Rainforest persistence and recruitment after Australia’s 2019–2020 fires in subtropical, temperate, dry and littoral rainforests
Andrew G. Baker A * , Claudia Catterall A and Matthew Wiseman B
A * , Claudia Catterall A and Matthew Wiseman B
A Forest Research Centre, School of Environment, Science and Engineering, Southern Cross University, Lismore, NSW 2480, Australia.
B NSW National Parks & Wildlife Service, 136 Summerland Way, Kyogle, NSW 2474, Australia.
Australian Journal of Botany 70(3) 189-203 https://doi.org/10.1071/BT21091
Submitted: 28 July 2021 Accepted: 22 February 2022 Published: 28 March 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Interactions between rainforest plants and fire occur when fires encroach into rainforest and when rainforest pioneers colonise fire-prone open forests. Although numerous studies show that many rainforest plants survive fire by resprouting and postfire seedling recruitment, data is lacking for several major Australian rainforest types. In this study, we examine fire-resilience traits among 228 taxa of woody rainforest plants in four rainforest classes (subtropical, warm temperate, dry and littoral rainforest) less than 1 year after being burnt in the extensive wildfires of 2019–2020. Among taxa with ≥ 5 records of complete crown scorch (126), resprouting occurred in 63% of taxa overall and 61% of late-successional taxa. Fire-cued seedling recruitment occurred in 62% of taxa overall and 48% of late-successional taxa. Surprisingly, species richness of woody plants increased 22% postfire due to high rates of persistence and emergence of new taxa into standing plant populations as seedlings. Stem density increased ∼400% postfire due to high rates of resprouting and reproduction through suckering and seedling recruitment, although there was a significant redistribution from medium to smaller stem size classes. Larger stems (>10 cm diameter at breast height) were not significantly reduced in rainforest stands. High resprouting rates in small rainforest plants (1 cm diameter at breast height, 1 m tall) suggests rapid attainment of resprouting capacity. Our findings demonstrate that most subtropical, dry, warm temperate and littoral rainforest plant taxa are resilient to rare fires, and suggest that rainforest plants that invade rarely-burnt open forests may quickly become resistant to removal by infrequent fires, with potential for increased populations through fire-enhanced seedling germination.
Keywords: basal resprouting, fire, fire-cued seeding, persistence niche, plant resilience traits, rainforest, resilience, succession, woody encroachment.
Introduction
Fire plays a major role in controlling rainforest boundaries, with rainforest communities usually limited to topographic positions that are sheltered from frequent fire (Bowman 2000; Wood et al. 2011; Ondei et al. 2017; Bond 2019). Yet the response of rainforest flora to fire is complex, and many rainforest species from tropical to temperate latitudes are capable of surviving occasional fires by resprouting and fire-cued seedling recruitment (e.g. Unwin 1983; Hill and Read 1984; Floyd 1990; Bowman 1991; Williams 2000; Marrinan et al. 2005; Campbell and Clarke 2006; Williams et al. 2012; Tolsma et al. 2019). Postfire resprouting and seedling recruitment are key life-history traits that confer resilience, profoundly influencing community composition and ecosystem function through differential species persistence after fire (Bond and Midgley 2001; Pausas et al. 2004). Together, these persistence traits provide a valuable basis for predicting postfire community assembly, and resilience of species and ecosystems to changing climate and fire regimes (Pausas et al. 2004; Pausas and Bradstock 2007).
Under typical weather conditions, fires originating in fire-prone ecosystems typically extinguish along rainforest margins (Wood et al. 2011; Collins et al. 2019), where the closed rainforest canopy reduces ecosystem flammability through a combination of reduced grassy fuels, lower temperatures, higher relative humidity, and higher moisture content and decomposition rates of litter fuels (Hoffmann et al. 2012b; Little et al. 2012; Alexander and Arthur 2014). However, these fuel and microclimate property differentials between open- and closed ecosystems are muted under extreme fire weather and drought (Collins et al. 2019), increasing the likelihood of fire encroachment into rainforest (Marrinan et al. 2005; Clarke et al. 2014; Nolan et al. 2020). In many regions of Australia, especially the south-east, climate change is projected to increase the number of extreme fire days, lightning ignitions, and the severity of drought (Bradstock 2010; Clarke and Evans 2019; Canadell et al. 2021), potentially leading to increased incidence of fires encroaching into rainforest. Understanding the resilience of rainforest flora to projected regional increases in fire activity is therefore crucial to inform future rainforest conservation and management (Bradstock 2016).
Conversely, in many high rainfall regions of Australia, rainforest pioneers (rainforest taxa capable of colonising non-rainforest communities) are expanding into fire-prone open forests due to declining fire frequency (Gilbert 1959; Russell-Smith et al. 2004; Stanton et al. 2014; Tasker et al. 2017; Fletcher et al. 2021). Global change is predicted to favour further rainforest expansion in some regions, especially northern and north eastern Australia where fire danger conditions are forecast to decrease or remain stable (e.g. Lim and Roderick 2009; Clarke et al. 2011). Elevated atmospheric carbon dioxide (CO2) is commonly invoked to explain rainforest expansion by favouring tree cover at the expense of flammable grasses (e.g. Bowman et al. 2010; Wigley et al. 2010). Other potential climate drivers include increased regional rainfall (Bowman et al. 2010), increased atmospheric humidity (Willett et al. 2007), and improved forest water-use efficiency in elevated CO2 atmospheres (Keenan 2015). While there is increasing evidence that invading rainforest pioneers displace open forest flora and fauna (Chapman and Harrington 1997; Woinarski et al. 2004; Baker et al. 2020a, 2020b) and disrupt ecosystem function (Hoffmann et al. 2012a; Baker et al. 2021), the threshold at which these invading rainforest plants become resistant to removal by subsequent fires is not well understood (Baker et al. 2020a). Resprouting capacity in woody plants often increases from a low level at the seedling and sapling stage, and peaks at the young to mature stage, before declining during late maturity to senescence (Bond and Van Wilgen 1996; Vesk 2006; Clarke et al. 2013; Jaureguiberry et al. 2020), although there is variation between growth forms and ecosystem type (Clarke et al. 2013).
During the unprecedented drought conditions of 2019–2020, extensive areas of both rainforest and non-rainforest vegetation were burnt within the Gondwana Rainforests of Australia World Heritage Area, posing a potentially major threat to these globally significant ecosystems (Nolan et al. 2020). We took advantage of this historically unprecedented event to examine postfire resprouting and seed germination among woody rainforest taxa across four rainforest classes. Postfire resprouting and seedling germination of rainforest plants has been widely reported in Australian rainforests (e.g. Williams 2000; Campbell and Clarke 2006; Clarke et al. 2015), yet to our knowledge, no study has quantified the impacts of fire on the subtropical, dry or littoral rainforest classes of Keith (2004), limiting understanding of community scale responses to changing climate and fire regimes. Further, fire-response data is lacking for most rainforest flora species (Kenny et al. 2004; OEH 2014), hampering efforts to define appropriate domains of fire frequency for open forests with rainforest plants in the understorey. Understanding postfire recovery traits of rainforest flora is crucial for predicting shifts in rainforest distribution and composition in response to changing climate and fire regimes. Motivated by the need to better understand the resilience of rainforests to fire, the aims of the present study were to (1) quantify the mortality, resprouting and seedling recruitment of woody rainforest taxa following wildfire in subtropical, temperate, dry and littoral rainforests on the North Coast of New South Wales; (2) compare prefire and postfire stem density and species richness; and (3) examine the effect of prefire stem diameter on postfire resprouting rates.
