Micropropagation of the therapeutic-honey plants Leptospermum polygalifolium and L. scoparium (Myrtaceae)
Ian D. Darby A , Shahla Hosseini Bai B , Helen M. Wallace A B and Stephen J. Trueman B C
B C
A Genecology Research Centre, University of the Sunshine Coast, Maroochydore DC, Qld 4558, Australia.
B Food Security Platform, Centre for Planetary Health and Food Security, School of Environment and Science, Griffith University, Nathan, Brisbane, Qld 4111, Australia.
C Corresponding author. Email: s.trueman@griffith.edu.au
Australian Journal of Botany 69(5) 310-317 https://doi.org/10.1071/BT21047
Submitted: 1 April 2021 Accepted: 23 June 2021 Published: 14 July 2021
Journal Compilation © CSIRO 2021 Open Access CC BY
Abstract
Demand for therapeutic honey is driving establishment of Leptospermum plantations. This study developed micropropagation methods for two species – Leptospermum polygalifolium Salisb. and L. scoparium J.R.Forst. & G.Forst. The study determined how shoot proliferation and adventitious rooting were influenced by the original explant position on the seedling and the concentration of benzyladenine (BA) in the proliferation medium. Hormone-free node culture was highly effective for both species. Nodal explants often formed roots in the absence of BA and developed elongated axillary shoots. Median shoot numbers of 584 and 659 were formed in 31–32 weeks from a single L. polygalifolium or L. scoparium seed, respectively. A low BA dose was effective for callogenesis and shoot proliferation of L. polygalifolium, but not L. scoparium. The median number of shoots produced from a single L. polygalifolium seed was 630 using 2.22-μM BA. This dose induced extremely high shoot numbers in some clones because explants often produced extensive callus and multiple short shoots. Shoots formed adventitious roots without indole-3-butyric acid and plantlets were acclimatised to nursery conditions. The original explant position did not influence shoot proliferation or adventitious rooting. Leptospermum polygalifolium and L. scoparium proved amenable to micropropagation, facilitating rapid establishment of nectar plantations.
Keywords: adventitious rooting, auxin, cytokinin, dihydroxyacetone, manuka honey, methylglyoxal, Myrtaceae, propagation, therapeutic honey, tissue culture, topophysis, Leptospermum.
Introduction
Mānuka honey has been harvested traditionally in New Zealand from hives of honeybees that forage on the mānuka tree, Leptospermum scoparium (Morgan et al. 2019; Bong et al. 2021; Schmidt et al. 2021). Mānuka honey contains high levels of the antimicrobial and wound-healing compound, methylglyoxal (MGO), which is converted gradually in the honey from dihydroxyacetone (DHA) that is found in L. scoparium nectar (Carter et al. 2016; Cokcetin et al. 2016; Grainger et al. 2016; Niaz et al. 2017; Schmidt et al. 2021). Honeybees that forage on L. scoparium and some other Leptospermum species in Australia also produce honey that contains high levels of MGO (Cokcetin et al. 2016; Pappalardo et al. 2016; Williams et al. 2018). Demand for therapeutic MGO-containing honey is greater than supply, so there is a drive to establish Leptospermum plantations to provide nectar for therapeutic honey production.
One of the limitations to plantation establishment has been the difficulty in propagating Leptospermum species as seedlings because the seed can be difficult to extract from the fruit or difficult to germinate (Shipton and Jackes 1986; Lyne and Crisp 1996; Battersby et al. 2017). Therefore, clonal propagation methods have been sought for propagating Leptospermum species by tissue culture and cuttings. Clonal propagation has been used to propagate limited numbers of elite clones in clonal plantation programs (Trueman 2006; Xavier et al. 2013; Trueman et al. 2018). These programs have the advantage that individual clones have been selected for desirable mature-age characteristics such as trunk straightness, wood volume or fruit yield (Aimers-Halliday and Burdon 2003; Mitchell et al. 2004; Wendling et al. 2014a, 2014b). Clonal plantation programs have the disadvantage that selected clones have often undergone maturation, reducing their propagation capacity and growth potential (Aimers-Halliday and Burdon 2003; Pijut et al. 2011; Wendling et al. 2014a, 2014b). Increasingly, clonal propagation is used instead to propagate multiple clones that are the progeny of selected mother trees in ‘vegetative family plantation programs’ (Lee 2007; Trueman et al. 2018). These programs have the disadvantage that not all clones share the full suite of desirable characteristics of the selected mother tree. However, a vegetative family program has the advantage that the clones are juvenile, often giving them much higher propagation capacity and growth potential than mature clones (McMahon et al. 2013, 2014; Wendling et al. 2014a, 2014b).
The current study is part of a program focussed on developing clonal propagation methods for juvenile germplasm of therapeutic-honey Leptospermum species. Little was known about the micropropagation capacity of most Leptospermum species, although an extensive array of micropropagation methods has been developed for other Myrtaceae species from the eucalypt genera, Corymbia and Eucalyptus. These methods employ cytokinins, typically benzyladenine (BA), to promote axillary bud outgrowth or adventitious shoot production (Trueman et al. 2018). Shoots of L. scoparium and its hybrids have been proliferated in vitro in full-strength Murashige and Skoog (MS) medium (Murashige and Skoog 1962) containing BA at 0.00–4.44 μM (Braun and Leung 1991; Seelye et al. 2001; Fan et al. 2013). Acclimatisation of plantlets to nursery conditions proved difficult for L. scoparium (Braun and Leung 1991) but was successful for plantlets of L. scoparium × L. rotundifolium backcrossed to L. rotundifolium (Seelye et al. 2001). Eucalypt shoots are usually proliferated in full-strength MS media or MS salts that are supplemented with BA at 0.44–6.67 μM (Niccol et al. 1994; Hervé et al. 2001; Glocke et al. 2005; Arya et al. 2009; Hung and Trueman 2012). However, eucalypt shoot proliferation can sometimes be prolific in media lacking BA (Brondani et al. 2013; Trueman et al. 2018). Hormone-free medium promotes extensive shoot elongation and the production of multiple nodes, whereas media containing BA promote callogenesis and the production of multiple short shoots, in cultures of Corymbia torelliana × C. citriodora (Trueman and Richardson 2007; Hung and Trueman 2010). Shoot proliferation potential in the hormone-free medium was influenced by the position on the seedling from which the original explant was taken, with proliferation being higher from the first node (i.e. cotyledonary node) and second node than more-apical nodes (Hung and Trueman 2011). These types of positional effects on the growth and development of propagules are termed ‘topophysis’, and an understanding of topophysic effects can lead to great improvements in the efficiency of a propagation system (George 1993; Mitchell et al. 2004; Hung and Trueman 2011; Wendling et al. 2015).
