Enright NJ, Fontaine JB, Merritt DJ, Ooi MKJ, Ruthrof KX, Miller BP (2019) Mechanisms of fire seasonality effects on plant populations. Trends in Ecology & Evolution 34, 1104–1117.
| Mechanisms of fire seasonality effects on plant populations.Crossref | GoogleScholarGoogle Scholar |
Mills JN (1983) Herbivory and seedling establishment in post-fire southern California chaparral. Oecologia 60, 267–270.
| Herbivory and seedling establishment in post-fire southern California chaparral.Crossref | GoogleScholarGoogle Scholar |
Moir ML (2021) Coextinction of Pseudococcus markharveyi (Hemiptera: Pseudococcidae): a case study in the modern insect extinction crisis. Austral Entomology 60, 89–97.
| Coextinction of Pseudococcus markharveyi (Hemiptera: Pseudococcidae): a case study in the modern insect extinction crisis.Crossref | GoogleScholarGoogle Scholar |
Moore N, Barrett S, Howard K, Craig MD, Bowen B, Shearer B, Hardy G (2014) Time since fire and average fire interval are the best predictors of Phytophthora cinnamomi activity in heathlands of south-western Australia. Australian Journal of Botany 62, 587–593.
| Time since fire and average fire interval are the best predictors of Phytophthora cinnamomi activity in heathlands of south-western Australia.Crossref | GoogleScholarGoogle Scholar |
Moreno JM, Oechel WC (1989) A simple method for estimating fire intensity after a burn in California chaparral. Acta Oecologica 10, 57–68.
Moreno JM, Oechel WC (1991) Fire intensity and herbivory effects on postfire resprouting of Adenostoma fasciculatum in southern California chaparral. Oecologia 85, 429–433.
| Fire intensity and herbivory effects on postfire resprouting of Adenostoma fasciculatum in southern California chaparral.Crossref | GoogleScholarGoogle Scholar |
Murphy BP, Bowman DMJS (2007) The interdependence of fire, grass, kangaroos and Australian Aborigines: a case study from central Arnhem Land, northern Australia. Journal of Biogeography 34, 237–250.
| The interdependence of fire, grass, kangaroos and Australian Aborigines: a case study from central Arnhem Land, northern Australia.Crossref | GoogleScholarGoogle Scholar |
Murphy BP, Bradstock RA, Boer MM, Carter J, Cary GJ, Cochrane MA, Fensham RJ, Russell-Smith J, Williamson GJ, Bowman DMJS (2013) Fire regimes of Australia: a pyrogeographic model system. Journal of Biogeography 40, 1048–1058.
| Fire regimes of Australia: a pyrogeographic model system.Crossref | GoogleScholarGoogle Scholar |
Nolan RH, Resco de Dios V, Boer MM, Caccamo G, Goulden ML, Bradstock RA (2016) Predicting dead fine fuel moisture at regional scales using vapour pressure deficit from MODIS and gridded weather data. Remote Sensing of Environment 174, 100–108.
| Predicting dead fine fuel moisture at regional scales using vapour pressure deficit from MODIS and gridded weather data.Crossref | GoogleScholarGoogle Scholar |
Nolan RH, Boer MM, Collins L, Resco de Dios V, Clarke H, Jenkins M, Kenny B, Bradstock RA (2020a) Causes and consequences of eastern Australia’s 2019–20 season of mega-fires. Global Change Biology 26, 1039–1041.
| Causes and consequences of eastern Australia’s 2019–20 season of mega-fires.Crossref | GoogleScholarGoogle Scholar |
Nolan RH, Blackman CJ, Resco de Dios V, Choat B, Medlyn BE, Li X, Bradstock RA, Boer MM (2020b) Linking forest flammability and plant vulnerability to drought. Forests 11, 779
| Linking forest flammability and plant vulnerability to drought.Crossref | GoogleScholarGoogle Scholar |
Nolan RH, Collins L, Leigh A, Ooi MKJ, Curran TJ, Fairman TA, Resco de Dios V, Bradstock R (2021) Limits to post-fire vegetation recovery under climate change. Plant Cell & Environment 44, 3471–3489.
| Limits to post-fire vegetation recovery under climate change.Crossref | GoogleScholarGoogle Scholar |
NSW Department of Environment and Conservation (2006) Wollemi Pine (Wollemia nobilis) Rocovery Plan. NSW Department of Environment and Conservation, Hurstville, NSW, Australia.
