Stem functional traits vary among co-occurring tree species and forest vulnerability to drought
George Matusick A * , Katinka X. Ruthrof A B and Giles E. S. J. Hardy A
A * , Katinka X. Ruthrof A B and Giles E. S. J. Hardy A
A Environmental and Conservation Sciences, Murdoch University, 90 South Street, Murdoch, WA 6150, Australia.
B Biodiversity and Conservation Science, Department of Biodiversity, Conservation and Attractions, Kensington, WA 6151, Australia.
Australian Journal of Botany 70(3) 204-214 https://doi.org/10.1071/BT21077
Submitted: 15 June 2021 Accepted: 10 March 2022 Published: 6 April 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Stem functional traits are critical for tree hydraulic infrastructure and have important consequences for forest function, particularly concerning vulnerability to drought.
Methods: Three stem traits, sapwood area, heartwood area, and bark area, were measured in two co-dominant forest species, Eucalyptus marginata Donn. Ex. Sm. and Corymbia calophylla (Lindl.) K.D.Hill & L.A.S.Johnson, in forest patches with low and high vulnerabilities to drought in south-western Australia. Patches of high drought vulnerability experienced die-off during a heatwave and drought in 2011, while patches of low vulnerability were largely not affected.
Key results: Sapwood area was significantly higher in C. calophylla than in E. marginata, and C. calophylla maintained more sapwood per unit DBH than did E. marginata, especially in larger trees. There was a 29% smaller sapwood area in high drought-vulnerability patches than in low drought-vulnerability patches (including both species). The relationship between sapwood area and DBH varied by tree size. Small trees had a greater sapwood area in high drought-vulnerable patches, whereas larger trees had more sapwood in low drought-vulnerable patches. It is unclear whether sapwood area relationships reflect differences in leaf area or tree age.
Conclusions: Observed differences in sapwood between species may help explain their differential tolerance to drought, whereas differences between drought-vulnerability sites may suggest adaptation in the studied species.
Implications: Understanding the traits associated with drought vulnerability will increase our prediction of forest response to drying and warming. Strong relationships between stem traits and DBH, developed here, may help future efforts to model water-use in the Northern Jarrah Forest.
Keywords: bark, die-off, eucalyptus, heartwood, heatwave, jarrah, marri, sapwood.
Introduction
Historical climate is a key factor in driving the evolution of functional traits in trees, and climate change in some regions is expected to progress at rates that exceed the ability of some species to adapt (Hoffmann and Sgrò 2011). Regions projected to experience the combination of increasing temperatures and decreasing precipitation may be especially at risk for changes in forest species composition (Allen et al. 2010), since excessive temperatures compound the effects of drought and shorten the time to tree die-off and mortality (Adams et al. 2009; Williams et al. 2013). A growing body of research suggests recent increases in temperature and changing precipitation patterns are pushing species past critical hydraulic tipping points, leading to widespread tree die-off and mortality (Allen et al. 2010, 2015; Mitchell et al. 2014; Goulden and Bales 2019). Given these changes, understanding which functional traits are important for survival and the plasticity of those traits is critical for determining which species and ecosystems are more vulnerable to projected climatic changes (Lopez-Iglesias et al. 2014; Skelton et al. 2015).
Climate-induced forest die-off events commonly cause structural state shifts in ecosystems (Fensham and Holman 1999; Matusick et al. 2016) and may also result in species compositional shifts when co-occurring species show differential effects and tolerances (Mueller et al. 2005; Chen et al. 2011; Fauset et al. 2012). Varying responses to extreme climatic events are explained by different physiological tolerances (Breshears et al. 2009), functional traits (Condit et al. 1996), and ecological strategies (Zeppel et al. 2015). For example, in the semi-arid Pinyon pine–juniper ecosystem of south-western United States, repeated drought events have preferentially killed the less drought-tolerant Pinus edulis, shifting composition towards juniper (Juniperus spp.) dominance in certain circumstances (Mueller et al. 2005). These differences in drought-tolerance are partially explained by functional traits, including higher resistance to xylem cavitation, lower tracheid diameter, and xylem conductance in Juniperus than in P. edulis, highlighting the trade-off between resistance to drought and water use (Linton et al. 1998).
Stem sapwood is a critical component of the hydraulic infrastructure in trees and can represent an important constriction in the soil–plant–atmosphere hydraulic pathway (Tyree and Ewers 1991; Ryan and Yoder 1997). The production and maintenance of sapwood are critical for facilitating water movement, and can be limited by drought (Scholz et al. 2011). For example, less sapwood was produced when Pinus laricio Poiret was treated with reduced precipitation, and this was attributed to reduced water transport capacity resulting in significantly less stand transpiration (Cinnirella et al. 2002). Additionally, the proportion of sapwood and heartwood can be a critical component of stem water storage, which buffers trees during daily and seasonal dry periods (Scholz et al. 2011), especially in water-limiting environments (Hu et al. 2018). For example, Pfautsch and Adams (2013) identified the importance of stem water storage in resisting the effects of extreme drought and heat events in Eucalyptus. A recent study has found that drought resistance in Eucalyptus is enhanced after being exposed to repeated drought, in part, by altering stem morphology (Pritzkow et al. 2021). These studies have collectively illustrated the importance of stem functional traits and trait plasticity in drought resistance. At larger scales, understanding stem sapwood × tree diameter relationships is critical for determining site-stand-level transpiration dynamics, which is necessary for deciphering forest-level adaptation to climate change. In water-limited ecosystems, such as those in semi-arid and Mediterranean climate regions, where seasonal drought naturally limits tree growth, size and survival, adjustment to sapwood and heartwood areas may be an important strategy for regulating water use and preventing drought-induced tree die-off and mortality (Martínez-Vilalta et al. 2009; Markesteijn et al. 2011). In these dry regions, where climate change is extending seasonal drought patterns and contributing to extreme drought and heat events (Hoerling et al. 2012), it is important to determine species adaptation potential and which strategies are being utilised among co-occurring species to estimate ecosystem compositional changes.
Bark thickness is another stem functional trait that could explain current and future tree species distributions with climate change. Bark is the organ used by trees to resist the effects of fires (Nolan et al. 2020) and bark attributes help explain species distributions in fire-prone regions (Lawes et al. 2021). Climate change is either currently altering or expected to alter the frequency and intensity of fire in many regions (Bowman et al. 2020). Therefore, understanding differences in bark attributes among co-occurring species is critical for predicting future species compositions under altered climate and fire regimes.
In the Mediterranean climate region of south-western Australia, a severe heatwave-compounded drought event corresponded with tree die-off and mortality in the Northern Jarrah Forest (NJF) ecosystem in 2011 (Matusick et al. 2013). Although there was little evidence of consistent compositional shifts 49 months following the event (Matusick et al. 2016), the two dominant overstorey species exhibited different responses (Matusick et al. 2013; Ruthrof et al. 2015). For example, the dominant tree species Eucalyptus marginata Donn ex Sm. was more likely to experience rapid crown die-off in response to the event than was the co-dominant species Corymbia calophylla (Lindl.) K.D.Hill & L.A.S.Johnson, whereas both species exhibited significant resprouting responses in stems experiencing die-off (Matusick et al. 2013). In effect, C. calophylla exhibited higher resistance to a drought and heatwave, whereas the pronounced resprouting response in E. marginata illustrated its resilience to extreme hot and dry conditions (Matusick et al. 2013; Ruthrof et al. 2015). The shift observed from single large old stems to multiple small young stems in E. marginata has implications for forest sapwood, heartwood area, and water use (Macfarlane et al. 2010). For example, areas of regrowth E. marginata used double the amount of water compared with old-growth forest. This was attributed to differences in the relative amounts of sapwood and heartwood area (and not sapwood velocity or density; Macfarlane et al. 2010). The shift from large stems to small stems also has implications on the effects of future fires, because small stems tend to have thinner bark and are less resistance to fire as a result (Nolan et al. 2020).
