Warm stratification and optimised temperatures improve conservation of the endangered orchid, Caladenia robinsonii (Orchidaceae)
Brendan Janissen A * , Ann C. Lawrie A and Tien Huynh A
A * , Ann C. Lawrie A and Tien Huynh A
A School of Science, RMIT University, PO Box 71, Bundoora, Vic. 3083, Australia.
Australian Journal of Botany 70(4) 275-291 https://doi.org/10.1071/BT21085
Submitted: 8 July 2021 Accepted: 24 May 2022 Published: 24 June 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Maximising seed germination and seedling development is critical for conservation of endangered plants around the world. Orchidaceae is one of the most threatened plant families and can be one of the most difficult to propagate ex situ. Three critical, but potentially limiting, factors are important for orchid germination, namely, conducive conditions, ‘ready-to-germinate’ seed and effective mycorrhizal fungi.
Aims: Our aim was to improve poor germination in vitro of a recalcitrant Australian endangered orchid, Caladenia robinsonii, and to predict the potential impacts of climate change on this species.
Methods: Three experiments were conducted to optimise germination in C. robinsonii, with a focus on temperature. In Experiment 1, on the basis of meteorological data, three constant temperatures (15°C, 20°C and 27°C) were tested. In Experiment 2, the optimal constant temperature was compared with diurnally varying temperatures of 22°C/18°C (12/12 h), with and without warm stratification at 30°C/27°C (12/12 h) for 1 week. In Experiment 3, the same diurnally varying temperature and warm stratification were tested using multiple orchid mycorrhizal fungal isolates cultured from wild and re-introduced populations of C. robinsonii.
Key results: Without warm stratification, germination was greatest at 20°C (21%), but only 4% of seedlings developed to the green-leaf stage, whereas fungal growth was greatest at 27°C. Stratification increased germination (79%) and development to the green-leaf stage (47%), but more so with subsequent incubation under constant 20°C than diurnal 22°C/18°C. Fungal isolate affected total germination (53–69%) and development to the green-leaf stage (26–41%); isolates from the wild population were less effective than were those from re-introductions.
Conclusions: Warm stratification and specific seasonal temperatures significantly improved germination, both factors being typical of seeds with physiological dormancy.
Implications: Mimicking in situ conditions can provide a strong basis for ex situ germination strategies and predicting future outcomes. Winter–spring flowering orchid seedlings are commonly observed in autumn, and warm stratification should be considered for improving germination of similar orchid species. However, future climate warming may reduce in situ seedling recruitment.
Keywords: Caladenia robinsonii, climate change, conservation, diurnal variation, fungi, orchid, stratification, temperature.
Introduction
The Orchidaceae contains one of the largest numbers of species in the world, with 28 484 species being currently accepted (WCSP 2017). However, it also has one of the largest numbers of threatened (rare, vulnerable, endangered) and extinct species (Wraith and Pickering 2018), with 785 species currently in the IUCN Red List (IUCN 2020). Terrestrial orchids dominate in temperate climates that contain seasons unsuitable for growth, during which the orchids persist in dormant underground tubers (Rasmussen 1995). Many terrestrial orchids in such habitats are among the most threatened plants in the world. An example is the genus Caladenia in Australia, in which 22% are threatened nationally and the genus contains many endangered species that have declining numbers (Janissen et al. 2021). Most of these species occur in dry woodlands and heaths, are winter-active and lie dormant during the hot summer (Dixon and Tremblay 2009). Mean global temperatures have already increased by 1°C between the industrial revolution and 2017 (Allen et al. 2018), but this hides much greater changes in some parts of the world. The Australian Bureau of Meteorology predicts that southern Australia will become hotter (+0.4–1.1°C) and drier (less rainfall in winter and spring) over the next 10 years (Bureau of Meteorology 2020a). This is expected to increase plant stress and is likely to accelerate the existing decline of these species.
Many of these endangered Caladenia species have active recovery/action plans (e.g. Todd 2000) that specify monitoring of wild populations, collection of seed, establishment of an ex situ population and translocation of some of these plants to augment the existing wild population and re-introduce the species to previously known habitats. Two key steps in this process are for seeds to germinate and for seedlings to develop green leaves. One such endangered Caladenia species is C. robinsonii G.W.Carr (Frankston spider-orchid), which was only re-discovered in 1991 as a wild population of about 40 plants in a temperate dry woodland. Since then, five groups of ex situ plants have been added to augment the natural population; even so, the total population is now only about 260 individuals (15 wild and 245 from introductions).
Seed germination in orchids requires the following three essential factors: environmental conditions (temperature, moisture, light/dark) conducive to germination, primed ‘ready-to-germinate’ (not dormant) seed and (unlike most other plants) a symbiotically effective fungus. This last factor is because orchid seeds have negligible endosperm and so lack food resources as the embryo germinates (Rasmussen 1995). Hyphae of symbiotic fungi enter a limited number of basal cells in the embryo, which later digests the hyphae, thus obtaining nutrition that supports seedling growth (Yamamoto et al. 2017). In Caladenia species, the testa on the seed splits (germination), allowing the seedling to put out rhizoids that are invaded by the fungus. The seedling forms a protocorm with a leaf primordium, which then develops into a green leaf and, with the fungus, supports plant growth and development (Clements et al. 1986; Rasmussen 1995).
Conservation efforts for C. robinsonii have been limited by poor germination in vitro; this is probably because one or more of the critical factors for germination previously mentioned is not being overcome. Previous symbiotic germination trials on C. robinsonii have achieved at best 18% germination (Raleigh 2005; Richard Thomson, Australian Native Orchid Society, pers. comm.) and seedlings were slow to develop beyond protocorm formation. By contrast, seed germination of two common species was much greater, namely, 65% for C. phaeoclavia (Raleigh 2005), 52% (Wright et al. 2011) and 66% (Raleigh 2005) for C. tentaculata. Once the ‘right’ combination of conducive environmental conditions, ‘ready-to-germinate’ seed and an effective symbiotic fungus has been found, germination can be high. For example, the germination of rare and common Caladenia taxa was up to 60% at 18°C (Swarts et al. 2010) and up to 94% at 23°C after 8 weeks in C. latifolia (Bustam et al. 2014). The poor germination in C. robinsonii demonstrates that the critical factors for germination of C. robinsonii and other endangered orchids are not well understood.
One critical factor is environmental conditions. The conditions necessary for optimum germination in C. robinsonii have not been investigated. Most studies on Caladenia species have used constant incubation temperatures in the range of 15–25°C (Huynh et al. 2009; Nikabadi et al. 2014; Oktalira et al. 2019). Only one study investigated the effects of different temperatures on the germination of two Caladenia species as well as one species each of Microtis and Pterostylis (Nikabadi et al. 2014). However, this study was also limited to constant temperatures (10°C, 15°C, 20°C and 25°C). The temperature required should ideally be modelled on conditions in autumn at the time just before seedlings are observed in the wild to mimic germination conditions in situ. Understanding germination responses to temperature in vitro can assist in predicting population responses to likely temperature ranges in situ. Over the next 30 years, climate change predictions are that temperatures will increase by 0.45–0.60°C (high–very high confidence), diurnal variation will increase by 0.3–0.45°C (high confidence) and annual rainfall will decrease by 60–120 mm (medium confidence; Bureau of Meteorology 2020a, 2020b). The length of droughts is also expected to increase (Bureau of Meteorology 2020a). These changes may make it more difficult for C. robinsonii to survive and recruit new seedlings (a vital step in conservation) in the next 30 years (Janissen et al. 2021), the same length of time since it was first found at the only extant site.