NSW Saving our Species (2020) Micromyrtus minutiflora. 2019–2020 annual report card. Available at https://www.environment.nsw.gov.au/-/media/OEH/Corporate-Site/Documents/Animals-and-plants/Threatened-species/Report-cards/2019-2020/02-site-managed-flora/micromyrtus-minutiflora-2019-20.PDF [verified 21 January 2022]
NSW Saving our Species (2021) Nightcap Oak. 2020–2021 annual report card. Available at https://www.environment.nsw.gov.au/-/media/OEH/Corporate-Site/Documents/Animals-and-plants/Threatened-species/Report-cards/2020-2021/02-site-managed-species/nightcap-oak-eidothea-hardeniana-2020-21.pdf [verified 26 July 2022]
NSW Scientific Committee (1998) Final Determination to list Diuris disposita D.L. Jones., a terrestrial orchid, as an Endangered species. Available at https://www.environment.nsw.gov.au/topics/animals-and-plants/threatened-species/nsw-threatened-species-scientific-committee/determinations/final-determinations/1996-1999/diuris-disposita-a-terrestrial-orchid-endangered-species-listing [verified 21 January 2022]
NSW Scientific Committee (2011) Determination to make a minor amendment to Part 3 of Schedule 1 (Endangered ecological communities) of the Act by inserting the Milton Ulladulla Subtropical Rainforest in the Sydney Basin Bioregion. Available at https://www.environment.nsw.gov.au/topics/animals-and-plants/threatened-species/nsw-threatened-species-scientific-committee/determinations/final-determinations/2011-2012/milton-ulladulla-subtropical-rainforest-in-the-sydney-basin-bioregion-minor-amendment-determination [Accessed 21 January 2022]
OEH (2014) NSW Flora Fire Response Database Version 2.1. Unpublished database. NSW Office of Environment and Heritage.
Ooi MKJ (2007) Dormancy classification and potential dormancy-breaking cues for shrub species from fire-prone south-eastern Australia. In ‘Seeds: biology, development and ecology’. (Eds S Adkins, S Ashmore, SC Navie) pp. 205–216. (CAB International)
Ooi MKJ, Auld TD, Denham AJ (2009) Climate change and bet-hedging: interactions between increased soil temperatures and seed bank persistence. Global Change Biology 15, 2375–2386.
| Climate change and bet-hedging: interactions between increased soil temperatures and seed bank persistence.Crossref | GoogleScholarGoogle Scholar |
Palmer HD, Denham AJ, Ooi MKJ (2018) Fire severity drives variation in post-fire recruitment and residual seed bank size of Acacia species. Plant Ecology 219, 527–537.
| Fire severity drives variation in post-fire recruitment and residual seed bank size of Acacia species.Crossref | GoogleScholarGoogle Scholar |
Parr CL, Andersen AN (2006) Patch mosaic burning for biodiversity conservation: a critique of the pyrodiversity paradigm. Conservation Biology 20, 1610–1619.
| Patch mosaic burning for biodiversity conservation: a critique of the pyrodiversity paradigm.Crossref | GoogleScholarGoogle Scholar |
Parson BC, Gosper CR (2011) Contemporary fire regimes in a fragmented and an unfragmented landscape: implications for vegetation structure and persistence of the fire-sensitive malleefowl. International Journal of Wildland Fire 20, 184–194.
| Contemporary fire regimes in a fragmented and an unfragmented landscape: implications for vegetation structure and persistence of the fire-sensitive malleefowl.Crossref | GoogleScholarGoogle Scholar |
Pausas JG, Bradstock RA, Keith DA, Keeley JE, (GLOBAL G, NETWORK4 COTEF) (2004) Plant functional traits in relation to fire in crown-fire ecosystems. Ecology 85, 1085–1100.
| Plant functional traits in relation to fire in crown-fire ecosystems.Crossref | GoogleScholarGoogle Scholar |
Pegg G, Shuey L, Giblin F, Price R, Entwistle P, Carnegie A, Firn J (2021) Fire and rust – impact of myrtle rust on post-fire regeneration. Report to Threatened Species Recovery Hub Project 8.3.5 update report, Brisbane, Qld, Australia. Available at https://www.nespthreatenedspecies.edu.au/media/kd2h4njq/8-3-5-fire-and-rust-impact-of-myrtle-rust-on-post-fire-regeneration-update-report_v3.pdf [verified 25 January 2022]
PlantNet (The NSW Plant Information Network System, Royal Botanic Gardens and Domain Trust, Sydney) (2022a) Hakea pachyphylla Sieber ex Spreng. Available at https://plantnet.rbgsyd.nsw.gov.au/cgi-bin/NSWfl.pl?page=nswfl&lvl=sp&name=Hakea∼pachyphylla [verified 21 January 2022]
PlantNet (The NSW Plant Information Network System, Royal Botanic Gardens and Domain Trust, Sydney) (2022b) Acacia bulgaensis Tindale & S.J.Davies. Available at https://plantnet.rbgsyd.nsw.gov.au/cgi-bin/NSWfl.pl?page=nswfl&lvl=sp&name=Acacia∼bulgaensis [verified 21 January 2022]
PlantNet (The NSW Plant Information Network System, Royal Botanic Gardens and Domain Trust, Sydney) (2022c) Rhodamnia rubescens (Benth.) Miq. Available at https://plantnet.rbgsyd.nsw.gov.au/cgi-bin/NSWfl.pl?page=nswfl&lvl=sp&name=Rhodamnia∼rubescens [verified 21 January 2022]
Prior LD, Bowman DMJS (2020) Classification of post-fire responses of woody plants to include pyrophobic communities. Fire 3, 15
| Classification of post-fire responses of woody plants to include pyrophobic communities.Crossref | GoogleScholarGoogle Scholar |
Prior LD, French BJ, Storey K, Williamson GJ, Bowman DMJS (2020) Soil moisture thresholds for combustion of organic soils in western Tasmania. International Journal of Wildland Fire 29, 637–647.