Methods
Field survey design and sampling
The study was undertaken in and adjacent to reserves of the Gondwana Rainforests of Australia World Heritage Area in the North Coast Bioregion of NSW. To examine patterns of postfire resprouting and seedling recruitment, 17 sites were surveyed across four rainforest classes (Keith 2004; Supplementary Table S1) burnt during the extensive 2019–2020 bush fires of eastern Australia (Fig. 1), including subtropical (6 sites), warm temperate (7), dry (1) and littoral (3) rainforest. Burnt cool temperate rainforest was not sampled due to prohibitively difficult access. Fire severity at the sites was generally low to moderate, resulting in substantial scorching of the midstorey and subcanopy, but with variable degrees of canopy scorch (0–90%). Near-complete canopy scorch occurred on one site each of subtropical and littoral rainforest. Sites were located in burnt areas of mature rainforest (lacking Eucalypts or sclerophylls; Lynch and Neldner 2000) where possible, although sclerophyll species occurred as scattered individuals on many sites, usually as emergent trees above a well-developed, closed rainforest canopy. Five sites were located in burnt rainforest ecotones with a canopy of sclerophyll trees (>30% crown cover) above a well-developed rainforest subcanopy, where fire had self-extinguished at the rainforest boundary without encroaching stands of mature rainforest. The minimum distance between any two plots was 200 m (mean = 4889 m, SE = 1727 m).
|
|
Sampling was undertaken over a 6-month period from June to November 2020, 6–12 months after fire, thus corresponding to the immediate postfire period (≤ 1 year; Clarke et al. 2015). Data for subtropical, warm temperate and dry rainforest was derived from sampling by the NSW National Parks & Wildlife Service (NPWS; Nicholson et al. 2020). At each site, vegetation was sampled once in ∼20 m × 20 m quadrats, with plot dimensions modified as necessary to maximise sampling of narrow ecotones or areas of consistent burn severity (mean plot area = 421 ± 16.3 m2). Within each plot, all woody stems (rainforest, sclerophyll, trees, shrubs, vines) were identified to species and examined for evidence of postfire resprouting, before assignment to one of three postfire response classes: fire killed (no postfire resprouting), live resprout (generation of new shoots from dormant buds after 100% crown scorch), and alive escape (crown survived with only minor scorching). For living stems, species identification was based on examination of live foliage and stem features. In the case of dead stems, most individuals retained dead leaves at the time of sampling, and these were used for identification to species. Identification of dead stems without foliage was frequently possible by examination of bark features, branching architecture and comparison to adjacent stems with live foliage. Stems lacking sufficient features to allow confident identification were assigned as unknown and excluded from analyses.
Resprouting location was identified as aerial (apical, stem), basal (arising from stem at ground level) or underground (lignotubers, rhizomes, root suckers). Root suckers were differentiated from seedlings by the general absence of cotyledons, juvenile leaf form and visible rootlets, and excavation of surrounding soil to confirm connection to a pre-existing root system. Where possible, root suckers were assigned to identified pre-existing stems, by identification of the proximal roots (pointing toward the parent plant), which are typically thinner than distal roots and allow determination of the direction of the parent plant. Prefire plant size was quantified by recording the height and diameter at breast height (DBH, 1.3 m above ground) of original stems (dead, alive). For finer stems burnt below 1.3 m, diameter was measured at the highest available location.
In addition to resprouting response, identification of fire-cued seedling recruitment (sensu Clarke et al. 2015; germination in the immediate postfire period; ≤ 1 year) was also assessed at each site. Seedlings were differentiated from root suckers by the presence of cotyledons, juvenile leaf form and visible rootlets, and excavation of surrounding soil to confirm a lack of connection to a pre-existing root system. For groups of plantlets of uniform size, occasional seedlings (∼1 in 10) were pulled out to confirm seedling status. All seedlings were identified to species and tallied. In rare cases where plants could not be assigned confidently to suckers or seedlings, they were excluded from further analysis.
All identified stems were classified into functional groups based on descriptions of the Royal Botanical Gardens Trust (RBGT 2021): rainforest (rainforest or its margins), sclerophyll (predominantly open forest or woodland). Rainforest taxa were also classified as early successional (pioneer, early secondary; Kooyman 1996) or late successional (later secondary, mature phase; Kooyman 1996). All analyses were limited to native woody rainforest taxa, except for a comparison of mean seedling densities between rainforest and sclerophyll taxa.
Postfire resprouting and seedling recruitment patterns in woody rainforest taxa
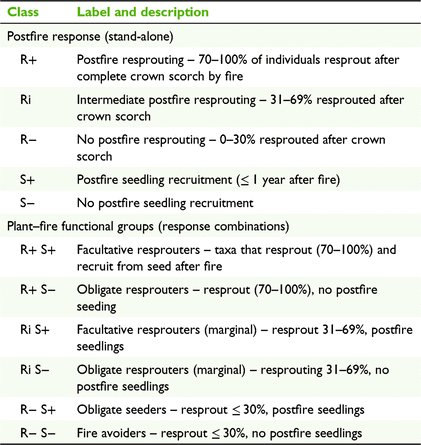
To examine fire-resilience traits, taxa with at least five individual records of 100% crown scorch were classified to postfire resprouting and seedling response classes according to Table 1. Resprouting and seedling traits were also combined for each species to allow assignment to the plant–fire functional groups (Table 1).
|
The prepared data were analysed in terms of the proportional abundance of resprouting and seeding responses of woody rainforest taxa across rainforest classes. We used the Kruskal–Wallis rank sum test and Conover–Iman post hoc tests with Bonferroni correction (P ≤ 0.05) to determine whether there were differences among fire-resilience traits between rainforest type and rainforest successional stage. Statistical analyses were processed using R software (v.3.4.2.; R Foundation for Statistical Computing, Vienna, Austria) and the package PMCMR (Pohlert 2018). Change in overall abundance of taxa across all sites was examined by comparing pre- and postfire counts of taxa with ≥ 10 prefire records.
Postfire plant density and species richness
To explore the effect of fire on the community structure and composition, we compared counts of prefire individuals (fire killed + live resprout + alive escape) and postfire individuals (live resprout + root suckers + alive escape + seedlings) to calculate changes in plant density, species richness and size class distribution. Root suckers were counted as individuals in the postfire individual total, as root suckers are a common means of postfire vegetative reproduction among rainforest taxa (Hoffmann 1998). To account for variation in plot area, we standardised data as follows: stem density (stems m−2) and species richness (number of taxa per plot, 421 m2). Pairwise differences between prefire and postfire stem density and species richness were determined using Conover–Iman post hoc tests with Bonferroni correction (P ≤ 0.05). Kruskal–Wallis rank sum tests were performed on prefire and postfire stem class distribution using the ‘kruskal.test’ function in R. To examine pathways of species turnover (persistence, immigration, extinction), all fire responses observed for each species at a site were grouped into combined classes (e.g. resprouting + seed germination), before calculating the proportion of individuals in each combined class across all species at a site prefire. Taxa with both postfire seedlings and prefire standing plants as potential parents were classed ‘local seed’, while those with postfire seedlings but no prefire plants were classed ‘new seed’.
Plant size effects on resprouting capacity
To examine the influence of plant size on resprouting capacity, stem diameter and height data across all woody rainforest plants were grouped into six DBH classes (0–1 cm, 1–2 cm, 2–3 cm, 3–5 cm, 5–10 cm, and >10 cm) and five height classes (0–1 m, 1–2 m, 2–5 m, 5–10 m and >10 m) respectively for analysis. The largest DBH class (> 10 cm) was not further subdivided as > 95% of stems in this class were <50 cm DBH. To determine proportion of taxa capable of resprouting with a DBH of ≤ 1 cm, taxa with at least five individual records of 100% crown scorch and resprouting rates ≥ 30% were classified as capable of resprouting in this size class.
Conover–Iman post hoc tests with Bonferroni correction (P ≤ 0.05) were used to determine whether there were differences between classes of stem diameter and height. All means are reported as mean ± s.e. (s.e.m.).