A second critical factor is readiness to germinate, where seeds may have high viability but be unable to germinate because of innate dormancy or immaturity. In some northern hemisphere orchids, seed dormancy has been broken by cold stratification (Zettler et al. 2001; Poff et al. 2016). For example, cold stratification increases the proportion of seeds germinating in Platanthera chapmanii from 25 to 35% (Poff et al. 2016). Similarly, germination in Calopogon tuberosus increased from <45% to >60% after cold stratification at 10°C for 8 weeks. However, cold stratification at the temperatures used in northern hemisphere summer-flowering orchids before spring germination is unlikely to be relevant for spring-flowering, winter-active orchids with seed that germinate in autumn at the end of a hot dry summer. In temperate terrestrial northern hemisphere orchids, the main growth period is spring–summer and the seed is released in autumn before a cold winter. Subjecting the seed to cold thus simulates the cold winter and breaks dormancy. By contrast, southern hemisphere temperate orchids that grow during autumn–winter–spring release seed in late spring. Seed must survive the hot summer and seedlings are seen in autumn. This raises the question of whether exposure to the hot temperatures of summer is important in germination of seed of C. robinsonii? Warm stratification has not been investigated for southern hemisphere taxa and was of particular interest in this study, to improve germination success in the endangered C. robinsonii.
A third critical factor is the presence of a symbiotically effective fungus for C. robinsonii. Caladenia associates with a single genus of mycorrhizal fungi, Serendipita (Weiß et al. 2016; Reiter et al. 2020). All isolates that germinated C. robinsonii to the green-leaf stage belong to operational taxonomic unit A (OTU A) by analysis of sequences of the nuclear ribosomal internal transcribed spacer (ITS) region of DNA (Reiter et al. 2020). A recent taxonomic study has classified fungi in OTU A as Serendipita australiana (Oktalira et al. 2019). Despite this uniformity in ITS, strains differ in effectiveness and only empirical testing of isolates on ‘ready-to-germinate’ seed in conducive conditions can find symbiotically effective isolates. Unless such a symbiotically effective fungus is present, seed will not germinate even if seeds are ready to germinate and conditions are conducive to germination. Testing for germination thus involves using combinations of seeds and fungi, modelling environmental conditions on those experienced in situ and those successful with similar Caladenia species.
The aim of this study was to improve the germination of C. robinsonii and investigate its likely response to climate change. Given the greater confidence in temperature than rainfall projections, this study aimed to (1) analyse meteorological data for the site to determine realistic temperature ranges experienced by C. robinsonii, (2) determine how both these and projected temperatures for the region are likely to affect orchid seed germination and orchid mycorrhizal fungi (OMF) growth in vitro, (3) determine the impact of fungal isolates on the efficacy of seed germination and seedling development, and (4) investigate the effects of warm stratification and diurnal variation in temperature on success in seed germination and seedling development.
Materials and methods
The study species
Caladenia robinsonii (Frankston spider orchid) is an endangered winter-active terrestrial orchid perennating by means of an underground tuber. It produces a single green hairy leaf and, in some years, a single flower stalk containing (normally) one to (rarely) three flowers. The flowers are cream to creamy-yellow with dark red markings and a lobed labellum. Aerial growth emerges during autumn (May); the plant is vegetative during winter (June–August), flowers in early to mid-spring (September–October), matures fruit (capsules) during mid–late spring (October–November), releases seed and senesces in late spring (November) and survives summer (December–February) and early autumn (March–April) as a dormant tuber underground. Many dust-like seeds are released in late spring and must survive over the hot summer; seedlings are seen in May, simultaneously with leaves emerging from dormant tubers of mature plants. Seeds thus undergo a prolonged period (3–4 months) of temperatures that routinely climb to over 30°C (Climate Data Online, available at www.bom.gov.au) before seedlings are observed soon after rains in April–May.
The only known extant population of C. robinsonii occurs in a woodland (EVC3 Damp Sands Herb-rich Woodland), with sandy soil, Eucalyptus canopy species and ground vegetation dominated by the shrubs Banksia marginata, Leptospermum myrsinoides, L. continentale and Xanthorrhoea australis (Todd 2000). Rainfall is distributed like that in a Mediterranean climate, in which the main rainfall is during winter–spring and the summer is hot and dry.
Re-introduction programmes have been underway since 2008 and all re-introductions have been within 200 m of the remnant population, within the same reserve. The first re-introduction (Re-introduction 1) in 2008 resulted in 4 or 32 plants (12%) by 2019, with natural recruitment occurring in 5 or 9 years. A further re-introduction programme in 2010 (Re-introduction 2) resulted in 50 of 94 (53%) emergent plants in 2020, plus 29 new recruits. Re-introduction 3 from 2012 resulted in 6 of 58 (10%) emergent plants in 2019, with no evidence of recruitment in any year. The two re-introduction programmes in 2017 resulted in 52 of 89 (58%) emergent plants, plus 43 new recruits in 2020 (Re-introduction 4) and 27 of 27 (100%) emergent plants, plus 33 new recruits in 2020 (Re-introduction 5). Re-introductions 2, 4 and 5 are, thus, the only populations with enough recruitment to keep their numbers stable so far.
Seed collection
Seed availability was dictated by management plans and governing bodies. Mature, undehisced capsules were collected and provided by The Department of Environment, Land, Water and Planning (DELWP) and the Australasian Native Orchid Society (ANOS) (www.anosvic.org.au). Seed was removed from the capsules, cleaned and stored in paper envelopes, dried over silica gel for 24 h and stored at 4°C until used (the standard treatment recommended by DELWP and ANOS). Three experiments were conducted to investigate the previously mentioned critical factors to optimise germination in C. robinsonii. Experiments were designed to accommodate the use of different batches of scarce seed (Table 1) and analysed independently. Seed viability was tested using the 2,3,5-triphenyltetrazolium chloride (TTC) method of Raleigh (2005) as modified from Van Waes and Debergh (1986) and scored after 4 days. The viabilities of seeds collected in 2017 and 2018 were 84.1 ± 2.1% and 90 ± 0.4% respectively. A chi-square test showed that there was no significant difference in viability among seed lots (P = 0.384).

|
Fungal isolation
Owing to the small size and distribution of C. robinsonii populations, mycorrhizal fungus collection was restricted to three plants under conditions imposed in DELWP Permit no. 10008397. For Experiment 1, in July 2018, one whole stem collar (swollen area below leaf base) from one randomly selected plant was collected from each of three Re-introduction sites 2, 4 and 5. For germination Experiments 2 and 3, in August 2019, one whole stem collar from one randomly selected plant was collected from each of two Re-introduction sites 2 and 3, as well as the wild site. Stem collars were surface-sterilised and pelotons cultured as described previously (Huynh et al. 2009), using fungal isolation medium (FIM; Clements et al. 1986). Briefly, stem collars were rinsed under running tap water for 10 min to remove debris before being immersed in 10% (V/V) Domestos® (www.unilever.com) for 3 min, followed by three washes in sterile H2O for 2 min each. Pelotons were released from the collar cells with a scalpel to macerate the tissue. In total, 10–15 pelotons were placed on each plate. Plates were sealed in zip-lock bags and incubated at 27°C for 4 weeks. White fungal isolates typical of OMF were subcultured into pure culture.
Temperature history of site and future predictions
To help inform the in vitro germination temperatures chosen and predict future scenarios, temperature data were obtained from the Bureau of Meteorology (Climate Data Online, available at www.bom.gov.au) using the closest weather station with continuous records for 1991–2020 (Cerberus (086361), 22 km from the orchid population). Monthly mean minimum and maximum temperatures between 1991 and 2020 were converted into 5-year averages to give insight into the temperature dynamics of each month. Rainfall was analysed using the same method as for temperature, but using data obtained from Rosebud Country Club (086213, 2 km from orchid population).
