Assessing the storage potential of seed collections to inform the management of wild species seed banks
S. Balasupramaniyam A B , D. J. Merritt B C , F. R. Hay D and E. L. Dalziell B C *
B C *
A
B
C
D
Abstract
The storage of seed in seed banks is a primary strategy for the ex-situ conservation of plant species globally. However, changing practices have meant that institutions storing seeds for decades have often stored older collections sub-optimally for at least some of the storage time.
Using banked seed collections at Kings Park and Botanic Garden (Perth, Western Australia), we aimed to assess the relative future longevity of several seed lots within 10 species. These seed lots had been stored for 4–34 years.
We conducted germination assessments on seeds from 44 collections. We conducted a rapid ageing experiment for species with multiple accessions that retained high viability by subjecting seeds to 60% relative humidity at 45°C to determine the potential remaining longevity.
Several collections of Brachyscome iberidifolia, Myriocephalus gueriniae, Olearia axillaris and O. pimeleoides banked in the 1980s and 1990s displayed 0% germination. Newer collections of B. iberidifolia, Hyalosperma cotula, O. axillaris, Panaetia lessonii, Podotheca angustifolia and Trachymene pilosa retained similarly high, consistent viability over time in storage. Rapid ageing of these collections showed that the time to 50% loss of viability (p50) varied significantly and was not necessarily lowest for the oldest seed collections.
Rapidly ageing seeds enabled us to determine that several species and individual collections have lower longevity and therefore need to be prioritised for more frequent viability monitoring, use, or recollection.
This method could be used in wild species seed banks globally for making more informed decisions about historical and ageing seed collections.
Keywords: comparative seed longevity, ex situ conservation, germination testing, plant conservation, rapid ageing, seed bank management, seed storage, seed viability, wild species.
Introduction
The storage of seeds in seed banks is a primary strategy for the ex situ conservation of plant species and associated genetic diversity (Walters and Pence 2021). Wild plant species may be threatened by many factors including habitat loss, changing climate, pests and diseases. Ex situ conservation programs aim to prevent species extinctions and support species survival in the natural habitat by ensuring the availability of genetic materials in specialised repositories such as seed banks, and providing propagation material for plant translocations and broader ecosystem restoration (Martyn Yenson et al. 2024). Monitoring the viability of seed collections can be a significant challenge for any seed bank (Fu et al. 2015; Hay and Whitehouse 2017), and for seed banks conserving wild species, the diversity of species creates some challenges with respect to establishing effective procedures and workflows (Hay and Probert 2013; Walters 2015). Seeds of wild species may be banked with little direct prior knowledge of seed storage behaviour or germination requirements, meaning that evidence-based curation is an ever-increasing challenge as the number and diversity of the collections grow. The increasing scale and pace of the threats to plant biodiversity mean that the long-standing efforts to upscale seed banking programs are vital for the capture and conservation of plant genetic diversity before this is lost (Liu et al. 2018). Management of collections must be informed by knowledge of seed biology and storage behaviour for seed banks to achieve the goal of preserving and providing high quality germplasm when required.
Periodic testing of the viability of seed collections is a core component of seed bank management. The goal of viability monitoring is to identify declining seed quality before the utility of the accession is compromised, without testing too frequently so as to consume valuable seed stocks unnecessarily (Hay and Whitehouse 2017). International genebank standards recommended viability testing intervals be set at 5 years for short-lived seeds or 10 years for long-lived seeds, or if there is knowledge of the anticipated seed longevity, at one-third of the time for viability to decrease to 85% of the initial viability (FAO 2014). However, the storage behaviour of wild seeds is diverse and seeds of different species vary in the degree of desiccation tolerance, survival of sub-zero storage temperatures and moisture/temperature relationships with longevity (Walters 2015). Effective viability monitoring can also be challenging due to uncertainties surrounding seed germination requirements and the resources required to test large numbers of accessions (Fu et al. 2015). Together with large, diverse collections, many seed banks have seed lots that were banked decades before, some of which may have variable storage histories and limited information regarding initial seed quality or viability. The seed bank at Kings Park and Botanic Garden holds ~14,000 accessions of 4000 species native to Western Australia (WA), with collections dating to the early 1960s. Originally established to provide seeds for the development of plant displays within the Western Australian Botanic Garden, the seed bank program has grown to include a greater focus on the conservation of WA’s flora and supporting the restoration of urban bushland under the management of the Botanic Gardens and Parks Authority. The storage conditions of many of the older collections have changed over time due to the improved understanding of seed banking practices and updates to infrastructure. For many collections, storage history is imperfect and there is a lack of data on the current and/or initial viability to conduct analyses of seed viability in relation to time in storage. Germination testing can provide data on current quality and utility but provides little information on future utility, i.e. longevity. Studies of seed longevity through seed storage experiments can provide additional data regarding the physiological seed quality and can be used to compare the longevity of different species and seed accessions (Hay and Whitehouse 2017; Merritt et al. 2021). In this regard, conducting seed storage experiments could be a useful additional seed bank curation tool to better delineate the quality of seed accessions of similar germination and provide information on the future storage potential to inform scheduling of future viability monitoring and recollection intervals (i.e. for longer vs shorter-lived accessions).