| Soil moisture thresholds for combustion of organic soils in western Tasmania.Crossref | GoogleScholarGoogle Scholar |
Prior LD, Foyster SM, Furlaud JM, Williamson GJ, Bowman DMJS (2022) Using permanent forest plots to evaluate the resilience to fire of Tasmania’s tall wet eucalypt forests. Forest Ecology and Management 505, 119922
| Using permanent forest plots to evaluate the resilience to fire of Tasmania’s tall wet eucalypt forests.Crossref | GoogleScholarGoogle Scholar |
Puno VI, Laurence MH, Guest DI, Liew ECY (2015) Detection of Phytophthora multivora in the Wollemi pine site and pathogenicity to Wollemia nobilis. Australasian Plant Pathology 44, 205–215.
| Detection of Phytophthora multivora in the Wollemi pine site and pathogenicity to Wollemia nobilis.Crossref | GoogleScholarGoogle Scholar |
Read J, Guerin J, Duval D, Moseby K (2021) Charred and chewed chalkies: effects of fire and herbivory on the reintroduction of an endangered wattle. Ecological Management & Restoration 22, 35–43.
| Charred and chewed chalkies: effects of fire and herbivory on the reintroduction of an endangered wattle.Crossref | GoogleScholarGoogle Scholar |
Ruffault J, Curt T, Moron V, Trigo RM, Mouillot F, Koutsias N, Pimont F, Martin-StPaul N, Barbero R, Dupuy J-L, Russo A, Belhadj-Khedher C (2020) Increased likelihood of heat-induced large wildfires in the Mediterranean Basin. Scientific Reports 10, 13790
| Increased likelihood of heat-induced large wildfires in the Mediterranean Basin.Crossref | GoogleScholarGoogle Scholar |
Russell-Smith J, Yates CP, Whitehead PJ, Smith R, Craig R, Allan GE, Thackway R, Frakes I, Cridland S, Meyer MCP, Gill AM (2007) Bushfires ‘down under’: patterns and implications of contemporary Australian landscape burning. International Journal of Wildland Fire 16, 361–377.
| Bushfires ‘down under’: patterns and implications of contemporary Australian landscape burning.Crossref | GoogleScholarGoogle Scholar |
Silva LGM, Doyle KE, Duffy D, Humphries P, Horta A, Baumgartner LJ (2020) Mortality events resulting from Australia’s catastrophic fires threaten aquatic biota. Global Change Biology 26, 5345–5350.
| Mortality events resulting from Australia’s catastrophic fires threaten aquatic biota.Crossref | GoogleScholarGoogle Scholar |
Splawinski TB, Greene DF, Michaletz ST, Gauthier S, Houle D, Bergeron Y (2019) Position of cones within cone clusters determines seed survival in black spruce during wildfire. Canadian Journal of Forest Research 49, 121–127.
| Position of cones within cone clusters determines seed survival in black spruce during wildfire.Crossref | GoogleScholarGoogle Scholar |
Tasker EM, Denham AJ, Taylor JE, Strevens TC (2011) Post-fire seed predation: does distance to unburnt vegetation matter? Austral Ecology 36, 755–766.
| Post-fire seed predation: does distance to unburnt vegetation matter?Crossref | GoogleScholarGoogle Scholar |
Tozer MG, Auld TD (2006) Soil heating and germination: investigations using leaf scorch on graminoids and experimental seed burial. International Journal of Wildland Fire 15, 509–516.
| Soil heating and germination: investigations using leaf scorch on graminoids and experimental seed burial.Crossref | GoogleScholarGoogle Scholar |
Ward M, Tulloch AIT, Radford JQ, Williams BA, Reside AE, Macdonald SL, Mayfield HJ, Maron M, Possingham HP, Vine SJ, O’Connor JL, Massingham EJ, Greenville AC, Woinarski JCZ, Garnett ST, Lintermans M, Scheele BC, Carwardine J, Nimmo DG, Lindenmayer DB, Kooyman RM, Simmonds JS, Somnter LJ, Watson JEM (2020) Impact of 2019–2020 mega-fires on Australian fauna habitat. Nature Ecology and Evolution 4, 1321–1326.
