Nutritional traits of riverine eucalypts across lowland catchments in southeastern Australia
Denise R. Fernando A , Fiona Dyer B , Susan Gehrig C , Sam Capon D , Anthony E. Fernando E , Amy George D , Cherie Campbell F , Alica Tschierschke B , Gary Palmer D , Micah Davies
A , Fiona Dyer B , Susan Gehrig C , Sam Capon D , Anthony E. Fernando E , Amy George D , Cherie Campbell F , Alica Tschierschke B , Gary Palmer D , Micah Davies  G , Andrew S. Kinsela H , Richard N. Collins H , Martin Nolan G and Tanya Doody
G , Andrew S. Kinsela H , Richard N. Collins H , Martin Nolan G and Tanya Doody  G *
G *
A Department of Ecology, Environment and Evolution, La Trobe University, Bundoora, Vic. 3085, Australia.
B Centre for Applied Water Science, University of Canberra, Canberra, ACT 2617, Australia.
C Flora, Flow & Floodplains, Mildura, Vic., Australia.
D Australian Rivers Institute, Griffith University, South Brisbane, Qld 4101, Australia.
E Ecolinc Science and Technology Innovations Centre, Maddingley, Vic. 3340, Australia.
F Centre for Applied Water Science, University of Canberra, Canberra, ACT 2617, Australia.
G CSIRO Land and Water, Glen Osmond, SA 5064, Australia.
H School of Civil and Environmental Engineering, University of New South Wales, Sydney, NSW 2052, Australia.
Australian Journal of Botany 69(8) 565-584 https://doi.org/10.1071/BT21002
Submitted: 2 January 2021 Accepted: 21 July 2021 Published: 28 October 2021
© 2021 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial 4.0 International License (CC BY-NC)
Abstract
Eucalyptus (Myrtaceae) trees are ubiquitous in riparian–floodplain zones of Australia’s south-eastern river catchments, where natural ecosystems continue to be affected. In the Murray–Darling Basin (MDB), provision of environmental flows to mitigate tree decline is informed by past field studies. However, broadscale empirical field data on tree nutrition and response to external changes remain scarce. This is the first study to gather soil and plant data across a large area of catchment lowlands to generate a low-resolution regional snapshot of tree nutrition and soil chemistry. Leaves and soils were sampled across and adjacent to the MDB; from and beneath mature trees of three key riverine eucalypts, Eucalyptus largiflorens, E. camaldulensis, and E. coolabah. Foliar sodium concentrations ranged from ∼500 mg kg−1 for E. coolabah up to ∼4500 mg kg−1 for E. largiflorens, with highest values at the River Murray sites. The results suggest E. largiflorens is highly salt tolerant by foliage accumulation given all trees sampled were in good condition. Further research into these species is needed to determine toxicity thresholds for elements such as sodium to aid early diagnosis of potential tree stress, which could provide an additional line of evidence for when environmental water is required to mitigate decline.
Keywords: environmental flows, eucalypt nutrition, Eucalyptus camaldulensis, Eucalyptus coolabah, Eucalyptus largiflorens, floodplain–riparian environments, Lake Eyre Basin, Murray–Darling Basin, Murray River, natural waterflows, plant biogeochemistry, salinity, water extraction.
Introduction
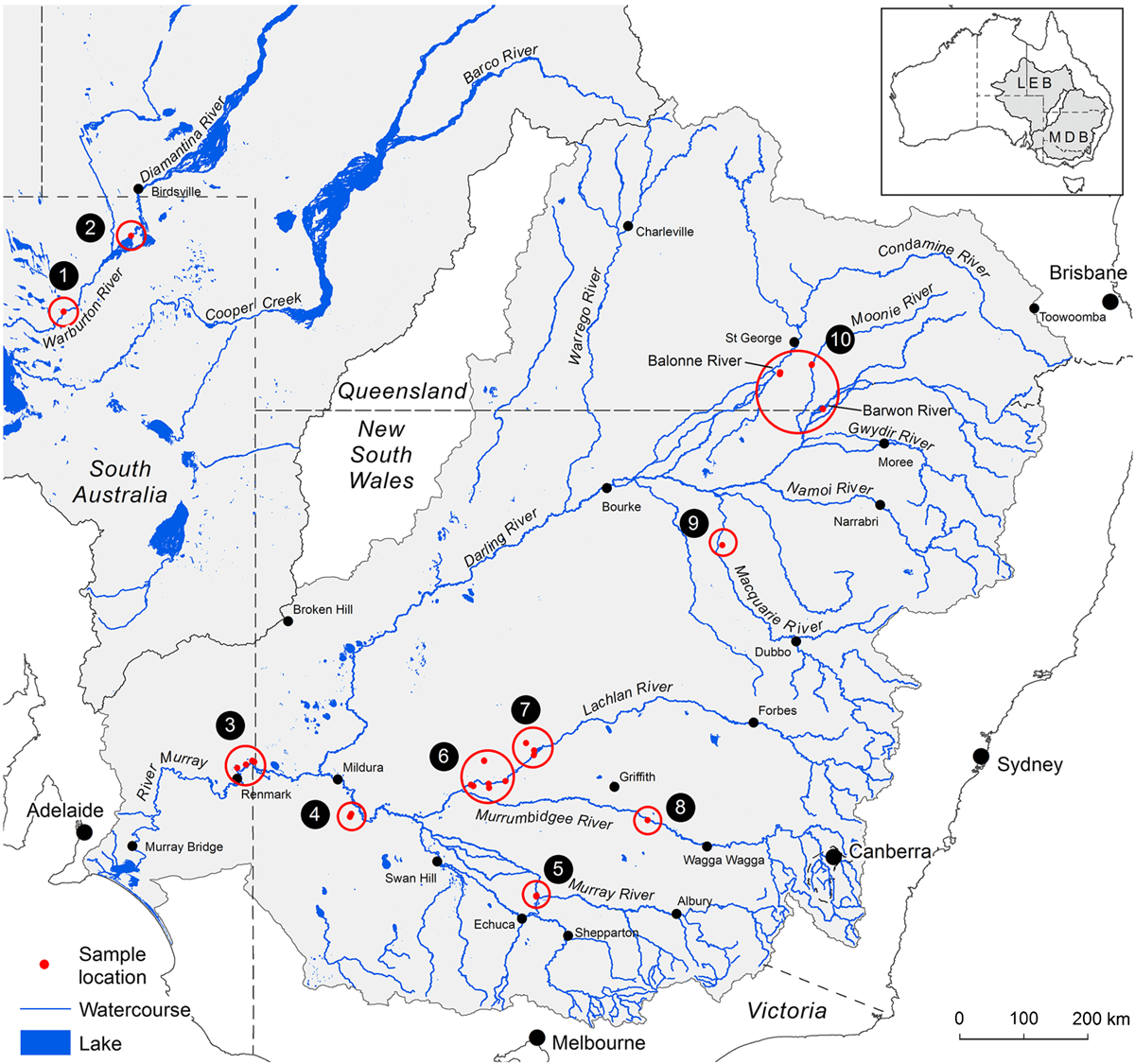
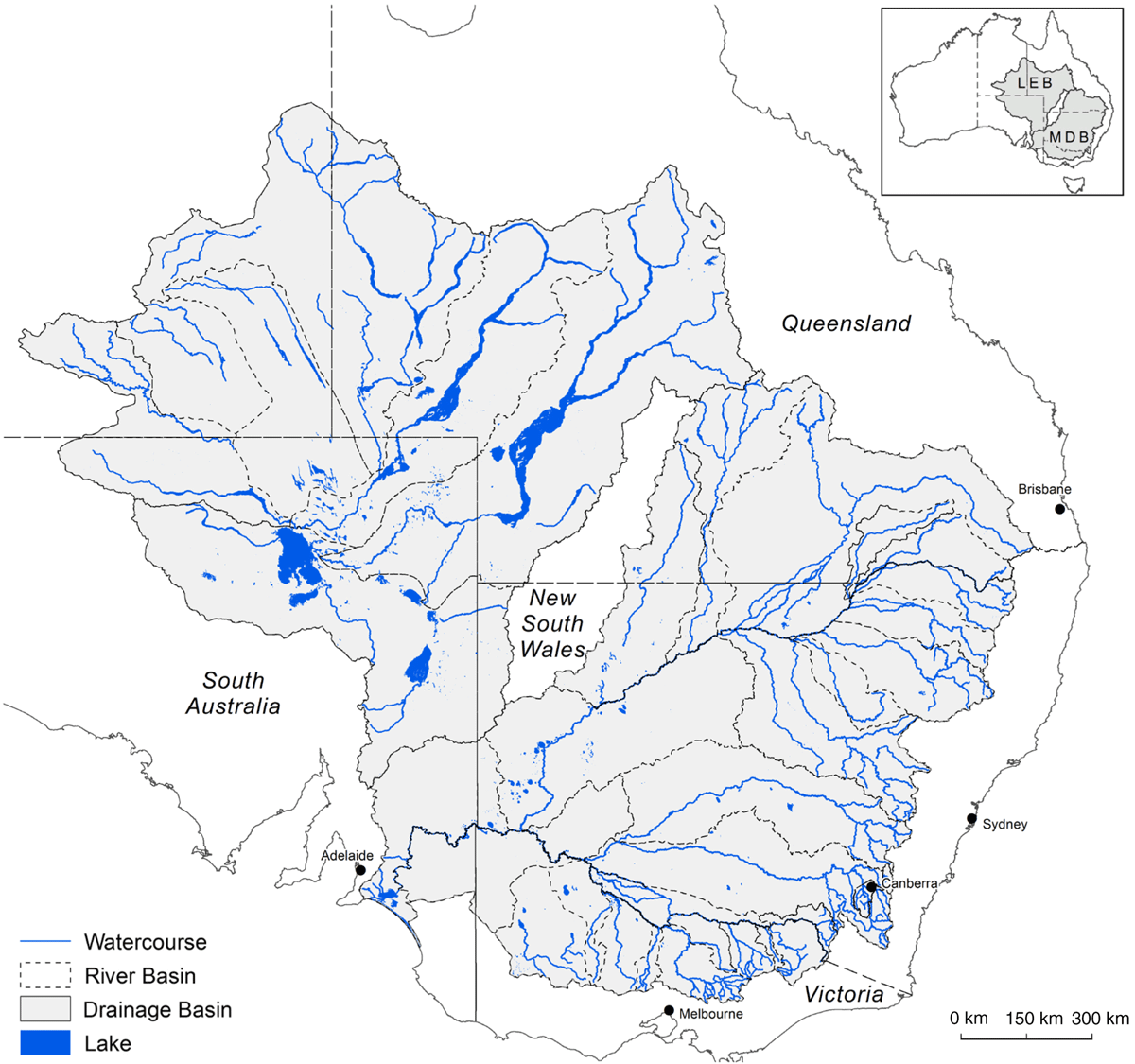
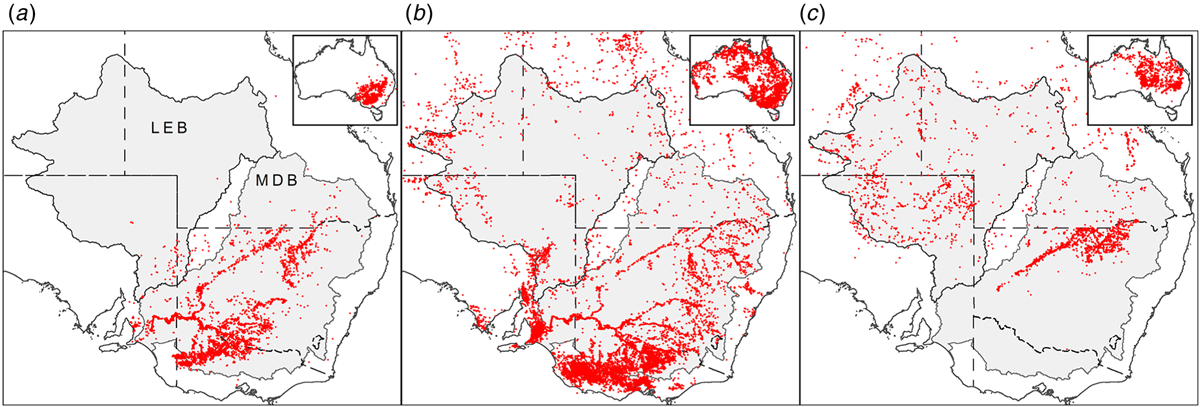
The condition of native trees reliant on natural waterflows within some of Australia’s key river basin systems such as the Murray–Darling Basin (MDB; Fig. 1) continues to be variously affected by diminishing environmental conditions, primarily attributed to climate change and water extraction (Pittock and Finlayson 2011; Swirepik et al. 2016; Grafton et al. 2018). The agriculturally productive MDB represents over a million square kilometres, incorporating a variety of ecosystems, from aquatic to riparian to floodplain. Trees native to these habitats are highly represented by the southern hemisphere genus Eucalyptus (Myrtaceae); mostly E. largiflorens Muell. (Black Box), E. camaldulensis Dehnh. (River Red Gum), and E. coolabah Blakely and Jacobs (Coolabah) (Fig. 2) (Overton et al. 2009; Roberts and Marston 2011; Doody et al. 2014; https://avh.chah.org.au/).
|
|