Results
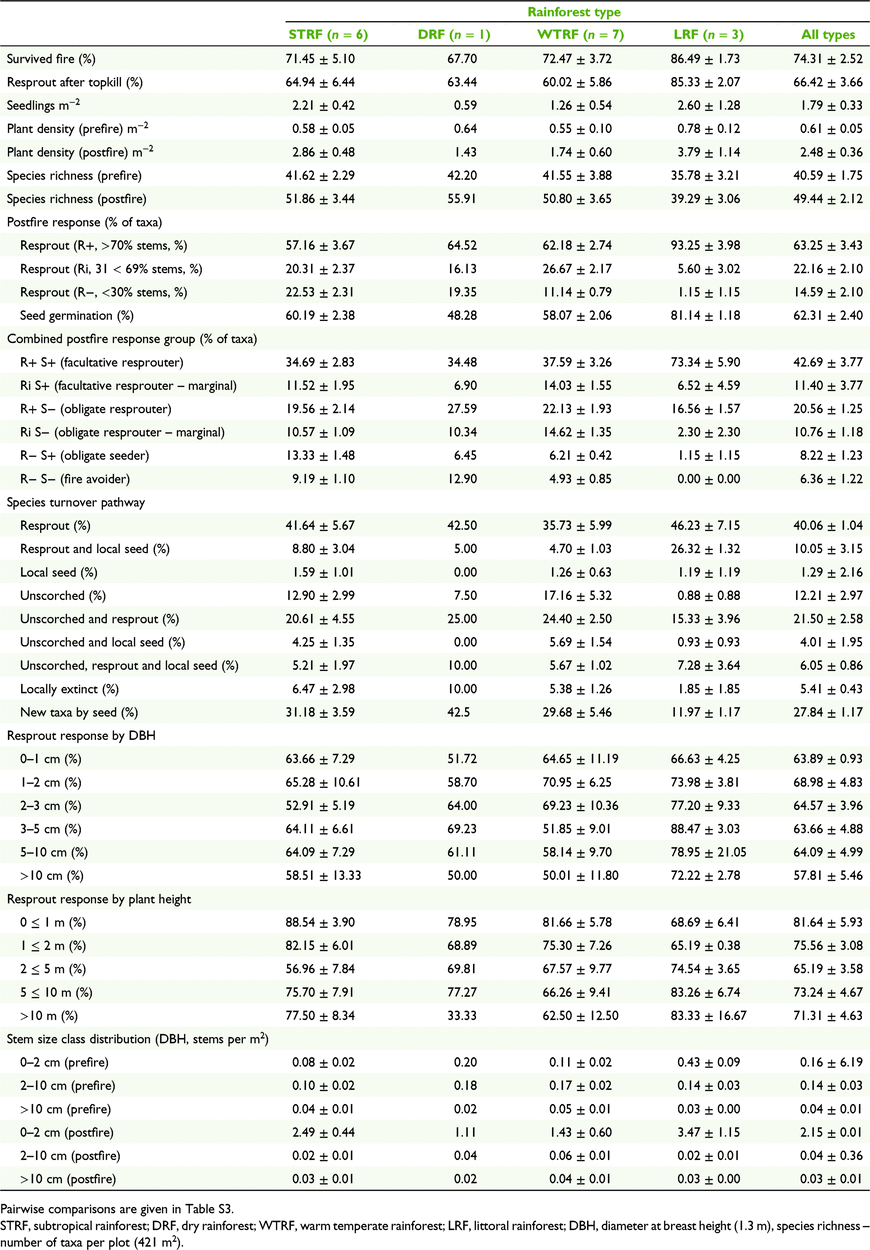
We recorded a total of 4 254 fire-affected standing plants and 12 719 seedlings from 228 taxa of woody rainforest plants. Growth forms included trees/shrubs (∼84%) and vines (∼16%). Approximately 9% of all fire-affected standing plants could not be identified. Overall response of standing woody rainforest plants to wildfire included individuals that were either killed by fire (25.7 ± 2.7%), resprouted after 100% canopy scorch (53.7 ± 4.4%) or survived with their crowns largely unscorched (20.7 ± 3.0%). Survival as live crowns varied by stem size (DBH), including >10 cm (63.5 ± 5.8%; H1 = 3.53, P = 0.06), 2–10 cm (27.4 ± 3.96%; H1 = 20.83, P < 0.001) and <2 cm (11.9 ± 3.08%; H1 = 24.43, P < 0.001). Of all individuals subject to complete canopy scorch, 66.4 ± 3.7% survived by resprouting (Table 2). Resprouting rates were not significantly different between rainforest classes (P > 0.05; Table S3). The most common position of resprouting was basal (52.3 ± 9.1%), followed by underground (34.8% ± 7.7%) and aerial stems (12.9 ± 2.2%). Postfire seedling germination of woody rainforest plants was observed at a mean density of 1.8 ± 0.4 seedlings m−2. Seedling densities were not significantly different between rainforest types (P > 0.05). Mean seedling density of sclerophyll taxa (e.g. Lophostemon, Allocasuarina, Eucalyptus) was 0.06 m−2, of which 56% were L. confertus, a common dominant of both sclerophyll and rainforest communities in the study region. Although excluded from the analysis, postfire weeds were absent from all but three sites with very low weed stem abundance of 0.2–1.7%.
|
Postfire mortality, resprouting and seedling recruitment of woody rainforest taxa
Of the 228 woody rainforest taxa assessed, 213 (93.4%) displayed a capacity to persist in situ through wildfire by either resprouting (188 taxa) or seed germination (118 taxa); 85 (37.2%) both resprouted and germinated from seed, while seven taxa (3.1%) showed no evidence of persistence mechanisms (Table S2). Among the 126 taxa of plants with ≥ 5 records of 100% crown scorch, resprouters (R+) comprised the highest proportion of attributed taxa (63%), intermediate resprouters (Ri) comprised 22% and non-resprouters (R−) comprised 15% (Fig. 2). Of these taxa, 62% persisted in situ through seed germination, with or without resprouting (seeders, S+).
Across all sites and rainforest types, facultative resprouters (R+ S+) comprised the highest proportion of taxa (43%); obligate resprouters (R+ S−) comprised 21%; marginal facultative resprouters (Ri S+) comprised 11%; marginal obligate resprouters (Ri S−) comprised 11%; obligate seeders (R− S+) comprised 8%; and fire avoiders (R− S−) comprised 6% (Table 2, Fig. 2). Among taxa attributed as early successional, 83% were seeders (S+), 72% resprouted via suckering; 67% were resprouters (R+), 50% were facultative resprouters (R+ S+), and no taxa were fire avoiders (R− S−). Among late-successional taxa, resprouters comprised 61%, 59% resprouted via suckering, seeders comprised 48%, facultative resprouters comprised 32%, and fire avoiders comprised 11%.
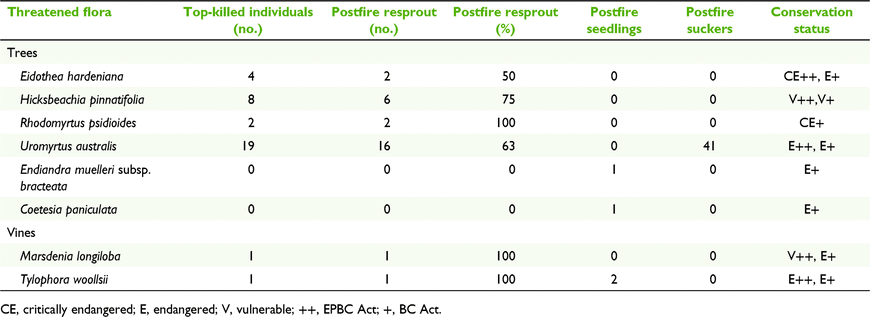
Eight woody rainforest taxa listed as threatened species under the Commonwealth Environment Protection and Biodiversity Conservation Act 1999 (EPBC Act) and/or the NSW Biodiversity Conservation Act 2016 (BC Act) were found to have postfire regeneration mechanisms (Table 3). Among these, seven taxa had the ability to resprout after 100% crown scorch by fire (≥ 1 record), three taxa showed postfire germination and one taxon increased stem density by root suckers.
|
Postfire stem density and species richness
The number of standing plant crowns declined by 79.3 ± 3.0% immediately postfire, being either returned to ground level by complete canopy scorch or killed. Including postfire seedlings and resprouts, the density of living woody rainforest plants was significantly higher (∼four times higher, P < 0.001) after wildfire (2.47 ± 0.36 plants m−2) than before wildfire (0.61 ± 0.05) (Table 2, Fig. 3). Postfire stem densities comprised ∼74% of prefire standing plants that remained alive after the fire (0.45 ± 0.04 plants m−2) plus the postfire emergence of additional seedlings (1.79 ± 0.35 m−2) and root suckers (0.23 ± 0.05 m−2). Postfire stem densities increased significantly in the 0–2 cm DBH class (H1 = 23.75, P < 0.001) and decreased in the 2–10 cm class (H1 = 22.45, P < 0.001), but did not change significantly in the >10 cm class (H1 = 1.334, P = 0.23) (Fig. 4). Species richness of woody rainforest plants was also significantly higher (21.8% higher, P = 0.006) after wildfire (49.44 ± 2.12) than before wildfire (40.59 ± 1.75) (Fig. 3). Species turnover following wildfire comprised ∼5% of taxa becoming locally extinct from plots, ∼95% of taxa persisting through resprouting, germination from local seed or remaining unscorched, and the addition of new taxa to standing plant populations through postfire seedling germination (∼11 new taxa per plot) increasing species richness by ∼28% (Fig. 5). Seedling density and resprouting rates were not significantly affected by fire severity (P > 0.05; Table S3).