Experiment 1
Experiment 1 used a mature undehisced seed pod collected in 2017 from a naturally pollinated flower located in Re-introduction 2; this collection was the only one authorised by the permit. Seed was prepared and plated on Miracloth® (50–100 seeds per plate) as in Huynh et al. (2009), except that seed was not pre-treated with Tween 80® and complex oatmeal agar (OMA) was used (Clements et al. 1986). Three fungal isolates from each of the three plants collected in 2018 were chosen randomly for germination experiments (Table 2). Seed on Miracloth® pieces were inoculated with fungi by punching sections 5 mm in diameter out of the growing edges of actively growing cultures of fungi on OMA. Plates were incubated at 15°C, 20°C or 27°C in darkness for 8 weeks before germination and seedling development were scored as a percentage of the total number of seeds. Germination and seedling development were scored as follows: Stage 1 (embryo imbibed and testa split – germination), Stage 2 (protocorm formation), Stage 3 (leaf primordium) or Stage 4 (green leaf). Each temperature–isolate combination was replicated five times.

|
After scoring at 8 weeks, seedlings that had developed a leaf primordium were transferred into glass jars containing a lower layer of OMA and an upper layer of autoclaved vermiculite mix containing ground oats (0.25% w/v), yeast extract (0.01% w/v) and MilliQ water (45%, v/v), with both the OMA and the vermiculite mix being approximately 1.5 cm deep. Seedlings were transferred complete with Miracloth® from the Petri dish to the mixed-media jars and incubated at the same temperatures under a 16 h photoperiod of 25 μmoles m−2 s−1 provided by ‘Fluora’ fluorescent lights.
The same fungal isolates were cultured without seed to test temperature effects on fungal growth. One 5 mm inoculation plug was placed in the centre of a Petri dish containing the same OMA medium. Cultures were incubated at 15°C, 20°C or 27°C in triplicate.
Experiment 2
Experiment 2 compared germination and seedling development under optimal constant temperatures (from Experiment 1) with those in diurnal fluctuations experienced in situ. Three mature undehisced capsules were collected in 2019 from naturally pollinated flowers, two from Re-introduction 3 and one from Re-introduction 5. Seeds were mixed and prepared as for Experiment 1, but inoculated with only one fungal isolate prior to incubation (Fungal isolate 2 from Re-introduction 2 from 2019, which had recently germinated seed, Table 3). Two factors were tested, namely warm stratification and diurnal variation. Warm stratification conditions, based on summer temperatures in situ, were 30°C/27°C (12/12 h) for 1 week in darkness. Seeds were divided into four batches and were subjected to one of four treatments, all in darkness. Batch A (warm stratified) was incubated for 1 week 30°C/27°C (12 h/12 h) followed by 7 weeks at 22°C/18°C (12 h/12 h). Batch B was warm stratified for 1 week followed by 7 weeks at constant 20°C. Batch C was incubated for 8 weeks at 22°C/18°C (12 h/12 h). Batch D was incubated for 8 weeks at 20°C. Each treatment was replicated 28 times (112 Petri dishes in total) and germination was scored as in Experiment 1. Diurnal temperatures were chosen on the basis of soil temperatures in situ. A remote data logger (HOBO® Micro Station (H21-USB)) was installed at the study site, with a temperature sensor (S-TMB-M0XX) buried in the top 10 cm of soil (www.hobodataloggers.com.au). Readings were taken every 6 h for 12 months.

|
Experiment 3
Experiment 3 compared germination and seedling development among 30 isolates from 2019 (Table 3) under both warm stratification and diurnal fluctuation in temperature. Three mature undehisced seed pods were collected in 2019 from naturally pollinated flowers, two from Re-introduction site 2 and one from Re-introduction site 3. Seeds were prepared and inoculated as for Experiment 1; they were inoculated with 1 of 30 isolates and then warm-stratified at 30/27°C (12/12 h) for 1 week in darkness before incubation at 22°C/18°C (12/12 h) in darkness for 7 weeks. Each of the 30 isolates was replicated five times (300 Petri dishes in total) and germination was scored as in Experiment 1. New isolates were used for the following two reasons: (1) a possible loss of effectiveness after long-term culture of 2018 isolates, and (2) to compare isolates from re-introduced populations with those from the wild population, because wild isolates were collected only in 2019 because of management restrictions.
Molecular analysis of fungi
All 38 of the C. robinsonii isolates, nine from 2018 and 29 from 2019 (one isolate could not be sequenced), were cultured in 50 mL of oatmeal broth (OMB) for molecular analysis. OMB was prepared by adding ground oatmeal (2.5% w/v) to 1 L water in a muslin bag and microwaved for 10 min, cooled and dispensed as liquid in 50 mL aliquots into glass jars before inoculation. Four inoculation plugs (5 mm × 5 mm from agar plate growths) per replicate were added to the OMB and incubated by shaking at 30 rpm at 27°C for 8 weeks. Mycelia were harvested and drained; 100 mg of each isolate was then ground into a fine powder by using liquid nitrogen in a mortar and pestle. DNA was extracted using a QIAGEN DNeasy® Plant Mini Kit according to the manufacturer’s instructions, and quantified using electrophoresis alongside a GeneRuler™ 100-bp DNA ladder (Fermentas, Germany).
DNA was amplified with universal primers ITS1 and ITS4 (White et al. 1990). Each 25 μL reaction contained 12.5 μL Promega GoTaq Green Master Mix, 8.5 μL nucleus-free water, 1 μL ITS1 (10 μM), 1 μL ITS4 (10 μM), 2 μL DNA (5–10 ng) or sterile nuclease-free water. A G-Storm thermocycler was programmed as follows: initial denaturation: 10 min 94°C; 35 cycles of: 30 s 94°C, 1 min 51°C and 1 min 72°C; final extension: 10 min 72°C. PCR products were separated by electrophoresis on a 1.4% agarose gel containing 10 μL per 100 mL of GelRed® (Biotium, USA) in 1 × TAE buffer at 100 V alongside a GeneRuler™ 100 bp DNA Ladder. Amplicons were imaged using a ChemiDoc MP system with Image Lab™ software (Bio-Rad, Australia).
The PCR products were purified using a QIAGEN QIAquick® purification kit (QIAGEN, Germany) according to the manufacturer’s instructions. Purified products were sequenced using BigDye v3.1 Cycle Sequencing Kit protocol (Applied Biosystems) according to the manufacturer’s instructions. DNA was precipitated using the Applied Biosystems ethanol precipitation protocol 1 (Perkin-Elmer 1995) and products were processed at Micromon (Monash University, www.monash.edu/researchinfrastructure/micromon).
Sequence alignment and phylogenetic analysis
Sequences obtained from Micromon were analysed using MEGA version X (Kumar et al. 2018). Because of the problems with stutter (caused by multiple thymine residues) with sequences from some isolates, manual construction guided by the intact sequences was required for 2018 isolates from Re-introductions 4 and 5, using the first clear 330 bp from the 5′ direction from ITS1 (forward) and the first clear 200 bp from the 5′ direction from ITS4 (reverse). Sequences were searched for matches by using Blastn at National Centre for Biotechnology Information (NCBI; www.ncbi.nlm.nih.gov) and the closest matches (≥99% identity) for each were downloaded. Evolutionary analysis was inferred using the maximum-likelihood method with the Tamura–Nei model (Tamura and Nei 1993) and nearest-neighbourhood-interchange (NNI) tree inference, then visualised as a phylogenetic tree by using MEGA version X (Kumar et al. 2018). Trametes versicolor was used as an outgroup. Sequence differences were compared using distance analysis (P-distance). Sequences were deposited into GenBank, with accession numbers MN069548–MN069556 (Table 2) and MT457361–MT457388 (Table 3).