Disjunct studies undertaken over past decades have offered useful insights (Treloar 1959; Stone and Bacon 1995; Akeroyd et al. 1998; Bramley et al. 2003; Jensen et al. 2008; Doody et al. 2009, 2015; Rahimi-Nasrabadi et al. 2013); however, their collective geographic coverage has historically focused on the southwest corner of the MDB, in South Australia. It is difficult to resolve causes and effects related to stress response in mature trees given the complexities of, (a) underlying interrelated drivers such as soil chemistry, climate, water flows and plant genetics (Fernando and Lynch 2015; Fernando et al. 2018), and, (b) resolving time lags between specific causes and their detectable effects. Although measurement of parameters such as new growth flush, leaf water potential and germination have been undertaken in discrete studies, these parameters variously depend on one or more factors such as time of collection and season.
Plant nutrition and soil chemistry are among the most important determinants of tree condition, but are generally overlooked in floodplain–riparian environments such as in the MDB. For example, many native tree-based studies have investigated water sources of the key native tree species in the basin (Mensforth et al. 1994b; Holland et al. 2005; Doody et al. 2009), tree water potential dynamics (Akeroyd et al. 1998; Zubrinich et al. 2000; Doody et al. 2009; Wallace et al. 2021), stomatal conductance (McEvoy 1992; Mensforth et al. 1994a), soil chloride concentrations and matric potential (Thorburn et al. 1993; Mensforth et al. 1994a; Wallace et al. 2020). An important study by McLennan et al. (2013) does however, provide some key insights into the potential use of plant biogeochemistry as an environmental monitoring tool, particularly in relation to molybdenum, sodium and potassium.
Soil characteristics, land topography and climate largely determine the natural distributions of tree species, and to some extent, their nutritional makeup, notwithstanding basic requirement by plants for certain essential macro- and micro-nutrients (Bedinger 1979; Medina and Cuevas 1994; Sakai and Ohsawa 1994; Marschner 2002). Therefore, any evaluation of nutritional data across a large geographically heterogenous area such as the MDB should take these external factors into consideration. Furthermore, genetic variation and local adaptations are similarly underpinned by such external factors. Scientific literature that presents empirical data on tree nutrition across river basin ecosystems remains scarce, possibly due in part to constraints of geographic scale, with evaluation of overall vegetation structure the most widely used approach to date (Bradley and Smith 1986; Sunil et al. 2010; Wallace et al. 2020). Although remote sensing techniques have previously been employed to assess MDB tree condition from far above canopy level (Cunningham et al. 2013), the relatedness of such data to in situ tree–soil interactions has not been sufficiently explored.
The management and accounting of water resources is an issue of mounting importance globally (Grafton et al. 2018), and to that extent, evaluation of tree nutrition on a broader scale, and with respect to hydrology, will ultimately assist future management practices. Fundamental parameters of tree condition besides canopy-related metrics from across the MDB have not been measured thus far, except in the far southwest corner (Slavich et al. 1999; Bramley et al. 2003; Miller et al. 2003; Smith and Smith 2014; Moxham et al. 2018). The low-resolution empirical study presented here assembled field data on the mineral-nutrient and aluminium (Al) concentrations in floodplain and riparian eucalypt foliage, and their host soils. Sampling from a vast area incorporating lowland sites across the MDB and an area outside was made possible by opportunistically collaborating with a network of researchers and seeking samples from their field sites. These sites were being studied for a variety of research objectives outside the scope of this present study. The sampling area also consisted of regions underlain by groundwater salinised through the complex interaction of several contributing factors (Herczeg et al. 2001). The objectives of this study were to (a) measure tree nutrition and soil chemistry by paired samplings of leaves and soils to identify new methods by which to inform water-management strategies, (b) compare foliage elemental concentrations for each tree species at different geographic locations, and (c) interpret field soil data obtained here against a surface-soil map of the regions studied.