| Impact of 2019–2020 mega-fires on Australian fauna habitat.Crossref | GoogleScholarGoogle Scholar |
Westlake SM, Mason D, Lázaro-Lobo A, Burr P, McCollum JR, Chance D, Lashley MA (2020) The magnet effect of fire on herbivores affects plant community structure in a forested system. Forest Ecology and Management 458, 117794
| The magnet effect of fire on herbivores affects plant community structure in a forested system.Crossref | GoogleScholarGoogle Scholar |
Westoby M, Falster DS, Moles AT, Vesk PA, Wright IJ (2002) Plant ecological strategies: some leading dimensions of variation between species. Annual Review of Ecology and Systematics 33, 125–159.
| Plant ecological strategies: some leading dimensions of variation between species.Crossref | GoogleScholarGoogle Scholar |
Weston P, Kooyman R (2002) Systematics of Eidothea (Proteaceae), with the description of a new species, E. hardeniana, from the Nightcap Range, north-eastern New South Wales. Telopea 9, 821–832.
| Systematics of Eidothea (Proteaceae), with the description of a new species, E. hardeniana, from the Nightcap Range, north-eastern New South Wales.Crossref | GoogleScholarGoogle Scholar |
Whelan RJ (1995) ‘The ecology of fire.’ (Cambridge University Press: Cambridge, UK)
Whelan RJ, Ayre DJ (2022) High adult mortality and failure of recruitment in a population of Banksia spinulosa following high-intensity fire. Austral Ecology 47, 1162–1167.
| High adult mortality and failure of recruitment in a population of Banksia spinulosa following high-intensity fire.Crossref | GoogleScholarGoogle Scholar |
Whight S, Bradstock R (1999) Indices of fire characteristics in sandstone heath near Sydney, Australia. International Journal of Wildland Fire 9, 145–153.
| Indices of fire characteristics in sandstone heath near Sydney, Australia.Crossref | GoogleScholarGoogle Scholar |
Yang X, Zhu Q, Tulau M, McInnes-Clarke S, Sun L, Zhang X (2018) Near real-time monitoring of post-fire erosion after storm events: a case study in Warrumbungle National Park, Australia. International Journal of Wildland Fire 27, 413–424.
| Near real-time monitoring of post-fire erosion after storm events: a case study in Warrumbungle National Park, Australia.Crossref | GoogleScholarGoogle Scholar |
Yates CJ, Gosper CR, Hopper SD, Keith DA, Prober SM, Tozer MG (2017) Mallee Woodlands and Shrublands – The mallee, muruk/muert and maalok vegetation of southern Australia. In ‘Australian vegetation’. 3rd edn. (Ed. DA Keith) pp. 570–598. (Cambridge University Press: Cambridge, UK)
Yates CJ, Barrett S, Dilly M, Hopper SD, Stewart B, Williams MR (2021) Modelling the impact of canker disease and fire regimes on the population dynamics and extinction risk of the Critically Endangered and granite endemic shrub Banksiaverticillata R.Br. Australian Journal of Botany 69, 274–284.
| Modelling the impact of canker disease and fire regimes on the population dynamics and extinction risk of the Critically Endangered and granite endemic shrub Banksiaverticillata R.Br.Crossref | GoogleScholarGoogle Scholar |
Young AM, Higuera PE, Duffy PA, Hu FS (2017) Climatic thresholds shape northern high-latitude fire regimes and imply vulnerability to future climate change. Ecography 40, 606–617.
| Climatic thresholds shape northern high-latitude fire regimes and imply vulnerability to future climate change.Crossref | GoogleScholarGoogle Scholar |
Zhang Y, Xiao X, Wu X, Zhou S, Zhang G, Qin Y, Dong J (2017) A global moderate resolution dataset of gross primary production of vegetation for 2000–2016. Scientific Data 4, 170165
| A global moderate resolution dataset of gross primary production of vegetation for 2000–2016.Crossref | GoogleScholarGoogle Scholar |
Zimmer HC, Auld TD, Benson J, Baker PJ (2014) Recruitment bottlenecks in the rare Australian conifer Wollemia nobilis. Biodiversity and Conservation 23, 203–215.
| Recruitment bottlenecks in the rare Australian conifer Wollemia nobilis.Crossref | GoogleScholarGoogle Scholar |
Zylstra PJ (2018) Flammability dynamics in the Australian Alps. Austral Ecology 43, 578–591.
| Flammability dynamics in the Australian Alps.Crossref | GoogleScholarGoogle Scholar |