This study aimed to develop micropropagation methods for two species – L. polygalifolium and L. scoparium – that are highly sought for establishing therapeutic honey plantations. The study aimed to determine the extent to which shoot proliferation and nursery acclimatisation are influenced by the concentration of BA in the proliferation medium. The study also aimed to assess the extent to which micropropagation capacity is affected by the position of the initial explant on the original seedling.
Materials and methods
Seed germination
Seeds of a local subtropical provenance of L. polygalifolium were provided by Dr Peter Brooks (University of the Sunshine Coast, Sippy Downs, Australia) and seeds of selected L. scoparium from southern Australia were provided by Mr Ted Allender (ERA Nurseries, Hamilton, Australia). Batches of ~100 seeds of each species were washed in 70% ethanol (v/v) for 1 min in 70-mL vials containing one drop of Tween 20. They were then rinsed in sterile distilled water. Each batch was then separated into batches of 25 seeds, and each new batch was transferred into a new vial containing 1% sodium hypochlorite with one drop of Tween 20. The vials were swirled on an orbital shaker at 240 rpm for 10 min. The seeds were rinsed in sterile distilled water. Seeds were placed on sterile paper to remove excess liquid between solutions. Seeds were plated (25 seeds per 90-mm-diameter Petri dish) onto germination medium consisting of half-strength MS basal salts (PhytoTechnology Laboratories, Shawnee Mission, KS) with 58.4-μM sucrose, solidified with 8 g L–1 of agar (Bacto Laboratories, Liverpool, Australia) and with pH adjusted to 5.8 before autoclaving at 121°C for 20 min. This procedure was repeated to create 90 dishes, each of 25 seeds, per species. The seeds were maintained at 28°C under a 16-h photoperiod (~100 μmol m–2 s–1 with fluorescent tubes) for 3–4 weeks.
Shoot induction (first passage)
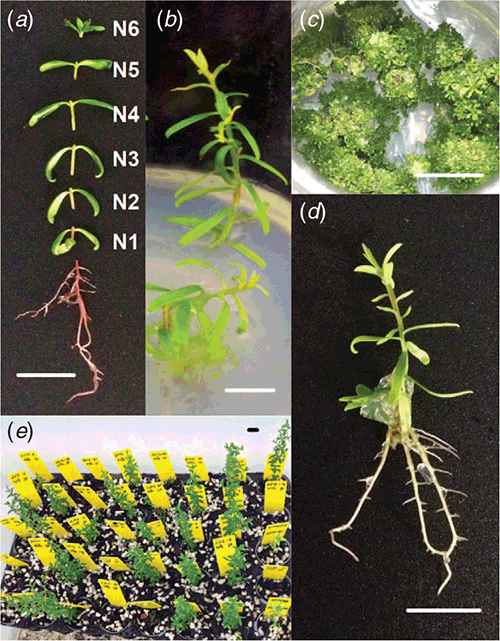
All shoots greater than 5-mm length were excised at the root collar and transferred (5 shoots per jar) into 375-mL glass jars containing 50 mL of shoot induction medium consisting of full-strength MS medium with vitamins (PhytoTechnology Laboratories) with 87.6-μM sucrose, solidified with 8 g L–1 of agar and with pH adjusted to 5.8 before autoclaving at 121°C for 20 min. The shoots were maintained at 28°C under a 16-h photoperiod (~100 μmol m–2 s–1 with fluorescent tubes) for 5 weeks. The shoots were then dissected into their individual nodes by cutting immediately above each node (Fig. 1a). The apical bud was regarded as a single node.
|
Shoot proliferation (second, third and fourth passages)
Each node from each seedling was transferred to a 375-mL glass jar containing full-strength MS medium with vitamins, with 87.6-μM sucrose and one of the following five hormone concentrations: 0.0-, 2.2-, 4.4-, 8.9- or 17.8-μM BA (Sigma, Saint Louis, MO, USA). All nodes from a single seedling were placed in the one jar (i.e. one clone per jar) for the first proliferation passage. The original position of each node on the seedling was marked on the outside of the jar, with ‘1’ being the cotyledonary node and ‘2,’ ‘3’, ‘4’, 5’ or ‘6’ representing progressively more-apical nodes. At least 15 clones per BA treatment were available for each species, and each clone number was marked on the outside of the jar. The shoots were maintained at 28°C under a 16-h photoperiod (~100 μmol m–2 s–1 with fluorescent tubes). The shoots were maintained for 7 weeks, then dissected into their nodes and transferred to fresh medium of the same BA concentration for a second proliferation passage of 8 weeks. They were then dissected into their nodes and transferred again to fresh medium of the same BA concentration for a third proliferation passage of 8 weeks. The number of shoots with roots and the number of nodes available for transfer from each of the original-seedling node positions was counted at the end of each proliferation passage, i.e. after 7, 15 and 23 weeks in proliferation medium. The percentage of shoots per clone with roots and the number of available nodes per clone was then calculated from the data obtained from each of the original-seedling node positions.