Plant size effects on resprouting capacity
Moderate to high proportions of rainforest plants resprouted across all classes of stem height (∼64–100%) and stem diameter (∼66–100%) (Table 2, Fig. 6). There were no significant differences in resprouting response between classes of stem height or stem diameter.
Discussion
Although numerous studies have documented postfire resprouting and seeding in Australian rainforests (e.g. Gilbert 1959; Floyd 1990; Williams 2000; Marrinan et al. 2005; Campbell and Clarke 2006; Williams et al. 2012), none have examined fire effects in subtropical, dry or littoral rainforests. By including previously unstudied rainforest classes in our study, our findings extend this literature to show that subtropical, dry and littoral rainforests have high fire resilience – comparable to that reported for other rainforest classes. Together with earlier studies, our findings suggest that high fire resilience, at least to infrequent fires, is a universal property of Australian rainforests.
Fire resilience of rainforest structure
In the short-term, single wildfires greatly reduced live above-ground biomass (∼80% of stems), resulting in profound structural changes in the rainforest communities examined. Despite this, most larger rainforest trees (> 10 cm DBH) survived beyond the scorch height of the fires and high proportions of woody rainforest stems and taxa have persisted through a pulse of resprouting and seed germination, resulting in a surprising net increase of stem density and species richness in the first year after fire. While the long-term trajectory of these forests remains unclear, the high density and richness of persisting woody rainforest taxa indicate a high potential for structural and compositional recovery in the longer term.
Previous studies show rainforest structure can regenerate quickly in the initial years after fire. Tropical Amazonian rainforest can develop a dense 4 m high canopy from basal shoots within 20 months after fire (Kauffman 1991). In Queensland wet sclerophyll forests, basal shoots of resprouting rainforest trees returned to prefire height (4–8 m) between 2 and 3 years after fire and postfire rainforest seedlings reached 8–12 m within 13 years (Williams et al. 2012). Further, in Eucalypt forest ∼15 km from the littoral rainforest sites in this study, postfire resprouts and seedlings of the rainforest trees Glochidion ferdinandi and Alphitonia excelsa formed a 12 m tall, closed canopy 16 years after severe wildfire (Baker et al. 2020a). Further insights into rainforest canopy recovery can be gained from rainforest plantings for ecological restoration. Canopy closure is routinely achieved within 3–5 years (Catterall and Harrison 2006; Freebody 2007) using industry standard planting densities of 0.31 plants m−2 (1.8 m spacings) (Freebody 2007; Parkes et al. 2012) – less than half the postfire density of resprouting woody rainforest plants and remote suckers observed in this study (0.68 plants m−2). Furthermore, canopy regeneration via resprouting is faster than from seedlings due to well-established root systems and starch reserves (Kauffman 1991; Bond and Midgley 2001), allowing resprouting saplings to rapidly refill their own gaps and those left by adjacent non-resprouters (Marrinan et al. 2005). Including seedlings, total postfire stem densities observed in the present study (2.47 plants m−2) are eight times higher than standard rainforest restoration planting densities.
The high postfire seedling densities we observed (∼1.8 seedlings m−2) are consistent with previous studies. Hill and Read (1984) found seedling densities of several rainforest taxa were higher in recently burnt than in unburnt wet sclerophyll forests. Williams et al. (2012) found rainforest plant recruitment in rainforest ecotones was most abundant (∼2.3 seedlings m−2) in the first year after fire. The long-term survival rate of seedlings recorded in this study is unknown, however studies of rainforest seedling recruitment in warm temperate rainforests and wet sclerophyll forests in northern NSW (Campbell et al. 2012, 2016) suggest seedling survival rates may be lower in the more open conditions found after fire, due to postfire herbivory and increased moisture stress. In wet sclerophyll forest in far north Queensland, Williams et al. (2012) found high postfire densities of Alphitonia petriei seedlings had declined by 97% within 13 years after fire, although they still maintained a subcanopy above 50% cover that was sufficient to shade out the grassy understorey. However, dendrochronological analysis in Victorian Mountain Ash forest (Simkin and Baker 2008) revealed that a rainforest subcanopy of Nothofagus cunninghamii resulted from widespread germination in the wake of the major 1939 ‘Black Friday’ fires, further demonstrating the capacity for postfire rainforest seedlings to make substantial long-term contributions to forest structure. Irrespective of long-term seedling survival rates on our study sites, the density of resprouting individuals and suckers (0.68 plants m−2) exceeds prefire stem density (0.61 m−2) and is therefore likely to enable canopy closure within ∼5 years after fire (Kauffman 1991; Freebody 2007; Williams et al. 2012).
Fire resilience of rainforest floristic composition
The increased postfire species richness, comprising high proportions of late-successional taxa, provides a strong basis for the long-term maintenance of floristic diversity in the rainforests examined. Assessment of species turnover pathways identified very high rates of taxa persistence (∼95%) through resprouting, local seed germination and/or escaping unscorched, while postfire seedling germination also added new taxa to standing plant populations, increasing species richness by ∼28%. Additionally, species richness is likely to increase over time as recovering structural complexity facilitates subsequent establishment of additional late-successional taxa. For example, Williams et al.(2012) reported a second suite of rainforest taxa that began to recruit additional seedlings around 8 years after fire, presumably as leaf litter and canopy closure increased. Although our results show a strong overall capacity for recovery of rainforest structure and diversity generally, differential postfire abundance among taxa will likely lead to compositional shifts, at least in the short term. The high rates of postfire resprouting and seeding recruitment among late-successional taxa contradicts the definition of these taxa as being restricted to ‘undisturbed rainforest’ (Kooyman 1996).
Very few differences were found in postfire response between the rainforest classes examined. A notable exception was the unique absence of fire avoiders from littoral rainforest. One possible explanation is that, unlike other classes, littoral rainforest plots were located in fire-prone Eucalypt forest with a closed rainforest subcanopy, where the periodic fires inherent to these forests may select for a higher proportion of fire-resilient rainforest taxa. Fire avoiders occurred in all other classes, albeit in low proportions (5–13%). Fire avoiders can neither survive fire by resprouting nor recruit by seed immediately postfire, so rely on seed dispersal from unburnt populations to re-colonise postfire (Clarke et al. 2015). Among the 11 fire avoiders identified in this study, eight were still capable of resprouting at low rates (16–29%), while nine were capable of long-distance seed dispersal by birds or wind, as indicated by seed morphology (RBGT 2021). Fires in the study locations were typically patchy within sites (as indicated by high proportions of unscorched crowns) and across the broader landscape (DPIE 2020), increasing the likelihood of fire-avoiding taxa persisting in unburnt refuges in the landscape and recolonising by bird- or wind-dispersed seed over time.
We found no evidence of a shift in floristic composition towards a more-flammable sclerophyll forest (e.g. Barker 1994; Tolsma et al. 2019). Firstly, scattered individuals of sclerophyll taxa (e.g. Allocasuarina, Corymbia, Eucalyptus, Lophostemon and Xanthorrhoea) already existed on or immediately adjacent to 14 of the 17 sites prefire, with the remaining three devoid of sclerophyll taxa postfire. Secondly, postfire sclerophyll seedling densities were extremely low, at around 1% of the density of rainforest plants, including on sites where Eucalypts dominated the canopy. Finally, regeneration of flammable ground-layer plants (e.g. graminoids) was negligible in all plots, and any minor regeneration is likely to be quickly eliminated as regenerating rainforest plants achieve canopy closure (Williams et al. 2012; Hoffmann et al. 2012a; Baker et al. 2021).