To determine whether three key stem functional traits are associated with the contrasting observed responses between E. marginata and C. calophylla, bark area, sapwood area, and heartwood area were assessed in forest patches that had experienced severe forest canopy die-off (defined as >70% canopy die-off) and minimal forest canopy die-off (exhibiting <10% canopy die-off) during drought in 2011 (Matusick et al. 2013). From here on, we differentiate these forest patches on their vulnerability to drought, and categorise them as having either high (experiencing severe canopy die-off) or low (experiencing minimal canopy die-off) drought vulnerability. Our objective was to examine whether these three functional traits varied among the two co-occurring species and two levels of drought vulnerability (high and low).
Materials and methods
Study area description
The NJF represents the northern portion of the Jarrah Forest ecosystem, spanning from latitude −30.761570, longitude 115.98676 to latitude −33.504507, longitude 117.082340, and is classified as open forest in the north and tall, more closed forest in the south (Specht et al. 1974). The upland forest overstorey is dominated by E. marginata (jarrah) and C. calophylla (marri), with E. wandoo (wandoo, Blakely) occurring predominately in the eastern region. The midstorey is composed of a mixture of small trees and the understorey is highly diverse (Myers et al. 2000; Gioia and Hopper 2017).
The climate of the NJF is considered a Mediterranean-type, characterised by cool, wet winters, and an extended summer drought period, lasting from 6 to 8 months. Historically, annual precipitation has ranged from 635 to ∼1300 mm (Gentilli 1989). A strong precipitation gradient occurs from the north-east (drier) to the south-west (wetter) across the NJF. A well-documented shift in precipitation has occurred in the south-west of Western Australia during the past 40+ years (Cai et al. 2011; Matusick et al. 2013). The mean maximum temperature in the town of Dwellingup, Western Australia (within the NJF), is 21.9°C, and high temperatures and the frequency of extreme heat events have been increasing in the study region (Matusick et al. 2016; Breshears et al. 2021).
In much of the NJF, an extensive lateritic duricrust overlies a predominately granitic basement. The laterites weather to yield gravel and sand, whereas the weathered granite below the duricrust is represented by a deep clay (Churchward and Dimmock 1989). Over time, the deep-rooted overstorey trees have tunnelled through the duricrust, forming root channels, allowing access to water stored in the underlying clay (Churchward and Dimmock 1989; Dell and Havel 1989). This globally unique hydrogeology, with a deeply weathered lateritic profile and large soil water storage capacity, supports tall trees, which are reliant on stored water during the annual summer drought as well as intermittently dry winters (Dell and Havel 1989; Schofield et al. 1989; Farrington et al. 1996).
Forest die-off
Corresponding with the ninth heatwave of the 2010/2011 summer period, significant changes were observed in a broad range of terrestrial and marine flora and fauna in south-western Australia (Ruthrof et al. 2018, 2021). This included patches of the NJF, which began experiencing die-off in February 2011 (Matusick et al. 2013). Forest patches with shallow soils, and limited access to stored water were most vulnerable to die-off, including those on upper slope positions surrounding granite rock outcrops (Brouwers et al. 2013). Patches experiencing die-off were larger and more densely clustered in xeric areas (Andrew et al. 2016). Structural characteristics, combined with the lack of the drought-susceptible midstorey species, B. grandis (see also Steel et al. 2019), strongly suggest that these patches have experienced previous periods of die-off and may be conditioned to living through large oscillations in moisture availability.
Each study site contained patches of high and low drought-vulnerable forest. A subset of eight study sites was selected randomly from a larger sample of sites (20) for sapwood measurements (see Matusick et al. 2013 for how study sites were identified). Briefly, forest patches with high vulnerability to drought were delineated using a differential GPS (Pathfinder Pro XRS receiver, Trimble Navigation Ltd, Sunnyvale, CA, USA). These drought-vulnerable patches were defined as having >70% of the forest canopy showing severe effects, including yellow, orange, and red leaves. A band of forest outside the high drought patches was considered to have a low vulnerability to drought (Matusick et al. 2013).
Stem trait sampling
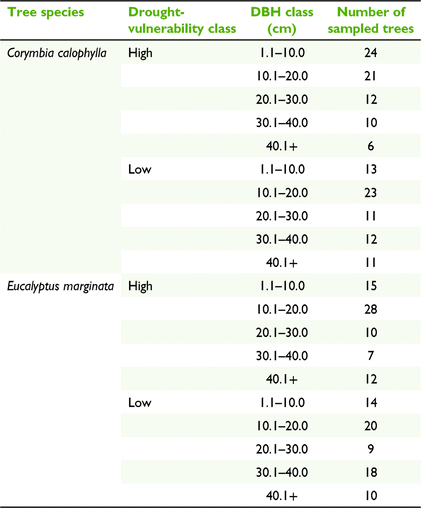
Twenty-six months following the die-off event, between 3 and 25 (average: 10) E. marginata and C. calophylla trees ranging in size between 1.3 and 92 cm diameter at breast height (DBH, 1.3 m height) were sampled within the high and low drought-vulnerable patches (total n = 143 for each species, Table 1). All samples were taken within a 21-day period. For each tree, the DBH was measured and stems were cut transversely at 1.3 m height and a slice of stem cross-section was collected for measurements. The width of the bark, sapwood and heartwood was taken on each collected cross-section at three random locations on the sample by using a digital calliper (mm). Sapwood was discriminated from heartwood primarily by using the distinct natural colouration differences. For samples difficult to discriminate, 1% methyl orange was used to enhance the differences between tissue types, as described in Macfarlane et al. (2010). The total amount of cross-sectional bark, sapwood, and heartwood area was calculated for each sample tree from these measurements. The ratio of sapwood to heartwood area was also calculated for each tree.
|
Statistical analysis
To determine whether bark, sapwood and heartwood areas, as well as the ratio of sapwood to heartwood area, vary by tree species and drought vulnerability (high and low) independently or interactively, variables were used in a linear mixed effects model in the MIXED procedure in SAS (SAS ver. 9.3, Cary, NC, USA). Bark, sapwood and heartwood area were log-transformed, whereas the ratio of sapwood to heartwood area was square-root-transformed to correct for normality and variance. Tree species, drought vulnerability, and the interaction between tree species and drought vulnerability were included as fixed effects, and site was included as the random factor in the mixed effects model. Diameter at breast height (DBH, log-transformed) was included as the covariate in each model. Tukey’s multiple-comparison tests were used to discriminate differences within and across factors.
The relationship between DBH and the measured stem functional traits were also of interest because of differences in forest structure mentioned previously. Simple linear regression equations were fitted for each drought vulnerability, tree species, and stem trait separately by using PROC REG, including log-transformed DBH and log-transformed stem traits to determine the strength of the relationship between DBH and stem traits.
Results
Bark area
Bark area varied between species, with E. marginata having slightly more bark than C. calophylla (12 303 mm2 vs 12 230 mm2); however, the difference was not statistically significant (F = 3.46, P = 0.0640). Drought vulnerability had no statistical effect on bark area (F = 1.12, P = 0.2912), nor was there a statistically significant interaction (F = 0.02, P = 0.8780). A very significant linear relationship between bark area and DBH was observed for both species (Fig. 1). Bark area was greater in smaller E. marginata trees and in larger C. calophylla trees.