The longevity of desiccation tolerant seeds varies by four orders of magnitude under high temperature and relative humidity storage conditions that have been widely used to rapidly age seeds for experimental purposes (45°C and 60% relative humidity (RH); Newton et al. (2014) and Hay et al. (2022)). The time for seed viability of Australian species to decline to 50% (p50) under such storage conditions has been documented to range between <1 day and >770 days (Probert et al. 2009; Merritt et al. 2014; Satyanti et al. 2018; Sommerville et al. 2023). These studies and others examining seed longevity across a range of storage conditions, including genebank conditions (Walters et al. 2005; Chau et al. 2019; Colville and Pritchard 2019), have identified longer- or shorter-lived seeds to be associated with certain plant families. Shorter-lived seeds have been found within, for example, the Apiaceae, Araliaceae, Asteraceae, Brassicaceae, Poaceae and Orchidaceae. Such associations identify families of interest for research into storage behaviour and longevity but wide variation in seed longevity is also observed within families and longevity can even be specific to different seed lots of a single species (Lee et al. 2019), making generalising storage behaviour and potential longevity in the absence of any prior data difficult.
In this study, we tested the germination of banked seed collections of species of Asteraceae and Araliaceae, as plant families putatively having species with shorter-lived seeds. We quantified the seed germination of each accession to identify any trends in viability decline amongst the accessions and in relation to time in storage. For species for which several accessions were determined to be of equal current viability, we conducted a seed storage (rapid ageing) experiment to differentiate the longevities of these accessions, hypothesising that seed longevity will be lower in older accessions due to prior time in storage, often under variable conditions. Our goal was to examine the utility of experimental seed ageing for identifying the storage potential of seed collections of unknown quality to inform the scheduling of future viability monitoring and/or use of such collections.
Materials and methods
Study species and seed accessions
Study species were selected through reviewing the Kings Park and Botanic Garden seed bank database. We focused on families previously identified as having species with shorter-lived seeds (e.g. as summarised in Merritt et al. (2021)), species with known germination requirements, and species for which several accessions had been collected and stored in different years. The first component of the study included selection of 44 accessions of 10 species native to Western Australia (WA) stored for up to 36 years to quantify seed viability in relation to time in storage (Table 1). Nine were species of Asteraceae, and one a species of Araliaceae; most were annual herbs – a plant life-form associated with shorter-lived seeds in an earlier study on Australian species (Merritt et al. 2014). Collections of Hyalosperma cotula, Olearia axillaris, Pithocarpa cordata, Podotheca angustifolia and Trachymene pilosa were sourced from urban bushland remnants (Kings Park, and Bold Park, Perth, WA) and collections of Brachyscome iberidifolia, Myriocephalus gueriniae, Olearia pimeleoides, Rhodanthe sterilescens and Panaetia lessonii from wild populations within WA. Collections were originally made with the intention of growing plants for bushland restoration and the development of botanic garden displays. All accessions were processed and banked within 12 months of collection.
| Species | Life form | Collection year | eRH (%) | |
|---|---|---|---|---|
| Brachyscome iberidifolia Benth. (Asteraceae) | Annual herb | 1982 | 43.3 | |
| 1999 | 51.2 | |||
| 2000 | 43.8 | |||
| 2003 | N/A | |||
| 2016 | 35.4 | |||
| Hyalosperma cotula Steetz (Asteraceae) | Annual herb | 2005 | 22.9 | |
| 2007 | 17.8 | |||
| 2009 | 19.0 | |||
| Myriocephalus gueriniae F.Muell. (Asteraceae) | Annual herb | 1990 | 72.3 | |
| 1995 | 45.6 | |||
| 1996 | 48.2 | |||
| 2003 | 46.4 | |||
| 2016 | 23.4 | |||
| Olearia axillaris (DC.) Benth. (Asteraceae) | Perennial shrub | 1992 | 43.1 | |
| 2007 | 18.3 | |||
| 2010 | 18.9 | |||
| 2014 | 21.9 | |||
| 2015 | 37.2 | |||
| Olearia pimeleoides (DC.) Benth. (Asteraceae) | Perennial shrub | 1987 | 33.5 | |
| 1989 | 32.6 | |||
| 2003 | 32.1 | |||
| Panaetia lessonii Cass. (Asteraceae) | Annual herb | 2000(a) | 34.4 | |
| 2000(b) | 40.5 | |||
| 2003(a) | 60.2 | |||
| 2003(b) | 41.8 | |||
| Pithocarpa cordata (DC.) Schmidt-Leb. & R.L.Barrett (Asteraceae) | Perennial shrub | 1992 | 37.8 | |
| 1999 | 47.2 | |||
| 2000 | 42.8 | |||
| 2005 | 22.5 | |||
| 2011 | 18.6 | |||
| Podotheca angustifolia (Labill.) Less. (Asteraceae) | Annual herb | 2003 | 40.7 | |
| 2005 | 33.2 | |||
| 2007 | 28.7 | |||
| 2011 | 23.4 | |||
| Rhodanthe sterilescens (F.Muell.) Paul G.Wilson (Asteraceae) | Annual herb | 1988 | 39.1 | |
| 1992 | 45.3 | |||
| 1998 | 45.5 | |||
| 2016 | 29.6 | |||
| Trachymene pilosa Sm. (Araliaceae) | Annual herb | 2001 | 49.5 | |
| 2003 | 40.8 | |||
| 2004 | 24.6 | |||
| 2005 | 34.9 | |||
| 2007 | 20.8 | |||
| 2014 | 15.3 |
eRH refers to the equilibrium RH of seeds at 15°C, determined at the time of removal from the seed bank freezer, after foil packets containing seeds had equilibrated to 15°C.