Fire resilience of rainforest pioneers in long-unburnt open forest
Beyond postfire recovery of rainforest, the fire resilience of rainforest plants also affects the persistence of invading rainforest pioneers in long-unburnt open forests (e.g. dry-, wet- and swamp-sclerophyll forest) when fire eventually returns. Invading rainforest pioneers rapidly outcompete shade-intolerant understorey plant communities in long-unburnt open forest (Rose and Fairweather 1997; Woinarski et al. 2004; Lewis et al. 2012; Baker et al. 2020a), although some displaced plant taxa persist as soil-stored seed (Baker et al. 2020a), awaiting the return of fire to restore open conditions through removal of the dense rainforest midstorey (Campbell et al. 2012). However, fire-resilient rainforest plants may quickly reassert competitive pressure postfire (e.g. Williams et al. 2012), potentially limiting long-term re-establishment of typical sclerophyll understorey plant communities, fauna habitat (Laurance 1997; Tasker et al. 2017) and understorey fuels that underpin open-forest flammability (Hoffmann et al. 2012a; Baker et al. 2021). Our results demonstrate resprouting potential among a wide diversity of rainforest taxa known to colonise long-unburnt open forests adjacent to the major rainforest classes of eastern Australia.
Additionally, the high rates of postfire resprouting (∼60–80% individuals) we observed in small rainforest plants (1 cm DBH, 1 m tall) suggests invading rainforest pioneers may become resilient to removal by fire soon after recruiting into open forests. This is consistent with Williams et al. (2012) who found 37% of seedlings from four rainforest taxa resprouted basally when burnt only 2 years after germination, including seedlings of Polyscias elegans, a facultative resprouter identified in this study. We found resprouting in small plants (DBH ≤1 cm) occurred in 14 of 17 (83%) woody rainforest taxa for which we had sufficient data, indicating that rapid attainment of this trait is widespread among different taxa. However, in the context of rainforest invasion, significant biodiversity declines can be driven by only one or two fire-resilient species (Rose and Fairweather 1997; Baker et al. 2020a).
The high proportion of early-successional taxa (83%) capable of exploiting the postfire environment through seedling recruitment recorded in this study is also highly relevant to rainforest-invasion processes in open forests. While resprouting provides a mechanism for the postfire persistence of invading-rainforest pioneers, seedling recruitment provides a potential mechanism for rainforest pioneer populations to increase after fire. Many taxa with postfire seedling germination are recognised pioneers, adapted to establishing in open conditions, so seedling survival rates are expected to be relatively high. We observed Euroschinus falcatus densities to increase over 100-fold due to seedling recruitment on sites that appear to be rainforest-invaded dry sclerophyll forest, based on the canopy species and their habit. These seedlings were observed around 1 year postfire and showed no evidence of postfire herbivory or moisture stress. Although Williams et al. (2012) reported a very low (3%) survival rate for Alphitonia petriei seedlings 13 years after fire, this still resulted in around one tree every 10 m−2, most of which were > 8 m tall and situated among other rainforest trees that also recruited postfire. More research is needed to determine long-term seedling survival rates in this context.
Management implications
Historical rainforest fire intervals in the study area (∼50–1000 years) provide a baseline for understanding tolerable fire regimes, with charcoal evidence of pre-European wildfires in subtropical rainforest (including with Brush Box emergents) in Nightcap National Park every ∼300–1000 years (Turner 1984), and intervals <50 years reported for warm temperate rainforests (Floyd 1990). Despite encroachment of the 2019–2020 fires into rainforests, there are no records to our knowledge of recurrent wildfire affecting any stand of mature rainforest of the Gondwana WHA since European colonisation, and thus no evidence of a shift from multi-centennial to decadal fire frequency. Although the postfire-regeneration capacity of rainforest observed in this study is likely to confer ecosystem resilience to at least centennial-scale, and potentially longer decadal-scale fire frequencies, fire frequency and resilience under future climate change remains uncertain. While this and previous studies demonstrate the resilience of rainforest to occasional fires, it is generally understood that there is a threshold frequency which can prevent rainforest resprouts and seedlings from maturing, and ultimately restrict rainforests to landscape positions where the topography creates fire refugia (Bowman 2000; Fensham et al. 2003). These thresholds urgently require further study. Conversely, in open forests, low fire frequency can facilitate invasion and displacement by rainforest, and in high rainfall regions, ultimately restrict open forest to landscape positions where frequent landscape fires persist (Russell-Smith et al. 2004; Stanton et al. 2014). Thus, where rainforest sits within a matrix of open forest, active management of fire frequency, such as through the restoration of Aboriginal burning, is needed to maintain rainforest boundaries and ensure the long-term conservation of the values inherent to both rainforest and open-forest plant communities (Bowman 2000; Bradstock 2016). Rainforest areas need to be protected as much as possible from potential increases in fire frequency with climate change. Planned fires in open forest immediately adjacent to rainforest have been shown to mitigate the spread of unplanned fires into rainforest (King et al. 2008), and may be needed to prevent the increased fire encroachment into rainforest predicted with climate change (Bradstock 2016).
More specifically, our findings prompt review of existing fire interval guidelines for the maintenance of open-forest biodiversity near rainforest. Firstly, our findings demonstrate that rainforest pioneers which invade dry open forests can become resistant to removal by fire, posing a major threat to the postfire recovery of shade-intolerant plant communities and associated fauna habitat. Therefore, to maintain dry sclerophyll open-forest biodiversity, fires should be of sufficient frequency to prevent widespread establishment of fire-resistant rainforest plants in forests where they were historically absent or rare.
Secondly, recommended minimum fire intervals for shrubby wet sclerophyll forests are partly based on assumptions that most understorey rainforest taxa are killed by fire and that to maintain their populations, minimum fire intervals need to be long enough to allow recolonisation by seed, and for recruits to complete their primary juvenile period before replenishing seed banks (Noble and Slatyer 1980; Kenny et al. 2004). Our findings demonstrate that a high proportion of rainforest plants are not removed by fire, but persist by resprouting, and may attain this capacity at a relatively young age. Such taxa may therefore be capable of maintaining resilient populations under shorter maximum fire intervals than is typically recommended (e.g. 25 years, Kenny et al. 2004). This study has generated fire-response data for a substantial number of additional rainforest flora (∼147; NSW Flora Fire Response Database v 2.1; OEH 2014), and their integration into future plant–fire vital attribute analyses (Noble and Slatyer 1980; Kenny et al. 2004) would enable more accurate identification of tolerable fire intervals for open forests with understorey rainforest plants.
We are mindful that the use of postfire counts (fire killed + live resprout + alive escape) as a proxy measure for prefire stem density and species richness may underrepresent non-resprouting stems that were completely incinerated or disintegrated postfire. Related findings should therefore be interpreted with caution. However, we argue that any effects would be limited to very small size classes, given that the diameter of live stems consumed by flaming combustion is typically < 3 mm (Hines 2010; Cruz et al. 2013) and small fire-killed stems (< 1 cm DBH) in our plots typically remained standing and retained scorched foliage, suggesting low postfire disintegration rates. Further, we found that the percentage change in density and species richness were not significantly altered by fire severity, indicating that stem loss may not have strongly influenced the overall trends observed. In rainforests, the seedling cohort typically comprises the highest stem densities, but lowest species richness (e.g. Green et al. 2014), indicating larger potential uncertainty in our results for stem density than for species richness.
Conclusions
This study adds to the large body of field evidence from other rainforest classes across Australia, which together demonstrate the high resilience of rainforest to occasional fire (Bowman 2000). We show that high fire resilience extends to previously unstudied major rainforest classes (subtropical, dry, littoral) and late-successional rainforest taxa. Although we found no evidence to indicate long-term floristic or structural decline in rainforests exposed to a single fire, the frequency at which fires prevent maturation of rainforest resprouts and seedlings or increase community flammability is unknown. In regions where fire frequency is predicted to increase with climate change, preventing recurrent fire incursions into rainforest may require fuel management in adjacent open forests, including through the restoration of more frequent Aboriginal burning.