Statistical analysis
Data were analysed using Minitab 19 statistical software (Minitab Inc., State College, Pennsylvania, USA, www.minitab.com). Temperature and rainfall trends were identified using regression analysis. A general linear model was used to analyse temperature history of the site; mean monthly maximum and minimum temperatures were used as the response variables, whereas month was the predictor. Differences between maximum or minimum temperatures for individual months over years 1991–2000 were analysed using ANOVA. For germination experiments, percentage total germination, seedling developmental stage and fungal growth were used as response variables, and temperature and isolate were used as predictors. Normality of data and residuals from ANOVA was tested using the Anderson–Darling method. Non-normal data were normalised using Johnson transformation before analysis. Tukey’s post hoc tests were performed to identify homogeneous subsets. Significance was accepted at P < 0.05.
Results
Temperature history of site and future predictions
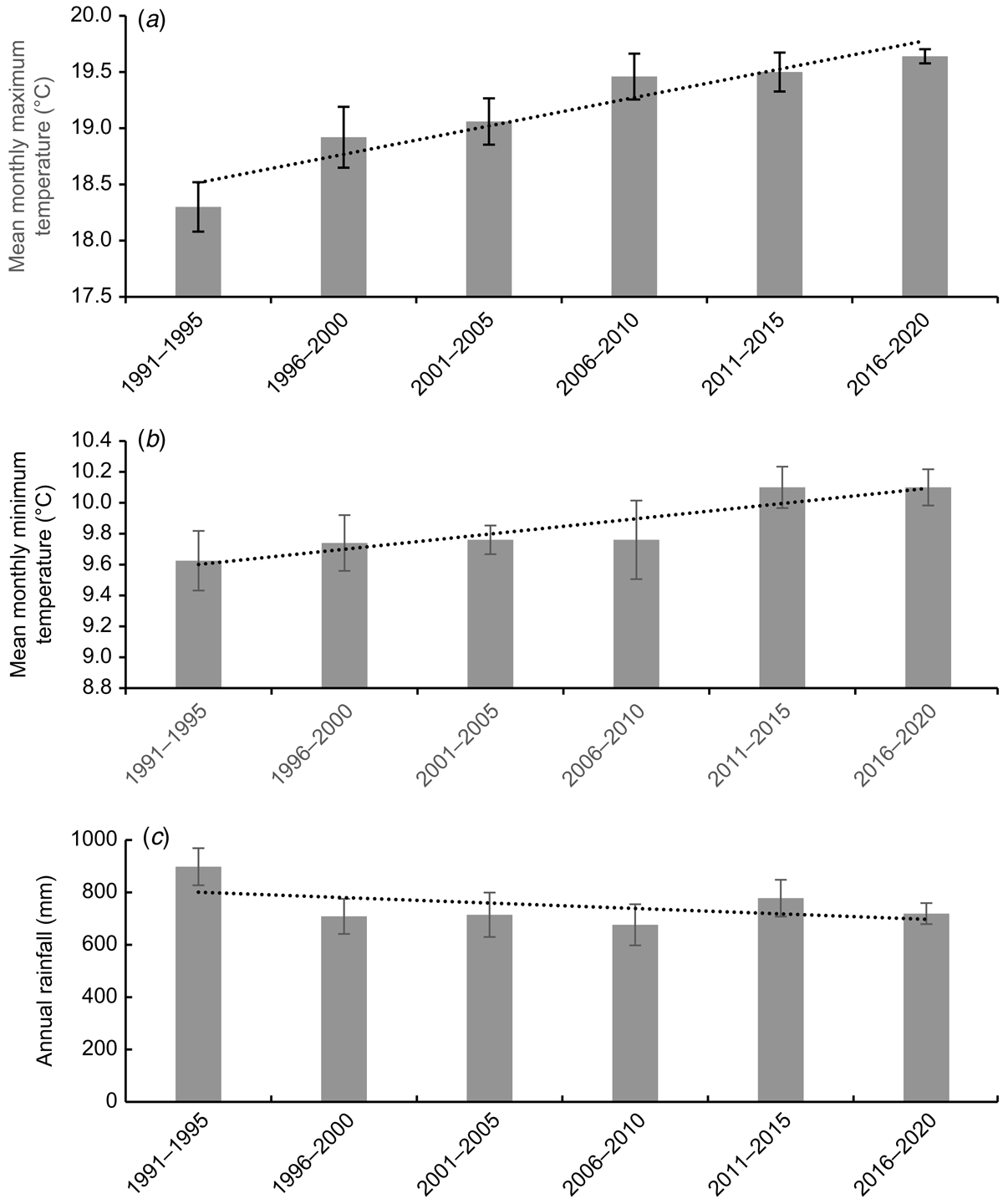
Analysis of 5-year averages for climate data (temperature and rainfall) at the sole C. robinsonii habitat during 1991–2019 (since the C. robinsonii population was discovered) showed significantly increasing trends in both maximum and minimum temperatures (R2 = 0.861, P < 0.02 and R2 = 0.844, P < 0.05 respectively) but there was no significant trend in annual rainfall (R2 = 0.234, P > 0.05; Fig. 1). More detailed analysis (Fig. 2) showed that the maximum temperature increased in only four individual months: October–November (spring; R2 = 0.861–0.785) and December–January (summer; R2 = 0.9367–0.8768). By contrast, the minimum temperature did not change significantly in any individual month.
|
|
|
In relation to the life cycle of C. robinsonii, the orchid finishes flowering and matures its fruits during October–November, seeds are dispersed in November–December at the start of the hot summer. The orchid emerges from dormancy in April–May, which is also when seedlings are observed in the field. Thus, after seeds are released into the soil and before seedlings appear, the mean maximum temperature is 21–26°C (with an increasing number of days >30°C) and the mean minimum temperature is 12.5–16.5°C and steady.
Experiment 1
In Experiment 1, total germination (hereafter referred to as germination) under different temperature treatments ranged from 0 to 21% at 8 weeks (Fig. 3). Germination was significantly affected by incubation temperature (F = 13.39, P < 0.01) and isolate (F = 2.06, P = 0.04), but there was no significant interaction between isolate and temperature (F = 1.92, P = 0.06). Germination was significantly greater at 15–20°C than at 27°C. No germinant developed past Stage 1 at 27°C and germinants developed to the vital Stage 4 (green leaf) only at 20°C. Only three isolates resulted in Stage 4 seedlings at this time: Isolates 26 and 32 from Re-introduction 4 and Isolate 11 from Re-introduction 5, although orchids with Isolates 10, 17 and 18 developed to Stage 4 after 8–16 weeks of incubation. The isolates resulting in the greatest development were not those resulting in the greatest germination. Re-introduction 4 isolates produced the most Stage 4 protocorms at 20°C.
Seedlings varied in stage of development at 16 weeks after inoculation. Only seedlings produced from five of the nine isolates developed further into functional plants with green leaves, including two isolates at 15°C and three isolates at 20°C. However, plant numbers were small, with only two to five plants reaching further development for each isolate. After 16 weeks, leaf length varied four-fold (8–39 mm) at 20°C, whereas it varied less than two-fold (13–18 mm) at 15°C (Fig. 4). Leaf length varied significantly with isolate (F = 3.92, P = 0.01). Isolates with the greatest development were not those with the greatest germination. The OMF isolates that produced developed seedlings at 15°C were different from those that did so at 20°C.