Immediately prior to the viability assessment we conducted, all seeds had been stored at −18°C in hermetically sealed aluminium foil packets. However, seeds of the different accessions had varied storage history depending on age. Seeds collected and stored prior to 1990 were originally processed and dried under ambient conditions, and stored in glass jars within a storage room at ambient temperatures. In 1990, air-conditioning was introduced to the seed store to maintain the room temperature at 23°C. In 1997, the existing seed collections were removed from glass jars, transferred to laminated aluminium foil packets and stored in chest freezers at −18°C, and all new collections from this time onwards were also stored at −18°C, following processing and drying under ambient conditions. In December 2005, a controlled environment room was installed to dry seeds at 15°C and 15–20% RH, with dried seeds hermetically sealed in laminated aluminium foil packets and stored in a walk-in freezer room at −18°C. As a result, many of the collections we have tested here, particularly the older collections, will have been stored under a range of conditions across the total storage life.
Seed germination testing
Laminated foil packets were retrieved from the freezer room and placed in the controlled environment drying room to equilibrate to 15°C before opening between December 2021 and May 2022. The equilibrium RH of the seeds was determined at 15°C using a hygrometer (Rotronic HygroPalm HP23AW, Switzerland) immediately upon opening of the foil packets. Samples of seeds of each accession were removed from the packets and examined under X-ray (Multifocus digital X-ray cabinet, Faxitron, Arizona). Only filled seeds, based on the presence of intact embryonic and endosperm tissue in the X-ray image, were selected for subsequent germination testing and experimentation.
Prior to germination testing, seeds were surface sterilised in a 2% (w/v) solution of calcium hypochlorite with two drops of surfactant (Tween 80®, Hurst Scientific, Perth) under partial vacuum (−80 kPa for 10 min two times, 10 min apart). Following surface sterilisation, seeds were rinsed three times in autoclave-sterilised reverse-osmosis water. For each accession, 100 seeds were tested for germination by placing 25 seeds into each of four Petri dishes containing solidified water-agar (0.7% w/v) with either karrikinolide (1 μM KAR1) (Trachymene pilosa) or gibberellic acid (1 mM GA3) (all other species). Petri dishes were incubated in a plant growth chamber (Contherm BIOSYN 6000CP, New Zealand) at 15°C with a 12/12 h light/dark regime (photo irradiance of 30 μM m−2 s−1 400–700 nm). Selected germination conditions were based on those found to be optimal through prior research. Germination was scored twice weekly for 28–35 days (depending on species), with seeds deemed to have germinated upon radicle emergence of >2 mm.
Seed longevity in experimental storage
Where multiple accessions of a species were found to have similar viability based on the germination testing (i.e. no significant differences in germination between accessions), a rapid seed ageing experiment was conducted to further differentiate the quality and potential future longevity of these accessions. A total of 24 accessions of six species were selected: B. iberidifolia (four accessions), H. cotula (three accessions), O. axillaris (three accessions), P. lessonii (four accessions), P. angustifolia (four accessions) and T. pilosa (six accessions). We included one accession for rapid ageing with significantly different germination from the other three accessions for P. lessonii, on account of this accession having the highest viability, yet being the oldest, collected in 2000(a).
The ageing experiments were conducted between June and October 2022. Seeds were aged following the comparative seed longevity testing protocol (Probert et al. 2009; Newton et al. 2014). Ten samples of at least 50 seeds of each accession were sealed into fine mesh nylon bags and placed in an air-tight electrical enclosure box (28 cm × 28 cm × 14 cm; NHP, Fibox, Australia), suspended above a non-saturated LiCl solution (385 g L−1) creating an RH of 47% at 20°C for 14 days. Seeds were subsequently transferred to a second electrical enclosure box with LiCl solution giving 60% RH (300 g L−1 LiCl) that was placed in a laboratory oven (WA Scientific Instruments, Australia) at 45°C for ageing. The RH inside the boxes was monitored using a data logger (T-TEC, Australia) to ensure that a constant environment was maintained. A sample of at least 50 seeds for each accession of each species was removed at intervals (e.g. 2, 5, 10, 20, 30, 40, 50, 75, 100 and 125 days) for germination testing in Petri dishes, as described above.
Statistical analyses
Germination data were analysed using R ver. 4.3.1 (R Core Team 2022) in RStudio version 2023.06.1 (RStudio Team 2022). Final percentage germination data within each species were analysed via a generalised linear model (GLM) with a logit link function and binomial error distribution, and with collection year as a factor for the seed viability testing experiment. Differences in maximum germination between collection years were initially assessed using a Wald chi-squared ANOVA using the package ‘car’ (Fox and Weisberg 2018) and when differences were detected, Holm’s adjusted pairwise T-tests were applied using the ‘estimate_contrasts’ function in the package ‘modelbased’ (Makowski et al. 2020) to confirm significant differences between collections.
In the seed storage experiment, data were analysed via probit analysis using Genstat (ver. 12, VSN International Ltd, UK), thereby fitting the seed viability equation (Ellis and Roberts 1980):
where ν is the viability in normal equivalent deviates (NED) of the seeds after p days in storage, Ki is the initial viability (NED) and σ is time (in days) for viability to fall by 1 NED. Probit analysis was undertaken simultaneously for all accessions within a species, first by fitting the full model (i.e. different estimates for each parameter), and then fitting reduced models where one or more parameters (Ki, σ) were constrained to the same value for each accession. An approximate F-test was subsequently used to determine the most suitable model.