In relation to rainforest-invaded open forests, our findings suggest that most rainforest pioneers can become resistant to removal by occasional fire, and that postfire seedling recruitment provides a potential mechanism for population increases after occasional fires. In high rainfall regions where global change factors are predicted to favour rainforest expansion (e.g. increased rainfall, increased CO2, reduced fire activity) our findings suggest that the maintenance of dry sclerophyll open forests within a matrix of rainforest may require relatively high fire frequencies.
Supplementary material
Supplementary material is available online.
Data availability
The data that support this study were obtained from the NSW National Parks & Wildlife Service by permission. Data will be shared upon reasonable request to the corresponding author with permission from NSW National Parks & Wildlife Service.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research was supported by an Australian Government Research Training Program (RTP) Scholarship. This research did not receive any additional specific funding.
Author contributions
AB conceived and designed the research, performed the experiments and data analysis and wrote the manuscript; CC and MW supervised the project and provided critical feedback on research design, analysis and manuscript.
Acknowledgements
We thank Nan and Hugh Nicholson, Andrew Murray, Barbara Stewart and Annette McKinley for undertaking surveys in subtropical, dry and warm temperate rainforest plots, which were funded and coordinated by the NSW National Parks and Wildlife Service (NPWS). We also thank Elizabeth Tasker (Department of Planning, Industry and Environment) for assistance with sampling design.
References
Alexander HD, Arthur MA (2014) Increasing red maple leaf litter alters decomposition rates and nitrogen cycling in historically oak-dominated forests of the eastern US. Ecosystems 17, 1371–1383.| Increasing red maple leaf litter alters decomposition rates and nitrogen cycling in historically oak-dominated forests of the eastern US.Crossref | GoogleScholarGoogle Scholar |
Baker AG, Catterall C, Benkendorff K, Fensham RJ (2020a) Rainforest expansion reduces understorey plant diversity and density in open forest of eastern Australia: understorey response to rainforest expansion. Austral Ecology 45, 557–571.
| Rainforest expansion reduces understorey plant diversity and density in open forest of eastern Australia: understorey response to rainforest expansion.Crossref | GoogleScholarGoogle Scholar |
Baker AG, Catterall C, Benkendorff K, Law B (2020b) No room to move: bat response to rainforest expansion into long-unburnt eucalypt forest. Pacific Conservation Biology 27, 13–26.
| No room to move: bat response to rainforest expansion into long-unburnt eucalypt forest.Crossref | GoogleScholarGoogle Scholar |
Baker AG, Catterall C, Benkendorff K (2021) Invading rain forest pioneers initiate positive fire suppression feedbacks that reinforce shifts from open to closed forest in eastern Australia. Journal of Vegetation Science 32, e13102
| Invading rain forest pioneers initiate positive fire suppression feedbacks that reinforce shifts from open to closed forest in eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Barker MJ (1994) The effects of fire on Tasmania’s west coast lowland rainforest. MSc Thesis, University of Tasmania, Hobart, Tas., Australia.
Bond WJ (2019) ‘Open ecosystems: ecology and evolution beyond the forest edge’, (Oxford University Press: Oxford, UK)
Bond WJ, Midgley JJ (2001) Ecology of sprouting in woody plants: the persistence niche. Trends in Ecology & Evolution 16, 45–51.
| Ecology of sprouting in woody plants: the persistence niche.Crossref | GoogleScholarGoogle Scholar |
Bond W, Van Wilgen B (1996) ‘Fire and plants.’ Population and community biology series 14. (Springer: Netherlands)
Bowman DMJS (1991) Recovery of some northern Australian monsoon forest tree species following fire. Proceedings of the Royal Society of Queensland 101, 21–25.
Bowman DMJS (2000) ‘Australian rainforests: islands of green in a land of fire’, (Cambridge University Press: Cambridge, UK)
Bowman DMJS, Murphy BP, Banfai DS (2010) Has global environmental change caused monsoon rainforests to expand in the Australian monsoon tropics? Landscape Ecology 25, 1247–1260.
| Has global environmental change caused monsoon rainforests to expand in the Australian monsoon tropics?Crossref | GoogleScholarGoogle Scholar |
Bradstock RA (2010) A biogeographic model of fire regimes in Australia: current and future implications: a biogeographic model of fire in Australia. Global Ecology and Biogeography 19, 145–158.
| A biogeographic model of fire regimes in Australia: current and future implications: a biogeographic model of fire in Australia.Crossref | GoogleScholarGoogle Scholar |
Bradstock RA (2016) Report on issues relevant to prescribed burning in the Nightcap National Park. Centre for Environmental Risk Management of Bushfires, University of Wollongong, Wollongong, NSW, Australia.
Campbell ML, Clarke PJ (2006) Response of montane wet sclerophyll forest understorey species to fire: evidence from high and low intensity fires. Proceedings of the Linnean Society of New South Wales 127, 63 https://search.informit.com.au/documentSummary;dn=044135229933707;res=E-LIBRARY
Campbell ML, Clarke PJ, Keith DA (2012) Seed traits and seed bank longevity of wet sclerophyll forest shrubs. Australian Journal of Botany 60, 96
| Seed traits and seed bank longevity of wet sclerophyll forest shrubs.Crossref | GoogleScholarGoogle Scholar |
Campbell ML, Keith DA, Clarke PJ (2016) Regulation of seedling recruitment and survival in diverse ecotonal temperate forest understories. Plant Ecology 217, 801–816.
| Regulation of seedling recruitment and survival in diverse ecotonal temperate forest understories.Crossref | GoogleScholarGoogle Scholar |
Canadell JG, Meyer CP, Cook GD, Dowdy A, Briggs PR, Knauer J, Pepler A, Haverd V (2021) Multi-decadal increase of forest burned area in Australia is linked to climate change. Nature Communications 12, 6921
| Multi-decadal increase of forest burned area in Australia is linked to climate change.Crossref | GoogleScholarGoogle Scholar | 34836974PubMed |
Catterall C, Harrison DA (2006) ‘Rainforest restoration activities in Australia’s tropics and subtropics’, (Rainforest Cooperative Research Centre: Cairns, Qld)
Chapman A, Harrington GN (1997) Responses by birds to fire regime and vegetation at the wet sclerophyll/tropical rainforest boundary. Pacific Conservation Biology 3, 213–220.
| Responses by birds to fire regime and vegetation at the wet sclerophyll/tropical rainforest boundary.Crossref | GoogleScholarGoogle Scholar |
Clarke H, Evans JP (2019) Exploring the future change space for fire weather in southeast Australia. Theoretical and Applied Climatology 136, 513–527.
| Exploring the future change space for fire weather in southeast Australia.Crossref | GoogleScholarGoogle Scholar |
Clarke HG, Smith PL, Pitman AJ (2011) Regional signatures of future fire weather over eastern Australia from global climate models. International Journal of Wildland Fire 20, 550–562.
| Regional signatures of future fire weather over eastern Australia from global climate models.Crossref | GoogleScholarGoogle Scholar |
Clarke PJ, Lawes MJ, Midgley JJ, Lamont BB, Ojeda F, Burrows GE, Enright NJ, Knox KJE (2013) Resprouting as a key functional trait: how buds, protection and resources drive persistence after fire. New Phytologist 197, 19–35.
| Resprouting as a key functional trait: how buds, protection and resources drive persistence after fire.Crossref | GoogleScholarGoogle Scholar |
Clarke PJ, Knox KJE, Bradstock RA, Munoz-Robles C, Kumar L (2014) Vegetation, terrain and fire history shape the impact of extreme weather on fire severity and ecosystem response. Journal of Vegetation Science 25, 1033–1044.
| Vegetation, terrain and fire history shape the impact of extreme weather on fire severity and ecosystem response.Crossref | GoogleScholarGoogle Scholar |
Clarke PJ, Lawes MJ, Murphy BP, Russell-Smith J, Nano CEM, Bradstock R, Enright NJ, Fontaine JB, Gosper CR, Radford I, Midgley JJ, Gunton RM (2015) A synthesis of postfire recovery traits of woody plants in Australian ecosystems. Science of The Total Environment 534, 31–42.
| A synthesis of postfire recovery traits of woody plants in Australian ecosystems.Crossref | GoogleScholarGoogle Scholar |
Collins L, Bennett AF, Leonard SWJ, Penman TD (2019) Wildfire refugia in forests: severe fire weather and drought mute the influence of topography and fuel age. Global Change Biology 25, 3829–3843.
| Wildfire refugia in forests: severe fire weather and drought mute the influence of topography and fuel age.Crossref | GoogleScholarGoogle Scholar | 31215102PubMed |
Cruz MG, McCaw WL, Anderson WR, Gould JS (2013) Fire behaviour modelling in semi-arid mallee-heath shrublands of southern Australia. Environmental Modelling & Software 40, 21–34.
| Fire behaviour modelling in semi-arid mallee-heath shrublands of southern Australia.Crossref | GoogleScholarGoogle Scholar |
DPIE (2020) ‘Google Earth Engine Burnt Area Map (GEEBAM)’, (NSW Department of Planning Infrastructure and Environment: Sydney) Available at https://datasets.seed.nsw.gov.au/dataset/google-earth-engine-burnt-area-map-geebam.