Root induction
A random subsample of 20 shoots >15 mm long (where available) in hormone-free medium was taken from each original seedling-node position of each clone after the third proliferation passage. These shoots were transferred randomly into one of four glass jars (five shoots per jar) containing 50 mL of half-strength MS medium with 58.4-μM sucrose, 8 g L–1 of agar and either 0.0-, 4.9-, 19.6- or 78.4-μM indole-3-butyric acid (IBA). The shoots were maintained in this medium for 24 h in darkness at 24°C. They were then transferred to hormone-free half-strength MS medium with 58.4-μM sucrose and 8 g L–1 of agar, where they were maintained for 6 days in darkness and then 3 weeks at ~100 μmol m–2 s–1 irradiance. The same process was applied to shoots from proliferation medium containing 2.2-μM BA, except that these shoots were transferred first to hormone-free proliferation medium for 5 weeks to allow shoot elongation before they were transferred to the IBA treatments.
Nursery acclimatisation
Plantlets were then transferred into 70-mL propagation tubes (Darby et al. 2021) containing eucalypt seedling mix consisting of a 75/25 (v/v) mixture of shredded pine bark and perlite, with 3 kg of 8–9-month slow-release Osmocote fertiliser (Scotts International, Heerlen, Netherlands), 3 kg of lime (Unimin, Lilydale, Australia), 1 kg of gypsum (Queensland Organics, Narangba, Australia), 1 kg of Micromax micronutrients (Scotts Australia, Baulkham Hills, Australia) and 1 kg of Hydroflow wetting agent (Scotts Australia) incorporated per square metre (Trueman et al. 2013a, 2013b, 2013c, 2013d, 2014). The plantlets were maintained at 28°C under a 16-h photoperiod (~50 μmol m–2 s–1) in a sealed 80-L plastic tub for a further 4 weeks. The tubs were then transferred to a polyethylene propagation chamber that was custom-built within a glasshouse. Plantlets were maintained within the sealed tub and under 50% shadecloth (~85 μmol m–2 s–1) for 3 days, and then the tub lid was opened progressively at 4-day intervals and the shadecloth removed to increase irradiance to ~380 μmol m–2 s–1 over a further 12 days. Mist irrigation was provided for 1 min every 10 min from 0700 to 1800 hours. The percentage of shoots from each jar that had survived and formed roots was recorded after 8 weeks in the glasshouse.
Statistical analyses
Shoot proliferation data was analysed by Kruskal–Wallis test because shoot number data was not normally distributed. Dunn’s tests with Bonferroni corrections were used when significant differences among the medians were detected by Kruskal–Wallis test. Medians are presented with 25th and 75th percentiles, 10th and 90th percentiles, and outliers. Rooting data was analysed by t-test or by random block ANOVA because adventitious rooting data was normally distributed. Clones were regarded as blocks. Means are presented with standard errors. Differences between medians or means were regarded as significant at P < 0.05.
Results
Shoot proliferation
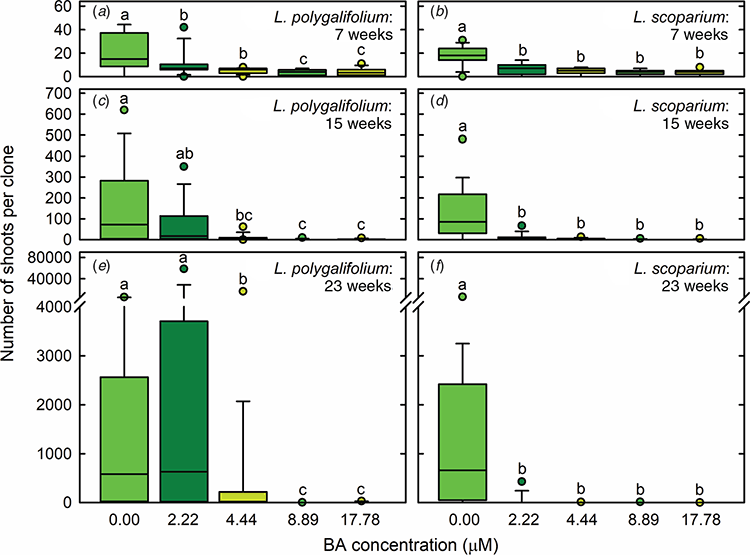
The highest production of both L. polygalifolium and L. scoparium shoots during the first proliferation passage occurred in hormone-free medium (Fig. 2a, b). Explants developed new nodes on elongating axillary shoots in the absence of BA (Fig. 1b). L. polygalifolium also produced multiple shoots during the second and third proliferation passages in medium containing 2.22-μM BA (Fig. 2c, e), with these being short shoots that developed either from or through callus (Fig. 1c). Extensive explant death occurred in media containing 4.44–17.78-μM BA for L. polygalifolium (Fig. 2c, e) and 2.22–17.78-μM BA for L. scoparium (Fig. 2d, f). As a result, the most effective BA concentrations for shoot proliferation were 0.00 or 2.22 μM for L. polygalifolium (Fig. 2e) and 0.00 μM for L. scoparium (Fig. 2f). The median (range) numbers of shoots produced per clone were 584 (0–5427) in 0.0-μM BA and 630 (0–58 635) in 2.2-μM BA for L. polygalifolium and 659 (0–5985) in 0.0-μM BA for L. scoparium.