Results
Seed viability testing
Final percentage seed germination varied significantly between accessions for all species except H. cotula, where all three accessions germinated to 95–96% (P = 0.93), P. angustifolia (100% germination in all four accessions (P = 1.0)), and T. pilosa (98–100% germination in all six accessions (P = 1.0; Fig. 1)). Germination of the four accessions of B. iberidifolia collected between 1999 and 2016 was also relatively high, ranging between 71 and 82%, with no significant difference between these accessions (P ≥ 0.41) and only the seeds collected in 1982 recording zero germination.
Cumulative germination curves for the 10 study species, with multiple accessions from different collection years. Seeds of Olearia pimeleoides collected in 1987 did not germinate – the line is obscured by data for the 1989 collection.
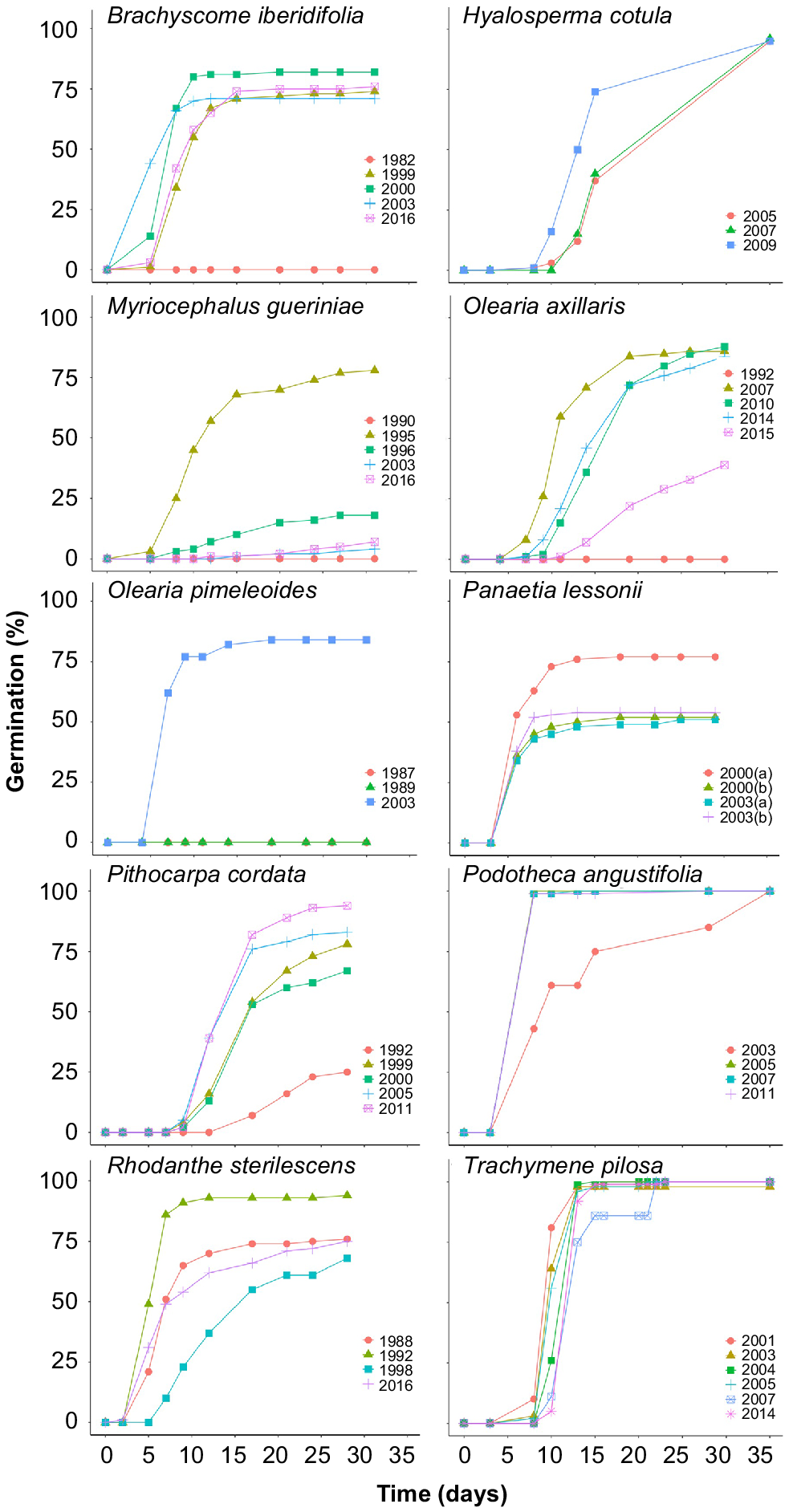
In the other species, seeds of at least one accession germinated to a high percentage (>75–80%) but there was also significant variation in germinability between different accessions. In most of these species (M. gueriniae, O. axillaris, O. pimeleoides and P. cordata), the lowest germination was associated with the oldest accession tested but a sequential decline in germination with accession age was not observed, and only in P. cordata and O. pimeleoides did the youngest accession germinate to the highest percentage. Seeds of M. gueriniae collected in 1995 germinated to 78%, significantly higher (all P < 0.014) than seeds collected in 1990, 1996, 2003 and 2016 (between 0 and 18% germination). Seeds of O. axillaris collected in 2007, 2010 and 2014 germinated to 84–88%, significantly higher (P < 0.001) than seeds collected in 1992 (0%) and 2015 (39%). Only seeds of O. pimeleoides collected in 2003 germinated (84%), with no germination of seeds collected in 1987 and 1989. P. cordata seeds collected in 2011 germinated to 94%, significantly higher (all P < 0.05) than in the four other accessions that ranged from 25% (1992 accession) to 83% (2005 accession). Seeds of R. sterilescens had relatively high germination across all accessions but there was a significant difference (all P < 0.004) in germination between seeds collected in 1992 (94%) and seeds collected in 1988, 1998 and 2016 (68–76%). Seeds of one of the accessions of P. lessonii collected in 2000 germinated to 77%, but those of the other accession collected in 2000, and those collected in 2003 and 2004 had significantly lower (all P < 0.003) germination (51–54%).