Fungal isolate diameter varied five-fold, from 4.3 to 20.7 mm (Fig. 5). Temperature had a significant effect on fungal growth (F = 2.44, P = 0.025), as did isolate (F = 109.1, P < 0.01). The largest radial growth was observed at 20°C and 27°C, and was almost twice that at 15°C. The effect of temperature was not consistent across all temperature–isolate combinations, because there was a significant interaction between temperature and isolate (F = 3.77, P < 0.01). For example, Isolate 24 from Re-introduction 2 had greater growth at 20°C than at 27°C, whereas Isolate 17 from Re-introduction 5 had greater growth at 27°C than at either 15°C or 20°C.
Experiment 2
In Experiment 2, total germination varied from 43% to 79% (Fig. 6). Warm stratification (F = 213.97, P < 0.05) and incubation temperature (F = 68.71, P < 0.05) had significant effects on total germination. There was a significant interaction between warm stratification and temperature (F = 74.35, P < 0.05). The greatest total germination was reached with warm stratification followed by incubation at constant 20°C, and the least without stratification in both constant and diurnally varied temperatures. Warm stratification increased total germination by 37% (from 43% to 79%) at constant 20°C, but only by 9% (from 44% to 53%) at diurnal 22°C/18°C. Diurnal variation in temperature decreased total germination by 26% (from 79% to 53%) compared with constant 20°C after warm stratification, but had no effect on seed not stratified (43% vs 44%).
Warm stratification and diurnal variation in temperature also had significant effects on the proportion of seedlings in Stage 1 (F = 35.52, P < 0.05) and Stage 4 (F = 26.39, P < 0.05), but not in Stage 2 (F = 1.4, P = 0.24) or Stage 3 (F = 0.27, P = 0.60). Warm stratification increased the proportion of seedlings in Stage 4 in both temperature treatments. Diurnal variation in incubation temperature reduced the proportion of seedlings in Stage 4, by increasing the proportion of seedlings not progressing beyond Stage 1.
Experiment 3
In Experiment 3, total germination ranged from 53% to 69% (Fig. 7). Total germination was not significantly affected by fungal isolate (F = 0.42, P = 0.50), fungal source (F = 0.01, P = 0.99) or their interaction (F = 0.34, P = 0.71). However, fungal isolate had a significant effect on the percentage of seeds reaching Stages 1 (F = 4.91, P =< 0.01), 3 (F = 6.97, P < 0.01) and 4 (F = 9.56, P =< 0.01), but not Stage 2 (F = 1.89, P = 0.06). The source of the fungal isolates also affected these proportions; isolates from Re-introductions 2 and 3 resulted in greater proportions of Stage 4 seedlings than did isolates from the wild population (F = 12.28, P < 0.01). The greatest percentages (26–41%) of seedlings in Stage 4 were reached by Isolates 2, 6 and 9 from Re-introduction 2 and Isolates 1 and 7 from Re-introduction 3.
Molecular analysis of fungi
For isolates from the 2018 collection, clear sequences were obtained for fungal isolates from Re-introduction 2 (2010), whereas isolates from Re-introductions 4 and 5 (2017) all had problems with stutter after eight consecutive occurrences of thymine in the 5′-prime direction and had a matching problem with adenine in the 3′ direction. After manual construction guided by the clear sequences from Re-introduction 2 isolates, using 330 bp from ITS1 (forward) and 200 bp from ITS4 (reverse), a total sequence length of approximately 530 bp, equal to that of the clear sequences, was obtained. Isolates from the 2019 collection all produced clear sequences, except for Isolate 8 from Re-introduction 2 and Isolate 4 from Re-introduction 3, which were excluded from analysis.
All C. robinsonii isolate sequences clustered within Clade A (97% bootstrap value), along with closest matches (97–99%) in GenBank to other Serendipita species (formerly Sebacina spp., Weiß et al. 2016; Fig. 8). All C. robinsonii isolates were closely similar; P-distances among populations were less than 0.005, except for Re-introduction 5 Isolate 17 (0.016). Mean P-distance among all isolates was 0.027. The sequences from Caladenia OMF branched into Clades A and B with a highly reliable bootstrap value (95%). Clade A also included closely matching sequences of fungi isolated from other rare or endangered Caladenia species, including C. venusta, C. patersonii, C. huegelii, C. amoena and C. rosella. Clade B contained less closely matching sequences of isolates from other Caladenia species, including C. latifolia, C. colorata, C. atroclavia and C. pectinata. C. colorata and C. atroclavia are endangered, whereas C. latifolia and C. pectinata are widely distributed on the south-western coast of Australia. Sequences within each clade were more similar to one another than to isolates from more distantly related common Caladenia species, such as, for example, C. tentaculata.
|
|
Discussion
This is the first study to discover the positive effects of warm stratification on germination and seedling development in orchids. Initially (Experiment 1), germination was only 21%, suggesting that one or more of the three critical factors (ready-to-germinate seed, conducive environment and effective fungus) had not been achieved. Germination and Stage 4 seedlings more than tripled after warm stratification, suggesting that seed dormancy was the major factor limiting germination and seedling development once other suitable factors were provided. The increased germination from 15% to 70% (and increase in green-leaved seedlings from 14% to 47%) achieved here will help recovery efforts of C. robinsonii by decreasing the quantity of seed required to raise ex situ plants used to augment the wild population. Warm stratification to make seeds ‘ready-to-germinate’ may be applicable to other endangered southern hemisphere species, especially Caladenia species, that have the same seasonal seed distribution, poor germination and seedling development. The second potential limitation, a subsequent conducive environment, had less effect. The data presented here suggest that the projected future warming of +2°C by 2040 (Bureau of Meteorology, CSIRO 2020) at the only known location for C. robinsonii will reduce seedling recruitment. The third potential limitation, of an effective symbiotic fungus, is necessary but seems unlikely to limit germination and seedling development in C. robinsonii, provided isolates are obtained from appropriate host plants.
Potentially critical factor: seed dormancy
Warm stratification could be the key to producing ‘ready-to-germinate’ seed and overcoming the first critical factor to germination. No previous research has considered the effects of warm stratification on germination in winter–spring flowering orchids even though seedlings are commonly observed in the wild in autumn, following the hot summer (Nikabadi et al. 2014; Duncan and Moloney 2018). By contrast, a cold winter normally precedes germination in summer-flowering orchids in the northern hemisphere. Cold stratification improved germination (Sharma et al. 2003; Poff et al. 2016), probably by breaking dormancy and producing ‘ready-to-germinate’ seed. For example, germination of the south-eastern USA orchid, C. tuberosus, was greatly improved from a maximum of about 43% to about 88% after cold stratification at 10°C for 8 weeks (Kauth et al. 2011). Given the improved germination observed in northern hemisphere orchids after cold stratification, it was logically predictable that warm stratification could be effective in southern hemisphere orchids.
This study has demonstrated the positive effects of warm stratification on germination in the southern hemisphere C. robinsonii, which is probably a result of the removal of physiological seed dormancy (Rasmussen 1995). This may have the potential to improve germination and seedling development in other threatened Caladenia species and also other orchid genera, such as Microtis, Prasophyllum, Pterostylis and Thelymitra, that germinate following hot summers (Dowling and Jusaitis 2012). A lack of ‘ready-to-germinate’ seed would also explain the differences observed between viability and germination achieved in Experiment 1 and as reported in other germination studies (Dowling and Jusaitis 2012; Lemay et al. 2015). Put simply, seeds probably had viable embryos but were not ‘ready to germinate’.