Root induction and nursery acclimatisation
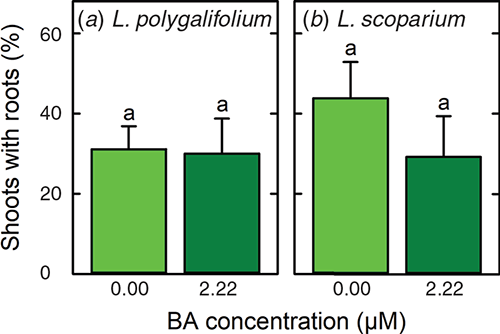
Extensive shoot death occurred when L. polygalifolium and L. scoparium were treated with 4.9-, 19.6- or 78.4-μM IBA (data not presented). However, rooting occurred in medium that lacked IBA (Fig. 1d), and many of these plantlets were acclimatised successfully to nursery conditions (Fig. 1e). The percentages of shoots that formed roots in the absence of IBA and acclimatised successfully to nursery conditions were 31 ± 6 and 30 ± 9% for L. polygalifolium shoots that had been proliferated in media containing 0.00- or 2.22-μM BA respectively (Fig. 3a). The percentages of shoots that formed roots and acclimatised successfully were 44 ± 9 and 29 ± 10% for L. scoparium shoots that had been proliferated in media containing 0.00- or 2.22-μM BA respectively (Fig. 3b).
|
Topophysic effects on proliferation, rooting and nursery acclimatisation
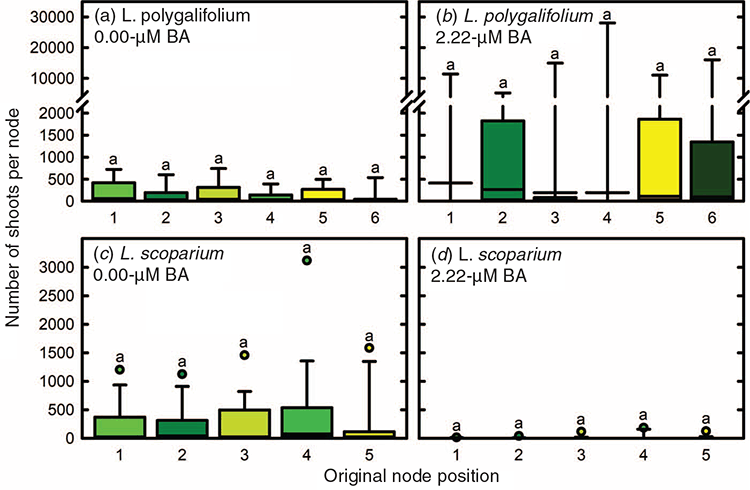
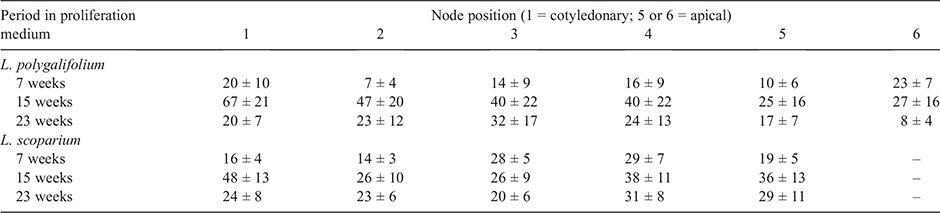
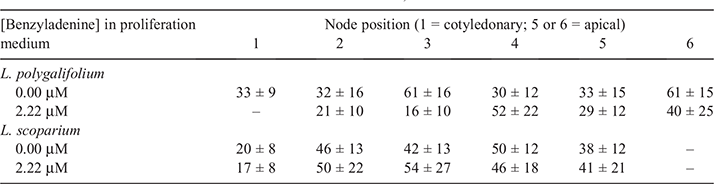
The position of the initial explant on the original seedling did not significantly affect the proliferation capacity of L. polygalifolium or L. scoparium shoots in media containing either 0.00- or 2.22-μM BA (Fig. 4). Many shoots formed roots in proliferation medium containing 0.00-μM BA (Table 1) but rooting was almost never observed in media containing BA (data not shown). Root formation in the hormone-free proliferation medium was not affected significantly by the original explant position (Table 1). Root induction and nursery acclimatisation of plantlets was also not influenced significantly by the original explant position (Table 2).
Discussion
Seedling shoots of L. polygalifolium and L. scoparium proved highly susceptible to shoot death in the presence of BA doses, 4.44–17.78 and 2.22–17.78 μM respectively that are commonly used for proliferation of eucalypt shoots. These doses are used for shoot proliferation of eucalypts including C. citriodora, C. torelliana × C. citriodora, Eucalyptus cloeziana, E. dunnii, E. erythronema and E. stricklandii (Tanabe et al. 1996; Glocke et al. 2005; Hung and Trueman 2012; Navroski et al. 2014; Oliveira et al. 2015; Trueman et al. 2018). However, hormone-free medium was highly effective for shoot proliferation of both L. polygalifolium and L. scoparium. Nodal explants often formed roots in this medium and they developed elongated axillary shoots from existing axillary buds. As a result, median shoot numbers of 584 and 659 could be formed in 31–32 weeks from plating of a single L. polygalifolium or L. scoparium seed, respectively. This type of node culture in BA-free medium has also proven highly effective for propagation of C. torelliana × C. citriodora (Trueman and Richardson 2007; Hung and Trueman 2010). A potential advantage of node culture is that shoot proliferation is not associated with the formation of callus, and so this method reduces the risk of releasing or inducing somaclonal variation in the propagated plant population (George 1993).