Sapwood area
Sapwood area varied by tree species (F = 22.88, P < 0.0001) and drought vulnerability (F = 7.41, P = 0.0069) independently. Corymbia calophylla had a larger sapwood area than did E. marginata (Fig. 2a). When controlling for DBH, trees located in low drought-vulnerability patches had a significantly greater sapwood area than did those in high drought-vulnerability patches (Fig. 2b). From the regression analysis, the relationship between sapwood area and DBH was strong for both species and across drought vulnerabilities. Corymbia calophylla had more sapwood than did E. marginata in smaller trees, but less in larger trees (Fig. 3a). Similar results were found between high and low drought-vulnerability patches. Smaller trees had more sapwood area in high-drought patches and larger trees had more sapwood area in low-drought patches (Fig. 3b).
Heartwood area
There was a significant statistical interaction between tree species and drought vulnerability (F = 4.54, P = 0.0340). Corymbia calophylla had a significantly smaller heartwood area than did E. marginata (F = 7.21, P = 0.0077) in high drought-vulnerability patches, but this relationship was not statistically significant in low drought-vulnerability patches (Fig. 4). The relationship between the heartwood area and DBH was strong for both species (Fig. 5). Eucalyptus marginata maintained more heartwood in smaller trees, but the relationship was similar between species in larger trees.
Sapwood to heartwood area ratio
A significant tree species effect was detected (F = 9.38, P = 0.0024). Corymbia calophylla had a much higher ratio than did E. marginata (2.4 vs 0.8). On average, C. calophylla had more sapwood than heartwood, whereas E. marginata had more heartwood than sapwood. Drought vulnerability was not statistically significant in the linear mixed model (F = 0.22, P = 0.6373).
Discussion
This study has shown differences in certain stem functional traits between co-occurring tree species in a forest considered to have high and low vulnerability to drought. The observed differences in sapwood are perhaps most applicable in terms of drought responses in trees, because sapwood is an essential component of hydraulic infrastructure. Corymbia calophylla had a greater sapwood area than did E. marginata and was observed to be more resistant to die-off than was E. marginata (Matusick et al. 2013; Ruthrof et al. 2015). Sapwood can function as a significant water-storage organ (i.e. hydraulic capacitance; Goldstein et al. 1998; Köcher et al. 2013), including in Eucalyptus (Pfautsch and Adams 2013), and represents a critical water source during dry periods (Stout and Sala 2003; Hartzell et al. 2017; Salomón et al. 2017). These findings, combined with a greater stomatal control in C. calophylla (Szota et al. 2011), suggest sapwood storage and stomatal control combine to enhance C. calophylla resistance to drought, compared with E. marginata (Ruthrof et al. 2015).
The two species in the current study have some similar, and some disparate, ecological characteristics. For example, C. calophylla flowers on a 3- to 5-year cycle (Robinson 1960) and E. marginata flowers intermittently every 4–6 years (Abbott and Loneragan 1986; Abbott et al. 1989). Corymbia calophylla has much larger fruit (29.6 mm width, cf. 12.8 mm for E. marginata; Gill et al. 1992), a higher number of seeds per fruit (3.1 cf. 0.8 seeds per fruit respectively; Gill et al. 1992), and unusually large seeds (94 mg cf. 12 mg respectively; McChesney et al. 1995), compared with E. marginata because. The size of fruit and seed may be driven by predation pressure, C. calophylla is a favoured food source by three cockatoo species (Biggs et al. 2011; Lee et al. 2013). Both of these serotinous species also invest in a bank of suppressed, lignotuberous seedlings (Schuster 1980), which grow rapidly (∼1 m/year) once a disturbance occurs, and canopy competition is decreased (Ward and Koch 1995). The survival rate of these seedlings is considerably higher in C. calophylla than in E. marginata (Abbott 1984). Wood density for the study species is similar, at 0.67 and 0.65 t/m3 respectively (Forest Products Commission 2020), which is known to explain drought response (Hoffmann et al. 2011). The physiological response to water deficit is among the biggest differences between the species. Szota et al. (2011) showed that although E. marginata showed a high tolerance to dehydration and was able to survive drought via resprouting C. calophylla had higher drought tolerance (via lower stomatal conductance, higher leaf osmotic potential and leaf water content). Observations made following the response of the species to the 2011 die-off event support the findings by Szota et al. (2011). They collectively have implications for the species under a drier future. Pfautsch and Adams (2013) highlighted the importance of stem water storage in the survival of E. regnans during hot and dry conditions, including the importance of refilling depleted stem water stores during the night. Under the expected drier and hotter future in south-western Australia (Andrys et al. 2017), the capacity of Eucalyptus species to refill stem water and access stored water will be critical for determining competitive relationships in natural ecosystems. If the increased sapwood area in C. calophylla proves to represent a greater opportunity to store and access water, we would expect C. calophylla to have a competitive advantage over E. marginata under both short, high-intensity heat and drought events and protracted periods of water deficits. Specifically, increased survival and a high probability of maintaining a dominant stem would advantage C. calophylla in re-populating forest patches experiencing die-off, particularly given the reproductive characteristics mentioned previously. Future work should conclusively determine the role of sapwood and stem water storage in the increased drought resistance of C. calophylla and search for evidence of compositional shifts driven by repeated die-off events to assist predictions of future climate changes.
Sapwood areas were greater, regardless of tree species, in forest with a low vulnerability to drought-induced die-off. In the absence of other measurements, it remains unclear what this finding means. Measurement of leaf area would enable calculation of the Huber value, which describes the relationship between leaf area and sapwood area (Tyree and Ewers 1991) and is an indicator of functional adaptation to environmental conditions in trees (Gotsch et al. 2010). Although we cannot directly measure the Huber value here, we can infer that those forests highly vulnerable to drought had maintained less leaf area in advance of the drought than had forests less vulnerable from indirect evidence, including the fact that this high drought-vulnerable forest had experienced die-off in the recent past, had higher levels of recent tree mortality and partial crown die-off (Matusick et al. 2013). Indeed, Eucalyptus adjusts their Huber value to account for differences with water supply to maintain homeostasis (Carter and White 2009; Zolfaghar et al. 2015). Lower levels of leaf area in a drought-prone forest may explain the observed differences in sapwood area, because less sapwood is required to meet transpirational demands in trees with smaller tree crowns and fewer leaves (Paine et al. 1990). However, the exact relationship between Huber value and forest vulnerability to die-off in the NJF has yet to be determined.
The findings presented here show that the relationship between DBH and sapwood area changed with tree size. Small trees contained greater sapwood areas in high-drought patches, whereas larger trees contained greater sapwood areas in low-drought patches. These results may reflect leaf area adjustments to water availability (Pook 1985), in relation to competitive position (Crous et al. 2021), or simply the effects of age differences (Roberts et al. 2001). In high drought-vulnerable forest, repeated die-off events have caused retraction in overstorey trees, tree mortality, and vigorous re-sprouting and growth of small stems (Matusick et al. 2013). This exchange of growth between large trees and small, young stems is also likely to be reflected in relative leaf area changes, where proportional leaf area has shifted from large trees to small trees. With a greater leaf area per unit DBH in smaller trees, than in larger trees, more sapwood area would be required to satisfy transpirational demands (assuming similar sap velocities; Köstner et al. 2002). Because low drought-vulnerability sites did not experience overstorey die-off, large trees remained dominant and small trees have remained suppressed (with potentially a lower leaf area:DBH ratio), explaining why proportional sapwood areas are greater in larger trees in low-drought patches. Alternatively, our findings are the result of an age-related phenomenon. Small stems in high drought-vulnerable patches are likely to be younger than are small stems in low drought-vulnerable patches, owing to recruitment opportunities following recent die-off events. If this is true, we might expect higher sapwood areas per unit DBH in younger stems, because proportional sapwood naturally decreases with age as heartwood increases (Santos et al. 2021).