Germination rates might be expected to be lower in endangered orchids than in common ones, but previous reports are inconsistent. In Caladenia, the germination of the endangered C. huegelii reached 100% at 20°C, compared with only 58% in the common C. latifolia (Nikabadi et al. 2014). This is different from the results of Bustam et al. (2014), who achieved 95% germination in C. latifolia after incubation at 23°C. By contrast, germination in endangered Southeast Asian Dendrobium chrysotoxum reached 85%, compared with 86% in the common D. findlayanum, suggesting that conservation status is not an indicator of germination success. Further investigation of these and other species using optimised conditions such as stratification may resolve apparent inconsistencies in reports of germination rates among rare and common orchids.
Caution should be observed when comparing germination data because of inconsistent definitions of germination. For example, some studies use different stages to classify germination, whereas others do not report stages at all. Because of these inconsistencies, it is difficult to compare germination across studies. To advance orchid conservation research, it is recommended that ‘germination’ be used only for Stage 1 (ruptured testa) and that seedling developmental stages be reported in all studies for greater transparency. Because only green-leaved seedlings progress to maturity, their proportion is equally, or more, important in species recovery and needs to be reported.
Potentially critical factor: fungal isolate
Differences among isolates were apparent both with and without stratification. However, no isolate resulted in zero germination under any of the conditions tested, suggesting that all were potentially capable of supporting seedling development, although to different extents (Reiter et al. 2020). Differences in the stages reached in seedling development were greater without (Experiment 1) than with (Experiment 3) stratification, suggesting that the outcome depends partly on the compatibility of the fungus and partly on the readiness of the orchid to develop, rather than just on the fungus.
The lesser seedling development with isolates from the wild plant than from the re-introduced plants was not expected. One explanation may be that isolates from re-introduced plants have already been pre-selected in vitro for their high ability to support seedling development before use (Wright et al. 2009). One perplexing observation is the large percentage of seedlings (50–98%) not progressing to the green-leaf stage, even after stratification. This requires further study to find out whether all germinated seeds eventually develop fully given longer time.
ITS sequence similarities (P-distance values of <0.016) suggest that all OMF isolates from C. robinsonii belong to OTU A (Reiter et al. 2020) of the genus Serendipita, as do OMF from other endangered and rare species of Caladenia (Huynh et al. 2009; Swarts et al. 2010; Mehra et al. 2017; Reiter et al. 2020). The dispersal of germinating intermixed with non-germinating isolates in Clade A supports evidence (Wright et al. 2010) that ITS sequence and taxonomic grouping are not related to an isolate’s ability to germinate seed. The close similarity of ITS sequences of OMF isolated from C. robinsonii and those from other endangered species (C. amoena, C. huegelii, C. patersonii, C. rosella and C. venusta) supports previous suggestions that symbiotic fungi from rare Caladenia are similar in their genetic diversity and OMF specificity (Swarts et al. 2010).
Potentially critical factor: conducive conditions
The importance of conducive conditions for germination was shown by the narrow range of temperature (15–20°C) in which C. robinsonii seeds germinated and developed into functional seedlings. The increased temperature projected for the region could start to limit germination, because germination at 27°C was minimal. This study demonstrated that there is a narrow overlap between the most favourable conditions for the orchid seed and OMF growth, and only within this overlap can most seeds germinate and seedlings develop. The OMF were uniformly less effective in a warmer temperature (27°C), possibly because of fungal growth overwhelming and ‘eating’ the orchid, as suggested previously (Rasmussen 1995). This suggests that maintaining this cooler range of temperature is vital to successful emergence and recovery in the wild. In situ autumn (emergence) temperatures in 2019 were within this range, suggesting that these temperatures are suitable for seedling recruitment.
Similarly, Nikabadi et al. (2014) found that germination was greater at 15°C and 20°C than at 25°C for Microtis media, Pterostylis sanguinea, C. latifolia and Dactylorhiza mafalis. Similar results have been observed in northern hemisphere orchids. For example, germination of European D. majailis declined outside its optimum temperature of 23–25°C (Rasmussen et al. 1990). Furthermore, Nikabadi et al. (2014) found reduced mycorrhizal development at 27°C, suggesting that seed germination may be greater when OMF growth is suboptimal (Nikabadi et al. 2014). In Thelymitra epipactoides, the sensitivity to temperature in germination was ascribed to the OMF provenance rather than the orchid (Reiter et al. 2018). The environmental conditions at the OMF provenance probably resulted in an increased temperature range where germination was achieved. A limiting factor in generalisation is the lack of research investigating temperature effects on orchid germination for both northern and southern hemisphere taxa.
Diurnal variation in temperature would be expected for the seed after dispersal; however, the extent to which this occurs depends on its depth within the soil. The differences in germination observed here in C. robinsonii between constant and diurnal temperatures suggests that seeds are exposed to limited fluctuating temperature in situ. This is consistent with the seed lying on the surface after dispersal and being exposed to temperatures likely to trigger warm stratification. Once the primed seed then becomes buried in the soil and invaded by OMF, diurnal temperature fluctuations would decline. The 25% decrease in germination of stratified seed in vitro in diurnal variation rather than a constant 20°C is consistent with this expectation. These observations differ from those of Kauth et al. (2011), who observed greater germination in vitro of C. tuberosus with diurnal fluctuation (12/12 h) than with constant 25°C, with varying effects being related to location of seed source. By contrast, Johnson and Kane (2012) observed no differences in germination in vitro for Bletia purpurea under constant and diurnal temperature treatments. There is clearly much value in optimising temperature for germination in rare orchids, because this reduces the wastage commonly encountered.
Conservation implications under climate change
The differences in temperature profile for greatest seed germination (15–20°C) and greatest growth of OMF isolates (27°C) have several implications for the conservation of C. robinsonii in view of the predicted changes of almost +2°C projected by 2040 (Bureau of Meteorology, CSIRO 2020). First, these differences suggest that seed germination is optimal within the overlapping temperature ranges for growth of the seedling and the OMF. The likely temperature rise is within the acceptable range, as found in three northern hemisphere orchids (McCormick et al. 2016, 2018). Second, the uniformly greater germination at 15°C/20°C than at 27°C suggests that the orchid controls the temperature responses for seed germination, unlike the case in T. epipactoides (Reiter et al. 2018). If in situ temperatures increase and so favour OMF growth, which is an evident trend at the C. robinsonii population, it may have serious implications for C. robinsonii recruitment and the mutualistic nature of the mycorrhiza (Johnson et al. 1997). These implications could be significantly greater for orchid species that associate with a narrow phylogenetic clade of OMF (Huynh et al. 2009; Swarts et al. 2009). More coordinated studies of the effects of climate factors (temperature and moisture) are needed not only on OMF abundance in soil (McCormick et al. 2016, 2018) but also simultaneously on orchid recruitment and survival in situ.
Conclusions
This is the first study to demonstrate the positive effects of warm stratification on germination and seedling development in the endangered Australian orchid C. robinsonii and may also improve conservation efforts for other threatened plants. Following stratification at 30°C/27°C, germination increased from 21% to 79% and seedlings with green leaves from 14% to 47%. Warm stratification is a simple process that could be implemented to assist recovery of other threatened orchids by improving in vitro germination. Germination and seedling development were unexpectedly less in symbiotic fungi isolated from wild plants than from re-introduced plants at the site. Incubation temperature significantly affected germination of the endangered orchid C. robinsonii and this demonstrated the importance of using in situ conditions to improve germination success in vitro. Furthermore, the plants produced may be more robust and more likely to survive re-introduction, which is vital given the predicted increase of almost +2°C as a result of climate change in the next 30 years at its current distribution. Mimicking in situ conditions, such as warm stratification and germination temperature, should be tested with other orchid species to see whether they are broadly effective at improving germination and, thus, should be adopted for future re-introduction programmes.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
This project was funded by the Government of Victoria through the Biodiversity On-Ground Action grant (grant number BOA2017CA009).