Materials and methods
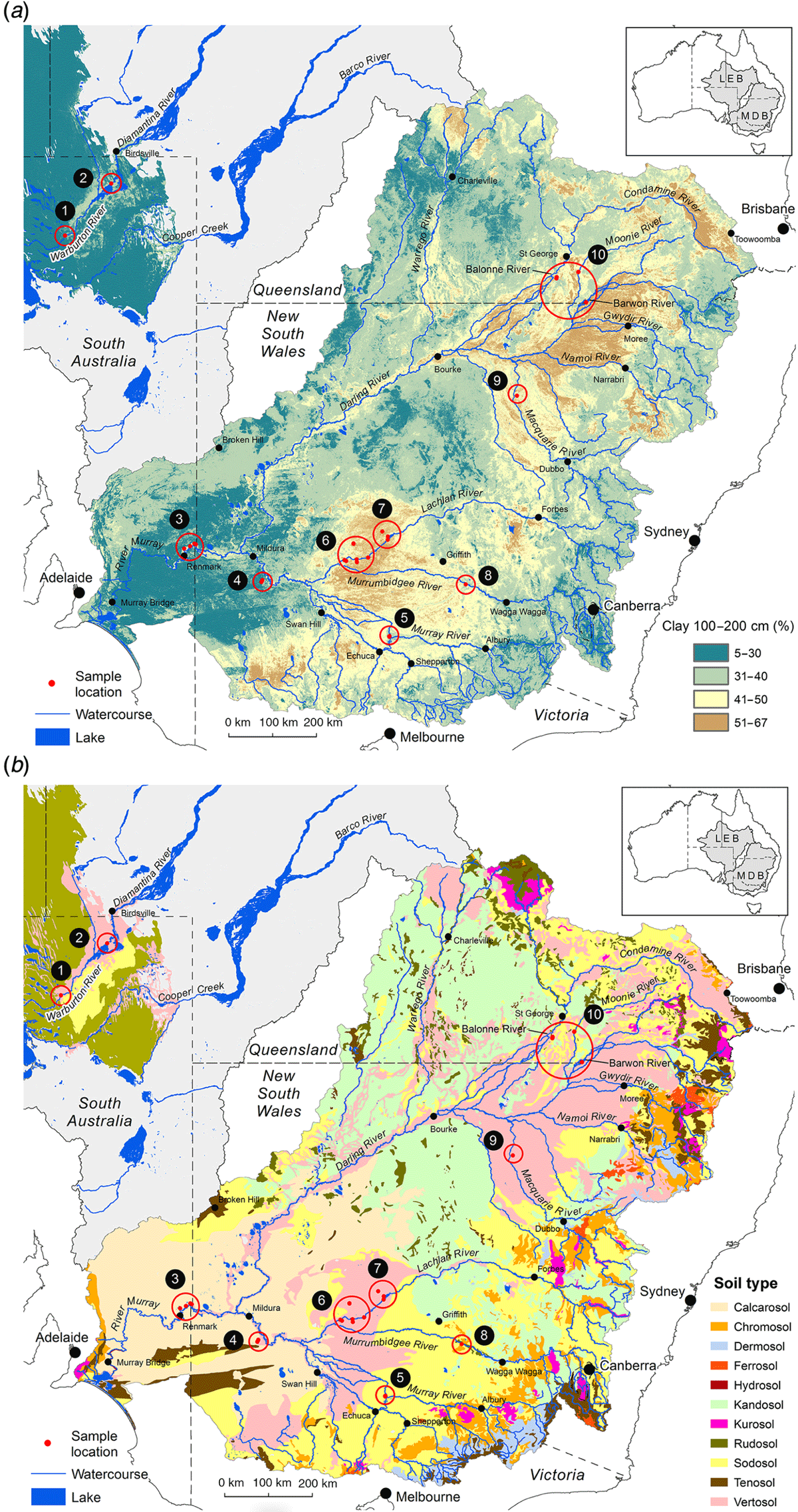
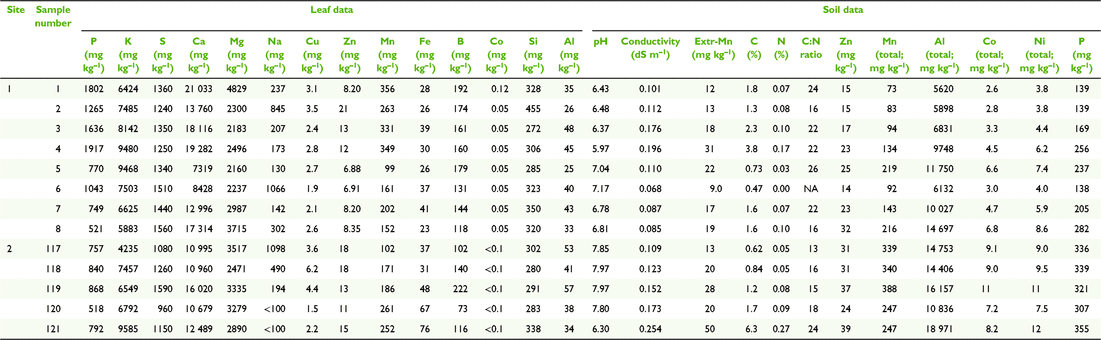
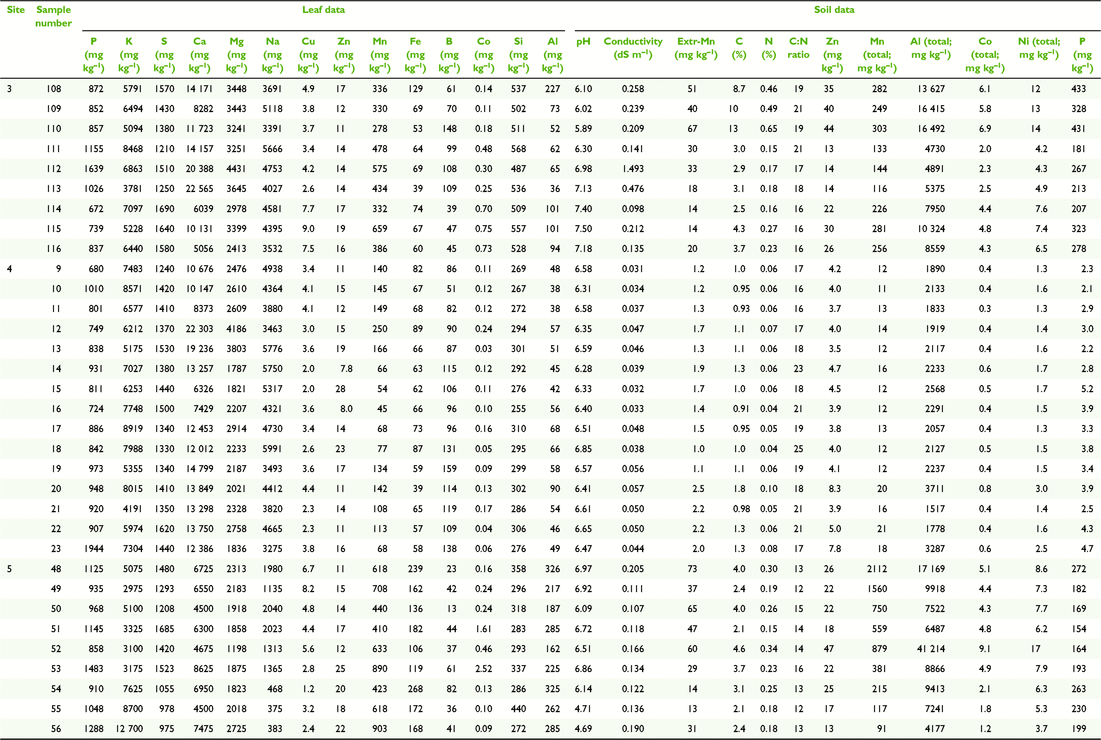
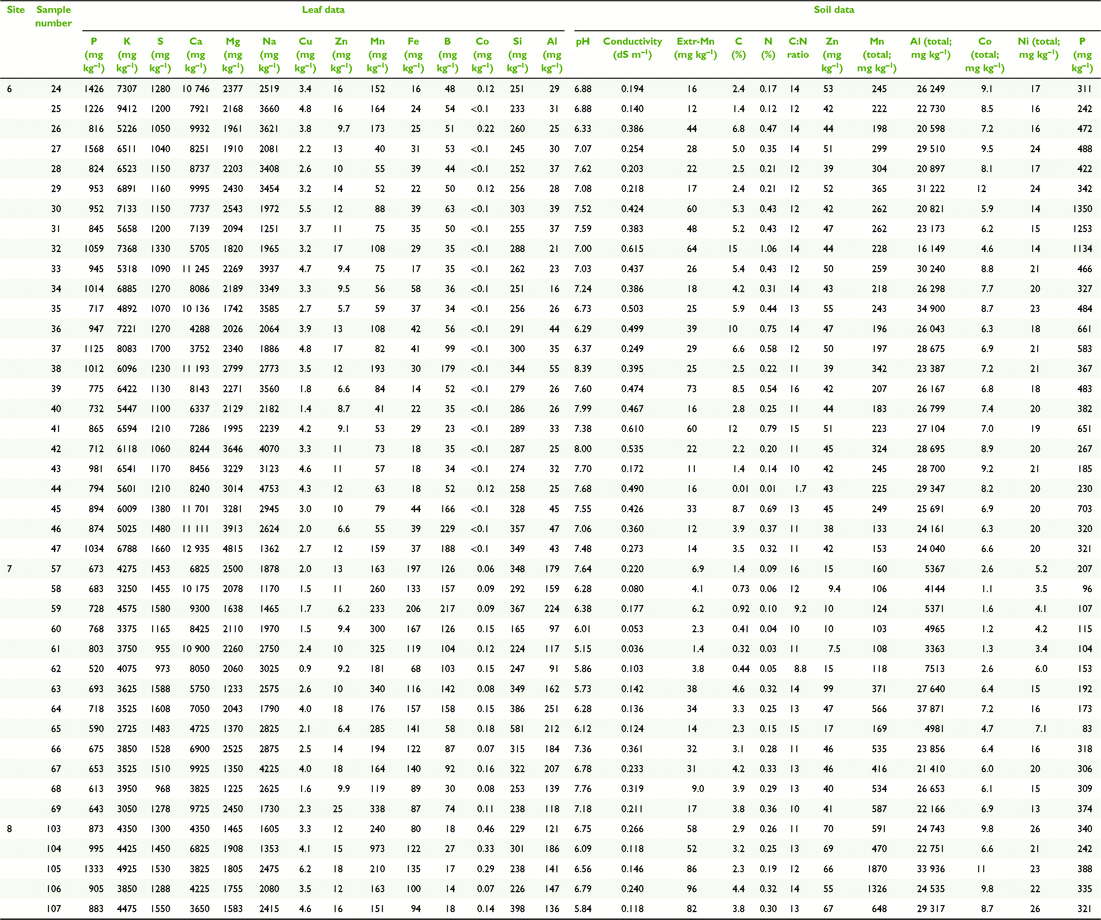
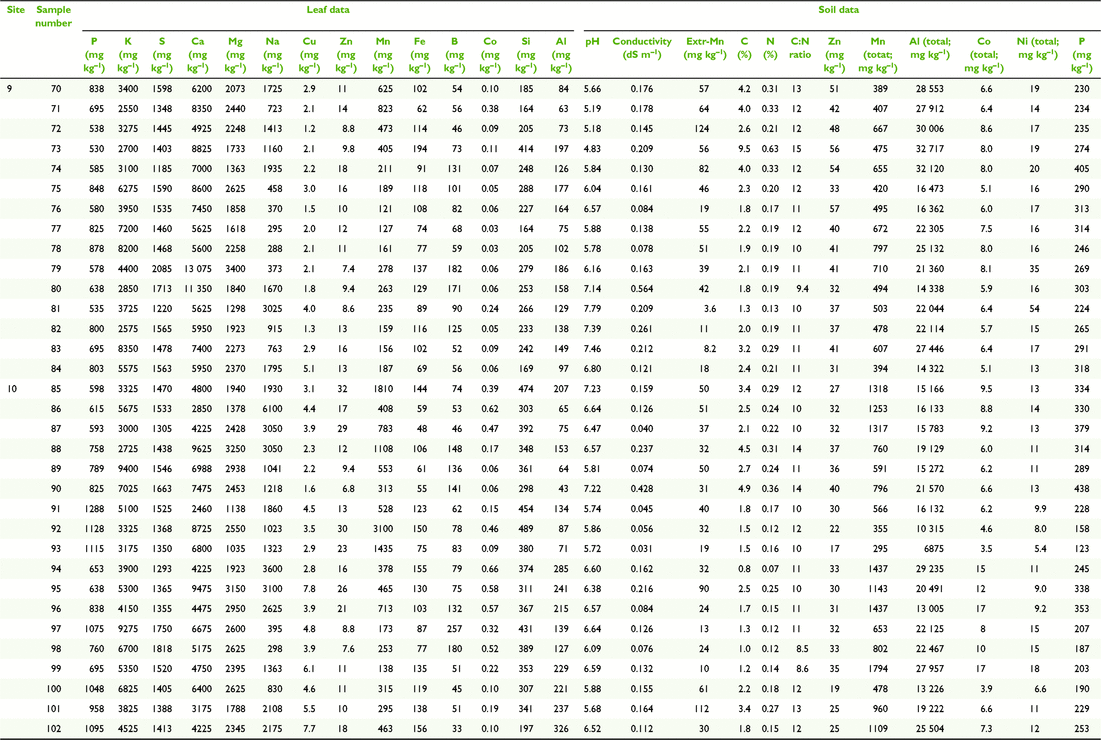
Paired plant and soil samples were accessed from locations 2–9 within the MDB, and two external locations 1 and 2 (Fig. 3 and Table 1) between May 2017 and July 2018. The latter sites in the Lake Eyre Drainage Basin were included to incorporate geographic outliers to the MDB, and additional E. coolabah trees, a species, whose distribution skirts the far northern MDB (Fig. 2c). As a great majority of these sites were already in use for a variety of research projects (Fig. 4), their inclusion in this present study enabled access to empirical sampling data from a wide geographic area otherwise unlikely to be investigated solely for the purposes of gathering tree nutritional and soil chemical data. The sites were grouped such that locations 1 and 2 were on the Diamantina River, locations 3–5 on the Murray River, locations 6–8 on the Murrumbidgee and Lachlan Rivers, and locations 9 and 10 in the Darling River. At each location, 10–20 mature leaves were collected from at least one of the tree species E. largiflorens (n = 57), E. camaldulensis (n = 40) and E. coolabah (n = 24). For each tree sampled, a paired soil sample was collected from the top 10-cm horizon within the dripline of that tree, by pooling 3–5 replicates. Leaves were collected in paper bags, and soil placed in plastic zip-lock bags. All samples were oven dried prior to being analysed as described by Fernando et al. (2018). For each leaf element, tissue-concentration data across species and sites were statistically examined for significant differences (P = 0.05) using one-way ANOVA and the post hoc Hochberg’s GT2 test. Variance was assumed to be equal. Two-way ANOVA was used to test for significant species and site effects on leaf elemental concentrations (P = 0.05). Calculations were undertaken using SPSS (ver. 27, IBM Corporation, New York, NY, USA).
|
Results
Soil composite maps of the study regions (Fig. 5) depict natural clay content shown here to vary between ∼5 and 67% down to 1–2-m depths, and 11 soil classes. According to these data, soil clay content on the Murray (3–5) and Diamantina (1, 2) sites are the lowest of all sites, with soil types similar within each group, and differing between them. The most notable difference in soil clay content was between the Murray–Diamantina soils and the Murrumbidgee–Lachlan soils (6–8) that are similar within each of those groups. The Darling sites (9, 10) have medium to high clay content and similar soil types.
|
In general, average total soil Al concentrations (Tables 2–5) were higher in the Murrumbidgee–Lachlan (22 671 ± 1339) and Darling (20 691 ± 1137) basins than in the Murray (7093 ± 1339) and Diamantina (11 217 ± 1209) basins. Soil pH in each of the four river-basin datasets ranged from strong-moderately acidic, to moderately alkaline values of pH 4.70–7.50 in the Murray basin, pH 5.15–8.39 in the Murrumbidgee–Lachlan basin, pH 4.83–7.79 in the Darling basin, and, pH 5.97–7.97 in the Diamantina basin.
|
|
|
|
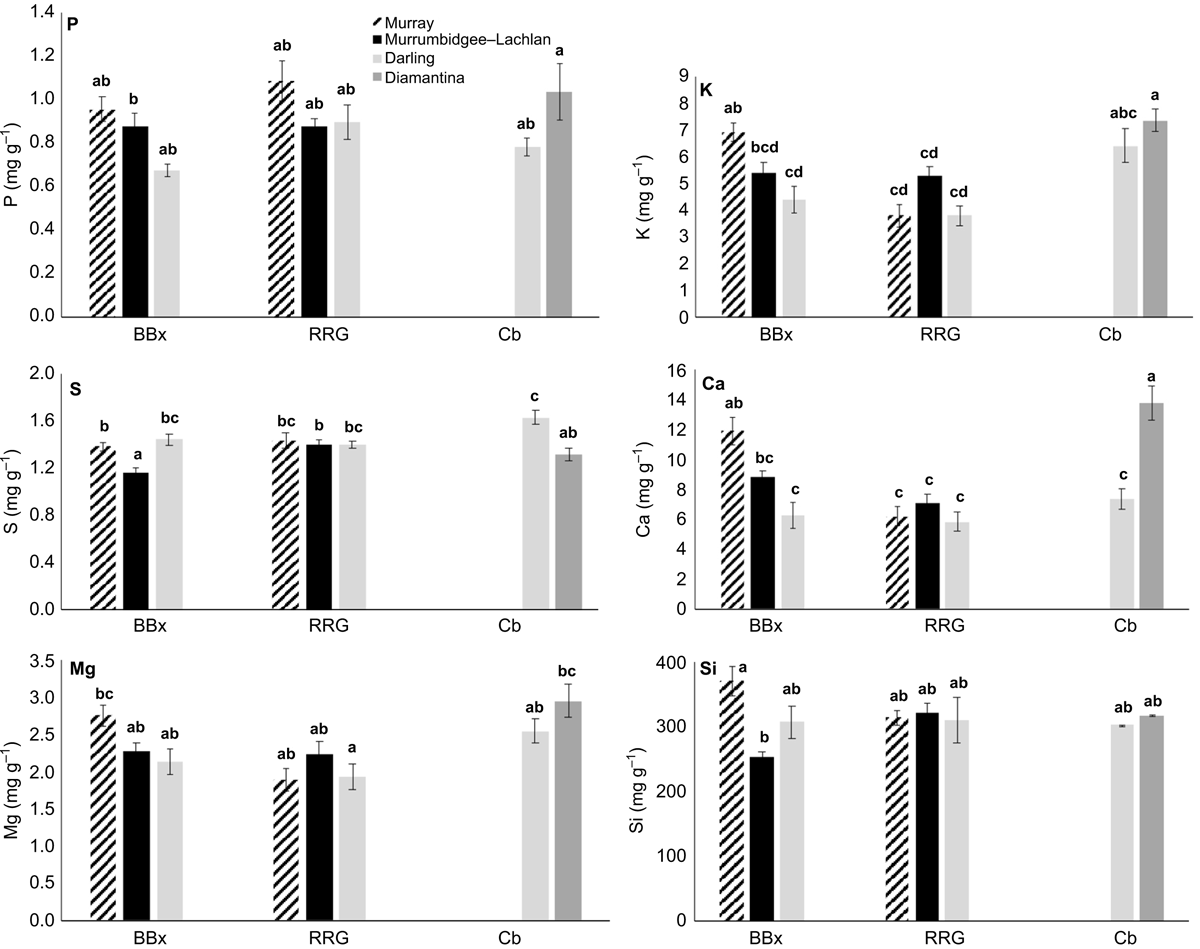
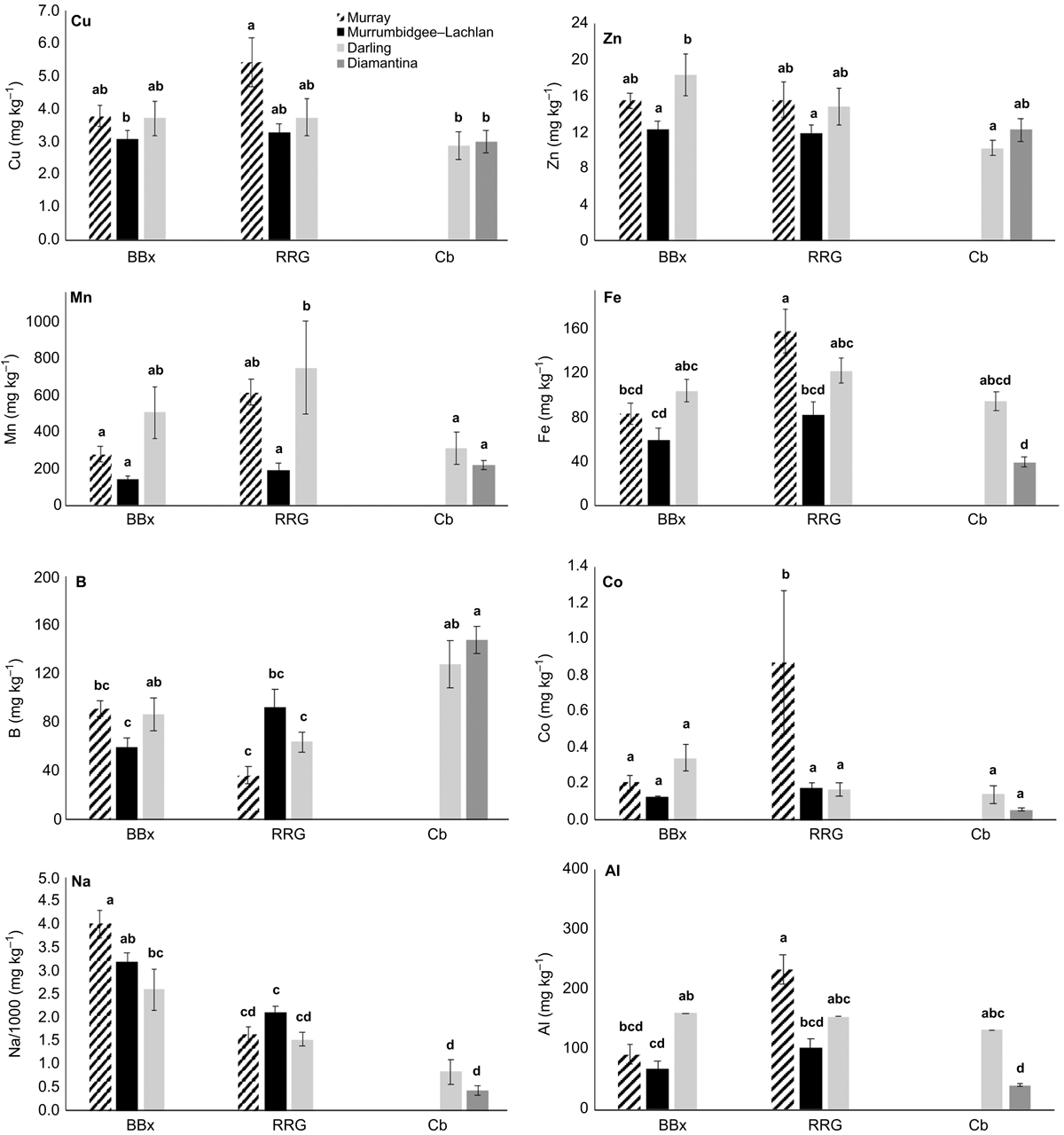
The leaf elemental concentration data (Fig. 6 and 7) showed some variation in the macronutrients P (P = 0.78), K (P < 0.001), S (P < 0.05) Mg (P < 0.01) and the beneficial element Si (P = 0.92) (Fig. 6) across all species and sites, with Ca concentrations in E. coolabah in the Diamantina basin sites and E. largiflorens in the Murray sites higher than those of all other trees studied (P < 0.001). Foliage micronutrient and Al concentration data (Fig. 7) suggest that for each species there was greater geographic and species-related variation compared with that of leaf macronutrients (Fig. 6). Foliar B concentrations were highest in E. coolabah (P < 0.001). Leaf