How functional traits, such as sapwood and heartwood areas, interact with drought and heatwave events has important consequences for stand function, particularly for water use and vulnerability to future drought events (Greenwood and Weisberg 2008). In the water-limited NJF, stand structural characteristics and the associated amounts of sapwood and heartwood are important regulators of stand water use (Macfarlane et al. 2010). Specifically, large trees contain greater quantities of non-water-conducting heartwood than do smaller trees, and site water use in the NJF can be attributed to these structural attributes (compared with sap velocity) (Macfarlane et al. 2010). Therefore, the replacement of large trees with higher densities of smaller trees (containing much less heartwood), as is seen following bauxite mining (Grigg and Grant 2009) and drought-induced die-off (Matusick et al. 2016), is likely to alter site water use by the forest canopy. In a drying climate, increasing site water use from disturbance-induced structural shifts may interact to result in repeated tree die-off and mortality events.
Since heartwood formation is an active process, trees may regulate the amount of sapwood by altering the rate of heartwood formation (Spicer 2005). Heartwood area, sapwood area, and transpirational demands are inherently linked, and some have postulated that drought and associated cavitation of the inner sapwood precedes and facilitates heartwood formation (Beauchamp et al. 2013; Chan et al. 2013). Therefore, heartwood formation can be a function of age (Santos et al. 2021), climate (Almeida et al. 2020), growth resources (Miranda et al. 2006, 2009), site quality (Climent et al. 1993), and stress (Paine et al. 1990). However, in this study, we found no differences in heartwood area between high and low drought-vulnerable forest. Instead, we found differences in heartwood area between competing species, with E. marginata having greater quantities of heartwood in smaller trees. Inherent genetic differences in rates of heartwood formation are common among Eucalyptus (Miranda et al. 2014). However, since trees were not aged, it remains unclear whether smaller E. marginata trees were of ages similar to those of similar-sized C. calophylla trees. Eucalyptus marginata can arrest its growth and development and persist as saplings for many years under intense competition (Abbott et al. 1989). Because age is an important determinant of heartwood quantity (Climent et al. 2002), this potential confounding variable needs to be understood prior to making conclusions regarding tissue allocation.
Bark thickness in eucalypt forests and woodlands is a function of stem diameter and confers greater resistance to fire damage (Wesolowski et al. 2014; Schubert et al. 2016), which is frequent in many eucalypt-dominated ecosystems (Murphy et al. 2013). It is therefore not surprising that co-occurring E. marginata and C. calophylla have similar bark areas when controlling for DBH, because they experience identical frequent fire regimes comingling in the upland NJF. Our findings suggest that smaller E. marginata trees have more bark than do C. calophylla trees, which may confer greater fire protection during the early stages of stand development.
This study has provided further information about three stem functional traits in C. calophylla and E. marginata in a forest with high and low vulnerability to drought; however, making strong conclusions regarding the role of these traits requires additional measurements to add appropriate physiological context. Examining individual functional traits in isolation is unlikely to explain the overall response of a species to drought. A suite of additional measurements on functional traits are needed to explain differences in species responses to drought. These include tree age, sapwood:leaf area ratio, and vulnerability to cavitation because it relates to sapwood:leaf area ratio, specifically at drought-vulnerable sites. Sapwood capacitance is not only a function of sapwood area, but also, critically, wood anatomy and density, which is commonly found to be associated with drought responses in trees (Hoffmann et al. 2011). This study does not infer how much sapwood is active within the trees, nor sap flow rates, which are critical for examining water use among forest patches (Doody et al. 2015). Additional information regarding natural embolism rates or sap flow rates could better estimate water use on drought-vulnerable sites. Further research encompassing wood density, stomatal conductance, rooting depth, water age and differences between species and how they relate to drought vulnerability is required. Importantly, these measurements should be undertaken on drought-vulnerable sites with limited connection to groundwater, which can buffer interseasonal and interannual surface soil dryness.
In conclusion, our study has shown that the two co-occurring canopy species in the NJF have different sapwood and heartwood areas. Specifically, C. calophylla had a larger sapwood area and a smaller heartwood area than did E. marginata (in high-drought patches), which may help explain observations of differential species responses to drought. Regression equations developed in this study may be helpful for projecting quantities of sapwood and heartwood across forest patches, which may be required for future modelling exercises and understanding of the spatial and temporal vulnerability of the NJF. This study has highlighted important and fruitful research pathways, investigating the differences among the co-occurring species to inform predictions regarding future forest composition. Greater knowledge about the responses to drought by co-occurring forest tree species will increase our predictive ability regarding forest responses in a drying and warming climate.
Data availability
The data will be made available upon request to the main author.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research was conducted as part of the Western Australian State Centre of Excellence for Climate Change Woodland and Forest Health, which is a partnership between private industry, community groups, Universities, and the Government of Western Australia.
Acknowledgements
We thank C. Marbus and Dr J. Fontaine (Murdoch University) for technical support.
References
Abbott I (1984) Emergence, early survival, and growth of seedlings of six tree species in Mediterranean forest of Western Australia. Forest Ecology and Management 9, 51–66.| Emergence, early survival, and growth of seedlings of six tree species in Mediterranean forest of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Abbott I, Loneragan O (1986) ‘Ecology of jarrah (Eucalyptus marginata) in the northern jarrah forest of Western Australia’, (Department of Conservation and Land Management: WA, Australia)
Abbott I, Dell D, Loneragan O (1989) The jarrah plant. In ‘The jarrah forest’. (Eds B Dell, JJ Havel, N Malajczuk) pp. 41–51. (Kluwer Academic Publishers: Dordrecht, Netherlands)
Adams HD, Guardiola-Claramonte M, Barron-Gafford GA, Villegas JC, Breshears DD, Zou CB, Troch PA, Huxman TE (2009) Temperature sensitivity of drought-induced tree mortality portends increased regional die-off under global-change-type drought. Proceedings of the National Academy of Sciences 106, 7063–7066.
| Temperature sensitivity of drought-induced tree mortality portends increased regional die-off under global-change-type drought.Crossref | GoogleScholarGoogle Scholar |
Allen CD, Macalady AK, Chenchouni H, Bachelet D, McDowell N, Vennetier M, Kitzberger T, Rigling A, Breshears DD, Hogg EH, Gonzalez P, Fensham R, Zhang Z, Castro J, Demidova N, Lim J-H, Allard G, Running SW, Semerci A, Cobb N (2010) A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Forest Ecology and Management 259, 660–684.
| A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests.Crossref | GoogleScholarGoogle Scholar |
Allen CD, Breshears DD, McDowell NG (2015) On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 6, 1–55.