Acknowledgements
BJ thanks RMIT University for a PhD scholarship. This research was conducted under DELWP Permit No. 10008397 as part of Biodiversity On-ground Action grants awarded to TH. All authors thank the Australasian Native Orchid Society, especially Richard Thomson and Russell Mawson, for guidance and assistance with orchid growth and field work. The authors also thank DELWP, especially Karen Lester, for co-operation and assistance with field work and ongoing support.
References
Allen MR, Dube OP, Solecki W, Aragon-Durand F, Cramer W, Humphreys S, Kainuma M, Kala J, Mahowald N, Mulugetta Y, Perez R, Wairiu M, Zickfeld K (2018) Framing and Context. In ‘Global Warming of 1.5°C. An IPCC Special Report on the impacts of global warming of 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty’. (Eds V Masson-Delmotte, P Zhai, H-O Pörtner, D Roberts, J Skea, PR Shukla, A Pirani, W Moufouma-Okia, C Péan, R Pidcock, S Connors, JBR Matthews, Y Chen, X Zhou, MI Gomis, E Lonnoy, T Maycock, M Tignor, T Waterfield) pp. 49–92. ((IPCC), Cambridge University Press: Cambridge, UK and New York, NY, USA)| Crossref |
Bureau of Meteorology (2020a) Climate change in Australia: projections for Australia’s NRM regions. Southern slopes (Victoria West). Available at https://www.climatechangeinaustralia.gov.au/en/climate-projections/future-climate/regional-climate-change-explorer/sub-clusters/?current=SSVWC&popup=true&tooltip=true
Bureau of Meteorology (2020b) Australian climate variability and change – Trend maps. Available at http://www.bom.gov.au/climate/change/index.shtml#tabs=Tracker&tracker=trend-maps
Bureau of Meteorology, CSIRO (2020) ‘State of the climate 2020.’ (Bureau of Meteorology) Available at http://www.bom.gov.au/state-of-the-climate/
Bustam BM, Dixon KW, Bunn E (2014) In vitro propagation of temperate Australian terrestrial orchids: revisiting asymbiotic compared with symbiotic germination. Botanical Journal of the Linnean Society 176, 556–566.
| In vitro propagation of temperate Australian terrestrial orchids: revisiting asymbiotic compared with symbiotic germination.Crossref | GoogleScholarGoogle Scholar |
Clements MA, Muir H, Cribb PJ (1986) A preliminary report on the symbiotic germination of European terrestrial orchids. Kew Bulletin 41, 437–445.
| A preliminary report on the symbiotic germination of European terrestrial orchids.Crossref | GoogleScholarGoogle Scholar |
Dixon K, Tremblay RL (2009) Biology and natural history of Caladenia. Australian Journal of Botany 57, 247–258.
| Biology and natural history of Caladenia.Crossref | GoogleScholarGoogle Scholar |
Dowling N, Jusaitis M (2012) Asymbiotic in vitro germination and seed quality assessment of Australian terrestrial orchids. Australian Journal of Botany 60, 592–601.
| Asymbiotic in vitro germination and seed quality assessment of Australian terrestrial orchids.Crossref | GoogleScholarGoogle Scholar |
Duncan M, Moloney PD (2018) Comparing wild and reintroduced populations of the threatened orchid Diuris fragrantissima (Orchidaceae) in south-eastern Australia. Australian Journal of Botany 66, 459–467.
| Comparing wild and reintroduced populations of the threatened orchid Diuris fragrantissima (Orchidaceae) in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Huynh TT, Thomson R, Mclean CB, Lawrie AC (2009) Functional and genetic diversity of mycorrhizal fungi from single plants of Caladenia formosa (Orchidaceae). Annals of Botany 104, 757–765.
| Functional and genetic diversity of mycorrhizal fungi from single plants of Caladenia formosa (Orchidaceae).Crossref | GoogleScholarGoogle Scholar | 19561011PubMed |
IUCN (2020) The IUCN red list of threatened species. Version 2020-3. Available at https://www.iucnredlist.org/search?query=Orchid&searchType=species
Janissen B, French G, Selby-Pham J, Lawrie AC, Huynh T (2021) Differences in emergence and flowering in wild, re-introduced and translocated populations of an endangered terrestrial orchid and the influences of climate and orchid mycorrhizal abundance. Australian Journal of Botany 69, 9–20.
| Differences in emergence and flowering in wild, re-introduced and translocated populations of an endangered terrestrial orchid and the influences of climate and orchid mycorrhizal abundance.Crossref | GoogleScholarGoogle Scholar |
Johnson TR, Kane ME (2012) Effects of temperature and light on germination and early seedling development of the pine pink orchid (Bletia purpurea). Plant Species Biology 27, 174–179.
| Effects of temperature and light on germination and early seedling development of the pine pink orchid (Bletia purpurea).Crossref | GoogleScholarGoogle Scholar |
Johnson NC, Graham J-H, Smith FA (1997) Functioning of mycorrhizal associations along the mutualism–parasitism continuum. New Phytologist 135, 575–585.
| Functioning of mycorrhizal associations along the mutualism–parasitism continuum.Crossref | GoogleScholarGoogle Scholar |
Kauth PJ, Kane ME, Vendrame WA (2011) Comparative in vitro germination ecology of Calopogon tuberosus var. tuberosus (Orchidaceae) across its geographic range. In Vitro Cellular & Developmental Biology – Plant 47, 148–156.
| Comparative in vitro germination ecology of Calopogon tuberosus var. tuberosus (Orchidaceae) across its geographic range.Crossref | GoogleScholarGoogle Scholar |
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution 35, 1547–1549.
| MEGA X: molecular evolutionary genetics analysis across computing platforms.Crossref | GoogleScholarGoogle Scholar | 29722887PubMed |
Lemay M-A, De Vriendt L, Pellerin S, Poulin M (2015) Ex situ germination as a method for seed viability assessment in a peatland orchid, Platanthera blephariglottis. American Journal of Botany 102, 390–395.
| Ex situ germination as a method for seed viability assessment in a peatland orchid, Platanthera blephariglottis.Crossref | GoogleScholarGoogle Scholar | 25784472PubMed |
McCormick MK, Taylor DL, Whigham DF, Burnett RK (2016) Germination patterns in three terrestrial orchids relate to abundance of mycorrhizal fungi. Journal of Ecology 104, 744–754.
| Germination patterns in three terrestrial orchids relate to abundance of mycorrhizal fungi.Crossref | GoogleScholarGoogle Scholar |
McCormick MK, Whigham DF, Canchani-Viruet A (2018) Mycorrhizal fungi affect orchid distribution and population dynamics. New Phytologist 219, 1207–1215.
| Mycorrhizal fungi affect orchid distribution and population dynamics.Crossref | GoogleScholarGoogle Scholar | 29790578PubMed |
Mehra S, Morrison PD, Coates F, Lawrie AC (2017) Differences in carbon source utilisation by orchid mycorrhizal fungi from common and endangered species of Caladenia (Orchidaceae). Mycorrhiza 27, 95–108.
| Differences in carbon source utilisation by orchid mycorrhizal fungi from common and endangered species of Caladenia (Orchidaceae).Crossref | GoogleScholarGoogle Scholar | 27639577PubMed |
Nikabadi S, Bunn E, Stevens J, Newman B, Turner SR, Dixon KW (2014) Germination responses of four native terrestrial orchids from south-west Western Australia to temperature and light treatments. Plant Cell, Tissue and Organ Culture (PCTOC) 118, 559–569.