Na concentrations among the three species were significantly different (P < 0.05) such that E. largiflorens > E. camaldulensis > E. coolabah. Foliar-Mn concentrations were generally elevated well above known plant nutritional requirements (Marschner 2002). Murray basin E. camaldulensis foliage (Fig. 7) accumulated the highest levels of Mn (P < 0.001), Fe (P < 0.01), Co (P < 0.05) and Al (P < 0.01), with significant species effects for foliar Zn (P < 0.01) and non-significant species effect for foliar Cu (P = 0.12). There were significant site effects for foliar Mn (P < 0.001), Fe (P < 0.001), Co (P < 0.001), Al (P < 0.001), Cu (P < 0.05), Zn (P < 0.01). Significant site-species interactions were detected for foliar Co (P < 0.001) and Al (P < 0.01).
Of note were foliage Na concentrations across all three eucalypts examined here (Fig. 7), with highest foliar Na concentrations of ∼4500 mg kg−1 were observed for E. largiflorens (P < 0.001 Murray and Murrumbidgee Lachlan sites, P < 0.01 Darling sites), with maximum observed E. camaldulesis leaf-Na concentrations of ∼2500 mg kg−1, and lowest foliar Na concentrations of ∼500 mg kg−1 recorded for E. coolabah.
Discussion
Empirical data from this study provide a static view of the nutritional status of three eucalypts native to several south-eastern Australian river catchments. Macronutrients, micronutrients, beneficial elements, and Al concentrations in foliage and in soil, as well as other soil traits were mapped on a geographic scale previously not undertaken for the region. The trade-off between the low resolution of this study and its large sampling area was considered worthwhile because enabled eucalypt mineral nutrition to be placed in broad ecological context to offer new insights warranting future detailed field research. The integration of plant and soil data was not meaningful due to cross-seasonal samplings and varying site-specific conditions of waterflows that can ultimately influence plant soil nutrient availability (CSIRO 1983; White 1997; Marschner 2002; Fernando et al. 2021a), although several noteworthy observations highlight lesser considered aspects of these riverine eucalypt tree–soil systems. For example, certain leaf nutrient concentrations appeared to be associated with species and geography. Soil chemical and physical properties, many of which underpin nutrient acquisition by trees, were found to be highly heterogeneous within and among the 10 field sites of varying soil types and clay content. Soil pH and redox potential are among key factors affecting nutrient availability to plants, and systems reliant on periodic flooding undergo changes associated with such events (Fernando et al. 2021a).
Comparison of foliage macronutrients obtained here (Fig. 6) against standard ‘normal’ plant nutrient values drawn from crop studies (Marschner 2002; Taiz et al. 2015) shows lower P and K concentrations in these eucalypts, comparable S concentrations, much higher Ca concentrations, comparable Mg levels, and relatively lower foliage Si concentrations. Published leaf nutritional data for eucalypts from field samples and forestry plantings show greater nutritional enrichment in the latter due to the application of fertilisers and other soil amendments (Judd et al. 1996). Overall, the macronutrient data for E. largiflorens, E. camaldulensis, and E. coolabah (Fig. 6) indicated ‘normal’ levels of P, K, S, and Mg assimilation (Marschner 2002) despite substantial variation in their soil type and chemistry, suggesting that these eucalypts are able to adequately access macronutrients from highly variable soils.