| On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene.Crossref | GoogleScholarGoogle Scholar |
Almeida MNFd, Vidaurre GB, Pezzopane JEM, Lousada JLPC, Silva MECM, Câmara AP, Rocha SMG, de Oliveira JCL, Campoe OC, Carneiro RL, Alvares CA, Tomazzelo-Filho M, de Figueiredo FdM, Oliveira RF (2020) Heartwood variation of Eucalyptus urophylla is influenced by climatic conditions. Forest Ecology and Management 458, 117743
| Heartwood variation of Eucalyptus urophylla is influenced by climatic conditions.Crossref | GoogleScholarGoogle Scholar |
Andrew ME, Ruthrof KX, Matusick G, Hardy GESJ (2016) Spatial configuration of drought disturbance and forest gap creation across environmental gradients. PLoS ONE 11, e0157154
| Spatial configuration of drought disturbance and forest gap creation across environmental gradients.Crossref | GoogleScholarGoogle Scholar | 27275744PubMed |
Andrys J, Kala J, Lyons TJ (2017) Regional climate projections of mean and extreme climate for the southwest of Western Australia (1970–1999 compared to 2030–2059). Climate Dynamics 48, 1723–1747.
| Regional climate projections of mean and extreme climate for the southwest of Western Australia (1970–1999 compared to 2030–2059).Crossref | GoogleScholarGoogle Scholar |
Beauchamp K, Mencuccini M, Perks M, Gardiner B (2013) The regulation of sapwood area, water transport and heartwood formation in Sitka spruce. Plant Ecology & Diversity 6, 45–56.
| The regulation of sapwood area, water transport and heartwood formation in Sitka spruce.Crossref | GoogleScholarGoogle Scholar |
Biggs EK, Finn HC, Taplin RH, Calver MC (2011) Landscape position predicts distribution of eucalypts feed trees for threatened black-cockatoos in the northern jarrah forest, Western Australia. Journal of the Royal Society of Western Australia 94, 541–548.
Bowman DMJS, Kolden CA, Abatzoglou JT, Johnston FH, van der Werf GR, Flannigan M (2020) Vegetation fires in the Anthropocene. Nature Reviews Earth & Environment 1, 500–515.
| Vegetation fires in the Anthropocene.Crossref | GoogleScholarGoogle Scholar |
Breshears DD, Myers OB, Meyer CW, Barnes F J, Zou CB, Allen CD, McDowell NG, Pockman WT (2009) Tree die-off in response to global change-type drought: mortality insights from a decade of plant water potential measurements. Frontiers in Ecology and the Environment 7, 185–189.
| Tree die-off in response to global change-type drought: mortality insights from a decade of plant water potential measurements.Crossref | GoogleScholarGoogle Scholar |
Breshears DD, Fontaine JB, Ruthrof KX, Field JP, Feng X, Burger JR, Law DJ, Kala J, Hardy GESJ (2021) Underappreciated plant vulnerabilities to heat waves. New Phytologist 231, 32–39.
| Underappreciated plant vulnerabilities to heat waves.Crossref | GoogleScholarGoogle Scholar |
Brouwers N, Matusick G, Ruthrof K, Lyons T, Hardy G (2013) Landscape-scale assessment of tree crown dieback following extreme drought and heat in a Mediterranean eucalypt forest ecosystem. Landscape Ecology 28, 69–80.
| Landscape-scale assessment of tree crown dieback following extreme drought and heat in a Mediterranean eucalypt forest ecosystem.Crossref | GoogleScholarGoogle Scholar |
Cai W, van Rensch P, Borlace S, Cowan T (2011) Does the Southern Annular Mode contribute to the persistence of the multidecade-long drought over southwest Western Australia? Geophysical Research Letters 38, L14712
| Does the Southern Annular Mode contribute to the persistence of the multidecade-long drought over southwest Western Australia?Crossref | GoogleScholarGoogle Scholar |
Carter JL, White DA (2009) Plasticity in the Huber value contributes to homeostasis in leaf water relations of a mallee Eucalypt with variation to groundwater depth. Tree Physiology 29, 1407–1418.
| Plasticity in the Huber value contributes to homeostasis in leaf water relations of a mallee Eucalypt with variation to groundwater depth.Crossref | GoogleScholarGoogle Scholar | 19797243PubMed |
Chan JM, Raymond CA, Walker JCF (2013) Development of heartwood in response to water stress for radiata pine in southern New South Wales, Australia. Trees 27, 607–617.
| Development of heartwood in response to water stress for radiata pine in southern New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Chen I-C, Hill JK, Ohlemüller R, Roy DB, Thomas CD (2011) Rapid range shifts of species associated with high levels of climate warming. Science 333, 1024–1026.
| Rapid range shifts of species associated with high levels of climate warming.Crossref | GoogleScholarGoogle Scholar | 21852500PubMed |
Churchward HM, Dimmock GM (1989) The soils and landforms of the northern jarrah forest. In ‘The jarrah forest: a complex Mediterranean ecosystem’. (Eds B Dell, JJ Havel, N Malajczuk) pp. 13–21. (Kluwer Academic Publishers: Dordrecht, Netherlands)
Cinnirella S, Magnani F, Saracino A, Borghetti M (2002) Response of a mature Pinus laricio plantation to a three-year restriction of water supply: structural and functional acclimation to drought. Tree Physiology 22, 21–30.
| Response of a mature Pinus laricio plantation to a three-year restriction of water supply: structural and functional acclimation to drought.Crossref | GoogleScholarGoogle Scholar | 11772552PubMed |
Climent J, Gil L, Pardos J (1993) Heartwood and sapwood development and its relationship to growth and environment in Pinus canariensis Chr.Sm ex DC. Forest Ecology and Management 59, 165–174.
| Heartwood and sapwood development and its relationship to growth and environment in Pinus canariensis Chr.Sm ex DC.Crossref | GoogleScholarGoogle Scholar |
Climent J, Chambel MR, Pérez E, Gil L, Pardos J (2002) Relationship between heartwood radius and early radial growth, tree age, and climate in Pinus canariensis. Canadian Journal of Forest Research 32, 103–111.
| Relationship between heartwood radius and early radial growth, tree age, and climate in Pinus canariensis.Crossref | GoogleScholarGoogle Scholar |
Condit R, Hubbell SP, Foster RB (1996) Assessing the response of plant functional types to climatic change in tropical forests. Journal of Vegetation Science 7, 405–416.
| Assessing the response of plant functional types to climatic change in tropical forests.Crossref | GoogleScholarGoogle Scholar |
Crous KY, Campany C, López R, Cano FJ, Ellsworth DS (2021) Canopy position affects photosynthesis and anatomy in mature Eucalyptus trees in elevated CO2. Tree Physiology 41, 206–222.
| Canopy position affects photosynthesis and anatomy in mature Eucalyptus trees in elevated CO2.Crossref | GoogleScholarGoogle Scholar |
Dell B, Havel JJ (1989) The jarrah forest, an introduction. In ‘The jarrah forest: a complex Mediterranean ecosystem’. (Eds B Dell, JJ Havel, N Malajczuk) pp. 1–10. (Kluwer Academic Publishers: Dordrecht, Netherlands)
Doody TM, Colloff MJ, Davies M, Koul V, Benyon RG, Nagler PL (2015) Quantifying water requirements of riparian river red gum (Eucalyptus camaldulensis) in the Murray-Darling Basin, Australia – implications for the management of environmental flows. Ecohydrology 8, 1471–1487.
| Quantifying water requirements of riparian river red gum (Eucalyptus camaldulensis) in the Murray-Darling Basin, Australia – implications for the management of environmental flows.Crossref | GoogleScholarGoogle Scholar |
Farrington P, Turner JV, Gailitis V (1996) Tracing water uptake by jarrah (Eucalyptus marginata) trees using natural abundances of deuterium. Trees 11, 9–15.