Seed longevity in experimental storage
Slope values of transformed survival curves could be constrained to a common value (i.e. common 1/σ) for seeds of H. cotula, O. axillaris, P. angustifolia and T. pilosa (Table 2) but could not be constrained for B. iberidifolia or P. lessonii (Table 2). Ki ranged from 0.1 for one of the P. lessonii seed lots collected in 2003 (20040681) to 4.38 for P. angustifolia seeds collected in in 2007 (Table 2). Ki was <1 for several collections of B. iberidifolia and P. lessonii. Ki could be constrained to a common intercept for H. cotula and O. axillaris. The p50 values for all species and accessions varied between <1 day and 58.78 days, with seeds of P. lessonii (0.56 days ≤ p50 ≥ 4.98 days), O. axillaris (5.57 days) and B. iberidifolia (4.67 days ≤ p50 ≥ 15.62 days) being much shorter-lived than seeds of H. cotula, P. angustifolia and T. pilosa, in which p50 values generally ranged between 40 and 50 days (Fig. 2, Table 2).
| Species | Accession | Collection year | Pre-ageing germination (2022, %) | Model | Ki ± s.e. (NED) | 1/σ ± s.e. (days−1) | σ (days) | p50 ± s.e. (days) | |
|---|---|---|---|---|---|---|---|---|---|
| Brachyscome iberidifolia | 20000426 | 1999 | 74 | Independent models | 0.70 (0.117) | 0.13 (0.015) | 7.69 | 5.34 (0.728) | |
| 20020768 | 2000 | 82 | 0.86 (0.112) | 0.09 (0.008) | 11.11 | 9.90 (0.966) | |||
| 20040734 | 2003 | 71 | 0.50 (0.111) | 0.11 (0.012) | 9.09 | 4.67 (0.861) | |||
| 20171454 | 2016 | 76 | 1.04 (0.129) | 0.07 (0.006) | 14.29 | 15.62 (1.23) | |||
| Hyalosperma cotula | 20060615 | 2005 | 95 | Ki and σ constrained | 2.31 (0.126) | 0.05 (0.003) | 20.00 | 42.48 (0.995) | |
| 20080060 | 2007 | 96 | |||||||
| 20100160 | 2009 | 95 | |||||||
| Olearia axillaris | 20061736 | 2007 | 86 | Ki and σ constrained | 0.84 (0.065) | 0.15 (0.01)) | 6.67 | 5.586 (0.349) | |
| 20100811 | 2010 | 88 | |||||||
| 20140749 | 2014 | 84 | |||||||
| Panaetia lessonii | 20010288 | 2000(a) | 77 | Independent models | 0.70 (0.104) | 0.14 (0.016) | 7.14 | 4.98 (0.623) | |
| 20010648 | 2000(b) | 52 | 0.17 (0.105) | 0.17 (0.027) | 5.88 | 1.01 (0.602) | |||
| 20030441 | 2003(a) | 51 | 0.10 (0.111) | 0.11 (0.016) | 9.09 | 0.90 (1.048) | |||
| 20040681 | 2003(b) | 54 | 0.14 (0.114) | 0.26 (0.046) | 3.85 | 0.56 (0.448) | |||
| Podotheca angustifolia | 20040253 | 2003 | 100 | σ constrained | 3.65 (0.201) | 0.08 (0.004)) | 12.50 | 49.40 (1.478) | |
| 20061247 | 2005 | 100 | 3.01 (0.172) | 40.30 (1.320) | |||||
| 20080478 | 2007 | 100 | 4.38 (0.239) | 58.78 (1.672) | |||||
| 20120326 | 2011 | 100 | 3.91 (0.213) | 52.36 (1.537) | |||||
| Trachymene pilosa | 20020589 | 2001 | 100 | σ constrained | 3.83 (0.186) | 0.08 (0.003) | 12.50 | 49.88 (1.476) | |
| 20040574 | 2003 | 98 | 3.48 (0.167) | 45.35 (1.361) | |||||
| 20061202 | 2004 | 100 | 2.12 (0.124) | 27.64 (1.170) | |||||
| 20061215 | 2005 | 100 | 3.31 (0.162) | 43.13 (1.325) | |||||
| 20080458 | 2007 | 100 | 3.82 (0.180) | 49.76 (1.443) | |||||
| 20150067 | 2014 | 100 | 3.85 (0.181) | 50.17 (1.452) |
Where Ki or σ could be constrained across accessions within a species, results of the constrained model are provided. Pre-ageing germination data are summarised from data for relevant accessions presented in Fig. 1.
Survival curves of rapidly aged (at 45°C and 60% RH) seeds fitted by probit analysis for different collections of six study species.