| Germination responses of four native terrestrial orchids from south-west Western Australia to temperature and light treatments.Crossref | GoogleScholarGoogle Scholar |
Oktalira FT, Whitehead MR, Linde CC (2019) Mycorrhizal specificity in widespread and narrow-range distributed Caladenia orchid species. Fungal Ecology 42, 100869
| Mycorrhizal specificity in widespread and narrow-range distributed Caladenia orchid species.Crossref | GoogleScholarGoogle Scholar |
Perkin-Elmer (1995) ‘ABI PRISM™ dye terminator cycle sequencing ready reaction kit with AmpliTaq® DNA Polymerase, Protocol Number 402078, Revision A.’ (The Perkin-Elmer Corporation)
Poff KE, Sharma J, Richards M (2016) Cold-moist stratification improves germination in a temperate terrestrial orchid. Castanea 81, 292–301.
| Cold-moist stratification improves germination in a temperate terrestrial orchid.Crossref | GoogleScholarGoogle Scholar |
Raleigh R (2005) Propagation and biology of Arachnorchis (Orchidacae) and their mycorrhizal fungi, PhD Thesis, RMIT Univeristy, Vic., Australia. Available at http://researchbank.rmit.edu.au/view/rmit:9881
Rasmussen HN (1995) ‘Terrestrial orchids: from seed to mycotrophic plant.’ (Cambridge University Press: New York, NY, USA)
Rasmussen H, Andersen TF, Johansen B (1990) Temperature sensitivity of in vitro germination and seedling development of Dactylorhiza majalis (Orchidaceae) with and without a mycorrhizal fungus. Plant, Cell & Environment 13, 171–177.
| Temperature sensitivity of in vitro germination and seedling development of Dactylorhiza majalis (Orchidaceae) with and without a mycorrhizal fungus.Crossref | GoogleScholarGoogle Scholar |
Reiter N, Lawrie AC, Linde CC (2018) Matching symbiotic associations of an endangered orchid to habitat to improve conservation outcomes. Annals of Botany 122, 947–959.
| Matching symbiotic associations of an endangered orchid to habitat to improve conservation outcomes.Crossref | GoogleScholarGoogle Scholar | 29897399PubMed |
Reiter N, Phillips RD, Swarts ND, Wright M, Holmes G, Sussmilch FC, Davis BJ, Whitehead MR, Linde CC (2020) Specific mycorrhizal associations involving the same fungal taxa in common and threatened Caladenia (Orchidaceae): implications for conservation. Annals of Botany 126, 943–955.
| Specific mycorrhizal associations involving the same fungal taxa in common and threatened Caladenia (Orchidaceae): implications for conservation.Crossref | GoogleScholarGoogle Scholar | 32574356PubMed |
Sharma J, Zettler LW, Van Sambeek JW, Ellersieck MR, Starbuck CJ (2003) Symbiotic seed germination and mycorrhizae of federally threatened Platanthera praeclara (Orchidaceae). The American Midland Naturalist 149, 104–120.
| Symbiotic seed germination and mycorrhizae of federally threatened Platanthera praeclara (Orchidaceae).Crossref | GoogleScholarGoogle Scholar |
Swarts ND, Sinclair EA, Krauss SL, Dixon KW (2009) Genetic diversity in fragmented populations of the critically endangered spider orchid Caladenia huegelii: implications for conservation. Conservation Genetics 10, 1199–1208.
| Genetic diversity in fragmented populations of the critically endangered spider orchid Caladenia huegelii: implications for conservation.Crossref | GoogleScholarGoogle Scholar |
Swarts ND, Sinclair EA, Francis A, Dixon KW (2010) Ecological specialization in mycorrhizal symbiosis leads to rarity in an endangered orchid. Molecular Ecology 19, 3226–3242.
| Ecological specialization in mycorrhizal symbiosis leads to rarity in an endangered orchid.Crossref | GoogleScholarGoogle Scholar | 20618899PubMed |
Tamura K, Nei M (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Molecular Biology and Evolution 10, 512–526.
| Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees.Crossref | GoogleScholarGoogle Scholar | 8336541PubMed |
Todd JA (2000) ‘Recovery plan for twelve threatened Spider-orchid Caladenia taxa of Victoria and South Australia 2000–2004.’ (Department of Natural Resources and Environment) Available at https://www.environment.gov.au/system/files/resources/5673cd4d-1802-4927-85e5-4bdbf947ba58/files/12-orchid.pdf
Van Waes JM, Debergh PC (1986) Adaptation of the tetrazolium method for testing the seed viability, and scanning electron microscopy study of some Western European orchids. Physiologia Plantarum 66, 435–442.
| Adaptation of the tetrazolium method for testing the seed viability, and scanning electron microscopy study of some Western European orchids.Crossref | GoogleScholarGoogle Scholar |
WCSP (2017) World checklist of selected plant families. Available at https://wcsp.science.kew.org/incfamilies.do
Weiß M, Waller F, Zuccaro A, Selosse M-A (2016) Sebacinales – one thousand and one interactions with land plants. New Phytologist 211, 20–40.
| Sebacinales – one thousand and one interactions with land plants.Crossref | GoogleScholarGoogle Scholar | 27193559PubMed |
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In ‘PCR protocols: a guide to methods and applications’. pp. 315–322. (Academic Press: San Diego, CA, USA)
Wraith J, Pickering C (2018) Quantifying anthropogenic threats to orchids using the IUCN Red List. Ambio 47, 307–317.
| Quantifying anthropogenic threats to orchids using the IUCN Red List.Crossref | GoogleScholarGoogle Scholar | 29043611PubMed |
Wright M, Cross R, Dixon K, Huynh T, Lawrie A, Nesbitt L, Pritchard A, Swarts N, Thomson R (2009) Propagation and reintroduction of Caladenia. Australian Journal of Botany 57, 373–387.
| Propagation and reintroduction of Caladenia.Crossref | GoogleScholarGoogle Scholar |
Wright MM, Cross R, Cousens RD, May TW, McLean CB (2010) Taxonomic and functional characterisation of fungi from the Sebacina vermifera complex from common and rare orchids in the genus Caladenia. Mycorrhiza 20, 375–390.
| Taxonomic and functional characterisation of fungi from the Sebacina vermifera complex from common and rare orchids in the genus Caladenia.Crossref | GoogleScholarGoogle Scholar | 20054590PubMed |
Wright M, Cross R, Cousens RD, May TW, McLean CB (2011) The functional significance for the orchid Caladenia tentaculata of genetic and geographic variation in the mycorrhizal fungus Sebacina vermifera s. lat. complex. Muelleria 29, 130–140.
Yamamoto T, Miura C, Fuji M, Nagata S, Otani Y, Yagame T, Yamato M, Kaminaka H (2017) Quantitative evaluation of protocorm growth and fungal colonization in Bletilla striata (Orchidaceae) reveals less-productive symbiosis with a non-native symbiotic fungus. BMC Plant Biology 17, 50
| Quantitative evaluation of protocorm growth and fungal colonization in Bletilla striata (Orchidaceae) reveals less-productive symbiosis with a non-native symbiotic fungus.Crossref | GoogleScholarGoogle Scholar | 28222700PubMed |
Zettler LW, Stewart SL, Bowles ML, Jacobs KA (2001) Mycorrhizal fungi and cold-assisted symbiotic germination of the federally threatened eastern prairie fringed orchid, Platanthera leucophaea (Nuttall) Lindley. The American Midland Naturalist 145, 168–175.
| Mycorrhizal fungi and cold-assisted symbiotic germination of the federally threatened eastern prairie fringed orchid, Platanthera leucophaea (Nuttall) Lindley.Crossref | GoogleScholarGoogle Scholar |