Most striking of the leaf micronutrient data (Fig. 7) were Na concentrations ranging between ∼4500 and ∼500 mg kg−1, well exceeding standard plant nutritional requirements (Marschner 2002; Taiz et al. 2015), most notably in E. largiflorens. Greatly elevated foliar Na concentrations in E. largiflorens on the Murray, Murrumbidgee–Lachlan and Darling sites consistently and significantly exceeded those in E. camaldulensis, which in turn significantly exceeded those of E. coolabah, pointing to species-wide salt tolerance by each of these species by varying or mixed strategies given these trees co-occurred at many sites. Strategies adopted by plants for tolerating soil-available toxins such as excess salt and metals commonly include: (a) uptake and sequestration in above-ground organs (Baker 1981), for example excessive foliage salt accumulation; (b) storage in roots with no or partial translocation to above-ground parts; and (c) uptake exclusion at the root–soil interface. In this study, strategy (a) was apparent for E. largiflorens (Fernando et al. 2021b), whereas strategy (b) and (c) may be occurring for E. coolabah, and a combinations of the above strategies may apply for E. camaldulensis. Recent studies of E. largiflorens on the Hattah Lakes system in the Murray basin (Fernando et al. 2018, 2021a) detected extremely high foliar Na concentrations, whereas its localised hybrid on the Chowilla Floodplain has already been noted for exceptional salt tolerance (Zubrinich et al. 2000; Koerber et al. 2013). Eucalyptus camaldulensis is known to harbour foliage Na concentrations as high as 1900 mg kg−1 (Hulme 2010). All three tree species in this present study are known to variously tolerate soil and groundwater salinity at specific locations (Roberts and Marston 2011), whereas isotope studies have demonstrated incidental salinity tolerance (Poss et al. 2000; Akeroyd et al. 2003; Costelloe et al. 2008). Species-wide salt tolerance by these eucalypts is consistent with adaptation to the presence of naturally saline groundwaters (Herczeg et al. 2001). There is evidence that in general, adventitious roots of eucalypts can occur close to the soil surface, enabling shallow access to water and nutrients (Robinson et al. 2006).
Past experiments and field studies of a wide range of western and eastern Australian eucalypts other than the focal species of this present study have examined salinity and waterlogging tolerance (van der Moezel et al. 1991; Marcar 1993; Adams et al. 2005; Woodward and Bennett 2005). They included exposing propagated plants to conditions of waterlogging and salt treatments to examine the interaction of stress effects, the association between foliar osmolytes and salt tolerance, and the effects of salt treatment on leaf content of abscisic acid, proline, and chlorophyll. An evaluation of how groundwater salinity interpreted by leaf-Na accumulation in E. largiflorens and E. camaldulensis (McLennan et al. 2013) found Na concentrations as high as 7300 mg kg−1. The canopy condition of E. camaldulensis and E. coolabah has previously been observed to go into decline when exposed to substrate salinity greater than 30 000 μSv (Overton et al. 2006; Payne et al. 2006; Costelloe et al. 2008). However, E. largiflorens trees are known to tolerate soil salinity levels as high as 55 000 μSv (Slavich et al. 1999; Overton et al. 2006). A recent a study of E. largiflorens at Hattah Lakes showed gradual lowering of foliar-Na concentrations across the flooding period, with a steep increase in foliar-Na after water levels had subsided (Fernando et al. 2021a). These findings suggest that the species tolerates high salinity substrates by foliage Na overaccumulation while potentially being able to ‘dilute’ concentrated leaf-Na upon surface inundation. Further detailed temporal studies are required to investigate the utility of such measurements in informing environmental flow management. These recent findings on salt-accumulating Hattah Lakes E. largiflorens trees led to application of SEM-EDS and XRF analytical microprobe techniques, which established that Na and Cl were strongly accumulated in leaf cell vacuoles of E. largiflorens, thus pointing to a likely salt tolerance strategy (Fernando et al. 2021b).