| Tracing water uptake by jarrah (Eucalyptus marginata) trees using natural abundances of deuterium.Crossref | GoogleScholarGoogle Scholar |
Fauset S, Baker TR, Lewis SL, Feldpausch TR, Affum-Baffoe K, Foli EG, Hamer KC, Swaine MD (2012) Drought-induced shifts in the floristic and functional composition of tropical forests in Ghana. Ecology Letters 15, 1120–1129.
| Drought-induced shifts in the floristic and functional composition of tropical forests in Ghana.Crossref | GoogleScholarGoogle Scholar | 22812661PubMed |
Fensham RJ, Holman JE (1999) Temporal and spatial patterns in drought-related tree dieback in Australian savanna. Journal of Applied Ecology 36, 1035–1050.
| Temporal and spatial patterns in drought-related tree dieback in Australian savanna.Crossref | GoogleScholarGoogle Scholar |
Forest Products Commission (2020) Timber species from the South West of Western Australia. (Forest Products Commission, The Government of Western Australia: Perth, WA, Australia) Available at https://www.wa.gov.au/sites/default/files/2020-08/SW-timber-poster_700x500.pdf [Accessed 25 October 2021]
Gentilli J (1989) Climate of the jarrah forest. In ‘The jarrah forest: a complex mediterranean ecosystem’. (Eds B Dell, JJ Havel, N Malajczuk) pp. 23–40. (Kluwer Academic Publishers: Dordrecht, Netherlands)
Gill AM, Brooker MIH, Moore PHR (1992) Seed weights and numbers as a function of fruit size and subgenus in some Eucalyptus species from south-western Australia. Australian Journal of Botany 40, 103–111.
| Seed weights and numbers as a function of fruit size and subgenus in some Eucalyptus species from south-western Australia.Crossref | GoogleScholarGoogle Scholar |
Gioia P, Hopper SD (2017) A new phytogeographic map for the Southwest Australian Floristic Region after an exceptional decade of collection and discovery. Botanical Journal of the Linnean Society 184, 1–15.
| A new phytogeographic map for the Southwest Australian Floristic Region after an exceptional decade of collection and discovery.Crossref | GoogleScholarGoogle Scholar |
Goldstein G, Andrade JL, Meinzer FC, Holbrook NM, Cavelier J, Jackson P, Celis A (1998) Stem water storage and diurnal patterns of water use in tropical forest canopy trees. Plant, Cell & Environment 21, 397–406.
| Stem water storage and diurnal patterns of water use in tropical forest canopy trees.Crossref | GoogleScholarGoogle Scholar |
Gotsch SG, Geiger EL, Franco AC, Goldstein G, Meinzer FC, Hoffmann WA (2010) Allocation to leaf area and sapwood area affects water relations of co-occurring savanna and forest trees. Oecologia 163, 291–301.
| Allocation to leaf area and sapwood area affects water relations of co-occurring savanna and forest trees.Crossref | GoogleScholarGoogle Scholar | 20058025PubMed |
Goulden ML, Bales RC (2019) California forest die-off linked to multi-year deep soil drying in 2012–2015 drought. Nature Geoscience 12, 632–637.
| California forest die-off linked to multi-year deep soil drying in 2012–2015 drought.Crossref | GoogleScholarGoogle Scholar |
Greenwood DL, Weisberg PJ (2008) Density-dependent tree mortality in pinyon–juniper woodlands. Forest Ecology and Management 255, 2129–2137.
| Density-dependent tree mortality in pinyon–juniper woodlands.Crossref | GoogleScholarGoogle Scholar |
Grigg AH, Grant CD (2009) Overstorey growth response to thinning, burning and fertiliser in 10–13-year-old rehabilitated jarrah (Eucalyptus marginata) forest after bauxite mining in south-western Australia. Australian Forestry 72, 80–86.
| Overstorey growth response to thinning, burning and fertiliser in 10–13-year-old rehabilitated jarrah (Eucalyptus marginata) forest after bauxite mining in south-western Australia.Crossref | GoogleScholarGoogle Scholar |
Hartzell S, Bartlett MS, Porporato A (2017) The role of plant water storage and hydraulic strategies in relation to soil moisture availability. Plant and Soil 419, 503–521.
| The role of plant water storage and hydraulic strategies in relation to soil moisture availability.Crossref | GoogleScholarGoogle Scholar |
Hoerling M, Eischeid J, Perlwitz J, Quan X, Zhang T, Pegion P (2012) On the increased frequency of Mediterranean drought. Journal of Climate 25, 2146–2161.
| On the increased frequency of Mediterranean drought.Crossref | GoogleScholarGoogle Scholar |
Hoffmann AA, Sgrò CM (2011) Climate change and evolutionary adaptation. Nature 470, 479–485.
| Climate change and evolutionary adaptation.Crossref | GoogleScholarGoogle Scholar | 21350480PubMed |
Hoffmann WA, Marchin RM, Abit P, Lau OL (2011) Hydraulic failure and tree dieback are associated with high wood density in a temperate forest under extreme drought. Global Change Biology 17, 2731–2742.
| Hydraulic failure and tree dieback are associated with high wood density in a temperate forest under extreme drought.Crossref | GoogleScholarGoogle Scholar |
Hu G, Liu H, Shangguan H, Wu X, Xu X, Williams M (2018) The role of heartwood water storage for sem-arid trees under drought. Agricultural and Forest Meteorology 256–257, 534–541.
| The role of heartwood water storage for sem-arid trees under drought.Crossref | GoogleScholarGoogle Scholar |
Köcher P, Horna V, Leuschner C (2013) Stem water storage in five coexisting temperate broad-leaved tree species: significance, temporal dynamics and dependence on tree functional traits. Tree Physiology 33, 817–832.
| Stem water storage in five coexisting temperate broad-leaved tree species: significance, temporal dynamics and dependence on tree functional traits.Crossref | GoogleScholarGoogle Scholar | 23999137PubMed |
Köstner B, Falge E, Tenhunen JD (2002) Age-related effects on leaf area/sapwood area relationships, canopy transpiration and carbon gain of Norway spruce stands (Picea abies) in the Fichtelgebirge, Germany. Tree Physiology 22, 567–574.
| Age-related effects on leaf area/sapwood area relationships, canopy transpiration and carbon gain of Norway spruce stands (Picea abies) in the Fichtelgebirge, Germany.Crossref | GoogleScholarGoogle Scholar | 12045028PubMed |
Lawes MJ, Woolley L-A, Van Holsbeeck S, Murphy BP, Burrows GE, Midgley JJ (2021) Bark functional ecology and its influence on the distribution of Australian half-butt eucalypts. Austral Ecology 46, 1097–1111.
| Bark functional ecology and its influence on the distribution of Australian half-butt eucalypts.Crossref | GoogleScholarGoogle Scholar |
Lee J, Finn H, Calver M (2013) Feeding activity of threatened black cockatoos in mine-site rehabilitation in the jarrah forest of south-western Australia. Australian Journal of Zoology 61, 119–131.
| Feeding activity of threatened black cockatoos in mine-site rehabilitation in the jarrah forest of south-western Australia.Crossref | GoogleScholarGoogle Scholar |
Linton MJ, Sperry JS, Williams DG (1998) Limits to water transport in Juniperus osteosperma and Pinus edulis: implications for drought tolerance and regulation of transpiration. Functional Ecology 12, 906–911.