Use of a low dose of BA (2.22 μM) was also effective for shoot proliferation of L. polygalifolium, but not L. scoparium. The median number of shoots produced from a single L. polygalifolium seed in this medium (630) was similar to the number produced in BA-free medium (i.e. 584). However, BA had the capacity to induce extremely high numbers of shoots in some clones. This was because explants in BA-containing medium often produced extensive callus and multiple short shoots, without forming roots. Similar callogenesis has been observed in cultures of C. torelliana × C. citriodora with the same BA dose (Trueman and Richardson 2007; Hung and Trueman 2010, 2011, 2012). Shoot formation appeared to occur through a combination of axillary shoot production, callogenesis and shoot regeneration, as it does in C. torelliana × C. citriodora (Trueman and Richardson 2007; Hung and Trueman 2010). The relative contributions of axillary shoots and adventitious shoots to total shoot proliferation are difficult to ascertain, especially because many Myrtaceae species have multiple axillary and accessory buds within each leaf axil (Burrows et al. 2008; Burrows 2013). Histological examination would be required to determine the cellular origin of the multiple shoots produced in the presence of BA (Dobrowolska et al. 2017; Trueman et al. 2018). The possibility that many of the shoots are formed via an intervening callus phase may explain the very high shoot proliferation in some clones. However, it also means that this method has the potential to release or induce somaclonal variation (George 1993; Tibok et al. 1995; Mo et al. 2009). This might not be a concern in a vegetative family propagation program that establishes nursery stock populations and plantations with a large and diverse range of juvenile clones.
Shoots of both L. polygalifolium and L. scoparium were converted successfully into plantlets and acclimatised to nursery conditions. Rooting and acclimatisation success were similar among shoots that had been produced along the hormone-free node-culture pathway and the BA-induced callogenic pathway, noting, though, that shoots from the latter pathway were maintained in the absence of BA for 5 weeks before root induction. The percentages of shoots that formed roots and acclimatised successfully were somewhat low (30–31% for L. polygalifolium and 29–44% for L. scoparium), and these percentages could not be increased using IBA doses that stimulate adventitious rooting in eucalypt shoots (Trueman et al. 2018) and in shoots of L. scoparium × L. rotundifolium backcrossed to L. rotundifolium (Seelye et al. 2001). Fortunately, the nursery stock plants formed from the L. polygalifolium and L. scoparium plantlets have very high capacity for rooted-cutting production (Darby et al. 2021). The stock plants produce shoots prolifically and the cuttings from these shoots have very high capacity for adventitious rooting. Rooting percentages of L. polygalifolium and L. scoparium cuttings are 88–93 and 65–76% respectively, which would be considered high for most eucalypt species (Trueman et al. 2017; Darby et al. 2021). These rooting percentages are also higher than, or similar to, the 70% threshold that is required by many commercial nurseries (Trueman 2006; Hunt et al. 2011; Rigby and Trueman 2015).
No evidence was found of topophysic effects on in-vitro rooting, shoot proliferation or ex vitro acclimatisation of L. polygalifolium or L. scoparium shoots. The first (cotyledonary) node and the second node have higher rooting, shoot elongation and shoot proliferation capacity than more apical nodes in hormone-free node cultures of C. torelliana × C. citriodora (Hung and Trueman 2011). Such topophysic effects are not evident in BA-induced callogenic cultures, where rooting and extensive shoot elongation do not occur. These topophysic effects in C. torelliana × C. citriodora cultures allow the separation of different seedling nodes into different propagation pathways to optimise shoot proliferation, clonal archiving and field testing. The current results indicate that the first five or six nodes of L. polygalifolium or L. scoparium have similar proliferation and acclimatisation capacity. Therefore, separation of explants on the basis of node position would not be beneficial unless subsequent maturation effects on plant performance, associated with higher node positions, became evident in the nursery or plantation (Aimers-Halliday and Burdon 2003; Mitchell et al. 2004; Trueman 2006; Wendling et al. 2014a, 2014b).
In conclusion, L. polygalifolium and L. scoparium have high capacity for mass propagation in a simple node-culture system that does not employ BA and does not induce callus formation. One of these species, L. polygalifolium, also has high capacity for micropropagation in a callogenic-culture system that uses a low dose of BA (i.e. 2.22 μM). Shoots from both systems can be converted into plantlets and acclimatised to nursery conditions. These plantlets have been found to have excellent capacity as nursery stock plants for rooted-cutting production (Darby et al. 2021). These results demonstrate that L. polygalifolium and L. scoparium can be propagated rapidly to establish high-value nectar plantations for therapeutic-honey production.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
Ian Darby was the recipient of a USC Postgraduate Scholarship.
Acknowledgements
Thanks to Peter Brooks (USC) and Ted Allender (ERA Nurseries) for providing seeds and Brittany Elliott, Tracey McMahon, Barbara Robinson and Michael Nielsen for technical assistance.
References
Aimers-Halliday J, Burdon RD (2003) Risk management for clonal forestry with Pinus radiata – Analysis and review. 2: Technical and logistical problems and countermeasures. New Zealand Journal of Forestry Science 33, 181–204.Arya ID, Sharma S, Chauhan S, Arya S (2009) Micropropagation of superior eucalyptus hybrids FRI-5 (Eucalyptus camaldulensis Dehn × E. tereticornis Sm) and FRI-14 (Eucalyptus torelliana F.V. Muell × E. citriodora Hook): a commercial multiplication and field evaluation. African Journal of Biotechnology 8, 5718–5726.
| Micropropagation of superior eucalyptus hybrids FRI-5 (Eucalyptus camaldulensis Dehn × E. tereticornis Sm) and FRI-14 (Eucalyptus torelliana F.V. Muell × E. citriodora Hook): a commercial multiplication and field evaluation.Crossref | GoogleScholarGoogle Scholar |
Battersby PF, Wilmshurst JM, Curran TJ, Perry GLW (2017) Does heating stimulate germination in Leptospermum scoparium (mānuka; Myrtaceae)? New Zealand Journal of Botany 55, 452–465.
| Does heating stimulate germination in Leptospermum scoparium (mānuka; Myrtaceae)?Crossref | GoogleScholarGoogle Scholar |
Bong J, Middleditch M, Loomes KM, Stephens JM (2021) Proteomic analysis of honey. Identification of unique peptide markers for authentication of NZ mānuka (Leptospermum scoparium) honey. Food Chemistry 350, 128442
| Proteomic analysis of honey. Identification of unique peptide markers for authentication of NZ mānuka (Leptospermum scoparium) honey.Crossref | GoogleScholarGoogle Scholar | 33388180PubMed |
Braun RH, Leung DWM (1991) Establishment and clonal propagation of in vitro plantlets of Leptospermum scoparium. New Zealand Natural Sciences 18, 39–43.