There currently are no guidelines for trace nutrient concentrations in Eucalyptus foliage, and comparison of field data here (Fig. 7) with standard plant nutritional data (Marschner 2002; Taiz and Zeiger 2002) suggests much higher Mn values in the field samples, comparable Fe levels, and higher foliage B concentrations in these three eucalypts. In glasshouse experiments E. camaldulensis has been shown to have the capacity to strongly accumulate metals including micronutrients, often to levels well above their known nutritional requirements (Marschner 2002; Reichman et al. 2004, 2006). For example, dosing trials of juvenile E. camaldulensis trees by Reichman et al. (2004) showed foliar Mn accumulation as high as 7000 mg kg−1, around sevenfold higher than concentrations observed in the present field dataset (Fig. 7). Foliar Cu accumulation in E. camaldulensis was similarly induced (Reichman et al. 2006) to around tenfold the maximum field data values observed here. Other growth experiments have found strong Pb accumulation by this species (Nawazi et al. 2016). Field studies of E. camaldulensis on ore deposits near Broken Hill at the north-western end of the MDB (Fig. 3) showed Zn, Pb, Ag, As and Au accumulation in foliage, suggesting that it may serve as an indicator species for undiscovered metal deposits (Hulme and Hill 2005). Although controlled experiments on plant uptake can induce responses not observed in the field, leaf micronutrient and Al data obtained in this study suggest E. camaldulensis trees sampled in the Murray and Darling basins in particular have a high capacity to accumulate foliar Cu, Zn, Mn, Fe, Co and Al (Fig. 7).
Plant acquisition of micronutrients from soil can be affected by several factors, which are often in dynamic states with the capability of interacting mutually (Leeper and Uren 1997; White 1997). Soil pH is one of these. It can be driven by wetness, microbial activity, plant root exudation, for example (Graham et al. 1988; Leeper and Uren 1997; White 1997; St Clair and Lynch 2004, 2010). The effect of soil pH on the plant bioavailability of micronutrients is generally more pronounced compared to that of macronutrients, particularly for plant acquisition of micronutrients whose accessibility increases with soil acidity (White 1997), although there are some recent examples to the contrary (Lambers and Oliveira 2019). The net effects of drivers of soil pH can expose a plant to a toxic or deficient supply of multiple micronutrients ions or non-nutrient metal ions, depending on the element, and the plant species or phenotypic response. Here, soil acidity within each of the four basin systems was highly variable given their soil pH values ranged from strongly acidic (down to pH ∼4–5.5), through moderately acidic (pH ∼5.5–6.5), neutral (pH ∼6.5–7.5), to moderately alkaline (pH ∼7.5–8.5) (Leeper and Uren 1997). Given these values represent a single sampling, and that it is possible that soil pH fluctuates with environmental conditions, the values obtained here may not strictly represent pH of corresponding soil solutions in situ. Nevertheless, they capture variation that warrants consideration when formulating management strategies tailored for local conditions. The net effects of the fluctuating accessibility to trees of various nutrients and other elements in soil requires continuous sampling and analysis.
The distribution patterns of soil clay content down to 1–2-m depth as mapped for this study (Fig. 5) should be interpreted as being highly generalised given that soil composition is commonly spatially heterogenous at a smaller scale than depicted here (CSIRO 1983; Leeper and Uren 1997; White 1997). The well documented association of Al with clays due to their aluminosilicate mineralogy was evident in this study (CSIRO 1983; Leeper and Uren 1997; White 1997). Highest total soil–Al concentrations (Tables 2–5) were detected in the Murrumbidgee–Lachlan and Darling sites that were broadly mapped here as having highest soil clay contents (Fig. 5). These soil clay maps (Fig. 5) do not capture capping clays of varying thickness overlaying sandy soils and lacustrine sediments on the Chowilla floodplain (site 9) in the Murray Basin (Fig. 3) (Overton and Jolly 2004; Overton et al. 2006; Overton and Doody 2010).
Notwithstanding the coarseness of this study and its one-off sampling approach that precluded measurement of tree–soil interactions, it highlights inherent heterogeneity in biotic and abiotic variables central to tree condition. An improved theoretical understanding of localised variation may ultimately benefit tree health in the longer term by better informing management strategies. Although further detailed work is clearly necessary for knowledge gathering on many fronts including tree phenotypic variation, soil chemistry and soil biota, extracting such empirical data is time-consuming, expensive and impractical to undertake on an extensive scale. Acknowledgement of the complexity of these systems is needed, with attention to localised drivers of tree condition, particularly in the management of valuable water resources. Modelling data useful for assessing large areas (Cunningham et al. 2009, 2013) are currently insufficiently combined with or compared with complementary basic on-ground field data. Such considerations would aid resolution of different localised tree–soil nutritional dynamics, particularly with respect to soil chemical changes due to wetting and drying. This study supports the need for continuous field data-gathering to inform management decisions, while also offering insight into broader patterns of in situ nutrient acquisition by mature E. largiflorens, E. camaldulensis and E. coolabah trees, which warrant further investigation.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
D. R. Fernando acknowledges funding from the Australian Research Council (ARC grant number DE120100510) and Eucalypt Australia; and in-kind contribution from the University of New South Wales.
Acknowledgements
The support and expertise of Parks Victoria and New South Wales National Parks and Wildlife Service staff Shane Southon, Rhett Cameron, Nicola Brookhouse, Mick Lalor and Amanda Lavender were invaluable. The authors thank Myra Tolhurst for welcoming us to her property during fieldwork at Macquarie Marshes. We thank Jane Roberts and Heather McGinness for their guidance and expert advice on this project. The authors thank Angus Fernando-Plant for fieldwork assistance.
References
Adams MA, Richter A, Hill AK, Colmer TD (2005) Salt tolerance in Eucalyptus spp.: identity and response of putative osmolytes. Plant, Cell & Environment 28, 772–787.| Salt tolerance in Eucalyptus spp.: identity and response of putative osmolytes.Crossref | GoogleScholarGoogle Scholar |
Akeroyd MD, Tyerman SD, Walker GR, Jolly ID (1998) Impact of flooding on the water use of semi-arid riparian eucalypts. Journal of Hydrology 206, 104–117.
| Impact of flooding on the water use of semi-arid riparian eucalypts.Crossref | GoogleScholarGoogle Scholar |
Akeroyd AD, Walker GR, Kendall MB (2003) Response of Eucalyptus largiflorens to floodplain salinisation. Water Science and Technology 48, 113–120.
| Response of Eucalyptus largiflorens to floodplain salinisation.Crossref | GoogleScholarGoogle Scholar |
Baker AJM (1981) Accumulators and excluders – strategies in the response of plants to heavy metals. Journal of Plant Nutrition 3, 643–654.
| Accumulators and excluders – strategies in the response of plants to heavy metals.Crossref | GoogleScholarGoogle Scholar |
Bedinger MS (1979) ‘Forests and flooding with special reference to the White River and Ouachita River basins, Arkansas’. (United States Geological Survey: Reston, VA, USA)
Bradley CE, Smith DG (1986) Plains cottonwood recruitment and survival on a prairie meandering river floodplain, Milk River, southern Alberta and northern Montana. Canadian Journal of Botany 64, 1433–1442.
| Plains cottonwood recruitment and survival on a prairie meandering river floodplain, Milk River, southern Alberta and northern Montana.Crossref | GoogleScholarGoogle Scholar |
Bramley H, Hutson J, Tyerman SD (2003) Floodwater infiltration through root channels on a sodic clay floodplain and the influence on a local tree species Eucalyptus largiflorens. Plant and Soil 253, 275–286.
| Floodwater infiltration through root channels on a sodic clay floodplain and the influence on a local tree species Eucalyptus largiflorens.Crossref | GoogleScholarGoogle Scholar |
Costelloe JF, Payne E, Woodrow IE, Irvine EC, Western AW, Leaney FW (2008) Water sources accessed by arid zone riparian trees in highly saline environments, Australia. Oecologia 156, 43–52.
| Water sources accessed by arid zone riparian trees in highly saline environments, Australia.Crossref | GoogleScholarGoogle Scholar | 18270743PubMed |
CSIRO (1983) ‘Soils an Australian viewpoint.’ (Academic Press: London, UK)
Cunningham S, MacNally R, Griffioen P, White M (2009) Mapping the condition of river red gum and black box stands in The Living Murray icon sites. Report Book Number 51/10. Murray–Darling Basin Authority, Canberra, ACT, Australia.