| Limits to water transport in Juniperus osteosperma and Pinus edulis: implications for drought tolerance and regulation of transpiration.Crossref | GoogleScholarGoogle Scholar |
Lopez-Iglesias B, Villar R, Poorter L (2014) Functional traits predict drought performance and distribution of Mediterranean woody species. Acta Oecologica 56, 10–18.
| Functional traits predict drought performance and distribution of Mediterranean woody species.Crossref | GoogleScholarGoogle Scholar |
Macfarlane C, Bond C, White DA, Grigg AH, Ogden GN, Silberstein R (2010) Transpiration and hydraulic traits of old and regrowth eucalypt forest in southwestern Australia. Forest Ecology and Management 260, 96–105.
| Transpiration and hydraulic traits of old and regrowth eucalypt forest in southwestern Australia.Crossref | GoogleScholarGoogle Scholar |
Markesteijn L, Poorter L, Paz H, Sack L, Bongers F (2011) Ecological differentiation in xylem cavitation resistance is associated with stem and leaf structural traits. Plant, Cell & Environment 34, 137–148.
| Ecological differentiation in xylem cavitation resistance is associated with stem and leaf structural traits.Crossref | GoogleScholarGoogle Scholar |
Martínez-Vilalta J, Cochard H, Mencuccini M, Sterck F, Herrero A, Korhonen JFJ, Llorens P, Nikinmaa E, Nolè A, Poyatos R, Ripullone F, Sass-Klaassen U, Zweifel R (2009) Hydraulic adjustment of Scots pine across Europe. New Phytologist 184, 353–364.
| Hydraulic adjustment of Scots pine across Europe.Crossref | GoogleScholarGoogle Scholar |
Matusick G, Ruthrof KX, Brouwers NC, Dell B, Hardy GESJ (2013) Sudden forest canopy collapse corresponding with extreme drought and heat in a Mediterranean-type eucalypt forest in southwestern Australia. European Journal of Forest Research 132, 497–510.
| Sudden forest canopy collapse corresponding with extreme drought and heat in a Mediterranean-type eucalypt forest in southwestern Australia.Crossref | GoogleScholarGoogle Scholar |
Matusick G, Ruthrof KX, Fontaine JB, Hardy GESJ (2016) Eucalyptus forest shows low structural resistance and resilience to climate change-type drought. Journal of Vegetation Science 27, 493–503.
| Eucalyptus forest shows low structural resistance and resilience to climate change-type drought.Crossref | GoogleScholarGoogle Scholar |
McChesney CJ, Koch JM, Bell DT (1995) Jarrah forest restoration in Western Australia: canopy and topographic effects. Restoration Ecology 3, 105–110.
| Jarrah forest restoration in Western Australia: canopy and topographic effects.Crossref | GoogleScholarGoogle Scholar |
Miranda I, Gominho J, Lourenço A, Pereira H (2006) The influence of irrigation and fertilization on heartwood and sapwood contents in 18-year-old Eucalyptus globulus trees. Canadian Journal of Forest Research 36, 2675–2683.
| The influence of irrigation and fertilization on heartwood and sapwood contents in 18-year-old Eucalyptus globulus trees.Crossref | GoogleScholarGoogle Scholar |
Miranda I, Gominho J, Pereira H (2009) Variation of heartwood and sapwood in 18-year-old Eucalyptus globulus trees grown with different spacings. Trees 23, 367–372.
| Variation of heartwood and sapwood in 18-year-old Eucalyptus globulus trees grown with different spacings.Crossref | GoogleScholarGoogle Scholar |
Miranda I, Gominho J, Araújo C, Pereira H (2014) Family effects in heartwood content of Eucalyptus globulus L. European Journal of Forest Research 133, 81–87.
| Family effects in heartwood content of Eucalyptus globulus L.Crossref | GoogleScholarGoogle Scholar |
Mitchell PJ, O’Grady AP, Hayes KR, Pinkard EA (2014) Exposure of trees to drought-induced die-off is defined by a common climatic threshold across different vegetation types. Ecology and Evolution 4, 1088–1101.
| Exposure of trees to drought-induced die-off is defined by a common climatic threshold across different vegetation types.Crossref | GoogleScholarGoogle Scholar | 24772285PubMed |
Mueller RC, Scudder CM, Porter ME, et al. (2005) Differential tree mortality in response to severe drought: evidence for long-term vegetation shifts. Journal of Ecology 93, 1085–1093.
| Differential tree mortality in response to severe drought: evidence for long-term vegetation shifts.Crossref | GoogleScholarGoogle Scholar |
Murphy BP, Bradstock RA, Boer MM, Carter J, Cary GJ, Cochrane MA, Fensham RI, Russell-Smith J, Williamson GJ, Bowman DMJS (2013) Fire regimes of Australia: a pyrogeographic model system. Journal of Biogeography 40, 1048–1058.
| Fire regimes of Australia: a pyrogeographic model system.Crossref | GoogleScholarGoogle Scholar |
Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403, 853–858.
| Biodiversity hotspots for conservation priorities.Crossref | GoogleScholarGoogle Scholar | 10706275PubMed |
Nolan RH, Rahmani S, Samson SA, Simpson-Southward HM, Boer MM, Bradstock RA (2020) Bark attributes determine variation in fire resistance in resprouting tree species. Forest Ecology and Management 474, 118385
| Bark attributes determine variation in fire resistance in resprouting tree species.Crossref | GoogleScholarGoogle Scholar |
Paine TD, Malinoski MK, Scriven GT (1990) Rating Eucalyptus vigor and the risk of insect infestation: leaf surface area and sapwood:heartwood ratio. Canadian Journal of Forest Research 20, 1485–1489.
| Rating Eucalyptus vigor and the risk of insect infestation: leaf surface area and sapwood:heartwood ratio.Crossref | GoogleScholarGoogle Scholar |
Pfautsch S, Adams MA (2013) Water flux of Eucalyptus regnans: defying summer drought and a record heatwave in 2009. Oecologia 172, 317–326.
| Water flux of Eucalyptus regnans: defying summer drought and a record heatwave in 2009.Crossref | GoogleScholarGoogle Scholar | 23070142PubMed |
Pook EW (1985) Canopy dynamics of Eucalyptus maculata Hook. III Effects of drought. Australian Journal of Botany 33, 65–79.
| Canopy dynamics of Eucalyptus maculata Hook. III Effects of drought.Crossref | GoogleScholarGoogle Scholar |
Pritzkow C, Szota C, Williamson V, Arndt SK (2021) Previous drought exposure leads to greater drought resistance in eucalypts through changes in morphology rather than physiology. Tree Physiology 41, 1186–1198.
| Previous drought exposure leads to greater drought resistance in eucalypts through changes in morphology rather than physiology.Crossref | GoogleScholarGoogle Scholar | 33530102PubMed |
Roberts S, Vertessy R, Grayson R (2001) Transpiration from Eucalyptus sieberi (L. Johnson) forests of different age. Forest Ecology and Management 143, 153–161.
| Transpiration from Eucalyptus sieberi (L. Johnson) forests of different age.Crossref | GoogleScholarGoogle Scholar |
Robinson A (1960) The importance of the marri as a food source to south-western Australian birds. The Western Australian Naturalist 7, 109–115.