Fensham RJ, Fairfax RJ, Butler DW, Bowman DMJS (2003) Effects of fire and drought in a tropical eucalypt savanna colonized by rain forest. Journal of Biogeography 30, 1405–1414.
| Effects of fire and drought in a tropical eucalypt savanna colonized by rain forest.Crossref | GoogleScholarGoogle Scholar |
Fletcher M-S, Hall T, Alexandra AN (2021) The loss of an indigenous constructed landscape following British invasion of Australia: an insight into the deep human imprint on the Australian landscape. Ambio 50, 138–149.
| The loss of an indigenous constructed landscape following British invasion of Australia: an insight into the deep human imprint on the Australian landscape.Crossref | GoogleScholarGoogle Scholar | 32378038PubMed |
Floyd AG (1990) ‘Australian rainforests in New South Wales’, (Surrey Beatty and Sons: Sydney, NSW, Australia)
Freebody K (2007) Rainforest revegetation in the uplands of the Australian Wet Tropics: the Eacham Shire experience with planting models, outcomes and monitoring issues. Ecological Management & Restoration 8, 140–143.
| Rainforest revegetation in the uplands of the Australian Wet Tropics: the Eacham Shire experience with planting models, outcomes and monitoring issues.Crossref | GoogleScholarGoogle Scholar |
Gilbert JM (1959) Forest succession in the Florentine Valley, Tasmania. In ‘Papers and Proceedings of the Royal Society of Tasmania’. vol. 93, pp. 129–152.
Gill AM, Bradstock RA (1992) A national register for the fire responses of plant species. Cunninghamia 2, 653–660. https://publications.csiro.au/rpr/pub?list=BRO&pid=procite:0d693cc0-5963-4818-a4ce-651aa6dbebc1
Green PT, Harms KE, Connell JH (2014) Nonrandom, diversifying processes are disproportionately strong in the smallest size classes of a tropical forest. Proceedings of the National Academy of Sciences of the United States of America 111, 18649–18654.
| Nonrandom, diversifying processes are disproportionately strong in the smallest size classes of a tropical forest.Crossref | GoogleScholarGoogle Scholar | 25512498PubMed |
Hill RS, Read J (1984) Post-fire regeneration of rainforest and mixed forest in Western Tasmania. Australian Journal of Botany 32, 481
| Post-fire regeneration of rainforest and mixed forest in Western Tasmania.Crossref | GoogleScholarGoogle Scholar |
Hines F, Fire and Adaptive Management Branch Victoria, Department of Sustainability and Environment Victoria (2010) ‘Overall fuel hazard assessment guide.’ (Fire Management Branch, Dept of Natural Resources and Environment: East Melbourne, Vic, Australia)
Hoffmann WA (1998) Post-burn reproduction of woody plants in a neotropical savanna: the relative importance of sexual and vegetative reproduction. Journal of Applied Ecology 35, 422–433.
| Post-burn reproduction of woody plants in a neotropical savanna: the relative importance of sexual and vegetative reproduction.Crossref | GoogleScholarGoogle Scholar |
Hoffmann WA, Geiger EL, Gotsch SG, Rossatto DR, Silva LCR, Lau OL, Haridasan M, Franco AC (2012a) Ecological thresholds at the savanna-forest boundary: how plant traits, resources and fire govern the distribution of tropical biomes. Ecology Letters 15, 759–768.
| Ecological thresholds at the savanna-forest boundary: how plant traits, resources and fire govern the distribution of tropical biomes.Crossref | GoogleScholarGoogle Scholar | 22554474PubMed |
Hoffmann WA, Jaconis SY, Mckinley KL, Geiger EL, Gotsch SG, Franco AC (2012b) Fuels or microclimate? Understanding the drivers of fire feedbacks at savanna-forest boundaries. Austral Ecology 37, 634–643.
| Fuels or microclimate? Understanding the drivers of fire feedbacks at savanna-forest boundaries.Crossref | GoogleScholarGoogle Scholar |
Jaureguiberry P, Cuchietti A, Gorné LD, Bertone GA, Díaz S (2020) Post-fire resprouting capacity of seasonally dry forest species – two quantitative indices. Forest Ecology and Management 473, 118267
| Post-fire resprouting capacity of seasonally dry forest species – two quantitative indices.Crossref | GoogleScholarGoogle Scholar |
Kauffman JB (1991) Survival by sprouting following fire in tropical forests of the eastern Amazon. Biotropica 23, 219–224.
| Survival by sprouting following fire in tropical forests of the eastern Amazon.Crossref | GoogleScholarGoogle Scholar |
Keenan RJ (2015) Climate change impacts and adaptation in forest management: a review. Annals of Forest Science 72, 145–167.
| Climate change impacts and adaptation in forest management: a review.Crossref | GoogleScholarGoogle Scholar |
Keith DA (2004) ‘Ocean shores to desert dunes: the native vegetation of New South Wales and the ACT,’ (Department of Environment and Conservation NSW: Hurstville, NSW, Australia)
Kenny B, Sutherland E, Tasker E, Bradstock R (2004) ‘Guidelines for ecologically sustainable fire management.’ (NSW National Parks and Wildlife Service: Hurstville, NSW, Australia)
King KJ, Bradstock RA, Cary GJ, Chapman J, Marsden-Smedley JB (2008) The relative importance of fine-scale fuel mosaics on reducing fire risk in south-west Tasmania, Australia. International Journal of Wildland Fire 17, 421–430.
| The relative importance of fine-scale fuel mosaics on reducing fire risk in south-west Tasmania, Australia.Crossref | GoogleScholarGoogle Scholar |
Kooyman RM (1996) ‘Growing rainforest: rainforest restoration and regeneration: recommendations for the humid sub-tropical region of northern New South Wales and south east Queensland.’ (Greening Australia: Brisbane, Qld, Australia)
Laurance WF (1997) A distributional survey and habitat model for the endangered northern bettong Bettongia tropica in tropical Queensland. Biological Conservation 82, 47–60.
| A distributional survey and habitat model for the endangered northern bettong Bettongia tropica in tropical Queensland.Crossref | GoogleScholarGoogle Scholar |
Lewis T, Reif M, Prendergast E, Tran C (2012) The effect of long-term repeated burning and fire exclusion on above- and below-ground Blackbutt (Eucalyptus pilularis) forest vegetation assemblages. Austral Ecology 37, 767–778.
| The effect of long-term repeated burning and fire exclusion on above- and below-ground Blackbutt (Eucalyptus pilularis) forest vegetation assemblages.Crossref | GoogleScholarGoogle Scholar |
Lim WH, Roderick ML (2009) ‘An atlas of the global water cycle.’ (ANU Press).
| Crossref |
Little JK, Prior LD, Williamson GJ, Williams SE, Bowman DMJS (2012) Fire weather risk differs across rain forest-savanna boundaries in the humid tropics of north-eastern Australia. Austral Ecology 37, 915–925.
| Fire weather risk differs across rain forest-savanna boundaries in the humid tropics of north-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Lynch AJJ, Neldner VJ (2000) Problems of placing boundaries on ecological continua – options for a workable national rainforest definition in Australia. Australian Journal of Botany 48, 511
| Problems of placing boundaries on ecological continua – options for a workable national rainforest definition in Australia.Crossref | GoogleScholarGoogle Scholar |
Marrinan MJ, Edwards W, Landsberg J (2005) Resprouting of saplings following a tropical rainforest fire in north-east Queensland, Australia. Austral Ecology 30, 817–826.
| Resprouting of saplings following a tropical rainforest fire in north-east Queensland, Australia.Crossref | GoogleScholarGoogle Scholar |
Nicholson N, Nicholson H, Murray A, McKinley A, Stewart B, Wiseman M (2020) Response of rainforest plants to the 2019–2020 bushfires: a case study from the Gondwanan rainforests of Richmond River Area. Unpublished report to the NSW National Parks and Wildlife Service. Landmark Ecological Services.