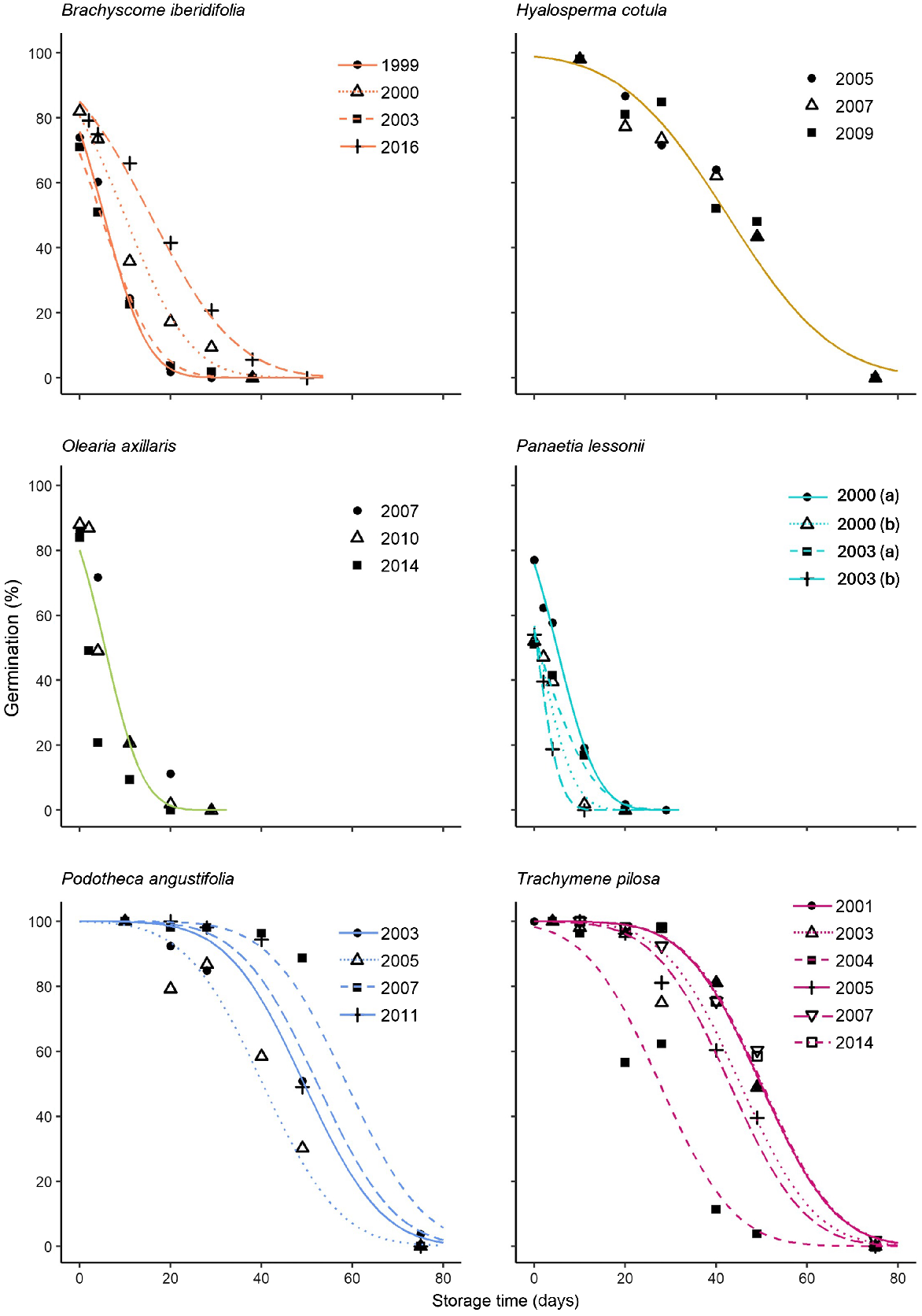
In most species except H. cotula and O. axillaris, p50 varied between the accessions tested and there was no consistent relationship between p50 and collection year. For example, for P. lessonii the oldest collection from 2000 had the greatest p50 and the greatest remaining longevity.
Discussion
Seed bank managers often contend with historical seed collections with incomplete records, changes to facilities, infrastructure and storage practices over long (e.g. decadal) timeframes, and resource constraints – all while new collections continue to accumulate (Hay et al. 2021). The Kings Park seed bank comprises many accessions for which there are no prior data or knowledge on seed viability or longevity. We tested the viability of multiple seed accessions, spanning time in storage of many decades, of a range of species hypothesised to be shorter-lived in storage. In accessions determined to have similar levels of viability, we applied a rapid ageing protocol to better discern differences in seed quality and longevity between the accessions. Viability testing of seeds retrieved from storage showed that for three species (Hyalosperma cotula, Podotheca angustifolia and Trachymene pilosa), germination was ≥95% across all accessions regardless of age, with storage periods ranging between 17 and 21 years. In the remaining species, there were significant differences in germination between accessions but no relationship was evident between collection year and seed viability. In some species (e.g. Brachyscome iberidifolia, Olearia axillaris and O. pimeleoides), accessions collected and banked in the 1980s or 1990s had poorer viability (0–20%) than more recent accessions, as expected. However, there were also examples of species for which older accessions retained high viability – in some cases, more so than those collected more recently. For example, Myriocephalus gueriniae seeds collected in 1995 germinated to 80%, far higher than all more recent accessions (all <20%). Similarly, for Rhodanthe sterilescens, seeds collected in 1992 had greater germination than the other accessions tested, including two accessions collected more recently in 1998 and 2016. This is despite seeds of the 1992 collection of R. sterilescens being subjected to poorer storage conditions than the more recently collected seeds stored continuously at −18°C. Notably, the range of conditions (temperature and RH) under which seeds were stored varies substantially amongst accessions, and notwithstanding the poor seed germination of many of the oldest accessions (i.e. those stored in an air-conditioned room at 23°C, up until moving to −18°C storage in 1997), there was no consistent relationship between storage conditions and current germination. Based on the eRH determined at the time of seed retrieval from storage for viability testing (Table 1), many accessions have been stored at relatively high moisture contents but these accessions did not have lower germination than seeds stored following drying to 15–20% RH. For example, seeds from all collections of Podotheca angustifolia and Trachymene pilosa germinated to ~100% despite RH (measured at the time of retrieval) varying substantially between accessions, ranging from 15 to 50% (Table 2). Such results demonstrate that storage history is not necessarily an accurate indicator of seed quality and is not instructive for decision-making on seed curation of these collections.