Brondani GE, Oliveira LS, Bergonci T, Brondani AE, França FAM, Silva ALL, Gonçalves AN (2013) Chemical sterilization of culture medium: a low cost alternative to in vitro establishment of plants. Scientia Forestalis 41, 257–264.
Burrows GE (2013) Buds, bushfires and resprouting in the eucalypts. Australian Journal of Botany 61, 331–349.
| Buds, bushfires and resprouting in the eucalypts.Crossref | GoogleScholarGoogle Scholar |
Burrows GE, Hornby SK, Waters DA, Bellairs SM, Prior LD, Bowman DMJS (2008) Leaf axil anatomy and bud reserves in 21 Myrtaceae species from northern Australia. International Journal of Plant Sciences 169, 1174–1186.
| Leaf axil anatomy and bud reserves in 21 Myrtaceae species from northern Australia.Crossref | GoogleScholarGoogle Scholar |
Carter DA, Blair SE, Cokcetin NN, Bouzo D, Brooks P, Schothauer R, Harry EJ (2016) Therapeutic manuka honey: no longer so alternative. Frontiers in Microbiology 7, 569
| Therapeutic manuka honey: no longer so alternative.Crossref | GoogleScholarGoogle Scholar | 27148246PubMed |
Cokcetin NN, Pappalardo M, Campbell LT, Brooks P, Carter DA, Blair SE, Harry EJ (2016) The antibacterial activity of Australian Leptospermum honey correlates with methylglyoxal levels. PLoS One 11, e0167780
| The antibacterial activity of Australian Leptospermum honey correlates with methylglyoxal levels.Crossref | GoogleScholarGoogle Scholar | 28030589PubMed |
Darby ID, Hosseini Bai S, Wallace HM, Trueman SJ (2021) Adventitious rooting of cuttings from the therapeutic honey plants, Leptospermum polygalifolium and L. scoparium. Rhizosphere 17, 100306
| Adventitious rooting of cuttings from the therapeutic honey plants, Leptospermum polygalifolium and L. scoparium.Crossref | GoogleScholarGoogle Scholar |
Dobrowolska I, Andrade GM, Clapham D, Egertsdotter U (2017) Histological analysis reveals the formation of shoots rather than embryos in regenerating cultures of Eucalyptus globulus. Plant Cell, Tissue and Organ Culture 128, 319–326.
| Histological analysis reveals the formation of shoots rather than embryos in regenerating cultures of Eucalyptus globulus.Crossref | GoogleScholarGoogle Scholar |
Fan TF, Liu HG, Wang TT, Du M, Zhang LS (2013) An efficient plant regeneration system for manuka (Leptospermum scoparium) from seedlings. Indian Journal of Horticulture 70, 423–427.
George EF (1993) ‘Plant Propagation by Tissue Culture. Part 1. The Technology.’ (Exegetics Ltd: Edington, UK)
Glocke P, Collins G, Sedgley M (2005) In vitro organogenesis from seedling explants of the ornamentals Eucalyptus erythronema, E. stricklandii and the interspecific hybrid E. erythronema × E. stricklandii cv. ‘Urrbrae Gem’. The Journal of Horticultural Science & Biotechnology 80, 97–104.
| In vitro organogenesis from seedling explants of the ornamentals Eucalyptus erythronema, E. stricklandii and the interspecific hybrid E. erythronema × E. stricklandii cv. ‘Urrbrae Gem’.Crossref | GoogleScholarGoogle Scholar |
Grainger MNC, Manley-Harris M, Lane JR, Field RJ (2016) Kinetics of conversion of dihydroxyacetone to methylglyoxal in New Zealand mānuka honey: part I – honey systems. Food Chemistry 202, 484–491.
| Kinetics of conversion of dihydroxyacetone to methylglyoxal in New Zealand mānuka honey: part I – honey systems.Crossref | GoogleScholarGoogle Scholar |
Hervé P, Jauneau A, Pâques M, Marien J-N, Boudet AM, Teulières C (2001) A procedure for shoot organogenesis in vitro from leaves and nodes of an elite Eucalyptus gunnii clone: comparative histology. Plant Science 161, 645–653.
| A procedure for shoot organogenesis in vitro from leaves and nodes of an elite Eucalyptus gunnii clone: comparative histology.Crossref | GoogleScholarGoogle Scholar |
Hung CD, Trueman SJ (2010) Nutrient responses differ between node and organogenic cultures of Corymbia torelliana × C. citriodora (Myrtaceae). Australian Journal of Botany 58, 410–419.
| Nutrient responses differ between node and organogenic cultures of Corymbia torelliana × C. citriodora (Myrtaceae).Crossref | GoogleScholarGoogle Scholar |
Hung CD, Trueman SJ (2011) Topophysic effects differ between node and organogenic cultures of the eucalypt Corymbia torelliana × C. citriodora. Plant Cell, Tissue and Organ Culture 104, 69–77.
| Topophysic effects differ between node and organogenic cultures of the eucalypt Corymbia torelliana × C. citriodora.Crossref | GoogleScholarGoogle Scholar |
Hung CD, Trueman SJ (2012) Cytokinin concentrations for optimal micropropagation of Corymbia torelliana × C. citriodora. Australian Forestry 75, 233–237.