Cunningham SC, White M, MacNally R, Griffioen P (2013) ‘Mapping floodplain vegetation types across the Murray–Darling Basin using remote sensing.’ (Murray–Darling Basin Authority: Canberra, ACT, Australia)
Doody TM, Holland KL, Benyon RG, Jolly ID (2009) Effect of groundwater freshening on riparian vegetation water balance. Hydrological Processes 23, 3485–3499.
| Effect of groundwater freshening on riparian vegetation water balance.Crossref | GoogleScholarGoogle Scholar |
Doody TM, Benger SN, Pritchard JL, Overton IC (2014) Ecological response of Eucalyptus camaldulensis (river red gum) to extended drought and flooding along the River Murray, South Australia (1997–2011) and implications for environmental flow management. Marine and Freshwater Research 65, 1082–1093.
| Ecological response of Eucalyptus camaldulensis (river red gum) to extended drought and flooding along the River Murray, South Australia (1997–2011) and implications for environmental flow management.Crossref | GoogleScholarGoogle Scholar |
Doody TM, Colloff MJ, Davies M, Koul V, Benyon RG, Nagler PL (2015) Quantifying water requirements of riparian river red gum (Eucalyptus camaldulensis) in the Murray–Darling Basin, Australia – implications for the management of environmental flows. Ecohydrology 8, 1471–1487.
| Quantifying water requirements of riparian river red gum (Eucalyptus camaldulensis) in the Murray–Darling Basin, Australia – implications for the management of environmental flows.Crossref | GoogleScholarGoogle Scholar |
Fernando DR, Lynch JP (2015) Manganese phytotoxicity: new light on an old problem. Annals of Botany 116, 313–319.
| Manganese phytotoxicity: new light on an old problem.Crossref | GoogleScholarGoogle Scholar | 26311708PubMed |
Fernando DR, Lynch JP, Reichman SM, Clark GJ, Miller RE, Doody TM (2018) Inundation of a floodplain lake woodlands system: nutritional profiling and benefit to mature Eucalyptus largiflorens (Black Box) trees. Wetlands Ecology and Management 26, 961–975.
| Inundation of a floodplain lake woodlands system: nutritional profiling and benefit to mature Eucalyptus largiflorens (Black Box) trees.Crossref | GoogleScholarGoogle Scholar |
Fernando DR, Fernando AE, Koerber GR, Doody TM (2021a) Tree–soil interactions through water release to a floodplain ecosystem: a case study of Black Box (Eucalyptus largiflorens) on loamy sands. Wetlands 41, 17–35.
| Tree–soil interactions through water release to a floodplain ecosystem: a case study of Black Box (Eucalyptus largiflorens) on loamy sands.Crossref | GoogleScholarGoogle Scholar |
Fernando DR, Lynch JP, Hanlon MT, Marshall AT (2021b) Foliar elemental microprobe data and leaf anatomical traits consistent with drought tolerance in Eucalyptus largiflorens (Myrtaceae). Australian Journal of Botany 69, 215–224.
| Foliar elemental microprobe data and leaf anatomical traits consistent with drought tolerance in Eucalyptus largiflorens (Myrtaceae).Crossref | GoogleScholarGoogle Scholar |
Grafton RQ, Williams J, Perry CJ, Molle F, Ringler C, Steduto P, Udall B, Wheeler SA, Wang Y, Garrick D, Allen RG (2018) The paradox of irrigation efficiency: higher efficiency rarely reduces water consumption. Science 361, 748–750.
| The paradox of irrigation efficiency: higher efficiency rarely reduces water consumption.Crossref | GoogleScholarGoogle Scholar | 30139857PubMed |
Graham RD, Hannam RJ, Uren NC (Eds) (1988) ‘Manganese in Soils and Plants. Proceedings of the International Symposium’, 22–26 August 1988, Glen Osmond, SA, Australia. (Springer)
Herczeg AL, Dogramaci SS, Leaney FWJ (2001) Origin of dissolved salts in a large, semi-arid groundwater system: Murray Basin, Australia. Marine and Freshwater Research 52, 41–52.
| Origin of dissolved salts in a large, semi-arid groundwater system: Murray Basin, Australia.Crossref | GoogleScholarGoogle Scholar |
Holland KL, Tyerman SD, Mensforth LJ, Walker GR (2005) Tree water sources over shallow, saline groundwater in the lower River Murray, south-eastern Australia: implications for groundwater recharge mechanisms. Australian Journal of Botany 54, 193–205.
| Tree water sources over shallow, saline groundwater in the lower River Murray, south-eastern Australia: implications for groundwater recharge mechanisms.Crossref | GoogleScholarGoogle Scholar |
Hulme KA (2010) ‘Eucalyptus camaldulensis (river red gum) biogeochemistry: an innovative tool for mineral exploration in the Curnamona Province and adjacent regions.’ (University of Adelaide: Adelaide, SA, Australia)
Hulme K, Hill S (2005) Mineralisation discovery through transported cover using River Redgums (Eucalyptus camaldulensis). In ‘Minerals explorations seminar.’ (Ed. RAD Gee) pp. 31–33. (CRC LEME: Perth, WA, Australia)
Jensen AE, Walker KF, Paton DC (2008) The role of seedbanks in restoration of floodplain woodlands. River Research and Applications 24, 632–649.
| The role of seedbanks in restoration of floodplain woodlands.Crossref | GoogleScholarGoogle Scholar |
Judd TS, Attiwill PM, Adams MA (1996) Nutrient concentrations in Eucalyptus: a synthesis in relation to differences between taxa, sites and components. In ‘Nutrition of eucalypts.’ (Eds PM Attiwill, MA Adams) pp. 123–153. (CSIRO Publishing: Melbourne, Vic., Australia)
Koerber GR, Anderson PA, Seekamp JV (2013) Morphology, physiology and AFLP markers validate that green box is a hybrid of Eucalyptus largiflorens and E. gracilis (Myrtaceae). Australian Systematic Botany 26, 156–166.
| Morphology, physiology and AFLP markers validate that green box is a hybrid of Eucalyptus largiflorens and E. gracilis (Myrtaceae).Crossref | GoogleScholarGoogle Scholar |
Lambers H, Oliveira RS (2019) ‘Plant physiological ecology.’ (Springer Nature: Cham, Switzerland).
| Crossref |
Leeper GW, Uren NC (1997) ‘Soil science: an introduction.’ (Melbourne University Press: Melbourne, Vic., Australia)
Marcar NE (1993) Waterlogging modifies growth, water use and ion concentrations in seedlings of salt-treated Eucalyptus camaldulensisE. tereticornis, E. robusta and E. globulus. Australian Journal of Plant Physiology 20, 1–13.
| Waterlogging modifies growth, water use and ion concentrations in seedlings of salt-treated Eucalyptus camaldulensisE. tereticornis, E. robusta and E. globulus.Crossref | GoogleScholarGoogle Scholar |
Marschner H (2002) ‘Mineral nutrition of higher plants.’ (Academic Press: London, UK)
McEvoy PK (1992) ‘Ecophysiological comparisons between Eucalyptus camaldulensis Denh., E. largiflorens F. Muell. and E. microcarpa (Maiden) Maiden on the River Murray floodplain.’ (University of Melbourne: Melbourne, Vic., Australia)
McLennan SM, Hill SM, Hatch M, Barovich K, Berens V (2013) Riparian eucalypt biogeochemical expression of groundwater salinity, Murray River, South Australia. Geochemistry 13, 159–168.
| Riparian eucalypt biogeochemical expression of groundwater salinity, Murray River, South Australia.Crossref | GoogleScholarGoogle Scholar |
Medina E, Cuevas E (1994) Mineral nutrition: humid tropical forests. Progress in Botany 55, 115–129.
| Mineral nutrition: humid tropical forests.Crossref | GoogleScholarGoogle Scholar |
Mensforth LJ, Thorburn PJ, Tyerman SD, Walker GR (1994a) Sources of water used in riaprian Eucalyptus camaldulensis overlying highly saline groundwater. Oecologia 100, 21–28.
| Sources of water used in riaprian Eucalyptus camaldulensis overlying highly saline groundwater.Crossref | GoogleScholarGoogle Scholar | 28307023PubMed |
Mensforth LT, Thorburn PT, Tyerman SD, Walker GR (1994b) Sources of water used by riparian Eucalyptus camaldulensis overlying highly saline groundwater. Oecologia 100, 21–28.
| Sources of water used by riparian Eucalyptus camaldulensis overlying highly saline groundwater.Crossref | GoogleScholarGoogle Scholar |
Miller AC, Watling JR, Overton IC, Sinclair R (2003) Does water status of Eucalyptus largiflorens (Myrtaceae) affect infection by the mistletoe Amyema miquelii (Loranthaceae)? Functional Plant Biology 30, 1239–1247.
| Does water status of Eucalyptus largiflorens (Myrtaceae) affect infection by the mistletoe Amyema miquelii (Loranthaceae)?Crossref | GoogleScholarGoogle Scholar | 32689105PubMed |
Moxham C, Duncan M, Moloney P (2018) Tree health and regeneration response of Black Box (Eucalyptus largiflorens) to recent flooding. Ecological Management and Restoration 19, 58–65.
| Tree health and regeneration response of Black Box (Eucalyptus largiflorens) to recent flooding.Crossref | GoogleScholarGoogle Scholar |
Nawazi MF, Gul S, Tanvir MA, Akhtar J, Chaudary S, Ahmad I (2016) Influence of NaCl-salinity on Pb-uptake behavior and growth of River Red gum tree (Eucalyptus camaldulensis Dehnh.). Turkish Journal of Agriculture and Forestry 40, 425–432.
| Influence of NaCl-salinity on Pb-uptake behavior and growth of River Red gum tree (Eucalyptus camaldulensis Dehnh.).Crossref | GoogleScholarGoogle Scholar |
Overton I, Doody T (2010) Ecosystem response modelling in the Chowilla floodplain, Lindsay and Wallpolla islands icon site. In ‘Ecosystem response modelling in the Murray–Darling Basin’. (Eds N Saintilan, I Overton) pp. 357–372. (CSIRO Publishing: Melbourne, Vic., Australia)
Overton IC, Jolly ID (2004) Integrated studies of floodplain vegetation health, saline groundwater and flooding on the Chowilla floodplain South Australia. CSIRO Land and Water Technical Report 20/04. (CSIRO: Canberra, ACT, Australia)
Overton IC, Jolly ID, Slavich PG, Lewis MM, Walker GR (2006) Modelling vegetation health from the interaction of saline groundwater and flooding on the Chowilla floodplain, South Australia. Australian Journal of Botany 54, 207–220.