Seed longevity determined via rapid ageing experiments (p50) varied between accessions of each species and older accessions did not have lower longevity, as originally hypothesised. There were incidences of p50 of older accessions being significantly greater than more recently banked accessions (e.g. Panaetia lessonii and Podotheca angustifolia) and for most species, longevity varied in a manner unrelated to prior time in storage. The differences in longevity between accessions of a species may be attributed to differences in both the initial viability (Ki) at the commencement of the ageing experiment and the rate of viability loss (1/σ), or both (Table 2). The rapid ageing experiment thus distinguished differences in initial viability amongst accessions that were not evident through the germination testing conducted when seeds were first removed from storage (i.e. the pre-ageing germination data presented in Table 2). For example, seeds of Podotheca angustifolia collected in 2005 had a Ki of 3.01, while the Ki of other accessions ranged between 3.65 and 4.38 despite germination testing showing all accessions to have 100% viability prior to the rapid ageing experiment. These data point to factors such as seed maturity at the point of collection, and post-harvest handling practices including cleaning, drying and banking procedures, and the influence of these on seed quality at the time of banking likely being more influential to the future longevity than prior time in storage.
Seeds of all accessions were determined to have p50 values < 50 days. These values are consistent with those of other species from seasonally dry regions of Australian with shorter-lived seeds, and with p50 data for other Australian Araliaceae and Asteraceae (Probert et al. 2009; Merritt et al. 2014, 2021). Based on p50 data from similar comparative longevity studies for a broad range of species from across the globe (Colville and Pritchard 2019), these data characterise the study species as having seeds with ‘short’ to ‘medium’ longevity (Mondoni et al. 2011). It should be noted, however, that because all accessions were stored for many years prior to the ageing experiments the p50 values do not account for the potential decline in longevity during storage. Whilst most species we selected for the ageing experiments comprised multiple accessions of relatively high (>85%) initial germination (except Brachyscome iberidifolia and Panaetia lessonii), meaning that the p50 values are broadly comparable to those presented in the studies of Probert et al. (2009) and Merritt et al. (2014) calculating p50 for freshly collected seeds would give additional weight to these species-level comparisons of seed longevity. All species had some accessions that retained high viability after ~15–20 years of storage, even with storage under sub-optimal conditions, providing confidence that seed banking is practical for germplasm conservation of these species over at least this timeframe.
We found many accessions to have a high RH at the time of retrieval from the seed bank for study. Many accessions were stored prior to the installation of the controlled environment drying room at Kings Park in December 2005, and the wide variation in RH between these accessions likely reflects the post-harvest processing practices of the time and the drying of seeds under ambient environmental conditions. One accession (Myriocephalus gueriniae collected in 1991) was measured at 72% eRH, indicating a likely failure of the seal or puncture to the laminated foil bag. Also, despite the installation of the seed drying room in December 2005, we identified 13 collections with an eRH above the target range (i.e. 15–20% RH), indicating incomplete drying of seeds and lack of procedural rigour by seed bank operational staff at the time of banking or poor dry-chain management procedures for accessions that were periodically removed to retrieve seeds.
Conclusions
Experimental ageing and the resultant p50 data provide insights into the initial viability and longevity of freshly collected seeds at the time of banking, and accessions that lose viability more rapidly can be noted for more frequent monitoring (Hay and Whitehouse 2017; White et al. 2023). We used this approach to distinguish seed quality factors for previously banked accessions of wild species postulated to be short-lived and subject to differing storage environments. Storage history was not an indicator of the likelihood of higher or lower germination of the accessions upon retrieval from the seed bank and based on the p50 data, is not informative regarding future storage potential. The rapid ageing experiment revealed differences in seed quality amongst accessions that were not evident in a single germination test. These data can be used to schedule future germination re-tests, and prioritise accessions for utilisation and species for recollection.
Declaration of funding
Project operating costs were supported by Murdoch University and an Australian Research Council Linkage Grant (LP200200680).
Acknowledgements
We are grateful to Joe Fontaine (Murdoch University) for support of the project, and Phil Withers (University of Western Australia) and Sean Tomlinson (Department of Biodiversity Conservation and Attractions) for collaboration on the Australian Research Council Linkage Grant (LP200200680). We thank Rebecca Jonas for assistance with preparing Fig. 2.