| Cytokinin concentrations for optimal micropropagation of Corymbia torelliana × C. citriodora.Crossref | GoogleScholarGoogle Scholar |
Hunt MA, Trueman SJ, Rasmussen A (2011) Indole-3-butyric acid accelerates adventitious root formation and impedes shoot growth of Pinus elliottii var. elliottii × P. caribaea var. hondurensis cuttings. New Forests 41, 349–360.
| Indole-3-butyric acid accelerates adventitious root formation and impedes shoot growth of Pinus elliottii var. elliottii × P. caribaea var. hondurensis cuttings.Crossref | GoogleScholarGoogle Scholar |
Lee DJ (2007) Achievements in forest tree genetic improvement in Australia and New Zealand 2: development of Corymbia species and hybrids for plantations in eastern Australia. Australian Forestry 70, 11–16.
| Achievements in forest tree genetic improvement in Australia and New Zealand 2: development of Corymbia species and hybrids for plantations in eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Lyne AM, Crisp MD (1996) Leptospermum jingera (Myrtaceae – Leptospermoideae): a new species from north-eastern Victoria. Australian Systematic Botany 9, 301–306.
| Leptospermum jingera (Myrtaceae – Leptospermoideae): a new species from north-eastern Victoria.Crossref | GoogleScholarGoogle Scholar |
McMahon TV, Hung CD, Trueman SJ (2013) In vitro storage delays the maturation of African mahogany (Khaya senegalensis) clones. Journal of Plant Sciences 8, 31–38.
| In vitro storage delays the maturation of African mahogany (Khaya senegalensis) clones.Crossref | GoogleScholarGoogle Scholar |
McMahon TV, Hung CD, Trueman SJ (2014) Clonal maturation of Corymbia torelliana × C. citriodora is delayed by minimal-growth storage. Australian Forestry 77, 9–14.
| Clonal maturation of Corymbia torelliana × C. citriodora is delayed by minimal-growth storage.Crossref | GoogleScholarGoogle Scholar |
Mitchell RG, Zwolinski J, Jones NB (2004) A review on the effects of donor maturation on rooting and field performance of conifer cuttings. Southern African Forestry Journal 201, 53–63.
| A review on the effects of donor maturation on rooting and field performance of conifer cuttings.Crossref | GoogleScholarGoogle Scholar |
Mo XY, Long T, Liu Z, Lin H, Liu XZ, Yang YM, Zhang HY (2009) AFLP analysis of somaclonal variations in Eucalyptus globulus. Biologia Plantarum 53, 741–744.
| AFLP analysis of somaclonal variations in Eucalyptus globulus.Crossref | GoogleScholarGoogle Scholar |
Morgan ER, Perry NB, Chagné D (2019) Science at the intersection of cultures – Māori, Pākehā and mānuka. New Zealand Journal of Crop and Horticultural Science 47, 225–232.
| Science at the intersection of cultures – Māori, Pākehā and mānuka.Crossref | GoogleScholarGoogle Scholar |
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum 15, 473–497.
| A revised medium for rapid growth and bio assays with tobacco tissue cultures.Crossref | GoogleScholarGoogle Scholar |
Navroski MC, Reiniger LRS, Araújo MM, Curti AR, Pereira MO (2014) In vitro establishment and multiplication of genotypes of Eucalyptus dunnii Maiden. Cerne 20, 139–146.
| In vitro establishment and multiplication of genotypes of Eucalyptus dunnii Maiden.Crossref | GoogleScholarGoogle Scholar |
Niaz K, Faheemmaqbool F, Bahadar H, Abdollahi M (2017) Health benefits of manuka honey as an essential constituent for tissue regeneration. Current Drug Metabolism 18, 881–892.
Niccol RJ, Regan PA, De Filippis LF (1994) Simplified protocol for the micropropagation of selected Eucalyptus and Banksia species. Australian Forestry 57, 143–147.
| Simplified protocol for the micropropagation of selected Eucalyptus and Banksia species.Crossref | GoogleScholarGoogle Scholar |
Oliveira LS, Brondani GE, Batagin-Piotto KD, Calsavara R, Gonçalves AN, Almeida M (2015) Micropropagation of Eucalyptus cloeziana mature trees. Australian Forestry 78, 219–231.
| Micropropagation of Eucalyptus cloeziana mature trees.Crossref | GoogleScholarGoogle Scholar |
Pappalardo M, Pappalardo L, Brooks P (2016) Rapid and reliable HPLC method for the simultaneous determination of dihydroxyacetone, methylglyoxal and 5-hydroxymethylfurfural in Leptospermum honeys. PLoS One 11, e0167006
| Rapid and reliable HPLC method for the simultaneous determination of dihydroxyacetone, methylglyoxal and 5-hydroxymethylfurfural in Leptospermum honeys.Crossref | GoogleScholarGoogle Scholar | 28030589PubMed |
Pijut PM, Wowste KE, Michler CH (2011) Promotion of adventitious root formation of difficult-to-root hardwood tree species. Horticultural Reviews 38, 213–251.
Rigby EM, Trueman SJ (2015) Umbrella fern: a new ornamental crop. Acta Horticulturae 221–226.
| Umbrella fern: a new ornamental crop.Crossref | GoogleScholarGoogle Scholar |
Schmidt C, Eichelberger K, Rohm H (2021) New Zealand mānuka honey – A review on specific properties and possibilities to distinguish mānuka from kānuka honey. Lebensmittel-Wissenschaft + Technologie 136, 110311
| New Zealand mānuka honey – A review on specific properties and possibilities to distinguish mānuka from kānuka honey.Crossref | GoogleScholarGoogle Scholar |
Seelye JF, Hofmann BL, Burge GK, Morgan ER (2001) In vitro propagation of Leptospermum hybrids. New Zealand Journal of Crop and Horticultural Science 29, 233–237.