Ruthrof KX, Matusick G, Hardy GEStJ (2015) Early differential responses of co-dominant canopy species to sudden and severe drought in a Mediterranean-climate type forest. Forests 6, 2082–2091.
| Early differential responses of co-dominant canopy species to sudden and severe drought in a Mediterranean-climate type forest.Crossref | GoogleScholarGoogle Scholar |
Ruthrof KX, Breshears DD, Fontaine JB, Froend RH, Matusick G, Kala J, Miller BP, Mitchell PJ, Wilson SK, van Keulen M, Enright NJ, Law DJ, Wernberg T, Hardy GESJ (2018) Subcontinental heat wave triggers terrestrial and marine, multi-taxa responses. Scientific Reports 8, 13094
| Subcontinental heat wave triggers terrestrial and marine, multi-taxa responses.Crossref | GoogleScholarGoogle Scholar | 30166559PubMed |
Ruthrof KX, Fontaine JB, Breshears DB, Field JP, Allen CD (2021) Extreme events trigger terrestrial and marine ecosystem collapses in the southwestern USA and southwestern Australia. In ‘Ecosystem collapse and climate change’. (Eds P Canadell, R Jackson) pp. 187–217. (Springer Nature: Switzerland)
Ryan MG, Yoder BJ (1997) Hydraulic limits to tree height and tree growth. BioScience 47, 235–242.
| Hydraulic limits to tree height and tree growth.Crossref | GoogleScholarGoogle Scholar |
Salomón RL, Limousin J-M, Ourcival J-M, Rodríguez-Calcerrada J, Steppe K (2017) Stem hydraulic capacitance decreases with drought stress: implications for modelling tree hydraulics in the Mediterranean oak Quercus ilex. Plant, Cell & Environment 40, 1379–1391.
| Stem hydraulic capacitance decreases with drought stress: implications for modelling tree hydraulics in the Mediterranean oak Quercus ilex.Crossref | GoogleScholarGoogle Scholar |
Santos LMH, Almeida MNFd, Silva JGMd, Vidaurre GB, Hein PRG, Silva GFd, Zanunxio AJV, Filho CVF, Campinhos EN, Mafia RG, Arantes MDC, Tomazello-Filho M, Oliveira MP, Rocha QS, Minini D, Melo ABd, Amorim AG (2021) Variations in heartwood formation and wood density as a function of age and plant spacing in a fast-growing eucalyptus plantation. Holzforschung 75, 979–988.
| Variations in heartwood formation and wood density as a function of age and plant spacing in a fast-growing eucalyptus plantation.Crossref | GoogleScholarGoogle Scholar |
Schofield NJ, Stoneman GL, Loh IC (1989) Hydrology of the jarrah forest. In ‘The jarrah forest: a complex mediterranean ecosystem’. (Eds B Dell, JJ Havel, N Malajczuk) pp. 179–201. (Kluwer Academic Publishers: Dordrecht, Netherlands)
Scholz FG, Phillips NG, Bucci SJ, Meinzer FC, Goldstein G (2011) Hydraulic capacitance: biophysics and functional significance of internal water sources in relation to tree size. In ‘Size- and age-related changes in tree structure and function’. (Eds FC Meinzer, B Lachenbruch, TE Dawson) pp. 341–361. (Springer: Dordrecht, Netherlands)
Schubert AT, Nano CEM, Clarke PJ, Lawes MJ (2016) Evidence for bark thickness as a fire-resistance trait from desert to savanna in fire-prone inland Australia. Plant Ecology 217, 683–696.
| Evidence for bark thickness as a fire-resistance trait from desert to savanna in fire-prone inland Australia.Crossref | GoogleScholarGoogle Scholar |
Schuster CJ (1980) ‘Regeneration in a jarrah (Eucalyptus marginata Sm.) and marri (E. calophylla R.Br.) forest following logging and burning’, (Forests Department of Western Australia)
Skelton RP, West AG, Dawson TE (2015) Predicting plant vulnerability to drought in biodiverse regions using functional traits. Proceedings of the National Academy of Sciences 112, 5744–5749.
| Predicting plant vulnerability to drought in biodiverse regions using functional traits.Crossref | GoogleScholarGoogle Scholar |
Specht RL, Roe ME, Boughton VH (1974) Conservation of major plant communities in Australia and Papua New Guinea. Australian Journal of Botany Supplementary Series 4, 1–667.
| Conservation of major plant communities in Australia and Papua New Guinea.Crossref | GoogleScholarGoogle Scholar |
Spicer R (2005) Senescence in secondary xylem: heartwood formation as an active development programme. In ‘Vascular transport in plants’. (Eds N Hollbrook, M Zwieniecki) pp. 457–475. (Elevier Academic Press: Burlington, MA, USA)
Steel EJ, Fontaine JB, Ruthrof KX, Burgess TI, Hardy GESJ (2019) Changes in structure of over- and midstory tree species in a Mediterranean-type forest after an extreme drought-associated heatwave. Austral Ecology 44, 1438–1450.
| Changes in structure of over- and midstory tree species in a Mediterranean-type forest after an extreme drought-associated heatwave.Crossref | GoogleScholarGoogle Scholar |
Stout DL, Sala A (2003) Xylem vulnerability to cavitation in Pseudotsuga menziesii and Pinus ponderosa from contrasting habitats. Tree Physiology 23, 43–50.
| Xylem vulnerability to cavitation in Pseudotsuga menziesii and Pinus ponderosa from contrasting habitats.Crossref | GoogleScholarGoogle Scholar |
Szota C, Farrell C, Koch JM, Lambers H, Veneklaas EJ (2011) Contrasting physiological responses of two co-occurring eucalypts to seasonal drought at restored bauxite mine sites. Tree Physiology 31, 1052–1066.
| Contrasting physiological responses of two co-occurring eucalypts to seasonal drought at restored bauxite mine sites.Crossref | GoogleScholarGoogle Scholar | 21908435PubMed |
Tyree MT, Ewers FW (1991) The hydraulic architecture of trees and other woody plants. New Phytologist 119, 345–360.
| The hydraulic architecture of trees and other woody plants.Crossref | GoogleScholarGoogle Scholar |
Ward SC, Koch JM (1995) Early growth of jarrah (Eucalyptus marginata Donn ex Smith) on rehabilitated bauxite minesites in south-west Australia. Australian Forestry 58, 65–71.
| Early growth of jarrah (Eucalyptus marginata Donn ex Smith) on rehabilitated bauxite minesites in south-west Australia.Crossref | GoogleScholarGoogle Scholar |
Wesolowski A, Adams MA, Pfautsch S (2014) Insulation capacity of three bark types of temperate Eucalyptus species. Forest Ecology and Management 313, 224–232.
| Insulation capacity of three bark types of temperate Eucalyptus species.Crossref | GoogleScholarGoogle Scholar |
Williams AP, Allen CD, Macalady AK, et al. (2013) Temperature as a potent driver of regional forest drought stress and tree mortality. Nature Climate Change 3, 292–297.
| Temperature as a potent driver of regional forest drought stress and tree mortality.Crossref | GoogleScholarGoogle Scholar |
Zeppel MJB, Harrison SP, Adams HD, Kelley DI, Li G, Tissue DT, Dawson TE, Fensham R, Medlyn BE, Palmer A, West AG, McDowell NG (2015) Drought and resprouting plants. New Phytologist 206, 583–589.
| Drought and resprouting plants.Crossref | GoogleScholarGoogle Scholar |
Zolfaghar S, Villalobos-Vega R, Zeppel M, Eamus D (2015) The hydraulic architecture of Eucalyptus trees growing across a gradient of depth-to-groundwater. Functional Plant Biology 42, 888–898.
| The hydraulic architecture of Eucalyptus trees growing across a gradient of depth-to-groundwater.Crossref | GoogleScholarGoogle Scholar | 32480731PubMed |