Noble IR, Slatyer RO (1980) The use of vital attributes to predict successional changes in plant communities subject to recurrent disturbances. Vegetatio 43, 5–21.
| The use of vital attributes to predict successional changes in plant communities subject to recurrent disturbances.Crossref | GoogleScholarGoogle Scholar |
Nolan RH, Boer MM, Collins L, de Dios VR, Clarke H, Jenkins M, Kenny B, Bradstock RA (2020) Causes and consequences of eastern Australia’s 2019–20 season of mega-fires. Global Change Biology 26, 1039–1041.
| Causes and consequences of eastern Australia’s 2019–20 season of mega-fires.Crossref | GoogleScholarGoogle Scholar | 31916352PubMed |
OEH (2014) ‘NSW flora fire response database (version 2.1)’, (NSW Office of Environment & Heritage: Hurstville, NSW, Australia)
Ondei S, Prior LD, Williamson GJ, Vigilante T, Bowman DMJS (2017) Water, land, fire, and forest: multi-scale determinants of rainforests in the Australian monsoon tropics. Ecology and Evolution 7, 1592–1604.
| Water, land, fire, and forest: multi-scale determinants of rainforests in the Australian monsoon tropics.Crossref | GoogleScholarGoogle Scholar | 28261468PubMed |
Parkes T, Delaney M, Dunphy M, et al. (2012) Big scrub: a cleared landscape in transition back to forest? Ecological Management & Restoration 13, 212–223.
| Big scrub: a cleared landscape in transition back to forest?Crossref | GoogleScholarGoogle Scholar |
Pausas JG, Bradstock RA (2007) Fire persistence traits of plants along a productivity and disturbance gradient in mediterranean shrublands of south-east Australia. Global Ecology and Biogeography 16, 330–340.
| Fire persistence traits of plants along a productivity and disturbance gradient in mediterranean shrublands of south-east Australia.Crossref | GoogleScholarGoogle Scholar |
Pausas JG, Bradstock RA, Keith DA, Keeley JE (2004) Plant functional traits in relation to fire in crown-fire ecosystems. Ecology 85, 1085–1100.
| Plant functional traits in relation to fire in crown-fire ecosystems.Crossref | GoogleScholarGoogle Scholar |
Pohlert T (2018) PMCMR: calculate pairwise multiple comparisons of mean rank sums. Available at https://cran.r-project.org/web/packages/PMCMR/. [Accessed 28 March 2019]
RBGT (2021) PlantNET (The NSW Plant Information Network System). Royal Botanical Gardens Trust, Sydney. Available at http://plantnet.rbgsyd.nsw.gov.au.
Rose S, Fairweather PG (1997) Changes in floristic composition of urban bushland invaded by Pittosporum undulatum in Northern Sydney, Australia. Australian Journal of Botany 45, 123
| Changes in floristic composition of urban bushland invaded by Pittosporum undulatum in Northern Sydney, Australia.Crossref | GoogleScholarGoogle Scholar |
Russell-Smith J, Stanton PJ, Whitehead PJ, Edwards A (2004) Rain forest invasion of eucalypt-dominated woodland savanna, Iron Range, north-eastern Australia: I. Successional processes. Journal of Biogeography 31, 1293–1303.
| Rain forest invasion of eucalypt-dominated woodland savanna, Iron Range, north-eastern Australia: I. Successional processes.Crossref | GoogleScholarGoogle Scholar |
Simkin R, Baker PJ (2008) Disturbance history and stand dynamics in tall open forest and riparian rainforest in the Central Highlands of Victoria. Austral Ecology 33, 747–760.
| Disturbance history and stand dynamics in tall open forest and riparian rainforest in the Central Highlands of Victoria.Crossref | GoogleScholarGoogle Scholar |
Stanton P, Stanton D, Stott M, Parsons M (2014) Fire exclusion and the changing landscape of Queensland’s Wet Tropics Bioregion 1. The extent and pattern of transition. Australian Forestry 77, 51–57.
| Fire exclusion and the changing landscape of Queensland’s Wet Tropics Bioregion 1. The extent and pattern of transition.Crossref | GoogleScholarGoogle Scholar |
Tasker E, Rennison B, Watson P, Baker L (2017) Vegetation change associated with reduced fire frequency in Border Ranges: loss of grassy forests and associated endangered fauna. In ‘2017 Bushfire Conference’, Sydney. (Nature Conservation Council of NSW: Sydney)
Tolsma A, Hale R, Sutter G, Kohout M (2019) Post-fire dynamics of Cool Temperate Rainforest in the O’Shannassy Catchment. Arthur Rylah Institute for Environmental Research Technical Report Series No. 298. Department of Environment, Heidelberg, Vic, Australia.
Turner J (1984) Radiocarbon dating of wood and charcoal in an Australian forest ecosystem. Australian Forestry 47, 79–83.
| Radiocarbon dating of wood and charcoal in an Australian forest ecosystem.Crossref | GoogleScholarGoogle Scholar |
Unwin GL (1983) Dynamics of the rainforest: eucalpyt forest boundary in the Herberton Highland North Queenland. MSc Thesis, James Cook University of North Queensland, Cairns, Qld., Australia.
Vesk PA (2006) Plant size and resprouting ability: trading tolerance and avoidance of damage? Journal of Ecology 94, 1027–1034.
| Plant size and resprouting ability: trading tolerance and avoidance of damage?Crossref | GoogleScholarGoogle Scholar |
Wigley BJ, Bond WJ, Hoffman MT (2010) Thicket expansion in a South African savanna under divergent land use: local vs. global drivers? Global Change Biology 16, 964–976.
| Thicket expansion in a South African savanna under divergent land use: local vs. global drivers?Crossref | GoogleScholarGoogle Scholar |
Willett KM, Gillett NP, Jones PD, Thorne PW (2007) Attribution of observed surface humidity changes to human influence. Nature 449, 710–712.
| Attribution of observed surface humidity changes to human influence.Crossref | GoogleScholarGoogle Scholar | 17928858PubMed |
Williams PR (2000) Fire-stimulated rainforest seedling recruitment and vegetative regeneration in a densely grassed wet sclerophyll forest of north-eastern Australia. Australian Journal of Botany 48, 651
| Fire-stimulated rainforest seedling recruitment and vegetative regeneration in a densely grassed wet sclerophyll forest of north-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Williams PR, Parsons M, Jensen R, Tran C (2012) Mechanisms of rainforest persistence and recruitment in frequently burnt wet tropical eucalypt forests. Austral Ecology 37, 268–275.
| Mechanisms of rainforest persistence and recruitment in frequently burnt wet tropical eucalypt forests.Crossref | GoogleScholarGoogle Scholar |
Woinarski JCZ, Risler J, Kean L (2004) Response of vegetation and vertebrate fauna to 23 years of fire exclusion in a tropical Eucalyptus open forest, Northern Territory, Australia. Austral Ecology 29, 156–176.
| Response of vegetation and vertebrate fauna to 23 years of fire exclusion in a tropical Eucalyptus open forest, Northern Territory, Australia.Crossref | GoogleScholarGoogle Scholar |
Wood SW, Murphy BP, Bowman DMJS (2011) Firescape ecology: how topography determines the contrasting distribution of fire and rain forest in the south-west of the Tasmanian Wilderness World Heritage Area. Journal of Biogeography 38, 1807–1820.
| Firescape ecology: how topography determines the contrasting distribution of fire and rain forest in the south-west of the Tasmanian Wilderness World Heritage Area.Crossref | GoogleScholarGoogle Scholar |