| In vitro propagation of Leptospermum hybrids.Crossref | GoogleScholarGoogle Scholar |
Shipton WA, Jackes BR (1986) Clonal propagation of Leptospermum spp. by tissue culture. Plant Cell Reports 5, 5–8.
| Clonal propagation of Leptospermum spp. by tissue culture.Crossref | GoogleScholarGoogle Scholar | 24247954PubMed |
Tanabe T, Murakami A, Tachikawa H, Izumi M, Shimizu T, Murakami K (1996) Practical micropropagation of eucalypt species for the pulp and paper industry. In ‘Environmental Management: the Role of Eucalypts and Other Fast Growing Species’. (Ed. KG Eldridge) pp. 53–64. (CSIRO Publishing: Melbourne, Vic., Australia)
Tibok A, Blackhall NW, Power JB, Davey MR (1995) Optimized plant regeneration from callus derived from seedling hypocotyls of Eucalyptus urophylla. Plant Science 110, 139–145.
| Optimized plant regeneration from callus derived from seedling hypocotyls of Eucalyptus urophylla.Crossref | GoogleScholarGoogle Scholar |
Trueman SJ (2006) Clonal propagation and storage of subtropical pines in Queensland, Australia. Southern African Forestry Journal 208, 49–52.
| Clonal propagation and storage of subtropical pines in Queensland, Australia.Crossref | GoogleScholarGoogle Scholar |
Trueman SJ, Richardson DM (2007) In vitro propagation of Corymbia torelliana × C. citriodora (Myrtaceae) via cytokinin-free node culture. Australian Journal of Botany 55, 471–481.
| In vitro propagation of Corymbia torelliana × C. citriodora (Myrtaceae) via cytokinin-free node culture.Crossref | GoogleScholarGoogle Scholar |
Trueman SJ, McMahon TV, Bristow M (2013a) Production of cuttings in response to stock plant temperature in the subtropical eucalypts, Corymbia citriodora and Eucalyptus dunnii. New Forests 44, 265–279.
| Production of cuttings in response to stock plant temperature in the subtropical eucalypts, Corymbia citriodora and Eucalyptus dunnii.Crossref | GoogleScholarGoogle Scholar |
Trueman SJ, McMahon TV, Bristow M (2013b) Production of Eucalyptus cloeziana cuttings in response to stock plant temperature. Journal of Tropical Forest Science 25, 60–69.
Trueman SJ, McMahon TV, Bristow M (2013c) Biomass partitioning in Corymbia citriodora, Eucalyptus cloeziana and E. dunnii stock plants in response to temperature. Journal of Tropical Forest Science 25, 504–509.
Trueman SJ, McMahon TV, Bristow M (2013d) Nutrient partitioning among the roots, hedge and cuttings of Corymbia citriodora stock plants. Journal of Soil Science and Plant Nutrition 13, 977–989.
| Nutrient partitioning among the roots, hedge and cuttings of Corymbia citriodora stock plants.Crossref | GoogleScholarGoogle Scholar |
Trueman SJ, McMahon TV, Grant EL, Walton DA, Wallace HM (2014) Designing food and habitat trees for urban koalas: graft compatibility, survival and height of tall eucalypt species grafted onto shorter rootstocks. Australian Journal of Botany 62, 196–204.
| Designing food and habitat trees for urban koalas: graft compatibility, survival and height of tall eucalypt species grafted onto shorter rootstocks.Crossref | GoogleScholarGoogle Scholar |
Trueman SJ, McMahon TV, Grant EL, Walton DA, Theilemann PH, McKinnon AJ, Wallace HM (2017) Designing food and habitat trees for urban koalas: tree height, foliage palatability and clonal propagation of Eucalyptus kabiana. Urban Forestry & Urban Greening 27, 196–202.
| Designing food and habitat trees for urban koalas: tree height, foliage palatability and clonal propagation of Eucalyptus kabiana.Crossref | GoogleScholarGoogle Scholar |
Trueman SJ, Hung CD, Wendling I (2018) Tissue culture of Corymbia and Eucalyptus. Forests 9, 84
| Tissue culture of Corymbia and Eucalyptus.Crossref | GoogleScholarGoogle Scholar |
Wendling I, Trueman SJ, Xavier A (2014a) Maturation and related aspects in clonal forestry – part I: concepts, regulation and consequences of phase change. New Forests 45, 449–471.
| Maturation and related aspects in clonal forestry – part I: concepts, regulation and consequences of phase change.Crossref | GoogleScholarGoogle Scholar |
Wendling I, Trueman SJ, Xavier A (2014b) Maturation and related aspects in clonal forestry – Part II: reinvigoration, rejuvenation and juvenility maintenance. New Forests 45, 473–486.
| Maturation and related aspects in clonal forestry – Part II: reinvigoration, rejuvenation and juvenility maintenance.Crossref | GoogleScholarGoogle Scholar |
Wendling I, Brooks PR, Trueman SJ (2015) Topophysis in Corymbia torelliana × C. citriodora seedlings: adventitious rooting capacity, stem anatomy, and auxin and abscisic acid concentrations. New Forests 46, 107–120.
| Topophysis in Corymbia torelliana × C. citriodora seedlings: adventitious rooting capacity, stem anatomy, and auxin and abscisic acid concentrations.Crossref | GoogleScholarGoogle Scholar |
Williams SD, Pappalardo L, Bishop J, Brooks PR (2018) Dihydroxyacetone production in the nectar of Australian Leptospermum is species dependent. Journal of Agricultural and Food Chemistry 66, 11133–11140.
| Dihydroxyacetone production in the nectar of Australian Leptospermum is species dependent.Crossref | GoogleScholarGoogle Scholar | 30289260PubMed |
Xavier A, Wendling I, Silva RL (2013) ‘Silvicultura clonal – princípios e técnicas.’ (Editora UFV: Viçosa, Brazil)