| Modelling vegetation health from the interaction of saline groundwater and flooding on the Chowilla floodplain, South Australia.Crossref | GoogleScholarGoogle Scholar |
Overton IC, Colloff MJ, Doody TM, Henderson B, Cuddy SM (2009) ‘Ecological outcomes of flow regimes in the Murray–Darling Basin.’ (CSIRO: Canberra, ACT, Australia)
Payne EGI, Costelloe JF, Woodrow IE, Irvine EC, Western AW, Herczeg AL (2006) Riparian tree water use by Eucalyptus coolabah in the Lake Eyre Basin. In ‘30th Hydrology & Water Resources Symposium: Past, Present & Future’, 4–7 December 2006, Launceston, Tas., Australia. (Engineers Australia) Available at https://search.informit.org/doi/book/10.3316/informit.0858257904
Pittock J, Finlayson CM (2011) Australia’s Murray–Darling Basin: freshwater ecosystem conservation options in an era of climate change. Marine and Freshwater Research 62, 232–243.
| Australia’s Murray–Darling Basin: freshwater ecosystem conservation options in an era of climate change.Crossref | GoogleScholarGoogle Scholar |
Poss JA, Grattan SR, Suarez DL, Grieve CM (2000) Stable carbon isotope discrimination: an indicator of cumulative salinity and boron stress in Eucalyptus camaldulensis. Tree Physiology 20, 1121–1127.
| Stable carbon isotope discrimination: an indicator of cumulative salinity and boron stress in Eucalyptus camaldulensis.Crossref | GoogleScholarGoogle Scholar | 11269964PubMed |
Rahimi-Nasrabadi M, Nazarian S, Farahani H, Reza G, Koohbijari F, Ahmadi F, Batooli H (2013) Chemical composition, antioxidant, and antibacterial activities of the essential oil and methanol extracts of Eucalyptus largiflorens F. Muell. International Journal of Food Properties 16, 369–381.
| Chemical composition, antioxidant, and antibacterial activities of the essential oil and methanol extracts of Eucalyptus largiflorens F. Muell.Crossref | GoogleScholarGoogle Scholar |
Reichman SM, Menzies NW, Asher CJ, Mulligan DR (2004) Seedling responses of four Australian tree species to toxic concentrations of manganese in solution culture. Plant and Soil 258, 341–350.
| Seedling responses of four Australian tree species to toxic concentrations of manganese in solution culture.Crossref | GoogleScholarGoogle Scholar |
Reichman SM, Menzies NW, Asher CJ, Mulligan DR (2006) Responses of four Australian tree species to toxic concentrations of copper in solution culture. Journal of Plant Nutrition 29, 1127–1141.
| Responses of four Australian tree species to toxic concentrations of copper in solution culture.Crossref | GoogleScholarGoogle Scholar |
Roberts J, Marston F (2011) ‘Water regime for wetland and floodplain plants: a source book for the Murray–Darling Basin.’ (National Water Commission, Commonwealth of Australia: Canberra, ACT, Australia)
Robinson N, Harper RJ, Smettem KRJ (2006) Soil water depletion by Eucalyptus spp. integrated into dryland agricultural systems. Plant and Soil 286, 141–151.
| Soil water depletion by Eucalyptus spp. integrated into dryland agricultural systems.Crossref | GoogleScholarGoogle Scholar |
Sakai A, Ohsawa M (1994) Topographical pattern of the forest vegetation on a river basin in a warm-temperate hilly region, central Japan. Ecological Research 9, 269–280.
| Topographical pattern of the forest vegetation on a river basin in a warm-temperate hilly region, central Japan.Crossref | GoogleScholarGoogle Scholar |
Slavich PG, Walker GR, Jolly ID, Hatton TJ, Dawes WR (1999) Dynamics of Eucalyptus largiflorens growth and water use in response to modified watertable and flooding regimes on a saline floodplain. Agricultural Water Management 39, 245–264.
| Dynamics of Eucalyptus largiflorens growth and water use in response to modified watertable and flooding regimes on a saline floodplain.Crossref | GoogleScholarGoogle Scholar |
Smith P, Smith J (2014) Floodplain vegetation of the River Murray in 1987–1988: an important pre-drought benchmark for subsequent studies. Cunninghamia 14, 97–151.
| Floodplain vegetation of the River Murray in 1987–1988: an important pre-drought benchmark for subsequent studies.Crossref | GoogleScholarGoogle Scholar |
St Clair SB, Lynch JP (2004) Base cation stimulation of mycorrhization and photosynthesis of sugar maple on acid soils are coupled by foliar nutrient dynamics. New Phytologist 165, 581–590.
| Base cation stimulation of mycorrhization and photosynthesis of sugar maple on acid soils are coupled by foliar nutrient dynamics.Crossref | GoogleScholarGoogle Scholar |
St Clair SB, Lynch JP (2010) The opening of Pandora’s Box: climate change impacts on soil fertility and crop nutrition in developing countries. Plant and Soil 335, 101–115.
| The opening of Pandora’s Box: climate change impacts on soil fertility and crop nutrition in developing countries.Crossref | GoogleScholarGoogle Scholar |
Stone C, Bacon PE (1995) Influence of herbivory on the decline of Black Box (Eucalyptus largiflorens. Australian Journal of Botany 43, 555–564.
| Influence of herbivory on the decline of Black Box (Eucalyptus largiflorens.Crossref | GoogleScholarGoogle Scholar |
Sunil C, Somashekar RK, Nagaraja BC (2010) Riparian vegetation assessment of Cauvery River Basin of South India. Environmental Monitoring and Assessment 170, 545–553.
| Riparian vegetation assessment of Cauvery River Basin of South India.Crossref | GoogleScholarGoogle Scholar | 20024615PubMed |
Swirepik JL, Burns IC, Dyer FJ, Neave IA, O’Brien MG, Pryde GM, Thompson RM (2016) Establishing environmental water requirements for the Murray–Darling Basin, Australia’s largest developed river system. River Research and Applications 32, 1153–1165.
| Establishing environmental water requirements for the Murray–Darling Basin, Australia’s largest developed river system.Crossref | GoogleScholarGoogle Scholar |
Taiz L, Zeiger E (2002) ‘Plant physiology’, 2nd edn. (Sinauer Associates, Inc: Sunderland, MA, USA)
Taiz L, Zeiger E, Moller IM, Murphy AS (2015) ‘Plant physiology and development.’ (Sinauer Associates: Sunderland, MA, USA)
Thorburn PJ, Hatton TJ, Walker GR (1993) Combining measurements of transpiration and stable isotopes of water to determine groundwater discharge from forests. Journal of Hydrology 150, 563–587.
| Combining measurements of transpiration and stable isotopes of water to determine groundwater discharge from forests.Crossref | GoogleScholarGoogle Scholar |
Treloar GK (1959) Some factors affecting seedling survival of Eucalyptus largiflorens F. Muell. Australian Forestry 23, 46–48.
| Some factors affecting seedling survival of Eucalyptus largiflorens F. Muell.Crossref | GoogleScholarGoogle Scholar |
van der Moezel PG, Pearce-Pinto GVN, Bell DT (1991) Screening for salt and waterlogging tolerance in Eucalyptus and Melaleuca species. Forest Ecology and Management 40, 27–37.
| Screening for salt and waterlogging tolerance in Eucalyptus and Melaleuca species.Crossref | GoogleScholarGoogle Scholar |
Viscarra Rossel R, Chen C, Grundy M, Searle R, Clifford D, Odgers N, Holmes K, Griffin T, Liddicoat C, Kidd D (2014) Soil and landscape grid national soil attribute maps – clay (3″ resolution) – release 1. v5. Data collection. (CSIRO: Canberra, ACT, Australia) Available at https://data.csiro.au/collections/collection/CI10168v005
Wallace TA, Gehrig S, Doody TM (2020) A standardised approach to calculating floodplain tree condition to support environmental watering decisions. Wetlands Ecology and Management 28, 315–340.
| A standardised approach to calculating floodplain tree condition to support environmental watering decisions.Crossref | GoogleScholarGoogle Scholar |
Wallace TA, Gehrig SL, Doody TM, Davies MJ, Walsh R, Fulton C, Cullen R, Nolan M (2021) A multiple lines of evidence approach for prioritising environmental watering of wetland and floodplain trees. Ecohydrology 14, e2272
| A multiple lines of evidence approach for prioritising environmental watering of wetland and floodplain trees.Crossref | GoogleScholarGoogle Scholar |
White RE (1997) ‘Principles and practices of soil science – the soil as a natural resource.’ (Blackwell Science: Melbourne, Vic., Australia)
Woodward AJ, Bennett IJ (2005) The effect of salt stress and abscisic acid on proline production, chlorophyll content and growth of in vitro propagated shoots of Eucalyptus camaldulensis. Plant Cell, Tissue and Organ Culture 82, 189–200.
| The effect of salt stress and abscisic acid on proline production, chlorophyll content and growth of in vitro propagated shoots of Eucalyptus camaldulensis.Crossref | GoogleScholarGoogle Scholar |
Zubrinich TM, Loveys B, Gallasch S, Seekamp JV, Tyerman SD (2000) Tolerance of salinised floodplain conditions in a naturally occurring Eucalyptus hybrid related to lowered plant water potential. Tree Physiology 20, 953–963.
| Tolerance of salinised floodplain conditions in a naturally occurring Eucalyptus hybrid related to lowered plant water potential.Crossref | GoogleScholarGoogle Scholar | 11303570PubMed |