References
Chau MM, Chambers T, Weisenberger L, Keir M, Kroessig TI, Wolkis D, Kam R, Yoshinaga AY (2019) Seed freeze sensitivity and ex situ longevity of 295 species in the native Hawaiian flora. American Journal of Botany 106(9), 1248-1270.
| Crossref | Google Scholar | PubMed |
Colville L, Pritchard HW (2019) Seed life span and food security. New Phytologist 224(2), 557-562.
| Crossref | Google Scholar | PubMed |
Ellis RH, Roberts EH (1980) Improved equations for the prediction of seed longevity. Annals of Botany 45(1), 13-30.
| Crossref | Google Scholar |
Fu Y-B, Ahmed Z, Diederichsen A (2015) Towards a better monitoring of seed ageing under ex situ seed conservation. Conservation Physiology 3, cov026.
| Crossref | Google Scholar |
Hay FR, Probert RJ (2013) Advances in seed conservation of wild plant species: a review of recent research. Conservation Physiology 1, cot030.
| Crossref | Google Scholar |
Hay FR, Whitehouse KJ (2017) Rethinking the approach to viability monitoring in seed genebanks. Conservation Physiology 5, cox009.
| Crossref | Google Scholar |
Hay FR, Whitehouse KJ, Ellis RH, Sackville Hamilton NR, Lusty C, Ndjiondjop MN, Tia D, Wenzl P, Santos LG, Yazbek M, Azevedo VCR, Peerzada OH, Abberton M, Oyatomi O, de Guzman F, Capilit G, Muchugi A, Kinyanjui Z (2021) CGIAR genebank viability data reveal inconsistencies in seed collection management. Global Food Security 30, 100557.
| Crossref | Google Scholar |
Hay FR, Davies RM, Dickie JB, Merritt DJ, Wolkis DM (2022) More on seed longevity phenotyping. Seed Science Research 32, 144-149.
| Crossref | Google Scholar |
Lee J-S, Velasco-Punzalan M, Pacleb M, Valdez R, Kretzschmar T, McNally KL, Ismail AM, Cruz PCS, Sackville Hamilton NR, Hay FR (2019) Variation in seed longevity among diverse Indica rice varieties. Annals of Botany 124, 447-460.
| Crossref | Google Scholar | PubMed |
Liu U, Breman E, Cossu TA, Kenney S (2018) The conservation value of germplasm stored at the Millennium Seed Bank, Royal Botanic Gardens, Kew, UK. Biodiversity and Conservation 27(6), 1347-1386.
| Crossref | Google Scholar |
Martyn Yenson AJ, Sommerville KD, Guja LK, Merritt DJ, Dalziell EL, Auld TD, Broadhurst L, Coates DJ, Commander L, Crawford AD, Emery NJ, Funnekotter B, Knapp Z, Makinson RO, Monks L, Wrigley D, Offord CA (2024) Ex situ germplasm collections of exceptional species are a vital part of the conservation of Australia’s national plant treasures. Plants, People, Planet 6(1), 44-66.
| Crossref | Google Scholar |
Merritt DJ, Martyn AJ, Ainsley P, Young RE, Seed LU, Thorpe M, Hay FR, Commander LE, Shackelford N, Offord CA, Dixon KW, Probert RJ (2014) A continental-scale study of seed lifespan in experimental storage examining seed, plant, and environmental traits associated with longevity. Biodiversity and Conservation 23, 1081-1104.
| Crossref | Google Scholar |
Merritt DJ, Whitehouse KJ, Hoyle GL, Crawford A, Wood JA, Satyanti A, Norton SL, Errington G, Martyn Yenson AJ (2021) Seed banking: orthodox seeds. In ‘Plant germplasm conservation in Australia: strategies and guidelines for developing, managing and utilising ex situ collections’. 3rd edn. (Eds AJ Martyn Yenson, C Offord, P Meagher, et al.) pp. 119–154. (Australian Network for Plant Conservation: Canberra, Australia)
Mondoni A, Probert RJ, Rossi G, Vegini E, Hay FR (2011) Seeds of alpine plants are short lived: implications for long-term conservation. Annals of Botany 107, 171-179.
| Crossref | Google Scholar | PubMed |
Probert RJ, Daws MI, Hay FR (2009) Ecological correlates of ex situ seed longevity: a comparative study on 195 species. Annals of Botany 104(1), 57-69.
| Crossref | Google Scholar | PubMed |
R Core Team (2022) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at http://www.R-project.org/
RStudio Team (2022) RStudio: integrated development for R. RStudio, Inc., Boston, MA, USA. Available at https://posit.co/products/open-source/rstudio/
Satyanti A, Nicotra AB, Merkling T, Guja LK (2018) Seed mass and elevation explain variation in seed longevity of Australian alpine species. Seed Science Research 28(4), 319-331.
| Crossref | Google Scholar |
Sommerville KD, Newby Z-J, Martyn Yenson AJ, Offord CA (2023) Are orthodox Australian rainforest seeds short-lived in storage? Australian Journal of Botany 71, 340-352.
| Crossref | Google Scholar |
Walters C (2015) Genebanking seeds from natural populations. Natural Areas Journal 35(1), 98-105.
| Crossref | Google Scholar |
Walters C, Pence VC (2021) The unique role of seed banking and cryobiotechnologies in plant conservation. Plants, People, Planet 3, 83-91.
| Crossref | Google Scholar |
Walters C, Wheeler LM, Grotenhuis JM (2005) Longevity of seeds stored in a genebank: species characteristics. Seed Science Research 15, 1-20.
| Crossref | Google Scholar |
White FJ, Hay FR, Abeli T, Mondoni A (2023) Two decades of climate change alters seed longevity in an alpine herb: implications for ex situ seed conservation. Alpine Botany 133, 11-20.
| Crossref | Google Scholar |


